Abstract
Objective
We report a case of pituitary metastasis (PM) presenting with acute anterior and posterior pituitary dysfunction following a two-decade-long oncologic course marked by disease progression.
Case Report
An elderly woman with a history of stage IIA invasive ductal carcinoma of the breast presented with confusion. Her laboratory evaluation was significant for panhypopituitarism and central diabetes insipidus, and magnetic resonance imaging findings were suggestive of PM. She was treated with hormone replacement, resulting in the reversal of her metabolic and cognitive derangements.
Discussion
PM is a rare complication of advanced malignancy. Although several malignancies may spread to the pituitary, the most common are breast cancer in women and lung cancer in men. Unlike pituitary adenomas, which predominantly involve the anterior pituitary, PM has a predilection for the posterior lobe and infundibulum due to direct access via systemic circulation. The clinical presentation of PM depends on the size of the metastatic deposit and other structures involved in the vicinity of the sella. Magnetic resonance imaging with gadolinium is the gold standard for the evaluation of sellar masses. The diagnosis of PM involves a thorough history, physical examination, biochemical evaluation of the hypothalamic-pituitary axis, and imaging studies.
Conclusion
Metastatic involvement of the pituitary is a rare condition seen in <2% of resected pituitary masses. The clinical presentation is heterogeneous and can include headache, visual impairment, and panhypopituitarism. Unfortunately, the presence of PM portends a poor prognosis, and the median survival rate after diagnosis is 6 to 13.6 months.
Key words: pituitary metastasis, panhypopituitarism, central diabetes insipidus (DI), metastasis, pituitary mass
Abbreviations: DI, diabetes insipidus; PM, pituitary metastasis
Introduction
Metastatic involvement of the pituitary gland and infundibular stalk is rare, accounting for <2% of all diagnosed pituitary masses. Although spread from several organs has been reported, breast and lung cancers are the most common primary tumors that metastasize to the pituitary gland. The clinical presentation varies and can include headache, visual impairment, diabetes insipidus (DI), and panhypopituitarism. The posterior lobe of the pituitary is more susceptible to metastatic involvement due to its direct blood supply from the systemic circulation, whereas the anterior lobe is relatively protected by the hypophyseal portal system. Additionally, due to its smaller size, the posterior lobe is more likely to be completely damaged by small tumor deposits, leading to an earlier dysfunction.
Case Report
An 81-year-old woman with a history of stage IIA (T2N0Mx) invasive ductal carcinoma of the left breast who was treated with left breast lumpectomy and axillary lymph node dissection in 1999, mastectomy for local recurrence in 2009, and palliative chemotherapy for subsequent pulmonary metastases, presented to the emergency room in January 2020 with 2 weeks of confusion. Laboratory results on admission were notable for hypernatremia with inappropriately low urine osmolality, consistent with DI. Pituitary evaluation further revealed secondary adrenal insufficiency, central hypothyroidism, hypogonadotropic hypogonadism, and mildly elevated prolactin (Table 1). A computed tomography scan of the brain showed two lytic lesions in the right occipital bone and vertex. Magnetic resonance imaging of the brain with and without gadolinium (Fig. 1 and 2) revealed a 9 × 10-mm, well-circumscribed, heterogeneously enhancing sellar mass with suprasellar extension, involving the infundibulum and hypothalamus, and exerting mass effect on the optic chiasm. The constriction of the mass at the diaphragma sellae without sellar expansion, leading to its dumbbell shape, was indicative of its rapid growth, and its heterogeneous enhancement likely represented internal hemorrhage. In the presence of co-existing panhypopituitarism with DI and calvarial lytic lesions, these findings were highly suggestive of pituitary metastasis (PM) from her widely metastatic breast cancer. During her hospitalization, the patient was treated with hydrocortisone, levothyroxine, and desmopressin, resulting in the normalization of her serum sodium and rapid improvement in her mental status. Following discharge, she completed fractionated stereotactic radiosurgery (3 fractions, 18 Gy total) to her pituitary lesion in March 2020. Unfortunately, she died in January 2021 during hospitalization for dyspnea from malignant pleural effusion.
Table 1.
Laboratory Tests at Initial Presentation
| Laboratory test | Level measured | Reference range |
|---|---|---|
| TSH | 3.03 U/L | 0.47-6.9 U/L |
| Free T4 | 0.351 ng/dL | 0.75-2 ng/dL |
| FSH | 0.2 mIU/mL | 25.8-134.8 mIU/mL |
| LH | <0.1 mIU/mL | 7.7-58.5 mIU/mL |
| Estradiol | <5 pg/mL | 5-138 pg/mL |
| 7AM ACTH | 3.4 pg/mL | 7.2-63.3 pg/mL |
| 7AM cortisol | 0.6 μg/dL | 6.2-29 μg/dL |
| IGF-1 | 88 ng/mL | 17-193 ng/mL |
| Prolactin | 30.47 mIU/mL | 4.79-23.3 mIU/mL |
| Before desmopressin | ||
| Sodium | 154 mEq/L | 135-145 mEq/L |
| Urine osmolality | 162 mOsm/kg | 50-1200 mOsm/kg |
| After desmopressin | ||
| Sodium | 143 mEq/L | 135-145 mEq/L |
| Urine osmolality | 469 mOsm/kg | 50-1200 mOsm/kg |
Abbreviations: ACTH = adrenocorticotropic hormone; FSH = follicle-stimulating hormone; IGF-1 = insulin-like growth factor 1; LH = luteinizing hormone; TSH = thyroid stimulating hormone; T4 = thyroxine.
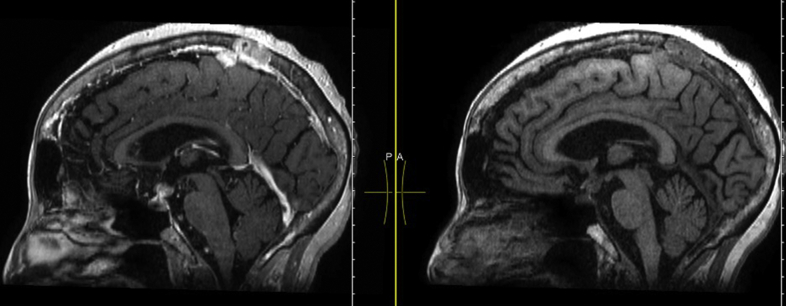
Fig. 1.
Magnetic resonance imaging of the brain/pituitary with and without gadolinium. Sagittal 3-dimensional view T1 before and after contrast showing a well-circumscribed, rim-enhancing mass measuring 9 × 10 mm likely involving the suprasellar cistern and sella. In addition, a 2-mm enhancing lytic skull lesion is noted in the right posterior temporal region along with a left posterior parasagittal lytic skull lesion at the apex consistent with metastases.
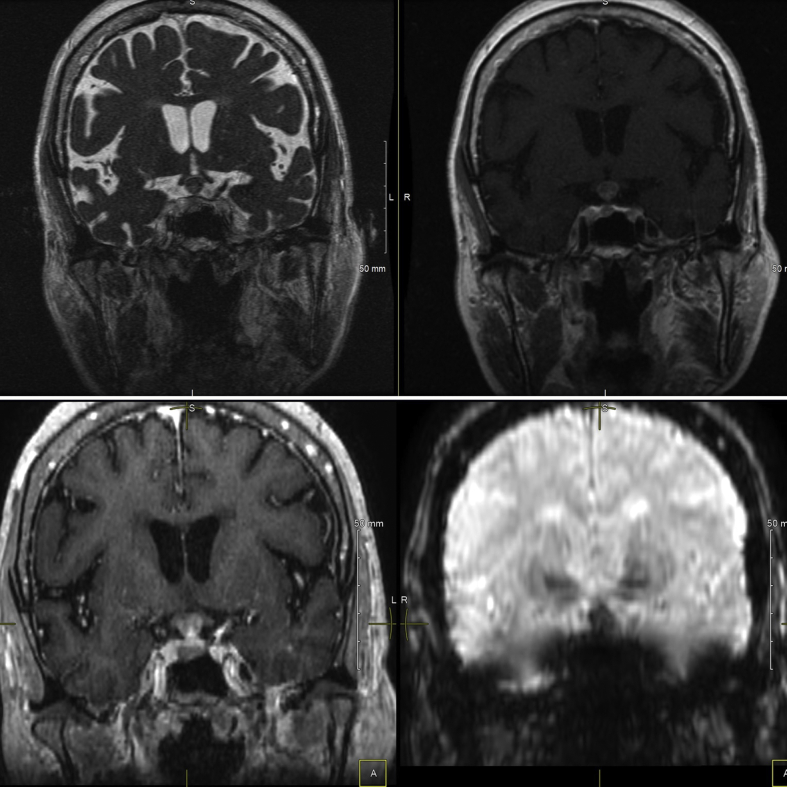
Fig. 2.
Magnetic resonance imaging of the brain/pituitary with and without gadolinium. Coronal view (from top left in the clockwise direction: coronal FIESTA image, coronal T1-weighted image, coronal susceptibility weighted image, coronal 3-dimensional view T1-weighted image) showing rim enhancement and blooming effect (hypointensity that becomes more pronounced on susceptibility-weighted imaging). Constriction at the diaphragma sellae, resulting in a dumbbell-shaped mass, can also be appreciated.
Discussion
PM was first reported by Ludwig Benjamin in 1857.1 Metastatic spread to the pituitary gland remains a rare complication of advanced malignancy. The most common malignancies associated with pituitary spread are breast cancer in women and lung cancer in men. However, thyroid (papillary thyroid cancer), kidney (renal cell cancer), prostate, skin (melanoma), and gastrointestinal tumors are also known to metastasize to the pituitary gland.2 When present, PM usually occurs in older individuals in the sixth and seventh decade of life.3
Several similarities exist between pituitary adenomas and PM, and it is important to distinguish between these two entities. While pituitary adenomas tend to affect the anterior lobe of the gland, PM from a distant tumor usually involves the posterior lobe and infundibulum due to direct access to this region via the systemic circulation and inferior hypophyseal artery.4 The anterior pituitary is relatively protected from distant metastasis due to its portal circulation. The smaller size of the posterior pituitary also makes it more susceptible to complete damage from tumor deposits.5 The pituitary may also be affected via direct expansion of a para-sellar malignancy and, in the case of central nervous system malignancies, leptomeningeal spread.6
As PM more frequently involves the posterior lobe of the pituitary, approximately 30% of patients present with DI, a condition that is rarely associated with pituitary adenomas. Depending on the size of the metastatic deposit, other structures in the vicinity of the sella may be affected, causing a variety of symptoms secondary to invasion or mass effect. These include visual impairment from compression of the optic nerve; ophthalmoplegia from the invasion of the cavernous sinus affecting the third, fourth, and sixth cranial nerves; and disruption of the hypothalamic-pituitary axes from damage to the anterior pituitary.7 While these complications occur at a higher frequency in PM than pituitary adenomas, their presence alone may not help with distinguishing between these two entities.
Magnetic resonance imaging with gadolinium is the gold standard for the evaluation of sellar masses. On imaging, PM to the sella may be distinguished from a pituitary adenoma by invasion into the surrounding structures, constriction of the tumor at the diaphragma sellae leading to a dumbbell shape, or hyperintensity of the optic tracts.8 Metastatic lesions affecting the infundibulum may appear as a nodular or irregular thickening or as an enhancement of the pituitary stalk as well as loss of the posterior pituitary bright spot.9 A fluorodeoxyglucose-positron emission tomography scan is of limited value in identifying central nervous system lesions and is not helpful in evaluating for PM. While pituitary biopsy provides a definitive histologic diagnosis, which can be particularly helpful in a minority of cases where the primary tumor is not immediately apparent, it carries a high risk as PM may bleed more compared with pituitary adenomas.10 Therefore, a pituitary biopsy may be considered in select patients who require surgical intervention nonetheless to relieve compressive symptoms from their PM.
PM may be treated with surgery, radiation therapy, and intrathecal chemotherapy, none of which, alone or in combination, are known to definitively improve life expectancy.11 These interventions are mostly palliative and used for local symptom management but risk further disrupting the pituitary function. In patients with limited metastatic disease, surgery can be considered but it may be challenging due to the high vascularity of the tumor tissue, as previously mentioned, and frequent invasion into the surrounding structures. In contrast, in patients with widespread metastatic disease, local radiation, chemotherapy, or both are preferred.
In summary, the diagnosis of PM can be challenging and involves a thorough history, physical examination, biochemical evaluation, and imaging in the appropriate clinical scenario. Once there is a suspicion for PM, the integrity of the hypothalamic-pituitary axis should be confirmed as soon as possible, and hormone deficiencies, if present, should be replaced immediately. Invasive therapies directed at PM should focus on palliation and reduction of mass effect as they do not seem to impact mortality. Ultimately, life expectancy after a diagnosis of PM depends on the type of the primary tumor and the burden of distant metastases and can vary from 6 to 13.6 months.5
Conclusion
While PM remain rare, their prevalence is expected to increase due to more sensitive biochemical tests and imaging techniques coupled with advances in cancer therapy that have increased life expectancy in these individuals. Our patient was significantly older than the median age reported in the literature. Her presentation with total anterior and posterior pituitary dysfunction after a two-decade-long oncologic course is also unusual. Unfortunately, consistent with the literature, the presence of PM portended a poor prognosis, and our patient died 12 months after this was identified.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Chiang M.F., Brock M., Patt S. Pituitary metastases. Neurochirurgia (Stuttg) 1990;33:127–131. doi: 10.1055/s-2008-1053571. [DOI] [PubMed] [Google Scholar]
- 2.Al-Aridi R., El Sibai K., Fu P., Khan M., Selman W.R., Arafah B.M. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: an analytical review. Pituitary. 2014;17(6):575–587. doi: 10.1007/s11102-013-0542-9. [DOI] [PubMed] [Google Scholar]
- 3.Schill F., Nilsson M., Olsson D.S., et al. Pituitary metastases: a nationwide study on current characteristics with special reference to breast cancer. J Clin Endocrinol Metab. 2019;104(8):3379–3388. doi: 10.1210/jc.2019-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kramer C.K., Ferreira N., Silveiro S.P., Gross J.L., Dora J.M., Azevedo M.J. Pituitary gland metastasis from renal cell carcinoma presented as a non-functioning macroadenoma. Arq Bras Endocrinol Metabol. 2010;54(5):498–501. doi: 10.1590/s0004-27302010000500011. [DOI] [PubMed] [Google Scholar]
- 5.Javanbakht A., D’Apuzzo M., Badie B., Salehian B. Pituitary metastasis: a rare condition. Endocr Connect. 2018;7(10):1049–1057. doi: 10.1530/EC-18-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimon I. Metastatic spread to the pituitary. Neuroendocrinology. 2020;110(9-10):805–808. doi: 10.1159/000506810. [DOI] [PubMed] [Google Scholar]
- 7.Komninos J., Vlassopoulou V., Protopapa D., et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89(2):574–580. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 8.Habu M., Tokimura H., Hirano H., et al. Pituitary metastases: current practice in Japan. J Neurosurg. 2015;123(4):998–1007. doi: 10.3171/2014.12.JNS14870. [DOI] [PubMed] [Google Scholar]
- 9.Lin D.S., Griffith B., Patel S., Rock J., Marin H. Pituitary metastasis from lung carcinoma presenting as a pituitary adenoma. Appl Radiol. 2018;47(7):34–36. [Google Scholar]
- 10.Dutta P., Bhansali A., Shah V.N., et al. Pituitary metastasis as a presenting manifestation of silent systemic malignancy: a retrospective analysis of four cases. Indian J Endocrinol Metab. 2011;15(suppl3):S242–S245. doi: 10.4103/2230-8210.84875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita A., Meyer F.B., Laws E.R., Jr. Symptomatic pituitary metastases. J Neurosurg. 1998;89(1):69–73. doi: 10.3171/jns.1998.89.1.0069. [DOI] [PubMed] [Google Scholar]