Abstract
Narcolepsy is a neurological disorder characterized by sleepiness and episodes of cataplexy. Cataplexy is an abrupt loss of muscle tone, most often triggered by sudden, strong emotions. A subset of cells in the medial medulla of the narcoleptic dog discharged at high rates only in cataplexy and rapid eye movement (REM) sleep. These cells were noncholinergic and were localized to ventromedial and caudal portions of the nucleus magnocellularis. The localization and discharge pattern of these cells indicate that cataplexy results from a triggering in waking of the neurons responsible for the suppression of muscle tone in REM sleep. However, most medullary cells were inactive during cataplexy but were active during REM sleep. These data demonstrate that cataplexy is a distinct behavioral state, differing from other sleep and waking states in its pattern of brainstem neuronal activity.
THE NARCOLEPTIC DOG EXHIBITS most of the symptoms of human narcolepsy. It has episodes of cataplexy, the loss of antigravity muscle tone triggered by emotional excitement. It also has periods of REM sleep just after sleep onset and increased sleepiness, as in the human condition (1). Canine and human cataplexy have similar pharmacological responses; both are exacerbated by α1, noradrenergic blockers (2) and improved by amphetamine, methylphenidate, and related drugs and by antidepressants (3–5). Both human and canine narcolepsy are genetically determined (4, 6).
It has been hypothesized that narcolepsy is a disease of REM sleep regulation (7). Accordingly, the cataplexy and sleep paralysis of narcolepsy represent a triggering during waking of mechanisms that normally suppress muscle tone during REM sleep. Similarly, the hypnagogic hallucinations of narcolepsy result from a release of the dream imagery of REM sleep into waking, and the REM sleep periods at sleep onset result from a loss of mechanisms that normally delay this state until after non-REM sleep. With the narcoleptic dog, one can investigate this hypothesis at the cellular level.
The suppression of muscle tone during REM sleep requires the integrity of the dorsolateral pons (8) and the medial medulla (9). Chemical stimulation studies have identified two distinct medullary regions that mediate muscle tone suppression: a rostroventromedial region corresponding to the ventral and caudal portions of the nucleus magnocellularis (NMC) and a caudomedial region corresponding to the nucleus paramedianus (10). A cell type within the medial medulla and dorsolateral pons has a high discharge rate during REM sleep and a low discharge rate during both active and quiet waking (11–14). This cell type is absent in adjacent pontine and medullary regions that are not required for atonia (15, 16). If cataplexy represents an abnormal activation of the atonia mechanism of REM sleep, then there should be a population of cells that is maximally active during both REM sleep and cataplexy in these regions. To search for these cells, we recorded the unit activity in the medial medulla of the narcoleptic dog during sleep-waking states and during cataplectic attacks.
Four narcoleptic dogs (Dobcrman-Labrador crossbreeds) were implanted through the interparietal bone with modified microdrives of the type that we have used in the freely moving cat (16). Each drive propelled two bundles of seven 32-μm microwires, and each animal had two microdrives. The microdrives passed through the transverse sinus, necessitating careful hemostasis during surgery, and then through the cerebellum and fourth ventricle, with the microwires projecting into the medulla. Stereotaxic coordinates were adjusted from the atlas of Lim and co-workers (17) on the basis of bone and x-ray landmarks.
The microwires were scanned for unit activity during REM sleep periods and during cataplectic episodes, as well as during quiet and active waking states. Cataplexies were elicited by play and by the presentation of preferred foods. Physostigmine (0.05 mg per kilogram of body weight) was administered in some cases to increase the frequency of cataplexy (1, 2, 5). Immunocytochemical processing for the identification of choline-acetyltransferase (CHAT)-containing cells was performed as previously described (18).
Two distinct cell types were observed in the medial medulla (19). The most common cell type was maximally active in REM sleep and waking but showed decreased discharge during cataplexy (Figs. 1A and 2A and Table 1). We call these “cataplexy-off’ cells (20). The discharge rates of these cells during active waking were significantly higher than, their discharge rates during quiet waking (P < 0.005; t test). Sixty percent (52 of 86) of the medial medullary cells we encountered were of this type.
Fig. 1.
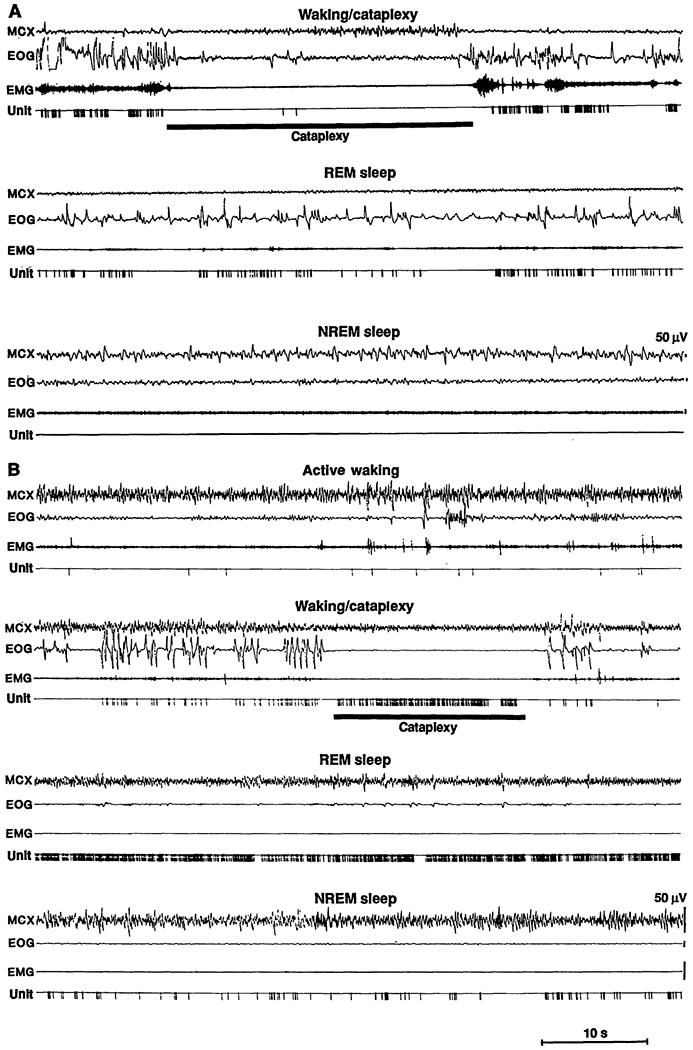
(A) Discharge pattern of a cataplexy-off cell. MCX, electroencephalogram from motor cortex; EOG, electro-oculogram; EMG, electromyogram from nuchal musculature; Unit, pulse output of window discriminator triggered by neuronal activity; NREM sleep, non-REM sleep. (B) Discharge patteren of a cataplexy-on cell.
Fig. 2.
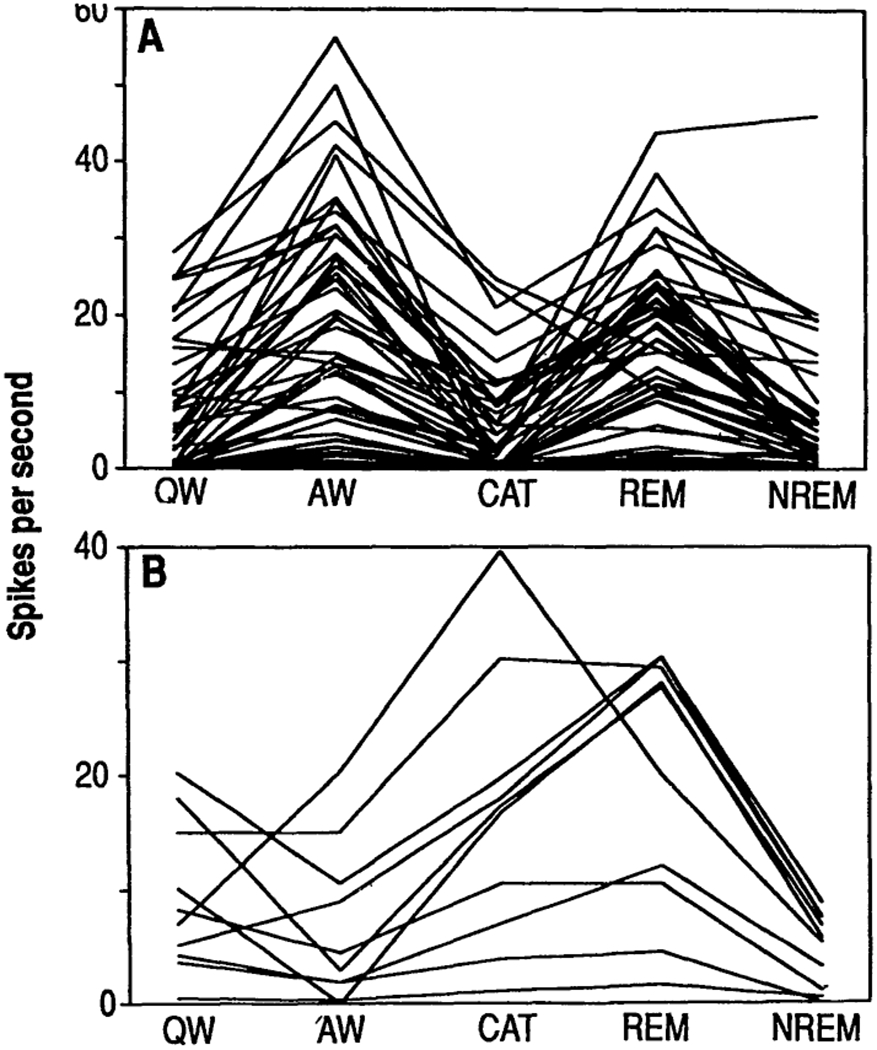
Discharge rates in (A) cataplexy-off and (B) cataplexy-on cells during sleep, waiking, and cataplexy states. QW, quiet waking; AW, active waking; CAT, cataplexy; REM, REM sleep, NREM, non-REM sleep.
Table 1.
Discharge rates (spikes per second) of medial medullary cells in sleep-wake states and cataplexy.
| Cell type | Quiet waking | Active waking | Cataplexy | REM | Non-REM | n |
|---|---|---|---|---|---|---|
| Cataplexy-off | 8.8 | 20.8 | 5.4 | 17.9 | 6.9 | 52 |
| Cataplexy-on | 9.2 | 6.7 | 16.4 | 19.5 | 4.6 | 10 |
| Other | 13.1 | 15.8 | 13.9 | 23.3 | 12.4 | 24 |
Twelve percent (10 of 86) of the cells had a very different pattern of activity. These cells were active in both REM sleep and cataplexy (Figs. 1B and 2B). Furthermore, their discharge patterns in both states were similar, with shortened modal interspike intervals and a reduction in interspike intervals over 300 ms (Fig. 3A). We call these cells “cataplexy-on” cells. They increased their discharge rate at or before the point of muscle tone decrease in cataplexy (Fig. 3B). In contrast to the cataplexy-off cells, which significantly increased their firing rate in active waking, the cataplexy-on cells fired at significantly higher rates in quiet waking than in active waking (Figs. 1 and 2 and Table 1) (21). Cataplexy-on cells were concentrated in the ventromedial and caudal portions of the NMC (Fig. 4). Immunocytochemical staining for CHAT was conducted at the sites of six of the cataplexy-on cells. All of the units were in regions devoid of CHAT. In the same sections, motoneurons of the nucleus ambiguus and of the facial nucleus were strongly labeled. All cataplexy-on cells had a high discharge rate in REM sleep; no cells were on during cataplexy but off during REM sleep. The remaining 28% (24 of 86) of the medial medullary cells did not show marked changes in discharge rate with cataplexy.
Fig. 3.
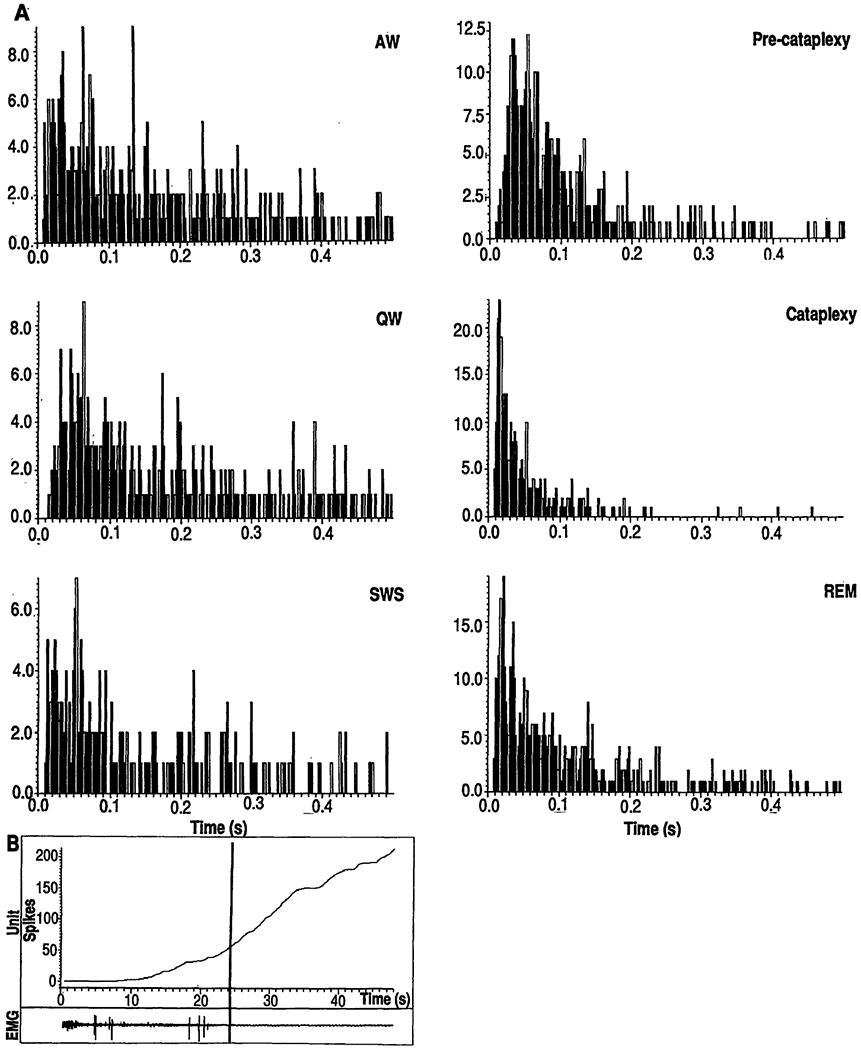
(A) Interspike interval histograms of a cataplexy-on cell as a function of state. The x axis is the Interspike interval in seconds; the y axis is the frequency of spikes separated by interval. SWS, slow wave sleep (non-REM sleep). (B) Cummulative histogram of the discharge of a cataplexy-on cell. Vertical line, point of a cataplexy onset. Lower trace shows a computer plot of the EMG during the period of the histogram.
Fig. 4.
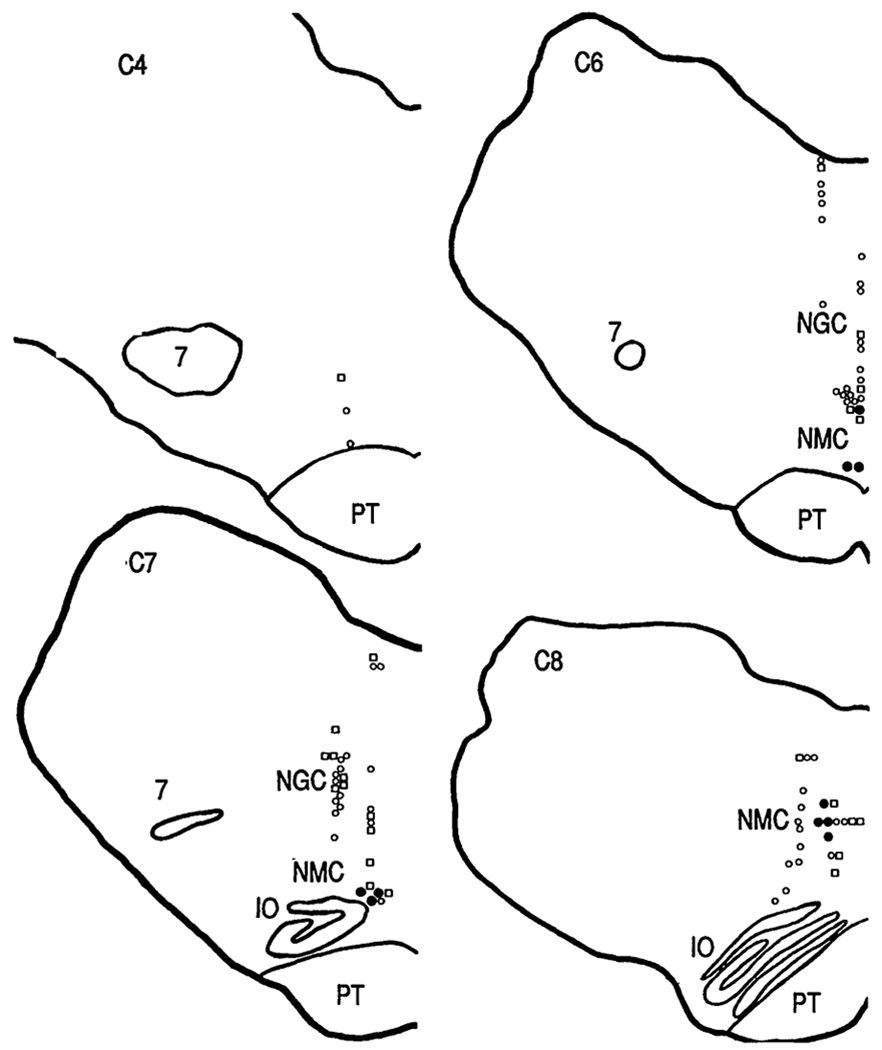
Anatomical distribution of cataplexy-related cells. C4, C6, C7, C8, anterior-posterior levels from the atlas of Lim et al. (17). Filled circles, cataplexy-on cells; open circles, cataplexy-off cells, squares, cells not changing rate by >50% with cataplexy. IO, inferior olive; NGC, nucleus gigantocellularis; NMC, nucleus magnocellularis; PT, pyramidal tract; 7, facial nucleus.
The existence of a cell population, with a common pattern of activity in cataplexy and REM sleep, that is localized to the area implicated in intact and decerebrate animals in atonia control (9–12, 14, 22) is consistent with the thesis that cataplexy and REM sleep atonia have a common basis. In the decerebrate animal, microinjection of glutamate and corticotropin-releasing factor (CRF) in the area where the cataplexy-on cells are concentrated rapidly induces atonia (10). Therefore, we hypothesize that the cataplexy-on cells have glutamate or CRF receptors (or both) or receive projections from colocalized neurons containing these receptors. The ventromedial and caudal portions of the NMC, where more than 40% of the cells were cataplexy-on cells (Fig. 4), have few CHAT-containing cells and none were seen in our CHAT-labeled sections containing cataplexy-on cells (23). Although glycine has been identified as the transmitter responsible for postsynaptic inhibition of motoneurons in REM sleep (24), the medullospinal cells that mediate atonia are likely to terminate on interneurons rather than directly on motoneurons (25). Thus, the transmitter utilized by the cataplexy-related cells is as yet unknown.
Because elevated numbers of muscarinic receptors have been found in the medial medulla of the narcoleptic dog (26), the cataplexy-on cells may be part of the pathological process responsible for cataplexy. Alternatively, they may be in the final common path triggering the suppression of muscle tone and may be responding normally to a pathological excitation from higher brainstem levels.
Although cataplexy-on cells had similar firing patterns during cataplexy and REM sleep, most medial medullary cells (cataplexy-off cells) had very different firing patterns in these two states. These cells had high discharge rates in REM sleep but were silent or had a greatly reduced discharge in cataplexy. Most cells in the medial pons of the narcoleptic animal also have this discharge pattern (27). The results in the normal cat (16, 28, 29) and our results in the narcoleptic dog indicate that cells active during waking and REM sleep, but inactive during cataplexy, are involved in the generation and expression of the phasic motor, autonomic, and sensory events that characterize both REM sleep and active waking states. The distinct discharge rates of most medullary and pontine cells during cataplexy and REM sleep indicate that the generalized phasic activation of brainstem neurons that characterizes REM sleep does not occur in cataplexy. This lack of brainstem activation may be related to the preservation of consciousness of the outside world that occurs during cataplectic states but not during REM sleep periods (7).
Acknowledgments
Supported by the Medical Research Service of the Veterans Administration; Public Health Service grants NS14610, HL41370, and NS23724; and the American Narcolepsy Association. We thank S. Goodman for advice on neurosurgical procedures.
Contributor Information
Jerome M. Siegel, Neurobiology Research, Veterans Affairs Medical Center, Sepulveda, CA 91343, and Department of Psychiatry and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024.
Robert Nienhuis, Neurobiology Research, Veterans Affairs Medical Center, Sepulveda, CA 91343, and Department of Psychiatry and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024.
Heidi M. Fahringer, Neurobiology Research, Veterans Affairs Medical Center, Sepulveda, CA 91343, and Department of Psychiatry and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024
Richard Paul, Neurobiology Research, Veterans Affairs Medical Center, Sepulveda, CA 91343, and Department of Psychiatry and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024.
Priyattam Shiromani, Department of Psychiatry, San Diego Veterans Affairs Medical Center, and University of California, La Jolla, CA 92093.
William C. Dement, Department of Psychiatry, Stanford University-School of Medicine, Palo Alto, CA 94305
Emmanuel Mignot, Department of Psychiatry, Stanford University-School of Medicine, Palo Alto, CA 94305.
Charles Chiu, Neurobiology Research, Veterans Affairs Medical Center, Sepulveda, CA 91343, and Department of Psychiatry and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024.
REFERENCES AND NOTES
- 1.Mitler ΜM, Nelson S Hajdukovic R, Psychiatr. Clin. N. Am 10, 593 (1987). [PubMed] [Google Scholar]
- 2.Guilleminault C et al. , Lancet ii (no. 8609), 511 (1988); [DOI] [PubMed] [Google Scholar]; Aldrich S and Rogers AE, Sleep 12, 254 (1989); [DOI] [PubMed] [Google Scholar]; Mignot E et al. Brain Res. 500, 1 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Mitler MM, Boyson BG, Campbell L, Dement WC, Exp. Neurol 45, 332 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker TL and Dement WC, in Brain Mechanisms of Sleep, McGinty DJ, Drucker-Colin R, Morrison A, Parmeggiani PL, Eds. (Raven, New York, 1985), p. 199. [Google Scholar]
- 5.Billiard M, Ann. Clin. Res 17, 220 (1985). [PubMed] [Google Scholar]
- 6.Honda Y, Doi Y, Juji T, Satake M, Folia Psychiatr. Neurol. Jpn 38, 360 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Wolpert EA, Dement WC, Mitchel SA, Fisher C, Electroenceph. Clin. Neurophysiol. 15, 599 (1963); [DOI] [PubMed] [Google Scholar]; Guilleminault C, in Narcolepsy, Guilleminault C, Dement WC, Passouant P, Eds. (Spectrum, New York, 1976), pp. 125–143. [DOI] [PubMed] [Google Scholar]
- 8.Jouvet M and Delorme F, C.R. Soc. Biol 159,895 (1965); [PubMed] [Google Scholar]; Henley K and Morrison AR, Acta Neurobiol. Exp 34, 215 (1974). [PubMed] [Google Scholar]
- 9.Schenkel E and Siegel JM, Neurosci. Lett 98, 159 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai YY and Siegel JM, J. Neurosci 8, 4790 (1988); [DOI] [PMC free article] [PubMed] [Google Scholar]; Soc. Neurosci. Abstr 17, 391 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netick A, Orem J, Dement WC, Brain Res. 120, 197 (1977). [DOI] [PubMed] [Google Scholar]
- 12.Siegel JM, Wheeler RL, McGinty DJ, ibid. 179, 49 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel JM, Exp. Neurol 65, 691 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai K, in The Reticular Formation Revisited, Hobson JA and Brazier MA, Eds. (Raven, New York, 1980), pp. 427–447. [Google Scholar]
- 15.Siegel JM, Tomaszewski KS, Wheeler RL, J. Neurophysiol 50, 717 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel JM and McGinty DJ, Science 199, 207 (1978);619453 [Google Scholar]; Siegel JM and Tomaszewski KS, J. Neurophysiol 50, 696 (1983).6619914 [Google Scholar]
- 17.Lim RKS, Liu C, Moffitt RL, A Stereotaxic Atlas of the Dog’s Brain (Thomas, Springfield, IL, 1960). [Google Scholar]
- 18.Shiromani PJ, Lai YY, Siegel JM, Brain Res. 517, 224 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataplexy-off ceUs were defined as those having a ≥50% decrease in their discharge rate during cataplexy relative to that during the active waking period immediately before cataplexy. Cataplexy-on ceUs were identified by the same 50% criterion. Cataplexy onset was defined by the abrupt loss of muscle tone recorded bilaterally in the nuchal muscles. Cataplexy durations ranged from 6 to 43 s (mean, 18.4 ± 10.5 s).
- 20.A two-way analysis of variance of state (quiet waking, active waking, cataplexy, REM sleep, non-REM sleep) by type (cataplexy-off, cataplexy-on, other) showed a significant state effect (P < 0.01) and a significant interaction effect (P < 0.05).
- 21.The ratio of quiet waking rates to active waking rates was significantly higher in cataplexy-on cells than in cataplexy-off cells (P < 0.001, two-tailed t test).
- 22.Tohyama M, Sakai K, Salvert D, Touret M, Jouvet M, Brain Res. 173, 383 (1979). [DOI] [PubMed] [Google Scholar]
- 23.Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC, Sleep 11, 1 (1988); [DOI] [PubMed] [Google Scholar]; Vincent SR and Reiner PB, Brain Res. Bull 18, 371 (1987); [DOI] [PubMed] [Google Scholar]; Sakai K et al. , ibid. 24, 437 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Chase ΜH, Soja PJ, Morales FR, J. Neurosci 9, 743 (1989); [DOI] [PMC free article] [PubMed] [Google Scholar]; Fort P, Luppi P, Wenthold R, Jouvet M, Acad CR. Sci. 311, 205 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankowska E, Lund S, Lundberg A, Pompeiano O, Arch. Ital. Biol 106, 124 (1968); [PubMed] [Google Scholar]; Mori S, Prog. Neurobiol. (N.Y.) 28, 161 (1987); [PubMed] [Google Scholar]; Morales FR, Fung SJ, Boxer PA, Chase MH, Sleep Res. 11, 26 (1982); [PubMed] [Google Scholar]; Chase MH, Int. Rev. Neurobiol 24, 213 (1983). [PubMed] [Google Scholar]
- 26.Kilduff TS et al. , Sleep 9, 102 (1986). [DOI] [PubMed] [Google Scholar]
- 27.Siegel JM and Nienhuis R, unpublished observations. Results based on 49 recorded pontine cells.
- 28.Siegel JM, Brain Res. Rev. 1, 69 (1979); [DOI] [PMC free article] [PubMed] [Google Scholar]; ___ and McGinty DJ, Science 193, 240 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki SS, Siegel JM, Wu MF, Brain Res. 484, 78 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]


