Summary
To understand the determinants of long-term immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the concurrent impact of vaccination and emerging variants, we follow a prospective cohort of 332 patients with coronavirus disease 2019 (COVID-19) over more than a year after symptom onset. We evaluate plasma-neutralizing activity using HIV-based pseudoviruses expressing the spike of different SARS-CoV-2 variants and analyze them longitudinally using mixed-effects models. Long-term neutralizing activity is stable beyond 1 year after infection in mild/asymptomatic and hospitalized participants. However, longitudinal models suggest that hospitalized individuals generate both short- and long-lived memory B cells, while the responses of non-hospitalized individuals are dominated by long-lived B cells. In both groups, vaccination boosts responses to natural infection. Long-term (>300 days from infection) responses in unvaccinated participants show a reduced efficacy against beta, but not alpha nor delta, variants. Multivariate analysis identifies the severity of primary infection as an independent determinant of higher magnitude and lower relative cross-neutralization activity of long-term neutralizing responses.
Keywords: SARS-CoV-2, humoral response, B cell memory, pseudovirus, neutralizing antibodies, variants of concern, severity, durability, half-life
Graphical abstract
Highlights
-
•
Long-term persistence (>12 months) of neutralizing antibodies against SARS-CoV-2
-
•
Severity of infection determines the magnitude and quality of neutralizing response
-
•
Vaccination boosts neutralizing response to natural infection
Pradenas and Trinité et al. analyze the kinetics of neutralizing antibodies in response to natural SARS-CoV-2 infection and evaluate the long-term (beyond 1 year) stability and breadth of the neutralizing response before subsequent vaccination.
Introduction
Immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection involve an undefined balance of innate and adaptive pathways1 resulting in the development of a seemingly long-lasting immunological memory.2,3 Although there is a general consensus on the key role of both T and B cells in the protection against SARS-CoV-2 infection and the development of coronavirus disease 2019 (COVID-19), the specific contribution of each arm of the immune system is still unclear.1 Neutralizing antibodies mediate their protective effect by binding to the spike (S) glycoprotein of SARS-CoV-2 and by blocking viral entry into target cells; however, additional effector functions promoting viral clearance or natural killer (NK)-mediated infected-cell killing seems to be also relevant in SARS-CoV-2 and other viral infections.4 Nevertheless, abundant experimental and epidemiological studies on SARS-CoV-2 indicate that neutralizing antibodies can serve as surrogate markers of protection,5, 6, 7 as they do for other viral infections.8,9
Given the relevance of antibodies, the early (1–3 months) and mid-term (3–12 months) humoral responses after SARS-CoV-2 infection have been thoroughly described.10, 11, 12, 13, 14 Current data outline a heterogeneous scenario in which infected individuals generate a wide range of neutralizing antibodies (from no seroconversion to rapid development of high titers) with no definitive association to age, gender, or disease severity.15, 16, 17 Various authors have also suggested complex kinetics of neutralizing activity decay.3,18,19 This is particularly relevant in the current context of viral evolution, as several variants of concern (VOCs) have shown total or partial resistance to neutralizing antibodies and partial resistance to polyclonal humoral responses elicited by infection or vaccination.20
To understand the dynamics of natural responses to infection, we focused on the longitudinal analysis of the neutralizing humoral response in a large cohort of mild/asymptomatic and hospitalized individuals infected by SARS-CoV-2. Our analysis includes one of the longest follow-up periods (up to 15 months) and shows that the long-term magnitude of neutralization is remarkably stable, being boosted by vaccines, and potentially threatened by VOCs. Clinical severity of primary infection was identified as the main factor determining the kinetics, magnitude, and quality of neutralizing antibodies.
Results
Cohort description
Our cohort included 332 participants with confirmed SARS-CoV-2 infection who were recruited between March 2020 and March 2021 in Catalonia (Northeastern Spain). Participants were grouped according to the epidemiological waves of the SARS-CoV-2 pandemic in Spain, defined by an early outbreak caused by the original 19B strain and the B.½0A variant (D614G) (from March to June 2020), a second wave dominated by the 20E (EU1) variant (from July to December 2020), and a third wave associated with the emergence of the B.1.1.7/20I Alpha variant covering the January to June 2021 period, until the recent introduction of the B.1.617.2 delta variant in June 2021 (Figure S1). A total of 212 participants were recruited during the first-wave period, 128 of whom had mild or absent symptomatology (WHO progression scale21 levels 1–3; non-hospitalized) and 84 of whom required hospitalization (WHO progression scale21 levels 4–10) with a wide range of severity from non-severe pneumonia to intensive care unit admission/death (Table 1). Comparable proportions of disease severity were observed in patients recruited in the second (n = 79) and third (n = 41) COVID-19 waves. In all cases, the hospitalization groups showed older age and lower female frequency when compared with non-hospitalized groups (mild or asymptomatic; Table 1).
Table 1.
Characteristics of individuals included in analysis
| March 20–June 20 |
July 20–December 20 |
January 21–March 21 |
p value | ||||
|---|---|---|---|---|---|---|---|
| Non-hospitalized (n = 128) | Hospita-lized (n = 84) | Non-hospitalized (n = 43) | Hospitalized (n = 36) | Non-hospitalized (n = 19) | Hospitalized (n = 22) | ||
| Gender (female), n (%) | 92 (72) | 39 (46) | 24 (56) | 12 (33) | 9 (47) | 5 (23) | 0.0006a |
| Age (years), median (IQR) | 47 (38–54) | 58 (48–67) | 43 (33–53) | 55 (45–63) | 46 (23–52) | 56 (49–62) | < 0.0001b |
| Severity | |||||||
| Asymptomatic, n (%) | 12 (9) | – | 7 (16) | – | 1 (5) | – | |
| Mild, n (%) | 116 (91) | – | 36 (84) | – | 18 (95) | – | |
| Hospitalized non-severe, n (%) | – | 31 (37) | – | 9 (25) | – | 2 (9) | |
| Hospitalized severe, n (%) | – | 41 (49) | – | 23 (64) | – | 20 (91) | |
| Hospitalized (intensive care unit), n (%) | – | 12 (14) | – | 4 (11) | – | 0 (0) | |
| Samples per participant median (IQR) | 3 (1–3) | 3 (2–4) | 2 (1–2) | 4 (2–4) | 1 (1–2) | 3 (2–4) | |
| Last sampling day, median (IQR) | 272 (180–360) | 311 (115–362) | 116 (21–186) | 183 (90–208) | 47 (17–108) | 32 (28–83) | |
| Length of follow up, days median (IQR) | 172 (0–256) | 185 (5–266) | 75 (0–166) | 169 (7–193) | 0 (0–80) | 26 (16–76) | |
IQR: IQR (25th and 75th percentiles).
Chi-square test.
Mann-Whiney test.
Longitudinal analysis of neutralization activity
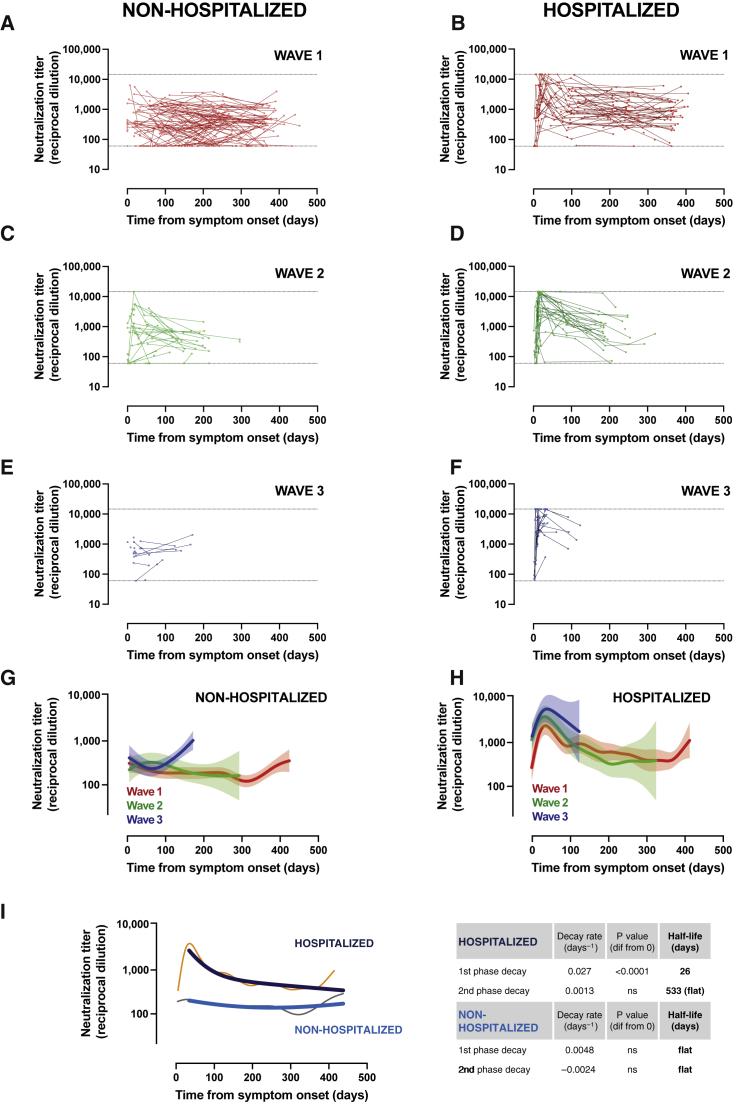
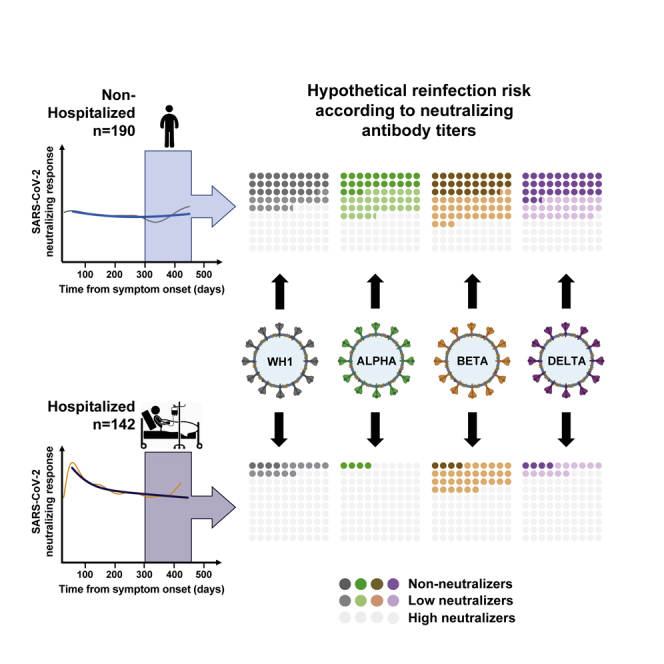
For comparative purposes, samples from all patients prior to vaccination, irrespective of the infection wave, were assayed for their plasma neutralization capacity of the original isolate WH1 sequence in a validated pseudovirus assay.13 Maximal follow-up periods for unvaccinated individuals infected during the first, second, and third waves were 458, 320, and 145 days, respectively. In line with previous analyses,15,17 irrespective of the infecting virus, hospitalized patients showed a rapid development of neutralizing activity over the first month after symptom onset and a transient decrease reaching a plateau (Figures 1B, 1D, and 1F). This was observed only in the first- and second-wave participants due to the limited follow-up of recently infected patients. In contrast, mildly affected or asymptomatic individuals developed a lower maximal neutralization titer with flatter behavior (Figures 1A, 1C, and 1E), although an early peak could be observed in some of the second-wave participants who had earlier sampling (Figure 1C). Longitudinal analysis using smoothing-splines mixed-effects models showed overlapping kinetics for the different waves in each clinical group (Figures 1G and 1H), although neutralizing activity tended to reach higher values at the peak (around 30 days) in hospitalized patients from the third wave (mostly infected by the Alpha variant; Figure 1H). According to recent data,22 we assumed the generation of early short-lived plasmablast/plasma cells and long-lived plasma and memory B cells and modeled data from all patients to a two-phase exponential decay. The longitudinal modeling revealed that hospitalized individuals had a significantly rapid first-phase decay (half-life of 26 days) and a flat slope in the second phase (half-life of 533 days; Figure 1I). Conversely, a flat slope (i.e., not significantly different from 0 in any phase) was observed in individuals with asymptomatic infection or mild disease (Figure 1I). These data confirm that, despite different individual patterns, a large fraction of infected individuals (87% in non-hospitalized and 96% in hospitalized) generate detectable long-lasting neutralizing antibodies (infective dose [ID]50 > 60).
Figure 1.
Longitudinal analysis of neutralizing activity
(A–F) Neutralization titer of 332 individuals according to disease severity (non-hospitalized or hospitalized groups) and date of infection (wave 1: March–June 2020, wave 2: July–December 2020, and wave 3: January–March 2021). Dots are single determinations, and lines indicate individual follow up. Dotted lines indicate the upper and lower limits of the neutralization assay.
(G and H) Longitudinal smoothing-splines mixed-effects models for the different groups shown in (A)–(F). Solid lines indicate the best fit, and light areas indicate confidence intervals (CIs).
(I) Non-linear models of the full dataset (n = 190 for non-hospitalized and n = 142 for hospitalized groups) were analyzed by smoothing-splines mixed-effects models (gray and orange narrow lines) or fitted to a non-linear two-phase exponential decay model (light and dark blue lines). Decay rate constants are described on the right side of the figure.
Impact of vaccination
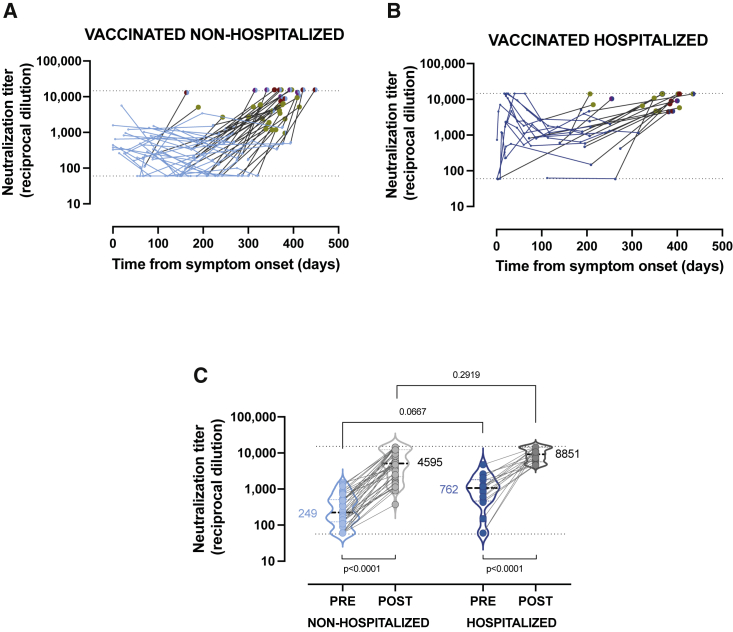
Massive vaccination campaigns across developed countries have positively impacted the course of the COVID-19 pandemic and have interfered with the follow-up of immune responses induced by natural infection. During routine follow-up visits, we identified 58 vaccinated individuals in our cohort. Participants showed a wide range of vaccination status in terms of type of vaccine (21% received BNT162b2 [Pfizer-BioNTech], 62% mRNA-1273 [Moderna], and 17% AZD1222 [AstraZeneca-Oxford]), number of doses (only 55% had received the full 2-dose schedule), and time from the last dose (BNT162b2 vaccinees analyzed at longer time points after vaccination). Despite these differences, vaccines boosted pre-existing neutralizing responses in all non-hospitalized (n = 40) and hospitalized (n = 18) participants (Figures 2A and 2B). A direct comparison of pre- and post-vaccination titers of neutralizing antibodies clearly confirms a highly significant increase in both groups (p < 0.0001). Pre-vaccination titers tended to be lower in non-hospitalized than in hospitalized individuals (geometric mean, 249 and 762, respectively, p = 0.0667) and reached comparable levels after vaccination (4,595 and 8,851, respectively; Figure 2C; p = 0.2919). However, the heterogeneous vaccine schedules and sampling times prevented further analysis.
Figure 2.
Impact of vaccination on convalescent plasma neutralizing activity
(A and B) Single measurements (dots) and individual evolution (lines) of the longitudinal analysis for vaccinated non-hospitalized (n = 40) and hospitalized (n = 18) individuals. Blue dots (light or dark) correspond to pre-vaccination measurements. Post-vaccine data are color-coded according to vaccine schedules: BNT162b2 (maroon), mRNA-1273 (red), and AZD1222 (purple). Full symbols indicate full schedule (two doses), while half circles indicate one single dose.
(C) Comparison of pre- and post-vaccination neutralizing antibody titers in both groups. Lower p values indicate paired comparison (Wilcoxon test) of pre- and post-values. Upper p value indicates Mann-Whitney comparison of pre-vaccination between groups. Geometric means of neutralization titer for each group are indicated. Dotted lines indicate the upper and lower limits of detection.
Impact of viral variants
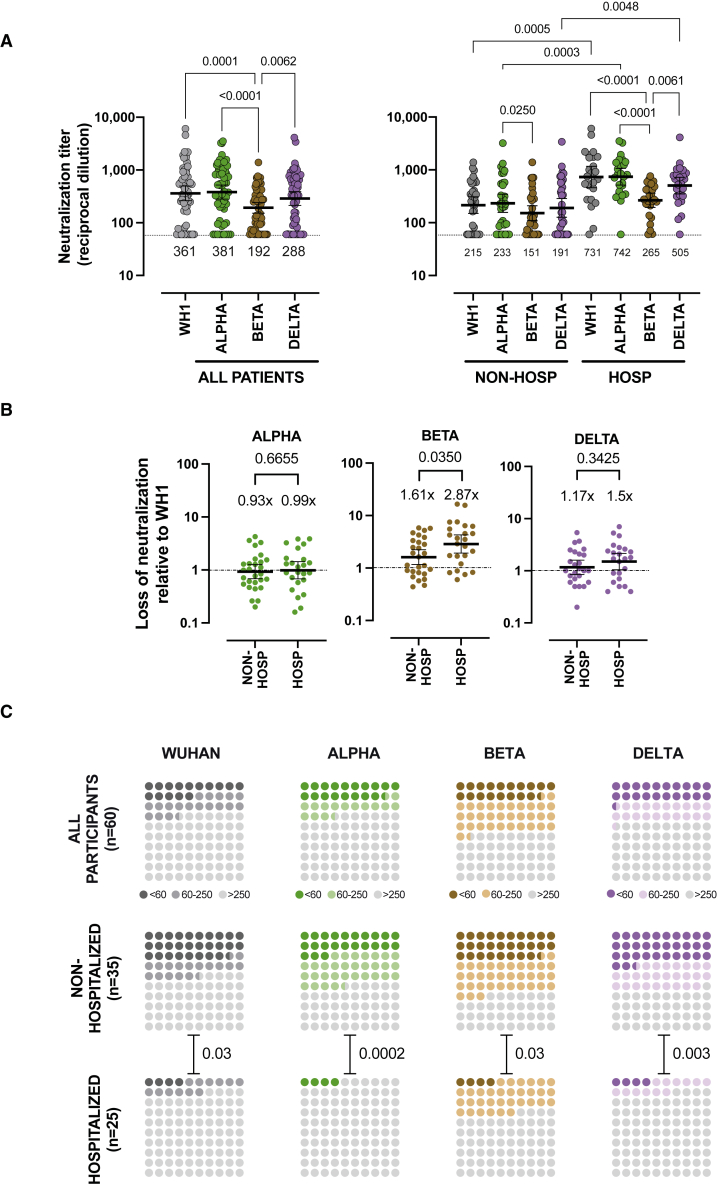
It is well known that SARS-CoV-2 VOCs show variable degrees of resistance to neutralizing responses elicited by natural infection or vaccination.20 Therefore, to evaluate the impact of the most relevant VOCs on long-term neutralizing activity, a subset of 60 unvaccinated individuals with follow-up periods beyond 300 days was analyzed against Alpha, Beta, and Delta variants. A global analysis showed that long-term neutralizing responses blocked the WH1 and the Alpha (B.1.1.7) variants with similar potency, while lower titers were measured against the Beta (B.1.351) variant (p = 0.0001), and an intermediate but not significant loss of neutralization was observed against the more transmissible Delta variant (Figure 3A). The analysis of non-hospitalized and hospitalized subgroups showed similar but not identical results. We observed a highly significant loss (p ≤ 0.0001) of neutralizing capacity against the Beta variant in hospitalized individuals (Figure 3A) but a lower loss in non-hospitalized individuals, reaching significance when compared with the Alpha but not with the original WH1 variant (p = 0.4020; Figure 3A). To quantitatively assess these differences, we compared the ratio of neutralization titers between the original WH1 sequence and the tested variants as a measure of the relative loss of neutralization for each individual. The comparison of this parameter among subgroups, using only values with measurable neutralization titers for both WH1 and the variant of interest, showed no differences between non-hospitalized and hospitalized groups for the Alpha/B.1.1.7 variant (with a mean fold change around 1). On the other hand, statistically significant differences were observed for the Beta/B.1.351 variant, which induced a higher relative loss of neutralization in hospitalized patients (p = 0.0350; Figure 3B), while the Delta/B.1.617.2 variant showed again intermediate behavior with a more pronounced decrease in hospitalized patients that did not reach significance (p = 0.3425; Figure 3B). As a consequence, the median magnitudes of neutralization against WH1, Alpha, and Delta were all superior in hospitalized individuals compared with in non-hospitalized individuals (p = 0.0005, 0.0003, and 0.0048 respectively), while statistical significance was lost for the Beta variant (p = 0.3107; Figure 3A).
Figure 3.
Impact of SARS-CoV-2 variants on long-term neutralizing activity
(A–C) Neutralization titers, against WH1, Alpha, Beta, and Delta spike variants, measured on convalescent plasmas collected more than 300 days after symptom onset from non-hospitalized (n = 35) and hospitalized (n = 25) patients (see Table S1 for details).
(A) Neutralization titers (ID50 expressed as reciprocal dilutions) from all patients (left) or divided into non-hospitalized and hospitalized patients (right). Bars and values below symbols indicate the geometric mean titer in each group. p values show the comparison of median titers among the four viruses (Friedman test with Dunn’s multiple comparison) or the comparison of the same variant between the two groups (Kruskal-Wallis test with Dunn’s multiple comparison). Only significant differences are shown. Dotted lines indicate lower limits of detection.
(B) Loss of neutralization titers against variants (indicated on top) compared with WH1 pseudovirus (lower values identify maintained neutralization). Samples with undetectable titers for both WH1 and the analyzed variant were removed from the analysis. Bars and values indicate the median ratio, and p values indicate the comparison of the two patient groups (Mann-Whitney test).
(C) Frequency of long-term non-neutralizers (ID50 < 60), low neutralizers (i.e., ID50 between 60 and 250 after 300 days post-symptom onset), and high neutralizers (ID > 250, light gray) in all patients and separately in hospitalized and non-hospitalized groups. p values show the comparison of frequency between both groups for each variant (chi-square test).
Following previous reports correlating protection with neutralization titers,7,23 we estimated the frequency of individuals with undetectable, low, and high neutralization titers using a previously described cutoff value of 250.2 The analysis showed that 33% of individuals had undetectable or low neutralization against the WH1 or the Alpha variant, increasing to 52% or 41% for the Beta and Delta variants, respectively. In all cases, the frequency of non-neutralizers and low neutralizers was higher in non-hospitalized individuals, reaching 63% against the Beta variant, compared with 36% in hospitalized patients (Figure 3C)
Factors determining long-term neutralizing activity
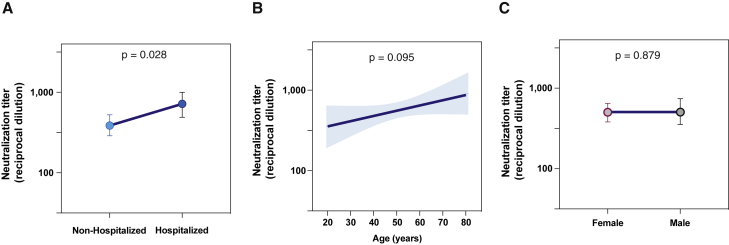
Despite similar long-term stability in non-hospitalized and hospitalized individuals, neutralizing activity was highly heterogeneous with the presence of non-neutralizer and highly neutralizer patients in both groups (see Figure 3). Therefore, we analyzed the factors that potentially define the magnitude of long-term neutralization (>300 days after infection) in unvaccinated infected patients. A multivariate analysis including severity group, age, and gender showed that only severity, as defined by hospitalization, was independently associated with the magnitude of responses (p = 0.0285; Figure 4A). Consistent with the close relationship between age and severity, age showed a significant effect in the univariate analysis that was lost in the multivariate model (p = 0.0951; Figure 4B), while gender had no impact (Figure 4C).
Figure 4.
Factors determining long-term neutralizing titer
(A–C) Factor effects by multivariate linear regression for samples collected more than 300 days post-symptom onset from 99 participants. Estimated effect (dots) and 95% CI (bars or bands) are plotted, and the p value is shown for each predictor covariate: (A) severity, (B) age, and (C) gender. Multivariate analyses were performed with R-3.6.3 software.
A similar approach was used to assess the impact of severity, age, and gender on variant cross-neutralization ratios (shown in Figure 3B). In this case, the multivariate analysis ruled out any impact of age and gender in the loss of neutralization against Alpha, Beta, and Delta variants, which pointed to severity as the main determinant, although it only reached significance for the Beta variant (p = 0.0259; Table S2).
Discussion
To our knowledge, this report has analyzed the neutralizing response against SARS-CoV-2 with the longest follow-up to date, with sampling more than 1 year after symptom onset, in a large cohort with a broad spectrum of clinical disease presentation (from asymptomatic to patients requiring intensive care) over different COVID-19 outbreaks in Catalonia. Longitudinal sampling allowed us to model accurate kinetics of neutralizing activity for the different waves (associated with different viral variants). The temporal patterns for each wave appear to repeat themselves independently of the infecting variant but with a strong impact of disease severity, as previously defined.13
In comparison to the apparent short-lasting immunity against seasonal human coronaviruses,24,25 the neutralizing response developed against SARS-CoV-2 shows a dynamic pattern similar to the ones described against other coronaviruses that cause severe acute respiratory illness, such as SARS-CoV and Middle East respiratory syndrome (MERS)-CoV. For these viruses, several studies detected neutralizing antibodies in the first days after diagnosis with a rapid increase peaking between 2 weeks and 1 month post-symptom onset. Thereafter, there was a decline and, subsequently, a “stabilization” that was maintained beyond 1 year after infection in most cases and was related to disease severity.26, 27, 28, 29, 30, 31, 32 Our data demonstrate the long-term persistence of neutralizing antibodies against SARS-CoV-2 in most individuals with COVID-19. Although long-term increases were observed in qualitative spline models, biexponential fitted models confirm a flat slope and therefore predict longer stability, as has also been described for SARS-CoV and MERS-CoV.33, 34, 35, 36, 37, 38 This raises an optimistic scenario, as neutralizing antibody levels are highly predictive of immune protection,5,7,39,40 although sporadic cases of reinfection have been reported even in the presence of neutralizing antibodies.41,42
Our results complement previous studies that evaluated mid-term immunity2,17,18,43,44 and are in line with current evidence showing a long-lasting neutralizing response for at least 1 year45, 46, 47 and the presence of receptor-binding domain (RBD)-specific memory B cells3,48 and long-lived bone marrow plasma cells.22 Although several mechanisms have been proposed that may lead to the long-term persistence of antibodies,49 the presence of long-lived plasma cells has received more support in recent years,50, 51, 52 and a biphasic model considering short- and long-lived plasma cells has been described.53,54 On this basis and considering the neutralizing capacity of plasma as a surrogate marker of the plasma-cell lifespan, we fitted our data to a two-phase exponential decay curve, probably reflecting both short- and long-lived plasma cells. Therefore, our data point to an initial and transient generation or expansion of short-lived SARS-CoV-2-specific plasmablast/plasma cells in hospitalized patients. While the selection of high-affinity B cells into germinal centers seems to be a hallmark for the generation of long-lived plasma cells,55 short-lived cells can be generated following an extrafollicular response,51 which does not necessarily imply immunoglobulin evolution through somatic hypermutation nor selection of high-affinity B cells. Interestingly, hospitalized patients showed a more limited cross-neutralizing response against the Beta variant relative to WH1, suggesting that B cell responses in severe disease, despite being higher in magnitude, could be less cross-neutralizing. Although the association of severity and magnitude of neutralizing responses has been pointed out for early responses in different studies,2,13,56 our data extend this observation to the long-term responses, also suggesting a discordant relationship between the magnitude and quality of antibodies in hospitalized individuals.
In the cohort studied, we observed that neutralizing activity is significantly boosted after vaccination, although the longevity of this response still needs to be determined. Based on our data on unvaccinated infected individuals, the vaccination of people who have overcome the SARS-CoV-2 infection should lead to a long-lasting protection. But this information must be interpreted carefully since new emerging variants of the virus could escape both natural and vaccine-induced immunity.57
To address the impact of VOCs, we tested neutralization titers against Alpha, Beta, and Delta variants. Despite showing lower titers, non-hospitalized individuals tended to have better relative cross-neutralization against all variants tested. Of note, the loss of neutralization titers observed in our study was lower than the values reported for vaccinated individuals.58 We did not observe changes for the Alpha variant, and the ratios calculated for the Beta and Delta variants compared with the WH1 isolate below 3-fold. This fact could be related to antibody evolution after recovery from infection and is consistent with data reporting increased cross-neutralization in vaccinated or unvaccinated COVID-19 convalescent individuals.48,58,59 Therefore, we exclusively observed a significant reduction of titers for the Beta variant, resulting in a high frequency of individuals with undetectable or low (<250) neutralizing capacities that were significantly higher in non-hospitalized individuals. When analyzing the clinical and demographic factors that could influence the long-term neutralizing antibody response, we did not observe any differences between women and men. In contrast, age shows a certain tendency (older participants present higher neutralizing activity) whose significance was evident in the univariate analysis but did not reach significance in the multivariate linear regression. This latter result could indicate that age by itself is not a determinant component but depends on other cofactors, as could be the severity of the disease, which is highlighted as the main determinant of the magnitude of long-term responses. This is in line with the evidence described so far,44,47 although it disagrees with another study describing antibody kinetics influenced by gender.46 Despite the clear effect of severity, there is still a high individual heterogeneity in the magnitude of neutralization achieved by participants in each group (non-hospitalized or hospitalized individuals) that needs further study to unveil additional determinants.
Limitations of the study
Our analysis provides one of the largest datasets on neutralizing activity (in number of participants and follow-up time) but is limited by the lack of parallel data on T cells and other immune-related factors. In addition, the long-term impact of vaccination is still an open question; therefore, beyond the clear boosting effect observed, we cannot draw further conclusions due to heterogeneous vaccine schedules and sampling times.
Our longitudinal analysis confirmed the early decay and long-term maintenance of neutralizing activity observed in other cohorts.10,12 Moreover, our data identified different dynamics of short- and long-lived responses after infection. In particular, severity of primary infection is associated with the emergence of short-lived antibodies (not observed in non-hospitalized individuals) and the generation of higher titers of less cross-neutralizing long-lived antibodies (beyond 1 year).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| pNL4-3.Luc.R-.E- | NIH ARP | Cat#3418 |
| SARS-CoV-2.SctΔ19 | This paper | N/A |
| pcDNA3.1(+) | GeneArt/Thermo Fisher Scientific | Cat#810330DE |
| pVSV-G | Clontech | Sánchez-Palomino et al48 |
| Chemicals, peptides, and recombinant proteins | ||
| Fetal Bovine Serum | Thermo Fisher Scientific | Cat#10270106 |
| Dulbecco’s Modified Eagle Medium | Thermo Fisher Scientific | Cat#41966052 |
| Expi293 Expression Medium | Thermo Fisher Scientific | Cat#A1435102 |
| Opti-MEM I Reduced Serum Medium | Thermo Fisher Scientific | Cat#31985070 |
| ExpiFectamine 293 Transfection Kit | Thermo Fisher Scientific | Cat#A14524 |
| Versene | Thermo Fisher Scientific | Cat#15040033 |
| Puromycin | Thermo Fisher Scientific | Cat#A1113803 |
| DEAE-Dextran | Sigma-Aldrich | Cat#D9885-100G |
| BriteLite Plus Luciferase | PerkinElmer | Cat#6066769 |
| Experimental models: Cell lines | ||
| Expi293F GnTI- cells | Thermo Fisher Scientific | Cat#A39240 |
| HEK293T/hACE2 cells | Integral Molecular | Cat#C-HA101 |
| Software and algorithms | ||
| GraphPad Prism v9.3.1 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| R v3.6.3 | R Foundation for Statistical Computing | https://www.r-project.org/ |
| “nlme” R Package | R Foundation for Statistical Computing | https://cran.r-project.org/web/packages |
| “sme” R Package | R Foundation for Statistical Computing | https://cran.r-project.org/web/packages |
| Other | ||
| GeneArt Gene Synthesis | Thermo Fisher Scientific | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Julià Blanco (jblanco@irsicaixa.es).
Materials availability
The plasmids pcDNA3.1 SARS-CoV-2.SctΔ19 are available upon request to the lead contact.
Experimental model and subject details
Study overview and subjects
The study KING was approved by the Hospital Ethics Committee Board from Hospital Universitari Germans Trias i Pujol (HUGTiP, PI-20-122 and PI-20-217) and was further amended to include vaccinated individuals. All participants provided written informed consent before inclusion.
Plasma samples were obtained from individuals of the prospective KING cohort of the HUGTiP (Badalona, Spain). The recruitment period lasted from March 2020 to March 2021, thus covering the three consecutive outbreaks of COVID-19 in Catalonia (Figure S1). The KING cohort included individuals with a documented positive RT-qPCR result from nasopharyngeal swab and/or a positive serological diagnostic test. Participants were recruited irrespective of age and disease severity including asymptomatic status in various settings, including primary care, hospital, and epidemiological surveillance based on contact tracing. We collected plasma samples at the time of COVID-19 diagnosis and at 3, 6 and 12 months after diagnosis. Additionally, hospitalized individuals were sampled twice a week during acute infection. Viral sequences were available from a subset of participants (n = 26, 8% of the cohort) and confirmed the expected prevalence of B.1 variant during the first wave (67% of sequences), 20E(EU1) variant during the second one (70% of sequences) and alpha variant after January 2021 (80% of sequences).
Cell lines
HEK293T cells overexpressing WT human ACE-2 (Integral Molecular, USA) were used as target in pseudovirus-based neutralization assay. Cells were maintained in T75 flasks with Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS and 1 μg/ml of Puromycin (Thermo Fisher Scientific, USA).
Expi293F cells (Thermo Fisher Scientific) are a HEK293 cell derivative adapted for suspension culture that were used for SARS-CoV-2 pseudovirus production. Cells were maintained under continuous shaking in Erlenmeyer flasks following manufacturer’s guidelines.
Method details
Pseudovirus generation and neutralization assay
HIV reporter pseudoviruses expressing SARS-CoV-2 S protein and Luciferase were generated. pNL4-3.Luc.R-.E- was obtained from the NIH AIDS Reagent Program.60 SARS-CoV-2.SctΔ19 was generated (GeneArt) from the full protein sequence of the original WH1 SARS-CoV-2 spike (Genbank MN908947.3) with a deletion of the last 19 amino acids in C-terminal,61 human-codon optimized and inserted into pcDNA3.1(+). A similar procedure was followed to generate expression plasmids for the alpha (69-70 del, Y144 del, N501Y, A570D, P681H, T716I, S982A and D1118H), beta (L18F, D80S, D215G, L242_244 Del, R246I, K417N, E484K, N501Y, D614G, A701V) and delta (T19R, 157-158del, L452R, T478K, D614G, P681R, D950N) variants of SARS-CoV-2 S protein59 according to consensus data (www.outbreak.info/). Expi293F cells were transfected using ExpiFectamine293 Reagent (Thermo Fisher Scientific) with pNL4-3.Luc.R-.E- and SARS-CoV-2.SctΔ19 (WH1, alpha, beta or delta), at an 8:1 ratio, respectively. Control pseudoviruses were obtained by replacing the S protein expression plasmid with a VSV-G protein expression plasmid as reported.62 Supernatants were harvested 48 hours after transfection, filtered at 0.45 μm, frozen, and titrated on HEK293T cells overexpressing WT human ACE-2 (Integral Molecular, USA). This neutralization assay has been previously validated in a large subset of samples and negative controls with a replicative viral inhibition assay.13
Neutralization assays were performed in duplicate. Briefly, in Nunc 96-well cell culture plates (Thermo Fisher Scientific), 200 TCID50 of pseudovirus were preincubated with three-fold serial dilutions (1/60–1/14,580) of heat-inactivated plasma samples for 1 hour at 37°C. Then, 2x104 HEK293T/hACE2 cells treated with DEAE-Dextran (Sigma-Aldrich) were added. Results were read after 48 hours using the EnSight Multimode Plate Reader and BriteLite Plus Luciferase reagent (PerkinElmer, USA). The values were normalized, and the ID50 (reciprocal dilution inhibiting 50% of the infection) was calculated by plotting and fitting all duplicate neutralization values and the log of plasma dilution to a 4-parameters equation in Prism 9.0.2 (GraphPad Software, USA).
Quantification and statistical analysis
Continuous variables were described using medians and the interquartile range (IQR, defined by the 25th and 75th percentiles), whereas categorical factors were reported as percentages over available data. Quantitative variables were compared using the Mann-Whitney test, and percentages using the chi-squared test. For the longitudinal analysis of neutralizing activity, patients were grouped into two severity groups according to the WHO progression scale21 asymptomatic or mild (levels 1-3), and hospitalized (levels 4-10).
Longitudinal kinetics of neutralization activity for hospitalized and mild groups were analyzed by nonlinear models in two ways, parametric and non-parametric models and stratifying by severity in both cases. We fitted a non-parametric model using smoothing-splines mixed-effects model using the “sme” package of R. The final part of this model, showing an increase in neutralization activity, is unreliable due to the small sample size available in that stretch. We also analyzed the observed decrease of neutralization after 30 days by a biexponential decay model [y=P1∗exp(-k1∗t) + P2∗exp(-k2∗t)] fitting a nonlinear mixed-effects model and using “nlme” package of R. In this case three samples were excluded due to their influence in the model fitting since were samples after 350 days with and important increase of neutralization with respect the previous determinations and although we cannot rule out their veracity, they had a great impact on the proper fit of the model due to the lack of sample size in the final part of the follow-up.
Differences in neutralization between both groups after 300 days since symptoms were analyzed. We also analyzed the effect of age and gender using a multivariate linear model adjusting by severity to avoid confusion effects, especially for age that are associated with severity. Statistical analyses were performed using R-3.6.3 (R Foundation for Statistical Computing) and Prism 9.0.2 (GraphPad Software).
Acknowledgments
This work was partially funded by Grifols, the Departament de Salut of the Generalitat de Catalunya (grants SLD016 to J.B. and SLD015 to J.C.), the Spanish Health Institute Carlos III and the European Regional Development Fund (grant PI17/01518 and PI20/00093 to J.B. and PI18/01332 to J.C.), CERCA Programme/Generalitat de Catalunya 2017 SGR 252, and the crowdfunding initiatives #joemcorono, BonPreu/Esclat, and Correos. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. E.P. was supported by a doctoral grant from the National Agency for Research and Development of Chile (ANID) (72180406). We are grateful to all participants and the technical staff of IrsiCaixa for sample processing. Francesc López-Seguí provided medical writing support during the preparation of the manuscript.
Author contributions
J.B. and B.C. designed and coordinated the study. E.P, B.T., S.M., F.T.-F., R.O., C.R., J.R., J.V.-A., J.S., and N.I.-U. performed and analyzed neutralization assays. E.P., B.T., and V.U. performed statistical analysis. M.N.-J., R.P., L.M., A.C., R.T., M.M., V.G., A.V., and J.C. selected patients and coordinated data. J.B. and E.P. drafted the manuscript, and all authors have made substantial contributions to the revision of the subsequent versions. All authors approved the submitted version of the manuscript and agreed both to be personally accountable for their own contributions and to ensure the accuracy or integrity of any part of the work.
Declaration of interests
Unrelated to the submitted work, J.B. and J.C. are founders and shareholders of AlbaJuna Therapeutics, SL. B.C. is founder and shareholder of AlbaJuna Therapeutics, SL, and AELIX Therapeutics, SL. The other authors declare no competing interests.
Published: January 24, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100523.
Supplemental information
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradenas E., Trinité B., Urrea V., Marfil S., Ávila-Nieto C., Rodríguez de la Concepción M.L., Tarrés-Freixas F., Pérez-Yanes S., Rovirosa C., Ainsua-Enrich E., et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med. 2021;2:313–320.e4. doi: 10.1016/j.medj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;80:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrillo J., Izquierdo-Useros N., Ávila-Nieto C., Pradenas E., Clotet B., Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2020;538:187–191. doi: 10.1016/j.bbrc.2020.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 6.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Warren F., et al. Antibody status and incidence of SARS-CoV-2 infection in Health care workers. N. Engl. J. Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addetia A., Crawford K.H.D.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J. Clin. Microbiol. 2020;58:1–11. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccin. Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AD J., AJ H., N M., M M., HC W. What level of hepatitis B antibody is protective? J. Infect. Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., et al. Antibody responses to SARS-CoV-2 in patients with Novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao A.T., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J. Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., Vanderheiden A., Nyhoff L., Davis C.W., Adekunle O., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinité B., Tarrés-Fraixas F., Rodon J., Pradenas E., Urrea V., Marfil S.S., Rodriguez de la Concepción M.L., Avila-Nieto C., Aguilar-Gurrieri C., Barajas A., et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021;11:2608. doi: 10.1038/s41598-021-81862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Guo X., Xin Q., Pan Y., Hu Y., Li J., Chu Y., Feng Y., Wang Q. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin. Infect. Dis. 2020;71:2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti–SARS-CoV-2 antibodies in Persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.-X., Lee W.S., Wragg K.M., Kelly H.G., Esterbauer R., et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Planchais C., Buchrieser J., Rajah M.M., Bishop E., et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J.C., Murthy S., Diaz J., Adhikari N., Angus D.C., Arabi Y.M., Baillie K., Bauer M., Berry S., Blackwood B., et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., Hansen L., Haile A., Klebert M.K., Pusic I., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;7867:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert P.B., Montefiori D.C., McDermott A., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2021;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 25.Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A.J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Abdely H.M., Midgley C.M., Alkhamis A.M., Abedi G.R., Lu X., Binder A.M., Alanazi K.H., Tamin A., Banjar W.M., Lester S., et al. Middle east respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg. Infect. Dis. 2019;25:753–766. doi: 10.3201/eid2504.181595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choe P.G., Perera R.A.P.M., Park W.B., Song K.H., Bang J.H., Kim E.S., Kim H.B., Ko L.W.R., Park S.W., Kim N.J., et al. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg. Infect. Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., Muth D., Sieberg A., Meyer B., Assiri A.M., et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie Y., Wang G., Shi X., Zhang H., Qiu Y., He Z., Wang W., Lian G., Yin X., Du L., et al. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 2004;190:1119–1126. doi: 10.1086/423286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park W.B., Perera R.A.P.M., Choe P.G., Lau E.H.Y., Choi S.J., Chun J.Y., Oh H.S., Song K.H., Bang J.H., Kim E.S., et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg. Infect. Dis. 2015;21:2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temperton N.J., Chan P.K., Simmons G., Zambon M.C., Tedder R.S., Takeuchi Y., Weiss R.A. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg. Infect. Dis. 2005;11:411–416. doi: 10.3201/eid1103.040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao W.-C., Liu W., Zhang P.-H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 34.Liu L., Xie J., Sun J., Han Y., Zhang C., Fan H., Liu Z., Qiu Z., He Y., Li T. Longitudinal profiles of immunoglobulin G antibodies against severe acute respiratory syndrome coronavirus components and neutralizing activities in recovered patients. Scand. J. Infect. Dis. 2011;43:515–521. doi: 10.3109/00365548.2011.560184. [DOI] [PubMed] [Google Scholar]
- 35.Alshukairi A.N., Zhao J., Al-Mozaini M.A., Wang Y., Dada A., Baharoon S.A., Alfaraj S., Ahmed W.A., Enani M.A., Elzein F.E., et al. Longevity of middle east respiratory syndrome coronavirus antibody responses in humans, Saudi Arabia. Emerg. Infect. Dis. 2021;27:1472–1476. doi: 10.3201/eid2705.204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson D.E., Tan C.W., Chia W.N., Young B.E., Linster M., Low J.H., Tan Y.-J., Chen M.I.-C., Smith G.J.D., Leo Y.S., et al. Lack of cross-neutralization by SARS patient sera towards SARS-CoV-2. Emerg. Microbes Infect. 2020;9:900–902. doi: 10.1080/22221751.2020.1761267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne D.C., Iblan I., Rha B., Alqasrawi S., Haddadin A., Al Nsour M., Alsanouri T., Ali S.S., Harcourt J., Miao C., et al. Persistence of antibodies against middle east respiratory syndrome coronavirus. Emerg. Infect. Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y., Cai C., Lai K., Chen R., Chan-Yeung M., et al. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11:49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y. Il, Kim S.M., Park S.J., Kim E.H., Yu K.M., Chang J.H., Kim E.J., Casel M.A.B., Rollon R., Jang S.G., et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg. Microbes Infect. 2021;10:152–160. doi: 10.1080/22221751.2021.1872352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Ding N., Ren L., Song R., Chen D., Zhao X., Chen B., Han J., Li J., Song Y., et al. COVID-19 reinfection in the presence of neutralizing antibodies. Natl. Sci. Rev. 2021;8:2021. doi: 10.1093/nsr/nwab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brehm T.T., Pfefferle S., von Possel R., Kobbe R., Nörz D., Schmiedel S., Grundhoff A., Olearo F., Emmerich P., Robitaille A., et al. Sars-cov-2 reinfection in a healthcare worker despite the presence of detectable neutralizing antibodies. Viruses. 2021;13:661. doi: 10.3390/v13040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont L., Snell L.B., Graham C., Seow J., Merrick B., Hallett S.R., et al. Neutralizing antibody activity in convalescent sera from infection in humans with SARS-CoV-2 and variants of concern. Nat. Microbiol. 2021;6:1433–1442. doi: 10.1038/s41564-021-00974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega N., Ribes M., Vidal M., Rubio R., Aguilar R., Williams S., Barrios D., Alonso S., Hernández-Luis P., Mitchell R.A., et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat. Commun. 2021;12:4740. doi: 10.1038/s41467-021-24979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muena N.A., García-Salum T., Pardo-Roa C., Serrano E.F., Levican J., José Avendaño M., Almonacid L.I., Valenzuela G., Poblete E., Salinas E., et al. Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are 1 robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines. medRxiv. 2021 doi: 10.1101/2021.05.17.21257197. [DOI] [Google Scholar]
- 46.Gallais F., Gantner P., Bruel T., Velay A., Planas D., Wendling M.-J., et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. eBioMedicine. 2021;71 doi: 10.1101/2021.05.07.21256823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haveri A., Ekström N., Solastie A., Virta C., Österlund P., Isosaari E., et al. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. European Journal of Immunology. 2021;51:3202–3213. doi: 10.1101/2021.07.13.21260426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:1–10. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slifka M.K., Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 50.Radbruch A., Muehlinghaus G., Luger E.O., Inamine A., Smith K.G.C., Dörner T., Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 51.Khodadadi L., Cheng Q., Radbruch A., Hiepe F. The maintenance of memory plasma cells. Front. Immunol. 2019;0:721. doi: 10.3389/fimmu.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manz R.A., Hauser A.E., Hiepe F., Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2004;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 53.Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 54.Miller J.J. An autoradiographic study of plasma cell and lymphocyte survival in rat popliteal lymph nodes. J. Immunol. 1964;92:673–681. [PubMed] [Google Scholar]
- 55.Suan D., Sundling C., Brink R. Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol. 2017;45:97–102. doi: 10.1016/j.coi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., Gruell H., Schlotz M., Ercanoglu M.S., Stumpf R., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Beltran W.F., Lam E.C., St. Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carreño J.M., Alshammary H., Singh G., Raskin A., Amanat F., Amoako A., Gonzalez-Reiche A.S., van de Guchte A., study group P., Srivastava K., et al. Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipients. EBioMedicine. 2021;73:103626. doi: 10.1016/j.ebiom.2021.103626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trinité B., Pradenas E., Marfil S., Rovirosa C., Urrea V., Tarrés-Freixas F., Ortiz R., Rodon J., Vergara-Alert J., Segalés J., et al. Previous SARS-CoV-2 infection increases B.1.1.7 cross-neutralization by vaccinated individuals. Viruses. 2021;13:1135. doi: 10.3390/v13061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 61.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sánchez-Palomino S., Massanella M., Carrillo J., García A., García F., González N., Merino A., Alcamí J., Bofill M., Yuste E., et al. A cell-to-cell HIV transfer assay identifies humoral responses with broad neutralization activity. Vaccine. 2011;29:5250–5259. doi: 10.1016/j.vaccine.2011.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.