Abstract
Patients with multisystem inflammatory syndrome in children (MIS-C) can develop clinical features resembling Kawasaki disease (KD). A full picture of MIS-C in East Asia which has higher incidence of KD than other regions remains unclear. We report on a 15-year-old Japanese boy with refractory MIS-C who was successfully treated with infliximab. A Japanese boy who was diagnosed with coronavirus disease 2019 (COVID-19) before a month developed MIS-C with fulfilling six principal symptoms of KD. Laboratory data showed extreme hyperferritinemia (11,404 ng/mL), besides lymphopenia and thrombocytopenia. The patient was refractory to initial therapy with intravenous immunoglobulin (IVIG; 2 g/kg), aspirin, and prednisolone. He was therefore administered a second IVIG (2 g/kg) and infliximab (5 mg/kg) on days 7 and 8 from the onset of fever, respectively, which resulted in an improvement of clinical symptoms. Only four Japanese cases with MIS-C were reported and all of them were responsive to IVIG. The hyperferritinemia in this case was distinctive from previously reported MIS-C cases in Japan and other cohorts and may be associated with refractoriness to IVIG therapy. Marked elevation of circulating ferritin levels is known to be induced by tumor necrosis factor-α, which plays a key role in the pathogenesis of both KD and MIS-C. Thus, for MIS-C patients with hyperferritinemia, early intervention with adjunctive infliximab may induce a more rapid resolution of inflammation and improve outcome. Because MIS-C may be heterogeneous with respect to immunopathology, genetic background, clinical phenotypes and response to therapies, optimized treatment strategies according to immunopathogenesis are required.
Keywords: Multisystem inflammatory syndrome in children, Infliximab, COVID-19, Hyperinflammation
1. Introduction
Beginning in Europe in April 2020 [1], many children with coronavirus disease 2019 (COVID-19) developed severe complications with Kawasaki disease (KD)–like presentations. The complications affect multiple organ systems, including cardiac, gastrointestinal, haematological, dermatological, neurological, and renal systems. In mid-May 2020, the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention (CDC) published each case definition for multisystem inflammatory syndrome in children (MIS-C) for disease surveillance [2,3]. Several treatment guidance for MIS-C recommended high-dose intravenous immunoglobulin (IVIG), 2 g/kg, and optional use of methylprednisolone and biological therapies including IL-1R antagonists (anakinra), IL-6R antagonist (tocilizumab), and tumor necrosis factor (TNF)-α antagonist (infliximab) [4,5]. Several systematic reviews of MIS-C have been reported [[6], [7], [8], [9]], in which most of the reviewed studies were conducted in the USA, Europe, Latin America, South and West Asia. A full picture of MIS-C in East Asia which has more than ten times higher incidence of KD than other regions [10] remain unclear. Herein, we report on a Japanese boy with refractory MIS-C who was successfully treated with infliximab.
2. Case report
A 15-year-old Japanese boy presented with fever and cough, and he was diagnosed with COVID-19 by polymerase chain reaction (PCR) test. His mother was also diagnosed with COVID-19 six days before. His past medical history revealed no major problems other than neck swelling at the age of 1.5 years. He spontaneously recovered from COVID-19 on the second day of fever without any sequelae. Twenty-seven days after the onset of COVID-19, he developed a fever of 39 °C and neck pain. A PCR test for COVID-19 was negative at that time. Two days later, he experienced headache and diarrhea. On day 4, he was admitted to the hospital because of continuous symptoms, and antimicrobial drugs were started. On day 5, bilateral bulbar conjunctival injection, red lips, body trunk erythema, and reddening of palms and soles were observed, and he fulfilled all clinical features of KD [11,12]. His blood examinations showed elevation of C-reactive protein (CRP; 7.1 mg/dL), d-dimer (13.0 μg/mL), ferritin (11,404 ng/mL), and decrease of platelet count (49,000/μL), serum sodium level (130 mEq/L). Thus, he was treated with IVIG; 2 g/kg, aspirin (ASA; 300 mg/day), prednisolone (PSL; 60 mg/day), and low-molecular-weight heparin (LMWH; 75 IU/kg/day) according to Japanese guideline for medical treatment of acute KD [12]. Because his symptoms did not improve, he was transferred to our hospital on day 6.
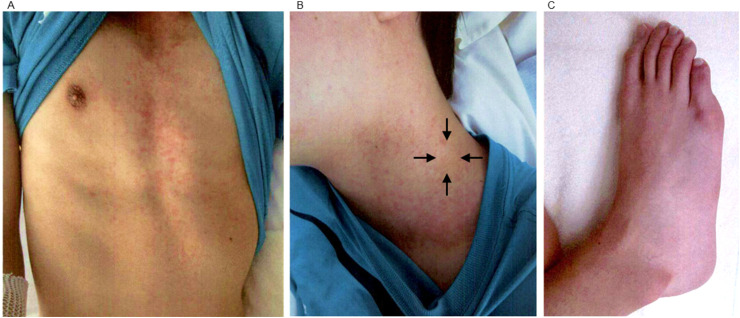
At admission, he presented with fever of 40.2 °C and diarrhea, and was associated with bilateral bulbar conjunctival injection, red lips, left tender cervical lymphadenopathy, erythema on body trunk and bilateral knees, and reddening of feet (Fig. 1 ). Blood examinations revealed elevation of neutrophil count (5680/μL), CRP (10.22 mg/dL), erythrocyte sedimentation rate (25 mm/hr), d-dimer (4.1 μg/mL), ferritin (6639 ng/mL), soluble interleukin-2 receptor (sIL2R; 4075 U/mL), brain natriuretic peptide (46.4 pg/mL) and decrease of absolute lymphocyte count (252/μL), platelet count (71,000/μL). A PCR test for COVID-19 was weakly positive (1.3 copies/μL), although the PCR test examined at the previous hospital was negative. Chest X-ray, chest computed tomography, and electrocardiogram showed no abnormal findings. Echocardiography revealed normal ventricular systolic function, no coronary arterial lesions, insignificant mitral valve regurgitation, and slight pericardial effusion. Therefore, he was diagnosed with MIS-C because he met the criteria recommended by the WHO [2], CDC [3], and the Royal College of Paediatrics and Child Health [13].
Fig. 1.
Erythema and reddening. (A) Erythema on trunk. (B) Left cervical lymphadenopathy, 2cm (black arrows). (C) Reddening of a foot.
We continued to administer IVIG, PSL, and LMWH, and increased dose of ASA to 900 mg/day. He developed severe headache and delirium in the night of day 6. Although the most of KD symptoms, including bulbar conjunctival injection, red lips, tender cervical lymphadenopathy, and erythema improved on the next day (day 7), his fever was persistent. Thus, we administrated second IVIG (2 g/kg) on day 7. On day 8, diarrhea disappeared, and fever subsided temporarily but then quickly returned. CRP and d-dimer levels decreased from their levels on Day 6, whereas ferritin remained at a high level (Table 1 ), suggesting possible hypercytokinemia. According to guidelines for IVIG refractory KD and MIS-C in Japan [14,15], we administered infliximab (5 mg/kg) through the intravenous route separated from the main route for 2 h, on day 8. Prior to the administration, we clarified that tuberculosis interferon-gamma release assay, hepatitis B surface antigen, hepatitis C virus antibody, human immunodeficiency virus antibody, and Treponema pallidum antibody were all negative. After infliximab administration, fever disappeared permanently, and other symptoms completely diminished. Laboratory data also showed improvement. On day 11, A COVID-19 PCR test was negative. Because the levels of aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) increased on day 8, we stopped ASA, and started dipyridamole treatment (75mg/day). We examined the patient by echocardiography every two or three days, and found stable ventricular systolic function, no coronary arterial lesions, insignificant mitral valve regurgitation, and tapered pericardial effusion. On day 13, we started tapering PSL treatment. Following the tapering of PSL, his condition stabilized, and laboratory results improved continuously. He was thus transferred to the previous hospital on day 15 and was discharged on day 23.
Table 1.
Blood examinations.
| Daya 5 | Day 6 | Day 8 | Day 10 | Day 15 | |
|---|---|---|---|---|---|
| White blood cell (103/μL) | 4.3 | 6.0 | 6.5 | 6.9 | 10.7 |
| Neutrophil (103/μL) | 3.7 | 5.7 | 6.0 | 4.1 | 6.3 |
| Lymphocyte (103/μL) | 0.2 | 0.3 | 0.4 | 2.3 | 3.3 |
| Hemoglobin (g/dL) | 15.8 | 13.9 | 15.2 | 14.2 | 14.5 |
| Platelet (104/μL) | 4.9 | 7.1 | 13.0 | 19.8 | 40.7 |
| Total protein (g/dL) | 6.4 | 7.4 | 9.6 | 8.2 | 9.1 |
| Albumin (g/dL) | 3.7 | 3.2 | 2.7 | 2.8 | 3.7 |
| Blood urea nitrogen (mg/dL) | 10.4 | 13.3 | 13.9 | 14.1 | 22.9 |
| Creatinine (mg/dL) | 0.85 | 0.83 | 0.64 | 0.60 | 0.65 |
| Sodium (mEq/L) | 130 | 136 | 134 | 136 | 137 |
| Potassium (mEq/L) | 3.8 | 3.5 | 4.2 | 4.7 | 4.1 |
| Chloride (mEq/L) | 92 | 99 | 100 | 102 | 100 |
| Lactate dehydrogenase (IU/L) | 514 | 464 | 504 | 403 | 233 |
| Creatinine kinase (CK; IU/L) | 101 | 75 | N/A | N/A | 28 |
| CK-myocardial band (IU/L) | N/A | 1 | N/A | N/A | N/A |
| Aspartate aminotransferase (IU/L) | 43 | 43 | 317 | 303 | 33 |
| Alanine aminotransferase (IU/L) | 32 | 37 | 297 | 558 | 174 |
| Total bilirubin (mg/dL) | 0.9 | 0.5 | 0.6 | 0.8 | 1.1 |
| Total cholesterol (mg/dL) | 135 | 122 | 124 | N/A | 198 |
| Triglyceride (mg/dL) | 115 | 96 | N/A | N/A | 206 |
| C-reactive protein (mg/dL) | 7.14 | 10.22 | 4.34 | 1.79 | 0.18 |
| Erythrocyte sedimentation rate (mm/h) | 5 | 25 | 69 | N/A | 44 |
| Prothrombin time-international normalized ratio | 1.17 | 0.99 | 0.97 | 1.03 | 0.94 |
| Fibrinogen (mg/dL) | 412 | 341 | 293 | 238 | N/A |
| D-dimer | 13.0 | 4.1 | 2.0 | 0.6 | 1.1 |
| Ferritin (ng/mL) | 11,404 | 6639 | 5155 | N/A | 1566 |
| Soluble interleukin-2 receptor (IU/mL) | N/A | 4074.6 | 3476.5 | N/A | 968.8 |
| Brain natriuretic peptide (pg/mL) | N/A | 46.4 | N/A | N/A | N/A |
| Troponin I (ng/mL) | N/A | 49 | N/A | N/A | N/A |
Days from onset of fever, N/A: not available. Bold values indicate out of normal ranges.
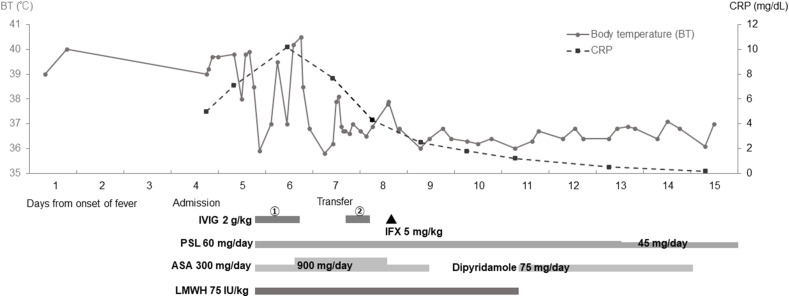
The clinical course of the patient is shown in Fig. 2 and the changes in laboratory data are shown Table 1.
Fig. 2.
Clinical and therapeutic courses of the patient. The grey line and dashed line indicate body temperature and C-reactive protein, respectively. CRP: C-reactive protein; IVIG: intravenous immunoglobulin; IFX: infliximab; PSL: prednisolone; ASA: aspirin; LMWH: low-molecular-weight heparin.
3. Discussion
MIS-C has been described as a severe KD-like disease accompanied with hyperinflammation or cytokine storm and has both partial similarity to and difference from KD. Although it was reported that only one-third or a quarter of MIS-C patients fulfilled criteria for complete KD [11,12], the patient was diagnosed as having MIS-C by fulfilling six principal clinical findings of KD. Several clinical features and laboratory findings, including higher age (>5 years), an antecedent infection of SARS-COV-2, gastrointestinal and neurological symptoms, lymphopenia and thrombocytopenia, are suggestive of MIS-C rather than KD [[16], [17], [18], [19]]. Moreover, extreme elevation of ferritin, D-dimer and sIL2R, which indicate hyperinflammation, may be noteworthy.
Recently, several comparative studies between MIS-C and KD/Kawasaki disease shock syndrome were reported [[16], [17], [18], [19]]. In particular, a systematic review and meta-analysis of laboratory data revealed that MIS-C patients had different hematology characteristics, including decreased white blood cell counts, absolute lymphocyte counts and platelet counts [16]; this indicated an association with SARS-CoV-2 infection and variation in potential immunopathogenesis. Indeed, at disease onset, the patient had relatively more severe lymphopenia and thrombocytopenia than the other cohorts (Table 2 ), but promptly improved by treatment (Table 1).
Table 2.
Comparison between MIS-C patients in Japan and other regions.
| This Case | MIS-C in Japan (n= 4) [[20], [21], [22], [23]] Median [range] | MIS-C in the worlda (n= 917) [8] Average (95% CI) | |
|---|---|---|---|
| Age at onset | 15 | 9.5 [[9], [10], [11], [12], [13], [14], [15], [16]] | 9.3 (8.4–10.1) |
| Sex, Male (%) | Male | 50 | 56.8 (52.1–61.5) |
| Clinical symptoms | |||
| Complete KD (%) | + | 75 | 44.3 (34.7–53.9) |
| Gastrointestinal (%) | + | 100 | 87.3 (82.9–91.6) |
| Neurologic (%) | + | 25 | 36.0 (22.8–49.2) |
| Cardiovascular (%) | – | 75 | 55.3 (42.4–68.2) |
| Laboratory values | |||
| White blood cell ( × 109/L) | 4.3 | 11.9 [8.8–14.1] | 11.8 (10.5–13.2) |
| Lymphocyte count ( × 109/L) | 0.17 | 0.26 [0.11–0.51] | 0.8 (0.7–1.0) |
| Platelet count ( × 109/L) | 49 | 144 [74–315] | 155.1 (143.2–167.1) |
| C-reactive protein (mg/dL) | 10.2 | 21.3 [19.2–23.0] | 23.5 (21.6–25.6) |
| Ferritin (ng/mL) | 11,404 | 909.5 [294–3685] | 711.0 (599.5–822.4) |
| d-Dimer (μg/mL) | 13.0 | 9.45 [3.6–24.3] | 3.5 (2.9–4.1) |
| Brain natrium peptide (pg/mL) | 46.4 | 599 [104–1271] | 2191.5 (1334.2–3048.7) |
| Treatment | |||
| Intravenous immunoglobulin (%) | + | 100 | 81.0 (75.0–86.9) |
| Corticosteroids (%) | + | 50 | 63.6 (53.4–73.8) |
| Aspirin (%) | + | 50 | 67.3 (48.8–85.7) |
| Infliximab (%) | + | 0 | 8.0 (2.9–13.1) |
| Mechanical ventilation (%) | – | 0 | 33.0 (24.5–41.5) |
| ECMO (%) | – | 0 | 6.3 (2.8–9.8) |
| Outcomes | |||
| ICU admission (%) | – | 25 | 79.1 (71.6–86.7) |
| Shock (%) | – | 25 | 65.8 (51.1–80.4) |
| Death (%) | – | 0 | 1.9 (1.0–2.8) |
| Coronary artery dilation or aneurysm (%) | – | 25 | 21.4 (12.8–30.1) |
MIS-C, multisystem inflammatory syndrome in children; KS, Kawasaki disease; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
Twelve studies were conducted in the United States, United Kingdom, France, Italy and Spain.
Only four case reports described Japanese MIS-C patients [[20], [21], [22], [23]] and suggested similarities and differences of clinical and laboratory phenotypes compared to patients in other regions or races. Although Japanese MIS-C patients had quite similar clinical symptoms, the frequencies of shock and ICU admission may be lower than in the other cohorts (Table 2). All four previously reported Japanese patients received 2 g/kg of IVIG, and three patients who fulfilled criteria for complete KD required 2 mg/kg/day of prednisolone with aspirin based on the guidelines for acute KD [12]. Even though one patient required inotropic support to stabilize circulation, no patients required invasive mechanical ventilation or extracorporeal membrane oxygenation. These data may indicate that MIS-C of appropriately treated Japanese patients had relatively milder phenotypes than in other regions. We hypothesized that a few factors behind this may be racial differences in susceptibility to COVID-19 or MIS-C opposite to KD, early intervention as KD, and less and later spread of infection.
Hyperferritinemia in this case was distinct from previously reported MIS-C cases in Japan and other cohorts by nearly less than 2000 ng/mL. Although elevation of serum ferritin level may be a useful biomarker to distinguish KD from other acute febrile illnesses using a cut-off value of 120.8 ng/mL [24] and to predict response to IVIG therapy [25], these levels were significantly lower in KD patients than patients with systemic juvenile idiopathic arthritis (sJIA), which has a cut-off value of 369.6 ng/mL [25]. A recent comparative study revealed that serum ferritin levels were higher in patients with MIS-C compared with patients with KD, but they were lower than in patients with macrophage activating syndrome (MAS) due to sJIA [26]. Although this case did not fulfill the criteria for MAS in sJIA [27], extreme hyperferritinemia in this case may be comparable to MAS and associated with refractory to IVIG therapy as opposed to other Japanese cases with good response to IVIG.
Ferritin is a ubiquitous protein that contributes to cellular iron homeostasis. Circulating ferritin levels are markedly elevated during inflammation and are known to be induced by TNF-α, which is released from activated macrophages [28]. TNF-α appears to play a key role in the pathogenesis of both KD and MIS-C [29,30]. Indeed, infliximab which is a chimeric monoclonal antibody that binds TNF-α and inhibits its downstream pro-inflammatory effects, has been successfully used to treat IVIG refractory KD [31] and MIS-C [32,33]. Cole et al. [32] reported that patients with severe MIS-C had better outcomes when they were initially treated with IVIG plus infliximab compared with IVIG alone. Abdel-Haq et al. [33] reported that patients with severe MIS-C who required critical care had higher serum ferritin levels and required second-line infliximab therapy. Therefore, we hypothesize that early intervention with adjunctive infliximab for MIS-C patients with hyperferritinemia, such as this case, may induce more rapid resolution of inflammation and improve outcome.
The whole picture of the hyperinflammatory syndrome in COVID-19 based on a dysregulated host innate immune response has been gradually revealed [32]. Various attempts have been made to build therapeutic strategies centered on therapies modulating the immune response to treat and prevent immunopathology in patients who progress to severe disease. It was suggested that adjunctive anti-inflammatory therapy besides IVIG should be considered early in the management of MIS-C patients with intense inflammation [34,35]. Almost 15–20% patients with MIS-C were treated with biopharmaceuticals, including IL-1R, IL-6, and TNF-α antagonist [[6], [7], [8], [9]] as second-line therapies subsequent to IVIG. As in previous reports [33,34], the patient who was refractory to IVIG therapy responded to infliximab, which has been used successfully to treat IVIG refractory KD, without adverse effects.
Previous reports that evaluated circulating cytokine profiles in MIS-C patients revealed differences in cytokine elevation patterns between MIS-C and KD or mild COVID-19 and between subgroups of MIS-C [18,35]. These data indicate that MIS-C contains several subgroups that may require different treatment strategies in accordance with each subgroup's immunopathology. It is only regrettable that evaluation of circulating cytokine profiles before treatment was not performed in this case. Further accumulation of MIS-C cases, especially IVIG refractory cases with evident hyperinflammation in each region or race, are required to develop and adjust treatment strategies for MIS-C that may be heterogeneous with respect to immunopathology, genetic background, clinical phenotypes, response to therapies, and natural history.
Ethical approval
Not applicable.
Authors’ contribution
Y.Y., K.T., H.I., K.H., Y.I. K.H., Y.T., M.M., M.Y., H.N., M.S., T.I., and T.U. contributed to treatment; Y.Y. and K.T. drafted the manuscript; M.S., H.K., and T.M. critically reviewed the manuscript and supervised the whole study process. All authors read and approved the final manuscript.
Patient consent
Authors obtained the written publication consent of the patient for the case details and images.
Declaration of competing interest
Hitoshi Irabu: Tokyo Medical and Dental University (TMDU) received unrestricted research grants for Ayumi Pharmaceutical Corporation, Chugai Pharmaceutical Co., Ltd., CSL BehringCSL Behring K. K., Japan Blood Products Organization, UCB Japan Co. Ltd., with TMDU paid the salary of Hitoshi Irabu. All other authors have declared no conflicts of interest.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multisystem inflammatory syndrome in children and adolescents with COVID-19 Scientific Brief. World Health Organization; 15 May 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Published. [Google Scholar]
- 3.Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) Health Alert Network (HAN); 14 May 2020. https://emergency.cdc.gov/han/2020/han00432.asp Published. [Google Scholar]
- 4.Multisystem inflammatory syndrome in children (MIS-C) interim guidance. American academy of Pediatrics; 15 November 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/ Updated. [Google Scholar]
- 5.Harwood R., Allin B., Jones C.E., Whittaker E., Ramnarayan P., Ramanan A.V., et al. Lancet Child Adolesc Health. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoste L., Van Paemel R., Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180:2019–2034. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimarães D., Pissarra R., Reis-Melo A., Guimarães H. Multisystem inflammatory syndrome in children (MISC): a systematic review. Int J Clin Pract. 2021 doi: 10.1111/ijcp.14450. [DOI] [PubMed] [Google Scholar]
- 8.Yasuhara J., Watanabe K., Takagi H., Sumitomo N., Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56:837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radia T., Williams N., Agrawal P., Harman K., Weale J., Cook J., et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uehara R., Belay E.D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T., Ayusawa M., Suzuki H., Abe J., Ito S., Kato T., et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition) Pediatr Int. 2020;62:1135–1138. doi: 10.1111/ped.14326. [DOI] [PubMed] [Google Scholar]
- 13.Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. Health RCoPaC; 2020. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance [Google Scholar]
- 14.Miura M., Ayusawa M., Ito SIkeda T., Kanai T., Kobayashi T., et al. The guidelines on acute stage Kawasaki disease treatment (in Japanese) Ped Cardiol Card Surg. 2020;36 S1.1‒S1.29. [Google Scholar]
- 15.Clinical consensus statement for multisystem inflammatory syndrome in children (MIS-C/PIMS) Japan Pediatric Society; 19 May 2021. https://www.jpeds.or.jp/uploads/files/202105120_mis-c_st.pdf Published. [Google Scholar]
- 16.Zhou C., Zhao Y., Wang X., Huang Y., Tang X., Tang L. Laboratory parameters between multisystem inflammatory syndrome in children and Kawasaki disease. Pediatr Pulmonol. 2021 doi: 10.1002/ppul.25687. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.S., Liu Y.C., Tsai C.C., Hsu J.H., Wu J.R. Similarities and differences between COVID-19-related multisystem inflammatory syndrome in children and Kawasaki disease. Front Pediatr. 2021;9:640118. doi: 10.3389/fped.2021.640118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteve-Sole A., Anton J., Pino-Ramirez R.M., Sanchez-Manubens J., Fumadó V., Fortuny C., et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Invest. 2021;131 doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki J., Abe K., Matsui T., Honda T., Yasukawa K., Takanashi J.I., et al. Kawasaki disease shock syndrome in Japan and comparison with multisystem inflammatory syndrome in children in European countries. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.625456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba T., Maruyama T., Katsuragi S., Maeda K., Kogaki S. Multisystem inflammatory syndrome associated with SARS-CoV-2 in a Japanese girl. Pediatr Int. 2021 doi: 10.1111/ped.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida M., Kashima Y., Mochizuki K., Sakamoto H., Mori K., Ebisawa S., et al. Multisystem inflammatory syndrome in children - a new syndrome complicated with acute heart failure following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Circ J. 2021;85:948–952. doi: 10.1253/circj.CJ-21-0243. [DOI] [PubMed] [Google Scholar]
- 22.Takasago S., Sakai A., Sugiyama M., Mizokami M., Hamada H., Ishizaka Y., et al. Case report: changes in cytokine kinetics during the course of disease in a Japanese patient with multisystem inflammatory syndrome in children. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.702318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda S., Kaneta M., Miyake M., Ohya T., Miyakawa K., Iwamoto M., et al. A case of multisystem inflammatory syndrome in children in a Japanese boy: with discussion of cytokine profile. Mod Rheumatol Case Rep. 2021;5:442–447. doi: 10.1080/24725625.2021.1920140. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.H., Song E.S., Yoon S., Eom G.H., Kang G., Cho Y.K. Serum ferritin as a diagnostic biomarker for Kawasaki disease. Ann Lab Med. 2021;41:318–322. doi: 10.3343/alm.2021.41.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuta M., Shimizu M., Inoue N., Kasai K., Nakagishi Y., Takahara T., et al. Serum ferritin levels as a useful diagnostic marker for the distinction of systemic juvenile idiopathic arthritis and Kawasaki disease. Mod Rheumatol. 2016;26:929–932. doi: 10.3109/14397595.2016.1159120. [DOI] [PubMed] [Google Scholar]
- 26.Otar Yener G., Paç Kısaarslan A., Ulu K., Atalay E., Haşlak F., Özdel S., et al. Differences and similarities of multisystem inflammatory syndrome in children, Kawasaki disease and macrophage activating syndrome due to systemic juvenile idiopathic arthritis: a comparative study. Rheumatol Int. 2021 doi: 10.1007/s00296-021-04980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravelli A., Minoia F., Davì S., Horne A., Bovis F., Pistorio A., et al. Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American College of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Ann Rheum Dis. 2016;75:481–489. doi: 10.1136/annrheumdis-2015-208982. 2016. [DOI] [PubMed] [Google Scholar]
- 28.Kwak E.L., Larochelle D.A., Beaumont C., Torti S.V., Torti F.M. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem. 1995;270:15285–15293. doi: 10.1074/jbc.270.25.15285. [DOI] [PubMed] [Google Scholar]
- 29.Jinkawa A., Shimizu M., Nishida K., Kaneko S., Usami M., Sakumura N., et al. Cytokine profile of macrophage activation syndrome associated with Kawasaki disease. Cytokine. 2019;119:52–56. doi: 10.1016/j.cyto.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Diorio C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J., Burudpakdee C., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori M., Hara T., Kikuchi M., Shimizu H., Miyamoto T., Iwashima S., et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8:1994. doi: 10.1038/s41598-017-18387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole L.D., Osborne C.M., Silveira L.J., Rao S., Lockwood J.M., Kunkel M.J., et al. IVIG compared to IVIG plus infliximab in multisystem inflammatory syndrome in children. Pediatrics. 2021 doi: 10.1542/peds.2021-052702. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Haq N., Asmar B.I., Deza Leon M.P., McGrath E.J., Arora H.S., Cashen K., et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. 2021;180:1581–1591. doi: 10.1007/s00431-021-03935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]