Abstract
A 47-year-old woman with systemic lupus erythematosus and previously repaired ascending aortic aneurysm presented with a new ascending aortic aneurysm. Genetic testing revealed a FLNA gene mutation. Her mother subsequently tested positive and was found to have an aneurysm on screening, illustrating the utility of genetic screening for aortopathies. (Level of Difficulty: Intermediate.)
Key Words: aorta, cardiovascular disease, genetic disorders, genetics, valve replacement, vascular disease
Abbreviations and Acronyms: MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus; TAA, thoracic aortic aneurysm
Central Illustration
History of Presentation
A 47-year-old woman presented to cardiology with progressively worsening dyspnea on exertion, occasional pressure-like chest pain occurring every few weeks, and rare slight lightheadedness for 2 months. Blood pressure was 122/66 mm Hg and pulse 60 beats/min. Physical examination revealed a soft grade II/VI mid- to late-peaking crescendo/decrescendo murmur best heard at the left upper sternal border with a prominent and split P2, stable from 6 months earlier.
Learning Objectives
-
•
To appropriately use genetic screening in evaluating patients with aortopathy so that focused imaging, surveillance, and family screening can be provided.
-
•
To describe the FNLA gene implications in health and disease to appropriately stratify risk.
-
•
To consider screening protocols of vascular territories based on cause of disease for early detection of aneurysms.
Past Medical History
The patient has a history of systemic lupus erythematosus (SLE) that manifested at age 31 years with arthralgias, immune thrombocytopenia, pleuritis, pericarditis, and a peak antinuclear antibody titer of 1:160. She also has a diagnosis of idiopathic thrombocytopenic purpura. She had a history of a patent ductus arteriosus ligation at age 5, and a second trimester miscarriage.
At age 31 years, 5 months after her SLE diagnosis, she was diagnosed with a large symptomatic ascending aortic aneurysm surgically repaired with a bioprosthetic aortic valve replacement. Replacement of the proximal arch under deep hypothermia was complicated by brain stem stroke, although visual and swallowing deficits improved over time. Annual surveillance included chest computed tomography angiography, and echocardiography. Surveillance imaging at age 40 years showed prior aortic valve and aortic arch replacement, with the aorta measuring 24 mm at the sinotubular junction, 31 mm at the arch, and 35 mm at the descending thoracic aorta.
Differential Diagnosis
The patient’s new symptoms were concerning for bioprosthetic aortic stenosis, recurrence of her ascending aortic aneurysm, or a new aneurysm.
Investigations
Transthoracic echocardiogram showed ejection fraction of 55%.
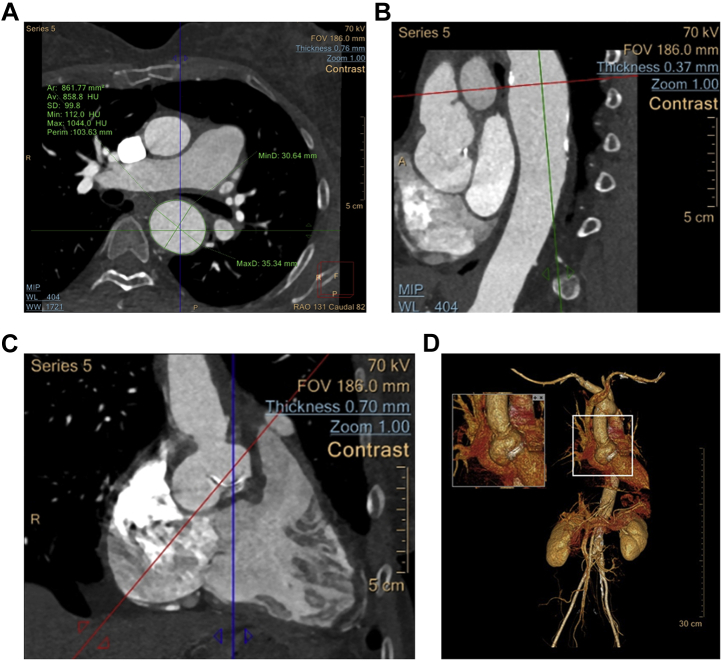
Evaluation with computed tomography angiogram demonstrated diffuse aneurysmal dilatation at the sinus of Valsalva measuring 34 × 21 mm most significant in the noncoronary cusp. The ascending aorta measured at the level of the right pulmonary artery approximately 26 × 25 mm and the descending thoracic aorta was aneurysmal, measuring up to 35 × 31 mm (Figure 1).
Figure 1.
Computed Tomography With Intravenous Contrast Visualizing the Thoracic Aortic Aneurysm
(A) Caudal, (B) sagittal, and (C) coronal views with measurements and scale as indicated. (D) A 3-dimensional reconstruction based on computed tomography of the patient’s aorta and surrounding structures with the sinus of Valsalva highlighted.
Cardiac catheterization demonstrated severe bioprosthetic aortic stenosis and dilated aortic noncoronary cusp.
Management
Because of her progressive aneurysms, our patient was not a candidate for transaortic valve replacement of her stenotic bioprosthetic aortic valve. She underwent surgical bio-Bentall replacement of her aortic root and ascending aorta with Valsalva aneurysm resection. She received a bioprosthetic 25 Inspiris pericardial tissue valve, chosen due to her history of immune thrombocytopenia.
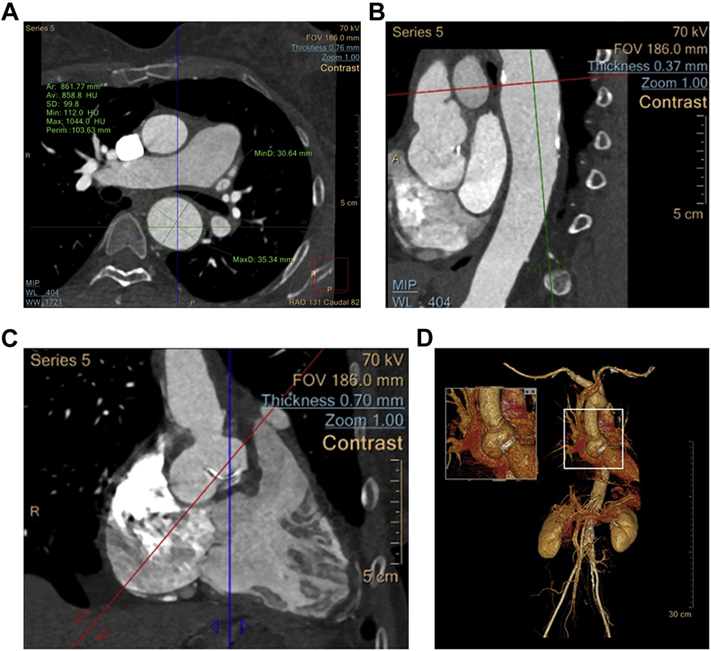
Initially, her aneurysms were attributed to her connective tissue disease and genetic aortopathy screening was not done. With this subsequent aneurysm, the patient asked about her daughter’s risk, prompting this assessment. Next-generation sequencing revealed an uncommon pathogenic heterozygous frameshift loss-of-function mutation of the FLNA gene (Coding DNA c.146dupT, variant Thr50HisfsX56). Family screening showed that her mother was also positive for this mutation and also has a significant ascending thoracic aortic aneurysm (TAA). Her father and her daughter are gene-negative, demonstrated in the pedigree in Figure 2. There was an MYLK variant of uncertain clinical significance (variant Pro1191Leu).
Figure 2.
Pedigree of FLNA Gene Positivity in This Patient’s Family
Maternal grandfather died at age 87 years of hip fracture. Maternal grandmother died at age 92 years of stomach cancer. Maternal aunt at age 73 years undergoing screening and has 3 daughters in their 50s.
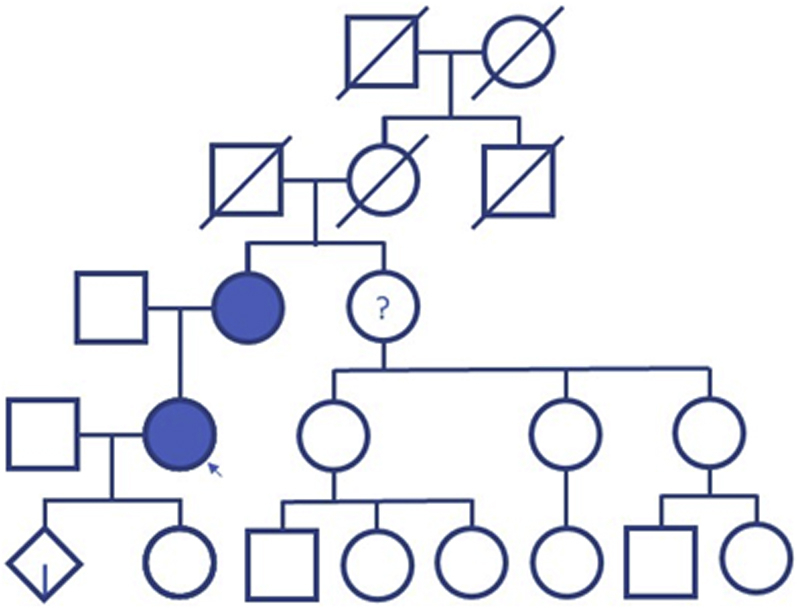
Screening brain magnetic resonance imaging (MRI) showed moderate bilateral white matter changes that may be microvascular and likely occurred at the time of her hemiarch replacement and ependymal nodularity involving the lateral ventricles (Figure 3).
Figure 3.
Magnetic Resonance Imaging
Magnetic resonance imaging of the brain revealing ependymal nodularity of the lateral ventricles (blue arrows).
Discussion
The FLNA gene codes for the protein filamin A, which contributes to cytoskeleton structures and extracellular matrix organization. Filamin A has numerous functions in skeletal and brain development, formation of heart tissue and blood vessels, blood clotting, skin elasticity, maintenance of lung tissue, and function of the digestive system. Many FLNA gene mutations are associated with various developmental dysplasias.1
More than 150 FLNA gene mutations cause an X-linked dominant condition called periventricular heterotopia, a result of failure of neurons to migrate during fetal brain development.2 Our patient’s nonspecific periventricular changes on brain MRI may represent periventricular heterotopia. Periventricular heterotopia usually initially presents as a seizure disorder in the teenage years. Our patient had neurologic symptoms of visual and swallowing deficits that were related to her initial surgical complications, but was otherwise neurologically asymptomatic.
Patients with the periventricular heterotopia phenotype also present with hyperextensible joints, skin laxity, and high risk of thoracic aortic disease. Our patient had a Breighton score of 8 of 9 for hyper-extensibility. It mainly affects female patients, who have a high rate of male fetus miscarriage.3 This suggests that affected males die prenatally. The sex of our patient’s miscarried fetus and cause of miscarriage are unknown.
Renard et al4 identified 30 genes with evidence of association with TAA, and categorized the FLNA gene mutation as a Category B (moderate, limited evidence), suggesting genetic testing of variants may determine cause of TAA. Prior studies of genetic testing in patients with TAA demonstrates a high yield of up to 22.5% with various genetic mutations,5 making a strong case for the routine use of genetic screening in these patients. There is variation in phenotypic expression of FLNA gene mutations. Nearly half of TAAs associated with FLNA gene mutations occur at the sinus of Valsalva and are often associated with patent ductus arteriosus.6 There is also a high prevalence of valvular disease,7 particularly myxomatous dystrophy.8 The pathology of our patient’s native valve is unknown. Although TAAs are well-reported, cerebrovascular disease is not. The frequency and extent of screening has not been established.
To our knowledge, this is the first documented case of the FLNA gene mutation and related aneurysm in a patient with lupus. Genetic screening in this patient was delayed due to the assumption of her aneurysm to be a presentation of her underlying connective tissue disease. Upon the discovery of the same mutation and a significant thoracic aneurysm in her mother, it is now convincing that the gene mutation was the true cause of her aneurysms. With genetic screening, we were able to detect and prevent complications of an aneurysm in the patient’s mother as well.
Follow-Up
It has been 2 years since her surgery. She continues to be followed up closely and has been undergoing annual echocardiography and MRI angiography of the brain and entire aorta to follow for aneurysms, which have thus far not presented.
Conclusions
In this case, genetic screening for our patient led to detection of a TAA in her mother. This case builds a strong argument for a universal approach to genetic screening of patients with aneurysms, including those with alternative explanations for etiology, and further screening of their family members. Recognition may lead to prevention of complications and better serve patients and their family members with appropriate screening and precautionary measures.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.NIH U.S. National Library of Medicine Genetics Home Reference. FLNA Gene. https://medlineplus.gov/genetics/gene/flna/#resources
- 2.NIH U.S. National Library of Medicine Genetics Home Reference. Periventricular heterotopia. https://medlineplus.gov/genetics/condition/periventricular-heterotopia/#inheritance
- 3.Karimi A., Milewicz D.M. Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol. 2016;32:26–34. doi: 10.1016/j.cjca.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renard M., Francis C., Ghosh R., et al. Clinical validity of genes for heritable thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2018;72:605–615. doi: 10.1016/j.jacc.2018.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziganshin B.A., Bailey A.E., Coons C., et al. Routine genetic testing for thoracic aortic aneurysm and dissection in a clinical setting. Ann Thorac Surg. 2015;100:1604–1611. doi: 10.1016/j.athoracsur.2015.04.106. [DOI] [PubMed] [Google Scholar]
- 6.Chen M.H., Choudhury S., Hirata M., Khalsa S., Chang B., Walsh C.A. Thoracic aortic aneurysm in patients with loss of function Filamin A mutations: clinical characterization, genetics, and recommendations. Am J Med Genet A. 2018;176:337–350. doi: 10.1002/ajmg.a.38580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Tourneau T., Le Scouarnec S., Cueff C., et al. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J. 2018;39:1269–1277. doi: 10.1093/eurheartj/ehx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyndt F., Gueffet J.P., Probst V., et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115:40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]