Summary
Detection of protein O-GlcNAcylation could be challenging. By using the host-cell factor 1 (HCF-1), a known O-GlcNAcylated protein, we immunoprecipitated HCF-1 from transfected HEK293T cells or endogenous HCF-1 from HeLa cells to detect its O-GlcNAc levels by Western blotting. We also take advantage of RNAi or chemical inhibitors to modulate OGT and OGA activities before HCF-1 immunoprecipitation.
For complete details on the use and execution of this protocol, please refer to Daou et al. (2011).
Subject areas: Cell Biology, Molecular Biology, Protein Biochemistry
Graphical abstract

Highlights
-
•
O-GlcNAcylation is an abundant and dynamic posttranslational modification of proteins
-
•
Immunoprecipitation and western blot detection of protein O-GlcNAcylation in cells
-
•
RNAi or inhibitors targeting OGT or OGA are used to modulate protein O-GlcNAcylation
Detection of protein O-GlcNAcylation could be challenging. By using the host-cell factor 1 (HCF-1), a known O-GlcNAcylated protein, we immunoprecipitated HCF-1 from transfected HEK293T cells or endogenous HCF-1 from HeLa cells to detect its O-GlcNAc levels by Western blotting. We also take advantage of RNAi or chemical inhibitors to modulate OGT and OGA activities before HCF-1 immunoprecipitation.
Before you begin
Overview
O-GlcNAcylation is an abundant post-translational modification of nuclear and cytoplasmic proteins and is catalyzed by the O-Linked N-acetylglucosamine (O-GlcNAc) transferase (OGT). It consists of the addition of a single N-acetylglucosamine residue (GlcNAc) to specific serine or threonine residues of target proteins to regulate their functions. This modification is removed by the O-GlcNAcase (OGA), highlighting the importance of O-GlcNAc turnover in the regulation of protein function. The attachment of O-GlcNAc to specific sites is often compared to phosphorylation, as both modifications target serine/threonine residues, which suggest an interplay between the two processes. O-GlcNAcylation is central to a wide range of cellular processes, including, transcription, translation, cell signaling, and cell cycle regulation. Changes in O-GlcNAc modification have also been implicated in various diseases such as diabetes, Alzheimer’s disease, and cancer (Butkinaree et al., 2010; Capotosti et al., 2011; Yang and Qian, 2017; Olivier-van Stichelen and Hanover, 2015; Ferrer et al., 2016; Bond and Hanover, 2015; Hart, 2019; Tramutola et al., 2018; Chang et al., 2020). Despite its abundance in cells, detecting O-GlcNAcylation of proteins can be challenging, as previously discussed in Gagnon et al. (2015). Using the chromatin-associated protein, the host-cell factor 1 (HCF-1), a known O-GlcNAcylated protein, we propose a protocol for the immunoprecipitation and immunodetection of protein O-GlcNAcylation following SDS-PAGE separation of total cell proteins. Several controls are included to ensure robust detection of protein O-GlcNAcylation.
This protocol gives complete details on the execution of the experimental approach for the detection of HCF-1 protein O-GlcNAcylation as published by Affar and colleagues (Daou et al., 2011). This protocol provides new useful updates that help firmly validate protein O-GlcNAcylation.
Experimental design and assessment of control conditions
Timing: 1–3 days
Before starting the experiment, it is important to make a chronologic design of the procedure and to think about all possible technical and biological controls, which are important to enable concluding whether the protein of interest is modified by O-GlcNAcylation in the cells.
To this end, an easy step to begin with is to determine O-GlcNAc levels by modulating OGT and OGA, which are the sole enzymes catalyzing and suppressing O-GlcNAcylation in mammals, respectively. We present here three approaches to evaluate protein O-GlcNAcylation. First, depletion of OGT expression using specific short-hairpin RNA (shRNA) that causes an overall decrease of total O-GlcNAc levels. Second, inhibition of OGA activity using specific chemical inhibitors such as PUGNAc or Thiamet G to increase the total levels of O-GlcNAcylation in cells (Yuzwa, 2008). Third, overexpression of the protein of interest with OGT to promote substrate O-GlcNAcylation. Additional controls include the overexpression of a catalytic dead mutant of OGT (OGTCD) or OGA. Other possible strategies targeting OGT/OGA activity are represented in Figure 1.
Figure 1.
Schematic representation of possible strategies to target OGT/OGA activities to modulate the process of O-GlcNAcylation
To determine the O-GlcNAcylation state of the protein of interest after modulating the intracellular O-GlcNAcylation process, immunoprecipitation of endogenously expressed or overexpressed protein is conducted and the degree of protein O-GlcNAcylation is determined using anti-O-GlcNAc antibodies in conjunction with Western Blotting (Figures 1 and 2).
Figure 2.
Pipelines of cell treatments to modulate HCF-1 O-GlcNAcylation in cells
To perform these experiments, we choose to use the human transformed embryonic kidney cells (HEK293T) and the cervical cancer derived cells (HeLa), which are widely used cell models to study protein signaling and are easily transfected for overexpression experiments.
Note: This protocol was optimized for the use of HeLa or HEK293T cells, as these are easily amenable to transfection. However, other cells lines could be used for transfection or for treatments with inhibitors such as: 3T3L1 murine adipocytes, IMR90 human pulmonary fibroblasts, pancreatic beta cells, MCF7 breast cancer cells, hPSC pluripotent stem cells, and many other cell types (Vosseller et al., 2002; Forma et al., 2018; Jo et al., 2016; Maury et al., 2013).
Before starting the experiment, make sure the cells are maintained in healthy conditions (passage every two days, low passage number, mycoplasma free). In addition, make sure that the plasmid constructs encoding a tagged form of the protein of interest or OGT/OGA as well as OGT shRNA constructs are highly purified.
In this protocol, we used HCF-1 as an example of protein modified by O-GlcNAcylation. HCF-1 is a transcription co-regulator that interacts with OGT (Kapuria et al., 2016; Yu et al., 2010), known to be modified by O-GlcNAcylation on its N-terminal region (Kapuria et al., 2016) and proteolytic processing domain (PPD) (Daou et al., 2011).
The design of this experiment will include three major steps (see graphical abstract):
-
1.Cell treatments:
-
a.HeLa cells transfection with OGT shRNA (results shown in Figure 3 of Daou et al., 2011) or treatment with OGA inhibitors (results shown in this protocol) for subsequent immunoprecipitation and detection of O-GlcNAcylation of the endogenous HCF-1 protein.
-
b.Overexpression of tagged HCF-1 in HEK293T cells and co-transfection with OGT, OGTCD mutant or OGA enzymes (results shown in Figure 3 of Daou et al., 2011).
-
a.
-
2.
Protein immunoprecipitation methods for endogenous and overexpressed HCF-1.
-
3.
Data acquisition and analysis by detecting O-GlcNAc levels following Western Blotting.
Cell treatments or transfection
Timing: 3 days
-
3.Treatments or transfection of HeLa cells for immunoprecipitation of HCF-1 protein under different conditions modulating O-GlcNAcylation.
-
a.In 15 cm dishes, seed 6 million HeLa cells in 20 mL of high glucose Dulbecco's Modified Eagle Medium (DMEM) with 5% Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (P/S) antibiotics. Incubate the cells at 37°C, 5% CO2 in a humidified atmosphere for at least 16 h. We will need 17 dishes for all the conditions (see the partition of these dishes in Figure 2).Note: we suggest seeding the cells in large 15 cm dishes to have enough material for the IP of endogenous proteins. Depending on the nature and intracellular stability of studied proteins, the number of cells treated or transfected could be reevaluated and optimized.
-
b.The following day, perform the treatment as described below:
-
i.Change the media of two dishes and treat these with 50 μM of PUGNAc for 3–9 h in DMEM medium containing 5% FBS.
-
ii.Change the media of two dishes and treat these with 50 μM of Thiamet G for 3–9 h in DMEM medium containing 5% FBS.
-
iii.Change the media of two dishes and treat these with DMSO, as the control condition, in DMEM medium containing 5% FBS.
-
iv.Wash two dishes three times with 20 mL each of Phosphate Buffer Saline (PBS) to discard residual media and then incubate the cells in 20 mL of Hank’s Balanced Salt Solution (HBSS) to induce nutrient starvation for 2 h.
-
v.To collect the cells, wash with 10 mL per 15 cm dish of ice-cold PBS. Empty the dishes, add 2 mL of ice-cold PBS, and then directly scrap the cells. Quickly collect the cells in a 15 mL tubes on ice.
-
vi.Centrifuge the cells for 5 min at 2000 RPM/400 g and remove the supernatant.
 Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed to cell lysis for the IP.
Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed to cell lysis for the IP.
-
i.
-
c.On the other hand, take four dishes and transfect them with shRNA targeting OGT. In addition, take another four dishes and transfect them with shRNA targeting GFP as a control. Keep one dish to be used later as a puromycin selection control to assess the transfection efficiency (see Figure 2). The transfection steps are described below:
-
i.In two 15 mL tubes, prepare Mix A-1 and Mix A-2 by adding 2 mL of Extreme DMEM to 60 μg of plasmid expressing either an shRNA targeting OGT or GFP, respectively and 2 μg of pBabe-puro plasmid to ensure puromycin resistance (see table below).
-
ii.In another 15 mL tube, prepare Mix B, which contains the lipofectamine 2000 transfection reagent. Add 4 mL of Extreme DMEM media to 240 μL of lipofectamine 2000 reagent (see table below).Note: The amount of plasmids and reagents used is sufficient to transfect four 15 cm dishes for each condition (the transfection ratio for one dish is: 15 μg of shRNA OGT/GFP plasmids, 0.5 μg of pBabe-puro plasmid, 30 μL of Lipofectamine 2000 and 1 mL of Extreme DMEM).
Mix A-1 Mix A-2 Mix B shRNA OGT plasmid 60 μg – – shRNA GFP plasmid – 60 μg – pBabe-puro plasmid 2 μg 2 μg – Lipofectamine 2000 – – 240 μL Extreme DMEM (to final volume) 2 mL 2 mL 4 mL -
iii.Take 2 mL of Mix B and add it to each tube containing Mix A to obtain a final volume of 4 mL of the transfection mixture. Vortex gently and incubate at 22°C–25°C for 30 min.
-
iv.Just before transfecting the cells, change culture media with 17 mL of serum free DMEM media supplemented with P/S. Add 1 mL of the transfection mixture dropwise into each dish. Shake gently and put in the incubator for 6–9 h.
-
v.Remove the transfection media, add fresh DMEM media containing 5% FBS and incubate the cells for 24 h.
-
vi.After 48h, add puromycin to the dishes to a final concentration of 2 μg/mL and wait until cells in the control dish are completely dead (about 24 h). At this point, a difference should be observed between the viable cells in the transfected dishes with shRNA OGT/GFP versus the control. Approximately 30%–50% of the cells should stay viable.
-
vii.Wash the cells twice with media to remove the dead cells, and harvest the cells according to step 1. b. v.
-
viii.Centrifuge the cells for 5 min at 4°C at 2000 RPM/400 g and remove the supernatant.
 Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed to cell lysis for the immunoprecipitation.
Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed to cell lysis for the immunoprecipitation.
-
i.
-
a.
-
4.In this step, V5-tagged HCF-1 protein is overexpressed in HEK293T cell line by co-transfection with wild type Myc-tagged OGT or OGA enzymes to perform either Western Blotting on total cell extracts as described in Daou et al. (2011) or immunoprecipitation of V5-tagged HCF-1 protein.Note: Here we described immunoprecipitation of V5-tagged HCF-1. However, other tag combinations can be used for the studied protein, such as HA, Flag and Myc.
-
a.In 10 cm dishes, seed 7 million HEK293T cells in 10 mL of DMEM supplemented with 5% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (P/S) antibiotics. Incubate the cells at 37°C with 5% CO2 in a humidified atmosphere for at least 16 h (See the partition of these dishes in Figure 2).
-
b.The day after, transfect the cells with either V5-tagged HCF-1 vector or a GFP expressing vector (as control). HCF-1 will be co-expressed with either Myc-tagged OGT or OGA constructs (see transfection conditions below):
-
i.In an Eppendorf tube, prepare mixes A-1 and A-2, as shown in the table below, by adding 0.5 mL of Extreme DMEM, and 10 μg of HCF-1 plasmid with either 10 μg of OGT or OGA, respectively. In the same way, prepare Mix A-3, the control transfection condition, which contains control vectors (empty vector or a vector expressing GFP).
-
ii.In another Eppendorf tube, prepare Mix B, which contains enough transfection reagent for all the conditions (we use the ratio of 20 μL of Polyethylenimine (PEI) per 7 μg of DNA mix).
Mix A-1 Mix A-2 Mix A-3 Mix B GFP control plasmid 20 μg – – – V5-tagged HCF-1 plasmid – 10 μg 10 μg – Myc-tagged OGT plasmid – 10 μg – – Myc-tagged OGA plasmid – – 10 μg – Polyethylenimine (PEI) – – – 171.42 μL Extreme DMEM (to final volume) 0.5 mL 0.5 mL 0.5 mL 1.5 mL -
iii.After 5 min of incubation, add 0.5 mL of Mix B to each 0.5 mL of Mixes A-1, A-2, and A-3.
-
iv.Mix vigorously, incubate at 22°C–25°C for 30 min and then delicately add, dropwise, the 1 mL transfection mix to each dish and incubate for 16 h.
-
i.
-
c.The next day, change to fresh media and check for GFP expression in the control condition, using the fluorescence microscope, to confirm the success of the transfection.
-
d.On the third day, harvest the cells according to step 1. b. v.
-
e.Centrifuge the cells for 5 min at 4°C at 2000 RPM/400 g and remove the supernatant.
 Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed directly to cell lysis for the immunoprecipitation.
Pause point: At this step, you can either snap freeze the pellet on dry ice or proceed directly to cell lysis for the immunoprecipitation. CRITICAL: Please see the simplified illustration in Figure 2 for the procedure to produce all the cell conditions for the experiment. All samples frozen on dry ice should be stored at −80°C.
CRITICAL: Please see the simplified illustration in Figure 2 for the procedure to produce all the cell conditions for the experiment. All samples frozen on dry ice should be stored at −80°C.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-O-linked β-D-N-acetylglucosamine | Abcam | Cat# AB2739; RRID: AB_303264 |
| Anti-β-Tubulin | Santa Cruz | Cat# SC-5286; RRID: AB_628411 |
| Anti-V5 | Abcam | Cat# AB2767; RRID:AB_471093 |
| Peroxidase-conjugated AffiniPure Fragment Goat Anti-Rabbit IgG (H +L ) | Jackson ImmunoResearch | Cat# 111-036-003; Lot #93036 |
| Peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG, Light Chain Specific | Jackson ImmunoResearch | Cat# 115-035-174; Lot #94404 |
| Anti-GAL4 | Santa Cruz | Cat# SC-577; RRID:AB_631554 |
| Anti-HCF-1 | Bethyl | Cat# A301-400A; RRID:AB_961015 |
| Chemicals, peptides, and recombinant proteins | ||
| β-Glycerophosphate (βGP) | BioShop | Cat# GYP001 |
| O-(2-Acetamido-2-deoxy-D-glucopyranosylidenamino) N-phenylcarbamate (PUGNAc) | Sigma-Aldrich | Cat# A7229 |
| 2-Mercaptoethanol | BioShop | Cat# MER002.500 |
| Anti-V5 agarose Beads | Sigma-Aldrich | Cat# A7345 |
| Bovine Serum Albumin | BioShop | Cat# ALB001 |
| Dithiothreitol (DTT) | BioShop | Cat# DTT001.10 |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Wisent | Cat# 319-005-CL |
| Ethylenediaminetetraacetic Acid (EDTA) | Sigma-Aldrich | Cat# E5134 |
| Extreme DMEM | Wisent | Cat# 390-000-CL |
| Fetal bovine serum (FBS) | Sigma-Aldrich | Cat# F1051 |
| Glycerol | BioShop | Cat# GLY001 |
| Glycine | Wisent | Cat# 800-045-IK |
| Kanamycin Sulfate | Bio Basic | Cat# KB0286 |
| L-Glutamine | BioShop | Cat# GLU102.250 |
| N-acetyl-D-glucosamine | Sigma-Aldrich | Cat# A8625 |
| Newborn calf serum (NCS) | Sigma-Aldrich | Cat# N4637 |
| Penicillin G | Bio Basic | Cat# PB0135 |
| Phenylmethylsulfonyl Fluoride (PMSF) | BioShop | Cat# PMS123100 |
| Pierce™ ECL | Thermo Fisher Scientific | Cat# 32209 |
| Polyethylenimine (PEI) | Sigma-Aldrich | Cat# 408727 |
| Ponceau | BioShop | Cat# PON001.50 |
| Potassium Chloride | BioShop | Cat# POC888.1 |
| Potassium Phosphate Monobasic | BioShop | Cat# PPM302.1 |
| Protease inhibitor cocktail (PIC) | Sigma-Aldrich | Cat# P8340 |
| Protein G Sepharose® beads | Sigma-Aldrich | Cat# P3296 |
| Skim Milk powder | Nelsea | n/a |
| Sodium Azide (NaN3) | Sigma-Aldrich | Cat# S2002 |
| Sodium Butyrate | Labo Mat | Cat# SP0188 |
| Sodium Chloride (NaCl) | Sigma-Aldrich | Cat# S3014 |
| Sodium Dodecyl Sulfate (SDS) | BioShop | Cat# SDS001.100 |
| Sodium Fluoride (NaF) | Sigma-Aldrich | Cat# S7920 |
| Sodium Orthovanadate (Na3VO4) | Sigma-Aldrich | Cat# S6508 |
| Sodium Phosphate Dibasic Heptahydrate | BioShop | Cat# SPD579.5 |
| Streptomycin | BioShop | Cat# STP101 |
| Thiamet G | Gift from Dr Vocaldo | Cat# n/a |
| Tris Hydroxymethyl Aminomethane (Tris) | BioShop | Cat# TRS001.5 |
| Triton X-100 | Sigma-Aldrich | Cat# X100-1GA |
| Trypsin/EDTA | Wisent | Cat# 325-043-CL |
| Trichloroacetic acid | BioShop | Cat# TCA001 |
| Sulfosalicylic acid | BioShop | Cat# SFS002 |
| Tween® 20 | Sigma-Aldrich | Cat# P1379-1L |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | Cat# CRL-3216; RRID:CVCL_0063 |
| HeLa | ATCC | Cat# CCL-2; RRID:CVCL_0030 |
| Recombinant DNA | ||
| V5 tagged HCF-1 vector | Daou et al. (2011) | n/a |
| pLenti GFP | Daou et al. (2011) | n/a |
| shRNA OGT vector | Daou et al. (2011) | n/a |
| shRNA GFP vector | Daou et al. (2011) | n/a |
| pBabe-puro | Daou et al. (2011) | n/a |
| Other | ||
| Azure c600 | Azure Biosystems | n/a |
| Cell culture Petri dish 100 × 20 mm | SARSTEDT | Cat# 83.3902 |
| Cell culture Petri dish 150 × 20 mm | SARSTEDT | Cat# 83.3903 |
| FiltroPur BT 50, bottle top filter 0.22 μm | SARSTEDT | Cat# 83.3941.101 |
| Mini-PROTEAN® Tetra Vertical Electrophoresis Cell | Bio-Rad Laboratories | Cat# 1658004 |
| PowerPac Universal Power Supply | Bio-Rad Laboratories | Cat# 1645070 |
| PVDF membranes | Bio-Rad Laboratories | Cat# 1620177 |
| Scrapers | SARSTEDT | Cat# 83.1830 |
Materials and equipment
High-salt lysis buffer composition for HCF-1 protein immunoprecipitation
| Reagent | Stock | Final concentration (mM or μM) | Amount (μL or mL) |
|---|---|---|---|
| Tris pH 7.5 | 1 M | 50 Mm | 5 mL |
| NaCl | 4 M | 300 mM | 7.5 mL |
| Triton X-100 | 10% | 0.5% | 5 mL |
| EDTA | 500 mM | 5 mM | 1 mL |
| DTT | 500 mM | 1 mM | 200 μL |
| PMSF | 100 mM | 1 mM | 1 mL |
| Anti-protease | 500 mM | 1:100 | 1 mL |
| NaF | 500 mM | 50 mM | 10 mL |
| βGP | 1 M | 10 mM | 1 mL |
| Na3VO4 | 200 mM | 1 mM | 500 μL |
| Sodium Butyrate | 200 mM | 10 mM | 1 mL |
| PUGNAc | 2 mM | 10 μM | 500 μL |
| ddH2O | n/a | n/a | 66.3 mL |
| Total | n/a | n/a | 100 mL |
Store at 4°C. The minimal buffer is stable for a year.
Low-salt lysis buffer composition for HCF-1 protein immunoprecipitation
| Reagent | Stock | Final concentration (mM or μM) | Amount (μL or mL) |
|---|---|---|---|
| Tris pH7.5 | 1 M | 50 mM | 5 mL |
| Triton X-100 | 10% | 0.5% | 5 mL |
| EDTA | 500 mM | 5 mM | 1 mL |
| DTT | 500 mM | 1 mM | 200 μL |
| PMSF | 100 mM | 1 mM | 1 mL |
| Anti-protease | 500 mM | 1:100 | 1 mL |
| NaF | 500 mM | 50 mM | 10 mL |
| βGP | 1 M | 10 mM | 1 mL |
| Na3VO4 | 200 mM | 1 mM | 500 μL |
| Sodium Butyrate | 200 mM | 10 mM | 1 mL |
| PUGNAc | 2 mM | 10 μM | 500 μL |
| ddH2O | n/a | n/a | 73.8 mL |
| Total | n/a | n/a | 100 mL |
Store at 4°C. The minimal buffer is stable for a year.
CRITICAL: The high and low salt lysis buffers containing only Tris, NaCl, Triton X-100 and EDTA are stable for many months at 4°C. However, all other components should be added just before use.
The Na3VO4 stock solution needs to be activated by adjusting pH to 10 with HCl, followed by repeated boiling steps on a hot plate for 10–15 min until the pH stabilizes at 10.
PMSF reagent is corrosive and toxic. PMSF half-life in lysis buffer is about 30 min. Make sure to add it at last to the lysis solution.
Laemmli loading buffer 2×
| Reagent | Stock | Final concentration | Amount (mL) |
|---|---|---|---|
| SDS | 10% | 4% | 200 mL |
| Glycerol | 100% | 20% | 100 mL |
| βGP | 1% | 0.004% | 2 mL |
| Tris-HCl pH 6.8 | 1 M | 125 mM | 62.5 mL |
| 2-mercaptoethanol | n/a | 1/20 | 25 mL |
| ddH2O | n/a | n/a | 110.5 mL |
| Total | n/a | n/a | 500 mL |
Store at 25°C. Stable for a year.
CRITICAL: 2-Mercaptoethanol is an acutely toxic reagent. It is corrosive and can cause irritation. Manipulate 2-Mercaptoethanol with care and under a chemical hood.
1X Running buffer
| Reagent | Stock | Final concentration | Amount (mL or L) | |
|---|---|---|---|---|
| Tris | 49.54 M | 6 g | 495 mM | 200 mL |
| Glycine | 0.384 M | 28.8 g | 384 mM | |
| SDS | 10% | 0.2% | ||
| ddH2O | n/a | n/a | 800 mL | |
| Total | n/a | n/a | 1 L | |
Storage at 25°C. Stable for about a year.
1X Transfer buffer
| Reagent | Stock | Final concentration | Amount (mL or L) | |
|---|---|---|---|---|
| Tris | 250 mM | 30.28 g | 25 mM | 100 mL |
| Glycine | 1.92 M | 144.125 g | 192 mM | |
| SDS | 10% | 0.05% | ||
| Methanol | 100% | 20% | 200 mL | |
| ddH2O | n/a | n/a | 700 mL | |
| Total | n/a | n/a | 1 L | |
Storage at 4°C. Stable for about a year.
CRITICAL: Methanol is a toxic reagent. Manipulate this reagent under the chemical hood.
Ponceau
| Reagent | Stock | Final concentration | Amount (g or L) |
|---|---|---|---|
| Ponceau Red | n/a | 0.2% | 2 g |
| Trichloroacetic acid | n/a | 3% | 30 g |
| Sulfosalicylic acid | n/a | 3% | 30 g |
| ddH2O | n/a | n/a | 1 L |
| Total | n/a | n/a | 1 L |
Storage at 25°C. Stable for about a year.
PBS-Tween 20 buffer
| Reagent | Stock | Final concentration | Amount (mL or L) |
|---|---|---|---|
| PBS | 10× | 1× | 100 mL |
| Tween 20 | 20% | 0.1% | 5 mL |
| ddH2O | n/a | n/a | 895 mL |
| Total | n/a | n/a | 1 L |
Storage at 25°C. Stable for about a year.
Step-by-step method details
Immunoprecipitation of exogenous and endogenous HCF-1
Timing: 3 days
The generated cell pellets from the different conditions described above will be lysed to perform immunoprecipitation of native HCF-1 protein.
-
1.Immunoprecipitation of endogenous HCF-1 protein:
-
a.Add to the cell pellets from steps 1.b.vi. and 1.c.viii., the amount of 10× the pellet volume of ice-cold high salt lysis buffer (see composition in the table). Make sure you have the same pellet volume in each condition. If not, calculate each pellet volume and add the equivalent volume to each pellet (Example: For 100 μL of cell pellet, add 900 μL of lysis buffer). In our conditions, we had 100 μL pellet for each condition.
-
b.Dissociate the pellet using the pipette or by vortexing.
-
c.Incubate on ice for 20–30 min.
-
d.Centrifuge the lysate for 20 min at 15000 RPM/21400 g at 4°C to separate the chromatin fraction (in the pellet) from the soluble fraction (supernatant).
-
e.Transfer the supernatant to a new tube and add 1:1 volume of low salt lysis buffer to have a final concentration of 150 mM of NaCl.
-
f.Keep a small fraction of the supernatant as an input (about 1/20) and mix it with 1:1 Laemmli sample buffer 2X (see composition in the table).
-
g.Split the rest of the cell lysate from each condition into two different tubes. One fraction will be used to immunoprecipitate endogenous HCF-1 protein using a specific antibody. The second fraction will serve as a control immunoprecipitation by using an unrelated antibody (anti-GAL4 in our case).Note: GAL4 protein is a transcription factor, which is not expressed in human cells. Using an unrelated antibody, as a control for protein immunoprecipitation, will insure specificity of the bait.
-
h.Add 5 μg of HCF-1 antibody to each IP condition from GFP shRNA, OGT shRNA, PUGNAc, Thiamet G, HBSS and DMSO lysates. Add 5 μg of GAL4 antibody to the immunoprecipitation control fractions.
-
i.Seal the tubes containing the cell lysate-antibody mix with Parafilm to prevent leakage. Place on a tube rotator overnight at 4°C.
-
j.The next day, prepare 25 μL of protein G-Sepharose beads for each condition in new tubes. The bead suspension should be blocked for at least 1 h in 1% BSA dissolved in the lysis buffer for an optimal immunoprecipitation.
-
k.Add the cell lysate-antibody mix to 25 μL of packed beads prepared in separate tubes.
-
l.Incubate for 4–6 h at 4°C.
-
m.Centrifuge the cell lysate-antibody-beads mix for 5 min at 4°C at 3000 RPM/900 g.
-
n.Discard the supernatant. At this step, HCF-1 protein should be coupled to the beads.
-
o.Wash the beads 5 times by adding 1 mL of wash buffer (same composition as the lysis buffer except for the anti-protease concentration that is reduced from 1:100 to 1:500). Invert the tube delicately after each wash and centrifuge 2 min at 4°C at 3000 RPM/900 g to pellet the beads.
-
p.Gently aspirate the last wash buffer and resuspend the beads in 60 μL Laemmli sample buffer 2×.
 Pause point: At this step, samples can be stored at −20°C for several weeks until the day of Western Blot.
Pause point: At this step, samples can be stored at −20°C for several weeks until the day of Western Blot.
-
a.
-
2.Immunoprecipitation of exogenous HCF-1 protein:
-
a.Take pellets from step 2.e. (V5-tagged HCF-1 overexpression in HEK293T)
-
b.Repeat steps 1-a to 1-f (from ‘immunoprecipitation of exogenous and endogenous HCF-1’ protocol) to lyse the cell pellets. In our case, we used 50 μL pellet for each condition.
-
c.Add 30 μL of anti-V5-coupled beads to the cell lysates, seal the Eppendorf tubes with Parafilm and incubate overnight on a tube rotator at 4°C.
-
d.The next day, centrifuge the beads for 5 min at 4°C at 3000 RPM /900 g and carefully discard the supernatant without touching the beads pellet.
-
e.Wash the beads at least 5 times with 1 mL of wash buffer (same composition as the lysis buffer except for the anti-protease mix concentration that is reduced from 1:100 to 1:500). Invert the tube delicately after each wash and centrifuge 2 min at 4°C at 3000 RPM/900 g to pellet the beads.
-
f.Gently aspirate wash buffer and resuspend the beads in 60 μL Laemmli sample buffer 2× by vortexing.
-
a.
Pause point: At this step, samples can be stored at −20°C for several weeks until the day of Western Blot.
CRITICAL: If the volumes of cell pellets are variable between conditions, we recommend performing a protein quantification using the Bradford method, right after step 1-e. (from ‘immunoprecipitation of exogenous and endogenous HCF-1’ protocol). After quantification, follow the immunoprecipitation steps with equivalent amounts of total proteins for each condition. Final lysate volumes can be adjusted using lysis buffer containing all inhibitors.
To increase precision, prepare a master mix by diluting the beads in the lysis buffer. Thus, you can aliquot the 25 μL beads in the new tubes in 1 mL volume using p1000 pipette.
When aliquoting, cut the extremity of the pipette tip to facilitate beads pipetting.
Do not vortex the beads until the final step of beads resuspension in sample buffer 2×. In addition, be careful when using the vacuum during the wash step, as the beads can be partially or totally lost. See Figure 3 for a simplified visualization of these steps.
Figure 3.
Detailed steps for immunoprecipitation
Protein O-GlcNAc level detection by Western Blotting
Timing: 2 days
At this step, we perform Western Blotting on immunoprecipitated proteins from step 2 (from ‘immunoprecipitation of exogenous and endogenous HCF-1’ protocol) or on total cell lysates quantified using the BCA or Bradford protein quantification methods to determine the amount of proteins to be loaded in the polyacrylamide gel, as previously described in (Daou et al., 2011).
-
3.Protein separation by SDS-PAGE electrophoresis in polyacrylamide gel:
-
a.Prepare or purchase a pre-made polyacrylamide gels of appropriate percentage according to the molecular weight of the protein of interest (here we use homemade gels at 8 or 10% polyacrylamide concentration).
-
b.Build your gel in the running electrophoresis apparatus and fill with 1× running buffer, as instructed by the manufacturer.
-
c.Carefully load your samples containing equal amounts of proteins from quantified cell lysates or from immunopurified protein samples. Include a molecular weight marker.
-
d.Run the gel at 100–150 V until protein marker reaches the bottom of the gel.
-
a.
-
4.Protein transfer on PVDF membranes:
-
a.Wet your PVDF membranes in methanol for few seconds.
-
b.Soak sponges and Whatman paper in transfer buffer.
-
c.Gently remove the gel from the running apparatus and prepare the transfer sandwich by building the following layers in the transfer cassette without making bubbles (see Figure 4): sponge ==» Whatman paper ==» gel ==»PVDF ==» Whatman paper ==» sponge.
-
d.Place the sandwich into a transfer apparatus and perform semi-dry or wet transfer at 0.5 A for 2 h or overnight at 35 V at 4°C. Proteins will migrate from the cathode (-) to the anode (+).
-
a.
-
5.Protein detection or immunoblotting:
-
a.After transfer, briefly rinse the membrane with distilled water and stain it with Ponceau S to make sure proteins were transferred from the gel to the PVDF membrane.
-
b.Rinse the membrane with distilled water and then with the PBS-tween buffer.
-
c.Incubate the membrane in the blocking solution for 1 h at 22°C–25°C or overnight at 4°C.Note: We use 5% of skim milk powder in PBS-tween as the blocking solution.
-
d.Wash 3 times with PBS-tween for 15 min each.
-
e.Incubate for at least 2 h at 4°C with primary antibody against HCF-1 protein or anti-O-GlcNAc. To make sure the anti-O-GlcNAc antibody used is specific, incubate a parallel membrane with the anti-O-GlcNAc antibody in the presence of 0.5–1 M of N-acetylglucosamine compound (see Figures 4 and 5).Note: Primary antibodies are prepared at 1:1000 dilution in PBS–tween buffer supplemented with 1% BSA and 1 mM sodium azide for long-term conservation.
-
f.Wash 3 times with PBS-tween for 15 min each.
-
g.Incubate with the secondary peroxidase-coupled antibody for 1 h.Note: Secondary antibodies are prepared at 1:1000 dilution in PBS-tween 5% skim milk powder.
-
h.Wash 3 times with PBS-tween for 15 min each.
-
i.Detect protein bands with ECL substrate using a chemiluminescence imager (we use Azure c600 machine from Azure Biosystems).
See Figure 4 for a simplified visualization of these steps. CRITICAL: Washing steps are important to minimize non-specific background. Do not let the membrane dry at any step during the process. Antibody concentration and incubation time may vary depending on the protein of interest. Use multiple exposure times with the machine to identify the optimal exposure for the best signal, making sure to avoid overexposure. When incubating with the ECL, make sure it is homogeneously spread over the membrane to avoid artifacts.
CRITICAL: Washing steps are important to minimize non-specific background. Do not let the membrane dry at any step during the process. Antibody concentration and incubation time may vary depending on the protein of interest. Use multiple exposure times with the machine to identify the optimal exposure for the best signal, making sure to avoid overexposure. When incubating with the ECL, make sure it is homogeneously spread over the membrane to avoid artifacts.
-
a.
Figure 4.
Specific protein O-GlcNAcylation detection by Western Blotting
Figure 5.
Summary of results of Daou's group
Expected outcomes
In this protocol, we described a method to study protein O-GlcNAcylation in cells. Some results derived from the experimental procedures described in this protocol are shown in our previous paper of Daou et al. (2011). We invite the reader to consult the figures of the paper to see the expected results of the procedures described in this protocol such as:
Co-transfection of HCF-1 with either OGT or OGA enzymes followed by Western Blotting on total cell extracts to evaluate the impact on HCF-1 O-GlcNAcylation levels. OGT expression with HCF-1 increases its O-GlcNAc levels, which is antagonized by OGA co-expression.
Depletion of OGT enzyme by shRNA followed by Western Blotting on total cell extract to detect O-GlcNAcylation levels of HCF-1. OGT depletion causes suppression of HCF-1 O-GlcNAcylation.
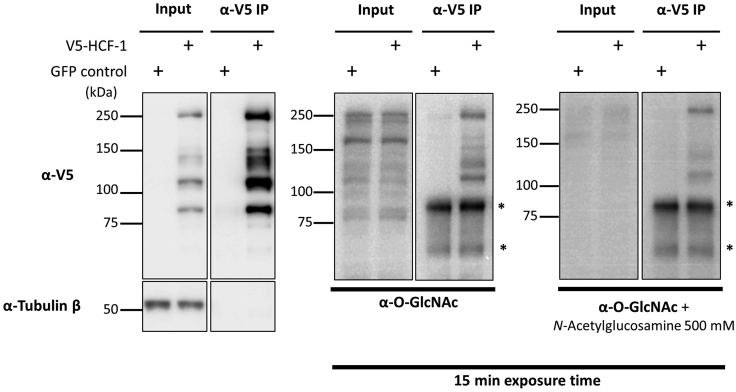
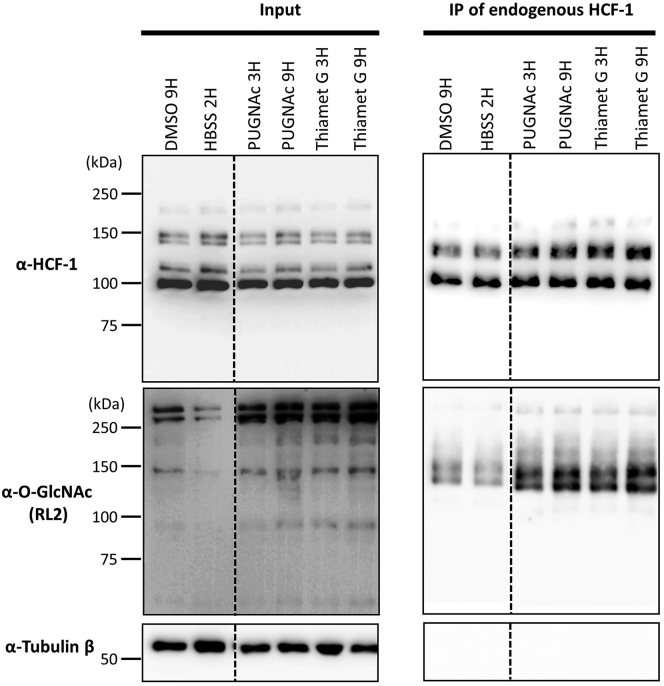
We show here a detailed protocol on how to perform immunoprecipitation of either endogenous or exogenous HCF-1 protein and to detect its O-GlcNAcylation using a specific antibody against O-GlcNAc by Western Blotting (Figures 6 and 7). We also show the impact of OGA modulation by chemical inhibitors or nutrient deprivation on the O-GlcNAcylation levels of the HCF-1 protein (Figure 8). HBSS treatment induces a decrease of total cell O-GlcNAcylation as shown by the detection of O-GlcNAc levels in the input fraction using the RL2 antibody. HCF-1 immunoprecipitation following HBSS treatment shows a significant reduction of its O-GlcNAcylation levels. However, PUGNAc and Thiamet G treatments increase total cell O-GlcNAcylation levels as shown by the detection of O-GlcNAc levels in the inputs. Moreover, HCF-1 shows elevated O-GlcNAcylation levels upon PUGNAc and Thiamet G treatments in comparison with DMSO condition.
Figure 6.
O-GlcNAcylation of Host Cell Factor 1 (HCF-1) following its overexpression
HEK293T cells were transfected with either GFP control vector or V5-HCF-1 vector. Cell pellets were collected three days after. Immunoprecipitation of V5-HCF-1 under native conditions was conducted and analysis was performed by Western Blotting. (Left panel) Immunodetection of V5 shows an enriched signal in the immunoprecipitated sample compared to input. (Left panel) Tubulin was detected in inputs and immunoprecipitation conditions (as a control). Note that GFP control also shows no signal in the V5 immunoprecipitation sample. (Right panel) Immunodetection of O-GlcNAc with the RL2 antibody, or detection following incubation with N-acetylglucosamine to show antibody specificity. Membranes were revealed in the same conditions of enhanced chemiluminescence (ECL).
Figure 7.
O-GlcNAcylation of endogenous HCF-1
Immunoprecipitation of native HCF-1 using an anti--HCF-1 antibody was conducted using HeLa and HEK293T cells. As a control, the immunoprecipitation was also done using an anti-GAL4 antibody. The obtained samples were incubated with the anti-HCF-1 and the anti-O-GlcNAc antibodies and the membranes were used for enhanced chemiluminescence detection.
Figure 8.
O-GlcNAcylation of endogenous HCF-1 after various treatments
HeLa cells were treated with either 50 μM PUGNAc, 50 μM Thiamet G, Hanks' Balanced Salt Solution (HBSS) or 0.1% DMSO. Cell pellets were collected at different time points and immunoprecipitation of endogenous HCF-1 under native conditions was conducted and analyzed by Western Blotting. DMSO control indicates normal HCF-1 expression. Under PUGNAc and Thiamet G conditions, two β-N-acetylglucosaminidase (OGA) inhibitors, O-GlcNAc detection of immunoprecipitated HCF-1 displays an enriched signal compared to DMSO control. Detection of Tubulin was used as a loading control as well as an immunoprecipitation specificity control. In the condition of HBSS treatment, O-GlcNAc levels decrease in the input and immunoprecipitated HCF-1 compared to DMSO control.
Altogether, these results prove that HCF-1 protein is modified by O-GlcNAcylation, as targeting OGT/OGA activity by the methods shown in Figure 1 results in modulating HCF-1 O-GlcNAcylation levels.
Note: Please see Figures legend in the end.
Limitations
The method we used for the detection of protein O-GlcNAcylation by western blot is limited by the success of the immunoprecipitation step. In addition, to detect protein O-GlcNAcylation levels, it is important that the same amount of the protein of interest be analyzed between conditions. This ensures that protein O-GlcNAcylation levels are compared, between the different treatments, for the same quantity of total proteins. This method is rather semi-quantitative and for accurate determination of protein O-GlcNAcylation and stoichiometry, mass spectrometry could be used.
Troubleshooting
Problem 1
Multiple bands detection for O-GlcNAc due to a non-specific antibody. (Step 5.i)
Potential solution 1
When incubating the membrane with anti-O-GlcNAc antibody, add an additional control condition for antibody specificity where you incubate another membrane with the anti-O-GlcNAc antibody in the presence of 0.5–1 M of N-acetylglucosamine. This compound will compete with the protein of interest and prevent the antibody from binding the O-GlcNAcylated protein. If the band of O-GlcNAcylated protein is specific, its intensity will decrease in the membrane incubated with N-acetyl-Glucosamine.
Problem 2
O-GlcNAcylation detection failure or weak signals detected. (Step 5.i)
Potential solution 2
Each protein detection by Western Blotting needs optimization depending on its expression levels in cells and quality of the antibodies. Load more proteins, incubate longer times with antibodies, use highly sensitive ECL reagents and increase the exposure time using the chemiluminescence imager. For an immunoprecipitation of exogenous proteins, try transfecting more plasmids and use more cells. You can also add OGA inhibitors to increase the signal. You can also try protein immunoprecipitation under denaturing conditions.
Problem 3
Identifying the O-GlcNAcylation residues within the protein of interest. (Step 5.i)
Potential solution 3
Western Blotting, coupled with specific antibodies, is a robust method to detect and quantify protein O-GlcNAcylation level. However, only mass spectrometry (MS) can identify the serine/threonine residues targeted by OGT within the protein of interest. The final purified products from the immunoprecipitation procedures described in this protocol can be used to perform MS to confirm the Western Blotting results.
Problem 4
Low efficiency of protein immunoprecipitation (Steps 1.i and 2.c)
Potential solution 4
If Protein G-Sepharose doesn’t bind well to the antibody-protein complex, try using beads with protein A or a mix of protein A/G. Incubate the protein A and/or G-Sepharose mix with the antibody-lysate for a longer period.
Problem 5
Ineffective modulation of O-GlcNAcylation levels with inhibitors, due to variable cell types responses to drugs or nutrient-deprivation treatments (step 1 of cell treatments or transfection).
Potential solution 5
Each cell type needs optimization for the time and concentration of drug treatment. When using another cell model, it is important to start by optimizing drug concentration and incubation time to determine the ideal parameters to modulate total O-GlcNAc levels in each cell model used before performing IP.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, [El Bachir Affar] (el.bachir.affar@umontreal.ca).
Further technical questions about the protocol should be addressed to the lead author, [Oumaima Ahmed] (ahmed.oumaima@umontreal.ca).
Materials availability
All reagents and materials mentioned above are commercially available. Please see the key resources table.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) to E.B.A. O.A. and L.M. hold PhD scholarships from the Fonds de recherche du Québec - Santé (FRQ-S). O.A. holds a PhD scholarship from the Cole Foundation. Certain forms and shapes displayed in figures were taken and modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License (http://smart.servier.com/).
Author contributions
Conceptualization and methodology: O.A., M.A., M.E., L.M., M.G.L., B.E., D.V., and E.B.A. Writing original draft and schematic figures: O.A. Experiment and data acquisition and figures: O.A., M.A., M.E., L.M., M.G.L., and B.E. Critical review and editing: O.A., L.M., M.A., M.E., B.E., M.G.L., D.J.V., and E.B.A.
Declaration of interests
D.J.V. is a founder of Alectos Therapeutics and is a consultant and a member of its scientific advisory board. The rest of the authors declare no competing interests.
Data and code availability
No data or code was generated or analyzed in this protocol.
References
- Bond M.R., Hanover J.A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 2015;208:869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkinaree C., Park K., Hart G.W. O-linked beta-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosti F., Guernier S., Lammers F., Waridel P., Cai Y., Jin J., Conaway J.W., Conaway R.C., Herr W. O-GlcNAc transferase catalyzes site-specific proteolysis of HCF-1. Cell. 2011;144:376–388. doi: 10.1016/j.cell.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Chang Y.H., Weng C.L., Lin K.I. O-GlcNAcylation and its role in the immune system. J. Biomed. Sci. 2020;27:57. doi: 10.1186/s12929-020-00648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou S., Mashtalir N., Hammond-Martel I., Pak H., Yu H., Sui G., Vogel J.L., Kristie T.M., Affar El B. Crosstalk between O-GlcNAcylation and proteolytic cleavage regulates the host cell factor-1 maturation pathway. Proc. Natl. Acad. Sci. U S A. 2011;108:2747–2752. doi: 10.1073/pnas.1013822108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer C.M., Sodi V.L., Reginato M.J. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J. Mol. Biol. 2016;428:3282–3294. doi: 10.1016/j.jmb.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forma E., Jozwiak P., Ciesielski P., Zaczek A., Starska K., Brys M., Krzeslak A. Impact of OGT deregulation on EZH2 target genes FOXA1 and FOXC1 expression in breast cancer cells. PLoS ONE. 2018;13:e0198351. doi: 10.1371/journal.pone.0198351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J., Daou S., Zamorano N., Iannantuono N.V., Hammond-Martel I., Mashtalir N., Bonneil E., Wurtele H., Thibault P., Affar El B. Undetectable histone O-GlcNAcylation in mammalian cells. Epigenetics. 2015;10:677–691. doi: 10.1080/15592294.2015.1060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G.W. Nutrient regulation of signaling and transcription. J. Biol. Chem. 2019;294:2211–2231. doi: 10.1074/jbc.AW119.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y.K., Park N.Y., Park S.J., Kim B.G., Shin J.H., Jo D.S., Bae D.J., Suh Y.A., Chang J.H., Lee E.K., et al. O-GlcNAcylation of ATG4B positively regulates autophagy by increasing its hydroxylase activity. Oncotarget. 2016;7:57186–57196. doi: 10.18632/oncotarget.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuria V., Rohrig U.F., Bhuiyan T., Borodkin V.S., van Aalten D.M., Zoete V., Herr W. Proteolysis of HCF-1 by Ser/Thr glycosylation-incompetent O-GlcNAc transferase:UDP-GlcNAc complexes. Genes Dev. 2016;30:960–972. doi: 10.1101/gad.275925.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury J.J., Chan K.K., Zheng L., Bardor M., Choo A.B. Excess of O-linked N-acetylglucosamine modifies human pluripotent stem cell differentiation. Stem Cell Res. 2013;11:926–937. doi: 10.1016/j.scr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Olivier-van Stichelen S., Hanover J.A. You are what you eat: O-linked N-acetylglucosamine in disease, development and epigenetics. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:339–345. doi: 10.1097/MCO.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramutola A., Sharma N., Barone E., Lanzillotta C., Castellani A., Iavarone F., Vincenzoni F., Castagnola M., Butterfield D.A., Gaetani S., et al. Proteomic identification of altered protein O-GlcNAcylation in a triple transgenic mouse model of Alzheimer's disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3309–3321. doi: 10.1016/j.bbadis.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Vosseller K., Wells L., Lane M.D., Hart G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U S A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Mashtalir N., Daou S., Hammond-Martel I., Ross J., Sui G., Hart G.W., Rauscher F.J., 3rd, Drobetsky E., Milot E., et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol. Cell Biol. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa S.A., Macauley M.S., Heinonen J.E., Shan X., Dennis R.J., He Y., Whitworth G.E., Stubbs K.A., McEachern E.J., Davies G.J., Vocadlo D.J. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nature Chemical Biology. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data or code was generated or analyzed in this protocol.