Summary
Background
The pathophysiological mechanisms underlying the association between red blood cell distribution width (RDW) and all-cause mortality are unknown. We conducted a data-driven discovery investigation to identify plasma proteins that mediate the association between RDW and time to death in community-dwelling adults.
Methods
At baseline, 962 adults (women, 54·4%; age range, 21–98 years) participated in the InCHIANTI, “Aging in the Chianti Area” study, and proteomics data were generated from their plasma specimens. Of these, 623 participants had proteomics data available at the 9-year follow-up. For each visit, a total of 1301 plasma proteins were measured using SOMAscan technology. Complete data on vital status were available up to the 15-year follow-up period. Protein-specific exponential distribution accelerated failure time, and linear regression analyses adjusted for possible covariates were used for mortality and mediation analyses, respectively (survival data analysis).
Findings
Baseline values of EGFR, GHR, NTRK3, SOD2, KLRF1, THBS2, TIMP1, IGFBP2, C9, APOB, and LRP1B mediated the association between baseline RDW and all-cause mortality. Changes in IGFBP2 and C7 over 9 years mediated the association between changes in RDW and 6-year all-cause mortality.
Interpretation
Cellular senescence may contribute to the association between RDW and mortality.
Funding
This study was funded by grants from the National Institutes of Health (NIH) and the National Institute on Aging (NIA) contract and was supported by the Intramural Research Program of the NIA, NIH. The InCHIANTI study was supported as a ‘targeted project’ by the Italian Ministry of Health and in part by the U.S. NIA.
Keywords: Aging, Proteomics, Red blood cell distribution width, Insulin-like growth factor-binding protein 2, Mortality
Research in context.
Evidence before this study
We searched PubMed for research articles with key words “red blood cell distribution width” (RDW) and “mortality” to identify publications related to the association between RDW and all-cause mortality and its possible underlying mechanisms. We found only research articles reporting higher RDW predicts higher risk of all-cause and cause-specific mortalities. However, the pathophysiological mechanisms underlying the association are unknown.
Added value of this study
We performed two parallel analyses to examine promising plasma proteins that mediate the association between RDW and time to death in a representative sample of community-dwelling adults (in the InCHIANTI, “Aging in the Chianti Area” study). This is the first study to identify circulation level of IGFBP2 as a significant mediator of the association between RDW and all-cause mortality in community dwelling adults with or without serious comorbidities. Interestingly, both IGFBP2 measured at single time point and change over time in IGFBP2 (9-year) mediated the association between RDW and mortality.
Implications of all the available evidence
The highlights of our new findings are two points. First, plasma IGFBP2 mediated the relationship between red blood cell distribution width (RDW) and all-cause mortality. Second, senescence-associated secretory proteins may also contribute to mechanisms underlying the RDW-mortality association.
Alt-text: Unlabelled box
Introduction
Red blood cell distribution width (RDW), which is typically included in a complete blood count, represents the degree of heterogeneity of erythrocyte volume (conventionally known as anisocytosis). There is a strong association between RDW and anaemia, with RDW differentiating iron-deficient anaemia from folate-deficient anaemia.1 In addition, a large body of evidence implicates high RDW as a strong risk factor for cardiovascular and all-cause mortality, both in healthy populations and a clinical series of patients with specific diseases.2,3
RDW is a powerful correlate for a long list of age-related conditions (e.g. cardiovascular disease, venous thromboembolism, cancer, diabetes, pneumonia, chronic obstructive pulmonary disease, and liver and kidney failure).2 Excessive oxidative stress, inflammation, and cell senescence in the erythropoietic compartment have been proposed as the conditions through which high RDW is associated with mortality,2,4,5 but empirical evidence supporting these mechanisms is limited. Thus, clinicians who detect high RDW in their patients have no direction on treatments that may prevent or at least delay these outcomes. Understanding the biological mechanisms by which RDW is linked to multiple health outcomes may help identify potential therapeutic targets.
Proteomic analysis has the potential to identify candidate biomarkers that connect elevated anisocytosis status with health consequences, including excess mortality. To minimise the identification of false positive findings due to the large number of proteins tested, two parallel analyses were performed. First, proteins measured at baseline were tested to examine whether they mediated the association between RDW and mortality over a 15-year follow-up. In the second analysis, to reduce the time gap between exposure and outcome events, we identified proteins for which the change between baseline and year 9 mediated an association between changes in RDW and 6-year mortality. The goal of this project was to identify common proteins that mediated an association between RDW and all-cause mortality in these two analyses and identify promising biomarkers connecting elevated anisocytosis and mortality in a representative sample of community-dwelling adults.
Methods
Study population and design
The Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, is a population-based prospective cohort study designed to investigate the aging process and identify mechanisms underlying the decline in physical function and mobility with age.6 The study recruited local residents living in the Chianti region in Tuscany, Italy. The details of the study have been published.6 For the analysis presented here, we selected 962 InCHIANTI participants (21–98 y) whose plasma proteomics data and RDW with concurrent data on BMI, education level, smoking status, and haemoglobin levels at the baseline visit were available. The mean follow-up time was 12·6 ± 3·2 years.
Measurement of plasma proteomics
Blood samples for routine testing and proteomics were taken in the early morning after an overnight fast. In accordance with the guidelines for protein biomarker work, all samples were stored at 4 °C, centrifuged for 4 h, immediately aliquoted, and frozen at −80 °C.7 Plasma proteins remain stable for 14–17 years in storage at −80 °C and for up to 25 freeze–thaw cycles.8,9 The present study measured plasma proteomics at baseline and the 9-year follow-up using the 1·3k HTS SOMAscan assay (SomaLogic, Boulder, CO).10,11 All proteomics data were expressed as abundance in relative fluorescence units and normalised according to the Trans-NIH Center for Human Immunology, Autoimmunity and Inflammation (CHI) pipeline. Data normalization procedures have been described elsewhere.10 The overall technical variability of the assay is low (median intraplate CV in the 3% to 4% range). Among the proteomic profiles of 1322 SOMAmers in plasma, the following SOMAmers were removed: 12 hybridisation controls, four viral proteins (HPV type 16, HPV type 18, isolate BEN, and isolate LW123) and five SOMAmer reagents that have been reported to be nonspecific (P05186/ALPL, P09871/C1S, Q14126/DSG2, Q93038/TNFRSF25, and Q9NQC3/RTN4). As a result, we used 1301 plasma proteins measured at both baseline and the 9-year follow-up visit for further analyses.
All-cause mortality over a 15-year follow-up period
To obtain information on vital status, the study utilised data from the Mortality General Registry operated by the Tuscany Region and death certificates deposited immediately after death at the registry office in the municipality of residence. In the present analysis, complete data on vital status were available until the 15-year follow-up visit.
Other covariates
The other covariates included sociodemographic variables (sex, age, and years of education), smoking status (current smoker or not), and baseline haemoglobin levels. Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0·1 cm. Body mass index (BMI) was included in the analysis.
Ethics
The study protocols complied with the Declaration of Helsinki and were approved by the Italian National Institute of Research and Care on Aging Ethical Committee, Ancona (baseline, protocol n° 14/CE, 28 February 2000) and by the Local Ethical Committee at Azienda Sanitaria of Florence (follow-up, protocol n° 5/04, 12 May 2004). Written informed consent was obtained from all participants after an extensive description of the study.
Statistics
Descriptive data are shown as mean ± SD for continuous variables and percentage for categorical variables. We checked the assumption of normality using the mean ± SD, Q-Q plot, and Shapiro-Wilk test. If we could not assume normality, log-transformed data were used for further analysis. Differences in age and sex between censored and deceased individuals were tested using the Student's t-test and chi-square test, respectively. All other characteristics evaluated at baseline between the same two groups were tested using age-adjusted linear regression models for continuous variables or logistic regression models for categorical variables. Time differences in RDW and 1301 proteins were tested using an age-adjusted linear mixed-effect model. All protein data above or below 3 SD from the mean values were considered outliers and excluded from the analysis.
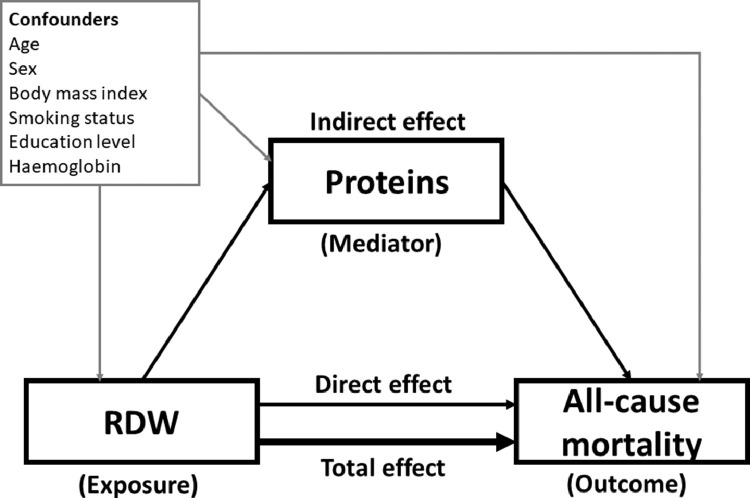
Figure 1 shows a hypothetical causal diagram. Because of strong evidence that higher RDW predicts a higher risk of all-cause mortality,3 we hypothesised that higher RDW reflects the dysregulation of some of the biological pathways that are primarily responsible for high risk of mortality and that the nature of such dysregulation may be revealed by the up/downregulation of specific plasma proteins.3 To test this hypothesis, we set RDW as the exposure, a specific protein as the mediator, and all-cause mortality as the outcome and used the mediation analysis approach proposed by Valeri and VanderWeele.12,13 We examined (i) the direct association between RDW and all-cause mortality independent of the protein, (ii) the indirect association between RDW and all-cause mortality mediated by one specific protein, and (iii) the total association between RDW and all-cause mortality (i.e., the sum of direct and indirect associations). This formula also provides the proportion of the total association of RDW mediated by the protein examined (%). The analysis relating exposure and outcome was conducted using an exponential distribution accelerated failure time (AFT) model. The relationships between exposure and mediators were explored using linear regression models. All models were protein-specific and adjusted for sex, baseline age, BMI, education level, smoking status, and haemoglobin level.
Figure 1.
Hypothesised causal diagram for the mediation of proteins in the association between RDW and all-cause mortality.
To test the hypothesis that changes in RDW between baseline and 9-year follow-up visits predict all-cause mortality, and that this association is mediated by changes in a specific set of proteins, we estimated annualised changes in RDW (Δ RDW) or proteins (Δ protein) as a ratio “(9-year follow-up visit – baseline visit)/exact time interval between baseline and 9-year follow-up visit”. We repeated mediation analysis using Δ RDW and Δ protein and the same set of covariates as described above. For this analysis, the time to death was calculated using the date of the 9-year follow-up visit as time 0, with the outcome as the 6-year all-cause mortality status.
Because anaemia status and specific medical conditions can affect the association between RDW, proteins, and all-cause mortality,3 we repeated the analyses limited to participants who were non-anaemic (n = 920 for the cross-sectional analysis; n = 601 for the longitudinal analysis) and free of chronic diseases (cancer, angina pectoris, myocardial infarction, congestive heart failure, stroke, and diabetes; n = 786 for the cross-sectional analysis; n = 529 for the longitudinal analysis) at baseline. anaemia was defined using the World Health organisation values of < 13·0 g/dL haemoglobin in men and < 12·0 g/dL in women.
SAS software version 9·4 for Windows (SAS Institute, Inc., Cary, NC) was used for all data processing and statistical analyses. We set the level of statistical significance as p < 0·05 (two-sided) to discover novel biomarkers as mediators without adjustment for multiplicity of testing. Thus, we did not report p-values for outcomes other than the primary analysis. Confidence intervals were two-sided with a 95% confidence level and were not adjusted for multiplicity of testing.
Role of funding source
This study was funded by grants from the National Institutes of Health (R01AG027012, R01AG057723, R01HL111271, and R21HL112662) and National Institute on Aging contract 263MD9164 and was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. The InCHIANTI study baseline (1998–2000) was supported as a ‘targeted project' ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821,336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1–1 and N.1-AG-1–2111); and the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5–0002) and supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the study participants. Among the 962 participants examined in the cross-sectional analysis, 367 (38·1%) died during the 15-year follow-up period. Among the 623 participants who had RDW and proteomics both at baseline and at the 9-year follow-up visits, 137 participants (22·0%) died during the following 6-year period (Appendix Table 1). The number of outliers varied across 1301 proteins (cross-sectional analysis, 9·0 [interquartile, IQR, 5–12); longitudinal analysis, 6·0 (IQR 4–8)). The RDW increased over time (β = 0·06, SE = 0·004). Among 1301 proteins, while circulation levels of 336 proteins increased, those of 186 proteins decreased (Appendix Table 2).
Table 1.
Participant characteristics at enrollment.
| Overall |
Censored |
Deceased |
||||
|---|---|---|---|---|---|---|
| (n = 962) |
(n = 595) |
(n = 367) |
||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 66.1 | 15.4 | 60.1 | 15.9 | 75.9 | 7.7 |
| Women (n,%) | 523 (54.4) | 341 (57.3) | 182 (49.6) | |||
| Body mass index (kg/m2) | 27.1 | 4.1 | 27 | 3.9 | 27.4 | 4.5 |
| Education level (6 years or higher,%) | 41.7 | 52.4 | 24.3 | |||
| current smoker (%) | 19.2 | 21 | 16.4 | |||
| haemoglobin (g/dl) | 13.9 | 1.3 | 13.9 | 1.2 | 13.8 | 1.4 |
| anaemia (%, n) | 42 (4.4) | 23 (3.9) | 19 (5.2) | |||
| Red cell distribution width (%) | 13.5 | 0.9 | 13.3 | 0.7 | 13.8 | 1.1 |
| Follow-up time (years)† | 12.6 | 3.2 | 14.4 | 0.2 | 9.6 | 3.6 |
Defined as the time between the date of baseline visit and the date of either death or 15-year follow-up visit.
Mediation analysis
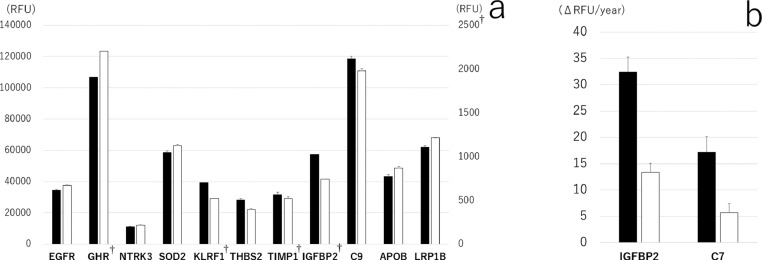
In the simple model that did not include proteins as independent variables, both baseline RDW and Δ RDW were associated with mortality: β = −0·08, 95% CI = (−0·08, −0·04) for baseline RDW; and β = −0·08, 95% CI = (−0·13, −0·03) for Δ RDW. Tables 2 and 3 show a list of proteins that mediated the association between RDW at baseline and 15-year all-cause mortality, as well as between changes in RDW and 6-year all-cause mortality (a full list of proteins is shown in Appendix Tables 3 and 4). Among the 1301 proteins measured at baseline, 11 proteins (epidermal growth factor receptor [EGFR], growth hormone receptor [GHR], NT-3 growth factor receptor [NTRK3], superoxide dismutase mitochondrial [SOD2], killer cell lectin-line receptor subfamily F member 1 [KLRF1], thrombospondin-2 [THBS2], metalloproteinase inhibitor 1 [TIMP1], insulin-like growth factor-binding protein 2 (IGFBP2), complement component C9 (C9), apolipoprotein B-100 (APOB), and low-density lipoprotein receptor-related protein 1 B (LRP1B)) mediated the association between RDW at baseline and 15-year all-cause mortality. These proteins mediated 47·9–53·0% of the association between baseline RDW and 15-year all-cause mortality. Meanwhile, changes in two proteins, IGFBP2 and complement component C7, mediated the association between changes in RDW and 6-year all-cause mortality. Δ IGFBP2 mediated 23·5% and Δ C7 mediated 25·8% of the association between Δ RDW and 6-year all-cause mortality, respectively. Of note, there were differences between censored and deceased individuals in protein abundance or changes in protein abundance (Figure 2).
Table 2.
Mediation of baseline proteins on the association between RDW and all-cause mortality (sorted by p-value in indirect association).
| Mediator |
Total association of RDW with all-cause mortality |
Direct association of RDW with all-cause mortality |
Indirect association of RDW with all-cause mortality through mediator |
Proportion mediated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Target | EntrezGene | β | 95%CI | p-value | β | 95%CI | p-value | β | 95%CI | p-value | ||||
| Symbol | |||||||||||||||
| 1 | ERBB1 | EGFR | 0.85 | 0.77 | 0.94 | 0.002 | 0.88 | 0.8 | 0.98 | 0.015 | 0.96 | 0.94 | 0.99 | 0.006 | 52.8% |
| 2 | Growth hormone receptor | GHR | 0.84 | 0.76 | 0.93 | 0.001 | 0.88 | 0.79 | 0.97 | 0.011 | 0.96 | 0.93 | 0.99 | 0.008 | 53.0% |
| 3 | TrkC | NTRK3 | 0.85 | 0.77 | 0.94 | 0.002 | 0.88 | 0.79 | 0.97 | 0.011 | 0.97 | 0.95 | 0.99 | 0.011 | 51.1% |
| 4 | Mn SOD | SOD2 | 0.86 | 0.78 | 0.96 | 0.005 | 0.89 | 0.8 | 0.98 | 0.022 | 0.97 | 0.95 | 0.99 | 0.014 | 51.9% |
| 5 | KLRF1 | KLRF1 | 0.85 | 0.77 | 0.94 | 0.001 | 0.87 | 0.78 | 0.96 | 0.007 | 0.98 | 0.96 | 0.997 | 0.02 | 49.6% |
| 6 | TSP2 | THBS2 | 0.85 | 0.77 | 0.94 | 0.002 | 0.86 | 0.78 | 0.96 | 0.005 | 0.99 | 0.97 | 0.998 | 0.024 | 48.6% |
| 7 | TIMP-1 | TIMP1 | 0.85 | 0.77 | 0.94 | 0.002 | 0.86 | 0.78 | 0.95 | 0.004 | 0.99 | 0.98 | 0.999 | 0.035 | 47.9% |
| 8 | IGFBP-2 | IGFBP2 | 0.85 | 0.77 | 0.94 | 0.002 | 0.86 | 0.78 | 0.96 | 0.005 | 0.99 | 0.97 | 0.999 | 0.035 | 48.4% |
| 9 | C9 | C9 | 0.85 | 0.77 | 0.94 | 0.002 | 0.86 | 0.78 | 0.96 | 0.005 | 0.98 | 0.97 | 0.999 | 0.035 | 49.0% |
| 10 | Apo B | APOB | 0.85 | 0.77 | 0.95 | 0.003 | 0.87 | 0.79 | 0.97 | 0.009 | 0.98 | 0.96 | 0.999 | 0.039 | 49.7% |
| 11 | LRP1B | LRP1B | 0.86 | 0.78 | 0.96 | 0.005 | 0.88 | 0.79 | 0.97 | 0.013 | 0.98 | 0.97 | 0.9996 | 0.044 | 49.7% |
Mediation analysis was performed using SAS macro (%macro mediation for survival data, Valeri & VanderWeele, 2015). Exposure-outcome association was examined by running protein-specific accelerated failure time model with exponential estimation. RDW-mediator (protein) associations were modeled with multivariable linear regression model. All models were adjusted for age, sex, body mass index, smoking status (current smoker or not), education level (6-year education or higher education or not), and haemoglobin. RDW and proteins were measured at baseline. Outcome was 15-year all-cause mortality.
Table 3.
Mediation of change in proteins on the association between change in RDW and all-cause mortality (sorted by p-value in indirect association).
| Mediator |
Total association of RDW with all-cause mortality |
Direct association of RDW with all-cause mortality |
Indirect association of RDW with all-cause mortality through mediator |
Proportion mediated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Target | EntrezGene | β | 95%CI | p-value | β | 95%CI | p-value | β | 95%CI | p-value | ||||
| Symbol | |||||||||||||||
| 1 | IGFBP-2 | IGFBP2 | 0.22 | 0.05 | 0.86 | 0.03 | 0.28 | 0.07 | 1.12 | 0.072 | 0.77 | 0.6 | 0.99 | 0.038 | 23.5% |
| 2 | C7 | C7 | 0.23 | 0.06 | 0.94 | 0.041 | 0.31 | 0.08 | 1.28 | 0.106 | 0.75 | 0.56 | 0.998 | 0.048 | 25.8% |
Mediation analysis was performed using SAS macro (%macro mediation for survival data, Valeri & VanderWeele, 2015). Exposure-outcome association was examined by running protein-specific accelerated failure time model with exponential estimation. RDW-mediator (protein) associations were modeled with multivariable linear regression model. All models were adjusted for age, sex, body mass index, smoking status (current smoker or not), education level (6-year education or higher education or not), and haemoglobin. RDW and proteins were measured at baseline. Outcome was 15-year all-cause mortality.
Figure 2.
A. Abundances in significant plasma proteins at baseline that mediated the association between baseline RDW and 15-year all-cause mortality. B. Abundances in significant changes in plasma proteins that mediated the association between change in RDW and 6-year all-cause mortality. Bar graphs represent least-squares means and standard errors from a linear regression model adjusted for age, sex, body mass index, smoking status, years of education, and haemoglobin level (black bars, deceased; white bars, censored). † secondary axis.
Appendix Table 5 shows the results of exposure-outcome (tested by the AFT model) and exposure-mediator (tested by linear regression model) analyses. RDW and protein that were measured at baseline independently predicted 15-year all-cause mortality in the AFT model, and RDW at baseline was associated with each protein. Meanwhile, in the AFT models for longitudinal analysis, Δ RDW did not predict 6-year all-cause mortality, and Δ IGFBP2 and Δ C7 predicted 6-year all-cause mortality.
Senescence-Associated secretory phenotype (SASP)
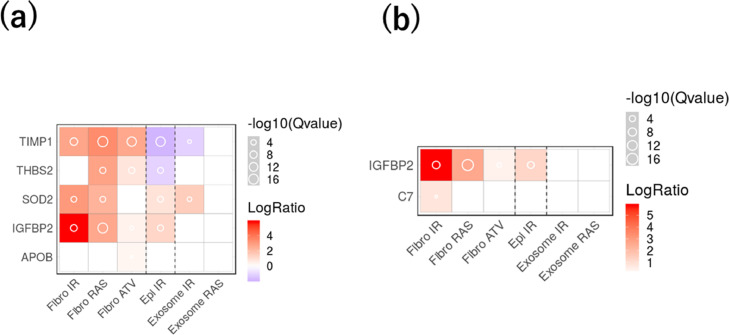
To better understand the candidate proteins observed to mediate the association between RDW and all-cause mortality, we checked whether those proteins have been reported as SASP factors.14 Of the observed proteins that mediated the association between baseline RDW and 15-year all-cause mortality, five have been reported as SASP and both proteins were observed to mediate the association between changes in RDW and 6-year all-cause mortality were reported as SASP (Figure 3).
Figure 3.
Senescence-Associated Secretory Phenotype (SASP) among significant proteins. a. SASP in proteins that mediated the association between baseline RDW and 15-year all-cause mortality. b. SASP in changes in protein that mediated the association between change in RDW and 6-year all-cause mortality.
In anaemia-free individuals at baseline, protein-specific survival analysis identified 10 proteins that mediated the association between RDW at baseline and all-cause mortality (Table 4). Of the 11 proteins identified in the full sample, nine proteins remained significant in anaemia-free individuals, while two proteins, APOB and LRP1B, did not, and one new protein, NTproBNP, became a mediator. In longitudinal analysis, while Δ C7 remained a mediator in the association between Δ RDW and 6-year all-cause mortality, Δ IGFBP2 was not a mediator in the association.
Table 4.
Mediation of proteins on the association between RDW and all-cause mortality in anemia-free adults (sorted by p-value in indirect association).
| Mediator |
Total association of RDW with all-cause mortality |
Direct association of RDW with all-cause mortality |
Indirect association of RDW with all-cause mortality through mediator |
Proportion mediated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Target | Entrez Gene |
β | 95%CI | p-value | β | 95%CI | p-value | β | 95%CI | p-value | ||||
| Cross-sectional analysis† | |||||||||||||||
| 1 | ERBB1 | EGFR | 0.85 | 0.76 | 0.94 | 0.002 | 0.88 | 0.79 | 0.98 | 0.02 | 0.96 | 0.94 | 0.99 | 0.01 | 52.8% |
| 2 | Growth hormone receptor | GHR | 0.84 | 0.75 | 0.93 | 0.002 | 0.87 | 0.79 | 0.97 | 0.01 | 0.96 | 0.93 | 0.99 | 0.01 | 53.0% |
| 3 | TrkC | NTRK3 | 0.85 | 0.76 | 0.94 | 0.003 | 0.87 | 0.78 | 0.97 | 0.01 | 0.97 | 0.95 | 0.99 | 0.02 | 51.0% |
| 4 | IGFBP-2 | IGFBP2 | 0.85 | 0.76 | 0.94 | 0.002 | 0.86 | 0.77 | 0.96 | 0.01 | 0.98 | 0.97 | 0.997 | 0.02 | 48.9% |
| 5 | Mn SOD | SOD2 | 0.86 | 0.77 | 0.95 | 0.004 | 0.88 | 0.79 | 0.98 | 0.02 | 0.97 | 0.95 | 0.996 | 0.02 | 51.2% |
| 6 | KLRF1 | KLRF1 | 0.84 | 0.76 | 0.94 | 0.001 | 0.86 | 0.78 | 0.96 | 0.01 | 0.98 | 0.96 | 0.997 | 0.02 | 49.7% |
| 7 | TSP2 | THBS2 | 0.85 | 0.76 | 0.94 | 0.002 | 0.86 | 0.77 | 0.96 | 0.01 | 0.98 | 0.97 | 0.998 | 0.03 | 48.4% |
| 8 | TIMP-1 | TIMP1 | 0.85 | 0.76 | 0.94 | 0.002 | 0.86 | 0.77 | 0.95 | 0.004 | 0.99 | 0.98 | 0.999 | 0.03 | 48.0% |
| 9 | N-terminal pro-BNP | NPPB | 0.85 | 0.77 | 0.95 | 0.003 | 0.86 | 0.78 | 0.96 | 0.01 | 0.99 | 0.97 | 0.999 | 0.03 | 48.4% |
| 10 | C9 | C9 | 0.85 | 0.76 | 0.94 | 0.002 | 0.86 | 0.77 | 0.96 | 0.01 | 0.98 | 0.96 | 0.999 | 0.04 | 49.0% |
| Longitudinal analysis‡ | |||||||||||||||
| 1 | C7 | C7 | 0.26 | 0.06 | 1.18 | 0.08 | 0.36 | 0.08 | 1.70 | 0.20 | 0.71 | 0.51 | 0.997 | 0.048 | 29.4% |
| 2 | IGFBP-2 | IGFBP2 | 0.22 | 0.05 | 0.96 | 0.04 | 0.28 | 0.06 | 1.24 | 0.09 | 0.78 | 0.61 | 1.01 | 0.055 | 23.2% |
Protein-specific accelerated failure time model with exponetial estimation. RDW-mediator (one protein) associations were modeled with multivariable linear regression model. All models were adjusted for age, sex, body mass index, smoking status (current smoker or not), education level (6-year education or higher education or not), and haemoglobin.
RDW and proteins were measured at baseline. Outcome was 15-year all-cause mortality.
Longitudinal changes in RDW and proteins were calculated from {(9-year follow-up visit - baseline visit)/exact time intervals in each participant}. Outcome was 6-year all-cause mortality from 9-year follow-up visit.
Table 5 shows the results of the mediation analyses in those with no major chronic diseases. Seven proteins (NT-2 growth factor receptor [NTRK2], KLRF1, EGFR, NTRK3, GHR, LRP1B, and IGFBP2) mediated the association between RDW at baseline and 15-year all-cause mortality. In longitudinal analysis, two proteins (Δ TFF3 and Δ IGFBP2) mediated the association between Δ RDW and 6-year all-cause mortality (indirect effect, TFF3; IGFBP2), but not Δ C7 (indirect effect).
Table 5.
Mediation of proteins on the association between RDW and all-cause mortality in adults without medical conditions (sorted by p-values in indirect association).
| Mediator |
Total association of RDW with all-cause mortality |
Direct association of RDW with all-cause mortality |
Indirect association of RDW with all-cause mortality through mediator |
Proportion mediated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Target | EntrezGene Symbol |
β | 95%CI | p-value | β | 95%CI | p-value | β | 95%CI | p-value | ||||
| Cross-sectional analysis† | |||||||||||||||
| 1 | TrkB | NTRK2 | 0.85 | 0.76 | 0.96 | 0.01 | 0.89 | 0.79 | 1.005 | 0.06 | 0.96 | 0.93 | 0.99 | 0.01 | 54.5% |
| 2 | KLRF1 | KLRF1 | 0.86 | 0.76 | 0.96 | 0.01 | 0.88 | 0.78 | 0.997 | 0.04 | 0.97 | 0.95 | 0.99 | 0.02 | 51.7% |
| 3 | ERBB1 | EGFR | 0.87 | 0.77 | 0.98 | 0.02 | 0.89 | 0.79 | 1.01 | 0.06 | 0.97 | 0.94 | 0.99 | 0.02 | 53.3% |
| 4 | TrkC | NTRK3 | 0.86 | 0.77 | 0.97 | 0.02 | 0.89 | 0.79 | 1.003 | 0.06 | 0.97 | 0.95 | 0.996 | 0.02 | 52.3% |
| 5 | Growth hormone receptor | GHR | 0.85 | 0.75 | 0.96 | 0.01 | 0.88 | 0.78 | 0.99 | 0.04 | 0.96 | 0.93 | 0.996 | 0.03 | 52.7% |
| 6 | LRP1B | LRP1B | 0.87 | 0.77 | 0.99 | 0.03 | 0.89 | 0.79 | 1.01 | 0.07 | 0.98 | 0.96 | 0.999 | 0.04 | 51.6% |
| 7 | IGFBP-2 | IGFBP2 | 0.87 | 0.77 | 0.98 | 0.02 | 0.88 | 0.78 | 0.99 | 0.04 | 0.99 | 0.97 | 0.9998 | 0.05 | 49.3% |
| Longitudinal analysis‡ | |||||||||||||||
| 1 | TFF3 | TFF3 | 0.36 | 0.08 | 1.66 | 0.19 | 0.55 | 0.11 | 2.78 | 0.47 | 0.66 | 0.44 | 0.98 | 0.04 | 43.9% |
| 2 | IGFBP-2 | IGFBP2 | 0.31 | 0.06 | 1.52 | 0.15 | 0.42 | 0.08 | 2.09 | 0.29 | 0.74 | 0.55 | 0.99 | 0.04 | 33.0% |
Protein-specific accelerated failure time model with exponetial estimation. RDW-mediator (one protein) associations were modeled with multivariable linear regression model. All models were adjusted for age, sex, body mass index, smoking status (current smoker or not), education level (6-year education or higher education or not), and haemoglobin.
RDW and proteins were measured at baseline. Outcome was 15-year all-cause mortality.
Longitudinal changes in RDW and proteins were calculated from {(9-year follow-up visit - baseline visit)/exact time intervals in each participant}. Outcome was 6-year all-cause mortality from 9-year follow-up visit.
Discussion
To the best of our knowledge, this is the first study to identify IGFBP2 as a mediator of the association between RDW and all-cause mortality in community-dwelling adults with or without serious comorbidities. Remarkably, from two parallel analyses, we found that both IGFBP2 and change over time in IGFBP2 mediated the association between RDW and mortality. Of note, from the results of the cross-sectional analysis, several proteins found to mediate the association between RDW and mortality have been described as SASP members, suggesting that cellular senescence, possibly at the level of haematopoietic cells, may be implicated in this association.
RDW has been suggested as an integrative biomarker for a multidimensional dysfunctional physiological status that is clinically revealed by anisocytosis.15 Major proposed mechanisms of anisocytosis are oxidative stress, inflammation, and senescence of erythropoietic cells.2,5 RDW-related genetic variants are enriched in telomere length, ribosomal RNA, and apoptosis but the role of these mechanisms has not been confirmed.16 In this study, we found that baseline RDW predicted all-cause mortality in our population similar to previous studies.3 A novel finding of this study is that although on average RDW increases with aging,17 those individuals who show a steeper increase in RDW over time also have an increased risk of mortality. This finding suggests that RDW should be monitored over time, as these changes, including the rate of change, may signal an impending deterioration of health status.
Our major finding is that circulating levels of IGFBP2 mediate the association between RDW and all-cause mortality in both cross-sectional and longitudinal analyses. IGFBP2 is a member of the IGF-binding protein family (IGFBP1–6). IGFBP2 regulates the ability of IGF to activate IGF-I receptors. IGFBP2 binds IGF1, IGF2, and insulin, with the greatest affinity for IGF2.18 IGFBP2 is an IGF system regulator, whose major functions are to promote proliferation, survival, differentiation, and motility in various cell types.19,20 It is the second most abundant IGF-binding protein and is negatively regulated by growth hormones. In the haematopoietic system, IGFBP2 supports survival and cycling of haematopoietic stem cells.21 Previous studies have shown that elevated IGFBP2 is associated with a higher risk of adverse changes in body composition and physical function.22 Several lines of evidence suggest that elevated IGFBPs, including IGFBP2 in senescent endothelial and epithelial cells, are implicated in the regulation of signal pathways for cellular senescence in age-related diseases.19,23,24 Epidemiological studies have found that IGFBP2 increases with age and is associated with age-related diseases such as cancer, dementia, and mortality in older adults.19,22,25, 26, 27, 28 Consistent with the literature, we found higher circulating levels of IGFBP2 or larger increases in IGFBP2 in individuals closer to death.29 Interestingly, these results hold when we restricted our analyses to disease-free individuals, while the mediating effect of change in IGFBP2 was just shy of significance when the analysis was restricted to anaemia-free individuals at baseline (p = 0·055). A possible explanation is that the same biological pathway that connects RDW with higher mortality is also likely to cause anaemia. Future studies should test the hypothesis that higher plasma IGFBP2 levels are associated with pathological changes in the haematopoietic system that eventually cause elevated RDW.
Notably, in addition to IGFBP2, we found that the plasma levels of several SASP proteins mediated the association between RDW and all-cause mortality.14 In particular, four proteins (TIMP1, THBS2, SOD2, and APOB) showed mediation in cross-sectional analyses and one protein (C7) in longitudinal analysis. Anisocytosis has been related to parenchymal hypoxia and chronic inflammatory status, both of which have been shown to precipitate cellular senescence.30, 31, 32, 33, 34 TIMP1 is a member of the TIMP family that inhibits matrix metalloproteinase enzymes involved in extracellular matrix maintenance and remodelling. TIMP1, which regulates the survival and proliferation of normal hematopoietic progenitor cells and increases expression level in response to inflammation, predicts cardiovascular-specific or all-cause mortality.35, 36, 37 THBS2 belongs to a group of matricellular proteins that are components of the extracellular matrix and is overexpressed in response to damage or during remodelling in cardiovascular diseases.38 Epidemiological studies have found elevated THBS2 level to predict incident mobility disability and all-cause mortality.39,40 SOD2 plays a role in one of the primary defence mechanisms for the buffering of reactive oxygen species and is involved in the oxidative stress pathway.41 SODs catalyse the dismutation of superoxide anion (O2−) into hydrogen peroxide (H2O2).42 Using a mouse model, it was demonstrated that failure to express SOD2 impaired mitochondrial complex II activity and induced cellular senescence.43 SOD2 is a key protein that controls the mitochondrial redox state and lower circulating levels of SOD2 with aging is associated with mitochondria dysfunction and age-related degeneration in the nervous system, and all-cause mortality.42,44,45 APOB is implicated in lipid metabolism, and is upregulated with oxidative stress and systemic inflammation.46 It has been shown that APOB predicts coronary heart disease-specific mortality.47 Thus, one working hypothesis suggested by these findings is that oxidative stress and inflammation may contribute to induce cellular senescence and subsequent production of SASP proteins that circulate in blood and both locally and systemically have negative effects on the hematopoietic system leading to impaired erythropoiesis and high RDW. Future studies should test the hypothesis that RDW is a biomarker of senescent cell accumulation in different organs.
Two complement components (C7 and C9) were found to be mediators of RDW and all-cause mortality. Working with other immune or defence systems, the complement system is an arm of innate immunity that removes pathogens, necrotic, and apoptotic cells.48 The complement system mediates inflammatory status and cell lysis.49 Both over-expression and under-expression of the complement system have been implicated in complement-mediated inflammatory status and chronic disease risk, especially Alzheimer's disease (AD).50, 51, 52, 53 While the SASP Atlas shows C7 as SASP but not C9, C7, and C9 have similar structures and both proteins form the membrane attack complex (MAC).14,54 Under oxidative stress in endothelial cells, such as brain neurons and kidneys, abnormally activated MAC is implicated in tissue injury due to downregulation of complementary complement inhibitors such as complement factor H.52,55 The observations that C9 measured at a single time point and longitudinal change in C7 mediate RDW and all-cause mortality suggest that even in the complement system involved in the MAC, the trajectory changes in protein abundance and the role in mortality may differ by protein.
Five additional proteins measured at baseline that were not identified in the SASP included EGFR, GHR, NTRK3, KLRF1, and LRP1B, to illuminate the underlying mechanisms between RDW at baseline and 15-year all-cause mortality. Of these proteins, EGFR and GHR, which were not identified in the SASP, have been associated with cellular senescence. For example, it was previously found that five cytokines (IL-1β, IL-13, MCP-2, MIP-3α, and SDF-1α) induced cellular senescence through EGFR activation in an EGF-independent pathway, suggesting that cellular senescence can be induced by proinflammatory cytokines via EGFR signalling.56 Further, in another study, GHR deficiency was demonstrated to protect against age‐related NLRP3 inflammasome activation and immune senescence.57 In addition, EGFR, LRP1B, NTRK3, and GHR have been implicated in nervous system development, neurodegeneration, and development of dementia/AD.15,58, 59, 60, 61, 62, 63, 64, 65 Elevated RDW is associated with poor cognitive performance and RDW predicts cardiovascular diseases, stroke, and dementia, suggesting that RDW may also reflect cerebrovascular pathology.2,15 Accordingly, LRP-1 mediates transport of β-amyloid out of the brain and is expressed in endothelial cells and pericytes of the blood brain barrier.66 Dysfunction of LRP1 significantly exacerbates accumulation of Aβ in the brain.67 Another protein, NKp80/KLRF1, is expressed on the surface of NK cells and indicates NK cell maturity as KLRF1 acquisition is associated with a decline in cytokine production potential.68,69 It has been demonstrated that these proteins are up/down regulated in response to oxidative stress or inflammatory status.70, 71, 72, 73, 74, 75 Since our study examined the association among RDW, protein expression, and all-cause mortality, further studies are needed to examine cause-specific mortality to identify the possible physiopathology in which these proteins play key roles.
Our study has several strengths. An important strength is the longitudinal design with highly representative population-based samples with demographic diversity and low attrition during follow-up. We examined a wide range of proteins to explore promising candidate proteins that might elucidate the mechanisms underlying the connection between the diversity of red blood cell size and all-cause mortality. Furthermore, in addition to examining proteins measured at one time point and their mediation effects on the association between RDW at baseline and 15-year all-cause mortality, we performed longitudinal analyses using annual changes in RDW and proteins using baseline and 9-year follow-up visits. The limitations of our study include the use of an aptamer-based platform for proteomics that provides relative quantification; therefore, replication using antibody-based arrays and/or targeted LC-MS/MS methods is needed, in addition to repeating our findings in other independent populations. Second, a conservative approach to identify promising proteins by using multi-test adjustment was not used, as this study used a hypothesis-free discovery approach, and our interest was not to increase a type II error, but to interpret promising proteins identified by using nominal p-values.76,77 Third, because protein-protein interactions can occur, a protein-specific analysis should not be independent. However, significant proteins were consistent in the sensitivity analyses, suggesting that our main results were robust. Fourth, while we accounted for possible comorbidity status including anaemia, other haematological status such as transfusion and sepsis may affect the association among RDW, proteins, and mortality; however, the InCHIANTI study did not evaluate these haematological statuses. Finally, causes of death were not available for all participants who died because cause-specific data have not yet been released by the Tuscany regional authorities.
Conclusion
The present study demonstrates that plasma IGFBP2 is a promising candidate protein that mediates the association between RDW and all-cause mortality in community-dwelling adults. We also found other promising proteins associated with cellular senescence that could play a mediating role in the observed associations. Future investigations are needed to examine the roles of these proteins in biological pathways that lead to premature death.
Contributors
LF directed and supervised the project. The SOMAscan assay was run by GF, JC, and MR conducted proteomic data normalisation and cleaning. YO and LF configured the concept and design of the study and manuscript preparation. YO and TT verified underlying data and conducted statistical analyses. YO, LF, TT, RDS, and EMS contributed to the data interpretation. YO prepared the manuscript, and all authors have contributed to and approved the final version of the manuscript.
Declaration of interests
All authors declare no conflicts of interest.
Acknowledgments
Acknowledgements
This study was funded by grants from the National Institutes of Health (R01AG027012 and R01AG057723 to Dr. Semba, and R01HL111271 and R21HL112662 to Dr. Ferrucci) and National Institute on Aging contract 263MD9164 (to Dr. Ferrucci) and was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. The InCHIANTI study baseline (1998–2000) was supported as a ‘targeted project' ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1–1 and N.1-AG-1–2111); and the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5–0002) and supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
Data sharing statement
Proteomics data are not publicly accessible due to the Italian law. However, the proteomic data generated from this study are available upon request through the submission of proposals at the InCHIANTI study website http://inchiantistudy.net/wp/. SAS programs are available as deposited in Zenodo (DOI: 10.5281/zenodo.5795636).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103816.
Contributor Information
Yusuke Osawa, Email: yosawa@keio.jp.
Luigi Ferrucci, Email: FerrucciLu@grc.nia.nih.gov.
Appendix. Supplementary materials
References
- 1.Feng G.H., Li H.P., Li Q.L., Fu Y., Huang R.B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. 2017;2(3):172–175. doi: 10.1136/svn-2017-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvagno G.L., Sanchis-Gomar F., Picanza A., Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 3.Patel K.V., Semba R.D., Ferrucci L., et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65(3):258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J., Borne Y., Engstrom G. The relationship between red cell distribution width and all-cause and cause-specific mortality in a general population. Sci Rep. 2019;9(1):16208. doi: 10.1038/s41598-019-52708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiefer C.R., Snyder L.M. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7(2):113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L., Bandinelli S., Benvenuti E., et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 7.Tuck M.K., Chan D.W., Chia D., et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman L.J., Li M., Yarbrough W.G., Slebos R.J., Liebler D.C. Global stability of plasma proteomes for mass spectrometry-based analyses. Mol Cell Proteom. 2012;11(6):M111. doi: 10.1074/mcp.M111.014340. 014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassis M.E., Niles R.K., Braten M.N., et al. Evaluating the effects of preanalytical variables on the stability of the human plasma proteome. Anal Biochem. 2015;478:14–22. doi: 10.1016/j.ab.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candia J., Cheung F., Kotliarov Y., et al. Assessment of Variability in the SOMAscan Assay. Sci Rep. 2017;7(1):14248. doi: 10.1038/s41598-017-14755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraemer S., Vaught J.D., Bock C., et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: a SOMAmer-based, streamlined multiplex proteomic assay. PLoS One. 2011;6(10):e26332. doi: 10.1371/journal.pone.0026332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valeri L., VanderWeele T.J. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26(2):e23–e24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 13.Vanderweele T.J. Mediation analysis with multiple versions of the mediator. Epidemiology. 2012;23(3):454–463. doi: 10.1097/EDE.0b013e31824d5fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basisty N., Kale A., Jeon O.H., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1) doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weuve J., Mendes de Leon C.F., Bennett D.A., Dong X., Evans D.A. The red cell distribution width and anemia in association with prevalent dementia. Alzheimer Dis Assoc Disord. 2014;28(2):99–105. doi: 10.1097/WAD.0b013e318299673c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilling L.C., Atkins J.L., Duff M.O., et al. Red blood cell distribution width: genetic evidence for aging pathways in 116,666 volunteers. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0185083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alis R., Fuster O., Rivera L., Romagnoli M., Vaya A. Influence of age and gender on red blood cell distribution width. Clin Chem Lab Med. 2015;53(2):e25–e28. doi: 10.1515/cclm-2014-0756. [DOI] [PubMed] [Google Scholar]
- 18.Oh Y., Muller H.L., Lee D.Y., Fielder P.J., Rosenfeld R.G. Characterization of the affinities of insulin-like growth factor (IGF)-binding proteins 1-4 for IGF-I, IGF-II, IGF-I/insulin hybrid, and IGF-I analogs. Endocrinology. 1993;132(3):1337–1344. doi: 10.1210/endo.132.3.7679979. [DOI] [PubMed] [Google Scholar]
- 19.Li P., Sun X., Cai G., Chen X. Insulin-like growth factor system and aging. J Aging Sci. 2017;5(1):1–5. [Google Scholar]
- 20.Li T., Forbes M.E., Fuller G.N., Li J., Yang X., Zhang W. IGFBP2: integrative hub of developmental and oncogenic signaling network. Oncogene. 2020;39(11):2243–2257. doi: 10.1038/s41388-020-1154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huynh H., Zheng J., Umikawa M., et al. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood. 2011;118(12):3236–3243. doi: 10.1182/blood-2011-01-331876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu D., Pawlikowska L., Kanaya A., et al. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the health, aging, and body composition study. J Am Geriatr Soc. 2009;57(7):1213–1218. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grillari J., Hohenwarter O., Grabherr R.M., Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp Gerontol. 2000;35(2):187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang S., Moerman E.J., Jones R.A., Thweatt R., Goldstein S. Characterization of IGFBP-3, PAI-1 and SPARC mRNA expression in senescent fibroblasts. Mech Ageing Dev. 1996;92(2–3):121–132. doi: 10.1016/s0047-6374(96)01814-3. [DOI] [PubMed] [Google Scholar]
- 25.McGrath E.R., Himali J.J., Levy D., et al. Circulating IGFBP-2: a novel biomarker for incident dementia. Ann Clin Transl Neurol. 2019;6(9):1659–1670. doi: 10.1002/acn3.50854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huth C., Bauer A., Zierer A., et al. Biomarker-defined pathways for incident type 2 diabetes and coronary heart disease—A comparison in the MONICA/KORA study. Cardiovasc Diabetol. 2020;19(1):1–14. doi: 10.1186/s12933-020-01003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Beld A.W., Carlson O.D., Doyle M.E., et al. IGFBP-2 and aging: a 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur J Endocrinol. 2019;180(2):109–116. doi: 10.1530/EJE-18-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanety H., Madjar Y., Dagan Y., et al. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77(1):229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 29.van den Beld A.W., Blum W.F., Brugts M.P., Janssen J.A., Grobbee D.E., Lamberts S.W. High IGFBP2 levels are not only associated with a better metabolic risk profile but also with increased mortality in elderly men. Eur J Endocrinol. 2012;167(1):111–117. doi: 10.1530/EJE-12-0160. [DOI] [PubMed] [Google Scholar]
- 30.Fridman A.L., Tainsky M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27(46):5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Diazguerrero N.E., Luna-Lopez A., Gutierrez-Ruiz M.C., Zentella A., Konigsberg M. Susceptibility of DNA to oxidative stressors in young and aging mice. Life Sci. 2005;77(22):2840–2854. doi: 10.1016/j.lfs.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Toussaint O., Medrano E.E., von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 33.Kang H.T., Lee K.B., Kim S.Y., Choi H.R., Park S.C. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One. 2011;6(8):e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres C., Lewis L., Cristofalo V.J. Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. J Cell Physiol. 2006;207(3):845–853. doi: 10.1002/jcp.20630. [DOI] [PubMed] [Google Scholar]
- 35.Forte D., Salvestrini V., Corradi G., et al. The tissue inhibitor of metalloproteinases-1 (TIMP-1) promotes survival and migration of acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling. Oncotarget. 2017;8(2):2261–2274. doi: 10.18632/oncotarget.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71(4):659–672. doi: 10.1007/s00018-013-1457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavusoglu E., Ruwende C., Chopra V., et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006;151(5):1101. doi: 10.1016/j.ahj.2006.02.029. e1-8. [DOI] [PubMed] [Google Scholar]
- 38.Schroen B., Heymans S., Sharma U., et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95(5):515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 39.Shi S., Tian B. Identification of biomarkers associated with progression and prognosis in bladder cancer via co-expression analysis. Cancer Biomark. 2019;24(2):183–193. doi: 10.3233/CBM-181940. [DOI] [PubMed] [Google Scholar]
- 40.Osawa Y., Semba R.D., Fantoni G., et al. Plasma proteomic signature of the risk of developing mobility disability: a 9-year follow-up. Aging Cell. 2020;19(4):e13132. doi: 10.1111/acel.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul A., Belton A., Nag S., Martin I., Grotewiel M.S., Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128(11–12):706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R., Yin C., Li X.X., et al. Reduced SOD2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging (Albany NY) 2016;8(6):1184–1200. doi: 10.18632/aging.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velarde M.C., Flynn J.M., Day N.U., Melov S., Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY) 2012;4(1):3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barcena de Arellano M.L., Pozdniakova S., Kuhl A.A., Baczko I., Ladilov Y., Regitz-Zagrosek V. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging (Albany NY) 2019;11(7):1918–1933. doi: 10.18632/aging.101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn J.M., Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxena R., Suneja S., Saxena R., Sharma D., Lal A.M. Systemic inflammation, oxidative stress and apolipoprotein B/A1 ratio in active psoriasis: bridging an apparent paradox. Int J Res Dermatol. 2015;1(1):10–13. [Google Scholar]
- 47.Sierra-Johnson J., Fisher R.M., Romero-Corral A., et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J. 2009;30(6):710–717. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schraufstatter I.U., Khaldoyanidi S.K., DiScipio R.G. Complement activation in the context of stem cells and tissue repair. World J Stem Cells. 2015;7(8):1090–1108. doi: 10.4252/wjsc.v7.i8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mocco J., Mack W.J., Ducruet A.F., et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99(2):209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 50.Bonifati D.M., Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44(5):999–1010. doi: 10.1016/j.molimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Kolev M.V., Ruseva M.M., Harris C.L., Morgan B.P., Donev R.M. Implication of complement system and its regulators in Alzheimer's disease. Curr Neuropharmacol. 2009;7(1):1–8. doi: 10.2174/157015909787602805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D.F., Fan Y., Xu M., et al. Complement C7 is a novel risk gene for Alzheimer's disease in Han Chinese. Natl Sci Rev. 2019;6(2):257–274. doi: 10.1093/nsr/nwy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricklin D., Lambris J.D. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190(8):3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blom A.M. The role of complement inhibitors beyond controlling inflammation. J Intern Med. 2017;282(2):116–128. doi: 10.1111/joim.12606. [DOI] [PubMed] [Google Scholar]
- 55.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang D., Sun D., Shi C., et al. Activation of epidermal growth factor receptor signaling mediates cellular senescence induced by certain pro-inflammatory cytokines. Aging Cell. 2020;19(5):e13145. doi: 10.1111/acel.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spadaro O., Goldberg E.L., Camell C.D., et al. Growth Hormone Receptor Deficiency Protects against Age-Related NLRP3 Inflammasome Activation and Immune Senescence. Cell Rep. 2016;14(7):1571–1580. doi: 10.1016/j.celrep.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen-Plotkin A.S., Hu W.T., Siderowf A., et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69(4):655–663. doi: 10.1002/ana.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox A.J., Hugenschmidt C.E., Raffield L.M., et al. Heritability and genetic association analysis of cognition in the diabetes heart study. Neurobiol Aging. 2014;35(8):1958. doi: 10.1016/j.neurobiolaging.2014.03.005. e3- e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Q.W., Wang C., Zhou Y., et al. Plasma epidermal growth factor decreased in the early stage of Parkinson's disease. Aging Dis. 2015;6(3):168–173. doi: 10.14336/AD.2014.0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otnaess M.K., Djurovic S., Rimol L.M., et al. Evidence for a possible association of neurotrophin receptor (NTRK-3) gene polymorphisms with hippocampal function and schizophrenia. Neurobiol Dis. 2009;34(3):518–524. doi: 10.1016/j.nbd.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Pellecchia M.T., Santangelo G., Picillo M., et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson's disease patients. J Neurol. 2013;260(2):438–444. doi: 10.1007/s00415-012-6648-6. [DOI] [PubMed] [Google Scholar]
- 63.Shang Z., Lv H., Zhang M., et al. Genome-wide haplotype association study identify TNFRSF1A, CASP7, LRP1B, CDH1 and TG genes associated with Alzheimer's disease in Caribbean Hispanic individuals. Oncotarget. 2015;6(40):42504–42514. doi: 10.18632/oncotarget.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinohara M., Tachibana M., Kanekiyo T., Bu G. Role of LRP1 in the pathogenesis of Alzheimer's disease: evidence from clinical and preclinical studies. J Lipid Res. 2017;58(7):1267–1281. doi: 10.1194/jlr.R075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu H.E., Teixeira A.L., Barroso L., et al. Epidermal growth factor and fibroblast growth factor-2 circulating levels in elderly with major depressive disorder. Psychiatry Res. 2019;272:141–143. doi: 10.1016/j.psychres.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 66.Donahue J.E., Flaherty S.L., Johanson C.E., et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112(4):405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 67.Seok H., Lee M., Shin E., et al. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep. 2019;9(1):4414. doi: 10.1038/s41598-019-40736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Truong K.L., Schlickeiser S., Vogt K., et al. Killer-like receptors and GPR56 progressive expression defines cytokine production of human CD4(+) memory T cells. Nat Commun. 2019;10(1):2263. doi: 10.1038/s41467-019-10018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freud A.G., Keller K.A., Scoville S.D., et al. NKp80 defines a critical step during human natural killer cell development. Cell Rep. 2016;16(2):379–391. doi: 10.1016/j.celrep.2016.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arimura K., Ago T., Kamouchi M., et al. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9(1):1–9. doi: 10.2174/156720212799297100. [DOI] [PubMed] [Google Scholar]
- 71.Ishitsuka K., Ago T., Arimura K., et al. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83(3):352–359. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Wang L., Huang Z., Huang W., et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci Rep. 2017;8:45917. doi: 10.1038/srep45917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moses E., Johnson M., East C., et al. OS077. The chromosome 2q22 preeclampsia susceptibility locus reveals shared novel risk factors for CVD. Pregnancy Hypertens. 2012;2(3):219–220. doi: 10.1016/j.preghy.2012.04.078. [DOI] [PubMed] [Google Scholar]
- 74.Pagani S., Bozzola E., Strisciuglio C., et al. Growth hormone receptor gene expression increase reflects nutritional status improvement in patients affected by crohn's disease. Front Pediatr. 2018;6:338. doi: 10.3389/fped.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peraldi M.N., Berrou J., Dulphy N., et al. Oxidative stress mediates a reduced expression of the activating receptor NKG2D in NK cells from end-stage renal disease patients. J Immunol. 2009;182(3):1696–1705. doi: 10.4049/jimmunol.182.3.1696. [DOI] [PubMed] [Google Scholar]
- 76.Amrhein V., Greenland S., McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305–307. doi: 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- 77.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.