Abstract
Background: Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with wide spectrum of symptoms and few effective therapies. Evidence is suggestive of an association between immune system dysfunction and autism spectrum disorders (ASD) among children with ASD. Immunoglobulins (Ig) are found to be increased in the circulation of individuals with autism. The prospective study was aimed to estimate and correlate the levels of IgG4 in blood and saliva of children with autism. Methodology: Blood and unstimulated saliva were collected from 172 children (55 ASD, 57 healthy control, and 60 suspected parasitic infection) aged 0-18 years. Routine blood investigations were done. Serum and salivary IgG4 levels were analyzed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit. Data were subjected to statistical analysis. Results: ELISA tests showed that the IgG4 levels in serum and saliva were significantly increased (P<0.05) in children with ASD as compared to normal control children. Both serum and saliva IgG4 levels showed a significant positive correlation (P<0.05). Conclusion: IgG4 can be used as a potential biomarker for the early detection of ASD. Further, saliva can be a diagnostic, noninvasive assessment tool for health monitoring of children with autism. Lay summary: The collection of saliva is easy and painless compared to other sample collection methods. The present study shows that, among children with autism, brain-reactive antibody, immunoglobulin G4 (gG4), is increased both in blood and saliva, and there is a significant correlation between the two levels. Therefore, the study recommends IgG4 as a potential biomarker for the early detection of autism, and saliva can be helpful in diagnosis and health monitoring of children with ASD.
Keywords: Autism, children, India, ELISA, immunoglobulin G, saliva
Introduction
Autism spectrum disorders (ASD) are a group of relatively common pervasive neurodevelopmental disorders affecting 1 in 68 children (1 in 42 boys and 1 in 189 girls) [1]. According to the Centers for Disease Control and Prevention, autism is defined as developmental disabilities characterized by impairments in social interaction and communication, with restricted, repetitive, and stereotyped patterns of behavior [2]. Presently, worldwide about 1/132 kids are affected by autism [3]. Although, there are many ongoing funded studies in India to evaluate incidence and prevalence, biomarkers, autism biology, and associated risk factors, studies at the national level to evaluate the actual burden of autism are lacking [4].
The etiology of autism involves both genetic and environmental factors that play key roles. Children with autism (<10%) are known to be associated with several genetic disorders, such as tuberous sclerosis, fragile X syndrome, neurofibromatosis, and Angelman syndrome [5,6]. Environmental factors such as drugs-thalidomide, valproic acid- and prenatal, perinatal, and early postnatal infections are also known to be associated with autism [7-9]. The mercury component in many vaccines (including the MMR vaccine) was thought to cause autism. However, there is no evidence for this. In addition, most modern vaccines do not contain mercury components, which reinforces that mercury has no role in autism. Viral infections such as congenital rubella and cytomegalovirus among neonates-who were later diagnosed with autism-were associated with ASD [10].
Diagnosis of ASD is based on clinical history followed by observing and interacting with the child. There are no specific clinical markers or laboratory tests that can be used for the diagnosis of autism. However, various standardized checklists, assessment tools, and criteria are used to make a diagnosis for ASD [11]. Diagnostic and Statistical Manual of Diseases, fifth edition (DSM-V) and International Classification of Diseases tenth edition (ICD-10) have two main criteria: (i) Deficits in social communication and interaction, and (ii) restricted and repetitive patterns of behavior, interests, and activities. Childhood Autism Rating Scale (CARS) is the CDC-recommended diagnostic tool and the widely used rating scale for diagnosing and measuring the severity of autism in India [12,13]. CARS helps in the identification of children with autism, segregating them from the other developmental disorders, and differentiating the different degrees of autism.
Immune and the nervous system interacts reciprocally; evidence suggests the possible role in developing ASD. Recent studies on ASD describe immune abnormalities, such as increased levels of inflammatory mediators, and the presence of autoimmune phenomena [14]. Immunoglobulin G (IgG) is the most prevalent antibody isotype in human circulation and consists of four subclasses. Each subclass of IgG has different biological properties. IgG1 and IgG3 are predominately responsible for protection against re-infection based on their ability to activate complement, which induces platelet binding and clearance of infectious agents from the body. In contrast, IgG2 and IgG4 do not bind complement. IgG4 is univalent like Immunoglobulin E and is implicated in allergy development. It is speculated that IgG4, produced in response to chronic exposure, can be a blocking antibody for immune regulation. The clinical implications of skewed antibody subclasses are unclear. Recently Bayram et al. reported no association between autism and positivity of antibodies such as, anti-GAD, anti-GluR, and anti-ganglioside [15]. Zaman et al. used libraries of synthetic compounds (called as peptoids), and found reduced levels (>50%) of IgG1 [16]. Croonenberghs et al. demonstrated increased serum IgG2 and IgG4 concentrations in children with autism, which were associated with specific behavioral outcomes [17]. Enstrom et al., also found increased IgG4 levels in children with autism disorder [18]. So-Nam Kim et al. used an autistic animal model to demonstrate significantly higher levels of serum IgG1, IgG2 and IgG3 as compared to a highly social control strain [19]. Thus, although it is known that there Is generation of the specific anti-brain autoantibody, the process is unclear. In addition, a detailed investigation is necessary to elucidate the relationship between immunological findings and behavioral impairments in autism. Identification of specific targets increases the therapeutic possibilities. Since it is proved that serum IgG4 is increased in ASD, it is expected to increase in saliva as well. Saliva is easily available, easy to collect and the procedure of collecting samples is noninvasive. It can also be collected from the newborn without causing anxiety in the parents, so early detection is possible. Therefore the present study is designed to estimate IgG4 levels in saliva, a noninvasive method, which can be one of the biomarkers of ASD. Serum and salivary IgG4 levels were compared among three groups of children: autistic disorder, typical development (normal controls), and parasitic infection.
Materials and methods
Study design and population
The prospective case-control study was conducted at the Yenepoya (Deemed to be University), Mangaluru, Karnataka, India, between May 2015 and June 2017. Study sites located in and around Mangaluru, and nearby districts of Karnataka and Kerala were identified. Surveys and camps were conducted at the identified study sites. A flow chart of the study design is given in Figure 1. The study sites/centers included, for group 1-registered special schools for children with ASD, group 2-healthy children from the nearby primary schools, and for group 3-parasite cases included children seeking healthcare support in different wards of Yenepoya Medical College Hospital, Yenepoya Dental College and local hospitals. A detailed list of study sites with the data of visit is attached in Supplementary Table 1. Special dental camps were organized at these study sites to provide oral hygiene and autism awareness programs to the parents/caregivers of children with ASD.
Figure 1.

Flowchart detailing the study design and methodology of the study.
Ethical concern
Ethical approval was obtained by the Institutional Ethical Committee of Yenepoya (Deemed to be University) (Ref no. YUEC 39/5/2/2014) prior to conducting the study. Whenever possible, oral assent was taken from the children. Written consent was taken, in the local understandable language, from either parents or guardian of every child or the institutional head prior to inclusion in the study.
Identifying participant and Inclusion and exclusion criteria
At the time of recruitment, a health professional performed a clinical and general physical examination, including height, weight, vital signs and symptoms, of all participants to ensure the health and review inclusion-exclusion criteria. All the participants were examined by a senior psychiatrist, physician, and a dentist. Children were recruited to the three study groups: group 1 children with autism, group 2 control subjects with normal intellectual functioning, and group 3, a positive control group of parasite infection.
Autism group
Children with autism, aged ≤18 years, were screened for autism using CARS (Childhood Autism Rating Scale). CARS score assessment Performa is a 15-item behavioral rating scale developed to identify children with autism and categorize these behaviors from mild to moderate to severe. Further, autistic children were classified based on the total score as non-autistic (15-29.5), mild-moderate (30 to 36.5), or severe (≥37-60).
Control group
Children from nearby primary schools and those visiting the hospital, with good mental and physical health-no history of stomach or gut problems such as chronic diarrhea, constipation, gas, heartburn, bloating, etc.
Parasitic group
Elevated IgG4 are reported in patients with gastrointestinal infection [20-22], therefore as a positive control, children with symptoms suggestive of infection like abdomen pain, vomiting, and diarrhea (requiring prescription of an anthelmintic drug) or history of parasite worms in stool were included in the group.
Exclusion
In all the groups, children with any history of fever in the last two weeks, severe malnutrition, and asthenia (as reported by the caregiver or Nursing staff), dental issues such as bleeding gums were excluded from the study.
Participant recruitment and data collection
The surveys and awareness camps covered the 1478 special children and 883 healthy controls and parasitic cases from surveys and awareness camps. A total of 423 children were identified to fulfill the study’s inclusion-exclusion criteria (Figure 1). A total of 333 consents were obtained from the children’s parents or guardians in the local understandable language prior to participation from different study sites. Finally, a total of 172 participants who met the study criteria were recruited in the study. Demographic information was obtained for each study participant, including age, gender, siblings, health status during pregnancy, nutritional status, height, weight, and medication history, and family history of ASD.
Sample collection
All the samples were collected from 10 AM to 2 PM.
Saliva
Passive drool samples were obtained by asking participants to pool saliva in their mouths and deposit it into a sterile polypropylene (12×75 mm) culture tube (5 mL capacity). A sterile absorbent cotton roll was placed in the mouth under challenging cases until it was saturated (approximately 30-40 sec) and deposited back into the container. Research staff met child-participants individually to supervise that, collection protocol was adhered to uniformly by all the participants, adequate sample was collected, the participants did not touch the saliva and/or materials that contained saliva, and to assure proper measures were taken for the storage of saliva.
In order to avoid the possibility of contaminating substances in the saliva, which could interfere with the immunoassay, it was recommended that the participants or caregiver with nil per os with following precautions for research participants:
• Do not eat a major meal within 60 min of sample collection.
• Avoid dairy products for 20 min before sample collection.
• Avoid foods with high sugar or acidity, or high caffeine content, immediately before sample collection, since they may compromise the assay by lowering pH of the saliva, and increasing bacterial growth.
Serum sample
The blood samples (4 mL) were collected by vein puncture method at appropriate conditions. A syringe with a 21G needle was used, and the collected blood distributed equally in three vacutainer (Sodium citrate, EDTA, and plain) tubes. Sodium citrate and EDTA tubes were inverted 4-5 times for mixing the tube contents.
Transport and processing of the sample
Saliva and blood samples were immediately placed in a sample transport box containing frozen ice packs and shipped to the research lab on the same day. Sodium citrate and EDTA tubes were processed at the Yenepoya Central laboratory facility for routine blood tests. Saliva and plain tube were centrifuged, aliquoted, and were stored at -20°C until assay. Details of sample processing is given alsewhere [23].
Testing of samples
Routine blood investigation
Routine blood investigations, such as hemoglobin levels (Sahil’s method), total count (Sysmex XN-1000 hematology analyzer, USA), and erythrocyte sedimentation rate (Westergren method) using Ves-Metric cube30 (Transasia Bio-Madical, India), were done at the Yenepoya Hospital central laboratory facility.
Measurement of IgG4
All samples were assayed in duplicate using the commercial enzyme-linked immunosorbent assay (ELISA) kit (Human IgG4 Ready-Set-Go Kit, eBioscience, San Diego, CA, USA; #88-50590). The assay procedure was followed as per the manufacturer’s instructions. The absorbance was measured at 450 nm in the ELISA plate reader (FLUOstar Omega; BMG Labtech, Ortenberg, Germany). The working range of the Human IgG4 Ready-Set-Go Kit is 2000-31.3 ng/mL.
Statistics analysis
Data were analyzed by SPSS version 15. An initial frequency count of all variables was done. The mean, ranges, and standard deviation of the age and IgG4 levels were compared using the ANOVA test. Correlation between IgG4 levels was compared using the Pearson correlation variable. IgG4 level group-wise comparison was performed using the Independent “T” test. One-way analysis of variance (ANOVA) was used to compare the intergroup, and post hoc multiple comparisons were carried out using Tukey’s honest significant difference (HSD) test. The level of significance was set at P≤0.05.
Results
Demographic details
Figure 1 indicates the method of sample collection for the study. Samples collected for the study (n=172) in the three study groups were: Group 1 ASD (n=55), Groups 2 Healthy control (n=57), and Group 3 Suspected parasite infection (n=60). Table 1 shows that all the three study groups had predominantly male participants as follows: Group 1 (80%) > Group 2 (77%) > Group 3 (66%). The differences in the mean age of children in groups 1, 2, and 3, were 10.7±4.2 y, 11.2±2.7 y, and 9.2±2.7 y, respectively, were non-significant (P>0.05).
Table 1.
Description of the participants in the study groups (n=172)
| Parameters | ASD (n=55) | Control (n=57) | Parasite (n=60) |
|---|---|---|---|
| Gender n (%) | |||
| Male | 42 (76.4) | 44 (77.2) | 40 (66.7) |
| Female | 13 (23.6) | 13 (22.8) | 20 (33.3) |
| Age (y) | |||
| Mean ± SD | 10.7±4.2 | 11.2±2.7 | 9.2±2.7 (NS) |
| Range | 2.5-18 | 6-18 | 3-14 |
ASD: autism spectrum disorder; SD: standard deviation. The data for gender is represented as the frequency with percentage in parenthesis, and age is represented as mean ± SD and range as interval of minimum and maximum.
As shown in Figure 2, the BMI of the participants in the three groups was significantly different (P=0.017). Groups 1 participants had the highest BMI (20.8±2.6) compared to the control (15.7±0.4) and parasite (15.4±0.4) group.
Figure 2.

Body mass index of the study groups. The data represented is mean ± SD of the body mass index of the participating children in the three study groups: Group 1 autism spectrum disorder (ASD), Group 2 healthy controls, and Group 3 suspected parasitic infection.
Clinical findings
Categorization of the participants in group 1 (ASD), based on the severity of autism, using CARS showed that majority of the participants (60%) had severe autism and the rest (40%) had mild to moderate autism (Table 2). Family history of ASD was found in three cases among group 1: two had cousins with autism, and one participant had an uncle with autism.
Table 2.
Categorization of the children with autism diagnosis based on the CARS (n=55)
| Sl. No. | ASD diagnosis using CARS | n (%) | |
|---|---|---|---|
|
| |||
| Category | Interval of CARS scores | ||
| 1. | Non-autistic | 15.0-29.5 | 0 (0.0) |
| 2. | Mild-moderate autism | 30.0-36.5 | 22 (40.0) |
| 3. | Severe autism | 37.0-60.0 | 33 (60.0) |
The data represented is frequency with percentage in parenthesis. Abbreviations used: CARS-Childhood Autism Rating Scale (CARS). Group 1 participants with autism were categorized based on the scores obtained from the assessment using the CARS.
Among ASD children, 34.5% (19/55) were had a history of medications with psychiatric drugs such as oxcarbazepine (1), serenace (Haloperidol) (1), sodium valproat e (6) atomoxetine (1) levera (Levetiracetam) (1), sizodon (Risperidone) (1) lamitor (Lamotrigine) (1), decorate (Divalproex) (1), alprax (Alprazolam) (1), and ayurvedic medicines (2) for the treatment of either epilepsy, hyperactivity or to control seizures. Dental carries were found in 18.2% (10/55), 3.5% (2/57), and 8.3 % (5/60) among ASD, control, and parasitic group children, respectively. History of parasitic infection was found in 7.2% (4/55) in autistic children. The number of siblings in the ASD group was 1 (IQR 2, 3) in ASD, while in the control group, it was 2 (IQR 2, 4).
Laboratory findings
Routine blood analysis showed no significant differences in the hemoglobin levels, total WBC count, and ESR among the three groups (Table 3). Although, the ESR levels were higher in ASD and parasitic samples, they were not significantly different (P>0.05).
Table 3.
Comparison of the blood parameters among the study groups (n=172)
| Sl. No. | Parameters | ASD (n=55) | Control (n=57) | Parasite (n=60) | P value |
|---|---|---|---|---|---|
| 1. | Hb (gm/dL) | 12.75±1.67 | 12.74±1.78 | 12.56±1.1 | 0.776 |
| 2. | Total WBC count | 11556.3±9773.1 | 8660.0±3571.1 | 8642.5±2966.5 | 0.067 |
| 3. | ESR (mm) | 12.7±9.4 | 9.7±7.1 | 14.8±14.2 | 0.089 |
| 4. | Platelets (×103/mm3) | 315.0±105.9 | 328.9±77.2 | 338.1±80.4 | 0.584 |
Data represented is mean ± SD. Abbreviations used: ASD: autism spectrum disorder; SD: standard deviation; ESR: erythrocyte sedimentation rate. Statistical test used: ANOVA; Level of significance: P<0.0, P>0.05 was considered non-significant.
IgG4 profile of saliva and serum
The levels of IgG4 in all samples were tested using the commercially available kit. The Table 4 shows that the IgG4 levels in serum (mg/dL) decreased as follows: Group 1 ASD (44.80±0.76) > Group 3 Suspected of parasitic infection (38.81±17.54) > Group 2 normal control (34.90±20.19) mg/dL and were significantly different (P=0.028). Although the levels of IgG4 (mg/dL) were less in saliva, it showed a similar pattern: Group 1 ASD (0.89±0.69) > Group 3 Suspected of parasitic infection (0.56±0.38) > Group 2 normal control (0.74±0.79) mg/dL, which were significantly different (P=0.033).
Table 4.
Comparison of salivary and serum IgG4 in the three study groups (n=172)
| Sample | ASD (n=55) | Control (n=57) | Parasite (n=60) | P value | |
|---|---|---|---|---|---|
| Saliva (mg/dL) | Mean ± SD | 0.89±0.69 | 0.56±0.38 | 0.74±0.79 | 0.033* |
| Range | 0.09-3.28 | 0.06-1.73 | 0.12-5.27 | - | |
| Serum (mg/dL) | Mean ± SD | 44.80±20.76 | 34.90±20.19 | 38.81±17.54 | 0.028* |
| Range | 11.43-88.88 | 1.82-79.61 | 6.68-93.59 | - | |
Data represented is group statistics as mean ± SD and the range as interval. Abbreviations used: ASD: autism spectrum disorder; SD: standard deviation. Statistical test used: One-Way ANOVA; Level of significance:
P<0.05 was considered as significant.
There was significant positive correlation between the IgG4 levels in the saliva and serum of Group 1 autistic and Group 2 healthy control children (Figure 3). There were significantly increased levels of the IgG4 subclass in children with 0.89 (SD ±0.69) mg/dl compared to healthy controls 0.56±0.38 mg/dL. There was a significant and positive correlation for IgG4 levels of salivary and serum in ASD (P<0.05) and normal children (P<0.05) (Figure 4). Also, compared with BMI, serum and saliva IgG4 levels did not show significant correction with any group (Supplementary Figure 1). Details of statistical analysis is given in Supplementary Table 2.
Figure 3.

Correlation between the salivary and serum IgG4 levels in (A) ASD, (B) Normal control and (C) Suspected parasitic infection groups. Abbreviations used: ASD-children with Autism spectrum disorders, Statistical tests used: Karl Pearson correlation Level of significance: P<0.05 was considered significant.
Figure 4.
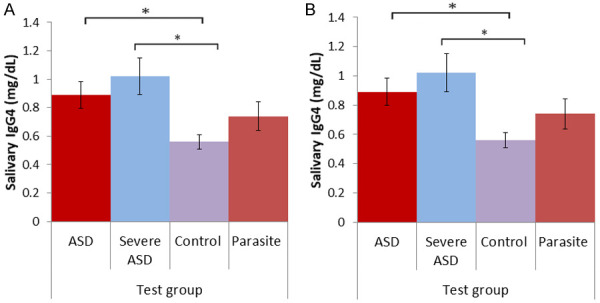
Comparison of the IgG4 levels in (A) saliva and (B) serum in the study groups. The data represented is mean ± SD of the IgG4 levels in the study groups. Abbreviations used: ASD-Autisim Spectrum disorders, Statistical tests used: ANOVA for multigroup comparison. Post hoc Tukey honest test for two group comparisons. Level of significance: P<0.05 was considered significant.
Discussion
A comprehensive study for the comparison of serum and salivary IgG4 among children with autism (ASD) is not reported till date. In the present study IgG4 levels in serum and saliva were compared between three groups: children with ASD, children suspected intestinal infection (positive control of IgG4) and typically developing children as controls. The findings reveal that the elevated levels of IgG4 in serum among autistic children, which had a positive correlation with the salivary IgG4. This is suggestive of the usefulness of salivary IgG4 levels for early detection of ASD, and confirms that saliva can be a noninvasive assessment tool for health monitoring among children with ASD.
The interaction of the central nervous system with the immune system during disease-associated anorexia is already reported [24]. In ASD, there is evidence that the brain communicates with the cells of the immune system, which results in active or passive (via the bloodstream) mobility. Early diagnosis and timely appropriate intervention is the key to the best treatment for ASD. Unfortunately, most children are not diagnosed until about four years of age, when communication and social disabilities become apparent [25]. Therefore, a reliable biomarker that can aid in the earlier diagnosis of children with ASD is the need of the hour.
The present study results suggest that the elevated IgG4 levels in children with ASD compared to typically developing children may be due to alterations in the autoimmune system. Earlier reports have also demonstrated increased IgG4 levels in autism [17,18]. The present study shows that increased IgG4 levels are associated with increasing severity of aberrant behaviors in autism. IgG4 is a unique IgG subclass as it has a less binding affinity with the receptors of immune cells and having a single binding site compared with other subclass IgG1, IgG2, and IgG3 [26]. These attribute different biological functions of IgG4 by shifting its protecting characteristic to blocking/inhibiting antibodies. The increased IgG4 in ASD children could be due to chronic self-antigen exposure, poor microbial antigen clearance, or some other immune abnormality. All these situations are equally indicative of its neuroactive activities in autism [18].
The novelty of the present study is that it shows a good correlation of saliva and serum IgG4 in both children with ASD and the healthy normal children for the first time. It also supports the earlier reports, which suggest saliva as an alternate diagnostic tool in ASD [27,28]. Diagnosis of the inflammatory markers and immunological parameters are generally done with blood and CSF samples. Blood as a diagnostic tool has an advantage because it directly measures the immune cell products. However, blood collection is an invasive procedure and is more costly as it requires a trained technician and the use of needles. In special children, blood collection is even difficult as these children may show anxiety and/or fear when they present for blood collection. Children with ASD sometimes show severe phobias towards it [29]. Therefore, although more children with autism were identified (n=193) to be suitable for participation in the study, only few of them (n=55) were recruited for the study. Alternate body fluids which can be collected by noninvasive techniques are saliva and urine; They are most suitable for participants having anxiety/fear towards body pricks for sample collection. Saliva is the easiest sample that can be collected noninvasively with minimal armamentarium and is associated with fewer compliance problems as compared to blood [30] and is very useful for pediatric care [31,32].
Limitations of the study: The present comparative study focused only on a few of biochemical parameters for the saliva and blood to have a diagnostic marker in autism. There are limited studies from India. However, the clinical profiles of the participants were not studied. Confirmation of the parasite group by laboratory also was not possible. Larger sample size and the clinical profiles of the participants are required to make conclusions regarding associations between the immunological profile, and age, clinical profile, or factors determining the severity autism.
Conclusions
The study concludes that the elevated serum and salivary IgG4 levels correlated significantly among children with autism. There was a significant correlation among the typically developing control children as well. Therefore, the study confirms the use of IgG4 as a potential biomarker for the early detection of ASD. Saliva, a noninvasive assessment tool, can be used for diagnosis and health monitoring.
Acknowledgements
We thank all the institutions, centers, medical staff, and faculty members from the Department of Paediatrics and Department of Pedodontics at Yenepoya Deemed to be University for extending their support for this study. The authors also acknowledge Ms. Kousalya Konila for supporting the initial stage of the project. The authors are grateful to Professor Vinitha Ramanath Pai for language editing, two referees, and the editor for comments. This study was funded by the Indian Council of Medical Research, New Delhi, India (No. Autism/14/2010-NCD-1) to S.S.B. B.S.K. received the “Low Income Countries Travel Award” by International Society for Autism Research (INSAR). This study was presented in part at the 17th Annual International Society for Autism Research (INSAR) 2018 Annual Meeting, Rotterdam, Netherlands (Abstract no. 167.119).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 2.Basco MR, Birchler GR, Kalal B, Talbott R, Slater MA. The clinician rating of adult communication (CRAC): a clinician’s guide to the assessment of interpersonal communication skill. J Clin Psychol. 1991;47:368–380. doi: 10.1002/1097-4679(199105)47:3<368::aid-jclp2270470308>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Raina SK, Kashyap V, Bhardwaj AK, Kumar D, Chander V. Prevalence of autism spectrum disorders among children (1-10 years of age)-findings of a mid-term report from Northwest India. J Postgrad Med. 2015;61:243–246. doi: 10.4103/0022-3859.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patra S, Kar SK. Autism spectrum disorder in India: a scoping review. Int Rev Psychiatry. 2021;33:81–112. doi: 10.1080/09540261.2020.1761136. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov HY, Stoyanova VK, Popov NT, Vachev TI. Autism spectrum disorder-a complex genetic disorder. Folia Med (Plovdiv) 2015;57:19–28. doi: 10.1515/folmed-2015-0015. [DOI] [PubMed] [Google Scholar]
- 6.Zachariah SM, Koshy B. Clinical features and diagnosis of autism spectrum disorder in children. Curr Med Issues. 2017;15:6–16. [Google Scholar]
- 7.Karimi P, Kamali E, Mousavi SM, Karahmadi M. Environmental factors influencing the risk of autism. J Res Med Sci. 2017;22:27. doi: 10.4103/1735-1995.200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalal BS, Pai VR, Upadhya D. Valproic acid reduces tumor cell survival and proliferation with inhibitors of downstream molecules of epidermal growth factor receptor pathway. J Pharmacol Pharmacother. 2018;9:11–16. [Google Scholar]
- 9.Kalal BS, Pai VR, Behera SK, Somashekarappa HM. HDAC2 inhibitor valproic acid increases radiation sensitivity of drug-resistant melanoma cells. Med Sci (Basel) 2019;7:51. doi: 10.3390/medsci7030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson M. Vaccination as a cause of autism-myths and controversies. Dialogues Clin Neurosci. 2017;19:403–407. doi: 10.31887/DCNS.2017.19.4/mdavidson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH Jr, Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE, Minshew NJ, Ozonoff S, Prizant BM, Rapin I, Rogers SJ, Stone WL, Teplin SW, Tuchman RF, Volkmar FR. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 12.Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013;12:92–98. doi: 10.1002/wps.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 14.Kalal BS, Bhat SS, Pai VR, Veena KM, Kakuje A. Role of the immune system in the biology of autism spectrum disorders. Int J Pharma Bio Sci. 2016;7:853–859. [Google Scholar]
- 15.Bayram AK, Kardas F, Demirci EO, Gokahmetoglu S, Ozmen S, Canpolat M, Oztop DB, Kumandas S, Gumus H, Per H. Lack of serum antineuronal antibodies in children with autism. Bratisl Lek Listy. 2016;117:77–79. doi: 10.4149/bll_2016_015. [DOI] [PubMed] [Google Scholar]
- 16.Zaman S, Yazdani U, Deng Y, Li W, Gadad BS, Hynan L, Karp D, Roatch N, Schutte C, Nathan Marti C, Hewitson L, German DC. A search for blood biomarkers for autism: peptoids. Sci Rep. 2016;6:19164. doi: 10.1038/srep19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 18.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SN, Jo GH, Kim HA, Heo Y. Aberrant IgG isotype generation in mice with abnormal behaviors. J Immunotoxicol. 2016;13:92–96. doi: 10.3109/1547691X.2015.1014581. [DOI] [PubMed] [Google Scholar]
- 20.Ansari NA, Kumar R, Raj A, Salotra P. Elevated levels of IgG3 and IgG4 subclass in paediatric cases of kala azar. Parasite Immunol. 2008;30:403–409. doi: 10.1111/j.1365-3024.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurniawan A, Yazdanbakhsh M, van Ree R, Aalberse R, Selkirk ME, Partono F, Maizels RM. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–3950. [PubMed] [Google Scholar]
- 22.Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, Bradley JE. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–996. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Nishana E, Bhat SS, Sahana KS, Hegde SK, Bhat V, Kalal BS. Estimation of salivary sCD14 in children with early childhood caries in association with pneumonia. Rep Biochem Mol Biol. 2019;8:132–138. [PMC free article] [PubMed] [Google Scholar]
- 24.Gabriel T, Paul S, Berger A, Massoubre C. Anorexia nervosa and autism spectrum disorders: future hopes linked to mucosal immunity. Neuroimmunomodulation. 2019;26:265–275. doi: 10.1159/000502997. [DOI] [PubMed] [Google Scholar]
- 25.Kalal BS, Pai VR, Bhat SS. Autism treatment challenges: need for accelerated research in pharmacological interventions. Clinical Biotechnology and Microbiology. 2016;1:9–10. [Google Scholar]
- 26.van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–3571. [PubMed] [Google Scholar]
- 27.Hicks SD, Rajan AT, Wagner KE, Barns S, Carpenter RL, Middleton FA. Validation of a salivary RNA test for childhood autism spectrum disorder. Front Genet. 2018;9:534. doi: 10.3389/fgene.2018.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngounou Wetie AG, Wormwood KL, Russell S, Ryan JP, Darie CC, Woods AG. A pilot proteomic analysis of salivary biomarkers in autism spectrum disorder. Autism Res. 2015;8:338–350. doi: 10.1002/aur.1450. [DOI] [PubMed] [Google Scholar]
- 29.Mental illness and the brain. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20369/NIoHUBSCSNCSSIBMNIoHUIa.
- 30.Castagnola M, Picciotti PM, Messana I, Fanali C, Fiorita A, Cabras T, Calo L, Pisano E, Passali GC, Iavarone F, Paludetti G, Scarano E. Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngol Ital. 2011;31:347–357. [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes LA, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Med (Zagreb) 2015;25:177–192. doi: 10.11613/BM.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding HT, Taur Y, Walkup JT. Gut microbiota and autism: key concepts and findings. J Autism Dev Disord. 2017;47:480–489. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


