Abstract
Oxidative stress is common in the whole process of broiler production, and breast muscle is one of the target organs most vulnerable to oxidative attack. When broilers are subjected to oxidative stress, the regulation of adenosine 5-monophosphate activated protein kinase (AMPK) is a critical path to maintain the dynamic balance of intracellular energy. However, whether calcium/calmodulin-dependent protein kinase (CaMKK) and liver kinase B1 (LKB1) are involved in the regulation of AMPK activation in broiler breast muscle under oxidative stress has not been elucidated. In this study, a total of 144 one-day-old male Ross 308 chicks were selected, with an average body weight of 43.44 ± 0.04 g. The broilers were divided into 3 groups with 6 replicates of 8 broilers each (control group, intraperitoneal injection of physiological saline group, and intraperitoneal injection of hydrogen peroxide [H2O2] group), the injection time was selected on the 16th and 37th day of the experimental period, the injection volumes were 1.0 mL/kg broiler body weight. The results of this experiment showed that H2O2 exposure reduced the average daily gain (ADG) and increased the feed to gain ratio (F/G), the level of corticosterone (CORT) and the activity of lactate dehydrogenase (LDH) in serum were increased after H2O2 exposure. H2O2 exposure also increased the contents of reactive oxygen species (ROS) and protein carbonyl, but decreased the activities of catalase (CAT), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) in breast muscle. After H2O2 exposure, the activity of pyruvate dehydrogenase (PDH) was decreased, the content of glycogen was reduced, and the contents of adenosine monophosphate (AMP) and lactate were increased in breast muscle. In addition, H2O2 exposure increased the content of Ca2+, upregulated the protein expression levels of CaMKK1 and p-AMPK, and increased the activities of hexokinase (HK) and LDH in breast muscle. These findings suggested that the activation of CaMKK/LKB1/AMPK signaling pathway would be associated with the accelerated glycolysis of broiler breast muscle under oxidative stress.
Key words: broiler, growth performance, hydrogen peroxide, glycolysis, adenosine 5-monophosphate activated protein kinase (AMPK) signaling pathway
INTRODUCTION
With the rapid development of the global economy and the improvement of living qualities of urban and rural residents, the demand for animal protein is increasing (Da Silva et al., 2017). Poultry meat is a high-quality animal protein and an essential source for maintaining human health and nutrition (Khan et al., 2019). With the intensive breeding and long-distance transportation, oxidative stress has become an increasingly severe and worsening problem in the global poultry industry (Guo et al., 2020). It is reported that oxidative stress leads to the decline of growth performance, deterioration of meat quality, and high mortality of broilers (Estevez, 2015). In addition, oxidative stress also decreases growth performance by impairing the normal physiological and metabolic processes of muscle (Zhang et al., 2011). However, the physiological and metabolic mechanism of oxidative stress impairs the growth performance of broilers remains unclear.
Oxidative stress induced by hydrogen peroxide (H2O2) reflects that excessive reactive oxygen species (ROS) attack the antioxidant defense system, leading to an imbalance in the redox system (Ahmad et al., 2012). When broilers are under oxidative stress, the supply of oxygen and nutrients would be weakened, and the energy supply mode of muscle cells would change to glycolysis (Barbut et al., 2008). The breast muscle of broilers is mainly composed of fast glycolytic muscle fibers, which are more vulnerable to oxidative stress (Wang et al., 2017). The enhancement of glycolysis leads to the continuous accumulation of lactate in muscle and the increase of muscle temperature. High carcass temperature at the early stage of postmortem, accompanied by the rapid decline of pH, would cause protein denaturation, further leading to the white color, tenderness, and water retention of meat (Kim et al., 2014).
In this condition, excessive ROS may change the structures of enzymes, affect the activities of enzymes, and cause damage to biomolecules (Betti et al., 2009). Previous studies reported that oxidative stress reduced the activities of crucial enzymes in the redox system in broiler breast muscle, resulted in the decrease of glycogen content and the increase of lactate level, and accelerated the glycolysis function after slaughter (Schieber and Chandel, 2014; Chen et al., 2017, 2020). ROS bind to amino acid residues of key glycolysis enzymes to form carbonyl functional group, which is an irreversible oxidative modification of protein known as carbonylation modification (Zheng and Bizzozero, 2010). Therefore, carbonylation modification may affect the activity or function of critical proteins in broiler muscles, regulate physiological metabolic processes, and affect growth performance.
Under the stimulation of adverse factors such as hypoxia, ischemia, and oxidative stress, adenosine 5-monophosphate activated protein kinase (AMPK) may be activated. The active AMPK inhibits anabolism and promotes catabolism by regulating its downstream target proteins to maintain the dynamic balance of intracellular energy (Xing et al., 2019). The regulation of AMPK on glycolytic metabolism is a critical way to promote catabolism. After AMPK activation, the capacity of glucose transport may be enhanced by upgrading the translocation of glucose transporter to the cell membrane. Lu et al. (2017) found that the activation of AMPK in broiler breast muscle would accelerate the process of glycolysis. In vivo, the activation of AMPK requires the phosphorylation of threonine 172 (thr172), which is directly regulated by the upstream kinases calcium/calmodulin-dependent protein kinase (CaMKK) and liver kinase B1 (LKB1) of AMPK (Carling, 2017). Zhang et al. (2017) found that transport stress could reduce the energy state in broiler muscle and accelerated glycolysis by promoting the phosphorylation of AMPK (thr172). However, whether CaMKK and LKB1 are involved in regulating AMPK activation in broiler muscle under oxidative stress has not been reported.
The purpose of this experiment was to explore the mechanism of oxidative stress induced by H2O2 promoting broiler glycolysis through CaMKK/LKB1/AMPK signaling pathway, which could provide scientific evidence for explaining the mechanism of glycolysis.
MATERIALS AND METHODS
Experimental Design and Broiler Management
The experimental animal management committee of Nanjing Agricultural University (Nanjing, P.R. China, GB/T 35892-2018) consulted the entire experimental process and experimental animals. A total of 144 one-day-old male Ross 308 chicks were selected, with an average body weight of 43.44 ± 0.04 g. The chicks were randomly divided into 3 treatments, 6 cages (replicates) for each treatment, and 8 chicks for each cage (cage size: 120 cm × 65 cm × 55 cm). All the chicks had free access to nutritional feed and clean water. In the control group, the broilers were not injected intraperitoneally; in the saline group, physiological saline (0.75%) were injected into the abdominal cavity of the broilers, the injection volumes were 1.0 mL/kg broiler body weight; in the H2O2 group, 10.0% H2O2 (Yonghua Chemical Co., Ltd, Suzhou, China) were injected into the abdominal cavity of broilers, the injection volumes were 1.0 mL/kg broiler body weight, and the H2O2 were dissolved in physiological saline. The basal diet in the entire experimental period met the nutrient requirements of Ross 308 broilers (Table 1). According to the previous research in our laboratory, the injection time was selected on the 16th and 37th day of the experimental period (Chen et al., 2017). The photoperiod and environmental temperature were set in strict accordance with the requirements of broiler welfare.
Table 1.
The composition and nutrient levels of the basal diets.
| 1 to 21 d | 22 to 42 d | |
|---|---|---|
| Ingredients (%) | ||
| Corn | 57.61 | 62.27 |
| Soybean meal | 31.00 | 23.00 |
| Corn gluten meala | 3.29 | 6.00 |
| Soybean oil | 3.11 | 4.00 |
| Limestone | 1.20 | 1.20 |
| Dicalcium phosphate | 2.00 | 2.00 |
| L-lysine | 0.34 | 0.35 |
| DL-methionine | 0.15 | 0.08 |
| Salt | 0.30 | 0.30 |
| Premixb | 1.00 | 1.00 |
| Calculated nutrient levels | ||
| MEc (MJ/kg) | 12.56 | 13.19 |
| CP (%) | 21.10 | 19.60 |
| Ca (%) | 1.00 | 0.95 |
| Available phosphorus (%) | 0.46 | 0.39 |
| Lysine (%) | 1.20 | 1.05 |
| Methionine (%) | 0.50 | 0.42 |
| Methionine + cysteine (%) | 0.85 | 0.76 |
| Analyzed nutrient levels | ||
| CP (%) | 20.84 | 19.23 |
| Ca (%) | 1.03 | 0.99 |
| Total phosphorus (%) | 0.64 | 0.61 |
The crude protein (CP) content was 60%.
Premix provided per kilogram of diet: vitamin A, 12,000 IU; cholecalciferol for vitamin D3, 2,500 IU; DL-α-tocopheryl acetate for vitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8.0 mg; nicotinamide, 40 mg; choline chloride, 400 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B 12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Abbreviation: ME, metabolizable energy.
Sample Collection
All the broilers were weighed on the 42nd day of the experimental period, recorded the broiler weight and feed consumption of each cage, a total of 18 cages, 2 broilers per cage (close to the average weight) were stunned by electric shock (50 V, alternating current, 400 Hz, 5 s), a total of 36 chicks were stunned, bloodletting was performed immediately after stunned by electric shock, blood samples were collected with centrifuge tubes, serum was obtained after centrifugation, and the pectoral muscles were collected by autopsy. Muscle slices were immediately frozen in liquid nitrogen. For the breast muscle fiber structure observation, sliced samples were fixed by polyformaldehyde solution.
Measurement of Carcass Traits
After complete bleeding of broilers, the whole body feathers were plucked, the dressing rate was calculated by dividing the de-feathered warm carcass weight by the live body weight. Removed the head, neck, feet and all internal organs of the broiler except the kidney and then weighed the carcass to calculate the rate of eviscerated yield. The rates of abdominal fat, breast muscles, and thigh muscles were calculated by dividing the weight of abdominal fat, bilateral breast muscles, and bilateral thigh muscles by the eviscerated weight respectively.
Analysis of Corticosterone, Creatine Kinase, and Lactate Dehydrogenase
The concentration of corticosterone (CORT), the activities of creatine kinase (CK), and lactate dehydrogenase (LDH) in serum were measured using commercial kits (Keygen Biotech, Co., Ltd, Nanjing, China). The method of enzyme linked immunosorbent assay (ELISA) was used to calculate the concentration of CORT in the sample by drawing the standard curve. The method of colorimetry was used to calculate the activity of CK. CK catalyzed adenosine triphosphate and creatine to produce creatine phosphate. Ammonium molybdate was added to produce phosphomolybdic acid and further reduced to molybdenum blue. The enzyme activity was calculated according to the amount of inorganic phosphorus. LDH catalyzed lactate to produce pyruvate, which reacted with 2,4-dinitrophenylhydrazine to produce pyruvate dinitrophenylhydrazone, which was brownish red in alkaline solution. The enzyme activity was calculated by colorimetry.
Measurement of Redox Status
The level of ROS was measured by the commercial kit (Keygen Biotech, Co., Ltd). The activities of catalase (CAT), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), glutathione peroxidase activity (GSH-Px), protein carbonyl level were determined using commercial kits (Keygen Biotech, Co., Ltd), following the manufacturer's instructions. The level of ROS was detected by 2,7-dichlorofluorescein diacetate (DCFH-DA) probe. The fluorescence value had the maximum peak at the excitation wavelength of 502 nm and the emission wavelength of 530 nm. The fluorescence intensity was directly proportional to the level of ROS. The reaction of CAT decomposing H2O2 was terminated by adding ammonium molybdate. The remaining H2O2 reacted with ammonium molybdate to produce a light yellow complex. The change of absorbance was measured at 405 nm and the activity of CAT was calculated. The superoxide anion radical in the sample oxidized hydroxylamine to form nitrite, which was purplish red under the action of chromogenic agent. The change of absorbance was measured at 550 nm and the activity of T-SOD was calculated. The antioxidant substances in the samples reduced Fe3+ to Fe2+. Fe2+ combined with phenanthrolines to form complexes. The activity of T-AOC was calculated by colorimetry. GSH-Px promoted the reaction between H2O2 and glutathione (GSH) to produce H2O and glutathione oxidized (GSSG). The activity of GSH-Px was determined by colorimetry. Protein carbonyl reacted with 2,4-dinitrophenylhydrazine to form red 2,4-dinitrophenylhydrazone, which had a characteristic absorption peak at 370 nm and the content of protein carbonyl was calculated by colorimetry.
Detection of Muscle Energy Metabolism Parameters
The contents of glycogen and lactate were performed using commercial kits (Nanjing Aoqing Biology Co., Ltd, Nanjing, China). The activities of Na+/K+-ATPase, Ca2+-ATPase, pyruvate dehydrogenase (PDH), aconitase (ACO), citrate synthase (CS), hexokinase (HK), pyruvate kinase (PK), phosphofructokinase (PFK), and lactate dehydrogenase (LDH) were evaluated using commercial kits (Nanjing Aoqing Biology Co., Ltd). Glycogen and lactate were determined by colorimetry. The contents of glycogen and lactate were calculated by standard curve. ATPase decomposed ATP to produce ADP and inorganic phosphorus. Determine the content of inorganic phosphorus to judge the activity of ATPase. The activities of PDH, ACO, CS, HK, PK, PFK, and LDH in breast muscle were determined by ultraviolet spectrophotometry.
Detection of Muscle Adenosine Phosphates
In this study, the method of measuring muscle adenosine phosphate referred to the method of Wang et al. (2017). The concentrations of pectoral muscle adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) were determined by high-performance liquid chromatography. 0.2 g frozen breast muscle sample was homogenized in 1.0 mL of precooled 7% perchloric acid, and then centrifuged at 15,000 g for 15 min at 4°C. The supernatant was neutralized with 1.03 M KOH and centrifuged at 15,000 g for 15 min at 4°C. After filtration with a 0.45 μm membrane, 10 μL sample solution was injected into the Waters-2695 Alliance HPLC system (Waters Corp., Milford, MA). Chromatographic analysis was performed on the Waters SunFire C18 column at 30°C. The mobile phase was methanol-phosphate buffer (14:86, volume ratio), the flow rate was 1 mL/min, and the UV detection wavelength was 254 nm.
Determination of Ca2+ Concentration
The concentration of Ca2+ was determined using the modified method of Parrish et al. (1981). The 2 g frozen breast muscle was homogenized with 10 mL cold solution (2 mM ATP and MgCl2, pH 7.0) at 11,000 rpm for 1 min. The supernatants were removed, 2 mL 5% trichloroacetic acid (TCA) and 0.5% strontium chloride mixed solution were added, centrifuged at 1,600 g for 15 min. Finally, the concentration of Ca2+ in the extracted supernatants was determined by atomic absorption spectrometry (170-10, Hitachi Co., Japan).
Hematoxylin and Eosin Analysis and Nuclear Factor Erythroid 2-Related Factor 2 Immunohistochemistry
Pectoral muscle specimens were preserved in 10% formaldehyde solution, and then specimens were embedded in paraffin. Cut 6 μm slice from each sample, stained with hematoxylin and eosin (H&E). Nrf2-positive cells were determined using the anti-Nrf2 antibody (Servicebio, Wuhan, China) and diaminobenzidine (DAB) staining kit (Servicebio). Assessment of the dewaxing sections and immunohistochemically stained sections were performed using a conventional light microscope (Olympus Corporation, Japan).
RNA Extraction and Gene Expression Analysis
TRIzol reagent (Takara Biotechnology Co. Ltd, Dalian, China) was used to extract total RNA from the breast muscle samples. Reverse transcription to cDNA was performed using Prime Script RT Master Mix (Takara Biotechnology Co. Ltd). The real-time PCR was performed using an ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA) with SYBR Premix Ex Taq kit. The primer sequences were shown in Table 2, and relative gene expression was calculated using the 2−ΔΔCt method (Wang et al., 2017).
Table 2.
The primer sequences used for real-time PCR analysis.
| Gene1 | Genbank number | Primer sequence (5′→3′) | Product size(bp) |
|---|---|---|---|
| AMPKα1 | NM_001039603.1 | Forward: CAAGTAGTGTCTCGCACGGT | 133 |
| Reverse: GACTGATAGCTGGTCCCACG | |||
| AMPKα2 | NM_001039605.1 | Forward: GGCACCTTCGGCAAAGTCAAG | 198 |
| Reverse: GAAGAAGTCTGTTGGCGTGC | |||
| LKB1 | NM_001045833.1 | Forward: CCAAGGCCATCTGCATGAAC | 135 |
| Reverse: TGGAGAGCTTGCGGATCTTG | |||
| CaMKKα | XM_015295973.3 | Forward: CAAACAGCGGCACGTTGGAG | 184 |
| Reverse: CTGCAGAGAGCTGGAATGCT | |||
| CaMKKβ | XM_025155530.2 | Forward: TGAAGCTGGCCTACAACGAG | 236 |
| Reverse: GTCATCCAGCACCTCCACAA | |||
| GAPDH | NM_204305.1 | Forward: TCAAATGGGCAGATGCAGGT | 291 |
| Reverse: TGATGGCATGGACAGTGGTC |
Abbreviations: AMPKα1, adenosine 5‘-monophosphate (AMP)-activated protein kinase α1; AMPKα2, adenosine 5‘-monophosphate (AMP)-activated protein kinase α2; CaMKKα, Calcium/Calmodulin dependent protein kinase α; CaMKKβ, Calcium/Calmodulin dependent protein kinase β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LKB1, Liver kinase B1.
Western Blot Analysis
Homogenization of frozen breast muscle samples by centrifugation to collect the supernatants. The supernatants were electrophoresed by sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS-PAGE), cut gel and the target protein was transferred to the polyvinylidene fluoride (PVDF) membrane, incubated with 5% bovine serum albumin and photographed. The antibodies for HK1 (#2024S), PK (#3106S), p-AMPKαThr172 (#2535) and horseradish-peroxidase-conjugated (#7074) were purchased from Cell Signaling Technology (Beverly, MA). The antibodies for PFK (GB112059) and GAPDH (GB11002) were purchased from Servicebio. The antibodies for LKB1 (bs-3250R) and CaMKK1 (bs-6253R) were purchased from Bioss biotechnology company (Beijing). After binding the antibodies, the PVDF membrane was developed with an ECL chemiluminescence reagent (EpiZyme Inc., Minhang District, Shanghai). The band density was quantified using Imager Bio-Rad ChemiDoc Touch and Image Quantity One software (GE, Uppsala, Sweden) (Xing et al., 2017).
Statistical Analyses
All data were analyzed using SPSS 22.0 software (SPSS, Inc., Chicago, IL). One-way ANOVA model and Duncan's multiple range test were used to evaluate differences. The data were expressed as means ± standard error of the mean (SEM), and P < 0.05 was considered statistically significant.
RESULTS
Growth Performance
As shown in Table 3, after exposure to H2O2, the average daily gain (ADG) was decreased in the H2O2 treatment (P < 0.05), and the feed to gain ratio (F/G) was increased in the H2O2 treatment than those in the control and the saline treatments (P < 0.05). The above data indicated that H2O2 exposure decreased the ADG and increased the F/G in broilers.
Table 3.
Effects of oxidative stress induced by H2O2 on the growth performance of broilers.
| Treatments2 |
|||||
|---|---|---|---|---|---|
| Items1 | Control | Saline | H2O2 | SEM | P value |
| ADFI (g/bird/day) | 107.41 | 104.05 | 105.05 | 1.81 | 0.765 |
| ADG (g/bird/day) | 57.08a | 55.72a | 49.54b | 1.16 | 0.008 |
| F/G (g/g) | 1.88b | 1.86b | 2.13a | 0.04 | 0.001 |
Means in a row without a common superscript letter significantly differ (P < 0.05). The results are represented as the mean value with pooled SEM (n = 6).
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; F/G, feed to gain ratio.
The control group was the noninjected treatment. The saline group: birds were injected with physiological saline buffer (0.75%). The H2O2 group: birds were given an injection of 10.0% H2O2.
Slaughter Performance
As shown in Table 4, there were no significant differences in the dressing percentage, eviscerated yield, abdominal fat percentage, breast muscle yield, and thigh muscle yield among the control, saline and H2O2 groups after H2O2 exposure.
Table 4.
Effects of oxidative stress induced by H2O2 on the slaughter performance of broilers.
| Treatments1 |
|||||
|---|---|---|---|---|---|
| Items | Control | Saline | H2O2 | SEM | P value |
| Dressing percentage (%) | 96.02 | 96.31 | 95.87 | 0.10 | 0.192 |
| Eviscerated yield (%) | 75.79 | 75.85 | 75.14 | 0.18 | 0.190 |
| Abdominal fat percentage (%) | 0.93 | 0.92 | 0.78 | 0.05 | 0.419 |
| Breast muscle yield (%) | 25.17 | 23.49 | 23.52 | 0.49 | 0.291 |
| Thigh muscle yield (%) | 17.31 | 17.82 | 18.03 | 0.21 | 0.360 |
The results are represented as the mean value with pooled SEM (n = 6).
The control group was the non-injected treatment. The saline group: birds were injected with physiological saline buffer (0.75%). The H2O2 group: birds were given an injection of 10.0% H2O2.
Assessment of Histomorphology and Immunohistochemical
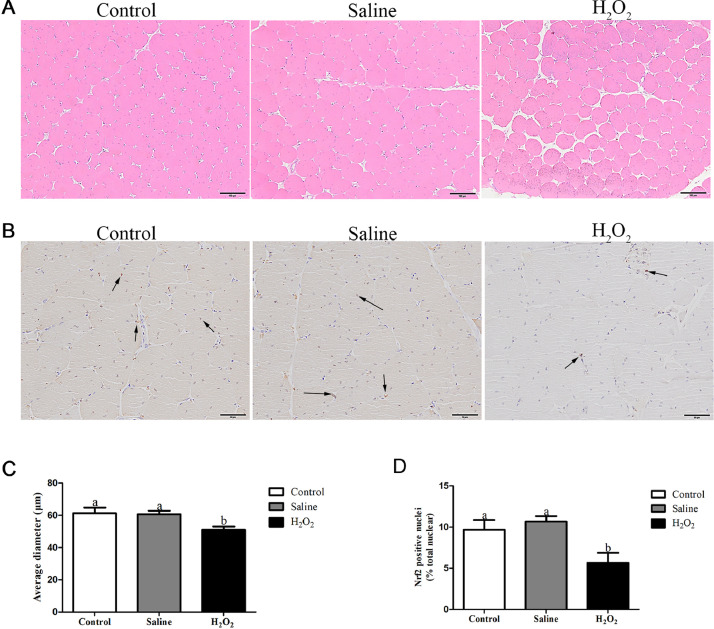
As shown in Figure 1, the structure of pectoral muscle fiber was clear and orderly in the control and saline treatments. In the H2O2 treatment, the average muscle fiber diameter was significantly reduced (P < 0.05, Figures 1A and 1C), the pectoral muscle fiber was misaligned.
Figure 1.
Effects of oxidative stress induced by H2O2 on the structure of muscle fibers and the percentage of Nrf2 positive cells in broiler breast muscle. (A) Hematoxylin & eosin staining images, scale bar: 100 μm. (B) Immunohistochemical images, scale bar: 50 μm. Black arrows indicate Nrf2-positive cells. (C, D) Quantitative analysis of muscle fiber diameter and Nrf2 positive cell percentage. Data are presented as mean ± SE (n = 6). Different letters indicate significant differences at P < 0.05.
After H2O2 exposure, the percentage of Nrf2-positive cells of breast muscle in the H2O2 group was significantly decreased (P < 0.05, Figures 1B and 1D) in comparison to the control and saline groups. The above data indicated that H2O2 exposure reduced the protein expression of Nrf2 and inhibited the growth of breast muscle fibers.
Serum Stress Indexes, ROS Generation and Redox Status
As shown in Table 5, after H2O2 exposure, compared with the control and saline groups, the level of CORT and the activity of LDH in serum of the H2O2 group were significantly increased (P < 0.05). H2O2 exposure significantly inhibited the activities of CAT, T-AOC, T-SOD, and GSH-Px (P < 0.05), and increased the levels of ROS and protein carbonyl (P < 0.05). These results indicated that H2O2 exposure led to stress response in broilers, the redox system was destroyed, and the accumulation of ROS and oxidation products led to oxidative damage in breast muscle.
Table 5.
Effects of oxidative stress induced by H2O2 on the serum oxidative stress indexes and redox status in broiler breast muscle.
| Treatments1 |
|||||
|---|---|---|---|---|---|
| Items2 | Control | Saline | H2O2 | SEM | P value |
| CORT (ng/mL) | 68.83b | 72.33b | 108.01a | 5.78 | 0.008 |
| CK (U/mL) | 2.57 | 2.41 | 2.78 | 0.08 | 0.179 |
| LDH (U/mL) | 2.13b | 2.26b | 2.60a | 0.06 | 0.004 |
| CAT (U/mg of protein) | 1.14a | 1.12a | 0.73b | 0.07 | 0.040 |
| T-AOC (U/mg of protein) | 0.56a | 0.53a | 0.43b | 0.01 | <0.001 |
| T-SOD (U/mg of protein) | 50.71a | 47.61a | 40.86b | 1.06 | <0.001 |
| GSH-Px (U/mg of protein) | 12.78a | 12.51a | 10.01b | 0.33 | <0.001 |
| ROS (% of Control) | 100.00b | 106.05b | 133.33a | 2.89 | <0.001 |
| Carbonyl (nmol/mg of protein) | 1.63b | 1.48b | 1.91a | 0.05 | <0.001 |
Means in a row without a common superscript letter significantly differ (P < 0.05). The results are represented as the mean value with pooled SEM (n = 6).
The control group was the non-injected treatment. The saline group: birds were injected with physiological saline buffer (0.75%). The H2O2 group: birds were given an injection of 10.0% H2O2.
Abbreviations: CAT, catalase; CORT, corticosterone; CK, creatine kinase; GSH-Px, glutathione peroxidase; LDH, lactate dehydrogenase; ROS, reactive oxygen species; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase.
Concentrations of Muscle ATP, ADP, AMP, Glycogen, and Lactate
As shown in Table 6, compared with the control and saline groups, H2O2 exposure increased the content of AMP and the ratio of AMP/ATP (P < 0.05), decreased the content of ATP by 23.80%. Additionally, H2O2 exposure decreased the content of glycogen (P < 0.05) and increased the contents of lactate and GP (P < 0.05). These results suggested that H2O2 exposure inhibited aerobic respiration, reduced the production of ATP, and enhanced glycolysis.
Table 6.
Effects of oxidative stress induced by H2O2 on the contents of adenosine phosphates, glycogen, and lactate in broiler breast muscle.
| Treatments1 |
|||||
|---|---|---|---|---|---|
| Items2 | Control | Saline | H2O2 | SEM | P value |
| ATP (μmol/g) | 4.41 | 4.23 | 3.36 | 0.29 | 0.302 |
| ADP (μmol/g) | 0.64 | 0.66 | 0.52 | 0.05 | 0.435 |
| AMP (μmol/g) | 0.46b | 0.43b | 0.63a | 0.03 | 0.043 |
| AMP/ATP ratio | 0.12b | 0.11b | 0.19a | 0.01 | 0.010 |
| Glycogen (μmol/g) | 4.07a | 4.11a | 3.64b | 0.08 | 0.038 |
| Lactate (μmol/g) | 119.36b | 112.73b | 131.74a | 2.83 | 0.016 |
| GP (μmol/g) | 127.51b | 120.94b | 139.02a | 2.81 | 0.025 |
Means in a row without a common superscript letter significantly differ (P < 0.05). The results are represented as the mean value with pooled SEM (n = 6).
The control group was the non-injected treatment. The saline group: birds were injected with physiological saline buffer (0.75%). The H2O2 group: birds were given an injection of 10.0% H2O2.
Abbreviations: ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; GP, glycolytic potential = 2[glycogen]+[lactate].
Activities of Key Enzymes of Aerobic Respiration and Glycolysis
As shown in Table 7, compared to the control and saline treatments, H2O2 exposure decreased the activity of PDH (P < 0.05) and decreased the activity of Ca2+-ATPase by 17.95% in breast muscle. Additionally, after H2O2 exposure, the activities of HK and LDH were increased in the H2O2 group (P < 0.05). These results indicated that H2O2 exposure inhibited the tricarboxylic acid cycle in aerobic respiration and enhanced glycolysis.
Table 7.
Effects of oxidative stress induced by H2O2 on the activities of key enzymes in aerobic respiration and glycolysis in broiler breast muscle.
| Treatments1 |
|||||
|---|---|---|---|---|---|
| Items2 | Control | Saline | H2O2 | SEM | P value |
| Na+/K+-ATPase (U/mg of protein) | 1.69 | 1.77 | 1.42 | 0.08 | 0.195 |
| Ca2+-ATPase (U/mg of protein) | 1.54 | 1.58 | 1.28 | 0.06 | 0.080 |
| PDH (U/mg of protein) | 9.51a | 9.56a | 4.76b | 0.57 | <0.001 |
| ACO (U/mg of protein) | 21.33 | 18.49 | 17.79 | 1.35 | 0.546 |
| CS (U/mg of protein) | 6.37 | 5.89 | 6.28 | 0.12 | 0.242 |
| HK (U/mg of protein) | 13.88b | 14.41b | 17.63a | 0.67 | 0.043 |
| PK (U/mg of protein) | 9.12 | 9.44 | 10.93 | 0.45 | 0.229 |
| PFK (U/mg of protein) | 130.81 | 125.68 | 132.66 | 4.13 | 0.784 |
| LDH (U/mg of protein) | 3.46b | 3.37b | 4.66a | 0.22 | 0.024 |
Means in a row without a common superscript letter significantly differ (P < 0.05). The results are represented as the mean value with pooled SEM (n = 6).
The control group was the noninjected treatment. The saline group: birds were injected with physiological saline buffer (0.75%). The H2O2 group: birds were given an injection of 10.0% H2O2.
Abbreviations: ACO, aconitase; CS, citrate synthase; HK, hexokinase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; PK, pyruvate kinase; PFK, phosphofructokinase.
Relative Expression of Gene and Protein in AMPK Pathway
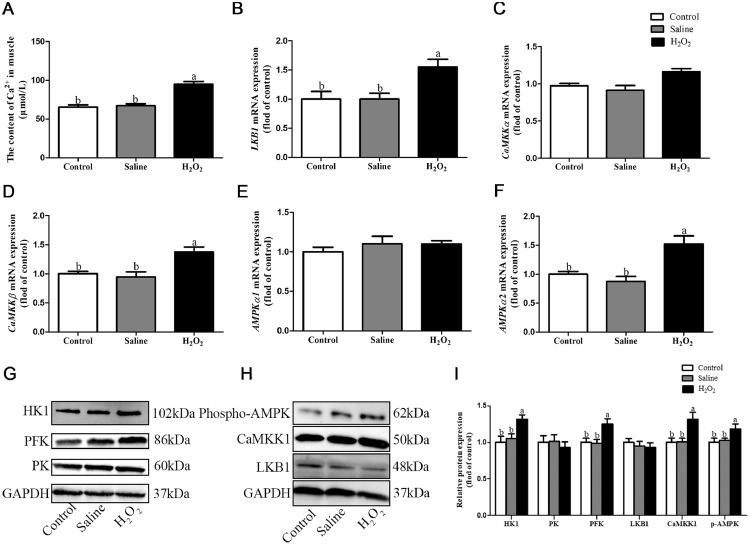
As shown in Figure 2, compared with the control and saline groups, H2O2 exposure increased the content of Ca2+ in breast muscle (P < 0.05, Figure 2A). H2O2 exposure upregulated mRNA expression levels of LKB1, CaMKKβ, and AMPKα2 (P < 0.05, Figures 2B, 2D, 2F), and the protein expression levels of CaMKK1, p-AMPK, HK1, and PFK (Figures 2G and 2H). These results suggested that H2O2 exposure enhanced glycolysis by activating CaMKK/LKB1/AMPK signaling pathway.
Figure 2.
Effects of oxidative stress induced by H2O2 on the content of Ca2+, gene and protein expression of LKB1, CaMKKα, CaMKKβ, AMPKα1, AMPKα2, HK1, PK, PFK, and GAPDH in broiler breast muscle. (A) Content of Ca2+ in breast muscle. (b–F) Relative mRNA expression levels of LKB1, CaMKKα, CaMKKβ, AMPKα1, and AMPKα2. (G, H) Relative protein expression levels of HK1, PK, PFK, LKB1, CaMKK1, and Phospho-AMPK. (i) Quantification of HK1, PK, PFK, LKB1, CaMKK1 and Phospho-AMPK. Data are presented as mean ± SE (n = 6). Different letters indicate significant differences at P < 0.05.
DISCUSSION
In this experiment, the effects of oxidative stress on broiler breast muscles were investigated using the model of intraperitoneal injection of H2O2, which was similar to the hypoxia of broilers in intensive culture. Oxidative stress may cause oxidative damage by increasing the level of ROS in the body. In addition, excessive ROS damage the aerobic respiration of broilers, and the energy supply mode of muscle cells change from aerobic respiration to glycolysis, resulting in the accumulation of lactate in muscle (Chen et al., 2020). The accumulation of lactate leads to the decrease of muscle pH and further denaturation of protein. Excessive ROS attack macromolecules in cells such as DNA, protein and lipid, and ultimately damage the health of broilers (Yin et al., 2013). The present study showed that intraperitoneal injection of H2O2 decreased the ADG and increased the F/G of broilers. Chen et al. (2018) showed that the oxidative stress induced by H2O2 significantly decreased the body weight (BW) gain, feed intake, and gain/feed ratio of broilers. Oxidative stress could lead to DNA damage, lipid peroxidation and protein degradation, affected the health and reduced production performance by damaging the liver, intestine and other tissues and organs (Yin et al., 2013). Therefore, the decrease of ADG in this study was mainly due to oxidative stress.
The level of serum CORT, and the activities of the CK and LDH are considered typical indicators of the stress response (Soleimani et al., 2011). Oxidative stress stimulates the hypothalamic pituitary adrenal axis and increases the concentration of blood corticosterone in poultry (Soleimani et al., 2011). CK and LDH are mainly stored in the muscle, when stress occurs, these 2 enzymes may be quickly released into the blood (Yu et al., 2009). In this study, after H2O2 exposure, the level of CORT, and the activities of the CK and LDH were increased, which was consistent with the findings of Chen et al. (2020). This finding suggested that broilers suffered strong physiological stress after intraperitoneal injection of H2O2. Oxidative stress may also increase the level of ROS and destroy the redox system in broiler muscle (Chen et al., 2017). In this study, the level of ROS was increased, and the activities of T-AOC, T-SOD, CAT, and GSH-Px were decreased in the breast muscle after intraperitoneal injection of H2O2, suggesting that the antioxidant system was destroyed and oxidative damage occurred. Wen et al. (2019) reached a similar conclusion, suggesting that oxidative stress induced by heat stress reduced the activity of GSH, and increased the content of malondialdehyde (MDA). ROS accumulated in response to oxidative stress impaired cell membrane integrity through protein oxidation, which significantly increased the risk of muscle oxidation (Estevez, 2015). Therefore, protein oxidation has been identified as the main cause affecting meat quality (Zhang et al., 2013). Protein carbonyl is known as a marker of oxidative stress, which reflects the degree of protein oxidation (Del Rio et al., 2005). Moreover, after oxidative exposure, the content of protein carbonyl in the H2O2 group was increased, which was in agreement with the report by Wan et al. (2017).
Oxidative stress may impair the meat quality of broilers from several aspects of physiological metabolism. The change of cell energy demand is the first metabolic change caused by oxidative stress (Akbarian et al., 2016). Zhang et al. (2017) reported that the increase of ROS in muscle could affect the conversion of energy substances by decreasing ATP generation and increasing the AMP/ATP ratio in muscle. In accordance with these studies, we observed that the content of ATP in the H2O2 group was decreased, and the content of AMP and the ratio of AMP/ATP in the H2O2 group were increased compared to the control and saline groups, which was consistent with the findings of Zhang et al. (2021a). Under stressful conditions, muscle glycogen primarily produces ATP through glycolysis, which provides energy for muscle contractions (Meton et al., 1999). It is generally recognized that when the cell produces ATP through glycolysis, it may generate lactate. When the lactate is accumulated in muscle, the pH value of muscle will drop rapidly (Kemp, 2005). In the present study, the content of glycogen in the H2O2 group was decreased, and the contents of lactate and GP in the H2O2 group were increased, which was in consistent with the findings of Chen et al. (2020).
When poultry is under oxidative stress, the mutual transformation between mitochondrial oxidative respiration and energy substances is difficult to maintain the internal energy balance, resulting in a sharp increase in energy consumption (Akbarian et al., 2016). Ca2+-ATPase catalyzes ATP hydrolysis on the inner side of the plasma membrane, and the released energy drives the intracellular Ca2+ out of the cells or into the endoplasmic reticulum cavity for storage, thereby maintaining a low concentration of free Ca2+ in the cells. The tricarboxylic acid cycle is the most effective way for the cell to obtain energy, as the key enzymes of aerobic respiration, the activities of PDH, ACO, and CS reflect the energy metabolism function of cells (Tang et al., 2021). In this study, the activities of Ca2+-ATPase and PDH were decreased after H2O2 exposure, which indicated that ROS damaged the aerobic respiration of muscle cells and inhibited energy metabolism. When aerobic respiration is inhibited, glycolysis would be activated for supplementary energy. The glycolysis pathway is mediated by several enzymes, and the key enzymes are the key to control the function of glycolysis. HK and PFK are the most important rate-limiting enzymes in the aerobic oxidation of glucose, PK, and LDH are the key enzymes in the terminal step of the muscle glycolysis metabolic reaction (Scheffler and Gerrard, 2007). The increase in the activities of HK, PK, PFK, and LDH in broiler muscle may indicate the increase in glycolysis rate, and the accumulation of lactate led to the rapid decline of pH (Shao et al., 2018). In this experiment, after oxidative exposure, the activity of HK in the breast muscle was increased, which was in agreement with the research by Zhang et al. (2021b). The energy supply was insufficient, which accelerated the glycolysis function of muscle cells. With the accumulation of metabolites pyruvate in the previous step, LDH was activated to convert pyruvate to lactate, which was consistent with the increase in LDH activity in breast muscle of H2O2-injected broilers in our study.
AMPK is a highly conserved heterotrimeric kinase that widely exists in eukaryotic cells. Under the stimulation of adverse factors such as nutritional deficiency, metabolic disorder, hypoxic ischemia, and oxidative stress, AMPK would be activated. The active AMPK may inhibit anabolism and promote catabolism by regulating its downstream target proteins, so as to maintain the dynamic balance of intracellular energy (Xing et al., 2019). In the field of meat science, previous studies confirmed that AMPK could play an important regulatory role in the process of postmortem muscle glycolysis, indicating that AMPK may affect meat quality by regulating the process of postmortem muscle glycolysis (Xing et al., 2015; Zhang et al., 2017). The activation of AMPK is directly regulated by upstream proteins, the main upstream proteins of AMPK are LKB1 and CaMKK (Carling, 2017). When the content of intracellular ATP is decreased, and the content of AMP is increased, LKB1 would be activated after forming a complex with 2 accessory proteins STRAD and MO25. At the same time, the AMP and γ subunit of AMPK bind to become the substrate of the LKB1 complex and promote the phosphorylation of the thr172 site of AMPK. CaMKK may sensitively sense the change of Ca2+ in cytoplasm, when the concentration of Ca2+ in the cytoplasm is increased, CaMKK may be activated, which promotes the activation of AMPK (Hardie et al., 2012). In our present study, after intraperitoneal injection of H2O2, the content of Ca2+ in breast muscle was increased, the mRNA expression levels of LKB1, CaMKKβ, and AMPKα2 were increased, and the protein expression levels of CaMKK1 and p-AMPK were increased. It seems that LKB1 and CaMKK coexist in the regulation of AMPK signaling pathway, and CaMKK may occupy a dominant position in this experiment.
CONCLUSIONS
In conclusion, the oxidative stress induced by intraperitoneal injection of H2O2 damaged the breast muscle redox system, impaired energy status and enhanced glycolysis by activating the CaMKK/LKB1/AMPK signaling pathway in breast muscle of broilers, eventually resulting in the decline of growth performance.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31872374, 32072780), the National Key Research and Development Program of China (2016YFD0500501, 2018YFD0500405), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2021]459), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_0601).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Ahmad H., Tian J.K., Wang J.J., Khan M.A., Wang Y.X., Zhang L.L., Wang T. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J. Agric. Food Chem. 2012;60:7111–7120. doi: 10.1021/jf3017207. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:1–14. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbut S., Sosnicki A.A., Lonergan S.M., Knapp T., Ciobanu D.C., Gatcliffe L.J., Huff-Lonergan E., Wilson E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008;79:46–63. doi: 10.1016/j.meatsci.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Betti M., Schneider B.L., Wismer W.V., Carney V.L., Zuidhof M.J., Renema R.A. Omega-3-enriched broiler meat: 2. Functional properties, oxidative stability, and consumer acceptance. Poult. Sci. 2009;88:1085–1095. doi: 10.3382/ps.2008-00158. [DOI] [PubMed] [Google Scholar]
- Carling D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Chen X., Gu R., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Induction of nuclear factor-kappa B signal-mediated apoptosis and autophagy by reactive oxygen species is associated with hydrogen peroxide-impaired growth performance of broilers. Animal. 2018;12:2561–2570. doi: 10.1017/S1751731118000903. [DOI] [PubMed] [Google Scholar]
- Chen Z.D., Xing T., Li J.L., Zhang L., Jiang Y., Gao F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2020;100:918–925. doi: 10.1016/j.psj.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.X., Zhang L., Li J.L., Gao F., Zhou G.H. Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-kappa B signal pathway. J. Agric. Food Chem. 2017;65:3986–3994. doi: 10.1021/acs.jafc.7b01267. [DOI] [PubMed] [Google Scholar]
- Da Silva D.C.F., De Arruda A.M.V., Goncalves A.A. Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J. Food Sci. Technol. 2017;54:1818–1826. doi: 10.1007/s13197-017-2612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Estevez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Guo K., Ge J., Zhang C., Lv M.W., Zhang Q., Talukder M., Li J.L. Cadmium induced cardiac inflammation in chicken (Gallus gallus) via modulating cytochrome P450 systems and Nrf2 mediated antioxidant defense. Chemosphere. 2020;249 doi: 10.1016/j.chemosphere.2020.125858. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G. Lactate accumulation, proton buffering, and pH change in is chemically exercising muscle. Am. J. Physiol-Reg. I. 2005;289:R895–R901. doi: 10.1152/ajpregu.00641.2004. [DOI] [PubMed] [Google Scholar]
- Khan U., Hussain J., Mahmud A., Khalique A., Mehmood S., Badar I.H., Usman M., Jaspal M.H., Ahmad S. Comparative study on carcass traits, meat quality and taste in broiler, broiler breeder and aseel chickens. Braz. J. Poult. Sci. 2019;21:001–010. [Google Scholar]
- Kim Y.H.B., Warner R.D., Rosenvold K. Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review. Anim. Prod. Sci. 2014;54:375–395. [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Meton I., Mediavilla D., Caseras A., Canto E., Fernandez F., Baanante I.V. Effect of diet composition and ration size on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata) Br. J. Nutr. 1999;82:223–232. [PubMed] [Google Scholar]
- Parrish F.C., Sebig C.J., Culler R.D., Zeece M.G. CAF activity, calcium concentration, and the 30,000-dalton component of tough and tender bovine longissimus muscle. J. Food Sci. 1981;46:308–311. [Google Scholar]
- Scheffler T.L., Gerrard D.E. Mechanisms controlling pork quality development: the biochemistry controlling postmortem energy metabolism. Meat Sci. 2007;77:7–16. doi: 10.1016/j.meatsci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D., Wang Q., Hu Y., Shi S.R., Tong H.B. Effects of cyclic heat stress on the phenotypic response, meat quality and muscle glycolysis of breasts and thighs of yellow-feather broilers. Ital. J. Anim. Sci. 2018;18:301–308. [Google Scholar]
- Soleimani A.F., Zulkifli I., Omar A.R., Raha A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–1440. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- Tang Q.G., Ding C., Xu Q.Q., Bai Y., Xu Q., Wang K.J., Fang M.Y. Mitochondrial fusion potentially regulates a metabolic change in Tibetan chicken embryonic brain during hypoxia. Front. Cell. Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.585166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.L., Zhang J.F., He J.T., Bai K.W., Zhang L.L., Wang T. Dietary enzymatically treated Artemisia annua L. supplementation alleviates liver oxidative injury of broilers reared under high ambient temperature. Int. J. Biometeorol. 2017;61:1629–1636. doi: 10.1007/s00484-017-1341-1. [DOI] [PubMed] [Google Scholar]
- Wang X.F., Li J.L., Cong J.H., Chen X.X., Zhu X.D., Zhang L., Gao F., Zhou G.H. Preslaughter transport effect on broiler meat quality and post-mortem glycolysis metabolism of muscles with different fiber types. J. Agric. Food Chem. 2017;65:10310–10316. doi: 10.1021/acs.jafc.7b04193. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen Y.P., Leng Z.X., Ding L.R., Wang T., Zhou Y.M. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agric. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- Xing T., Gao F., Tume R.K., Zhou G.H., Xu X.L. Stress effects on meat quality: a mechanistic perspective. Compr. Rev. Food Sci. Food Saf. 2019;18:380–401. doi: 10.1111/1541-4337.12417. [DOI] [PubMed] [Google Scholar]
- Xing T., Wang M.F., Han M.Y., Zhu X.S., Xu X.L., Zhou G.H. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Animal. 2017;11:1599–1607. doi: 10.1017/S1751731116002809. [DOI] [PubMed] [Google Scholar]
- Xing T., Xu X.L., Jiang N.N., Deng S.L. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer. Anim. Sci. J. 2015;87:299–307. doi: 10.1111/asj.12426. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W.K., Wu X.S., Yang G., Wang J., Li T.J., Ding J.N., Cai L.C., Su D.D. Oxidative stress-mediated signaling pathways: a review. J. Food Agric. Environ. 2013;11:132–139. [Google Scholar]
- Yu J.M., Tang S., Bao E.D., Zhang M., Hao Q.Q., Yue Z.H. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci. 2009;83:474–478. doi: 10.1016/j.meatsci.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Zhang B.L., Liu N., He Z., Song P.Y., Hao M.L., Xie Y.X., Li J.H., Liu R.J., Sun Z.W. Guanidino-acetic acid: a scarce substance in biomass that can regulate postmortem meat glycolysis of broilers subjected to pre-slaughter transportation. Front. Bioeng. Biotechnol. 2021;8 doi: 10.3389/fbioe.2020.631194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.Y., Zhai C.Y., Luo X., Lin H., Zhang M.H., Zhu L.X., Nair M.N., Ahn D.U., Liang R.R. An early-postmortem metabolic comparison among three extreme acute heat stress temperature settings in chicken breast muscle. J. Food Sci. Tech. Mys. 2021;58:4823–4829. doi: 10.1007/s13197-021-05230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang X.F., Li J.L., Zhu X.D., Gao F., Zhou G.H. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J. Agric. Food Chem. 2017;65:6991–6999. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]
- Zhang W.G., Xiao S., Ahn D.U. Protein oxidation: basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013;53:1191–1201. doi: 10.1080/10408398.2011.577540. [DOI] [PubMed] [Google Scholar]
- Zhang W.G., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 2011;59:969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]
- Zheng J., Bizzozero O.A. Traditional reactive carbonyl scavengers do not prevent the carbonylation of brain proteins induced by acute glutathione depletion. Free Radic. Res. 2010;44:258–266. doi: 10.3109/10715760903456092. [DOI] [PMC free article] [PubMed] [Google Scholar]