Abstract
Despite potency against a variety of cancers in preclinical systems, melittin (MEL), a major peptide in bee venom, exhibits non-specific toxicity, severe hemolytic activity, and poor pharmacological properties. Therefore, its advancement in the clinical translation system has been limited to early-stage trials. Herein, we report a biohybrid involving a bottlebrush-architectured poly(ethylene glycol) (PEG) and MEL. Termed pacMEL, the conjugate consists of a high-density PEG arrangement, which provides MEL with steric inhibition against protein access, while the high molecular weight of pacMEL substantially enhances plasma pharmacokinetics with a ~ 10-fold increase in the area under the curve (AUC∞) compared to free MEL. The pacMEL also significantly reduces hepatic damage and unwanted innate immune response, and all but eliminated hemolytic activities of MEL. Importantly, pacMEL passively accumulates at subcutaneously inoculated tumor sites and exhibits stronger tumor-suppressive activity than molecular MEL. Collectively, pacMEL makes MEL a safer and a more appealing drug candidate.
Keywords: melittin, bottlebrush polymer, drug delivery, PEGylation, narrow therapeutic index drugs
Graphical Abstract

INTRODUCTION
High binding constants and exquisite specificity with target protein of peptides often give rise to great potencies and few off-target effects compared with conventional small molecule drugs.1–3 Still, only a small fraction of peptides with in vitro potency eventually achieves regulatory approval, largely due to the intrinsic low protease stability, rapid renal clearance, and side effects. For instance, melittin (MEL), a lytic peptide with promising antitumor activities, is severely limited by its difficult pharmacological properties and extensive hemolytic activity. Consisting of 26 amino acids, MEL is the main component of bee venom (~ 50% dried weight). MEL can disrupt the phospholipid bilayer by generating transmembrane pores and cause the apoptosis/necrosis of cells. Importantly, MEL is regarded as one of the most potent anti-cancer agents, as resistance against physical pore-formation on the cell membrane is very difficult to develop by the cancer cell.4–8 However, at therapeutic concentrations, which are barely above the sub-lytic concentrations, MEL causes significant side effects including hemolysis, coagulopathy, allergic reactions, and pain at the injection site, which would render MEL a narrow therapeutic index drug, with slight changes in dosage inducing therapeutic failures or severe adverse drug reactions.9,10
Observing these obstacles, we recognize that most of them are associated with unwanted interactions during initial systematic circulation, such as those with proteins in the coagulation pathway (coagulopathy), the red blood cell membrane (hemolysis), and immune cells (allergic reactions).11–13 Suppressing these interactions may therefore reduce associated side effects, establish a new biodistribution profile that potentially redirects bioactivity of MEL to tissues of interest, and improve therapeutic index. Covalent conjugation with the synthetic polymer poly(ethylene glycol) (PEG) is a clinically successful strategy to reduce unwanted interactions between the therapeutic agent and the biological environment, which has been adopted in more than 20 approved peptide/protein biopharmaceuticals since the approval of Adagen, a PEGylated adenosine deaminase.14 PEG works by creating a large hydration shell shielding the conjugated species.15,16 Conventional PEGylation with linear or slightly branched PEG has not been able to fully overcome the various toxicity challenges associated with MEL, in part due to insufficient shielding and the strong lytic properties of the peptide.17 Recently, the rapid progress in polymer synthetic approaches allows polymers with more complex architectures to be obtained, such as the bottlebrush polymers, which can be easily synthesized by ring-opening metathesis polymerization (ROMP).18–20 Consisting of multiple PEG chains (usually 15–30) densely arranged on a central polymer backbone, the bottlebrush PEG creates significantly greater spatial congestion than linear or slightly branched PEG, which makes it particularly suitable for steric shielding of highly promiscuous binders, membrane disruptive agents, and/or enzymatically vulnerable molecules (such as unmodified oligonucleotides).21–26 Additionally, the recently reported degradable polynorbornene-based bottlebrush polymers further alleviate the concerns over using these large molecules as drug delivery vectors.27,28
Herein, we report a bottlebrush PEG-MEL conjugate as a non-immunogenic, non-hemolytic, and long-circulating anti-tumor prodrug to treat non-small cell lung carcinoma (NSCLC) in a xenograft mouse model. Abbreviated as pacMEL (polymer-assisted compaction of MEL), the conjugate links ~ 2 molecules of MEL covalently to the backbone of the bottlebrush PEG via a bioreductively cleavable disulfide linker or a non-cleavable bond. (Scheme 1) With reduced interactions with blood components, the pacMEL exhibits markedly prolonged plasma pharmacokinetics (~ 21 × longer elimination half-life compared with free MEL) and elevated plasma availability (~ 10 × greater area under the curve, AUC∞), which promotes the passive targeting of tumor xenografts via the enhanced permeability and retention (EPR) effect. At a dosage where free MEL has no discernible effect on tumor growth, pacMEL is able to exert a significant tumor-suppressive activity. Importantly, pacMEL does not exhibit the typical toxicity profile of MEL, such as hemolytic activity, cytotoxicity, and liver damage. Collectively, we demonstrate a safer and pharmacologically superior form of the highly toxic peptide MEL, achieved using predominately PEG. The findings reported here make MEL a more appealing therapeutic agent for translational consideration and should expand the possibilities for the delivery of other types of narrow-therapeutic index drugs.
Scheme 1.

Chemical structures and schematic illustrations of PEGylated MELs.
RESULTS AND DISCUSSION
Preparation and characterization of pacMEL
To shield MEL from the biological environment, we designed a high grafting density bottlebrush PEG (degree of polymerization ~ 30, Mw 271 kDa, Ð 1.27) with long side chains (Mn 10 kDa, Ð 1.05) as the carrier platform, based on previous physiochemical and pharmacological findings.23,25,26 The pacMEL was synthesized via click reaction between azide-functionalized bottlebrush polymer and dibenzocyclooctyne-functionalized MEL.29–31 Unreacted PEG macromonomers and MEL were removed during the purification via aqueous gel permeation chromatography (GPC, Figure 1A and S4C). Because MEL works by forming a tetrameric complex and then binds to the phospholipid bilayer, it must be released from the polymer to regain activity.25 Thus, a cysteine modification at the C terminus of MEL was used to form a bioreductively cleavable disulfide linkage with the diblock bottlebrush copolymer (pacMELClv). We also synthesized two negative controls: a non-cleavable conjugate, pacMELNClv, and a conjugate of low PEG density (Mn = 40 kDa, Y-shaped PEG, each arm is 20 kDa, Ð 1.05), YPEG-MEL (Scheme 1).
Figure 1.

(A and B) Aqueous GPC chromatograms and agarose gel electrophoresis of purified PEGylated MELs and free MEL. Note that the GPC peaks for the conjugates display high molecular shoulders (more notable for the YPEG-MEL), which suggest that the MEL component may cause slight aggregation. This peak asymmetry is absent for the parent brush polymer (Figure S2). (C) TEM image of pacMELClv negatively stained with 1% uranyl acetate. (D) Size distributions of PEGylated MELs (DLS intensity average; experiments were carried out at a scattering angle of 90° at room temperature in Nanopure™ water). (E) ζ potential of free MEL and PEGylated MELs in Nanopure™ water.
The number of peptides per bottlebrush was determined to be 3.3 for pacMELNClv and 2.6 for pacMELClv (Figure 1B, for details see SI). The average MEL loading number for pacMELClv was verified by treatment with TCEP and subsequent GPC analysis (Figure S4B and C) to be 2.1 MEL per conjugate, which is approximately consistent with the dye-labeling method. Transmission electron microscopy (TEM) shows that the pacMELClv exhibits a globular structure with a dry-state diameter of 23 ± 3 nm, which is in line with dynamic light scattering (DLS) analysis (Dh of 34.1 ± 0.4 nm, Figure 1C and D). The observed globular morphology of the pacMEL is the result of the collapse of the hydrophobic polynorbornene backbone in aqueous solution, which has been reported in other studies and simulations.32,33 Considering the number of the side chains (DPn ~ 30) in our design, the polymer used in the pacMEL has entered the bottlebrush regime and should offer stronger steric inhibition of external macromolecules than the slightly branched star polymers.21,34 The YPEG-MEL exhibits an expected smaller cumulant Dh of 21.0 ± 0.5 nm. ζ potential measurements indicate that PEGylation reduces the overall positive charge of MEL, especially by the bottlebrush PEG; the two pacMELs both exhibit near-neutral ζ potential (0.3 mV) compared with natural MEL (~ 8 mV, Figure 1E).
Reductive cleavage and proteinase stability
We first tested the bioreductive release of pacMELClv, which offers a secondary tumor-targeting effect by releasing active MEL more readily at tumor sites, in addition to the primary EPR effect. Glutathione levels are reported to be elevated in several human cancers, including NSCLC, which is used as a model to investigate the efficacy of pacMEL formulations (vide infra).35,36 Dithiothreitol (DTT, 10 mM in phosphate-buffered saline, PBS) was used to simulate the intracellular reducing environment in cancer cells, and agarose gel electrophoresis was used to monitor the release kinetics of MEL from the conjugate (Figure 2B). Using band densitometry analysis, a release profile is plotted as a function of incubation time. It is found that 50% MEL is released from the pacMELClv during the first hour, and 80% release is achieved in ~ 4 h (Figure 2C). The faster release kinetics relative to clearance (vide infra) suggests that the majority of conjugated MEL will become bioavailable as opposed to remaining in the prodrug form after clearance.
Figure 2.

(A) Schematic illustration of the bioreductive release of MEL from pacMELClv and degradation of the MEL component by α-chymotrypsin (Protein Data Bank ID 2CHA). (B) Agarose gel electrophoresis (2%) of pacMELClv treated with 10 mM DTT with varying treatment times. (C) The release profile for pacMELClv was determined by gel band densitometry analysis. (D) Polyacrylamide gel electrophoresis showing α-chymotrypsin degradation of pacMELClv, YPEG-MEL, and free MEL. The percentage of MEL degradation after 120 min of treatment is shown in (E). **P < 0.01, and ****P < 0.0001 (two-tailed t-test).
Next, we studied whether the bottlebrush PEG can shield the MEL better than conventional PEG using a proteinase assay. In this assay, α-chymotrypsin, which can catalyze the hydrolysis of peptide bonds on the C-terminal side of tryptophan and leucine,37 was used to treat the MEL-containing samples, and cleaved vs. intact MEL can be quantified after polyacrylamide gel electrophoresis (PAGE, Figure 2D). Upon the addition of α-chymotrypsin, free MEL and YPEG-MEL were digested rapidly; the bands corresponding to intact MEL diminished significantly after 15 min. In contrast, the majority (~ 60%) of pacMELClv remain intact after 2 h (Figure 2E). These results suggest that the bottlebrush PEG can offer markedly better steric shielding than slightly branched PEG, and in principle can more effectively inhibit unwanted interactions between MEL and various proteins/cells in the circulation system. Of note, α-chymotrypsin is a relatively small protein with a molecule weight of 25 kDa;38 larger proteins are expected to be blocked against to an even greater extent by the bottlebrush polymer.
Cellular uptake, cytotoxicity, and blood compatibility
One may intuitively assume that the dense PEGylation associated with the bottlebrush polymer diminishes all forms of interactions with the cells including endocytosis. Nonetheless, our recent studies on bottlebrush PEGylated oligonucleotides suggest that the hydrophobic backbone of the bottlebrush polymer is responsible for a moderate amount of endocytosis, and in fact, the conjugates exhibit ~10-fold higher cell uptake compared to naked oligonucleotides.39,40 It is therefore of curiosity to compare the cell uptake of free and PEGylated MEL forms. A human NSCLC cell line (NCI- H358) and an ovarian carcinoma cell line (SKOV3) are selected as models to study the cellular uptake activities. The uptake of free MEL in both cell lines is a fast and accelerating process (Figure 3 A and B, Figure S5A and B). It has been suggested that such unusual uptake is initiated first by electrostatic interaction of the cationic peptide with the negatively charged cell membrane, followed by plasma membrane poration, which enables subsequent fast and direct plasma membrane permeation.41,12 For the PEGylated MELs, cell uptake rates are slower and more linear, which is consistent with an endocytosis mechanism.42–44 Interestingly, the pacMELs are taken up more readily than YPEG-MEL, although the MEL component in the former is less exposed than in the latter. This phenomenon may be attributed to the hydrophobic effect of the polymer backbone, which promotes cell uptake.45 The distinct cellular uptake characteristics of naked and PEGylated MELs are also corroborated by confocal microscopy. Whereas free MEL exhibits very high cell-associated signals and a more diffused appearance of MEL’s intracellular distribution (suggesting cytosol access, Figure 3C), PEGylated MELs show reduced uptake and a punctate pattern indicative of endosomal localization.46
Figure 3.

(A and B) Cellular uptake kinetics (as determined by flow cytometry) of cells treated with free MEL and PEGylated MELs (1 μM of MEL for 1–4 h). (C) Confocal microscopy images of NCI-H358 cells treated with samples containing 1 μM FITC-labeled MEL for 4 hours. Cell nuclei are stained with DAPI (blue). The scale bar is 20 μm. Imaging settings were kept identical for all samples. However, signals for PEGylated samples were boosted 200% post-imaging in order for details to be visible on-screen.
Because the cytotoxicity of MEL relies on the formation of its tetramer and the resulting lytic activity, the intracellular release of pacMELClv under bioreductive conditions can be evaluated by comparing its cytotoxicity with the non-cleavable pacMELNClv and free MEL. We examined the cell viability using NCI-H358 and SKOV-3 cells after treatment with MEL-containing samples for 48 h. Remarkably, the cleavable conjugate, pacMELClv, retained almost the entirety of the bioactivity of the free peptide (Figure 4A, S5C), suggesting efficient uptake and conversion from prodrug to active drug. It may be possible that the initial lytic activity of released MEL accelerated subsequent uptake of the conjugate over the course of 48 h. In contrast, the non-releasable pacMELNClv exhibits no evident cytotoxicity. On the other hand, YPEG-MEL, which is non-cleavable, displays reduced but still apparent cytotoxicity, indicating that the normal PEGylation strategy cannot completely abolish the lytic activity of MEL.
Figure 4.

(A) Cell viability of NCI-H358 cells treated with MEL-containing samples and brush PEG for 48 h. (B) Activated partial thromboplastin times (aPTT) of human plasma treated with MEL-containing samples and controls. (C and D) Hemolysis assay of human red blood cells treated with MEL containing samples and controls. The percentage of hemolysis is determined by spectrophotometric measurement of hemoglobin present in the supernatant of centrifuged red blood cell suspensions. ***P < 0.001 and ****P < 0.0001 (two-tailed t-test).
Currently, the majority of peptide-based drugs are administrated through parenteral routes, including subcutaneous, intravenous and intramuscular administration, as a result of their low oral bioavailability.47,48 Therefore, to adopt MEL as a biopharmaceutical for passive solid tumor targeting, its behavior in the bloodstream is of great importance. It has been reported that MEL inhibits the activity of serine proteases, and thus delaying blood coagulation.11 We tested the anti-coagulation properties of MEL and PEGylated MELs in human plasma using the activated partial thromboplastin time (aPTT) assay (Figure 4B). Free MEL shows pronounced interference with the coagulation cascade, doubling the coagulation times at 20 μM MEL concentration. In contrast, pacMELClv displays a slight (statistically insignificant) increase in clotting times, while the bottlebrush PEG itself exhibits no measurable change. In comparison, both the YPEG-MEL and a physical mixture of MEL/bottlebrush PEG result in similar anticoagulation effects as naked MEL, indicating the architectural features of the bottlebrush are important in creating the steric hindrance necessary for inhibiting protein access.
Next, we examined MEL-induced hemolysis, a critical drawback of the peptide, by measuring the hemoglobin released from red blood cells (RBCs) (Figure 4C, D, and S5D). Free MEL, expectedly, shows near-complete hemolysis of RBCs at 60 μM, with no visible precipitant after centrifugation. Even at 1 μM, 59% hemolysis was observed. All PEGylated samples exhibit greatly decreased hemolytic levels. YPEG-MEL (1 μM) displays reduced albeit non-negligible hemolysis (16%). Strikingly, both cleavable and non-cleavable pacMELs (1 μM) show almost no detectable hemolysis (<1%). The hemolytic activity of MEL for the cleavable conjugate can be partly restored by coincubating with 10 mM DTT (~ 65% lysis of RBCs, consistent with the expected level of MEL release), and fully restored when coincubation time with DTT is increased to 8 h (DTT itself has no hemolytic property). The observation that pacMELClv shows essentially no hemolysis is consistent with the fact that mature RBCs do not endocytose, and thus cannot release MEL intracellularly.49 These results, together with enzymatic degradation, cytotoxicity, and blood coagulation studies (vide supra) paint an overall picture in which traditional PEGylation using linear or slightly branched PEG (e.g., YPEG) leaves the payload somewhat open to interaction with its intended or unintended target. The bottlebrush architecture, in contrast, imparts much stronger shielding to the payload against interactions with surrounding macromolecular or cellular species, making it more effective at suppressing unwanted side effects.
Pharmacokinetics, biodistribution, and in vivo antitumor efficacy
We anticipate that the biological stealth character of PEG and its dense arrangement in the pacMEL will reduce the non-specific interactions between MEL and serum proteins and thereby minimize clearance by the mononuclear phagocyte system (MPS).50 In addition, the large overall size of the pacMEL (~ 300 kDa) should bypass rapid renal clearance.51,52 These properties should in turn enhance plasma pharmacokinetics, which has been shown to favorably correlate with nanomedicine’s ability to passively accumulate in the mice tumor tissues through extravasation from the microvasculature (EPR effect).53–55 To evaluate the plasma pharmacokinetics of the pacMELs, we administered pacMELs, free MEL, and the bottlebrush polymer at equal peptide/polymer concentrations to immuno-competent C57BL/6 mice via the tail vein. The MEL component is labeled with a Cy5.5 dye; in the case of the free brush polymer, the dye is directly attached to the polymer (~1.0 Cy5.5 per bottlebrush polymer). Blood samples at a series of predetermined timepoint up to 48 hours were collected and analyzed (Figure 5A). Fitting the pharmacokinetic data using a two-compartment model, it is apparent that all bottlebrush polymer-containing samples exhibit a similar profile, in which the particles quickly distribute into tissues with distribution half-lives (t1/2α) around 15 min, and are cleared very slowly with elimination half-lives (t1/2β) around 15 h. There was still ~ 20% of injected dose remaining in blood circulation after 48 h of injection. In contrast, naked MEL was rapidly cleared, likely via the kidney, with t1/2α and t1/2β of 0.48 min and 43 min, respectively, which suggests low drug utilization. As a result, pacMELs experience substantially improved blood availability compared to free MEL (~ 10-fold higher the area under the curve [AUCpacMEL,∞/AUCMEL,∞ = 10]). While YPEG-MEL exhibited longer blood circulation times than the free MEL, the improvement is not evident when compared with the pacMELs or the bottlebrush polymer. Given the inferior in vitro and pharmacokinetics performance, YPEG-MEL is not included in subsequent in vivo tests. All pharmacokinetic parameters are summarized in Table S1.
Figure 5.
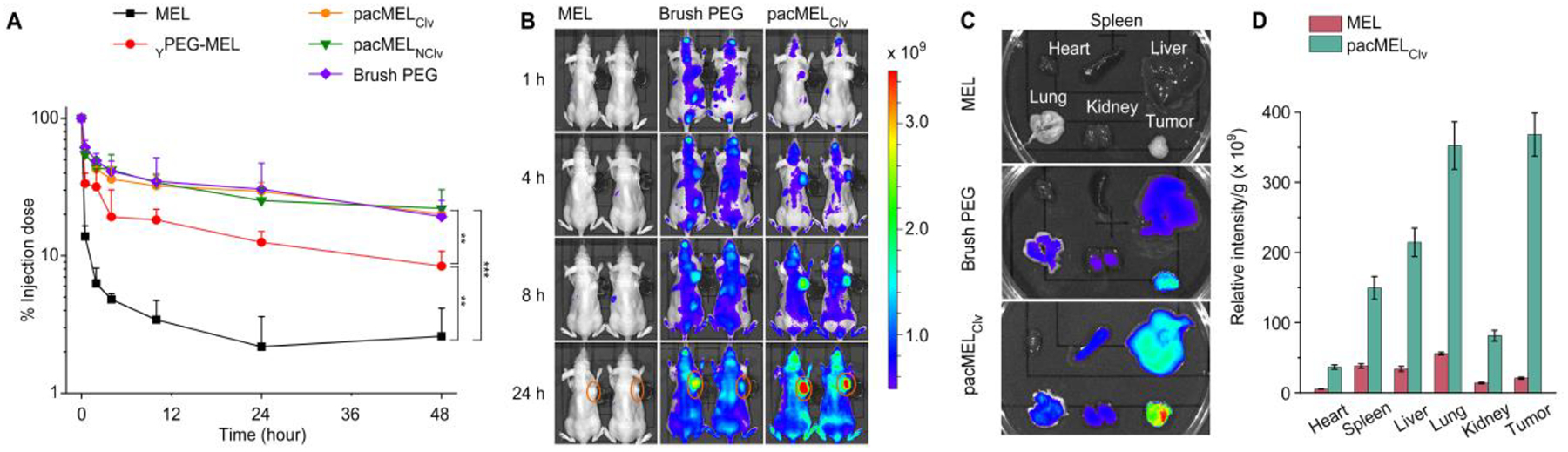
(A) Plasma pharmacokinetics of MEL-containing samples and free bottlebrush polymer in C57BL/6 mice. (B) Near-IR imaging of BALB/c mice bearing NCI-H358 xenografts 24 h after i.v. injection with Cy5.5-labeled free MEL, pacMELClv, and bottlebrush polymer. Tumors are highlighted with orange circles. Of note, the fluorescent intensities for the bottlebrush polymer and pacMELClv are not directly comparable due to non-equal fluorescence at equal molar concentrations (1 Cy5.5 per brush polymer and ~ 2 Cy5.5 per pacMELClv). (C and D) Ex vivo imaging of tumor and other major organs, and biodistribution profile determined from image analysis.
Fluorescent imaging of both live animals and dissected major organs/tumor 24 h post-injection shows generally much stronger signals in mice injected with bottlebrush polymer-containing samples than those receiving free MEL (Figure 5B and C). Importantly, pacMELs resulted in significantly improved tumor uptake (18.4-fold higher compared to free MEL), likely via the EPR effect (Figure 5D). Notably, the free MEL-treated group shows low tumor vs. spleen (0.55) and tumor vs. liver (0.61) ratios, as determined by the relative fluorescent intensities, suggesting potential systemic toxicity, while these values for pacMELClv are 2.46 (spleen) and 1.42 (liver). Similar results are observed for the bottlebrush polymer (Figure S6). We further examined the tumor penetration depth by confocal microscopy of cryosectioned tumor slices that are stained with 4’,6-diamidino-2-phenylindole (DAPI, Figure 6A). It is found that significant MEL signals are present throughout the sliced tumor section of pacMELClv-treated mice, similar to that of bottlebrush PEG-treated mice. These data clearly show that the bottlebrush polymer can effectively alleviate the fast clearance of naked MEL, achieve pro-longed systematic circulation, and impart the tumor accumulation and penetration properties to the embedded MEL.
Figure 6.

(A) Confocal microscopy images of NCI-H358 tumor cryosections following i.v. injection, showing deep tumor penetration. Blue: cell nuclei stained with DAPI. The scale bar is 100 μm. (B) Tumor volume change in the course of a 30 day-treatment.
To evaluate the antitumor efficacy of pacMEL, we established xenografts of NCI-H358 cells in athymic nude mice. Mice received tail-vein injections of PBS, free MEL, pacMELClv (normal and ¼ dose), and pacMELNClv every 3 days at a dosage of 3 mg/kg (MEL-basis). Dosage is determined based on reported median lethal dose (LD50) of intravenously (i.v.) delivered naked MEL for mice, which is 4 mg/kg.56 By day 30, tumors in vehicle-treated mice have grown to 453% of their original sizes. Despite a near-LD50 dosage, free MEL did not lead to statistically meaningful repression of tumor growth relative to the negative control, likely because tumor-associated MEL levels have yet to reach cytotoxic concentrations. In contrast, pacMELClv at the identical MEL dosage resulted in a significant decrease in tumor growth (165%, Figure 6B), which is attributed to improved pharmacokinetics and tumor localization. At ¼ dosage, however, the conjugate was not as effective. Although the pacMELClv does not lead to tumor stasis or regression at the tested concentrations, there is a clear dosage response, and the conjugate may have a higher mean tolerable dosage (MTD) to allow for more effective treatment, given its modified biodistribution profile and much reduced hemolytic activity. Indeed, while naked MEL caused alarming increases in alanine trans-aminase (ALT) and aspartate aminotransferase (AST) in C57BL/6 mice, indicative of liver damage, pacMELClv-treated groups exhibited no such increases and no noticeable changes other important biochemical and hematological parameters (Figure 7A and S7). Staining sectioned major organs with hematoxylin and eosin (H&E) revealed no abnormal histological changes for mice treated with all samples (Figure S8). It has been shown that, despite causing elevated liver enzymes and sometimes deaths, naked MEL may not produce observable histological evidence of toxicity.57 Further, free MEL induces activation of interleukin-6 (IL-6), as determined in a cytokine analysis using enzyme-linked immunosorbent assay (ELISA, Figure 7B). The pacMELs and the bottlebrush polymer, on the other hand, do not induce evident differences in the levels of IL-6, IL-10, IL-12, and tumor necrosis factor α (TNF-α). Collectively, these results show that the bottlebrush polymer is able to impart a more desirable pharmacological and safety profile to MEL while enhancing bioavailability and specific toxicity to the tumor.
Figure 7.

(A) Hepatic damage indicators (ALP, ASP, ALT, and total bilirubin) in the serum and (B) cytokine levels (IL-6, IL-10, IL-12, and TNF-α) in immunocompetent mice (C57BL/6) after 3 doses of pacMELClv and controls (3 mg/kg MEL basis). **P < 0.01, ***P < 0.001, ****P < 0.0001 (two-tailed t-test).
CONCLUSION
MEL, as well as other narrow therapeutic index agents with strong non-specific interactions, face a difficult compromise: the combined challenges of poor accumulation in target tissues and rapid clearance by the liver and/or the kidney require larger doses of the agent, but increasing doses would lead to elevated toxicity and/or immunological responses. These challenges limit the therapeutic window between a therapy that is efficacious and one that is toxic, increase the costs to patients, and very often limit the places in the body where effective therapies can be developed. The results reported herein provide tantalizing evidence that the bottlebrush polymer may be able to solve this conundrum. The high-density arrangement of the PEG side chains of the bottlebrush polymer provides extraordinary steric shielding to the embedded MEL compared to “normal” PEG, allowing the conjugate to bypass non-specific interactions with various proteins and cells while in circulation, thereby reducing coagulopathy, hemolysis, and capture by the MPS system. The large size of the pacMEL enables effective evasion from renal clearance, which significantly prolongs blood circulation times and gives rise to better utilization of the active drug, a more favorable biodistribution profile, and enhanced passive targeting of tumor tissues. Importantly, these pharmacological improvements are realized using a well-defined, single-entity molecular agent that consists predominantly of PEG, which is a safe material having been adopted in a large number of biopharmaceuticals. The simplicity makes these structures more amenable to large-scale manufacturing, quality control, and room-temperature storage compared with self-assembled, dynamic carrier systems that rely on non-covalent supramolecular interactions (such as extracellular vesicles, micelles, etc), which should elevate the translational potential of the conjugate.
In summary, we report a novel form of PEGylated MEL that utilizes the high grafting density of the bottlebrush polymer to achieve improved steric shielding. The conjugate exhibits vastly superior biopharmaceutical properties compared to naked MEL as well as PEGylated MEL containing a conventional, slightly branched PEG. This approach makes MEL a much more attractive anticancer drug candidate by abolishing its excessive hemolytic activity, prolonging plasma pharmacokinetics, and improving tumor uptake. Of significance, the bottlebrush system should be broadly applicable to a variety of therapeutic candidates whose biopharmaceutical properties and therapeutic indices are considered unsatisfactory.
MATERIALS AND METHODS
Animal protocols were approved by the Institutional Animal Care and Use Committee of Northeastern University and carried out in accordance with the approved guidelines.
Biodistribution.
Fluorescence whole-animal and ex vivo tissue imaging were used to assess the biodistribution of test agents in mice. NCI-H358 cells were used to establish subcutaneous xenografts. Cells (2.0 × 106) suspended in PBS solution (200 μL) were inoculated in female athymic nude mice subcutaneously. When the tumor size reaches ~ 200 mm3, mice were randomly divided into 3 groups (n = 2) and administrated with an equivalent dose of MEL (3 mg/kg) or bottlebrush polymer (equal molar concentration as pacMELClv) via tail vein injection in 200 μL PBS solution. Fluorescence imaging was performed using an animal imaging system (IVIS Lumina II imaging system, Caliper Life Sciences Inc. MA, USA) at 1, 4, 8, and 24 h after injection. Mice were euthanized 24 hours after injection and major organs were collected for imaging and biodistribution analysis. Tumor tissues were frozen in optimal cutting temperature compound (Fisher Scientific Inc., USA) and cut into 8 μm thick sections using a cryostat for agents’ tumor penetration depth study. DAPI was used to stain the nuclei and the tumor sections were imaged using confocal microscopy (Carl Zeiss Ltd., Cambridge, UK).
Pharmacokinetics.
Immunocompetent mice (C57BL/6) were randomly divided into 4 groups (n = 5): free MEL, pacMELs, and bottlebrush polymer (MEL is labeled with Cy5.5; equal MEL basis [6 nmol]; identical polymer molar amount as pacMELClv). Samples were injected intravenously via the tail vein and blood samples were collected from the submandibular region at varying time points (30 min, 2, 4, 10, 24, and 48 h) using vacutainer blood collection tubes containing sodium heparin (Becton, Dickinson and Company, US). Plasma at different time points was obtained by centrifugation at 3,000 rpm 4 °C for 15 min. The clear plasma samples were then transferred into a 96-well optical bottom plate (Fisher Scientific Inc., US) for fluorescence readout (ex = 620 nm, em = 680 nm) using a Synergy Neo2 microplate reader (BioTek Instruments Inc., US). The average values of each time point were then plotted against the standard curve prepared by sequential dilution with freshly collected mouse plasma.
Tumor growth inhibition.
Mouse xenograft model (NCI-H358 cell line) was established in athymic nude mice (vide supra). When tumor size reaches ~ 100 mm3, tumor-bearing mice were randomly divided into 5 groups (n = 4): free MEL (3 mg/kg), pacMELClv (dose 3 mg/kg), low-dose pacMELClv (0.75 mg/kg), brush polymer (identical polymer molar amount as normal dose pacMELs), and PBS control. Samples and controls were injected via the tail vein every third day for thirty days. The weight of mice (Fig. S9) and volume of tumors were recorded before each injection and at the end of 10 treatments. Tumor growth inhibition was evaluated by measuring the tumor volume at different time points (V = 0.5 × a × b2; a: long diameter, b: short diameter).
Innate immune response.
Innate immune response was evaluated in female C57BL/6 mice. The mice were randomly divided into 6 groups (n = 5): free MEL (3 mg/kg), pacMELs (equal MEL basis), bottlebrush polymer (identical polymer amount as pacMELs), and negative (vehicle, PBS) and positive (lipopolysaccharide, 15 μg per mouse) controls. Blood samples were collected for cytokine analysis 4 h after injection. TNF-α, IL-6, IL-10, and IL-12 were analyzed by the corresponding ELISA kits (R&D Systems Inc., MN, USA).
Hemanalysis and biochemical analyses.
Blood analyses were assessed in female C57BL/6 mice. Mice were randomly divided into 3 groups (n = 6): free MEL (3 mg/kg), pacMELClv (equal MEL basis), and PBS control. Samples and control were injected via the tail vein once every 3 days for a total of 3 doses. Blood samples were collected 2 days after the last injection (day 11) from the submandibular region. Ethylenediaminetetraacetic acid (EDTA)-treated whole blood samples were used to perform the Complete Blood Count (CBC) panel test, and serum samples were collected for hepatic damage evaluation.
Statistics.
All experiments were repeated at least three times unless otherwise indicated. Data are presented as means ± SD. Statistical significance was evaluated by using a two-tailed t-test when only two groups were compared. Statistical significance was set at **P < 0.01, ***P < 0.001 or ****P < 0.0001.
Supplementary Material
ACKNOWLEDGMENT
We thank the Institute for Chemical Imaging of Living System at Northeastern University for consultation and imaging support.
Funding Sources
Research reported in this publication was supported by the National Institute of General Medical Sciences (1R01GM121612), the National Cancer Institute (1R01CA251730), and the National Science Foundation (DMR award number 2004947).
Footnotes
Supporting Information.
This material is available free of charge via the Internet at http://pubs.acs.org.
Synthesis and characterization of PEGylated MELs; enzymatic degradation of MEL containing samples; in vitro experiments; in vivo antitumor activity and initial biocompatibility evaluation of MEL-containing samples.
REFERENCE
- (1).Henninot A; Collins JC; Nuss JM The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem 2018, 61, 1382–1414. [DOI] [PubMed] [Google Scholar]
- (2).Muttenthaler M; King GF; Adams DJ; Alewood PF Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov 2021, 1–17. [DOI] [PubMed] [Google Scholar]
- (3).Fosgerau K; Hoffmann T Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [DOI] [PubMed] [Google Scholar]
- (4).Choi JH; Jang AY; Lin S; Lim S; Kim D; Park K; Han S-M; Yeo J-H; Seo HS Melittin, a Honeybee Venom-derived Antimicrobial Peptide, May Target Methicillin-resistant Staphylococcus Aureus. Mol. Med. Rep 2015, 12, 6483–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gajski G; Garaj-Vrhovac V Melittin: A Lytic Peptide with Anticancer Properties. Toxicol. Pharmacol 2013, 36, 697–705. [DOI] [PubMed] [Google Scholar]
- (6).Schweizer F Cationic Amphiphilic Peptides with Cancer-Selective Toxicity. Eur. J. Pharmacol 2009, 625, 190–194. [DOI] [PubMed] [Google Scholar]
- (7).Fadnes B; Rekdal Ø; Uhlin-Hansen L The Anticancer Activity of Lytic Peptides Is Inhibited by Heparan Sulfate on the Surface of the Tumor Cells. BMC Cancer 2009, 9, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Soman NR; Lanza GM; Heuser JM; Schlesinger PH; Wickline SA Synthesis and Characterization of Stable Fluorocarbon Nanostructures as Drug Delivery Vehicles for Cytolytic Peptides. Nano Lett. 2008, 8, 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Memariani H; Memariani M; Moravvej H; Shahidi-Dadras M Melittin: A Venom-Derived Peptide with Promising Anti-Viral Properties. Eur. J. Clin. Microbiol. Infect. Dis 2020, 39, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bacalum M; Radu M Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther 2015, 21, 47–55. [Google Scholar]
- (11).Lee J; Park J; Yeom J; Han EH; Lim Y-H Inhibitory Effect of Bee Venom on Blood Coagulation via Anti-Serine Protease Activity. J. Asia. Pac. Entomol 2017, 20, 599–604. [Google Scholar]
- (12).Lee M-T; Sun T-L; Hung W-C; Huang HW Process of Inducing Pores in Membranes by Melittin. PNAS 2013, 110, 14243–14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nishikawa H; Kitani S Gangliosides Inhibit Bee Venom Melittin Cytotoxicity but Not Phospholipase A2-Induced Degranulation in Mast Cells. Toxicol. Appl. Pharmacol 2011, 252, 228–236. [DOI] [PubMed] [Google Scholar]
- (14).Vellard M The Enzyme as Drug: Application of Enzymes as Pharmaceuticals. Curr. Opin. Biotechnol 2003, 14, 444–450. [DOI] [PubMed] [Google Scholar]
- (15).Harris JM; Chess RB Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug. Discov 2003, 2, 214–221. [DOI] [PubMed] [Google Scholar]
- (16).Pasut G; Veronese FM State of the Art in PEGylation: The Great Versatility Achieved after Forty Years of Research. J. Control. Release 2012, 161, 461–472. [DOI] [PubMed] [Google Scholar]
- (17).Peng HT; Huang H; Shek PN; Charbonneau S; Blostein MD PEGylation of Melittin: Structural Characterization and Hemostatic Effects. J. Bioact. Compat. Polym 2010, 25, 75–97. [Google Scholar]
- (18).Leitgeb A; Wappel J; Slugovc C The ROMP Toolbox Upgraded. Polymer. 2010, 51, 2927–2946. [Google Scholar]
- (19).Xie M; Dang J; Han H; Wang W; Liu J; He X; Zhang Y Well-Defined Brush Copolymers with High Grafting Density of Amphiphilic Side Chains by Combination of ROP, ROMP, and ATRP. Macromolecules. 2008, 41, 9004–9010. [Google Scholar]
- (20).Medina JM; Ko JH; Maynard HD; Garg NK, Expanding the ROMP Toolbox: Synthesis of Air-Stable Benzonorbornadiene Polymers by Aryne Chemistry. Macromolecules, 2017, 50, 2, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Xia Y; Li Y; Burts AO Ottaviani MF, Tirrell DA, Johnson JA, Turro NJ, Grubbs RH, EPR Study of Spin Labeled Brush Polymers in Organic Solvents. J. Am. Chem. Soc 2011, 133, 19953–19959. [DOI] [PubMed] [Google Scholar]
- (22).Vohidov F; Andersen JN; Economides KD; Shipitsin MV; Burenkova O; Ackley JC; Vangamudi B; Nguyen HV-T; Gallagher NM; Shieh P; Golder MR; Liu J; Dahlberg WK; Ehrlich DJC; Kim J; Kristufek SL; Huh SJ; Neenan AM; Baddour J; Paramasivan S; de Stanchina E; KC G; Turnquist DJ; Saucier-Sawyer JK; Kopesky PW; Brady SW; Jessel MJ; Reiter LA; Chickering DE; Johnson JA; Blume-Jensen P Design of BET Inhibitor Bottlebrush Prodrugs with Superior Efficacy and Devoid of Systemic Toxicities. J. Am. Chem. Soc 2021, 143, 4714–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jia F; Lu X; Tan X; Wang D; Cao X; Zhang K Effect of PEG Architecture on the Hybridization Thermodynamics and Protein Accessibility of PEGylated Oligonucleotides. Angew. Chem. Int. Ed 2017, 56, 1249–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jia F; Lu X; Wang D; Cao X; Tan X; Lu H; Zhang K Depth-Profiling the Nuclease Stability and the Gene Silencing Efficacy of Brush-Architectured Poly(Ethylene Glycol)–DNA Conjugates. J. Am. Chem. Soc 2017, 139, 10605–10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wang D; Lin J; Jia F; Tan X; Wang Y; Sun X; Cao X; Che F; Lu H; Gao X; Shimkonis JC; Nyoni Z; Lu X; Zhang K Bottlebrush-Architectured Poly(Ethylene Glycol) as an Efficient Vector for RNA Interference in Vivo. Sci. Adv 2019, 5, eaav9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lu X; Tran T-H; Jia F; Tan X; Davis S; Krishnan S; Amiji MM; Zhang K Providing Oligonucleotides with Steric Selectivity by Brush-Polymer-Assisted Compaction. J. Am. Chem. Soc 2015, 137, 12466–12469. [DOI] [PubMed] [Google Scholar]
- (27).Shieh P; Nguyen HV-T; Johnson JA Tailored Silyl Ether Monomers Enable Backbone-Degradable Polynorbornene-Based Linear, Bottlebrush and Star Copolymers Through ROMP. Nat. Chem 2019, 11, 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shieh P; Zhang W; Husted KEL; Kristufek SL; Xiong B; Lundberg DJ; Lem D; Veysset J; Sun Y; Nelson KA; Plata DL; Johnson JA Cleavable Comonomers Enable Degradable, Recyclable Thermoset Plastics. Nature. 2020, 583, 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Johnson JA; Lu YY; Burts AO; Xia Y; Durrell AC; Tirrell DA, Grubbs RH Drug-Loaded, Bivalent-Bottle-Brush Polymers by Graft-through ROMP. Macromolecules 2010, 43, 10326–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Bates CM; Chang AB; Momčilović N; Jones SC; Grubbs RH ABA Triblock Brush Polymers: Synthesis, Self-Assembly, Conductivity, and Rheological Properties. Macromolecules 2015, 48, 4967–4973. [Google Scholar]
- (31).Xu L; Kuan SL; Weil T Contemporary Approaches for Site-Selective Dual Functionalization of Proteins. Angew. Chem. Int. Ed 2021, 60, 13757–13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhang M; Müller AHE Cylindrical Polymer Brushes. J. Polym. Sci., Part A: Polym. Chem 2005, 43, 3461–3481. [Google Scholar]
- (33).Jia F; Kubiak JM; Onoda M; Wang YRJ Macfarlane, Design and Synthesis of Quick Setting Non-Swelling Hydrogels via Brush Polymers. Adv. Sci 2021, 2100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pesek SL; Li X; Hammouda B; Hong K; Verduzco R Small-Angle Neutron Scattering Analysis of Bottlebrush Polymers Prepared via Grafting-Through Polymerization. Macromolecules 2013, 46, 17, 6998–7005 [Google Scholar]
- (35).Blair SL; Heerdt P; Sachar S; Abolhoda A; Hochwald S; Cheng H; Burt M Glutathione Metabolism in Patients with Non-Small Cell Lung Cancers. Cancer Res. 1997, 57, 152–155. [PubMed] [Google Scholar]
- (36).Gamcsik MP; Kasibhatla MS; Teeter SD; Colvin OM Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ingles D; Knowles J Specificity and Stereospecificity of α-Chymotrypsin. Biochem. J 1967, 104, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kumar A; Venkatesu P Overview of the Stability of α-Chymotrypsin in Different Solvent Media. Chem. Rev 2012, 112, 4283–4307. [DOI] [PubMed] [Google Scholar]
- (39).Lu X; Jia F; Tan X; Wang D; Cao X; Zheng K Effective Antisense Gene Regulation via Noncationic, Polyethylene Glycol Brushes. J. Am. Chem. Soc 2016, 138, 9097–9100. [DOI] [PubMed] [Google Scholar]
- (40).Wang D; Lu X; Jia F; Tan X; Sun X; Cao X; Wai F; Zhang C; Zhang K Precision Tuning of DNA- and Poly(Ethylene Glycol)-Based Nanoparticles via Coassembly for Effective Antisense Gene Regulation. Chem. Mater 2017, 29, 9882–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yang L; Harroun TA; Weiss TM; Ding L; Huang HW Barrel-Stave Model or Toroidal Model? A Case Study on Melittin Pore. Biophys. J 2001, 81, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Cao X; Lu X; Wang D; Jia F; Tan X; Corley M; Chen X; Zhang K Modulating the Cellular Immune Response of Oligonucleotides by Brush Polymer-Assisted Compaction. Small 2017, 13, 1701432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Choi CHJ; Hao L; Narayan SP; Auyeung E; Mirkin CA Mechanism for the Endocytosis of Spherical Nucleic Acid Nanoparticle Conjugates. PNAS 2013, 110, 7625–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McMahon HT; Boucrot E Molecular Mechanism and Physiological Functions of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol 2011, 12, 517–533. [DOI] [PubMed] [Google Scholar]
- (45).Yu C; He B; Xiong M-H; Zhang H; Yuan L; Ma L; Dai W-B; Wang J; Wang X-L; Wang X-Q; Zhang Q The Effect of Hydrophilic and Hydrophobic Structure of Amphiphilic Polymeric Micelles on Their Transport in Epithelial MDCK Cells. Biomaterials 2013, 34, 6284–6298. [DOI] [PubMed] [Google Scholar]
- (46).Urbé S; Mills IG; Stenmark H; Kitamura N; Clague MJ Endosomal Localization and Receptor Dynamics Determine Tyrosine Phosphorylation of Hepatocyte Growth Factor-Regulated Tyrosine Kinase Substrate. Mol. Cell. Biol 2000, 20, 7685–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bruno BJ; Miller GD; Lim CS Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Deliv 2013, 4, 1443–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Patel A; Patel M; Yang X; Mitra AK Recent Advances in Protein and Peptide Drug Delivery: A Special Emphasis on Polymeric Nanoparticles. Protein Pept. Lett 2014, 21, 1102–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Wadhwa R; Aggarwal T; Thapliyal N; Kumar A; Priya; Yadav P; Kumari V; Reddy BSC; Chandra P; Maurya PK Red Blood Cells as an Efficient in Vitro Model for Evaluating the Efficacy of Metallic Nanoparticles. 3 Biotech 2019, 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Halma C; Daha MR; Es L. a. V. In Vivo Clearance by the Mononuclear Phagocyte System in Humans: An Overview of Methods and Their Interpretation. Clin. Exp. Immunol 1992, 89, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ruggiero A; Villa CH; Bander E; Rey DA; Bergkvist M; Batt CA; Manova-Todorova K; Deen WM; Scheinberg DA; McDevitt MR Paradoxical Glomerular Filtration of Carbon Nanotubes. PNAS 2010, 107, 12369–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Meibohm B; Zhou H Characterizing the Impact of Renal Impairment on the Clinical Pharmacology of Biologics. J. Clin. Pharmacol 2012, 52, 54S–62S [DOI] [PubMed] [Google Scholar]
- (53).Fang J; Nakamura H; Maeda H The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev 2011, 63, 136–151. [DOI] [PubMed] [Google Scholar]
- (54).Greish K Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. Methods Mol. Biol 2010, 624, 25–37. [DOI] [PubMed] [Google Scholar]
- (55).Danhier F To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [DOI] [PubMed] [Google Scholar]
- (56).Habermann E; Zeuner G Comparative Studies of Native and Synthetic Melittins. Naunyn-Schmiedebergs’ Arch. Pharmacol 1971, 270, 1–9. [DOI] [PubMed] [Google Scholar]
- (57).Saeed WSE; Khalil E. a. G. Toxic Effects and Safety of Bee Venom Protein [Melittin] in Mice: Search for Natural Vaccine Adjuvants. J. Nat. Prod. Resour 2017, 3, 111–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


