Abstract
Response evaluation criteria in solid tumours (RECIST) v1.1 are currently the reference standard for evaluating efficacy of therapies in patients with solid tumours who are included in clinical trials, and they are widely used and accepted by regulatory agencies. This expert statement discusses the principles underlying RECIST, as well as their reproducibility and limitations. While the RECIST framework may not be perfect, the scientific bases for the anticancer drugs that have been approved using a RECIST-based surrogate endpoint remain valid. Importantly, changes in measurement have to meet thresholds defined by RECIST for response classification within thus partly circumventing the problems of measurement variability. The RECIST framework also applies to clinical patients in individual settings even though the relationship between tumour size changes and outcome from cohort studies is not necessarily translatable to individual cases. As reproducibility of RECIST measurements is impacted by reader experience, choice of target lesions and detection/interpretation of new lesions, it can result in patients changing response categories when measurements are near threshold values or if new lesions are missed or incorrectly interpreted. There are several situations where RECIST will fail to evaluate treatment-induced changes correctly; knowledge and understanding of these is crucial for correct interpretation. Also, some patterns of response/progression cannot be correctly documented by RECIST, particularly in relation to organ-site (e.g. bone without associated soft-tissue lesion) and treatment type (e.g. focal therapies). These require specialist reader experience and communication with oncologists to determine the actual impact of the therapy and best evaluation strategy. In such situations, alternative imaging markers for tumour response may be used but the sources of variability of individual imaging techniques need to be known and accounted for. Communication between imaging experts and oncologists regarding the level of confidence in a biomarker is essential for the correct interpretation of a biomarker and its application to clinical decision-making. Though measurement automation is desirable and potentially reduces the variability of results, associated technical difficulties must be overcome, and human adjudications may be required.
Keywords: tumour, biomarker, imaging, response, RECIST
Introduction
Imaging plays a major role in the evaluation of tumour response to cancer treatments. It provides an objective in-vivo measurement of tumour burden, and helps oncologists determine whether a treatment should be pursued, interrupted or adapted.
Response evaluation criteria in solid tumours (RECIST) v1.1 currently is the reference standard for evaluating efficacy of therapies in patients with solid tumours who are included in clinical trials, and it is widely used and accepted by regulatory agencies (1). However, many publications question both the reproducibility and the clinical relevance of RECIST. This paper is an expert statement aiming to answer some of the questions regarding the principles underlying RECIST and its reproducibility compared to other biomarkers, as well as the limitations to its application and continued role in an era where other biomarkers exist that are more explicitly geared towards tumour-specific properties.
How Were RECIST Thresholds Established?
RECIST has instituted several overarching principles underpinning its approach to tumour response evaluation. Primarily, RECIST defines which lesions are measurable in a reliable manner. Among these, it defines a maximal number of lesions (‘target lesions’) to be measured to yield a quantitative value representative of tumour burden. The remainder are considered ‘non-target lesions’ and are evaluated qualitatively. On follow-up scans, new lesions indicate progression ( Table 1 ). The threshold for response is defined as a decrease of at least 30% of sum of diameters (SOD) of target lesions compared to baseline, AND no progression of non-target lesions AND no new lesions. The threshold for progressive disease (PD) is defined as an increase of at least 20% of SOD of target lesions compared to nadir AND/OR unequivocal progression of non-target lesions AND/OR appearance of new lesions.
Table 1.
RECIST categories of response.
| Overall Response | Target Lesions | Non Target Lesions | New Lesions |
|---|---|---|---|
| Definition | •Lesions with longestdiameter≥10 mm and limits that are sufficiently well defined for their measurement to be considered reliable •Lymph nodes: measurement of short axis, target lesion if short-axis measures≥15 mm • Maximum number of selected target lesions 5/patient and 2/organ |
•Lesions that are too small (< 10 mm) •Lesions for which measurement is considered unreliable as their limits are difficult to define (bone or leptomeningeal lesions, ascites, pleural or pericardial effusion, lymphangitic carcinomatosis etc.) •Measurable lesions not selected as target lesions •Lymph nodes: measurement of short axis, non-target lesion if 10 mm ≤ short-axis diameter < 15 mm •Levels of tumour markers > normal (if relevant and predefined) |
|
| Complete response (CR) | • Disappearance of all target lesions and all nodes have short axis < 10 mm | • Disappearance of all non-target lesions and normalisation of tumour marker levels | • No |
| Partial response (PR) | •≥ 30% decrease in the sum of target lesions taking as reference the baseline sum | •No progression | • No |
| Stable disease (SD) | •Neither response nor progression | • Persistence of one or more non-target lesions and/or tumour marker levels > normal |
• No |
| Progressive disease (PD): response is PD if at least one category of lesions meets progression criteria | •≥ 20% increase in the sum of target lesions taking as reference the smallest sum measured during follow-up (nadir) and ≥ 5 mm in absolute value | • ‘Unequivocal’ progression (assessed qualitatively) in lesion size (an increase in size of a single lesion is not sufficient) | • Yes [appearance of new unequivocally metastatic lesion(s)] |
The first publication addressing thresholds for determining treatment efficacy was published by Moertel and Hanley in 1976 (2). In this study, 16 observers were asked to measure by clinical examination using a calliper the diameters of solid spheres of variable sizes arranged randomly underneath a mattress. Authors suggested the product of two diameters should be used, as this would be more reliable if lesions were not spherical. For this ‘clinical’ estimate, a 50% reduction in the product of two diameters was shown to have an acceptable measurement error estimated between 7-8%. Interestingly, the authors specifically stated that “the purpose was not to predict long-term efficacy but to determine what change in bidimensional size could be confidently considered a change”. Progression, on the other hand, was defined as an increase in the product of diameters ≥ 25%, but the authors could not justify this cut-off, other than by specifying it “should not necessarily be regarded as influencing the management of the patient”.
In 1979, the World Health Organization (WHO) provided recommendations for the evaluation of cancer treatments in clinical trials on imaging. Criteria were based not only on the bidimensional measurement of lesions on clinical examination, but also CT or standard radiography (3), transposing results of Moertel and Hanley’s study and setting cut-offs for definition of response to -50% and of progression to +25%. However, many technical aspects were not detailed, such as the number of lesions to be measured or what constituted a measurable lesion.
In 2000, a working group of European, American and Canadian cancer research organizations (EORTC, NCI, NCIC) defined the Response Evaluation Criteria In Solid Tumours – RECIST (4). They used data from over 4,600 patients enrolled in 14 clinical trials to formulate criteria based on imaging. RECIST used unidimensional measurement of lesions, justified by an extensive comparison of methods of measurement (1D vs. 2D) (5). Moreover, this working group specified conditions of measurement, number of lesions, and detailed how to document progression. Regarding cut-off values for response and progression, the -50% value for response for bidimensional measurement was altered to -30% for unidimensional measurements, and the +25% value for progression for bidimensional measurement was altered to +20% for unidimensional measurements ( Table 2 ).
Table 2.
Relationship between diameter and corresponding volume.
| Diameter (“long axis”) | Percentage of variation | Corresponding volume | Percentage of variation |
|---|---|---|---|
| 20 mm | 4.2 cm3 | ||
| 26 mm |
 +30% +30% |
9.2 cm3 |
 +120% +120% |
| 34 mm |
 +30% +30% |
20.6 cm3 |
 + 120% + 120% |
| 27 mm |
 -20% -20% |
10.3 cm3 |
 -50% -50% |
Repeated measurements are given for a theoretical lesion including diameter measured in a single dimension (long axis), percent changes between measurements, and the corresponding volume assuming the lesion is a sphere and percent changes in volume.
RECIST was then revised in 2009 (version 1.1) (1), introducing specific rules for measurement of small axis of lymph nodes and reducing the number of target lesions to five per patient. This new version was also based on data analysis, including a literature review and a simulation using a database of over 6,500 patients and 18,000 lesions. The number of target lesions for example, was chosen by determining the minimum number for which response rates and time to progression were not altered from RECIST 1.0 results (6, 7).
Statement #1
RECIST thresholds were chosen to produce a comparable classification of patients in a given category of response when comparing trials or even when comparing patients, taking into account tumour measurement variability.
Do RECIST Categories Predict Outcome?
RECIST criteria were originally tested and validated to provide an objective and reproducible assessment of treatment effect in cancer patients, without any references to patient outcome (8). Yet it seems intuitive that when a tumour decreases in size, a patient will have a better outcome, and vice versa. There is evidence to support this, including some large studies, which pool data from various trials. In over 500 patients with metastatic colorectal cancer treated with combination chemotherapy, a decrease in size resulted in a decreased hazard ratio for overall survival (OS) (9). In a meta-analysis of 24 phase I trials, a linear relationship was shown between change in tumour size and survival (10). In a pooled analysis of over 2,700 patients with metastatic renal cell carcinoma treated with anti-angiogenic agents, tumour shrinkage of ≥ 30% resulted in improved OS and progression-free survival (PFS) (11). In addition, the authors demonstrated that tumour shrinkage between 60% and 100% at 6-month follow-up represented an independent prognostic factor for OS. Litière et al. also demonstrated in an even larger pooled analysis of over 23,000 patients treated with targeted agents, chemotherapy or a combination thereof (12), that a decrease in tumour size was consistently associated with a lower hazard ratio, while an increase in size was associated with a higher hazard ratio.
Tumour response according to RECIST can only be quantified by a decrease in size or number of target lesions, as non-target lesions are not taken into consideration for partial response (PR). Regarding progression however, it is important to consider non-target lesions, as unequivocal progression of non-target lesions or emergence of new lesions defines tumour progression. In over 3,700 patients from 13 trials in the RECIST trial database, the presence of new lesions and progression of non-target lesions were most strongly associated with worse OS (hazard ratios range 1.5–2.3) regardless of tumour type, whereas percentage tumour growth in target lesions contributed less in a multivariate model of OS (13).
Finally, in two separate studies (14, 15), An et al. compared the predictive ability of RECIST categories vs. longitudinal tumour measurement–based continuous metrics and alternative categorical response metrics such as slope (absolute change in tumour size) and percent change (relative change in tumour size) to predict OS. The databases consisted respectively of almost 2,100 patients from 13 trials and over 1,500 patients from 3 trials with breast cancer, non–small cell lung cancer (NSCLC) or colorectal cancer. Although there seemed to be a slightly better performance for continuous variables, it was not statistically significant, which led the authors to conclude there was no evidence that growth rate or a continuous evaluation of percent change would improve prediction of outcome. However, it may be noted that timing of evaluations, particularly when considering non-continuous variables, may have an impact on their performance and results.
Statement #2
Tumour size changes correlate to outcome at a statistical (cohort) level.
How Reproducible Is RECIST?
When considering whether RECIST evaluates tumour response correctly, metrology principles guide us to consider two aspects (16): is the measurement “true” (when compared to a “real” value, which defines its accuracy), and is the measurement “precise” (i.e. repeatable and reproducible)?
Assessing accuracy of change in size measurements would require obtaining “true” values of change in size. As it is not possible to surgically excise all tumours for comparison with imaging, and often inaccurate to compare ex vivo with in vivo measurements, the true value of an imaging biomarker must be derived from data obtained through a combination of primary tumour excision and phantom studies.
Precision refers to the variability of the measurement process and can be evaluated by repeatability (when measurement conditions do not change) and reproducibility (when measurement conditions vary). The precision of RECIST and of response categories has been studied extensively. Table 3 lists the documented reproducibility of RECIST and factors that may impact it. Overall, SOD reproducibility is in the order of +/-20% in multi-observer studies, and +/-10% in single observer studies (17). Important factors associated with RECIST measurement reproducibility are the choice and number of target lesions ( Figure 1 ) and the experience of the reader(s). Where multiple target lesions are used, their selection affects variability: agreement ranges from 0.58 when different targets are chosen to 0.97 when the same targets are used (23). Variability also increases with the number of target lesions selected. For this reason, it has been recommended that a central review in clinical trials should include two readers and one adjudicator (29). Finally, reader experience has major impact on variability, from the selection of the correct reference examination (baseline vs. previous CT) to the detection and proper interpretation of new lesions (20, 21, 25, 26). Measurements of well-demarcated lesions and bigger lesions are also more reproducible (17–19), which vindicates RECIST recommendations for the choice of target lesions.
Table 3.
RECIST reproducibility and factors impacting it.
| Biomarker | Reproducibility | Factors impacting reproducibility | ||
|---|---|---|---|---|
| 95% limits of agreement | Kappa | Other | ||
| RECIST (measurement) CT (size) |
Per lesion - Intra-obs: -18% to 16% - Inter-obs: -22% to 25% (1 (17) Per sum of diameters - Intra-obs: -10% to 13% - Inter-obs: -20% to 20% Interval change in tumour burden (% change between time points) - -31% to 30% Repeatability (same image on repeat CT taken within 15 minutes) - -4% to +4% (18) |
With target lesion selection - Intra-obs: 0.957 (19) - Inter-obs 0.954 (19) Target response classification - Inter-obs: 0.48 (20) to 0.66 (21) Non-target response classification - Inter-obs: 0.58 (20) |
Lesion size ICC (22) -Pre-treatment: 0.72 -Post-treatment: 0.85 -Interval change: 0.70 |
-Selection of target lesions differs in 21 to 33% (17, 23, 24) -Practical training (ref 40)/expertise (21) -Same observer (17, 20) -Well delineated lesions (17, 19) -Lesions size (greater variability for smaller lesions) (18, 19) -Adjudication could reduce easily avoidable inconsistencies (20, 25) |
| RECIST (overall response) | With target lesion selection - Inter-obs: 0.97 (23) Without target lesion selection - Inter-obs: 0.51 (20), 0.53 (24, 26) to 0.58 (23) |
-30% of patients classified differently in a cohort of 39 pts with 2 readers (26) | -Arbitrary nature of CR/PR/SD/PD categories (10) -Inconsistencies mainly due to interpretation of new lesions (20, 26) -Choice of target lesions |
|
| 3D measurement | - Intra-obs: 0.4 to 33% according to automated volume measurement method (27) | Whole body volumetry - Inter-obs: 0.95 30) |
-Discordant classification in overall response in 10 to 21% of patients according to automated volume measurement method (27) | -Time consuming (28) -Do not resolve the discrepancies linked to the choice of target lesions (24) |
95% limits of agreement are derived from the Bland-Altman method comparing two measurements of the same variable. Kappa coefficients measure agreement between qualitative observations. ICC measures the reliability of measurements by comparing the variability of different ratings of the same subject to the total variation across all ratings and all subjects.
Intra-obs, intra-observer; Inter-obs, inter-observer; ICC, Intra-class coefficient; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
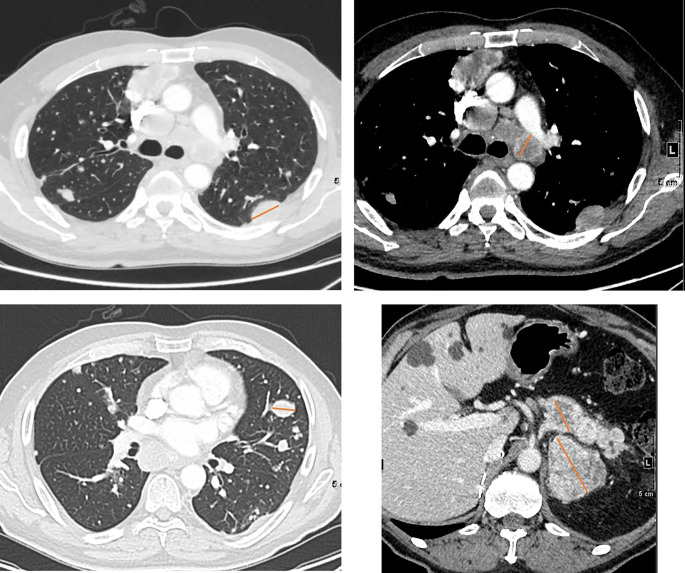
Figure 1.
Selecting target lesions in a 58 yo patient with metastatic renal cell carcinoma. Multiple lung, lymph node, pancreatic and adrenal metastases are present. Lymph nodes should be sampled from different locations where possible. Selection of target lesions at baseline from multiple organ sites is important for response evaluation at a patient level.
Statement #3
RECIST reproducibility is impacted by reader experience, choice of target lesions, lesion characteristics, and detection/interpretation of new lesions. At an individual level, this can result in patients being categorised incorrectly when values of SOD are near thresholds or when new lesions are either missed or incorrectly interpreted.
How Reproducible Are Other Biomarkers?
Table 4 summarises repeatability and reproducibility of some of the other biomarkers suggested or used as alternatives to RECIST for evaluating response. With the abundance of suggested candidate biomarkers in the published literature, the purpose here is not to be comprehensive, but to give a general overview of some of the most frequently explored options for providing a level of comparison with RECIST.
Table 4.
Reproducibility and factors impacting it of other imaging biomarkers.
| Biomarker | Reproducibility | Factors impacting reproducibility | ||
|---|---|---|---|---|
| ICC | Coefficient of Variation | Other | ||
| Metabolic activity (18-FDG PET) Semiquantitative: SUV (SUVmax, SUVmean; SUVpeak), SUL (SULmax, SULmean, SULpeak); MTV, TLG Response criteria: PERCIST (30)/EORTC (31) |
SUVmax (4 observers) (22) - Pre-treatment: 0.93 - Post-treatment: 0.91 - Interval change: 0.94 - SUVmean repeatability (32) - 0.91 (meta-analysis) SUVpeak - -31% to 30% |
SUVmax (4 observers) (22) - Pre-treatment: 6.3% - Post-treatment: 18.4% - Interval change: 16.7% |
Repeatability standard deviation (33) - SUVmax: 1.01 - SUVmean: 0.28 |
Technical factors: Scanner calibration/injected activity calibration (34, 35) Incorrect decay correction (36) Tracer extravasation (37, 38) Residual activity in syringe (34) Synchronization of clocks (34) Biological factors: Blood glucose levels (38) Inflammation (34) Patient preparation (38) Injection-acquisition interval (39, 40) BMI/metabolic syndrome (41) Drug interaction/corticosteroids (38) Physical factors: Acquisition parameters/matrix size (34, 36) Reconstruction algorithm (39, 42, 43) Partial volume effect (44) Normalization factor for SUV (34, 45) Use of contrast agents (34) ROI/VOI definition (39, 42) Semiautomated/manual contouring (46) Movement artifacts/respiratory movements (34) Recovery effect/motion blur (47) Image noise (44, 48) Background activity/visual assessment (42, 49) Lesion size/location (50) |
| Vascularity (DCE MRI) | DCE-MRI ktrans - Intra-obs: 0.98 (51) DCE CT (arterial flow, blood volume, permeability) - Intra-obs: 0.72-0.89 - Inter-obs: 0.70-0.91 (52) DCE and DSC-MRI intersoftware reproducibility ICC 0.31 to 0.58 (53) |
DCE MRI - model-free parameters (ex: AUC60, peak…): 12-24% - modelled parameters (ex: distribution volume, blood flow, mean transit time): 21-29% (54) DSC MRI normalised rCBVmax - repeatability: 50%, - reproducibility: 6% (55) DCE-CT (blood flow, blood volume, mean transit time, permeability) - within subject: 18% to 25%; DCE-MRI (Ktrans, k(ep), v(e)) - within subject 16% to 23% (56), |
- Parameter extraction model (54) - Segmentation: 3D vs 2D regions of interest (52) - Software (53) |
|
| Cellularity (MR) ADC |
ADC mean value - Intra-obs: 0.91 (57) – 0.99 (51) - Inter-obs: 0.92 (57) ROI segmentation method (Inter-obs) - Manual method: 0.69 - Semi-automated volumetric method: 0.96 (58) |
Repeatability - ADC total = 4.8% (57), 7.1% (59) to 13.3% (60) Different post-processing platforms - 2.8% (59) Different sites - multicentric: 9% (61) - ice-water phantom: 1.6% (61) - breast fibroglandular tissue: 7.0% (61) |
Repeatability (single centre) - ≤ ± 0.1x10-3 mm2/s (62) |
- Field homogeneity gradient linearity (63) - QA procedure by trained operators assessing artifacts, fat suppression, and signal-to-noise ratio (57) - Segmentation: 2D vs. 3D, manual vs. semi-automatic (58) - Choice of measurement: mean/min/max/percentiles of ADC (64) - Lesion size (59) |
SUVmax is measured as the maximum single voxel value of SUV, SUVmean is the average value of SUV in all voxels above a threshold, SUVpeak (is the average value of SUV in a region of interest positioned so as to maximize the enclosed average.
SUV, standardized uptake value; SUL, lean body mass corrected SUV; MTV, metabolic tumour volume; TLG, total lesion glycolysis; PERCIST, PET Response Criteria in Solid Tumours; EORTC, European Organization for Research and Treatment of Cancer; wCV, within-subject coefficient of variation; BMI, body mass index; ROI, region of interest; VOI, volume of interest; ICC inter correlation coefficient; DCE dynamic contrast enhanced; DSC-MRI dynamic susceptibility contrast magnetic resonance imaging; ADC, apparent diffusion coefficient; QA quality assurance; 3D, three-dimensional; 2D, bi-dimensional; AUC60, area under the curve at 60s; rCBV, relative cerebral blood volume; Ktrans, transfer constant; k(ep), wash-out transfer constant; v(e), extracellular volume.
A first alternative to measuring a single size dimension as a response biomarker, would be to measure volume of a single or several lesions as an indicator of tumour bulk. This seems particularly important when lesions are irregular in shape, or when they change orientation and are therefore not identically represented on standard axial follow-up scans. Volumetric response on first follow-up CT has been shown to better predict OS than RECIST response (65). Tumour volume response has been utilised in lung (66), cervical (67), and other solid malignancies (68). Despite a trend towards better intra- and inter-observer reproducibility, the routine use of volume has been hampered by the need for manual segmentation, which is user-dependent and time-consuming and does not improve the discrepancies linked to the choice of target lesions (24, 28). Aside from tumour bulk, metabolic activity of tumours through functional imaging (e.g. positron emission tomography - PET)) is highly predictive of response in lymphoma (69), lung cancer (70), and metastatic melanoma (71). Other radioligands are utilised for response or recurrence detection, e.g. 18F-fluoroestradiol (FES) in hormone-dependent breast cancer (72) and 18F- or 68Ga Prostate-Specific Membrane Antigen (PSMA) ligands in prostate cancer (73). Additionally, radiolabelled ligands of various metabolites and biologically active molecules can assess proliferation, hypoxia, angiogenesis, apoptosis and gene transfection (74).While parameters used for the quantification and measurement of tumour metabolism by PET are generally based on semi-quantitative assessments, these can be made relatively reproducible and harmonised throughout the world through standardised imaging protocols and dedicated initiatives promoted by the international scientific societies (75, 76), such as the accreditation program developed by the EANM Research Ltd. (EARL) (34, 77).
Other alternate imaging biomarkers include perfusion and diffusion imaging. As tumours are commonly characterised by neo-angiogenesis, perfusion and permeability derived from dynamic-contrast enhanced studies (e.g. with MR or CT) have been contenders for measuring early response (78), and vascularity can be quantified using most imaging techniques, such as MRI, CT, ultrasound and PET. The utility of biomarkers of vascularity has been demonstrated particularly where anti-angiogenic agents such as bevacizumab have been part of the therapeutic strategy (79). However, their quantitation, which depends on measuring or estimating an arterial input function, is susceptible to large potential variations (80), and the reproducibility of such data is often low, thus limiting their clinical utility (81). Another biomarker reflecting tissue cellularity, the apparent diffusion coefficient (ADC) from DW-MRI, has proven a robust quantitative measure with good repeatability and reproducibility across vendor platforms (82), and has the potential to detect therapeutic response earlier than size measurements. It is increasingly being introduced routinely into scanning protocols, as it does not require injection of an extrinsic contrast agent and is simple and fast to acquire and analyse. Increasing automation with artificial intelligence (AI) systems may aid the translation of biomarkers indicative of tumour characteristics other than bulk into routine clinical workflows. Unfortunately, tightened legal rules are slowing down the process of their adoption (83).
Although historically dependent on imaging, response assessment for malignancies may now also include liquid biopsies [quantification of circulating tumour cells or DNA (CTC, ctDNA)], as well as histological sampling. ctDNA shedding is influenced by the overall tumour burden (cells) and may thus inform the use of imaging in relation to likely tumour size (84), because ctDNA estimations require less workflow and infrastructure than repeated monitoring with imaging. Initial clinical evaluations showed that ctDNA detected response earlier than imaging-based assessment (85). The simplest clinical implementation of ctDNA may be in postoperative monitoring of disease recurrence (86) but even here reproducibility and standardisation issues remain limiting. In one study, ctDNA quantities based on measurement of some target genes (e.g. TERT) were, on average, more than two-fold higher than those of other assays (e.g. ERV3) (87). In another, quantities of cell-free DNA for the different isolation methods for detection of EGFR variants in NSCLC varied between medians of 1.6 ng/mL and 28.1 ng/mL (88). Moreover, concordance between tissue and plasma variant detection for leading platforms has been shown to range from 70% to 90% (89). Thus, ctDNA extraction/isolation methods (87, 88) may need to be standardised before routine clinical use.
Finally, histopathology may also be a method for tumour response evaluation. However, serial histological sampling is not routinely used for response assessment and has thus far shown agreement with imaging-based responses only in a few studies (90). Histopathological evaluation of response is performed usually after neoadjuvant therapy, when the organ is surgically resected. Qualitative or semi-quantitative histopathological evaluation also presents variable reproducibility according to organs, methods and published studies (91–94). Agreement between pathologists yielded kappa values ranging from 0.21 for extent in prostate cancer (92), to 0.49 for multiple well-trained observers in cervical cancer (93), 0.64 for a 5-point tumour regression grade in rectal cancer (90) and 0.83 for a central review in bladder cancer (91). As with macroscopic imaging, reader experience (94), and central review (92) improve reproducibility.
Statement #4:
Alternative biomarkers for tumour response yield reproducibility generally comparable to RECIST. Each technique has its sources of variability, and it is important to understand inherent variability and limitations of individual biomarkers. It is critical that imaging experts communicate their level of confidence in any chosen biomarker.
What Are Common RECIST Limitations?
Challenging Organs: Bone
Bone metastases were considered unmeasurable in the initial RECIST initiative, because of the lack of sensitivity of existing techniques to bone marrow infiltration (4). On CT it is the bone’s osteolytic or osteosclerotic reaction to the presence of tumour, or its response to therapy (flare lesions) that is visualised rather than the tumour itself (95, 96). With the updated RECIST 1.1. version, bone metastases with soft tissue masses ≥10 mm are recognized as measurable target lesions (1). Nevertheless, bone lesions without soft tissue involvement, whether lytic, mixed or sclerotic, remain unmeasurable by RECIST. Since the early 1990s, bone marrow MRI has been shown to be superior to bone scintigraphy and CT for the assessment of bone metastatic disease. Bone marrow replacement by neoplastic foci is detected and quantified on T1-weighted and fat-suppressed T2-weighted MRI sequences (97, 98), more recently complemented with diffusion-weighted imaging (DWI) sequences (99, 100). However, to date, RECIST 1.1 has not validated quantitative bone MRI for tumour response assessment. Positron Emission Tomography Response Criteria in Solid Tumours (PERCIST), introduced in 2009 (30, 101), enables response to be measured in 18F-fluorodeoxyglucose (18F-FDG) avid bone metastatic lesions based on their metabolic activity in the absence of any obvious anatomic changes. Finally, PSMA-PET appears promising for identifying bone marrow invasion due to prostate cancer, regardless of the impact on the bone mineral content (102, 103).
Challenging Diseases: GIST and mCRC
As RECIST is not organ-specific, it might not capture the key parameters that are associated with survival outcomes in certain cancer types, and under certain types of treatment. In gastrointestinal malignancies, the hepatic tumour burden and its response commonly outperform other sites of metastatic disease for survival prediction. A study in metastatic colorectal cancer (mCRC) showed that the depth and uniformity of response in liver metastases represented a highly useful and clinically relevant indicator for therapy monitoring (104). Organ-specific response patterns may also occur under immunotherapy possibly due to varying immune microenvironments in organs or the lymphatic system (105–107). Thus, choice of target lesions would largely impact the response observed according to the organ, as well as the predictive ability of RECIST. In this case also, reader experience and knowledge of the disease is crucial for proper target lesion selection.
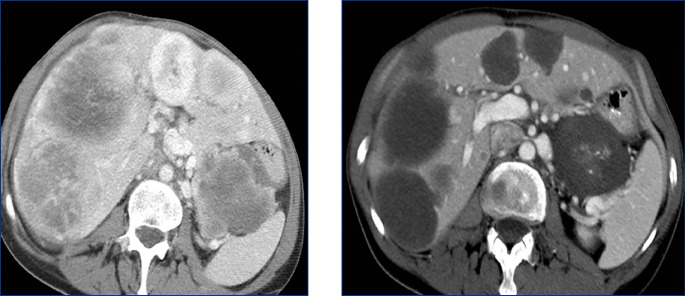
Response to therapy in patients with advanced GIST was drastically improved by the introduction of imatinib, a tyrosine-kinase inhibitor. Imatinib treatment has been shown to induce necrosis with a marked decrease in vascularity of GIST lesions, resulting in a decrease in CT density often before any significant decrease in size is seen, thus leading to underestimation of the initial tumour response (108, 109) ( Figure 2 ). A paradoxical increase in volume is occasionally observed, simulating progression (110). Choi et al. therefore proposed adapted criteria for GIST, combining changes in tumour density on contrast-enhanced CT expressed in Hounsfield units (HU) and/or size to determine tumour response (109): PR is defined as a decrease of ≥10% in the SOD or a decrease of ≥ 15% in tumour density of target lesions, whereas PD is defined as a ≥ 10% increase in size and not meeting the PR criteria by tumour density. PD may also occur if new intra-tumoural nodules are present or existing intra-tumoural nodules show an increase in size, factors which are not catered for in RECIST. In patients treated with imatinib, Choi criteria showed a significantly better correlation with survival rates than RECIST (111).
Figure 2.
Response unrelated to tumour size in a 66 yo patient treated with imatinib for a gastrointestinal stromal tumour (GIST). Compared to the baseline image (left), after treatment (right) the tumour shows a dramatic decrease in density rather than in size.
Challenging Treatments: Focal Therapies
Treatment of tumour lesions with ablative therapies, such as radiofrequency ablation, microwave ablation or cryoablation, results in a larger defect than the original lesion and such treated lesions are not considered measurable unless there is progression at this site (1), such as the development of a new measurable nodule within the ablation defect. Distinguishing normal post-ablation changes from residual disease and recurrence can be challenging (112).
Intravascular therapies are also a challenge for the use of RECIST. Trans-arterial radioembolization (TARE) induces inflammatory changes with a generally delayed morphologic response (112). A reduction of 18F-FDG uptake on early PET-CT has been found to be helpful in predicting further outcome of these patients (113). As a consequence, both TARE and intra-arterial therapies such as trans-arterial chemoembolization (TACE) in hepatocarcinoma require modified RECIST (mRECIST) criteria derived from arterial and portal venous enhancement phases of CT or MRI (114), and which take into account both lesion size and vascularity.
High-intensity focused ultrasound (HIFU), under the guidance of ultrasound or MRI, has also been used as a non-invasive technique for tissue ablation in prostate cancer and more recently in recurrent gynecological malignancy (115). The use of HIFU for hepatic tumour lesions is still in the exploratory stage. As for other ablative therapies and for similar reasons (116), RECIST 1.1 appears to be unsuitable for local response evaluation following HIFU applied to liver lesions.
Finally, tumour lesions in a previously irradiated area (via CyberKnife, stereotactic radiotherapy or traditional fractionated radiation therapy) are not considered measurable (1) and must be excluded from RECIST evaluation due to the inflammatory or fibrotic changes that may be observed, thus making evaluation of size unreliable.
Statement #5
There are several scenarios in which RECIST criteria fail to evaluate treatment-induced changes correctly. Informed appreciation that RECIST criteria are not applicable to all tumour sites and situations is thus crucial for proper interpretation and again dependent on reader experience.
When Is RECIST Response Assessment Misleading?
Pseudo-Progression
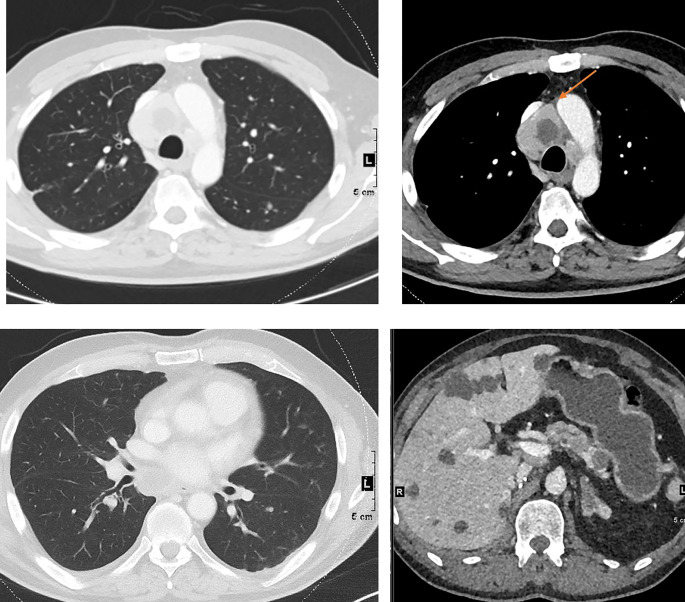
During immunotherapy, RECIST may describe progression that can be misleading and is thus classified as “pseudo-progression”. In fact, in around 5 to 10% of patients with metastatic disease treated with check-point inhibitors, an initial increase of tumour burden has been observed, followed by actual response or long-term stabilisation of disease (117–119). This phenomenon relates to the mechanism of action of immunotherapy, which stimulates the immune response and initially induces inflammation and tumour swelling, thus delaying visible tumour shrinkage. For this reason, adaptations of RECIST criteria for assessing treatment response to immunotherapy (iRECIST) have been developed. The first ascertainment of progression by iRECIST is considered as “immune unconfirmed progressive disease”(or iUPD), and requires, if possible, a subsequent evaluation 4 to 8 weeks later in order to confirm true progression (120) ( Figure 3 ).
Figure 3.
Pseudoprogression on immunotherapy in a 56 yo patient with metastatic non-small cell lung cancer. The baseline image (left) shows lung and peritoneal nodules (arrows). After 4 wks of antiPDL1 therapy (middle), CT shows an increase in previous lesions and the appearance of new lung nodules. Disease was considered immune unconfirmed progressive disease. Six weeks later (right) a dramatic response in all previous lesions was seen classifying the patients as a complete responder and endorsing an earlier diagnosis of pseudoprogression.
Mixed Response/Progression
In some patients, the tumour bulk does not respond homogeneously, with some lesions increasing and others decreasing. Mixed or heterogeneous response is defined as an increase in size of some tumour lesions and decrease of others in the same patient during treatment. This lesion-specific response has been attributed to the emergence of drug-resistant clones and indicates that tumour heterogeneity is likely causing treatment failure (121, 122). Mixed response has the same incidence in patients treated with targeted cancer agents and those undergoing chemotherapy alone or even combined with targeted agents (12, 28).
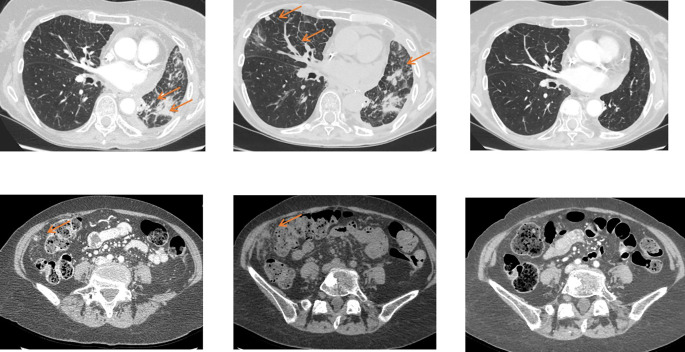
Since RECIST records overall patient response rather than individual lesion response, the choice of target lesions critically affects the objective assessment of overall patient response in patients with mixed response in individual lesions ( Figure 4 ) (12). As lesions escaping treatment control will weigh negatively on patient prognosis (123), their presence should be annotated in order to offer the best alternative treatment for the patient.
Figure 4.
Mixed response to treatment in the same patient illustrated in Figure 1 . Eight weeks after targeted therapy lung, adrenal and pancreatic metastases decreased, whereas one mediastinal lymph node (top right, arrow) increased.
Lesion cavitation, necrosis and residual non-viable masses represent other forms of response than decrease in size and may complicate RECIST assessment (124). Tumour necrosis with cavitation is present in approximately 14-24% of NSCLC patients undergoing anti-angiogenic drug therapy (125–127). When cavitation is present, lesion size may not change significantly and RECIST may therefore under-estimate the effect of therapy. Conversely, cavitation also risks missing progression if there is tumour regrowth inside the cavity. While alternative criteria have been proposed in such cases, e.g. subtracting the longest cavitation diameter from the largest lesion diameter (such as Crabb criteria) (126), these are not commonly used.
When residual tissue is present after therapy, evaluation with RECIST criteria is subject to pitfalls. First, an asymmetric shrinkage of the tumour may result in a similar longest diameter and consequent stable disease (SD) rating not reflecting the real response to treatment ( Figure 2 ). Second, it may be difficult to distinguish between viable tumour and fibrosis. In such cases, best response assessment, an important endpoint in phase 2 studies (partial vs. complete response; PR vs. CR) may be affected (126). According to RECIST guidelines, in equivocal cases, residual lesions should be evaluated by either biopsy or PET(-CT) ( Figure 2 ). This may well then allow upgrading PR to CR. However, false positive PET findings are not uncommon (128). Alternatively, other advanced imaging tests, such as DWI-MRI or perfusion imaging (e.g. from MR or CT) could be used.
Statement #6
Some patterns of response/progression cannot be correctly documented by RECIST. These require specialist reader experience and communication with oncologists to determine appropriate evaluation approaches and/or therapeutic options.
Should (Could) RECIST be Automated?
The core assumption of RECIST is that a single diameter on the cross-sectional imaging slice presenting the largest cross-section of a given lesion (or sum thereof) is a surrogate for tumour burden. This assumes that lesions are grossly spherical and that their size represents their overall activity. To streamline the determination of this single diameter and make it less subject to possible human-induced variability, semi- or fully-automated 2D or even 3D segmentation techniques can be applied to target lesions, which can also be semi- or fully-automatically tracked between scans acquired at different time points (129–134). The 2D or 3D mask resulting from the segmentation process then readily permits the automated and accurate extraction of the largest diameter from the segmented lesion. With 3D segmentation, the full volume of a target lesion can be provided alongside an automatically extracted largest diameter, which may not be oriented in the 2D plane of the source images in a broader RECIST interpretation, together with any other geometric metric of relevance. Using the largest 3D diameter would allow RECIST to be used beyond 2D constraints, and can account for non-orthogonal motion of target lesions between scans at different time-points. While segmentation and tracking can now plausibly be fully automated, especially with newer approaches using machine learning, and such capabilities are already implemented in several commercially available clinical systems, some challenges remain with key RECIST operations, such as the proper selection of target lesions and dealing with new or disappeared lesions. These are currently still best addressed or verified with a human (e.g. a radiologist) in the loop (20, 135).
Statement #7
Though automation is desirable to streamline the process and potentially reduce the variability of results within the RECIST paradigm, remaining technical challenges must be overcome to ensure proper repeatability, and human adjudication is still required.
RECIST in Novel Drug Development
RECIST measurements play a pivotal role in the development of novel oncological drugs (136). In most registered randomised controlled trials (RCTs), studies are powered to meet primary endpoints such as OS/PFS, which determines the number of patients recruited. A study of RCTs between 2006 and 2015 looking for evidence of clinical efficacy of novel oncology drugs in order to gain US Food and Drug Administration (FDA) approvals had PFS as primary endpoint in 28 out of 42 RCTs (66%), and OS in 14 (33%). In 2012, 12 novel anticancer drugs were approved by the FDA; only three drugs showed improvement of overall survival (137). Similarly, a study of drugs approved by the European Medicine Agency (EMA) between 2009 and 2013 also showed that only 18 of 68 (26%) novel drug uses were supported by OS data, whereas PFS was used in 31 (46%) (138). In the vast majority of trials, PFS is determined using the RECIST1.1 framework, or iRECIST for immune-oncology trials. It is acknowledged however, that in some disease types other criteria are used: e.g. Lugano criteria for 18F-FDG PET/CT or RECIL in lymphoma (139, 140) and RANO criteria for brain tumours (141, 142). The fact that PFS can predict OS outcome in large patients cohorts with commonly occurring cancers, reinforces the use of RECIST criteria in clinical trials (143). Moreover, rapid progress in drug development will make the reliance on OS as endpoint for novel drugs in oncology increasingly challenging because treatment options on progression on trial, including in-trial cross-over, are increasing.
Statement #8
Although the RECIST framework might not be perfect, the scientific basis for the anticancer drugs that have been approved using a RECIST-based surrogate endpoint remains valid.
RECIST: Only as Good as its Users?
RECIST criteria were developed for clinical trials and thresholds chosen to produce a comparable classification of patients, taking into account tumour measurement variability. These criteria are widely used in clinical trials and accepted by regulatory agencies. Despite some limitations, the scientific basis for the anticancer drugs that have been approved using a RECIST-based surrogate endpoint remains valid. Reader experience, choice of target lesions and detection of new lesions impact RECIST reproducibility, which necessitates adequate training of radiologists using these criteria. Automation is not currently sufficiently reliable to replace human experience. Unfortunately, some organ-, disease- or drug-specific patterns of response/progression cannot be correctly documented by RECIST.
This expert statement includes that RECIST remains a tool for radiologists that needs to be used with discrimination and good understanding of its purpose and limitations. Training of radiologists is essential to improve its application and reproducibility. RECIST conclusions should not go against common (or informed) sense. Furthermore, RECIST criteria have the advantage of simplicity, availability, cost-effectiveness, and intuitiveness. Overall, therefore, RECIST provides a common language between oncologists and imaging experts (e.g. radiologists), provided there is full understanding of how measurements are made, what they represent, and their inherent limitations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors contributed to conception and design. CC and LF wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the numerous colleagues for their insightful discussions and comments that have facilitated the work in this manuscript.
References
- 1. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 2. Moertel CG, Hanley JA. The Effect of Measuring Error on the Results of Therapeutic Trials in Advanced Cancer. Cancer (1976) 38(1):388–94. doi: [DOI] [PubMed] [Google Scholar]
- 3. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer (1981) 47(1):207–14. doi: [DOI] [PubMed] [Google Scholar]
- 4. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI: J Natl Cancer Inst (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 5. James K, Eisenhauer E, Christian M, Terenziani M, Vena D, Muldal A, et al. Measuring Response in Solid Tumors: Unidimensional Versus Bidimensional Measurement. JNCI: J Natl Cancer Inst (1999) 91(6):523–8. doi: 10.1093/jnci/91.6.523 [DOI] [PubMed] [Google Scholar]
- 6. Bogaerts J, Ford R, Sargent D, Schwartz LH, Rubinstein L, Lacombe D, et al. Individual Patient Data Analysis to Assess Modifications to the RECIST Criteria. Eur J Cancer (2009) 45(2):248–60. doi: 10.1016/j.ejca.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 7. Moskowitz CS, Jia X, Schwartz LH, Gönen M. A Simulation Study to Evaluate the Impact of the Number of Lesions Measured on Response Assessment. Eur J Cancer (2009) 45(2):300–10. doi: 10.1016/j.ejca.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Booth CM, Eisenhauer EA. Progression-Free Survival: Meaningful or Simply Measurable? JCO (2012) 30(10):1030–3. doi: 10.1200/JCO.2011.38.7571 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki C, Blomqvist L, Sundin A, Jacobsson H, Byström P, Berglund Å, et al. The Initial Change in Tumor Size Predicts Response and Survival in Patients With Metastatic Colorectal Cancer Treated With Combination Chemotherapy. Ann Oncol (2012) 23(4):948–54. doi: 10.1093/annonc/mdr350 [DOI] [PubMed] [Google Scholar]
- 10. Jain RK, Lee JJ, Ng C, Hong D, Gong J, Naing A, et al. Change in Tumor Size by RECIST Correlates Linearly With Overall Survival in Phase I Oncology Studies. J Clin Oncol (2012) 30(21):2684–90. doi: 10.1200/JCO.2011.36.4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grünwald V, McKay RR, Krajewski KM, Kalanovic D, Lin X, Perkins JJ, et al. Depth of Remission is a Prognostic Factor for Survival in Patients With Metastatic Renal Cell Carcinoma. Eur Urol (2015) 67(5):952–8. doi: 10.1016/j.eururo.2014.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Litière S, Isaac G, De Vries EGE, Bogaerts J, Chen A, Dancey J, et al. RECIST 1.1 for Response Evaluation Apply Not Only to Chemotherapy-Treated Patients But Also to Targeted Cancer Agents: A Pooled Database Analysis. J Clin Oncol (2019) 37(13):1102–10. doi: 10.1200/JCO.18.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Litière S, de Vries EGE, Seymour L, Sargent D, Shankar L, Bogaerts J. The Components of Progression as Explanatory Variables for Overall Survival in the Response Evaluation Criteria in Solid Tumours 1.1 Database. Eur J Cancer (2014) 50(10):1847–53. doi: 10.1016/j.ejca.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 14. An M-W, Mandrekar SJ, Branda ME, Hillman SL, Adjei AA, Pitot HC, et al. Comparison of Continuous Versus Categorical Tumor Measurement-Based Metrics to Predict Overall Survival in Cancer Treatment Trials. Clin Cancer Res (2011) 17(20):6592–9. doi: 10.1158/1078-0432.CCR-11-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. An M-W, Dong X, Meyers J, Han Y, Grothey A, Bogaerts J, et al. Evaluating Continuous Tumor Measurement-Based Metrics as Phase II Endpoints for Predicting Overall Survival. J Natl Cancer Inst (2015) 107(11):djv239. doi: 10.1093/jnci/djv239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan DC, Obuchowski NA, Kessler LG, Raunig DL, Gatsonis C, Huang EP, et al. Metrology Standards for Quantitative Imaging Biomarkers. Radiol (2015) 277(3):813–25. doi: 10.1148/radiol.2015142202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon SH, Kim KW, Goo JM, Kim D-W, Hahn S. Observer Variability in RECIST-Based Tumour Burden Measurements: A Meta-Analysis. Eur J Cancer (2016) 53:5–15. doi: 10.1016/j.ejca.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 18. Oxnard GR, Zhao B, Sima CS, Ginsberg MS, James LP, Lefkowitz RA, et al. Variability of Lung Tumor Measurements on Repeat Computed Tomography Scans Taken Within 15 Minutes. J Clin Oncol (2011) 29(23):3114–9. doi: 10.1200/JCO.2010.33.7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McErlean A, Panicek DM, Zabor EC, Moskowitz CS, Bitar R, Motzer RJ, et al. Intra- and Interobserver Variability in CT Measurements in Oncology. Radiol (2013) 269(2):451–9. doi: 10.1148/radiol.13122665 [DOI] [PubMed] [Google Scholar]
- 20. Beaumont H, Evans TL, Klifa C, Guermazi A, Hong SR, Chadjaa M, et al. Discrepancies of Assessments in a RECIST 1.1 Phase II Clinical Trial - Association Between Adjudication Rate and Variability in Images and Tumors Selection. Cancer Imaging (2018) 18(1):50. doi: 10.1186/s40644-018-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellomi M, De Piano F, Ancona E, Lodigiani AF, Curigliano G, Raimondi S, et al. Evaluation of Inter-Observer Variability According to RECIST 1.1 and Its Influence on Response Classification in CT Measurement of Liver Metastases. Eur J Radiol (2017) 95:96–101. doi: 10.1016/j.ejrad.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 22. Jacene HA, Leboulleux S, Baba S, Chatzifotiadis D, Goudarzi B, Teytelbaum O, et al. Assessment of Interobserver Reproducibility in Quantitative 18F-FDG PET and CT Measurements of Tumor Response to Therapy. J Nucl Med (2009) 50(11):1760–9. doi: 10.2967/jnumed.109.063321 [DOI] [PubMed] [Google Scholar]
- 23. Kuhl CK, Alparslan Y, Schmoee J, Sequeira B, Keulers A, Brümmendorf TH, et al. Validity of RECIST Version 1.1 for Response Assessment in Metastatic Cancer: A Prospective, Multireader Study. Radiol (2019) 290(2):349–56. doi: 10.1148/radiol.2018180648 [DOI] [PubMed] [Google Scholar]
- 24. Keil S, Barabasch A, Dirrichs T, Bruners P, Hansen NL, Bieling HB, et al. Target Lesion Selection: An Important Factor Causing Variability of Response Classification in the Response Evaluation Criteria for Solid Tumors 1.1. Invest Radiol (2014) 49(8):509–17. doi: 10.1097/RLI.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 25. Karmakar A, Kumtakar A, Sehgal H, Kumar S, Kalyanpur A. Interobserver Variation in Response Evaluation Criteria in Solid Tumors 1.1. Acad Radiol (2019) 26(4):489–501. doi: 10.1016/j.acra.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 26. Suzuki C, Torkzad MR, Jacobsson H, Aström G, Sundin A, Hatschek T, et al. Interobserver and Intraobserver Variability in the Response Evaluation of Cancer Therapy According to RECIST and WHO-Criteria. Acta Oncol (2010) 49(4):509–14. doi: 10.3109/02841861003705794 [DOI] [PubMed] [Google Scholar]
- 27. Rothe JH, Grieser C, Lehmkuhl L, Schnapauff D, Fernandez CP, Maurer MH, et al. Size Determination and Response Assessment of Liver Metastases With Computed Tomography–Comparison of RECIST and Volumetric Algorithms. Eur J Radiol (2013) 82(11):1831–9. doi: 10.1016/j.ejrad.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann M, Kuhl CK, Engelke H, Bettermann G, Keil S. Factors That Drive Heterogeneity of Response-To-Treatment of Different Metastatic Deposits Within the Same Patients as Measured by RECIST 1.1 Analyses. Acad Radiol (2020) 28(8):e235–9. doi: 10.1016/j.acra.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 29. Ford R, Schwartz L, Dancey J, Dodd LE, Eisenhauer EA, Gwyther S, et al. Lessons Learned From Independent Central Review. Eur J Cancer (2009) 45(2):268–74. doi: 10.1016/j.ejca.2008.10.031 [DOI] [PubMed] [Google Scholar]
- 30. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J Nucl Med (2009) 50 Suppl 1:122S–50S. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of Clinical and Subclinical Tumour Response Using [18F]-Fluorodeoxyglucose and Positron Emission Tomography: Review and 1999 EORTC Recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer (1999) 35(13):1773–82. doi: 10.1016/S0959-8049(99)00229-4 [DOI] [PubMed] [Google Scholar]
- 32. de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, et al. Repeatability of 18F-FDG Uptake Measurements in Tumors: A Metaanalysis. J Nucl Med (2012) 53(5):701–8. doi: 10.2967/jnumed.111.095299 [DOI] [PubMed] [Google Scholar]
- 33. Burger IA, Huser DM, Burger C, von Schulthess GK, Buck A. Repeatability of FDG Quantification in Tumor Imaging: Averaged SUVs Are Superior to SUVmax. Nucl Med Biol (2012) 39(5):666–70. doi: 10.1016/j.nucmedbio.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 34. Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL Harmonization Strategies in PET Quantification: From Daily Practice to Multicentre Oncological Studies. Eur J Nucl Med Mol Imaging (2017) 44(Suppl 1):17–31. doi: 10.1007/s00259-017-3740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurland BF, Peterson LM, Shields AT, Lee JH, Byrd DW, Novakova-Jiresova A, et al. Test-Retest Reproducibility of 18F-FDG PET/CT Uptake in Cancer Patients Within a Qualified and Calibrated Local Network. J Nucl Med (2019) 60(5):608–14. doi: 10.2967/jnumed.118.209544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brendle C, Kupferschläger J, Nikolaou K, la Fougère C, Gatidis S, Pfannenberg C. Is the Standard Uptake Value (SUV) Appropriate for Quantification in Clinical PET Imaging? - Variability Induced by Different SUV Measurements and Varying Reconstruction Methods. Eur J Radiol (2015) 84(1):158–62. doi: 10.1016/j.ejrad.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 37. Miyashita K, Takahashi N, Oka T, Asakawa S, Lee J, Shizukuishi K, et al. SUV Correction for Injection Errors in FDG-PET Examination. Ann Nucl Med (2007) D 21(10):607–13. doi: 10.1007/s12149-007-0068-1 [DOI] [PubMed] [Google Scholar]
- 38. Boellaard R, Oyen WJG, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands Protocol for Standardisation and Quantification of FDG Whole Body PET Studies in Multi-Centre Trials. Eur J Nucl Med Mol Imaging (2008) 35(12):2320–33. doi: 10.1007/s00259-008-0874-2 [DOI] [PubMed] [Google Scholar]
- 39. Zhuang M, García DV, Kramer GM, Frings V, Smit EF, Dierckx R, et al. Variability and Repeatability of Quantitative Uptake Metrics in 18F-FDG PET/CT of Non-Small Cell Lung Cancer: Impact of Segmentation Method, Uptake Interval, and Reconstruction Protocol. J Nucl Med (2019) 60(5):600–7. doi: 10.2967/jnumed.118.216028 [DOI] [PubMed] [Google Scholar]
- 40. Laffon E, de Clermont H, Marthan R. A Method of Adjusting SUV for Injection-Acquisition Time Differences in (18)F-FDG PET Imaging. Eur Radiol (2011) 21(11):2417–24. doi: 10.1007/s00330-011-2204-5 [DOI] [PubMed] [Google Scholar]
- 41. Kamimura K, Nagamachi S, Wakamatsu H, Higashi R, Ogita M, Ueno S, et al. Associations Between Liver (18)F Fluoro-2-Deoxy-D-Glucose Accumulation and Various Clinical Parameters in a Japanese Population: Influence of the Metabolic Syndrome. Ann Nucl Med (2010) 24(3):157–61. doi: 10.1007/s12149-009-0338-1 [DOI] [PubMed] [Google Scholar]
- 42. Krak NC, Boellaard R, Hoekstra OS, Twisk JWR, Hoekstra CJ, Lammertsma AA. Effects of ROI Definition and Reconstruction Method on Quantitative Outcome and Applicability in a Response Monitoring Trial. Eur J Nucl Med Mol Imaging (2005) 32(3):294–301. doi: 10.1007/s00259-004-1566-1 [DOI] [PubMed] [Google Scholar]
- 43. Lasnon C, Quak E, Le Roux P-Y, Robin P, Hofman MS, Bourhis D, et al. EORTC PET Response Criteria Are More Influenced by Reconstruction Inconsistencies Than PERCIST But Both Benefit From the EARL Harmonization Program. EJNMMI Phys (2017) 4(1):17. doi: 10.1186/s40658-017-0185-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akamatsu G, Ikari Y, Nishida H, Nishio T, Ohnishi A, Maebatake A, et al. Influence of Statistical Fluctuation on Reproducibility and Accuracy of SUVmax and SUVpeak: A Phantom Study. J Nucl Med Technol (2015) 43(3):222–6. doi: 10.2967/jnmt.115.161745 [DOI] [PubMed] [Google Scholar]
- 45. Paquet N, Albert A, Foidart J, Hustinx R. Within-Patient Variability of (18)F-FDG: Standardized Uptake Values in Normal Tissues. J Nucl Med (2004) 45(5):784–8. [PubMed] [Google Scholar]
- 46. JH O, Lim SJ, Wang H, Leal JP, Shu H-KG, Wahl RL, et al. Quantitation of Cancer Treatment Response by 2-[18F]FDG PET/CT: Multi-Center Assessment of Measurement Variability Using AUTO-PERCISTTM . EJNMMI Res (2021) 11(1):15. doi: 10.1186/s13550-021-00754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Apostolova I, Wiemker R, Paulus T, Kabus S, Dreilich T, van den Hoff J, et al. Combined Correction of Recovery Effect and Motion Blur for SUV Quantification of Solitary Pulmonary Nodules in FDG PET/Ct. Eur Radiol (2010) 20(8):1868–77. doi: 10.1007/s00330-010-1747-1 [DOI] [PubMed] [Google Scholar]
- 48. Lodge MA, Chaudhry MA, Wahl RL. Noise Considerations for PET Quantification Using Maximum and Peak Standardized Uptake Value. J Nucl Med (2012) 53(7):1041–7. doi: 10.2967/jnumed.111.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams MC, Turkington TG, Wilson JM, Wong TZ. A Systematic Review of the Factors Affecting Accuracy of SUV Measurements. AJR Am J Roentgenol (2010) 195(2):310–20. doi: 10.2214/AJR.10.4923 [DOI] [PubMed] [Google Scholar]
- 50. Quak E, Le Roux P-Y, Hofman MS, Robin P, Bourhis D, Callahan J, et al. Harmonizing FDG PET Quantification While Maintaining Optimal Lesion Detection: Prospective Multicentre Validation in 517 Oncology Patients. Eur J Nucl Med Mol Imaging (2015) 42(13):2072–82. doi: 10.1007/s00259-015-3128-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gürses B, Altınmakas E, Böge M, Aygün MS, Bayram O, Balık E. Multiparametric MRI of Rectal Cancer-Repeatability of Quantitative Data: A Feasibility Study. Diagn Interv Radiol (2020) 26(2):87–94. doi: 10.5152/dir.2019.19127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lundsgaard Hansen M, Fallentin E, Axelsen T, Lauridsen C, Norling R, Svendsen LB, et al. Interobserver and Intraobserver Reproducibility With Volume Dynamic Contrast Enhanced Computed Tomography (DCE-CT) in Gastroesophageal Junction Cancer. Diagn (Basel) (2016) 6(1):E8. doi: 10.3390/diagnostics6010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conte GM, Castellano A, Altabella L, Iadanza A, Cadioli M, Falini A, et al. Reproducibility of Dynamic Contrast-Enhanced MRI and Dynamic Susceptibility Contrast MRI in the Study of Brain Gliomas: A Comparison of Data Obtained Using Different Commercial Software. Radiol Med (2017) 122(4):294–302. doi: 10.1007/s11547-016-0720-8 [DOI] [PubMed] [Google Scholar]
- 54. Aronhime S, Calcagno C, Jajamovich GH, Dyvorne HA, Robson P, Dieterich D, et al. DCE-MRI of the Liver: Effect of Linear and Nonlinear Conversions on Hepatic Perfusion Quantification and Reproducibility. J Magn Reson Imaging (2014) 40(1):90–8. doi: 10.1002/jmri.24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smits M, Bendszus M, Collette S, Postma LA, Dhermain F, Hagenbeek RE, et al. Repeatability and Reproducibility of Relative Cerebral Blood Volume Measurement of Recurrent Glioma in a Multicentre Trial Setting. Eur J Cancer (2019) 114:89–96. doi: 10.1016/j.ejca.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 56. Ng CS, Waterton JC, Kundra V, Brammer D, Ravoori M, Han L, et al. Reproducibility and Comparison of DCE-MRI and DCE-CT Perfusion Parameters in a Rat Tumor Model. Technol Cancer Res Treat (2012) 11(3):279–88. doi: 10.7785/tcrt.2012.500296 [DOI] [PubMed] [Google Scholar]
- 57. Newitt DC, Zhang Z, Gibbs JE, Partridge SC, Chenevert TL, Rosen MA, et al. Test-Retest Repeatability and Reproducibility of ADC Measures by Breast DWI: Results From the ACRIN 6698 Trial. J Magn Reson Imaging (2019) 49(6):1617–28. doi: 10.1002/jmri.26539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kwee RM, Dik AK, Sosef MN, Berendsen RCM, Sassen S, Lammering G, et al. Interobserver Reproducibility of Diffusion-Weighted MRI in Monitoring Tumor Response to Neoadjuvant Therapy in Esophageal Cancer. PLoS One (2014) 9(4):e92211. doi: 10.1371/journal.pone.0092211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weller A, Papoutsaki MV, Waterton JC, Chiti A, Stroobants S, Kuijer J, et al. Diffusion-Weighted (DW) MRI in Lung Cancers: ADC Test-Retest Repeatability. Eur Radiol (2017) 27(11):4552–62. doi: 10.1007/s00330-017-4828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koh D-M, Blackledge M, Collins DJ, Padhani AR, Wallace T, Wilton B, et al. Reproducibility and Changes in the Apparent Diffusion Coefficients of Solid Tumours Treated With Combretastatin A4 Phosphate and Bevacizumab in a Two-Centre Phase I Clinical Trial. Eur Radiol (2009) 19(11):2728–38. doi: 10.1007/s00330-009-1469-4 [DOI] [PubMed] [Google Scholar]
- 61. Sorace AG, Elkassem AA, Galgano SJ, Lapi SE, Larimer BM, Partridge SC, et al. Imaging for Response Assessment in Cancer Clinical Trials. Semin Nucl Med (2020) 50(6):488–504. doi: 10.1053/j.semnuclmed.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Galbán CJ, Ma B, Malyarenko D, Pickles MD, Heist K, Henry NL, et al. Multi-Site Clinical Evaluation of DW-MRI as a Treatment Response Metric for Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. PLoS One (2015) 10(3):e0122151. doi: 10.1371/journal.pone.0122151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Newitt DC, Tan ET, Wilmes LJ, Chenevert TL, Kornak J, Marinelli L, et al. Gradient Nonlinearity Correction to Improve Apparent Diffusion Coefficient Accuracy and Standardization in the American College of Radiology Imaging Network 6698 Breast Cancer Trial. J Magn Reson Imaging (2015) 42(4):908–19. doi: 10.1002/jmri.24883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Newitt DC, Amouzandeh G, Partridge SC, Marques HS, Herman BA, Ross BD, et al. Repeatability and Reproducibility of ADC Histogram Metrics From the ACRIN 6698 Breast Cancer Therapy Response Trial. Tomogr (2020) 6(2):177–85. doi: 10.18383/j.tom.2020.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hayes SA, Pietanza MC, O’Driscoll D, Zheng J, Moskowitz CS, Kris MG, et al. Comparison of CT Volumetric Measurement With RECIST Response in Patients With Lung Cancer. Eur J Radiol (2016) 85(3):524–33. doi: 10.1016/j.ejrad.2015.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee JH, Lee HY, Ahn M-J, Park K, Ahn JS, Sun J-M, et al. Volume-Based Growth Tumor Kinetics as a Prognostic Biomarker for Patients With EGFR Mutant Lung Adenocarcinoma Undergoing EGFR Tyrosine Kinase Inhibitor Therapy: A Case Control Study. Cancer Imaging (2016) 16 16:5. doi: 10.1186/s40644-016-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. deSouza NM, Soutter WP, Rustin G, Mahon MM, Jones B, Dina R, et al. Use of Neoadjuvant Chemotherapy Prior to Radical Hysterectomy in Cervical Cancer: Monitoring Tumour Shrinkage and Molecular Profile on Magnetic Resonance and Assessment of 3-Year Outcome. Br J Cancer (2004) 90(12):2326–31. doi: 10.1038/sj.bjc.6601870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fenerty KE, Folio LR, Patronas NJ, Marté JL, Gulley JL, Heery CR. Predicting Clinical Outcomes in Chordoma Patients Receiving Immunotherapy: A Comparison Between Volumetric Segmentation and RECIST. BMC Cancer (2016) 16(1):672. doi: 10.1186/s12885-016-2699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schmitz C, Hüttmann A, Müller SP, Hanoun M, Boellaard R, Brinkmann M, et al. Dynamic Risk Assessment Based on Positron Emission Tomography Scanning in Diffuse Large B-Cell Lymphoma: Post-Hoc Analysis From the PETAL Trial. Eur J Cancer (2020) 124:25–36. doi: 10.1016/j.ejca.2019.09.027 [DOI] [PubMed] [Google Scholar]
- 70. Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) Response After Radical Radiotherapy/Chemoradiotherapy for Non-Small Cell Lung Cancer Correlates With Patterns of Failure. Lung Cancer (2005) 49(1):95–108. doi: 10.1016/j.lungcan.2004.11.024 [DOI] [PubMed] [Google Scholar]
- 71. Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U, et al. The Role of Interim 18F-FDG PET/CT in Prediction of Response to Ipilimumab Treatment in Metastatic Melanoma. Eur J Nucl Med Mol Imaging (2018) 45(8):1289–96. doi: 10.1007/s00259-018-3972-9 [DOI] [PubMed] [Google Scholar]
- 72. Evangelista L, Guarneri V, Conte PF. 18f-Fluoroestradiol Positron Emission Tomography in Breast Cancer Patients: Systematic Review of the Literature & Meta-Analysis. Curr Radiopharm (2016) 9(3):244–57. doi: 10.2174/1874471009666161019144950 [DOI] [PubMed] [Google Scholar]
- 73. Tan N, Oyoyo U, Bavadian N, Ferguson N, Mukkamala A, Calais J, et al. PSMA-Targeted Radiotracers Versus 18F Fluciclovine for the Detection of Prostate Cancer Biochemical Recurrence After Definitive Therapy: A Systematic Review and Meta-Analysis. Radiol (2020) 296(1):44–55. doi: 10.1148/radiol.2020191689 [DOI] [PubMed] [Google Scholar]
- 74. Wester H-J. Nuclear Imaging Probes: From Bench to Bedside. Clin Cancer Res (2007) 13(12):3470–81. doi: 10.1158/1078-0432.CCR-07-0264 [DOI] [PubMed] [Google Scholar]
- 75. Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM Procedure Guidelines for Tumour PET Imaging: Version 1.0. Eur J Nucl Med Mol Imaging (2010) 37(1):181–200. doi: 10.1007/s00259-009-1297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Graham MM, Wahl RL, Hoffman JM, Yap JT, Sunderland JJ, Boellaard R, et al. Summary of the UPICT Protocol for 18F-FDG PET/CT Imaging in Oncology Clinical Trials. J Nucl Med (2015) 56(6):955–61. doi: 10.2967/jnumed.115.158402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Makris NE, Huisman MC, Kinahan PE, Lammertsma AA, Boellaard R. Evaluation of Strategies Towards Harmonization of FDG PET/CT Studies in Multicentre Trials: Comparison of Scanner Validation Phantoms and Data Analysis Procedures. Eur J Nucl Med Mol Imaging (2013) 40(10):1507–15. doi: 10.1007/s00259-013-2465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jun W, Cong W, Xianxin X, Daqing J. Meta-Analysis of Quantitative Dynamic Contrast-Enhanced MRI for the Assessment of Neoadjuvant Chemotherapy in Breast Cancer. Am Surg (2019) 85(6):645–53. doi: 10.1177/000313481908500630 [DOI] [PubMed] [Google Scholar]
- 79. Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Horita Y, et al. Pharmacokinetic Parameters From 3-Tesla DCE-MRI as Surrogate Biomarkers of Antitumor Effects of Bevacizumab Plus FOLFIRI in Colorectal Cancer With Liver Metastasis. Int J Cancer (2012) 130(10):2359–65. doi: 10.1002/ijc.26282 [DOI] [PubMed] [Google Scholar]
- 80. Rata M, Collins DJ, Darcy J, Messiou C, Tunariu N, Desouza N, et al. Assessment of Repeatability and Treatment Response in Early Phase Clinical Trials Using DCE-MRI: Comparison of Parametric Analysis Using MR- and CT-Derived Arterial Input Functions. Eur Radiol (2016) 26(7):1991–8. doi: 10.1007/s00330-015-4012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shukla-Dave A, Obuchowski NA, Chenevert TL, Jambawalikar S, Schwartz LH, Malyarenko D, et al. Quantitative Imaging Biomarkers Alliance (QIBA) Recommendations for Improved Precision of DWI and DCE-MRI Derived Biomarkers in Multicenter Oncology Trials. J Magn Reson Imaging (2019) 49(7):e101–21. doi: 10.1002/jmri.26518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Winfield JM, Tunariu N, Rata M, Miyazaki K, Jerome NP, Germuska M, et al. Extracranial Soft-Tissue Tumors: Repeatability of Apparent Diffusion Coefficient Estimates From Diffusion-Weighted MR Imaging. Radiol (2017) 284(1):88–99. doi: 10.1148/radiol.2017161965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. FDA: Food and Drug Agency . Health C for D and R. Artificial Intelligence and Machine Learning in Software as a Medical Device. FDA; (2021). Available at: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device. [Google Scholar]
- 84. Avanzini S, Kurtz DM, Chabon JJ, Moding EJ, Hori SS, Gambhir SS, et al. A Mathematical Model of ctDNA Shedding Predicts Tumor Detection Size. Sci Adv (2020) 6(50):eabc4308. doi: 10.1126/sciadv.abc4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nabet BY, Esfahani MS, Moding EJ, Hamilton EG, Chabon JJ, Rizvi H, et al. Noninvasive Early Identification of Therapeutic Benefit From Immune Checkpoint Inhibition. Cell (2020) 183(2):363–76. doi: 10.1016/j.cell.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dasari A, Morris VK, Allegra CJ, Atreya C, Benson AB, Boland P, et al. ctDNA Applications and Integration in Colorectal Cancer: An NCI Colon and Rectal-Anal Task Forces Whitepaper. Nat Rev Clin Oncol (2020) 17(12):757–70. doi: 10.1038/s41571-020-0392-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Devonshire AS, Whale AS, Gutteridge A, Jones G, Cowen S, Foy CA, et al. Towards Standardisation of Cell-Free DNA Measurement in Plasma: Controls for Extraction Efficiency, Fragment Size Bias and Quantification. Anal Bioanal Chem (2010) 406(26):6499–512. doi: 10.1007/s00216-014-7835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fleischhacker M, Schmidt B, Weickmann S, Fersching DM, Leszinski GS, Siegele B, et al. Methods for Isolation of Cell-Free Plasma DNA Strongly Affect DNA Yield. Clin Chim Acta (2011) 412(23‐24):2085–8. doi: 10.1016/j.cca.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 89. Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol (2018) 36(16):1631–41. doi: 10.1200/JCO.2017.76.8671 [DOI] [PubMed] [Google Scholar]
- 90. Tapia C, Aung PP, Roy-Chowdhuri S, Xu M, Ouyang F, Alshawa A, et al. Decrease in Tumor Content Assessed in Biopsies is Associated With Improved Treatment Outcome Response to Pembrolizumab in Patients With Rare Tumors. J Immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2020-000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ryan R, Gibbons D, Hyland JMP, Treanor D, White A, Mulcahy HE, et al. Pathological Response Following Long-Course Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Histopathol (2005) 47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x [DOI] [PubMed] [Google Scholar]
- 92. Voskuilen CS, Oo HZ, Genitsch V, Smit LA, Vidal A, Meneses M, et al. Multicenter Validation of Histopathologic Tumor Regression Grade After Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Carcinoma. Am J Surg Pathol (2019) 43(12):1600–10. doi: 10.1097/PAS.0000000000001371 [DOI] [PubMed] [Google Scholar]
- 93. Jaraj SJ, Camparo P, Boyle H, Germain F, Nilsson B, Petersson F, et al. Intra- and Interobserver Reproducibility of Interpretation of Immunohistochemical Stains of Prostate Cancer. Virchows Arch (2009) 455(4):375–81. doi: 10.1007/s00428-009-0833-8 [DOI] [PubMed] [Google Scholar]
- 94. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance-Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver Reproducibility of Cervical Cytologic and Histologic Interpretations: Realistic Estimates From the ASCUS-LSIL Triage Study. JAMA (2001) 285(11):1500–5. doi: 10.1001/jama.285.11.1500 [DOI] [PubMed] [Google Scholar]
- 95. Stattaus J, Hahn S, Gauler T, Eberhardt W, Mueller SP, Forsting M, et al. Osteoblastic Response as a Healing Reaction to Chemotherapy Mimicking Progressive Disease in Patients With Small Cell Lung Cancer. Eur Radiol (2009) 19(1):193–200. doi: 10.1007/s00330-008-1115-6 [DOI] [PubMed] [Google Scholar]
- 96. Messiou C, Cook G, Reid AHM, Attard G, Dearnaley D, de Bono JS, et al. The CT Flare Response of Metastatic Bone Disease in Prostate Cancer. Acta Radiol (2011) 52(5):557–61. doi: 10.1258/ar.2011.100342 [DOI] [PubMed] [Google Scholar]
- 97. Ciray I, Lindman H, Aström KG, Bergh J, Ahlström KH. Early Response of Breast Cancer Bone Metastases to Chemotherapy Evaluated With MR Imaging. Acta Radiol (2001) 42(2):198–206. doi: 10.1080/028418501127346503 [DOI] [PubMed] [Google Scholar]
- 98. Tombal B, Rezazadeh A, Therasse P, Van Cangh PJ, Vande Berg B, Lecouvet FE. Magnetic Resonance Imaging of the Axial Skeleton Enables Objective Measurement of Tumor Response on Prostate Cancer Bone Metastases. Prostate (2005) 65(2):178–87. doi: 10.1002/pros.20280 [DOI] [PubMed] [Google Scholar]
- 99. Padhani AR, Lecouvet FE, Tunariu N, Koh D-M, De Keyzer F, Collins DJ, et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body Magnetic Resonance Imaging-Based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur Urol (2017) 71(1):81–92. doi: 10.1016/j.eururo.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Van Nieuwenhove S, Van Damme J, Padhani AR, Vandecaveye V, Tombal B, Wuts J, et al. Whole-Body Magnetic Resonance Imaging for Prostate Cancer Assessment: Current Status and Future Directions. J Magn Reson Imaging (2020). doi: 10.1002/jmri.27485 [DOI] [PubMed] [Google Scholar]
- 101. JH O, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiol (2016) 280(2):576–84. doi: 10.1148/radiol.2016142043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, et al. 68Ga-PSMA-11 PET/CT-Derived Metabolic Parameters for Determination of Whole-Body Tumor Burden and Treatment Response in Prostate Cancer. Eur J Nucl Med Mol Imaging (2018) 45(11):1862–72. doi: 10.1007/s00259-018-4042-z [DOI] [PubMed] [Google Scholar]
- 103. Schmidkonz C, Cordes M, Goetz TI, Prante O, Kuwert T, Ritt P, et al. 68Ga-PSMA-11 PET/CT Derived Quantitative Volumetric Tumor Parameters for Classification and Evaluation of Therapeutic Response of Bone Metastases in Prostate Cancer Patients. Ann Nucl Med (2019) 33(10):766–75. doi: 10.1007/s12149-019-01387-0 [DOI] [PubMed] [Google Scholar]
- 104. Zhou J, Li Q, Cao Y. Spatiotemporal Heterogeneity Across Metastases and Organ-Specific Response Informs Drug Efficacy and Patient Survival in Colorectal Cancer. Cancer Res (2021) 81(9):2522–33. doi: 10.1158/0008-5472.CAN-20-3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, et al. Site-Specific Response Patterns, Pseudoprogression, and Acquired Resistance in Patients With Melanoma Treated With Ipilimumab Combined With Anti-PD-1 Therapy. Cancer (2020) 126(1):86–97. doi: 10.1002/cncr.32522 [DOI] [PubMed] [Google Scholar]
- 106. Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-Level Response Dynamics to Programmed Cell Death Protein (PD-1) Blockade. J Clin Oncol (2019) 37(36):3546–55. doi: 10.1200/JCO.19.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S, et al. Organ-Specific Response to Nivolumab in Patients With Non-Small Cell Lung Cancer (NSCLC). Cancer Immunol Immunother (2018) 67(12):1825–32. doi: 10.1007/s00262-018-2239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT Evaluation of the Response of Gastrointestinal Stromal Tumors After Imatinib Mesylate Treatment: A Quantitative Analysis Correlated With FDG PET Findings. AJR Am J Roentgenol (2004) 183(6):1619–28. doi: 10.2214/ajr.183.6.01831619 [DOI] [PubMed] [Google Scholar]
- 109. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of Computed Tomography and Positron Emission Tomography in Patients With Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J Clin Oncol (2007) 25(13):1753–9. doi: 10.1200/JCO.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- 110. Fournier L, Ammari S, Thiam R, Cuénod C-A. Imaging Criteria for Assessing Tumour Response: RECIST, mRECIST, Cheson. Diagn Interv Imaging (2014) 95(7–8):689–703. doi: 10.1016/j.diii.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 111. Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We Should Desist Using RECIST, at Least in GIST. J Clin Oncol (2007) 25(13):1760–4. doi: 10.1200/JCO.2006.07.3411 [DOI] [PubMed] [Google Scholar]
- 112. Maas M, Beets-Tan R, Gaubert J-Y, Gomez Munoz F, Habert P, Klompenhouwer LG, et al. Follow-Up After Radiological Intervention in Oncology: ECIO-ESOI Evidence and Consensus-Based Recommendations for Clinical Practice. Insights Imaging (2020) 11(1):83. doi: 10.1186/s13244-020-00884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sabet A, Meyer C, Aouf A, Sabet A, Ghamari S, Pieper CC, et al. Early Post-Treatment FDG PET Predicts Survival After 90Y Microsphere Radioembolization in Liver-Dominant Metastatic Colorectal Cancer. Eur J Nucl Med Mol Imaging (2015) 42(3):370–6. doi: 10.1007/s00259-014-2935-z [DOI] [PubMed] [Google Scholar]
- 114. Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Imseeh G, Giles SL, Taylor A, Brown MRD, Rivens I, Gordon-Williams R, et al. Feasibility of Palliating Recurrent Gynecological Tumors With MRGHIFU: Comparison of Symptom, Quality-of-Life, and Imaging Response in Intra and Extra-Pelvic Disease. Int J Hyperthermia (2021) 38(1):623–32. doi: 10.1080/02656736.2021.1904154 [DOI] [PubMed] [Google Scholar]
- 116. Ji Y, Zhu J, Zhu L, Zhu Y, Zhao H. High-Intensity Focused Ultrasound Ablation for Unresectable Primary and Metastatic Liver Cancer: Real-World Research in a Chinese Tertiary Center With 275 Cases. Front Oncol (2020) 10:519164. doi: 10.3389/fonc.2020.519164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res (2009) 15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 118. Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of Responses in Metastatic NSCLC During PD-1 or PDL-1 Inhibitor Therapy: Comparison of RECIST 1.1, irRECIST and iRECIST Criteria. Eur J Cancer (2018) 88:38–47. doi: 10.1016/j.ejca.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 119. Park HJ, Kim KW, Pyo J, Suh CH, Yoon S, Hatabu H, et al. Incidence of Pseudoprogression During Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis. Radiol (2020) 297(1):87–96. doi: 10.1148/radiol.2020200443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol (2017) 18(3):e143–52. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Russo M, Crisafulli G, Sogari A, Reilly NM, Arena S, Lamba S, et al. Adaptive Mutability of Colorectal Cancers in Response to Targeted Therapies. Sci (2019) 366(6472):1473–80. doi: 10.1126/science.aav4474 [DOI] [PubMed] [Google Scholar]
- 122. Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, et al. Radiologic and Genomic Evolution of Individual Metastases During HER2 Blockade in Colorectal Cancer. Cancer Cell (2018) 34(1):148–62.e7. doi: 10.1016/j.ccell.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 123. Dong Z-Y, Zhai H-R, Hou Q-Y, Su J, Liu S-Y, Yan H-H, et al. Mixed Responses to Systemic Therapy Revealed Potential Genetic Heterogeneity and Poor Survival in Patients With Non-Small Cell Lung Cancer. Oncologist (2017) 22(1):61–9. doi: 10.1634/theoncologist.2016-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. de Castro J, Cobo M, Isla D, Puente J, Reguart N, Cabeza B, et al. Recommendations for Radiological Diagnosis and Assessment of Treatment Response in Lung Cancer: A National Consensus Statement by the Spanish Society of Medical Radiology and the Spanish Society of Medical Oncology. Clin Transl Oncol (2015) 17(1):11–23. doi: 10.1007/s12094-014-1231-5 [DOI] [PubMed] [Google Scholar]
- 125. Ferretti GR, Reymond E, Delouche A, Sakhri L, Jankowski A, Moro-Sibilot D, et al. Personalized Chemotherapy of Lung Cancer: What the Radiologist Should Know. Diagn Interv Imaging (2016) 97(3):287–96. doi: 10.1016/j.diii.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 126. Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor Cavitation: Impact on Objective Response Evaluation in Trials of Angiogenesis Inhibitors in Non-Small-Cell Lung Cancer. J Clin Oncol (2009) 27(3):404–10. doi: 10.1200/JCO.2008.16.2545 [DOI] [PubMed] [Google Scholar]
- 127. Marom EM, Martinez CH, Truong MT, Lei X, Sabloff BS, Munden RF, et al. Tumor Cavitation During Therapy With Antiangiogenesis Agents in Patients With Lung Cancer. J Thorac Oncol (2008) 3(4):351–7. doi: 10.1097/JTO.0b013e318168c7e9 [DOI] [PubMed] [Google Scholar]
- 128. Kong BY, Menzies AM, Saunders CAB, Liniker E, Ramanujam S, Guminski A, et al. Residual FDG-PET Metabolic Activity in Metastatic Melanoma Patients With Prolonged Response to Anti-PD-1 Therapy. Pigment Cell Melanoma Res (2016) 29(5):572–7. doi: 10.1111/pcmr.12503 [DOI] [PubMed] [Google Scholar]
- 129. Rubin DL, Willrett D, O’Connor MJ, Hage C, Kurtz C, Moreira DA. Automated Tracking of Quantitative Assessments of Tumor Burden in Clinical Trials. Transl Oncol (2014) 7(1):23–35. doi: 10.1593/tlo.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barash Y, Klang E. Automated Quantitative Assessment of Oncological Disease Progression Using Deep Learning. Ann Transl Med (2019) 7(Suppl 8):S379. doi: 10.21037/atm.2019.12.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kickingereder P, Isensee F, Tursunova I, Petersen J, Neuberger U, Bonekamp D, et al. Automated Quantitative Tumour Response Assessment of MRI in Neuro-Oncology With Artificial Neural Networks: A Multicentre, Retrospective Study. Lancet Oncol (2019) 20(5):728–40. doi: 10.1016/S1470-2045(19)30098-1 [DOI] [PubMed] [Google Scholar]
- 132. Baidya Kayal E, Kandasamy D, Yadav R, Bakhshi S, Sharma R, Mehndiratta A. Automatic Segmentation and RECIST Score Evaluation in Osteosarcoma Using Diffusion MRI: A Computer Aided System Process. Eur J Radiol (2020) 133:109359. doi: 10.1016/j.ejrad.2020.109359 [DOI] [PubMed] [Google Scholar]
- 133. Tang Y, Yan K, Xiao J, Summers RM. “One Click Lesion RECIST Measurement and Segmentation on CT Scans”. In: Martel AL, Abolmaesumi P, Stoyanov D, Mateus D, Zuluaga MA, Zhou SK, et al. editors. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020, vol. p . Cham: Springer International Publishing; (2020). p. 573–83. (Lecture Notes in Computer Science). [Google Scholar]
- 134. Moawad AW, Fuentes D, Khalaf AM, Blair KJ, Szklaruk J, Qayyum A, et al. Feasibility of Automated Volumetric Assessment of Large Hepatocellular Carcinomas’ Responses to Transarterial Chemoembolization. Front Oncol (2020) 10:572. doi: 10.3389/fonc.2020.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Iannessi A, Beaumont H, Liu Y, Bertrand A-S. RECIST 1.1 and Lesion Selection: How to Deal With Ambiguity at Baseline? Insights Imaging (2021) 12(1):36. doi: 10.1186/s13244-021-00976-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ruchalski K, Braschi-Amirfarzan M, Douek M, Sai V, Gutierrez A, Dewan R, et al. A Primer on RECIST 1.1 for Oncologic Imaging in Clinical Drug Trials. Radiol Imaging Cancer (2021) 3(3):e210008. doi: 10.1148/rycan.2021210008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer Drugs in the United States: Justum Pretium–the Just Price. J Clin Oncol (2013) 31(28):3600–4. doi: 10.1200/JCO.2013.49.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of Evidence of Benefits on Overall Survival and Quality of Life of Cancer Drugs Approved by European Medicines Agency: Retrospective Cohort Study of Drug Approvals 2009-13. BMJ (2017) 359:j4530. doi: 10.1136/bmj.j4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group Consensus Response Evaluation Criteria in Lymphoma (RECIL 2017). Ann Oncol (2017) 28(7):1436–47. doi: 10.1093/annonc/mdx097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJB, Jaeckle K, Junck L, et al. Response Assessment in Neuro-Oncology (a Report of the RANO Group): Assessment of Outcome in Trials of Diffuse Low-Grade Gliomas. Lancet Oncol (2011) 12(6):583–93. doi: 10.1016/S1470-2045(11)70057-2 [DOI] [PubMed] [Google Scholar]
- 142. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response Assessment Criteria for Brain Metastases: Proposal From the RANO Group. Lancet Oncol (2015) 16(6):e270–278. doi: 10.1016/S1470-2045(15)70057-4 [DOI] [PubMed] [Google Scholar]
- 143. Shafrin J, Brookmeyer R, Peneva D, Park J, Zhang J, Figlin RA, et al. The Value of Surrogate Endpoints for Predicting Real-World Survival Across Five Cancer Types. Curr Med Res Opin (2016) 32(4):731–9. doi: 10.1185/03007995.2016.1140027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.