Abstract
This report describes a case of spontaneous corneal perforation in a patient with rheumatoid arthritis (RA) and highlights the efficacy of treatment with amniotic membrane transplantation (AMT). A 58-year-old African-American woman with RA presented with complaints of redness and blurred vision in the right eye. Spontaneous corneal perforation and iris prolapse were determined in the paracentral corneal region of the right eye. Two-layer AMT surgery was performed. The anterior chamber began to improve 2 weeks after the AMT. Treatment for RA was scheduled with the rheumatology department.
Keywords: Amniotic membrane transplantation, corneal perforation, dry eye syndrome, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is the most common systemic autoimmune disease and affects an estimated 0.9% of the general population (1). Ocular findings of RA include episcleritis, scleritis, peripheral ulcerative keratitis, and corneal melting (2). Ocular surface inflammation in RA may cause dry eye that is more severe than idiopathic dry eye (2). About half of the patients with RA have dry eye symptoms, and one-fourth have Sjögren’s syndrome, the most frequent ophthalmologic pathology associated with RA (2–4). Complications of recurrent corneal ulceration, peripheral ulcerative keratitis, and corneal melting can occur (2). These ocular comorbidities in patients with RA are often positively correlated with the duration of RA history (2).
Spontaneous rupture of the cornea is rare in healthy eyes without a history of trauma; however, inflammatory and ulcerative corneal diseases make it susceptible to breakage and can be a predisposing factor for perforation (5). Spontaneous corneal perforation with corneal melting may occur in rare cases of RA (2). This case report is a description of a case of spontaneous corneal perforation associated with RA and treatment with amniotic membrane transplantation (AMT).
Case Report
A 58-year-old African-American woman presented at the emergency department with the complaints of redness and blurred vision in the right eye ongoing for 3 weeks. She had been diagnosed with RA 15 years earlier and type 2 diabetes mellitus (DM) 3 years earlier. An ophthalmological examination yielded a best-corrected visual acuity (BCVA) of finger counting from 1 meter in the right eye and 20/25 in the left eye. A corneal perforation of about 1 mm in diameter and iris prolapse at the nasal paracentral region of the right eye as well as corneal thinning and opacity at the center of the left eye were detected on slit-lamp examination. There was no history of ocular trauma or infection in the eyes. The diagnosis was spontaneous corneal perforation related to RA. Surgical intervention was planned, given that the patient would not benefit from medical measures, and she was referred to the rheumatology department. Primary suturation was attempted, but the corneal defect could not be closed completely because of the fragility of the remaining corneal tissue. Despite performing the suturation away from the edge of the wound, gathering the edges and achieving complete closure of the defect with a 10-0 nylon suture was not feasible. Two layers of amniotic membrane, which was prepared in sterile conditions and stored in a frozen state (-80 ºC) in our clinic, was used to cover the corneal surface and preserve the anterior chamber (Fig. 1A). While the first layer of the amniotic membrane was sutured in place to close the corneal defect, the second layer was sutured to the conjunctiva (approximately 2 mm from the limbus) to cover the whole cornea. Follow-up consisted of a therapeutic contact lens, fluocortolone per oral 1mg/kg/day (Ultralan; Bayer, Schering Pharma AG, Berlin, Germany) and topical treatments of 8x1 moxifloxacin hydrochloride 0.5% ophthalmic solution (Vigamox; Alcon Lab, Inc., Fort Worth, TX, USA) and 12x1 sodium hyaluronate 0.15% (Eyestil; SIFI SpA, Rome, Italy) after surgery. The rheumatology department initiated anti-tumor necrosis factor (infliximab) therapy for the RA. Figure 1B illustrates the anterior segment of the right eye 2 weeks after AMT. A second AMT procedure was performed 1 month after the first surgery due to amniotic membrane resorption. Two months later, the patient had a full anterior chamber and corneal integrity except for an anterior synechia at the inferonasal part of the pupillary edge (Fig. 2A). The BCVA was 20/63 at the sixth month after the first AMT surgery, despite the development of a cataract in the right eye (Fig. 2B), and the appearance of the left eye remained as observed in the initial examination (Fig. 3).
Figure 1.
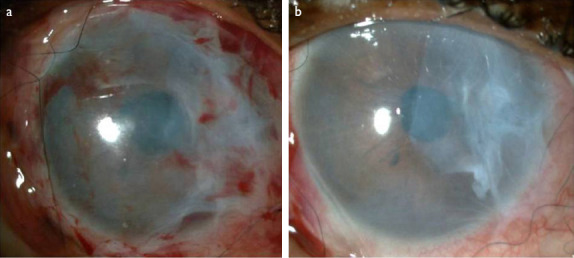
(a) Anterior segment of the right eye immediately after amniotic membrane transplantation (AMT) surgery. Two layers of amniotic membrane cover the corneal surface. (b) Anterior segment appearance of the right eye 2 weeks after AMT surgery.
Figure 2.
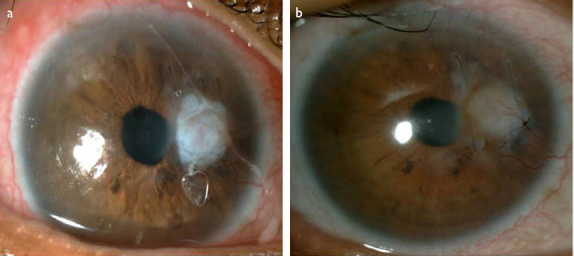
(a) Anterior segment appearance of the right eye 2 months after first amniotic membrane transplantation (AMT) surgery. Full anterior chamber integrity is seen except an anterior synechia at the inferonasal pupillary edge. (b) Anterior segment appearance of the right eye 6 months after the first AMT surgery. Development of the cataract is visible.
Figure 3.
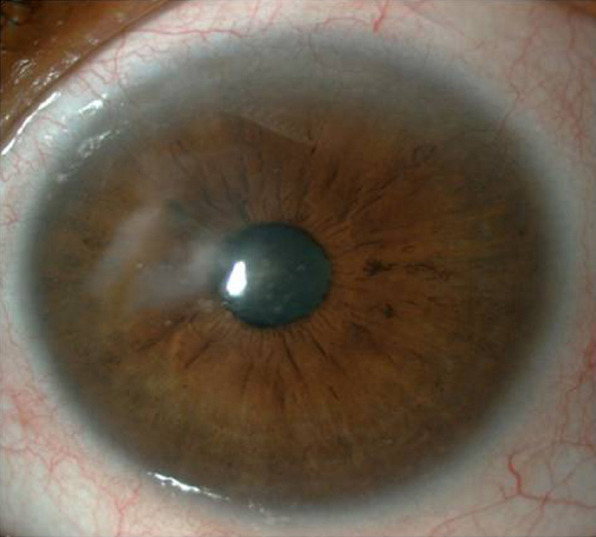
Anterior segment of the left eye.
Discussion
The corneal findings of RA are due to cellular and humoral immune system abnormalities (2). The levels of collagenases and inhibitors (e.g., matrix metalloproteinases and tissue inhibitor of metalloproteinases) in the corneal tissue are altered in RA (6). This imbalance may eventually lead to clinical conditions, including superficial punctate keratitis and corneal ulcers, if it is not treated (2, 4). Mechanisms underlying perforation are keratolysis, aqueous tear deficiency, and ulceration of superficial layers of the corneal epithelium. Therefore, dry eye in patients with RA should be treated aggressively with frequent lubrication, punctual occlusion, tarsorrhaphy, or immunosuppressive agents under the supervision of a rheumatologist (4).
Systemic corticosteroids, with a dose of 1 mg/kg/day prednisone equivalent per oral, are the treatment of choice for corneal involvement such as peripheral ulcerative keratitis (7). Conditions such as perforation can necessitate treatment with an intravenous bolus of methylprednisolone 1g/day for 3 days (7). We did not administer intravenous corticosteroid treatment in this case because the patient had DM. Cyclophosphamide therapy is also used when other options prove ineffective (7). Additional treatment agents include methotrexate, azathioprine, mycophenolate mofetil, and biological agents (2, 7). Healing of RA-associated keratolysis was reported after infliximab treatment in 3 cases, which is consistent with our case findings (8).
Surgical options include the use of tissue adhesives, lamellar keratoplasty, penetrating keratoplasty, and AMT (4, 7, 9–11). These treatment modalities have both advantages and disadvantages. Although very useful for temporary use, tissue adhesives are toxic to the cornea (12). Lamellar keratoplasty and penetrating keratoplasty are more definitive, but require a donor cornea and have a risk of rejection (12). AMT was preferred in our case due to the disadvantages of other treatment modalities and the small area of corneal perforation, and most importantly, amniotic membrane was the only treatment option available in our clinic in an emergency situation. Smaller, lamellar or peripherally located corneal defects can be managed with medical interventions, such as pressure patching or a bandage contact lens. We did not use these options before surgical intervention in our patient because she had a 1-mm diameter defect in the paracentral cornea as well as iris prolapse. We did not want to use a pedicle conjunctival flap in a patient with both DM and RA.
Ocular surface reconstruction, conjunctival reconstruction, pterygium surgery, bullous keratopathy, glaucoma surgery, oculoplastic procedures, and persistent epithelial defects like corneal perforations are the primary indications for AMT (11, 12). AMT has been a well-known method to manage spontaneous corneal perforations since 1997 (13). It also has anti-inflammatory properties that are useful for RA. The success rate for corneal perforations depends on the perforation size (12). Perforations >1.5 mm in size have a higher risk of treatment failure. Therefore, AMT is especially useful for small perforations (12).
RA is a connective tissue disease that can manifest with varied clinical presentations depending on the affected area. In our case, RA caused spontaneous corneal perforation due to dry eye. Physicians must be alert to any condition with persistent red eye and loss of vision in RA patients, and AMT can be a successful treatment modality for spontaneous perforation when combined with effective medical therapy, such as the methotrexate and infliximab used in our case. The use of amniotic membrane as a barrier appears to be a safe and simple method in emergency situations where tissue adhesives such as cyanoacrylate are not available.
Footnotes
Disclosures
Informed consent: Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
References
- 1.Widdifield J, Paterson JM, Bernatsky S, Tu K, Tomlinson G, Kuriya B, et al. The epidemio-logy of rheumatoid arthritis in Ontario, Canada. Arthritis Rheumatol. 2014;66:786–93. doi: 10.1002/art.38306. [DOI] [PubMed] [Google Scholar]
- 2.Artifoni M, Rothschild PR, Brézin A, Guillevin L, Puéchal X. Ocular inflammatory diseases associated with rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:108–16. doi: 10.1038/nrrheum.2013.185. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol. 2005;140:808–13. doi: 10.1016/j.ajo.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Lekskul M, Fracht HU, Cohen EJ, Rapuano CJ, Laibson PR. Nontraumatic corneal perfora-tion. Cornea. 2000;19:313–9. [Google Scholar]
- 5.McMonnies CW. Mechanisms for acute corneal hydrops and perforation. Eye Contact Lens. 2014;40:257–64. doi: 10.1097/ICL.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 6.Riley GP, Harrall RL, Watson PG, Cawston TE, Hazleman BL. Collagenase (MMP-1) and TIMP-1 in destructive corneal disease associated with rheumatoid arthritis. Eye (Lond) 1995;9:703–18. doi: 10.1038/eye.1995.182. [DOI] [PubMed] [Google Scholar]
- 7.Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007;33:835–54. doi: 10.1016/j.rdc.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JW, Pflugfelder SC. Therapy of progressive rheumatoid arthritis-associated corneal ulceration with infliximab. Cornea. 2005;24:742–4. doi: 10.1097/01.ico.0000154391.28254.1d. [DOI] [PubMed] [Google Scholar]
- 9.Tanrıverdi C, Köşker M, Acar U, Burcu A, Onat MM, Örnek F. İlerleyici periferik kornea incelmesinde tektonik kornea yama greftinin etkiniği. Turk J Ophthalmol. 2014;44:440–4. [Google Scholar]
- 10.Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Röck T, Bartz-Schmidt KU, Landenberger J, Bramkamp M, Röck D. Amniotic Membrane Transplantation in Reconstructive and Regenerative Ophthalmology. Ann Transplant. 2018;23:160–5. doi: 10.12659/AOT.906856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Ares MT, Touriño R, López-Valladares MJ, Gude F. Multilayer amniotic membrane transplantation in the treatment of corneal perforations. Cornea. 2004;23(6):577–83. doi: 10.1097/01.ico.0000121709.58571.12. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–12. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]


