Abstract
Background:
Preoperative variables can predict short term left ventricular assist device (LVAD) survival, but predictors of extended survival remain insufficiently characterized.
Method:
Patients undergoing LVAD implant (2012–18) in the Intermacs registry were grouped according to time on support: short-term (<1 year (Y), n=7483), mid-term (MT, 1–3 Ys, n=5976) and long-term (LT, ≥3 Ys, n=3015). Landmarked hazard analyses (adjusted hazard ratio, HR) were performed to identify correlates of survival after 1 and 3 Ys of support.
Results:
After surviving 1 Y of support, additional LVAD survival was less likely in older (HR 1.15 per decade), Caucasian (HR 1.22) and unmarried (HR 1.16) patients (p< 0.05). After 3 Y of support, only 3 preoperative characteristics (age, race, and history of bypass surgery, p< 0.05) correlated with extended survival. Postoperative events most negatively influenced achieving LT survival. In those alive at 1Y or 3Ys, the occurrence of postoperative renal (creatinine HR MT=1.09; LT HR=1.10 per mg/dL) and hepatic dysfunction (AST HR MT=1.29; LT HR=1.34 per 100 IU), stroke (MT HR=1.24; LT HR=1.42), infection (MT HR=1.13; LT HR=1.10), and/or device malfunction (MT HR=1.22; LT HR=1.46) reduced extended survival (all p ≤0.03).
Conclusion:
Success with LVAD therapy hinges on achieving long term survival in more recipients. After 1 year, extended survival is heavily constrained by the occurrence of adverse events and postoperative end-organ dysfunction. The growth of destination therapy intent mandates that future LVAD studies be designed with follow up sufficient for capturing outcomes beyond 24 months.
Keywords: LVAD, survival, risk factors, complications
Continuous flow left ventricular assist device (cfLVAD) therapy improves survival in carefully selected patients with stage D heart failure (HF) with reduced ejection fraction(1–3). One-year survival in the 2020 Society of Thoracic Surgeons (STS) Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) report was 82%.(4) While long-term survival has improved over time, only 43% of Intermacs patients are alive on durable cfLVAD therapy at 5 years.(4) Although the hazard for mortality after cfLVAD is highest in the first 90 days after implant, the hazard for major adverse events (AEs) during prolonged cfLVAD support continues to increase, contributing to both longer-term patient morbidity and mortality.(5) With destination therapy (DT) and bridge to candidacy indications representing the vast majority of U.S. cfLVAD implants in the contemporary era(4), it is imperative that the field continues to identify patient-specific and device-specific factors impairing truly long-term survival. This will, in turn, define patient management strategies and novel device technologies that mitigate identified risks and improve long-term outcomes.
Several studies have identified predictors of cfLVAD mortality, but analyses have either focused on 12–24 month patient survival or were potentially skewed by factors more likely to influence operative, and not long term, survival(1–3, 6, 7). Short-term success with cfLVAD therapy is heavily influenced by patient selection, as well as surgical technique and the occurrence of early perioperative complications. Preoperative laboratory tests (such as creatinine, albumin, INR and bilirubin), Intermacs profile, and hemodynamic signs of right HF have been shown to help predict operative mortality.(6–9) However, no study has consistently shown that these same variables can forecast outcomes in operative survivors years after device implantation.
The aim of the analysis herein is describe the pre- and post-operative characteristics of patients enrolled into the Intermacs registry that impact cfLVAD survival beyond 1- and 3-years of support. In addition, we aim to investigate the differential impact of AEs on subsequent survival in cfLVAD patients achieving the 1-year and 3-year support milestones. We hypothesize that preoperative patient characteristics will poorly predict survival after 1 year of cfLVAD support, and that extended survival during cfLVAD support will be greatly influenced by AEs that accrue during support.
Patients and Methods
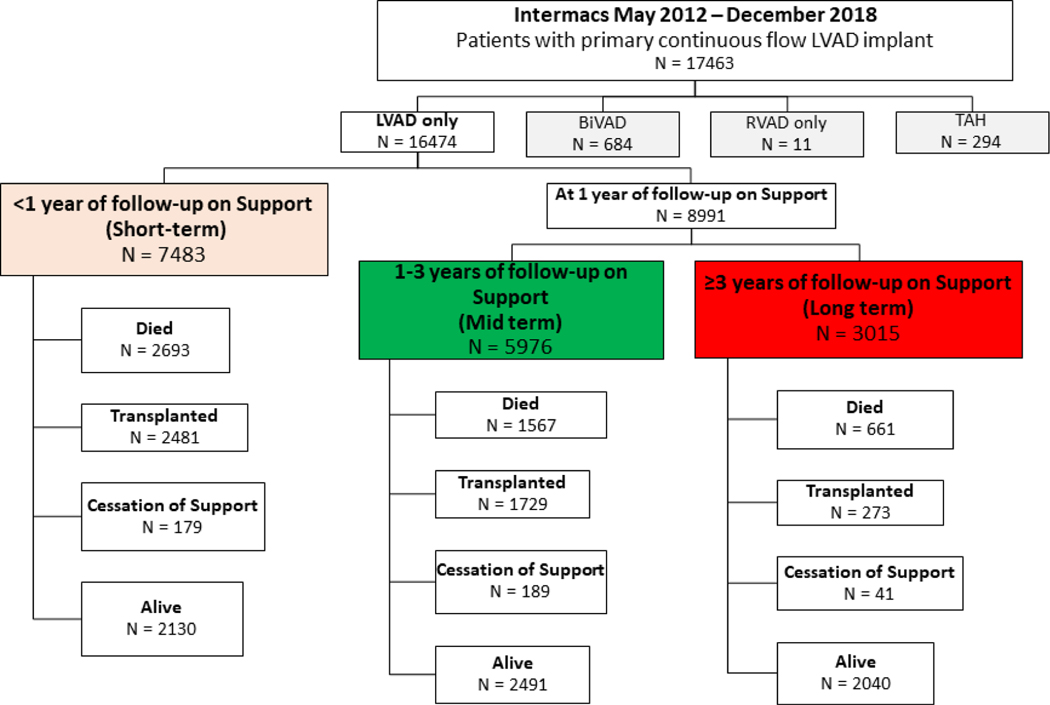
This was a retrospective cohort analysis of adult patients (age ≥19 years) undergoing primary cfLVAD implant (n=17463) from May 2012 through December 2018 in Intermacs. Follow-up ended June 2019. Patients undergoing biventricular support (n=684) during the index operation and those on isolated right ventricular assist device support (RVAD, n=11) and total artificial heart support (n=294) were excluded. The final cohort consisted of 16,474 patients on durable cfLVAD support. Patients were then placed into groups according to duration of cfLVAD support (Figure 1):
Figure 1. Intermacs Subgroups According to Duration of cfLVAD support.
Patients were sub-grouped according to survival on cfLVAD support. Patients on mid-term support (MT) were alive on support 1–3 years and those on long-term support (LT) were alive on support for ≥3 years. Y= years. cfLVAD= continuous flow left ventricular assist device
Short-term (ST) (supported <1 year): The ST group included patients alive with a device in place for <1 year, those who died within 1 year of cfLVAD implant, or those who underwent device explant for transplant or recovery within 1 year.
Mid-term (MT) (supported 1–3 years): Patients in the MT group were on cfLVAD between 1–3 years. Patients included those who were alive on device support for 1–3 years or those that died or had their device explanted for transplant or recovery between years 1–3.
Long-term (LT) (supported ≥3 years): Patients were alive on device support at the 3-year landmark.
Frequencies of major AEs were tallied for each patient and grouped according to the patient’s duration of cfLVAD support. Intermacs definitions from STS Intermacs User’s Guide® version (v) 5.0 were used for all AEs except right HF(10). Events classified according to v3.0 or v4.0 were individually mapped to v5.0 definitions as shown in supplementary Table 1. Because the outcome of interest was longer-term survival, AEs were focused on non-operative complications. Thus, AEs included stroke (hemorrhagic or ischemic cerebrovascular events, not including transient ischemic events), major bleeding from mucocutaneous sources (gastrointestinal or dental/nasopharyngeal causes), major infection and (separately) device-related infection (including pump and/or driveline infection and/or blood culture positivity), right HF, and device malfunction (major or minor). For the purposes of this analysis, right HF was defined as need for right ventricular assist device (RVAD) implantation outside the initial operative intervention or clinical signs of right HF (elevated right atrial pressure, dilated vena cava, edema, or ascites) with simultaneous requirement for inotrope support on, or after, the 1-month postoperative follow-up. The major causes of deaths were tallied for each survival group. Patients with a cause of death listed as right HF, ischemic cardiomyopathy, end stage cardiomyopathy, and heart disease were combined into a single “heart failure” cause of death category.
The primary goal was to identify factors impeding extended survival, defined as survival after achieving 1- and/or 3-years of cfLVAD support (evaluated separately). Secondly, we aimed to characterize the impact of major AEs on extended cfLVAD survival. Finally, we wished to identify the most common causes of death within the group of patients dying in the MT and LT periods.
Statistical analysis
Data analysis was performed by the University of Alabama at Birmingham, which is the Intermacs data coordinating center for STS. Patient characteristics were summarized using descriptive statistics. Data are summarized as mean ± standard deviation or standard error for continuous variables and percentage for categorical variables. Comparisons between groups were performed using the Student t-test for continuous variables and Chi-square test for categorical variables.
Estimated survival was calculated for the overall sample and for patient groups using the methods of Kaplan-Meier. Log rank testing was used for group survival comparisons. Due to inherent differences in times at risk for events and terminated risk due to transplant/explant in the INTERMACS registry, survival for the purposes of further analyses was landmarked at 1 and 3 years, as outlined above. Patients were censored if they reached the end of the study period. Then, risk factors for post-implant death were identified by conducting multi-phase parametric hazard modeling for each landmarked time point at 1 and 3 years. This method has been used extensively to identify the changing hazard profiles post-surgery and the association of risk factors with different phases of risk.(11) Up to three phases of risk (early declining phase, constant phase, and late phase) were evaluated. For our analysis, a constant phase best fits the shape of the hazard for post-LVAD death. Potential covariates were chosen a priori. All potential covariates had less than 20% missing and missing values were set to the mean. Postoperative laboratory values included in the analyses were those entered into the Intermacs registry closest to the 1- and 3-year survival landmarks. Postoperative AEs were included in modelling if events occurred within the first 1 year (MT+LT) or 3 years (LT) of support. Due to the notable differences in right HF definitions captured across Intermacs eras (treated as an event then later a condition), this variable was not reliably mappable across Intermacs eras and subsequently omitted from multivariable analyses. Stepwise selection was used to identify statistically significant risk factors for the final multivariable model, with a p-value of 0.05 for covariates to enter and remain in the model. Hazard ratios were expressed with 70% confidence intervals. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
This analysis was reviewed and approved by the STS Research Center for Intermacs. The results and conclusions herein represent those of the authors and not necessarily STS.
Results
The 1-, 3-, and 5-year survivals of the 16,474 patients undergoing cfLVAD support were 82%, 61%, and 43%, respectively. Of the patients (n=8991) who were alive with a device in place at 1 year, the 3-, 5-, and 6-year survivals were 75%, 53%, and 45%, respectively. Patients (n=3015) who were alive with a device in place at 3 years had survivals of 70% and 60% at 5 and 6 years, respectively.
Patients were categorized according to duration on cfLVAD support, regardless of patient outcome to allow for general comparisons of preoperative characteristics. There were 7483 patients in the ST group, 5976 in the MT group (alive on support 1–3 years), and 3015 in the LT group (alive on support ≥3 years) (Figure 1). The baseline characteristics of the groups according to survival status are presented in supplementary table 2.
Characteristics of Patients Dying during Short-, Medium-, and Long-Term Support Intervals
Of 4921 total mortalities during the 6-year period of analysis, there were 2693 (55%) deaths within 1 year, 1567 (32%) deaths with between 1–3 years of support, and 661 (13%) deaths in individuals with ≥3 years of cfLVAD support. Table 1 shows the baseline characteristics of cfLVAD patients who died within each of the 3 time periods. Patients dying in the ST interval displayed a greater burden of typical high risk characteristics, including preoperative renal and hepatic function, preoperative shock (Intermacs Profile 1, ECMO, ventilator support and/or renal replacement therapy), and they had high frequencies of concomitant surgery with longer cardiopulmonary bypass times than patients dying after 1 and 3 years of cfLVAD support.
Table 1.
Preoperative characteristics and operative data of patients dying during the short-term (<1 year), mid-term (1–3 years), and long-term (≥3 years) support periods.
| Short-Term (n=2693) | Mid-Term (n=1567) | Long-Term (n=661) | p | |
|---|---|---|---|---|
|
| ||||
| Age, years | 61.7 ± 0.22 | 60.4 ± 0.30 | 60.5 ± 0.50 | 0.0004 |
|
| ||||
| Male | 78.1% | 78.8% | 81.7% | 0.13 |
|
| ||||
| BSA, m2 | 2.07 ± 0.01 | 2.10 ± 0.01 | 2.11 ± 0.01 | 0.001 |
|
| ||||
| BMI, kg/m2 | 28.6 ± 0.14 | 29.4 ± 0.21 | 29.0 ± 0.27 | 0.01 |
|
| ||||
| BTT, listed | 16.9% | 13.6% | 11.2% | 0.0001 |
|
| ||||
| Caucasian Race | 72.0% | 68.6% | 72.6% | 0.04 |
|
| ||||
| Severe Diabetes | 10.9% | 12.6% | 11.5% | 0.24 |
| PVD | 6.5% | 7.2% | 5.5% | 0.30 |
| Solid Organ Cancer | 6.3% | 7.6% | 6.8% | 0.28 |
| Pulmonary HTN | 22.3% | 26.0% | 27.4% | 0.003 |
| Pulmonary Disease | 11.8% | 13.9% | 10.6% | 0.04 |
| Hepatitis | 1.3% | 2.5% | 2.6% | 0.005 |
|
| ||||
| Prior cardiac operation | 40.5% | 37.5% | 40.7% | 0.13 |
| CABG | 28.1% | 26.4% | 29.7% | 0.24 |
| CAD | 6.0% | 5.3% | 5.4% | 0.60 |
|
| ||||
| Intermacs Profile | ||||
| 1 | 20.3% | 11.0% | 9.8% | < .0001 |
| 2 | 36.4% | 35.6% | 33.7% | 0.44 |
| 3 | 30.0% | 37.3% | 35.4% | <0.0001 |
| 4–7 | 13.2% | 16.1% | 21.0% | <0.0001 |
|
| ||||
| TCS | 36.5% | 23.5% | 20.8% | <0.0001 |
| IABP | 35.0% | 30.0% | 25.0% | <0.0001 |
| ECMO | 7.8% | 2.7% | 1.8% | <0 .0001 |
|
| ||||
| Inotropes | 84.3% | 82.8% | 79.8% | .02 |
|
| ||||
| Dialysis | 5.3% | 2.2% | 2.0% | < .0001 |
|
| ||||
| Ventilator | 11.2% | 6.1% | 5.9% | < .0001 |
|
| ||||
| Creatinine, mg/dL | 1.52 ± 0.01 | 1.45 ± 0.02 | 1.39 ± 0.02 | < .0001 |
| BUN, mg/dL | 33.4 ± 0.37 | 30.9 ±.47 | 30.9 ± 0.47 | < .0001 |
| Albumin, g/dL | 3.30 ± 0.01 | 3.41 ± 0.02 | 3.46 ± 0.03 | < .0001 |
| INR | 1.35 ± 0.01 | 1.29 ± 0.01 | 1.32 ± 0.02 | 0.004 |
| AST, IU | 56.93 ± 2.67 | 42.90 ± 2.35 | 41.20 ± 3.67 | 0.0001 |
| Bilirubin, mg/dL | 1.61 ± 0.05 | 1.21 ± 0.03 | 1.21 ± 0.04 | < .0001 |
|
| ||||
| RA, mmHg | 14.00 ±−0.21 | 13.84 ± 0.28 | 13.37 ± 0.42 | 0.43 |
| PA Systolic, mmHg | 49.97 ± 0.31 | 49.93 ± 0.40 | 49.78 ± 0.62 | 0.97 |
| PA Diastolic, mmHg | 24.55 ± 0.19 | 24.62 ± 0.23 | 24.33 ± 0.37 | 0.81 |
| CO, L/min | 4.29 ± 0.03 | 4.25 ± 0.04 | 4.15 ± 0.06 | 0.13 |
|
| ||||
| History alcohol abuse | 6.1% | 7.2% | 8.6% | 0.05 |
| History drug use | 5.4% | 6.8% | 5.6% | 0.13 |
| Noncompliance | 2.9% | 4.4% | 3.3% | 0.03 |
| Married | 66.8% | 63.8% | 66.8% | 0.13 |
| Current Smoker | 4.8% | 7.0% | 4.7% | 0.01 |
|
| ||||
| Operative details | ||||
|
| ||||
| Bypass time, min | 104.28 ± 1.12 | 92.16 ± 1.16 | 97.85 ± 2.02 | < .0001 |
|
| ||||
| Concomitant Surgery | 47.8% | 38.5% | 39.9% | < .0001 |
|
| ||||
| Centrifugal HD Flow (n=5211) | 30.6% | 18.6% | 12.6% | <0.001 |
| Centrifugal ML flow (n=1160) | 4.0% | 0.1% | 0% | |
| Axial Flow (n=10098) | 65.4% | 81.2% | 87.4% | |
mean ± standard error shown. ALT= alanine aminotransferase; BMI= body mass index; BSA= body surface area; BTT= bridge to transplant; CAD= coronary artery disease; CABG= coronary artery bypass grafting; CKD= chronic kidney disease; CO= cardiac output; ECMO= extracorporeal membrane oxygenation; HD= hydrodynamic; HTN= hypertension; IABP= intra-aortic balloon pump; ML= full Magnetic levitation; PVD= peripheral vascular arterial disease; RA= right atrial; PA= pulmonary artery; TCS= temporary circulatory support
The preoperative differences between patients dying after 1- and 3-years of cfLVAD support were fewer. Patients dying after 3 years of support had a lower burden of pulmonary disease and were more likely to be male, implanted for DT, and had less preoperative renal dysfunction than patients who died after 1 year of support. Importantly, patients dying after 3 years of support were overall more likely than either ST or MT deaths to be categorized as Profiles 4–7, least likely to be Profile 1, and had the lowest frequency of preoperative ventilator, balloon pump, or temporary circulatory/ECMO use. Finally, patients who died after 3 years of support were more likely to have received an axial flow cfLVAD than those dying in the MT and ST. The latter statistic may in part be related to the later timeline of DT approval for centrifugal devices.
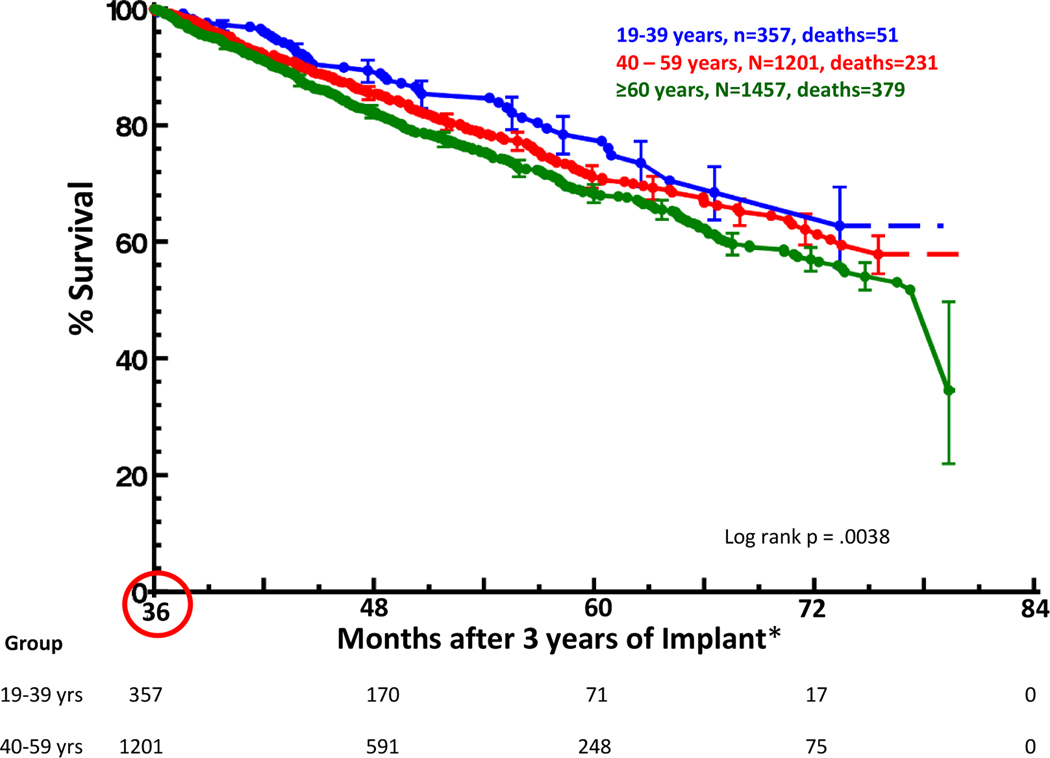
Age was statistically different between the patients who died in the ST, MT, and LT. Thus, further analyses within the entire cohort were undertaken and survival according to age group in those alive with a device in place at 3 years is shown in Figure 2. Individuals under the age of 40 years at the time of cfLVAD implant had a 5-year survival [70% confidence interval] of 78% [75–81%] while individuals aged 40–59 years and those ≥60 years had survivals of 71% [69–73%] and 68% [67–70%] at 5 years, respectively (p<0.05).
Figure 2. Overall Survival by Age Group for the Cohort of Patients Alive with a Device in place at 3 years after Continuous Flow Left Ventricular Assist Device Implant.
*Time 0 (circled) for the graph is 36 months post implant. Survial at 5 years was 78% [75–81%] for ages 19–39 years, 71% [69–73%] for ages 40–59 years, and 68% [67–70%] for ages ≥60 years. [70% confidence interval] shown.
Adverse Events and Impact on Survival after 1 Year of Support
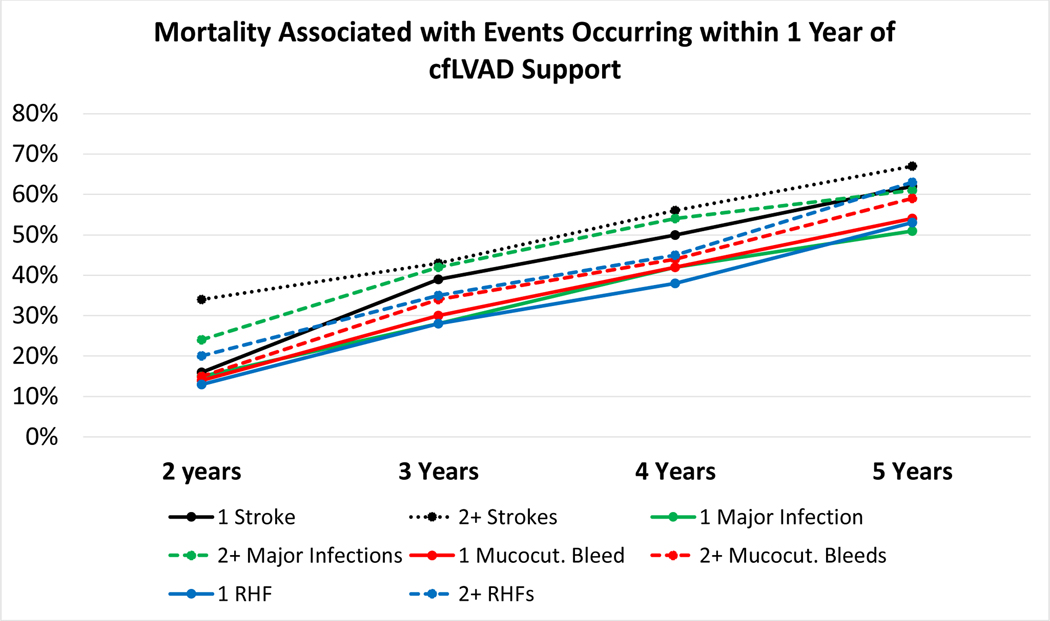
For those patients alive and on cfLVAD support at 1 year, the frequencies of adverse events occurring in the first year following cfLVAD implant are shown in supplemental Figure 1. The impact of these AEs and/or their recurrence on subsequent patient survival is shown in Table 2 and Figure 3. Additional survival beyond the one year milestone decreased as the number of episodes of right HF (milrinone or RVAD ≥1 month after cfLVAD implant), stroke, gastrointestinal-mucocutaneous bleeding, device malfunction, and infection increased in the first year after device implant. Patients with ≥2 episodes of stroke or pump-related infection were least likely to survive to 5 years. While patients with a significant burden of gastrointestinal-mucocutaneous bleeding during the first year of VAD support had better 5-year survival (44% [42–46%]) than those with recurrent strokes (38% [35–41%]) or pump-related infections (41% [39–43%]), survival was inferior when compared to those without any gastrointestinal-mucocutaneous bleeding events (56% [55–57%]) during year 1 of cfLVAD support (all p<0.05).
Table 2.
Survival after 1 year of support in those with adverse events. Survival is shown for those patients who were alive on support at 1 year according to the occurrence of no events, 1 event or ≥2 events of right heart failure, mucocutaneous/gastrointestinal bleeding, stroke or infection occurring within 65 days of left ventricular assist device implant.
| Events within 1st Year of Support | Survival (%) (70% confidence interval) | ||
|---|---|---|---|
| 3 years | 5 years | p | |
|
| |||
| Overall Survival | 75% (74.2–75.3) | 53% (51.8–53.6) | -- |
|
| |||
| Right heart failure | <0.001 | ||
| None (n=7060) | 76% (75.3–76.6) | 55% (53.7–55.8) | |
| 1 episode (n=1531) | 72% (70.2–73.2) | 47% (45.1–49.8) | |
| ≥2 episodes (n=400) | 65% (62.3–68.2) | 37% (33.0–42.0) | |
|
| |||
| Mucocutaneous bleed | <0.001 | ||
| None (n=6834) | 77% (76.3–77.6) | 56% (54.9–57.0) | |
| 1 episode (n=1181) | 70% (68.7–72.0) | 46% (43.6–48.6) | |
| ≥2 episodes (n=976) | 66% (64.0–67.7) | 41% (38.6–43.7) | |
|
| |||
| Stroke, any | <0.001 | ||
| None (n=8257) | 76% (75.4–76.6) | 54% (53.0–54.9) | |
| 1 episode (n=645) | 61% (58.8–63.8) | 38% (34.6–41.4) | |
| ≥2 episodes (n=89) | 57% (49.9–62.3) | 40% (32.6–48.0) | |
|
| |||
| Major Infection, any | <0.001 | ||
| None (n=5657) | 80% (79.1–80.4) | 57% (56.1–58.5) | |
| 1 episode (n=2083) | 72% (70.3–72.8) | 49% (46.9–50.9) | |
| ≥2 episodes (n=1251) | 58% (56.7–60.0) | 39% (37.0–41.1) | |
|
| |||
| Infection, pump-related | <0.001 | ||
| None (n=7222) | 78% (77.4–78.6) | 56 (54.5–56.6) | |
| 1 episode (n=1349) | 66% (63.9–67.2) | 44% (41.8–46.4) | |
| ≥2 episodes (n=420) | 49% (46.1–52.2) | 30% (26.7–34.3) | |
|
| |||
| Device malfunction | <0.001 | ||
| None (n=7641) | 76% (75.4–76.7) | 53% (52.4–54.5) | |
| 1 episode (n=1106) | 68% (66.1–69.6) | 50% (47.3–52.4) | |
| ≥2 episodes (n=244) | 67% (61.9–69.5) | 39% (32.9–46.4) | |
Events defined per Intermacs User’s Guide® version 5.0 except right heart failure (use of inotrope or right ventricular assist device ≥1 month after continuous flow left ventricular assist device implant).
Figure 3. Impact of Adverse Events on Survival in those Alive and on cfLVAD Support at 1 Year.
Adverse events occurring within 1 year of cfLVAD implant were categorized according to frequency with corresponding survival after 1 year shown. RHF=right heart failure. cfLVAD= continuous flow left ventricular assist device
Correlates of Extended Survival in Patients Alive with a Device in Place at 1 and 3 Years
The demographics and characteristics of patients included in the 1 year and 3 year landmarked survival analyses are presented in Supplementary table 3 online. Multivariable predictors of failure to achieve additional survival (expressed as a constant phase hazard ratio) in the subgroup of patients alive and on device support at 1 and/or 3 years are presented in Table 3. Extended survival beyond 1 year was less likely in patients who were of older age, obese, Caucasian race and those with signs of right heart dysfunction on preoperative cardiopulmonary hemodynamics. Important preoperative comorbidities in those dying after 1 year of LVAD support include a history of preoperative pulmonary disease (HR 1.19, p= 0.01), hepatitis (HR 1.54, p=0.002), solid organ cancer (1.26, p=0.01), and prior coronary artery bypass grafting (CABG, HR 1.24, p <0.0001). Of the social risks, patients who smoked at the time of cfLVAD (HR 1.44, p<0.0001) and those who were not married (HR 1.16, p=0.002) were less likely to survive beyond a year of cfLVAD support. While preoperative laboratory values and Intermacs profile were not predictive of survival (see bottom of table 3 and supplementary table 4), patients suffering renal, hepatic, and nutritional insufficiency within the first year following cfLVAD support were less likely to achieve extended survival beyond 1 year of support on adjusted analyses. Finally, the occurrence of AEs within the first year of support greatly reduced the likelihood of achieving extended survival in those alive and on cfLVAD support at 1 year. For each episode of stroke occurring in the first year, adjusted mortality increased 42% while each episode of device infection or device malfunction increased the constant hazard for mortality by 19% and 22%, respectively (all p <0.0001, table 3). Gastrointestinal bleeding was not a significant predictor of mortality in adjusted analysis.
Table 3.
Multivariate correlates of mortality in those alive with a device in place at 1 and/or 3 years following implant. The hazard ratio shown represents the constant hazard of death after surviving 1 or 3 years on device support. Detailed characteristics of patients included in the 1 year and 3 year landmarked survival analyses are presented in online supplementary table 3.
| Mortality Risk in those on Support at 1 Year | Mortality Risk in those on Support at 3 Years | |||
|---|---|---|---|---|
| Risk Factors for Death | Constant Phase Hazard Ratio (n=8991 at risk, n=2228 deaths) | p | Constant Phase Hazard Ratio (n=3015 at risk, n=661 deaths) | p** |
|
| ||||
| Demographics | ||||
| Age (per decade, with 50–60 years of age as reference) | 1.15 | <0.0001 | 1.08 | 0.02 |
| BMI, per kg/m2 | 1.01 | 0.0059 | ||
| Race: Caucasian | 1.22 | <0.0001 | 1.41 | 0.0002 |
| Not married | 1.16 | 0.0023 | ||
| Clinical Status | ||||
| History of solid organ cancer | 1.26 | 0.0051 | ||
| History of hepatitis | 1.54 | 0.0017 | ||
|
| ||||
| History of coronary artery bypass | 1.24 | < 0.0001 | 1.29 | 0.0002 |
| History of pulmonary disease | 1.19 | 0.0075 | ||
| Current smoker of tobacco | 1.44 | <0.0001 | ||
|
| ||||
| Pre-implant cardiopulmonary hemodynamics | ||||
| Pulmonary artery systolic, per 10 mmHg | 0.96 | 0.0092 | ||
| Right atrial pressure, per 1 mmHg | 1.01 | 0.0001 | ||
|
| ||||
| Clinical events within 1 or 3 years of LVAD implant * | ||||
| Stroke count (per event) | 1.42 | <0.0001 | 1.24 | 0.01 |
| Infection count (per event) | 1.13 | <0.0001 | 1.10 | <0.0001 |
| Pump related infection count (per event) | 1.19 | <0.0001 | ||
|
| ||||
| Device malfunction count (per event) | 1.22 | <0.0001 | 1.46 | 0.02 |
| Postoperative laboratory values obtained closest to 1 or 3-year follow up* | ||||
| Total bilirubin, per mg/dL | 1.19 | <0.0001 | ||
|
| ||||
| BUN, per 10 mg/dL | 1.07 | <0.0001 | ||
| AST, per 100 unit | 1.29 | <0.0001 | 1.34 | 0.01 |
| Creatinine, per mg/dL | 1.09 | 0.0008 | 1.10 | 0.03 |
|
| ||||
| Albumin, per g/dL | 0.66 | <0.0001 | 0.63 | <0.0001 |
For the 1 year model, adverse events occurred within 1 year of device implant and labs closest to 1 year follow-up were used. For the 3 year model, events occurred up to 3 years following implant and labs closest to 3 year follow-up were used.
Unless specified, p >0.05. Right heart failure not included in models as discussed in methods.
Variables entered into the models with an exit p>0.05 included the following with full statistical output for 1 year model in Supplementary table 3: Intermacs profile; sex; body surface area; preoperative INR, blood urea nitrogen (BUN), albumin, creatinine, total bilirubin, sodium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, hemoglobin, white cell count, blood type; history of alcohol abuse, drug use, noncompliance; history of stroke, valve surgery, severe diabetes, coronary disease, defibrillator present, or pulmonary hypertension; device intent; preop inotrope use; preop. systolic blood pressure, diastolic blood pressure, cardiac output, heart rate; preop mitral, tricuspid, and/or aortic insufficiency; preop. ventilator use, dialysis, extracorporeal membrane oxygenation, temporary circulatory support, and/or balloon pump; centrifugal flow pump type; concomitant surgery; cardiopulmonary bypass time; postop mucocutaneous bleed; postoperative hemorrhagic and/or ischemic stroke type; postoperative LDH and/or ALT; postop visual analog scale (VAS).
Abbreviations: BMI= body mass index
After at least 3 years of cfLVAD support, postoperative organ dysfunction and device complications had the greatest impact on extended survival (table 3). Specifically, additional survival beyond 3 years was less likely in patients with elevated postoperative creatinine, aspartate aminotransferase (AST) and/or reduced albumin at 3 years of follow-up. Further, each episode of stroke (HR 1.24 per event, p=0.01), major infection (HR 1.10 per event, p <0.0001), and device malfunction (HR 1.46 per event, p=0.02) occurring in the first 3 years of support conferred markedly inferior extended survival. The only preoperative characteristics that correlated with adverse cfLVAD survival beyond 3 years were age, a history of CABG, and Caucasian race.
Causes of Death by Survival Group
Table 4 shows the most common causes of death in each survival period. Patients on cfLVAD support ≥3 years were most likely to die from neurological complications, accounting for 15.7% of deaths in this group. “Other” was listed as the second (15.4%) most common cause of death. Complications such as HF (including right HF), infection, and respiratory failure were also notable contributors to mortality in patients on cfLVAD support ≥ 3 years. The most common causes of death in MT and ST patients included multi-system organ failure, neurologic complications, and withdrawal of care.
Table 4.
Causes of death in patients on short-term (<1 years), mid-term (1–3 years) and long-term (≥3 years) continuous flow left ventricular assist device support. Top 7 causes of death shown.
| Death with device in place <1 year (n=2693 total) | Death with device in place 1–3 years (n=1567 total) | Death with device in place ≥ 3 year (n=661 total) |
|---|---|---|
| Multisystem Organ failure (n=577, 21.4 %) |
Neurologic (n=296, 18.9%) |
Neurologic (n=104, 15.7%) |
| Neurologic (n=520, 19.3%) |
Withdrawal of Care (n=204, 13.0%) |
Other (n=102, 15.4%) |
| Withdrawal of care (n=379, 14.1%) |
Other (n=199, 12.7%) |
Heart Failure (n=92, 13.9%)* |
| Heart Failure (n=260, 9.7%)* |
Heart Failure (n=190, 12.1%)* |
Withdrawal of care (n=75, 11.3%) |
| Other (n=186, 6.9%) |
Multisystem Organ Failure (n=159, 10.1%) |
Multisystem Organ Failure (n=69, 10.4%) |
| Respiratory (n=155, 5.8%) |
Major Infection (n=106, 6.8%) |
Major Infection (n=59, 8.9%) |
| Major Infection (n=148, 5.5%) |
Respiratory (n=89, 5.7%) |
Respiratory (n=35, 5.3%) |
Heart failure includes right heart failure, CHF, end-stage cardiomyopathy, ischemic cardiomyopathy, and heart disease coding. LVAD= left ventricular assist device
Discussion
In this analysis, several important factors impeding longer term survival on cfLVAD support were identified. First, the most notable impediments to extended survival in those alive and on support at 1 and 3 years were the occurrence of postoperative end-organ dysfunction and device-related AEs, specifically stroke, infection, and device dysfunction. Second, while preoperative characteristics including older age, comorbidities (pulmonary disease, prior CABG, hepatitis, cancer), and BMI continue to influence outcomes in those who survived the first year of support, the only preoperative characteristics that impacted survival after reaching 3 years of cfLVAD support were older age, prior CABG, and Caucasian race. Finally, while age does correlate with reduced survival, many carefully selected patients over age 60 achieved support durations over 3 years. Taken together, these results suggest that preoperative correlates of risk are important and necessary for predicting short-term survival but are insufficient for forecasting longer-term success on cfLVAD support. Since nearly half of the patients destined for permanent support are alive at 5 years, the results suggest that clinical trial follow-up durations (presently at 24 months in most studies) are insufficient for capturing the journey of most patients. These findings are especially applicable to young patients reaching the 3 year support landmark, as 78% of these individuals were alive 5 years after device implant in Intermacs. Additionally, the results herein support the need for future clinical trials to accurately and completely capture all the drivers of morbidity and mortality that can impact both short- and longer-term success on cfLVAD support. Present clinical trial outcomes focus on composite endpoints that are limited to stroke and device exchange. In those alive and on support at 1 and 3 years, the impact of infection and mucocutaneous bleeding on morbidity, mortality, and quality of life should also be considered, especially if the field extends cfLVAD support into the less ill patient population.
In this analysis, AEs that accrued during cfLVAD had a marked impact on achieving extended survival. Each episode of stroke increased the hazard for mortality by 42% in those alive on support at 1 year and by 24% for those alive on support at 3 years. Prior analyses have demonstrated the substantial impact of stroke on survival but have focused on shorter support durations and/or incident stroke events. In a recent analysis from Intermacs (2014–2018) (12) and a sub-study from the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3)(13), the occurrence of incident postoperative stroke led to a significant reduction in survival at 2 years of support. The findings herein demonstrate that stroke occurring in patients alive out to 3 years has a negative impacted on added survival and recurrent neurologic events (ischemic or hemorrhagic) in patients on longer term support portends poor outcomes. Similarly, requirements for device exchange due to cfLVAD thrombosis have been shown to confer worse outcomes in the first two years of cfLVAD support (14–16). In this analysis, the occurrence of any pump malfunction reduced survival beyond 1 and 3 years by 22% and 46%, respectively. While stroke and device malfunction appear to occur at lower frequencies in patients supported with third generation centrifugal flow devices (1, 17), results from Intermacs or clinical trials with extended follow-up are warranted given the marked impact of these AEs on long term success. Furthermore, dedicated studies aimed specifically at stroke mitigation through improvements in patient management are necessary.
The contributions of other AEs, such as mucocutaneous bleeding, right HF, and infection, to reduced long-term survival were also unveiled in this analysis. Longer term survival is reduced in those with early (within 1 year) or later (within 3 years) events and those with recurrent events (figure 3). Each episode of infection was associated with a 10–13% increase in the adjusted continuous hazard for long-term mortality after 1 and 3 years of cfLVAD support, and survival in those with recurrent right HF and mucocutaneous bleeding events was significantly reduced on unadjusted analyses. Others have shown through AE pattern mining that there is a potential for interdependence between AEs that occur during cfLVAD support, such that the occurrence of one AE may be a risk for subsequent adverse outcomes of the same or different type.(18) Even in this large national registry, the ability to capture the full impact of these complications with sufficient granularity for morbidity and mortality assessment was limited (infection, right HF, mucocutaneous bleeding) or not possible (aortic insufficiency). Likewise, the impact of these AEs on patient quality of life remains poorly characterized(17, 19). Thus, future studies of long-term cfLVAD support should focus on the impact of individual, recurrent, and coincident complications on mortality, functional capacity, and patient reported outcome measures.
Finally, this analysis showed that the ability to forecast longer-term survival in the preoperative setting was very limited. Older age is commonly identified as a risk factor for adverse outcome after cfLVAD implant and age likely correlates with an increased burden of comorbidities that can impact survival. Importantly, however, the results herein also show that advanced age should not be the only exclusion for device implant. Nearly 60% of patients in this analysis with extended survival on cfLVAD support were over age 60 at implant, suggesting that careful selection is driving success in this patient demographic for whom transplant options may be fewer. Over two thirds of patients ≥60 years of age who were alive and on cfLVAD support at 3 years remained alive and on support at 5 years. Reasons for inferior longer term survival in Caucasians and those with prior CABG are not clear. Other studies have also correlated worse cfLVAD outcomes in those with prior cardiac surgery(20), but the impact of race on cfLVAD survival has not been consistent(21–23). Whether the correlations herein are also associated with causation cannot be gleaned from this study. We hypothesize that findings may be reflective of unmeasured concomitant medical or social risks and not directly due to race or CABG alone. Finally, while Intermacs Profile was not an independent predictor of survival in those on longer-term LVAD support, it is important to note that the highest frequencies of Profiles 4–7 were in the long-term survival group. These findings are favorable for extension of cfLVAD support into the less ill but randomized trials with modern devices are warranted. Since patients categorized as Profiles 4–7 are, by definition, less ill, it will be important to address the comorbidity burdens to ensure the field’s tenured success in this population.
Limitations
It is important to acknowledge the limitations of the present analysis. We lacked sufficient data on postoperative functional capacity and patient reported outcome measures to examine the impact of morbidities on quality of life during long-term cfLVAD support. This may be particularly important for mucocutaneous bleeding, which tends to be recurrent. Intermacs compiles thousands of pre- and postoperative variables but detailed descriptions and tallying of many preoperative comorbidities (such as “severe diabetes” and “pulmonary disease”) and psychosocial health risks are presently lacking. For example, preoperative pulmonary disease and unmarried social status were associated with increased mortality in those alive on support at 1 year but the severity of pulmonary disease and impact of other social support systems (partnered, family support) are not known. In Intermacs User’s Guide® version 6.0, we can anticipate the assignment of definitions for medical comorbidities (pulmonary, diabetic, and renal dysfunction) which may help in the elucidation of postoperative risk in future study. The new Intermacs version will also better capture urgent transplant due to device complications, a granular endpoint lacking in this analysis. Importantly, right HF was excluded from the multivariable analysis due to the complexity of mapping the different definitions applied over time within Intermacs. Likewise, the impact of aortic insufficiency leading to right HF and/or readmission was not able to be examined herein. Certainly, the impact of right HF and/or aortic insufficiency on morbidity and mortality during long-term cfLVAD support is under-appreciated in this analysis given the high frequency of multi-system organ failure and HF as documented causes of death. Finally, the data herein largely captures longer term support on HeartMate II (65% of sample) and HVAD (30% of sample) technologies implanted temporally according to Food and Drug Administration indications for use. While stroke and pump thrombosis risks appear lower with centrifugal flow technologies (1, 24), it is not clear that the newer technologies confer a clinically significant reduction in infection, bleeding, and/or right HF risks. Future Intermacs reports will shed light on these questions the impact of new technologies and contemporary experience on long-term survival.
Conclusion
While careful preoperative patient risk stratification is important, longer-term survival after cfLVAD implant is most greatly influenced by the patient’s postoperative course and the development of device-related complications and end-organ dysfunction. Our analysis shows that the “Achilles heel” of current-era cfLVAD support continues to be stroke, infection, and cfLVAD malfunction. While clinical trial data from newer cfLVAD systems have shown improvements in hemocompatibility (notably pump thrombosis and stroke), real-world data with extended patient follow-up will be important for understanding all the contributors to extended survival and quality of life on cfLVAD support. Further advances in device engineering leading to improved system (motor, outflow, and controller) durability, hemocompatibility, and the advent of fully implantable technology will be required for cfLVAD support to truly compete with cardiac transplant for long term survival. Until then, careful preoperative patient selection and close clinical monitoring are obligatory for early detection and treatment of complications that can impair long term cfLVAD success.
Supplementary Material
Acknowledgements:
Intermacs data prior to 2018 was supported by NHLBI grant HHSN2682011000250. Drs. Cowger, Cogswell, and Molina, Shah, and Gosev are consultants and/or speakers for Abbott, Inc and their institutions receive clinical trial funds. Dr. Cogswell is an advisor for Medtronic and her husband is a Medtronic employee. Dr. Kanwar is on the Abiomed advisory board. Dr. Cowger is also on the advisory board and/or steering committee for Medtronic (for HVAD), Procyrion and Cordella-Endotronix and her institution receives clinical trial funds from Medtronic. Dr. Shah also receives funds from Medtronic, Merck, Bayer, NuPulse, and Ortho Clinical Diagnostics. He receives support from NIH K23-1K23HL143179-01A1. Dr. Pagani is on the scientific advisory board for FineHeart and has an institutional contract for research with Abbott. Dr. Kirklin and Susan Myers receive partial salary support from the STS in their role in the DCC. Drs. Hariri and Dardas have no conflicts of interest to report.
Grant support: Intermacs data prior to Jan 1 2018 was supported by grant HHSN2682011000250 of the NHLBI
Conflict of Interest as of January 2021:
Hariri: none
Dardas: none.
Kanwar: Abiomed advisory board.
Cogswell: Abbott Lab (speaker’s bureau), Medtronic – advisory board, husband’s employment
Gosev: Abbott (consultant, speaker)
Molina: Abbott (consultant, speaker)
Myers: Employed by the DCC for STS Intermacs.
Kirklin: I serve as Director of the Data Coordinating Center for the STS Intermacs Database and receive partial salary support through funds paid to my institution.
Shah: Merck, Bayer, Abbott, Medtronic, AHA / Enduring Hearts (grant support), Procyrion, NuPulseCV, Ortho Clinical Diagnostics (consultant); Dr. Shah is supported under NIH K23- 1K23HL143179-01A1
Pagani: FineHeart, Inc., Scientific Advisory Board; National Heart, Lung and Blood Institute; PumpKIN Trial, Member Data Safety Monitoring Board; Abbott, Inc., Institutional contractual clinical trial research
Cowger: Abbott and Medtronic (consultant, speaker, institutional clinical trial funds); Procyrion (consultant, steering committee member, institutional clinical trial funds); Cordella Endotronix (consultant, steering committee member, institutional clinical trial funds)
Abbreviations:
- AE
Adverse event
- cfLVAD
continuous flow left ventricular assist device
- HF
heart failure
- Intermacs
Interagency Registry for Assisted Circulatory Support
- LT
long term
- MT
mid term
- STS
Society of Thoracic Surgeons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr., Yuzefpolskaya M, Salerno CT, et al. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019;380(17):1618–27. [DOI] [PubMed] [Google Scholar]
- 2.Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018;6(9):792–802. [DOI] [PubMed] [Google Scholar]
- 3.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376(5):451–60. [DOI] [PubMed] [Google Scholar]
- 4.Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg. 2021;111(3):778–92. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–6. [DOI] [PubMed] [Google Scholar]
- 6.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61(3):313–21. [DOI] [PubMed] [Google Scholar]
- 7.Kanwar MK, Lohmueller LC, Kormos RL, Teuteberg JJ, Rogers JG, Lindenfeld J, et al. A Bayesian Model to Predict Survival After Left Ventricular Assist Device Implantation. JACC Heart Fail. 2018;6(9):771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birati EY, Hanff TC, Maldonado D, Grandin EW, Kennel PJ, Mazurek JA, et al. Predicting Long Term Outcome in Patients Treated With Continuous Flow Left Ventricular Assist Device: The Penn-Columbia Risk Score. Journal of the American Heart Association. 2018;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowger JA, Grafton G. Candidate Selection for Durable Mechanical Circulatory Support. Cardiol Clin. 2018;36(4):487–94. [DOI] [PubMed] [Google Scholar]
- 10.STS Intermacs Database: Appendix A Adverse Event Definitions [January 15, 2020]. Available from: https://www.uab.edu/medicine/intermacs/intermacs-documents.
- 11.Blackstone E, Naftel D, Turner M. The Decomposition of Time-Varying Hazard Into Phases, Each Incorporating a Separate Stream of Concomitant Information. Journal of the American Statistical Association. 1986;81(295):615–24. [Google Scholar]
- 12.Kirklin JK, Naftel DC, Myers SL, Pagani FD, Colombo PC. Quantifying the impact from stroke during support with continuous flow ventricular assist devices: An STS INTERMACS analysis. J Heart Lung Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 13.Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, et al. Comprehensive Analysis of Stroke in the Long-Term Cohort of the MOMENTUM 3 Study. Circulation. 2019;139(2):155–68. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Heart Lung Transplant. 2015;34(12):1515–26. [DOI] [PubMed] [Google Scholar]
- 15.Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014;33(1):35–43. [DOI] [PubMed] [Google Scholar]
- 16.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, et al. Association of Clinical Outcomes With Left Ventricular Assist Device Use by Bridge to Transplant or Destination Therapy Intent: The Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) Randomized Clinical Trial. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Movahedi F, Kormos RL, Lohmueller L, Seese L, Kanwar M, Murali S, et al. Sequential pattern mining of longitudinal adverse events after Left Ventricular Assist Device implant. IEEE J Biomed Health Inform. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowger J. Quality of Life and Functional Capacity Assessment After Mechanical Circulatory Support: Divergent Study Results Exemplify the Need for Standardized and Dedicated Studies on Non-Mortality End-Points. J Card Fail. 2016;22(10):806–7. [DOI] [PubMed] [Google Scholar]
- 20.Mehta P, Imamura T, Juricek C, Sarswat N, Kim G, Raikhelkar J, et al. Combined Left Ventricular Assist Device and Coronary Artery Bypass Grafting Surgery: Should We Bypass the Bypass? ASAIO J. 2020;66(1):32–7. [DOI] [PubMed] [Google Scholar]
- 21.Birks EJ, McGee EC Jr., Aaronson KD, Boyce S, Cotts WG, Najjar SS, et al. An examination of survival by sex and race in the HeartWare Ventricular Assist Device for the Treatment of Advanced Heart Failure (ADVANCE) Bridge to Transplant (BTT) and continued access protocol trials. J Heart Lung Transplant. 2015;34(6):815–24. [DOI] [PubMed] [Google Scholar]
- 22.Meeteren JV, Maltais S, Dunlay SM, Haglund NA, Beth Davis M, Cowger J, et al. A multi-institutional outcome analysis of patients undergoing left ventricular assist device implantation stratified by sex and race. J Heart Lung Transplant. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Ueyama H, Malik A, Kuno T, Yokoyama Y, Briasouli A, Shetty S, et al. Racial disparities in in-hospital outcomes after left ventricular assist device implantation. J Card Surg. 2020;35(10):2633–9. [DOI] [PubMed] [Google Scholar]
- 24.Teuteberg JJ, Cleveland JC Jr., Cowger J, Higgins RS, Goldstein DJ, Keebler M, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg. 2020;109(3):649–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.