Summary
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant emerged in November 2021 and consists of several mutations within the spike. We use serum from mRNA-vaccinated individuals to measure neutralization activity against omicron in a live-virus assay. At 2–4 weeks after a primary series of vaccinations, we observe a 30-fold reduction in neutralizing activity against omicron. Six months after the initial two-vaccine doses, sera from naive vaccinated subjects show no neutralizing activity against omicron. In contrast, COVID-19-recovered individuals 6 months after receiving the primary series of vaccinations show a 22-fold reduction, with the majority of the subjects retaining neutralizing antibody responses. In naive individuals following a booster shot (third dose), we observe a 14-fold reduction in neutralizing activity against omicron, and over 90% of subjects show neutralizing activity. These findings show that a third dose is required to provide robust neutralizing antibody responses against the omicron variant.
Keywords: SARS-CoV-2, antibody, mRNA vaccines, neutralization assay, live-virus, vaccine induced immunity, Omicron, Omicron variant, B.1.1.529, booster dose
Graphical abstract
Highlights
-
•
Omicron has a significant impact on vaccine-induced neutralizing antibody (nAb) titers
-
•
Naive vaccinated individuals lost nAb titers against omicron variant after 6 months
-
•
A booster (third dose) is required to maintain neutralizing activity against omicron
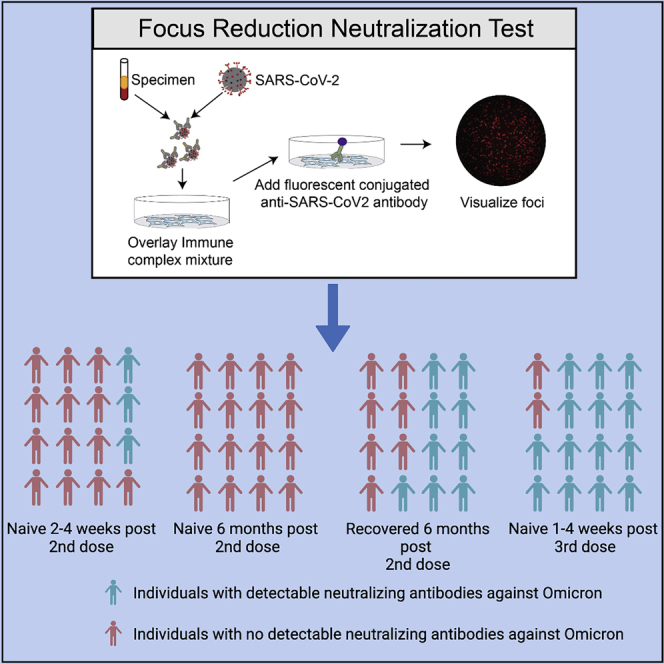
Here in this study, Edara et al. report that 6 months after the primary series (initial two doses) of mRNA vaccination, the majority of the subjects lost detectable neutralizing antibody titers against omicron. The data suggest that a third booster dose is necessary to sustain neutralizing activity against omicron.
Introduction
The BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines generate potent and durable neutralizing antibody responses against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1, 2, 3 The global emergence of SARS-CoV-2 variants with mutations in the spike protein, the principal antigenic target of the mRNA vaccines, has raised concern regarding the effectiveness of these vaccines. We previously found that mRNA vaccine-induced antibody responses have reduced neutralizing activity against the B.1.351 (beta) and, to a lesser extent, B.1.617.2 (delta) variants.4, 5, 6, 7
The B.1.1.529 (omicron) variant emerged in November 2021 and has rapidly spread throughout the world.8 We isolated the B.1.1.529 variant from a residual mid-turbinate swab collected from a returning traveler from South Africa (hCoV-19/USA/GA-EHC-2811C/2021). Relative to the WA1/2020 virus (nCoV/USA_WA1/2020; spike 614D), the B.1.1.529 variant contains many mutations within the spike protein (A67V, Δ69–70, T95I, G142D, Δ143–145, Δ211, N211I, +214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F). As a comparator, we included the B.1.351 (beta) variant in our neutralization assay. The beta variant has mutations within the spike protein at amino acid residues L18F, D80A, D215G, L241-, L242-, A243-, K417N, E484K, N501Y, D614G, and A701V. We used an in vitro live-virus focus reduction neutralization test (FRNT)9 on Vero-TMPRSS2 cells to perform a cross-sectional analysis of neutralizing antibody response in serum (Tables S1–S4) from three naive mRNA-vaccinated cohorts, which includes 2–4 weeks after the primary series (n = 24; n = 11 Moderna; n = 13 Pfizer), 6 months after the primary series (n = 25; n = 8 Moderna; n = 17 Pfizer), and 1–4 weeks after a third dose (n = 52; n = 17 Moderna third dose; n = 35 Pfizer third dose), and a COVID-19-recovered then mRNA-vaccinated cohort (6 months after second dose; n = 37; n = 13 Moderna; n = 24 Pfizer).
Results
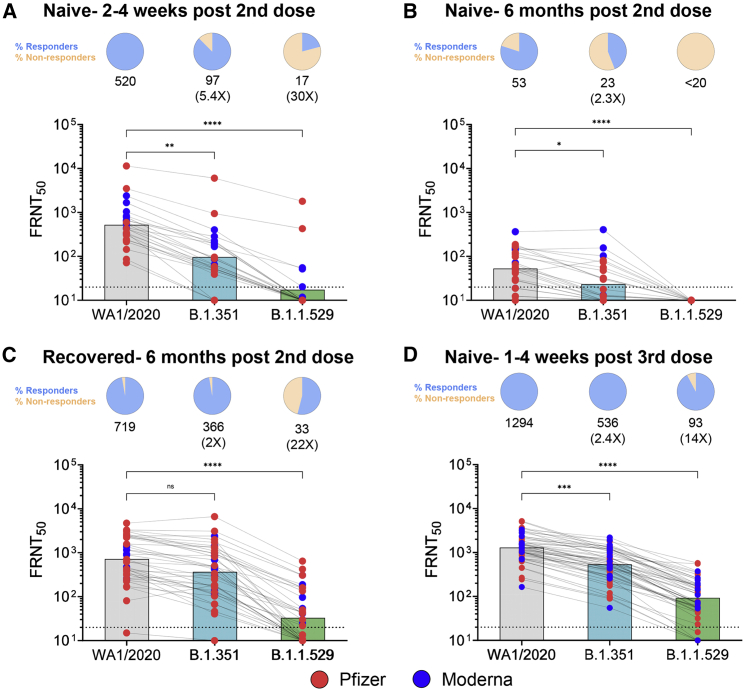
In Moderna- (Figure S1) or Pfizer-vaccinated (Figure S2) individuals, we noticed profoundly reduced neutralizing activity against the B.1.1.529 variant as compared with either the WA1/2020 strain or B.1.351 variant. Following primary mRNA vaccination in naive individuals, the FRNT geometric mean titers (GMTs) are 520 for WA1, 97 for B.1.351, and 17 for B.1.1.529 and correspond to a 5.4-fold and 30-fold reduction as compared with WA1, respectively (Figure 1A). Further, only 21% of the subjects show neutralizing antibody titers against the B.1.1.529 variant. Individuals who do not neutralize at the limit of detection at 50% are plotted at 10, which was used for geometric mean and fold-change calculations, and these samples are considered as undetectable or non-responders against the respective variant.
Figure 1.
Neutralization antibody responses against WA1/2020, B.1.351, and B.1.1.529 SARS-CoV-2 variants post-mRNA vaccination
(A–D) Data from the following cohorts are shown: (A) naive individuals 2–4 weeks after the second dose (n = 24), (B) naive individuals 6 months after the second dose (n = 25), (C) recovered individuals who received the primary mRNA vaccination series (n = 37), and (D) naive vaccinated individuals who received a third dose (n = 52). In (A)–(D), the FRNT50 GMTs for WA1/2020, B.1.351, and B.1.1.529 are shown with respective fold changes in comparison with the WA1/2020. A pie chart above each graph shows the frequency of individuals who have titers above (Responders) or below (Non-responders) the limit of detection (LOD). The connecting lines between the variants represent matched serum samples. The horizontal dashed lines along the x axis indicate the LOD (FRNT50 GMT = 10). Blue circles represent individuals who received the Moderna mRNA-1273 vaccine as the primary vaccine series, and the red circles represent individuals who received the Pfizer-BioNTech BNT162b2 vaccine as the primary vaccine series. Normality of the data was determined using Shapiro-Wilk normality test. Non-parametric pairwise analysis for neutralization titers was performed by Wilcoxon matched pairs signed rank test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Next, we examined the durability of neutralizing antibody responses in vaccinated subjects 6 months after receiving the second dose. This cohort was divided between individuals with no known prior COVID-19 exposure and those who had recovered from COVID-19 and then received the Moderna or Pfizer vaccine. In naive vaccinated subjects, the GMTs at 6 months are 53 for WA1, 23 for B.1.351, and <20 for B.1.1.529 (Figure 1B). This corresponds to a 2.3-fold reduction against B.1.351 as compared with WA1; however, none of these subjects show any detectable neutralizing activity against the B.1.1.529 variant. In recovered individuals who received the vaccine, the GMTs are 719 for WA1, 366 for B.1.351, and 33 for B.1.1.529 and correspond to a 2-fold and 22-fold reduction as compared with WA1, respectively (Figure 1C). In contrast with naive vaccinated subjects, 55% of recovered vaccinated subjects retain neutralizing activity against the B.1.1.529 variant 6 months after the second dose.
Next, we examined the impact of the B.1.1.529 variant on neutralizing antibody responses following a single booster dose (third dose). Most subjects received the third dose approximately 8 months (median 268 days) after the second dose. Roughly 85% of these subjects received a homologous third dose (primary vaccine versus booster dose), and a few subjects received 100 μg of mRNA-1273 as the third dose. Sampling occurred 1–4 weeks after the booster dose. Across all subjects who received a booster dose (Figure 1D), the GMTs are 1,294 for WA1, 536 for B.1.351, and 93 for B.1.1.529. This corresponds to a reduction of 2.4-fold and 14-fold in neutralizing activity as compared with WA1, and over 90% of subjects retain neutralizing activity against the B.1.1.529 variant.
Discussion
In agreement with recent studies, our findings show that the B.1.1.529 variant has a significant impact on the neutralizing activity against mRNA vaccine-induced responses.10,11 By 6 months, all naive vaccinated and a majority of recovered vaccinated subjects lost neutralizing activity against the B.1.1.529 variant. However, following a booster dose (third dose), through 1 month, a vast majority of subjects retained neutralizing activity against B.1.1.529. These findings support the need for a booster dose to maintain neutralizing activity against the B.1.1.529 variant.
Limitations of the study
Limitations of this study include: (1) small sample size, (2) selection bias for the third dose (Pfizer third dose received Emergency Use Authorization [EUA] approval prior to Moderna), (3) this is a cross-sectional analysis of subjects who received either the primary or booster mRNA vaccines, and (4) we are not able to link the clinical outcomes with the neutralization findings.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AF647-CR3022 | Dr. Jens Wrammert (Emory University, Atlanta, GA) | N/A |
| Bacterial and virus strains | ||
| nCoV/USA_WA1/2020 | Dr. Vineet D. Menachery (UTMB, Galveston, TX) | N/A |
| B.1.351 | Dr. Andy Pekosz (John Hopkins University, Baltimore, MD) | hCoV-19/South Africa/KRISP-K005325/2020 |
| B.1.1.529 | Mid-turbinate nasal swab | hCoV19/EHC_C19_2811C |
| Biological samples | ||
| Serum/Plasma samples | Emory Hope clinic and Emory Children’s Center | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Methylcellulose | Sigma-Aldrich | Cat. #: M0512-250G |
| Experimental models: Cell lines | ||
| VeroE6 C1008 cells | ATCC | Cat# CRL-1586, RRID:CVCL_0574 |
| Software and algorithms | ||
| GraphPad Prism (v9) | N/A | N/A |
| Viridot | Katzelnick et al. | https://github.com/leahkatzelnick/Viridot |
| Deposited data | ||
| Additional supplemental items are available from Mendeley data | data.mendeley.com | https://doi.org/10.17632/j6ds96cd5g.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Mehul Suthar (mehul.s.suthar@emory.edu).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Experiment model and subject details
Cell lines
VeroE6-TMPRSS2 cells were generated and cultured as previously described.7 Briefly, VeroE6-TMPRSS2 cells were generated by transfecting VERO E6 cells (ATCC CRL-1586) with pCAGGS plasmid in which chicken actin gene promoter drives the expression of an open reading frame comprising Puromycin N-acetyl transferase, GSG linker, 2A self-cleaving peptide of thosea asigna virus (T2A), human transmembrane serine protease 2 (TMPRSS2). Two days post-transfection, cells were trypsinzed and transferred to a 100 mm dish containing complete DMEM medium (1x DMEM, Thermo Fisher, # 11965118, 10% FBS, 1x penicillin/streptomycin) supplemented with puromycin (Thermo Fisher, #A1113803) at a final concentration of 10 μg/ml. Approximately ten days later, individual colonies of cells were isolated using cloning cylinders (Sigma) and expanded in medium containing puromycin. Clonal cell lines were screened for expression of TMPRSS2 by flow cytometry. VeroE6-TMPRSS2 cells were cultured in complete DMEM in the presence of Gibco Puromycin 10mg/mL (# A11138-03). VeroE6-TMPRSS2 cells were used to propagate all virus stocks.
Viruses
nCoV/USA_WA1/2020 (WA/1), closely resembling the original Wuhan strain and resembles the spike used in the mRNA-1273 and Pfizer BioNTech vaccine, was propagated from an infectious SARS-CoV-2 clone as previously described.12 icSARS-CoV-2 was passaged once to generate a working stock. The B.1.351 variant isolate, kindly provided by Dr. Andy Pekosz (John Hopkins University, Baltimore, MD), was propagated once to generate a working stock. hCoV19/EHC_C19_2811C (herein referred to as the B.1.1.529 variant) was derived from a mid-turbinate nasal swab collected in December 2021. This SARS-CoV-2 genome is available under GISAID accession number EPI_ISL_7171744. Using VeroE6-TMPRSS cells, the B.1.1.529 variant was plaque purified directly from the nasal swab, propagated once in a 12-well plate, and expanded in a confluent T175 flask to generate a working stock. All viruses used in this study were deep sequenced and confirmed as previously described.7
Plasma/serum study samples
At Emory University, collection and processing were performed under the University Institutional Review Board protocols #00045821, #00002061, #00001080 and #00022371 at the Emory Hope Clinic and Emory Children’s Center. Naïve and non-naïve adults ≥18 years were enrolled if they met eligibility criteria for these umbrella protocols and provided informed consent. Convalescent samples were a convenience sample of individuals with confirmed mild or moderate COVID-19 (March-August 2020).6 These participants subsequently received vaccination with 2 doses of Pfizer BNT162b2 or Moderna mRNA1273 and their sera or plasma samples were collected 6 months after vaccination (Table S3). Three other cohorts of naïve participants were enrolled after receiving either mRNA vaccines and their sera or plasma were collected at the following timepoints: 1) 2-4 weeks after primary series (Table S1); 2) 6 months after primary series (Table S2); and 3) 1-4 weeks after single dose boost (85% were homologous boosts, Table S4). Additional information for the plasma/serum samples was provided in the supplemental tables.
Method details
Focus reduction neutralization test
FRNT assays were performed as previously described.6,7,9 Briefly, samples were diluted at 3-fold in 8 serial dilutions using DMEM (VWR, #45000-304) in duplicates with an initial dilution of 1:10 in a total volume of 60 μl. Serially diluted samples were incubated with an equal volume of WA1/2020, B.1.351, or B.1.1.529 (100-200 foci per well based on the target cell) at 37°C for 45 minutes in a round-bottomed 96-well culture plate. The antibody-virus mixture was then added to VeroE6-TMPRSS2 cells and incubated at 37°C for 1 hour. Post-incubation, the antibody-virus mixture was removed and 100 μl of pre-warmed 0.85% methylcellulose overlay was added to each well. Plates were incubated at 37°C for 18 hours and the methylcellulose overlay was removed and washed six times with PBS. Cells were fixed with 2% paraformaldehyde in PBS for 30 minutes. Following fixation, plates were washed twice with PBS and permeabilization buffer (0.1% BSA, 0.1% Saponin in PBS) was added to permeabilize cells for at least 20 minutes. Cells were incubated with an anti-SARS-CoV spike primary antibody directly conjugated to Alexaflour-647 (CR3022-AF647) overnight at 4°C. Cells were washed three times in PBS and foci were visualized on Cytation7.
Quantification and statistical analysis
Antibody neutralization was quantified by counting the number of foci for each sample using the Viridot program.13 The neutralization titers were calculated as follows: 1 - (ratio of the mean number of foci in the presence of sera and foci at the highest dilution of respective sera sample). Each specimen was tested in duplicate. The FRNT-50 titers were interpolated using a 4-parameter nonlinear regression in GraphPad Prism 9.2.0. Samples that do not neutralize at the limit of detection at 50% are plotted at 10 and was used for geometric mean and fold-change calculations. Normality of the data was determined using Shapiro Wilk normality test. Non-parametric pairwise analysis for neutralization titers were performed by Wilcoxon matched pairs signed rank test. ∗p<0.05; ∗∗p<0.01; ∗∗∗p<0.001; ∗∗∗∗p<0.0001.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; P51OD011132, 3U19AI057266-17S1, 1U54CA260563, HHSN272201400004C, and NIH/NIAID CEIRR under contract 75N93021C00017 to Emory University), intramural funding from the National Institute of Allergy and Infectious Diseases, The Oliver S. and Jennie R. Donaldson Charitable Trust, Emory Executive Vice President for Health Affairs Synergy Fund award, Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children’s Healthcare of Atlanta, Emory-UGA Center of Excellence for Influenza Research and Surveillance (Atlanta, GA, USA), COVID-Catalyst-I3 Funds from the Woodruff Health Sciences Center and Emory School of Medicine, and Woodruff Health Sciences Center 2020 COVID-19 CURE Award. We thank Jessica Proulx from University of North Texas Health Science Center at Fort Worth for providing the BioRender illustration. The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
V.-V.E., M.S.S., K.E.M., M.E., S.L.F., L.L., K.M.M., K.F., and M.E.D.-G. contributed to acquisition of the data. V.-V.E. and M.S.S. contributed to analysis and interpretation of the data. G.M., L.E.N., S.B., G.A., A.N., H.S., M.L., L.B., M.G., J.R.-T., A.R.H., S.G., A.G., M.S., A.P., J.J.W., and D.C.D. contributed to the acquisition, analysis, and interpretation of the data. N.R. served as the principal investigator of the clinical protocol for acquisition of patient samples and contributed to interpretation of the data. J.W. and N.R. contributed to the acquisition, analysis, and interpretation of the data. V.-V.E. and M.S.S. contributed to the acquisition, analysis, and interpretation of the data, as well as the conception and design of the work and writing of the manuscript.
Declaration of interests
M.S.S. serves on the advisory board for Moderna and Ocugen.
Published: February 15, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100529.
Supplemental information
Data and code availability
-
•
Data have been deposited at data.Mendeley.com and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
The paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., Campbell T.B., Clark J., Jackson L.A., Fichtenbaum C.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegu A., O'Connell S.E., Schmidt S.D., O'Dell S., Talana C.A., Lai L., Albert J., Anderson E., Bennett H., Corbett K.S., et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021 doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthar M.S., Arunachalam P.S., Hu M., Reis N., Trisal M., Raeber O., Chinthrajah S., Davis-Gardner M.E., Manning K., Mudvari P., et al. Durability of immune responses to the BNT162b2 mRNA vaccine. bioRxiv. 2021 doi: 10.1101/2021.09.30.462488. 2021.2009.2030.462488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edara V.V., Norwood C., Floyd K., Lai L., Davis-Gardner M.E., Hudson W.H., Mantus G., Nyhoff L.E., Adelman M.W., Fineman R., et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe. 2021;29:516–521.e3. doi: 10.1016/j.chom.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edara V.V., Pinsky B.A., Suthar M.S., Lai L., Davis-Gardner M.E., Floyd K., Flowers M.W., Wrammert J., Hussaini L., Ciric C.R., et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N. Engl. J. Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Response Team SARS-CoV-2 B.1.1.529 (omicron) variant - United States, December 1-8, 2021. Morb. Mortal. Wkly. Rep. 2021;70:1731–1734. doi: 10.15585/mmwr.mm7050e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanderheiden A., Edara V.V., Floyd K., Kauffman R.C., Mantus G., Anderson E., Rouphael N., Edupuganti S., Shi P.-Y., Menachery V.D., et al. Development of a rapid focus reduction neutralization test assay for measuring SARS-CoV-2 neutralizing antibodies. Curr. Protoc. Immunol. 2020;131:e116. doi: 10.1002/cpim.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria-Rose N.A., Shen X., Schmidt S.D., O’Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., Tang H., et al. Booster of mRNA-1273 vaccine reduces SARS-CoV-2 omicron escape from neutralizing antibodies. medRxiv. 2021 doi: 10.1101/2021.12.15.21267805. 2021.2012.2015.21267805. [DOI] [Google Scholar]
- 11.Schmidt F., Muecksch F., Weisblum Y., Silva J.D., Bednarski E., Cho A., Wang Z., Gaebler C., Caskey M., Nussenzweig M.C., et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X., Muruato A., Lokugamage K.G., Narayanan K., Zhang X., Zou J., Liu J., Schindewolf C., Bopp N.E., Aguilar P.V., et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27:841–848.e3. doi: 10.1016/j.chom.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzelnick L.C., Escoto A.C., McElvany B.D., Chávez C., Salje H., Luo W., Rodriguez-Barraquer I., Jarman R., Durbin A.P., Diehl S.A., et al. Viridot: an automated virus plaque (immunofocus) counter for the measurement of serological neutralizing responses with application to dengue virus. PLoS Negl. Trop. Dis. 2018;12:e0006862. doi: 10.1371/journal.pntd.0006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data have been deposited at data.Mendeley.com and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
The paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.