Abstract
Background: The pretreatment of dexamethasone on the efficacy and immune-related adverse events of immunotherapy involving programmed cell death 1/programmed cell death 1 ligand 1 (PD1/PDL1) inhibitors is an effective option for the first-line treatment of advanced non-small-cell lung cancer (NSCLC). With the immunosuppressive effect, corticosteroids may be used to reduce the efficacy of PDL1 blockade, as well as prevent overactive immune responses, thereby reducing the occurrence of immune-related adverse events (irAEs). This study quantitatively summarized the current evidence, and compared the efficacy and toxicity of therapies involving chemotherapy plus PDL1 inhibitors plus dexamethasone pretreatment (I+C+D) with chemotherapy plus PDL1 inhibitors (I+C) and therapies involving PDL1 inhibitors or chemotherapy alone (I or C). Methods: The protocol of this study was registered with PROSPERO (CRD42021227281). By using a network meta-analysis approach, the different treatments were compared and ranked based on their effectiveness and rates of irAEs at the different grades. Risk rates were determined through direct meta-analysis and indirect treatment comparison. Results: 12 randomized clinical trials were included with a total of 7155 NSCLC patients. Network meta-analysis generated 15 comparisons. The combination treatment of I+C+D showed a longer progression-free survival and overall survival, while I+C was less toxic, and the toxicity of I+C+D or that of I+C had been significantly decreased, compared to that of monotherapy with either drug. According to the ranking analysis, I+C+D is consistently proved to be the most effective therapeutic strategy, while I+C is linked to the lowest rate of irAEs, with the rate of grade value of ≥3 irAEs. Conclusion: The combination treatment of I+C+D is the most effective approach for the first-line treatment of NSCLC patients treated with I+C, I, or C.
Keywords: Dexamethasone, immunotherapy, network meta-analysis
Introduction
Till now, there is no unanimous conclusion regarding the effects of steroids on the efficacy of immunotherapy drugs in non-small cell lung cancer (NSCLC) [1-3]. The KEYNOTE-407 clinical trial reported that pembrolizumab combined with either paclitaxel/carboplatin or nanoparticle albumin-bound paclitaxel (nab-p)/carboplatin in the treatment of lung squamous cell carcinoma did not reveal a significant difference in overall response rate (ORR) between the two groups [4]. In terms of survival benefit, progression-free survival (PFS) in the nab-p group was even lower than that observed in the paclitaxel group [4].
It was demonstrated in a previous study on the effect of hormones on the treatment of NSCLC with programmed cell death 1 (PD1) or programmed cell death 1 ligand 1 (PDL1) antibodies that, at the beginning of treatment with PDL1 antibody, ORR, PFS, and overall survival (OS) of patients treated with ≥10 mg prednisone were lower than the recorded values of the patients receiving <10 mg prednisone [5], indicating that treating physicians should be cautious about the application of the immunotherapy by using hormones to patients with lung cancer. However, the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines recommend the administration of dexamethasone to treat nausea and vomiting during radiotherapy and chemotherapy [6-8]. In addition, pretreatment with dexamethasone was performed according to the recommended therapy by using pemetrexed and paclitaxel.
Corticosteroids are widely applied in the oncology field as adjuvant-based anti-tumor therapy to relieve tumor-related manifestations. However, they are less effective in the context of cancer-related palliative treatment, symptomatic brain metastasis, cancer-related shortness of breath, bone metastasis pain, cancer-related fever, and previous chemotherapy- or radiotherapy-induced pneumonia. Considering their immunosuppressive effects and potential inhibition of T cell functions, corticosteroids may affect the efficacy of anti-PD1/PDL1 monoclonal antibodies [7-9]. In comparison with PDL1 inhibitor monotherapy, combination chemotherapy for the first-line treatment of NSCLC decreased the rates of most immune-related adverse events (irAEs), such as pneumonitis and endocrine and skin reactions, and the overall rate of adverse events [10]. However, the study results of pretreatment with dexamethasone on the efficacy and irAEs in treatment for advanced NSCLC remain elusive and controversial.
To fill this gap, an indirect meta-analysis was performed to compare these rates for the following treatment regimens in the present study: PD1 inhibitors plus chemotherapy plus dexamethasone (I+C+D); PD1 inhibitors plus chemotherapy (I+C); and PD1 inhibitors alone (I).
Materials and methods
Literature search and selection
Online databases, including PubMed (National Library of Medicine, Bethesda, MD, USA), Web of Science (Thompson Scientific, Philadelphia, PA, USA), MEDLINE, and the Cochrane Library, were searched for the eligible studies related to the following terms and relevant variants in English: NSCLC, first-line, front line, PD1/L1, nivolumab, pembrolizumab, atezolizumab, durvalumab, sintilimab, tislelizumab, toripalimab, camrelizumab, avelumab, Imfinzi (Tecentriq) OR (atezolizumab) OR (lambrolizumab) OR (Keytruda) OR (pembrolizumab) OR (Opdivo) OR (Nivolumab) (Dexamethasone) (Dexpak) OR (Dexasone).
This study also screened abstracts presented at the 2018 ASCO, International Conference on Oncology, European Society for Medical Oncology (ESMO), World Conference on Lung Cancer (WCLC), and American Association for Cancer Research, with all the articles or conference abstracts reported before November 30, 2020.
With the study protocol approved by the Tianjin Cancer Hospital (Tianjin, China), this study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was registered with PROSPERO (CRD42021227281).
The articles were identified by viewing the titles, abstracts, and full texts, thus leaving eligible studies meeting the following criteria: (1) randomized controlled trials (RCTs); (2) comparison studies of first-line therapies (I, I+C, or I+C+D) for advanced NSCLC; and (3) studies that reported the outcome of adverse events of interest. Non-comparative studies, case reports, review articles, commentary articles, letters, editorials, and expert opinions were excluded. All materials for any trials, including full-text articles, supplementary appendices, and conference abstracts (WCLC, ESMO, and ASCO), were used as resources. The grade of irAEs was determined based on the Common Terminology Criteria for Adverse Events (version 4.0) of the National Cancer Institute. The most recently updated data from each trial were chosen for analysis.
Data extraction and quality assessment
With each article independently evaluated by two investigators (WK and LYW) and any conflict adjudicated by a more senior investigator (PZY), data extraction was performed by using a Microsoft Excel database (Microsoft Corporation, Redmond, WA, USA). The researchers extracted the reported number of each irAE, the total group numbers of the experimental groups and the controls from the eligible studies, with the primary outcomes of the pooled rates of irAEs, indirect relative risks, PFS, and OS with I+C+D versus I. Following terms were included in the study characteristics: treatment lines, trial phase, blinding method, and the numbers and treatments of experimental and control groups (Table 1).
Table 1.
Include trial references
| Trial references | Phase no. patients | Therapy line | Study arm | No. of patients | Meta-analysis comparison |
|---|---|---|---|---|---|
| IMpower 130 Atezolizumab/PDL1 | Phase III RCT 679 | First line | Atezolizumab + carboplatin + nab-paclitaxel (4-6 cycles) followed by atezolizumab (maintenance therapy) | 451 | I+C |
| Carboplatin + nab-paclitaxel (4-6 cycles) followed by pemetrexed (maintenance therapy) or best supportive care | 288 | C | |||
| IMpower 131 Atezolizumab/PDL1 | Phase III RCT 683 | First line | Atezolizumab + carboplatin + nab_x005f paclitaxel (4-6 cycles) followed by atezolizumab (maintenance therapy) | 343 | I+C |
| Atezolizumab + carboplatin + paclitaxel pretreatment with dexamethasone | 338 | I+C+D | |||
| Carboplatin + nab-paclitaxel (4-6 cycles) followed by best supportive care | 340 | C | |||
| CheckMate-026 Nivolumab/PD1 | Phase III RCT 541 | First line | Nivolumab (every 2 weeks) | 271 | I |
| Platinum-based chemotherapy regimens (4 cycles) followed by pemetrexed (maintenance therapy) | 270 | C | |||
| ORIENT-11 Sintilimab/PD1/CHINA | Phase III RCT 397 | First line | Sintilimab + pemetrexed + platinum + pretreatment with dexamethasone | 252 | I+C+D |
| Platinum + pemetrexed | 126 | C | |||
| Camel Camrelizumab/PD1/CHINA | Phase III RCT 419 | First line | Camrelizumab + 4-6 cycles of carboplatin plus pemetrexed + pretreatment with dexamethasone | 205 | I+C+D |
| Platinum + pemetrexed | 207 | C | |||
| RATIONALE 304 Tislelizumab/PD1/CHINA | Phase III RCT 332 | First line | Tislelizumab + 4-6 cycles of carboplatin plus pemetrexed + pretreatment with dexamethasone | 222 | I+C+D |
| Platinum + pemetrexed | 110 | C | |||
| RATIONALE 307 Tislelizumab/PD1/CHINA | Phase III RCT 360 | First line | Tislelizumab + paclitaxel and carboplatin + pretreatment with dexamethasone | 120 | I+C+D |
| Tislelizumab + nab-paclitaxel and carboplatin | 120 | I+C | |||
| Paclitaxel and carboplatin IV Q3W | 121 | C | |||
| KEYNOTE 024 Pembrolizumab/PD1 | Phase III RCT 305 | First line | Pembrolizumab (35 cycles) | 154 | I |
| Platinum-based chemotherapy regimens (4-6 cycles) | 151 | C | |||
| KEYNOTE 042 Pembrolizumab/PD1 | Phase III RCT 1274 | First line | Pembrolizumab (35 cycles) | 637 | I |
| Platinum-based chemotherapy regimens (4-6 cycles) followed by pemetrexed (maintenance therapy) | 637 | C | |||
| KEYNOTE 407 Pembrolizumab/PD1 | Phase III RCT 559 | First line | Pembrolizumab (35 cycles) followed by carboplatin + paclitaxel (4 cycles) + pretreatment with dexamethasone | 169 | I+C+D |
| Pembrolizumab (35 cycles) followed by carboplatin +nab-paclitaxel (4 cycles) | 109 | I+C | |||
| Placebo (35 cycles) followed by carboplatin + nab-paclitaxel/or paclitaxel (4 cycles) | 280 | C | |||
| KEYNOTE 189 Pembrolizumab/PD1 | Phase III RCT 616 | First line | Pembrolizumab (35 cycles) followed by platinum + pemetrexed (4 cycles) + pretreatment with dexamethasone | 410 | I+C+D |
| Placebo (35 cycles) followed by platinum + pemetrexed (4 cycles) | 206 | C | |||
| IMpower 132 Atezolizumab/PDL1 | Phase III RCT 578 | First line | Atezolizumab + platinum + pemetrexed (4-6 cycles) followed by atezolizumab + pemetrexed (maintenance therapy) + pretreatment with dexamethasone | 292 | I+C+D |
| Platinum + pemetrexed (4-6 cycles) followed by pemetrexed (maintenance therapy) | 286 | C | |||
| 7,115 |
Abbreviations: C, chemotherapy; D, dexamethasone; I, PDL1 inhibitors; PDL1, programmed cell death 1 ligand 1.
Statistical analysis
A pair-wise meta-analysis was performed by using STATA version 15, with the odds ratio set with 95% confidence intervals (CIs) for dichotomous outcomes [11-13]. Heterogeneity was analyzed by I-squared statistics with a significance limit at I-squared >50%, suggesting high heterogeneity. Based on the rank probability of each treatment strategy, the surface under the cumulative ranking (SUCRA), indicating the rank of the included treatments, was applied [14,15]. For each treatment regimen, a vector of cumulative probability network plots of overall or subgroup populations for the three first-line therapies was completed based on the network package on STATA SE (version 15) [16,17]. The number of trials and the sample size was taken into consideration of the network plots. The heterogeneity among the studies was evaluated by using the Cochran I2 statistic among effect estimates [13-15]. An I2 statistic >50% indicated statistical heterogeneity among the studies. The fixed-effects model was preferred owing to its having no significant statistical heterogeneity. In addition, the random-effects model was used, for the meta-analysis, to calculate pooled estimates, with pooled rates of their 95% CIs, P values, and indirect relative risks of their 95% CIs determined to assess the risk of irAEs for each estimate. A two-sided P-value <0.05 denoted statistical significance.
The median ranks and SUCRA were estimated for all treatment regimens to determine the hierarchy of safety profiles. SUCRA was the percentage of drug safety on the adverse events that would be ranked first without uncertainty. SUCRA values of 1 or 0 are indicative of certainty that the safety profile of a drug is the best and worst, respectively [17-19].
Since consistency (defined as agreement) between direct and indirect results is the key to robust findings, the presence of inconsistency was first evaluated by node splitting analysis in the entire network on particular comparisons [18-20]. Subsequently, the loop-specific approach was adopted to evaluate the presence of inconsistency in each loop [17]. The values of the two odds ratios (RR) from direct and indirect evidence with 95% CI were calculated a P-value of <0.05 denoted significant inconsistency. Stratified analysis was performed by applying the meta-analysis package of R software (3.6.0), using the metaprop, forest, funnel, and metabias commands.
Results
Study selection and quality assessment
A total of 12 relevant RCTs published online or reported as abstracts during the international conferences held in 2020 (ASCO, ESMO, and WCLC) were included (a total of 7115 patients). All the studies were phase III international multicenter trials investigating PDL1 inhibitors, chemotherapy, or their combination for the first-line treatment of advanced NSCLC. The demographics of all the included studies are shown in Table 1. The results of a comparability analysis at baseline were shown that no significant baseline characteristics among groups (P>0.05). As shown in Figure 1, network plots of overall and subgroup populations are based on the connection of four types of first-line treatments (I+C+D, I+C, I, and C groups).
Figure 1.

The network plot of the effectiveness of four different treatment regimens.
Circles, with their sizes proportioned to the numbers of studies included, represent the interventions denoted as nodes in the network, while lines, with their thicknesses indicating the number of randomized clinical trials (RCTs) included in each comparison, represent direct comparisons within the frame of RCTs. No statistical inconsistency was shown in the three loops, indicting no differences observed between direct and indirect estimates for each comparison.
PFS and OS
PFS was analyzed by using data obtained from 12 studies [18-30], with three of them involving three treatment arms (Keynote 407, IMpower 131, and RationalE 307) and nine others including two arms (I+C+D: eight trials, N=2,008; I+C: four trials, N=1,023; I: three trials of PDL1 inhibitors alone [CHEKMATE 026, KEYNOTE 024, KEYNOTE 042], N=1,062; C: all 12 trials, N=3,022). Meta-analyses of efficacy were feasible for the following three comparisons: I+C+D versus C (eight trials); I+C versus C (four trials); and I versus C (three trials). Regarding all the subjects involved, the use of I+C+D for the treatment of patients with metastatic NSCLC meant a statistically significant improvement in PFS (hazard ratio [HR]: 0.50, 95% CI: 0.37-0.72). A network meta-analysis was conducted to investigate the treatment modalities included in the network plot (Figure 1).
Based on the above comparisons, the results obtained from network meta-analyses were consistent with those from standard pairwise meta-analyses (Figure 2). Overall, I+C+D and I+C therapies were more effective than C in terms of PFS. The combination therapy of I+C+D showed a statistically significant (HR: 0.66; 95% CI: 0.32-1.30) OS advantage over I+C (HR: 0.72; CI: 0.46-1.10) or I (HR: 0.79; CI: 0.30-2.1) (Figure 3). Besides, multiple comparisons of the rates are shown in Table 2. The rank probabilities of the four treatment regimens in terms of the best treatment are presented in Table 2, with I+C+D 0.46 (0.32, 0.65) shown to be the best therapeutic option.
Figure 2.
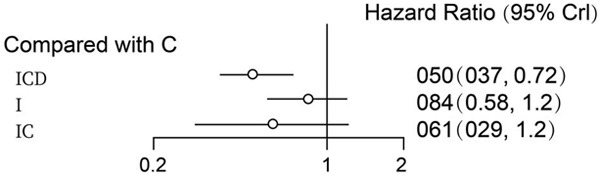
Progression-free survival of chemotherapy plus ICD/IC/I. I+C+D and I+C therapies were more effective than C in terms of PFS.
Figure 3.
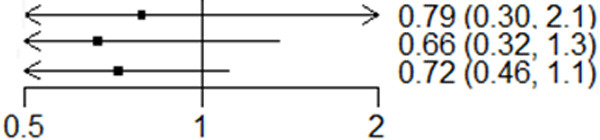
Overall survival of chemotherapy plus ICD/IC/I. The combination therapy of I+C+D showed a statistically significant OS advantage over I+C and I.
Table 2.
The rank probabilities of four treatment regimens
| I+C+D | I+C | I | C |
|---|---|---|---|
| I+C+D | 1.02 (0.53, 3.13) | 3.14 (1.90, 5.19) | 3.70 (1.93, 7.07) |
| 0.46 (0.32, 0.65) | IC | 1.43 (1.01, 2.04) | 1.69 (0.99, 2.90) |
| 0.62 (0.19, 0.53) | 0.70 (0.49, 0.99) | I | 1.18 (0.67, 2.08) |
| 0.57 (0.14, 0.52) | 0.59 (0.34, 1.01) | 0.85 (0.48, 1.50) | C |
Overall incidence of irAEs
The contribution of direct comparisons to determine the network meta-analysis estimates for mixed and indirect evidence was summarized. Overall, the rate of all-grade irAEs for the I+C+D and I+C regimens was the least parameter in all studies [18-30]. However, I+C was less toxic than I+C+D, although this difference did not reach statistical significance (HR: 1.07; 95% CI: 0.80-1.44). The combination therapy of I+C+D did not show increased toxicity in comparison with I+C or I. The combination therapy of I+C+D or that of I+C exhibited significantly decreased toxicity compared to monotherapy with either agent (I or C): I+C+D versus C (RR: 0.92; 95% CI: 0.56-1.51); I+C versus C (RR: 0.62; 95% CI: 0.37-1.04) (Figure 4).
Figure 4.
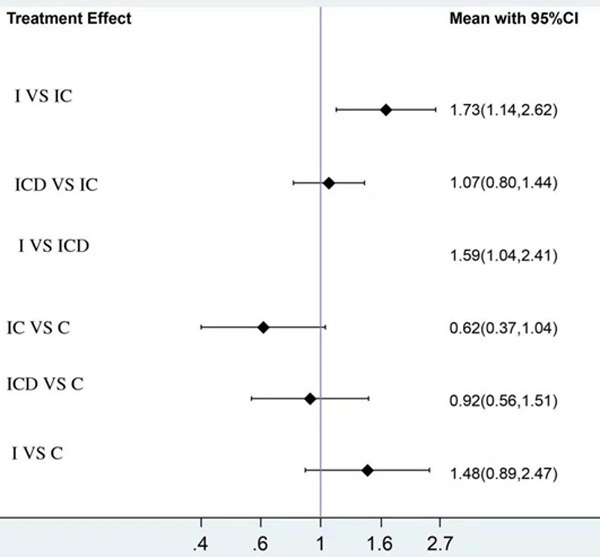
Toxicity of chemotherapy plus ICD/IC/I. The combination therapy of I+C+D or that of I+C exhibited significantly decreased toxicity compared to monotherapy with either agent (I or C).
I+C therapy was superior, with a significantly lower incidence of SAE (severe adverse events), (World Health Organization P G3 events), to I+C+D (RR: 1.49; 95% CI: 0.97-2.28) and I (RR: 0.46; CI: 0.32-0.66) therapies. Treatment with I was associated with higher grade 3-5 toxicity than I+C+D (RR: 0.72; 95% CI: 0.65-3.55) (Figure 5).
Figure 5.
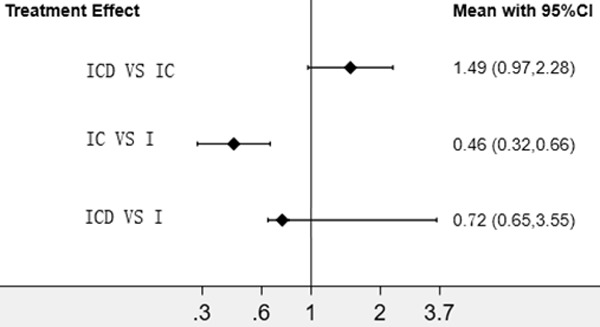
SAE of chemotherapy plus ICD/IC/I. I+C therapy was superior, with a significantly lower incidence of SAE.
Ranking findings
Ranking analysis for irAEs performed with SUCRA suggested that the combination regimen I+C is the best treatment option (SUCRA: 66.5%), followed by I+C+D (SUCRA: 59.1%) (Figure 6).
Figure 6.
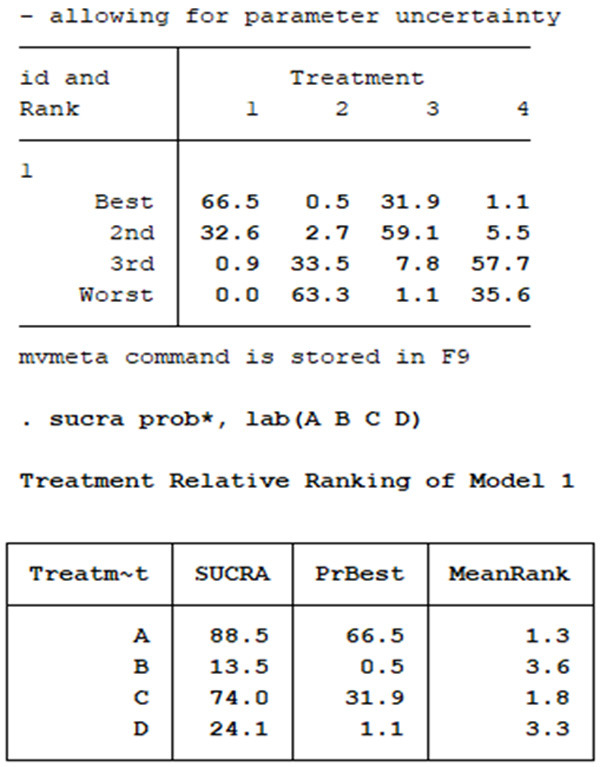
Ranking analysis for irAEs performed with SUCRA. I+C is the best treatment option (SUCRA: 66.5%), followed by I+C+D (SUCRA: 59.1%).
The rank probabilities of four strategies in terms of the best PFS , and the SUCRA ranking of four examined strategies based on the cumulative efficacy rank probability are both presented in Figure 7. The I+C+D regimen ranked first (SUCRA: 100%), followed by the I+C regimen (SUCRA: 95.2%). All three treatments significantly increased the PFS of patients and demonstrated satisfactory performance (Figure 7).
Figure 7.
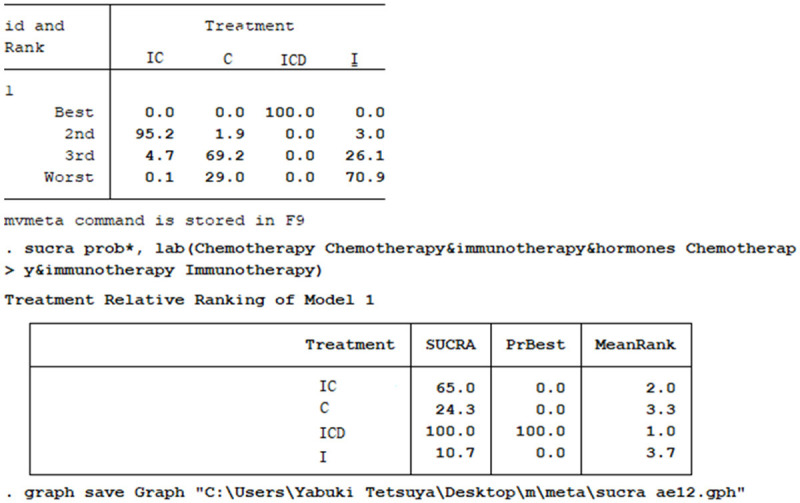
The rank probabilities of four strategies in terms of the best PFS. I+C+D is the best treatment option (SUCRA: 100%), followed by I+C (SUCRA: 95.2%).
The findings on efficacy and irAEs were incorporated into a bivariate ranking plot (Figure 8), with the ideal treatment (highest performance = best efficacy + lowest rate of irAEs) supposed to appear in the upper right corner of the plot. Although both I+C+D and I+C combination regimens improved treatment efficacy and tolerability, remarkably, the latter was associated with better results for these parameters than the former.
Figure 8.
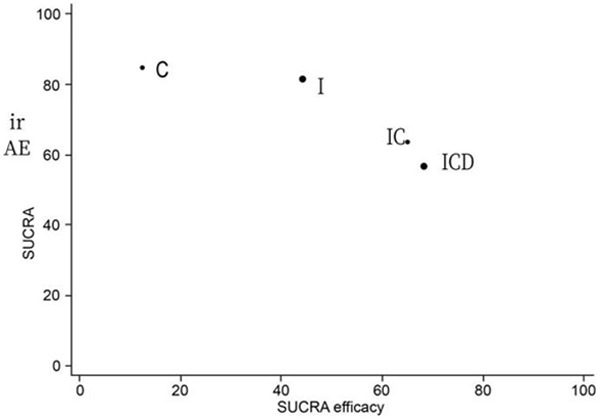
Ranking plot simultaneously representing the efficacy (X-axis) and the rate of irAEs (Y-axis) of the four examined treatment regimens, with the optimal treatment characterized by both high efficacy and tolerability and supposed to appear in the right upper corner of the graph. Both I+C+D and I+C combination regimens improved treatment efficacy and tolerability.
Discussion
Summary of key findings
This study indicated that the combination therapy of I+C+D decreased the rate of most irAEs associated with PDL1 inhibitors for the first-line treatment of NSCLC. The first-line treatment of NSCLC was developed by applying the pretreatment with dexamethasone in combination with immune checkpoint inhibitors. Utilizing network meta-analysis, differences in the effectiveness and toxicity of treatment regimens were determined, with the analyses based on the available evidence, suggesting that the combination regimen I+C+D might be the most effective and least toxic therapeutic strategy compared with other options (Figure 8). Despite a variety of PD1 antibodies developed and similar clinical trials carried out in China, the grade of evidence still revealed many problems.
Data for I+C+D treatments (e.g., sintilimab, tislelizumab, toripalimab, or camrelizumab) were predominantly collected from Chinese cohorts. In the RATIONALE 304 trial, the OS outcome was not reached. A comparison was carried out in the SHR-1210-307 trial between camrelizumab plus carboplatin plus paclitaxel and chemotherapy as first-line treatment for advanced squamous NSCLC, with an I+C+D regimen included. Reactive cutaneous capillary endothelial proliferation was the most common adverse event (approximately 75%) related to camrelizumab [22]. The therapeutic effect was not influenced by pretreatment with hormone 1 day prior to chemotherapy and infusion of immunotherapy and chemotherapy the following day, but with the reduced occurrence of side effects [19-24]. However, the side effects of immunotherapy were not affected by the second administration of immunotherapy after the application of high dose hormone. Similarly, we found that I+C+D had the best therapeutic effect, without significantly increasing the toxicity in comparison with I+C or I. Therefore, the combination may serve as a safe and effective alternative for NSCLC treatment.
Comparison with other studies
Our findings are consistent with those of previous studies. Wang et al. compared treatments with a PDL1 inhibitor alone with combined chemotherapy for the first-line treatment of NSCLC, reporting a decreased rate of most irAEs (e.g., pneumonitis and endocrine and skin reactions), and the overall rate of adverse events associated with combined therapy [31], indicating that the application of checkpoint inhibitors plus chemotherapy was different from chemotherapy alone for the first-line treatment of NSCLC, with significantly prolonged OS and PFS [32]. In the present study, we also identified that the combination of I+C+D is the most effective approach for the first-line treatment of NSCLC patients comparing to I+C, I, or C. Of note, the efficacy may be due to the poor prognosis of these patients, thus making it necessary to reduce or discontinue hormonal therapy before initiating immunotherapy [5]. In line with the results of our investigation, Wang et al. suggested the superiority of the combination treatment of I+C+D or I+C.
Limitations
While this study demonstrated that the combination treatment of I+C+D is linked to greater effectiveness and lower toxicity than the combination treatment of I+C (Figure 4), the evidence regarding the effects on PFS was mostly collected from the Chinese population, leading to a result that these conclusions may not be generalizable to other populations. Hence, the present findings are warranted to be verified with RCTs directly comparing the two therapeutic strategies in more diverse populations.
The administration of treatments after progression can mask the effectiveness. Patients who progressed, including those in the control arm, were allowed to receive treatment as those in the experimental group; this may impact the difference in OS observed between the two arms. Meanwhile, regarding toxicity, there was no available information on whether adverse events were observed before or after the treatment switch.
Research and clinical implication
Most safety assessments of PD1/PDL1 inhibitors are derived from comparisons with chemotherapy. Although indirect comparisons were used in this study, it had provided a head-to-head comparison of I+C+D and I+C. To strengthen the present conclusions, trials assessing the effectiveness and safety of treatment regimens combining hormones, PD1/PDL1 inhibitors, and chemotherapy should be conducted in the future. The results of both all-grade and high-grade outcomes were not stable in the sensitivity analysis. It should be noted that the rates of irAEs are more likely to be higher for PD1 than for PDL1, in keeping with their combination with chemotherapy. Moreover, the immune-related all-grade and high-grade outcomes were also significant for both nivolumab and pembrolizumab therapies.
Conclusions
In the absence of RCTs directly comparing I+C+D, I+C, I, and C therapies, the findings in this research suggest that I+C+D is an effective therapeutic strategy for patients with NSCLC, despite its lower tolerability compared with that observed for single agents. The present results are warranted to be verified in further investigations, particularly phase III RCT, comparing with I+C+D and I+C regimens.
Disclosure of conflict of interest
None.
References
- 1.Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, Zhang W, Song H, Bailey R, Davis D, Reid CM, Park DM, Gilbert MR. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, Riudavets M, Caramella C, Martinez G, Benitez JC, Martín-Romano P, El-Amarti L, Hendriks L, Ferrara R, Naltet C, Lavaud P, Gazzah A, Adam J, Planchard D, Chaput N, Besse B. Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI) Cancers (Basel) 2020;12:2827. doi: 10.3390/cancers12102827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, El-Amarti L, Caramella C, Bernal GM, Hendriks L, Ferrara R, Naltet C. Impact of early introduction of steroid on immune-checkpoint inhibitors (ICI) in patients with advanced non-small cell lung cancer treated. Ann Oncol. 2019;30:xi16. [Google Scholar]
- 4.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS, Mazières J, Hermes B, Cicin I, Medgyasszay B, Beatrix B. Pembrolizumab (pembro)+ chemotherapy (chemo) in metastatic squamous NSCLC: final analysis and progression after the next line of therapy (PFS2) in KEYNOTE-407. Ann Oncol. 2019;30:v918–v919. [Google Scholar]
- 5.Jove M, Vilariño N, Nadal E. Impact of baseline steroids on efficacy of programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) blockade in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(Suppl 4):S364–S368. doi: 10.21037/tlcr.2019.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeki T. The version 2.0 practice guideline for anti-emetic and standard therapy. Gan To Kagaku Ryoho. 2019;46:1683–1685. [PubMed] [Google Scholar]
- 7.Lee JH, Yu CJ, Chen KY, Shih JY, Lin YL, Yang CH. Pemetrexed for heavily pretreated patients with advanced non-small cell lung cancer. J Formos Med Assoc. 2010;109:338–344. doi: 10.1016/S0929-6646(10)60061-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: a comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;63:292–298. doi: 10.1016/j.intimp.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med. 1997;16:753–768. doi: 10.1002/(sici)1097-0258(19970415)16:7<753::aid-sim494>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 11.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 12.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 14.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101:447–459. [Google Scholar]
- 15.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 17.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, Soo R, Conter HJ, Kozuki T, Huang KC, Graupner V, Sun SW, Hoang T, Jessop H, McCleland M, Ballinger M, Sandler A, Socinski MA. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Socinski M, Creelan B, Horn L, Reck M, Paz-Ares L, Steins M, Felip E, Van den Heuvel M, Ciuleanu T, Badin F. NSCLC, metastatic CheckMate 026: a phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)-positive NSCLC. Ann Oncol. 2016;27:vi577. [Google Scholar]
- 19.Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, Chen G, Mei X, Yang Z, Ma R, Bi M, Ren X, Zhou J, Li B, Song Y, Feng J, Li J, He Z, Zhou R, Li W, Lu Y, Wang Y, Wang L, Yang N, Zhang Y, Yu Z, Zhao Y, Xie C, Cheng Y, Zhou H, Wang S, Zhu D, Zhang W, Zhang L. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (oncology program by InnovENT anti-PD-1-11) J Thorac Oncol. 2020;15:1636–1646. doi: 10.1016/j.jtho.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, Wang Q, Cheng Y, Wu F, Chen J, Lin X, Wang Y, Huang J, Cui J, Cao L, Liu Y, Zhang Y, Pan Y, Zhao J, Wang L, Chang J, Chen Q, Ren X, Zhang W, Fan Y, He Z, Fang J, Gu K, Dong X, Zhang T, Shi W, Zou J CameL Study Group. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9:305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 21.Calvani J, Elia R, Battistella M, Delyon J, Vivier-Chicoteau J, Gornet JM, Lebbé C, Baroudjian B, Bertheau P. An unusual digestive complication under anti-PD-1 (pembrolizumab) Ann Pathol. 2020;40:320–323. doi: 10.1016/j.annpat.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, Zhao J, Yu Y, Hu C, Yang K, Feng G, Ying K, Zhuang W, Zhou J, Wu J, Leaw SJ, Zhang J, Lin X, Liang L, Yang N. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:709–717. doi: 10.1001/jamaoncol.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 24.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 26.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, Hermes B, Cicin I, Medgyasszay B, Rodríguez-Cid J, Okamoto I, Lee S, Ramlau R, Vladimirov V, Cheng Y, Deng X, Zhang Y, Bas T, Piperdi B, Halmos B. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Garon EB, Novello S, Rubio-Viqueira B, Boyer M, Kurata T, Gray JE, Yang J, Bas T, Pietanza MC, Garassino MC. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 28.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S, Orlandi F, Sanborn RE, Szalai Z, Ursol G, Mendus D, Wang L, Wen X, McCleland M, Hoang T, Phan S, Socinski MA. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16:653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Liang H, Wang W, Zhao S, Cai X, Zhao Y, Li C, Cheng B, Xiong S, Li J, He J, Liang W. Immune-related adverse events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1 inhibitor alone in first-line treatment for advanced non-small cell lung cancer: a meta-analysis of randomized control trials. Cancer. 2021;127:777–786. doi: 10.1002/cncr.33270. [DOI] [PubMed] [Google Scholar]
- 30.Addeo A, Banna GL, Metro G, Di Maio M. Chemotherapy in combination with immune checkpoint inhibitors for the first-line treatment of patients with advanced non-small cell lung cancer: a systematic review and literature-based meta-analysis. Front Oncol. 2019;9:264. doi: 10.3389/fonc.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal GM, Mezquita L, Auclin E, Ferrara R, Planchard D, Masip JR, Lahmar J, Boucher M, Caramella C, Adam J. Baseline corticosteroids (CS) could be associated with absence of benefit to immune checkpoint inhibitors (ICI) in advanced non-small cell lung cancer (NSCLC) patients. 2017;28:v472. [Google Scholar]
- 32.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J. Clin. Oncol. 2019;37:1927–1934. doi: 10.1200/JCO.19.00189. [DOI] [PubMed] [Google Scholar]


