Abstract
Peptides deduced from the C-terminal end (residues 191 to 227) of pestivirus envelope protein Erns were used to develop enzyme-linked immunosorbent assays (ELISAs) to measure specifically antibodies against different types of pestiviruses. The choice of the peptide was based on the modular structure of the Erns protein, and the peptide was selected for its probable independent folding and good exposure, which would make it a good candidate for an antigenic peptide to be used in a diagnostic test. A solid-phase peptide ELISA which was cross-reactive for several types of pestivirus antibodies and which can be used for the general detection of pestivirus antibodies was developed. To identify type-specific pestivirus antibodies, a liquid-phase peptide ELISA, with a labeled, specific classical swine fever virus (CSFV) peptide and an unlabeled bovine viral diarrhea virus peptide to block cross-reactivity, was developed. Specificity and sensitivity of the liquid-phase peptide ELISA for CSFV were 98 and 100%, respectively. Because the peptide is a fragment of the Erns protein, it can be used to differentiate between infected and vaccinated animals when a vaccine based on the E2 protein, which is another pestivirus envelope protein, is used.
Classical swine fever virus (CSFV), Bovine viral diarrhea virus (BVDV), and Border disease virus (BDV) belong to the genus Pestivirus of the Flaviviridae family (7). CSFV is restricted to swine, whereas BVDV and BDV have been isolated from several species such as cattle, swine, sheep, deer, and giraffes (17). Although pigs can be infected by all these pestiviruses (17, 22), only CSFV induces severe disease and is often fatal. The disease is characterized by fever and leukopenia and can run an acute, chronic, or subclinical course. Although effective live-attenuated vaccines are available, pigs are not vaccinated against CSFV in the European Union (EU) because vaccinated and infected pigs are serologically indistinguishable. Outbreaks of classical swine fever (CSF) in the EU are controlled by eradication of all pigs from infected farms and farms in the vicinity. Because of this strategy, more than 10 million pigs had to be killed and destroyed during the 1997 to 1998 CSF epizootic in The Netherlands at a cost of more than 2 billion U.S. dollars (18). It is for this reason that there is a great demand for a marker vaccine which can provide protective immunity and which induces an antibody response in the vaccinated pigs which can be distinguished from the antibody response caused by a natural CSFV infection.
Pestiviruses are enveloped, plus-stranded RNA viruses whose genome comprises one long open reading frame (4, 14, 15). Translation into a hypothetical polyprotein is accompanied by processing into mature proteins. The structural proteins include a nucleocapsid protein, C, and three envelope glycoproteins, Erns, E1, and E2 (23). Envelope proteins Erns and E2 are able to induce neutralizing antibodies (3, 9, 10).
Glycoprotein E2 is a good candidate to incorporate in a vaccine because it is the most immunogenic protein of pestiviruses and elicits high titers of neutralizing antibodies after infection (20, 26). Vaccination of target animals with E2 has been shown to give protection against a lethal homologous challenge (2, 9). When E2 is used for vaccination of pigs, serological diagnosis of a natural pestivirus infection in pigs has to be performed with a second antigenic viral protein. For this purpose the Erns glycoprotein can be used as an antigen in a diagnostic test. This is called the diva vaccine or marker vaccine approach (24). Obviously, the application of these marker vaccines depends on sensitive tests and, for CSFV, the test also has to be very specific because pigs can be infected with the other antigenically closely related pestiviruses: BVDV and BDV. The diagnostic test for a CSFV marker vaccine should therefore detect CSFV-specific antibodies only and no other pestivirus-cross-reactive antibodies. A serological test based on epitopes present on the complete Erns protein has been developed (A. J. de Smit, G. van de Wetering, E. C. Colijn, M. Hulst, J. A. Kramps, A. van der Blink, and R. J. M. Moormann, unpublished data). In this study we describe a new test which is based on a peptide containing a different epitope, located on a small C-terminal fragment of the Erns protein.
MATERIALS AND METHODS
Peptide synthesis.
Peptides were selected from the C-terminal region, (residues 191 to 227) of CSFV Erns, strain Alfort 187, BVDV Erns, strain M96751, and BDV, strain X818 (1, 4, 19). Peptide sequences were as follows: CSFV, acetyl-ENARQGAARV TSWLGRQLRI AGKRLEGRSK TWFGAYA-COOH and biotin-ENARQGAARV TSWLGRQLRI AGKRLEGRSK TWFGAYA-COOH; BVDV, acetyl-EGARQGTAKL TTWLGKQLGI LGKKLENKSK TWFGAYA-COOH and biotin-EGARQGTAKL TTWLGKQLGI LGKKLENKSK TWFGAYA-COOH; BDV, biotin-ENARQGAAKL TSWLGKQLGI MGKKLEHKSK TWFGANA-COOH. Peptides were synthesized according to standard procedures on an Applied Biosystems 430A synthesizer using Fastmoc chemistry (6). An extra CSFV peptide and an extra BVDV peptide, which were N-terminally acetylated instead of biotinylated, were synthesized.
Serum samples.
The following serum samples were incorporated in the study to evaluate the peptide ELISAs. (i) Negative field serum samples (n = 96) were randomly obtained from slaughtered adult pigs. Sera were all tested negative in both the CSFV E2 (5) and the pan-pestivirus antibody-specific Ceditest enzyme-linked immunosorbent assays (ELISAs) (11, 16). (ii) Pestivirus serum antibody-positive but CSFV-negative serum samples (n = 96) were randomly obtained from slaughtered adult pigs. Swine sera were tested negative in Ceditest CSFV E2-specific ELISA and positive in the Ceditest ELISA (11, 16). (iii) CSFV antibody-positive field serum samples (n = 95) were obtained from a pig farm (VR) that was infected during the CSF epizootic in The Netherlands in 1997 to 1998. Eighty-one of the 95 samples were confirmed positive in the virus neutralization test, and 75 of the 95 samples were positive in the CSFV E2 ELISA. (iv) Sequential serum samples were collected during a vaccination-and-challenge experiment with 11 specific-pathogen-free pigs that were vaccinated with CSFV E2 glycoprotein and challenged with virulent CSFV strain Paderborn 2 weeks after a single vaccination with the E2 subunit vaccine. (v) A panel of swine sera were experimentally infected with BVDV (n = 5; sera 4 to 8). Sera 4 and 5 were infected with BVDV, strain den Otter; sera 6 to 8 were infected with strain Wisman. (vi) A panel of swine sera were experimentally infected with CSFV (n = 5; sera 9 to 13). Serum 9 was infected with strain Brescia, sera 10 to 12 were infected with weakly virulent strain van Zoelen, and serum 13 was infected with weakly virulent strain Henken. (vii) A panel of bovine sera were experimentally infected with BVDV (n = 8; sera 1 to 5, r4590-51, r4590-52, and 841). Samples 1, 2, 4, and 6 were field infections, 3 was infected with strain Appel, and 5, r4590-51, r4590-52, and 841 were infected with strain Oregon. (viii) A reference panel was obtained from the European reference laboratory for CSFV. The panel included sera from swine that were experimentally infected with CSFV (n = 14), BDV (n = 1), or BVDV (n = 12). Three sera were obtained from swine with experimental mixed infections of BVDV and BDV (n = 1) and CSFV and BVDV (n = 2). (ix) Pools of hyperimmunesera (HIS) against CSFV and BVDV were obtained.
sp-ELISA.
For the solid-phase peptide ELISA (sp-ELISA), a format similar to that for the previously developed respiratory syncytial virus G peptide ELISA (13) was chosen. One microgram of acetylated pestivirus peptide in 50 μl of carbonate buffer (pH 9.0, 37°C) was used to coat each well of a high-binding-capacity flat-bottom microplate (Greiner), and the wells were dried overnight. The optimal dilution of the peptide to coat ELISA plates was chosen in such a manner that maximum binding was obtained, as determined in a checkerboard titration. Swine or bovine test sera were serially diluted. Wells were washed six times between each incubation. Mouse anti-swine immunoglobulin G (IgG) (23.3.1b) conjugated to horseradish peroxidase (HRP) (26) was diluted 1:1,000. Rabbit anti-bovine IgG conjugated to HRP (P0159; Dako, Glostrup, Denmark) was diluted 1:1,000. Conjugates and test sera were incubated for 1 h at 37°C in low-salt ELISA buffer (8.1 mM Na2HPO4, 2.79 mM KH2PO4, 0.5 M NaCl, 2.68 mM KCl, 1 mM Na2EDTA, 0.05% [vol/vol] Tween 80, pH 7.2) containing 4% horse serum. The chromogen substrate consisted of ABTS (2,2′-azinobis[3-ethylbenzthiazolinesulfonic acid])-H2O2. Incubation was performed for 30 min at 22°C. Optical density (OD) was measured at 405 nm (Titertek multiscan).
Liquid-phase peptide ELISA (lp-ELISA).
For avidin-coated microtiter plates, 400 ng of ImmunoPure avidin (no. 21121; Pierce, Rockfort, Ill.) in 100 μl of carbonate buffer (pH 9) was used to coat each well of a high-binding-capacity flat-bottom microplate (Greiner). Plates were covered and incubated overnight at 37°C. After being coated the plates were kept frozen until use.
Before use, the avidin-coated plates were incubated with 100 μl of phosphate-buffered saline (pH 7) with 10% horse serum per well for 2 h at 37°C on a shaker.
Meanwhile, swine or bovine test serum (diluted 1:50) was incubated with a mixture of 10 ng of biotinylated CSFV peptide and 30 ng of acetylated BVDV peptide in 100 μl of low-salt ELISA buffer with 4% horse serum for 1 h at 37°C.
Avidin-coated plates were washed, and 100 μl of the test serum and peptide mixture was transferred to the wells and incubated for 45 min at 37°C. Subsequently, plates were washed and incubated with 100 μl of mouse anti-swine IgG (23.3.1b) conjugated to HRP (26) and diluted 1:1,000 or with rabbit anti-bovine IgG conjugated to HRP (P0159; Dako) and diluted 1:500. The chromogen substrate consisted of ABTS-H2O2. Incubation was performed for 30 min at 22°C. OD was measured at 405 nm (Titertek multiscan). The cutoff value was arbitrarily chosen as an OD of 0.5, which is approximately three times the average background of negative sera.
Detection of CSFV antibody.
Detection of CSFV Erns antibodies in sera was performed with an ELISA developed at the Institute for Animal Science and Health (de Smit et al., unpublished data) and a commercially available ELISA (Chekit-MARKER CSFV ELISA; Hoechst-Roussel Vet). Both ELISAs use the same test principle, namely, “blocking” of Erns antigen in a liquid phase. ELISAs were performed according to the manufacturer's description. The presence of CSFV antibodies in serum of the pigs was determined by an E2 ELISA (5) and a pestivirus ELISA (16). Serum samples were tested for the presence of neutralizing antibodies against CSFV, BVDV (strain Oregon or NADL), and BDV (strain F) (27), with the neutralization peroxidase-linked assay (21).
RESULTS
Selection of the peptide.
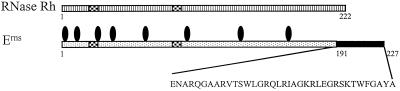
Two stretches of the pestivirus Erns protein show sequence homology with RNase Rh, a new class of microbial RNase of Rhizopus niveus, a member of the T2/S RNase superfamily (8). The crystal structure of RNase Rh has been determined (12), and the three-dimensional (3D) structure confirmed that both stretches with sequence homology to Erns constitute the active site of the RNase. The alignment, which is shown schematically in Fig. 1, showed that the 37 C-terminal residues of Erns do not align with RNase Rh and seem to form a separate region (Langedijk et al., unpublished data). A 3D model was built by homology modeling using the alignment of RNase Rh and pestivirus Erns (Langedijk et al., unpublished data). The 3D structure of Erns residues 1 to 190 corresponds to that of the RNase domain that is similar to RNase Rh. The C-terminal region is a separate domain that shows similarity to membrane active peptides.
FIG. 1.
Schematic representation of alignment of pestivirus Erns with RNase Rh, which indicates the modular organization of Erns. Erns consists of an RNase domain (dotted) and a C-terminal membrane active domain (black). The C-terminal domain (residues 191 to 227) is used as the antigen in the developed ELISA. Checkered boxes, strongly homologous RNase active-site domains; ellipses, potential glycosylation sites.
With the aid of the structural model it is possible to define antigenic regions on the surface of the protein which can be mimicked by single linear peptides. These would preferably be small subdomains, which fold relatively independently. A peptide consisting of the C-terminal 37 residues (191 to 227) is the best candidate because of its location on the outer rim on the surface of the Erns model structure, because it forms a small functional domain which folds independently from the rest of the protein, and because it is not masked by any potential carbohydrates.
We have evaluated the applicability of the peptides in diagnostics by the development of different diagnostic assays: an indirect ELISA in which the antigen is recognized as bound to a solid phase and an indirect ELISA in which the antigen is recognized in the liquid phase.
sp-ELISA.
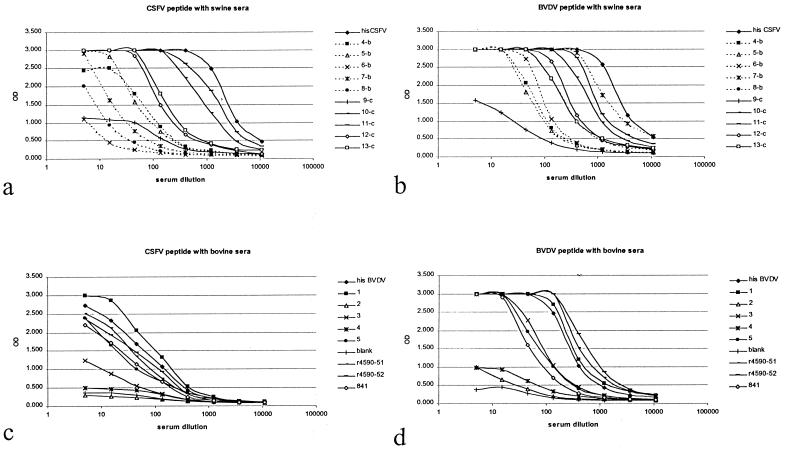
The reactivities of BVDV-positive swine sera (sera 4 to 8) and CSFV-positive swine sera (sera 9 to 13) were tested for reactivity in the CSFV sp-ELISA and the BVDV sp-ELISA (Fig. 2a and b). The reactivities of bovine sera 1 to 5, r4590-51, r4590-52, and 841 were tested in the CSFV sp-ELISA and the BVDV sp-ELISA (Fig. 2c and d).
FIG. 2.
(a) Reactivities (OD) in CSFV sp-ELISA of dilutions of BVDV-specific swine sera (4-b to 8-b), CSFV-specific swine sera (9-c to 13-c), and CSFV-specific HIS. (b) Reactivities in BVDV sp-ELISA of dilutions of BVDV-specific swine sera (4-b to 8-b), CSFV specific swine sera (9-c to 13-c) and CSFV-specific HIS. (c) Reactivities in CSFV sp-ELISA of dilutions of BVDV-specific bovine sera (1 to 5, r4590-51, r4590-52, and 841) and BVDV-specific HIS. (d) Reactivities in BVDV sp-ELISA of dilutions of BVDV-specific bovine sera (1 to 5, r4590-51, r4590-52, and 841) and BVDV-specific HIS. Blank, negative control serum.
Reactivities of the sera with the peptides were good, which suggests that the peptides indeed correspond to an immunodominant region of Erns. This agrees with the prediction of the immunodominant character of the subdomain. However, the CSFV sera and the BVDV sera are cross-reactive for both peptides. Although the panel of CSFV-specific swine sera reacted better than the panel of BVDV-specific swine sera in the CSFV ELISA (Fig. 2a), the reactivities of both panels of sera in the BVDV ELISA were similar (Fig. 2b). Similarly, the panel of BVDV-specific bovine sera showed high reactivity in the BVDV peptide ELISA (Fig. 2d), but the sera also cross-reacted considerably in the CSFV ELISA (Fig. 2c).
lp-ELISA.
Because of the high cross-reactivity in the sp-ELISA, an ELISA in which the antigen was recognized in the liquid phase (lp-ELISA) was developed in order to measure specific binding. Moreover, by labeling the homologous peptide of the pestivirus of interest (CSFV peptide), an unlabeled heterologous peptide of the cross-reactive pestivirus (BVDV peptide) could be used to block unspecific cross-reactivity.
In the lp-ELISA for detection of antibodies against CSFV, the test serum was incubated with a mixture of biotinylated CSFV peptide and acetylated BVDV peptide (without biotin). Most likely, CSFV-specific antibodies preferentially bind the biotinylated CSFV peptide and BVDV-specific antibodies preferentially bind the nonbiotinylated BVDV peptide. Subsequently, the mixture was transferred to an avidin-coated microtiter plate and biotinylated CSFV peptide together with the specific antibodies was caught by avidin. The antibodies complexed to the biotinylated CSFV peptide can be detected with an antiswine-peroxidase conjugate and subsequent incubation with substrate.
The reactivities of BVDV antibody-positive swine sera (sera 4 to 8) and CSFV-positive swine sera (sera 9 to 13) were tested in the CSFV lp-ELISA (Fig. 3). This test format showed a high specificity compared with that for the sp-ELISA (compare Fig. 2a with 3a). Next, the reactivities of BVDV antibody-positive bovine sera were tested in the CSFV lp-ELISA (compare Fig. 2c with 3b).
FIG. 3.
(a) Reactivities (OD) in CSFV lp-ELISA of dilutions of BVDV-specific swine sera (4 to 8) and CSFV-specific swine sera (9 to 13) and CSFV-specific HIS. (b) Reactivities in CSFV lp-ELISA of dilutions of BVDV-specific bovine sera (1 to 6, r4590-51, r4590-52, and 841) and BVDV-specific HIS.
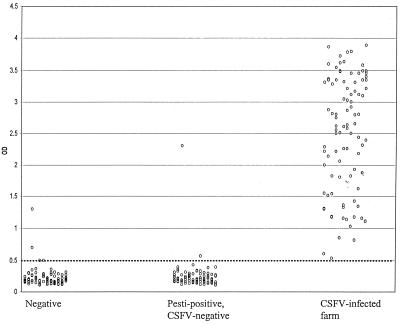
To determine the specificity of the lp-ELISA, 96 pestivirus-specific antibody-negative field serum samples were tested in the lp-ELISA for CSFV Erns antibody. Only 2 of 96 samples showed a positive response (cutoff was chosen arbitrarily as an OD of >0.5). Based on these limited data with these small serum panels, the specificity of the lp-ELISA for CSFV Erns antibody amounts to 98% (94/96 × 100%) (Fig. 4).
FIG. 4.
Reactivities of several panels of sera in the CSFV lp-ELISA, performed as described in Materials and Methods. Negative field serum samples (n = 96) were randomly obtained from slaughtered adult pigs and were all tested negative in standard pestivirus ELISA. Pestivirus-positive but CSFV-negative serum samples (n = 96) were randomly obtained from slaughtered adult pigs. CSFV-positive field serum samples (n = 95) were obtained from an infected farm (VR) that was infected during the CSF epizootic in The Netherlands in 1997 to 1998.
To determine the pestivirus type specificity of the lp-ELISA, 96 field sera that contain antibodies directed against pestiviruses other than CSFV (BVDV and BDV) were tested in the lp-ELISA. Only 2 of 96 samples showed a positive response (OD > 0.5). Based on these data, the relative specificity of the lp-ELISA for CSFV Erns antibody in non-CSFV pestivirus-positive sera approximates 98% (94/96 × 100%) (Fig. 4).
To determine the sensitivity of the lp-ELISA, 95 positive field serum samples (of which 81 were confirmed positive by a CSFV neutralization test) from a CSFV-infected farm (VR) obtained during the CSF epizootic in The Netherlands in 1997 to 1998 were tested in the lp-ELISA. All serum samples showed a positive response (OD > 0.5). Based on these limited data, the relative sensitivity of the lp-ELISA for CSFV antibodies amounts to 100% (Fig. 4).
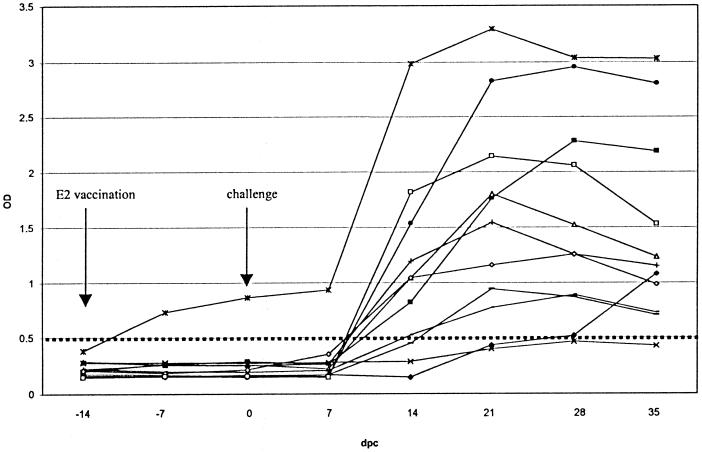
An interesting application of the lp-ELISA would be a diagnostic test that can be used to detect CSFV infection of E2-vaccinated pigs. Therefore, sera of E2-vaccinated pigs should be unreactive in the lp-ELISA and should be positive if pigs become infected after a CSFV challenge. Successive serum samples that were collected during a challenge experiment with 11 E2-vaccinated pigs that were infected with CSFV were tested in the lp-ELISA (Fig. 5). The results show that all sera but one from E2-vaccinated pigs were negative prior to CSFV challenge. All animals (except 1) seroconverted 14 to 28 days after challenge. The peptide ELISA detected more seroconversions 21 days after challenge (9 out of 11) than the Erns-based ELISA (4 out of 12) (de Smit et al., unpublished data).
FIG. 5.
Reactivity of successive serum samples collected during a vaccination-and-challenge experiment in the CSFV lp-ELISA. Eleven pigs were vaccinated with E2 14 days before challenge. dpc, days postchallenge.
Finally, the performance of the lp-ELISA was compared with that of the E2-based Ceditest ELISA and two other Erns-based ELISAs (see Materials and Methods). The reactivities of a panel of European reference sera (see Materials and Methods) were tested in all four ELISAs (Table 1). Although the E2-based Ceditest ELISA was superior (one false negative), the lp-ELISA performed better (three false negatives) than the other ELISAs, which were based on epitope blocking using complete Erns (five false negatives, one false positive and one false negative, and six false positives).
TABLE 1.
Comparison of reactivities of reference sera in different CSFV diagnostic tests
| Serum no.a | DPIb | Inoculum | Results of indicated ELISA
|
|||
|---|---|---|---|---|---|---|
| E2c | ERNS
|
Peptide (OD)e | ||||
| Ad | Bd | |||||
| 1 | 43 | CSFV Visbek/Han 95 | + | + | + | 0.171 |
| 2 | 16 | CSFV Visbek/Han 95 | + | + | + | 0.477 |
| 3 | 20 | CSFV Visbek/Han 95 | + | + | + | 1.796 |
| 4 | 20 | CSFV Visbek/Han 95 | + | − | + | 3.165 |
| 5 | 14 | CSFV Visbek/Han 95 | + | + | + | 0.7 |
| 6 | 21 | CSFV Alfort 187 | + | − | − | 0.186 |
| 7 | 29 | CSFV Diepholz1/Han94 | + | + | + | 2.25 |
| 8 | 29 | CSFV Diepholz1/Han94 | + | − | + | 2.619 |
| 9 | 29 | CSFV Diepholz1/Han94 | + | − | + | 1.857 |
| 10 | 34 | CSFV Visbek/Han95 | + | − | + | 3.87 |
| 11 | 55 | CSFV Visbek/Han95 | + | + | + | 1.122 |
| 12 | 93 | CSFV C-strain | + | + | + | 0.543 |
| 13 | 69 | CSFV Diepholz1/Han94 | + | + | + | 2.146 |
| 14 | 28 | CSFV Diepholz1/Han94 | − | + | + | 1.103 |
| 15 | BVDV NADL | − | − | − | 0.103 | |
| 16 | BDV | − | − | − | 0.45 | |
| 17 | BVDV 2214 | − | + | + | 0.161 | |
| 18 | BVDV NADL | − | − | − | 0.118 | |
| 19 | BVDV NADL + BDV | − | − | + | 0.136 | |
| 20 | BVDV + CSFV Alfort 187 | + | + | + | 3.208 | |
| 21 | BVDV Osloss | − | − | − | 0.146 | |
| 22 | BVDV Osloss | − | − | − | 0.151 | |
| 23 | BVDV Osloss | − | − | − | 0.151 | |
| 24 | BVDV Osloss | − | − | − | 0.162 | |
| 25 | BVDV Osloss | − | − | − | 0.172 | |
| 26 | BVDV Osloss | − | − | + | 0.164 | |
| 27 | BVDV Osloss | − | − | + | 0.149 | |
| 28 | BVDV Osloss | − | − | + | 0.163 | |
| 29 | BVDV Osloss | − | − | + | 0.215 | |
| 30 | CSFV Alfort 187 + BVDV Osloss | + | + | + | 0.567 | |
Sera 21 to 29 were obtained from the same animal.
DPI, days postinfection.
Ceditest E2 ELISA (5).
Antibody-blocking ELISA based on complete Erns (see Materials and Methods).
OD values of >0.5 were considered positive results.
To illustrate the compatibility of the peptide ELISA with other pestiviruses, the CSFV-specific peptide ELISA was changed into a BVDV ELISA and a BDV ELISA by exchanging the biotinylated CSFV peptide for a biotinylated BVDV peptide or a biotinylated BDV peptide, respectively, and exchanging the acetylated BVDV peptide for the acetylated CSFV peptide. The amount of peptide used was the same as that for the CSFV lp-ELISA, and all assay conditions were kept similar. The panel of swine sera that were experimentally infected with BVDV (n = 5; sera 4 to 8) or CSFV (n = 5; sera 9 to 13) were tested in the three different lp-ELISAs for the three different pestivirus types. Table 2 shows that BVDV-positive sera react best in the BVDV-specific peptide ELISA and that the CSFV-positive sera react best in the CSFV ELISA although the CSFV sera cross-react to some extent with the BVDV peptide. As expected on the basis of the sequence homology, the BVDV and BDV ELISAs show less differentiation. The BVDV and BDV ELISAs both contained acetylated CSFV peptide as the competing antigen. Some improvement may be possible when acetylated BDV and BVDV peptides are used as competing antigens in the BVDV and BDV ELISAs, respectively.
TABLE 2.
Comparison of reactivities (OD at 405 nm) in different pestivirus peptide ELISAs
| Serum,a virus (dilutionb) | OD from peptide ELISA for pestivirus:
|
||
|---|---|---|---|
| CSFV | BVDV | BDV | |
| 4, BVDV | 0.236 | 1.346 | 0.781 |
| 5, BVDV | 0.129 | 0.724 | 0.388 |
| 6, BVDV | 0.106 | 3.211 | 0.824 |
| 7, BVDV | 0.250 | 4.000 | 4.000 |
| 8, BVDV | 0.104 | 4.000 | 2.325 |
| 9, CSFV | 0.487 | 0.367 | 0.349 |
| 10, CSFV | 2.090 | 1.612 | 0.343 |
| 11, CSFV | 2.555 | 0.610 | 0.246 |
| 12, CSFV | 1.133 | 0.453 | 0.412 |
| 13, CSFV | 1.253 | 0.573 | 0.436 |
| HIS, CSFV (1:500) | 2.263 | 0.471 | 0.624 |
| HIS, CSFV (1:1,000) | 1.419 | 0.267 | 0.356 |
| HIS, CSFV (1:2,000) | 0.871 | 0.166 | 0.180 |
| Negative | 0.152 | 0.150 | 0.153 |
Numbers refer to sera in Fig. 2a and b and 3a.
All sera were diluted 1:50 except for the HIS sera.
DISCUSSION
The use of peptides as antigens in serological diagnosis has major advantages because peptides are cheap and easy to produce in a reproducible manner. However, peptide-based or single-epitope-based diagnostics may lack sensitivity because of the lack of antigenic information. Most peptides that have been used in serology represent continuous epitopes because it is difficult to detect antibodies against complex discontinuous epitopes using small linear peptides and because it is difficult to predict discontinuous epitopes based on the amino acid sequence of a protein. In addition, it is difficult to mimic the antigenic surface of large globular proteins accurately with a small linear peptide. The choice and design of the peptide are of major importance for a successful peptide ELISA. We have shown previously that a structure-based approach for the selection of a candidate antigenic peptide can be successful and can even offer antigenic peptides superior to the complete surface protein (13). The same approach of sequence analysis and homology modeling was used for pestivirus Erns to identify a region that can be used for the structure-based design of antigenic peptides. In this study we developed an ELISA which is based on a fragment of the Erns protein which may be a small, independently folding, biologically active protein module. Erns can be considered an RNase domain with an independent C-terminal domain of 37 residues, which is responsible for translocating the RNase domain across the plasma membrane (Langedijk et al., submitted). Analysis of the hypothetical Erns structure showed that this C-terminal domain would be the best peptide candidate to harbor intact epitopes.
Peptides based on the C-terminal domains of CSFV and BVDV, with a length of 37 residues, were synthesized and tested for antigenicity. As expected, the peptides appeared to harbor an immunodominant epitope(s). Most pestivirus-positive sera reacted with the peptides, but, due to the high homology and conserved mutations, the sp-ELISA was not specific for pestivirus types. Although the peptide is immunodominant, the intrapestivirus homology of 70% and the conserved nature of the nonidentical residues posed a serious cross-reactivity problem. To solve this problem, an ELISA in which the antigenic peptide was recognized in liquid phase and in which antibodies specific for the other pestivirus were blocked with the homologous peptide was developed. After optimization of the specific peptide and blocking-peptide concentrations and incubation time, an ELISA that showed good specificity and that was comparably specific to, or even more specific than, the existing ELISAs based on complete Erns was established. This result is remarkable when the variation between CSFV strains within the peptide (approximately 85% homology) and the homology between pestivirus types (70%) are compared. Only four residues (11%), at positions 9, 10, 21, 28 of the peptide, are unique for all CSFV strains. Despite these seemingly difficult conditions, the peptide ELISA is surprisingly specific and is able to detect CSFV antibodies against all tested strains (Table 1). It remains to be seen how the peptide ELISA performs for very distinct CSFV strains, which have some resemblance with other pestiviruses. If such results are inconclusive, the OD obtained in the CSFV peptide ELISA can be compared with the OD obtained in the BVDV peptide ELISA (Table 2). The highest OD will be indicative of the virus responsible for infection. Because the peptide ELISA detects antibodies to a different epitope than the existing ELISAs based on complete Erns, it can also be used as a confirmation test for Erns-specific antibodies. When new genetic sequence information about the circulating antigenic subpopulations is gathered, the peptides in the ELISA can be modified easily when the circumstances call for another antigen.
It is very likely that the lp-ELISA can be optimized further when it is changed into an antibody-blocking format like that of the other three ELISAs in Table 1.
The peptide ELISA is less sensitive than the E2-based ELISA (Table 1). Perhaps the peptide sequence did not match the sequence of the infecting virus strain, but it is also likely that the E2 protein is more immunodominant. However, this E2 ELISA cannot be used to differentiate between E2-vaccinated and infected animals. During screening we noticed that some individual animal sera react differently in the E2 and Erns ELISAs. Some sera reacted in the Erns ELISA or Erns peptide ELISA and not in the E2 ELISA and vice versa. For instance, all CSFV antibody-positive field sera (95) were tested positive with the lp-ELISA although only 81 were confirmed positive with the virus neutralization test. This is not understood. We know that virus neutralization titers always correspond to anti-E2 antibody titers. Therefore, it is possible that, for some reason, antibodies against the Erns peptide appear sooner, or more frequently, than anti-E2 antibodies in this particular case. Both ELISAs may be reliable tests for screening at a herd level, which is a common practice with outbreaks. The lp-ELISA also performs well in detecting infections of E2-vaccinated animals. Although the replication of CSFV in these vaccinated animals is very low due to the presence of neutralizing antibodies, it is possible to detect virus-specific seroconversion in 10 out of 11 animals (Fig. 5).
In conclusion, a structure-based approach was used to develop a very sensitive and specific CSFV peptide ELISA which uses an unlabeled peptide to block cross-reactive antibodies and which can be used to detect CSFV infections in E2-vaccinated pigs.
ACKNOWLEDGMENTS
We thank Hélène Winkelman-Goedhart and Gerard van de Wetering for technical assistance and the EU reference laboratory for the use of the serum panel.
REFERENCES
- 1.Becher P, Shannon A D, Tautz N, Thiel H J. Molecular characterization of border disease virus, a pestivirus from sheep. Virology. 1994;198:542–551. doi: 10.1006/viro.1994.1065. [DOI] [PubMed] [Google Scholar]
- 2.Bouma A, de Smit A J, de Kluijver E P, Terpstra C, Moormann R J. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet Microbiol. 1999;66:101–114. doi: 10.1016/s0378-1135(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 3.Bruschke C J, Moormann R J, van Oirschot J T, van Rijn P A. A subunit vaccine based on glycoprotein E2 of bovine virus diarrhea virus induces fetal protection in sheep against homologous challenge. Vaccine. 1997;15:1940–1945. doi: 10.1016/s0264-410x(97)00125-4. [DOI] [PubMed] [Google Scholar]
- 4.Colett M S, Larson R, Gold C, Strick D, Anderson D K, Purchio A F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988;165:191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- 5.Colijn E O, Bloemraad M, Wensvoort G. An improved ELISA for the detection of serum antibodies directed against classical swine fever virus. Vet Microbiol. 1997;59:15–25. doi: 10.1016/s0378-1135(97)00178-8. . (Erratum, 63:81–83, 1998.) [DOI] [PubMed] [Google Scholar]
- 6.Fields C G, Lloyd D H, Macdonald R L, Otteson K M, Noble R L. HBTU activation for automated Fmoc solid-phase peptide synthesis. Pept Res. 1991;4:95–101. [PubMed] [Google Scholar]
- 7.Francki R I B, Faquet D L, Knudson D L, Brown F. Fifth report of the International Committee on the Taxonomy of Viruses. Arch Virol Suppl. 1991;2:223–233. [Google Scholar]
- 8.Horiuchi H, Yanai K, Takagi M, Yano K, Wakabayashi E, Sanda A, Mine S, Ohgi K, Irie M. Primary structure of a base non-specific ribonuclease from Rhizopus niveus. J Biochem (Tokyo) 1988;103:408–418. doi: 10.1093/oxfordjournals.jbchem.a122284. [DOI] [PubMed] [Google Scholar]
- 9.Hulst M M, Westra D F, Wensvoort G, Moormann R J. Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J Virol. 1993;67:5435–5442. doi: 10.1128/jvi.67.9.5435-5442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konig M, Lengsfeld T, Pauly T, Stark R, Thiel H J. Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J Virol. 1995;69:6479–6486. doi: 10.1128/jvi.69.10.6479-6486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramps J A, van Maanen C, van de Wetering G, Stienstra G, Quak S, Brinkhof J, Ronsholt L, Nylin B. A simple, rapid and reliable enzyme-linked immunosorbent assay for the detection of bovine virus diarrhoea virus (BVDV) specific antibodies in cattle serum, plasma and bulk milk. Vet Microbiol. 1999;64:135–144. doi: 10.1016/s0378-1135(98)00265-x. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara H, Nonaka T, Mitsui Y, Ohgi K, Irie M, Nakamura K T. The crystal structure of ribonuclease Rh from Rhizopus niveus at 2.0 A resolution. J Mol Biol. 1996;255:310–320. doi: 10.1006/jmbi.1996.0025. [DOI] [PubMed] [Google Scholar]
- 13.Langedijk J P, Middel W G, Schaaper W M, Meloen R H, Kramps J A, Brandenburg A H, van Oirschot J T. Type-specific serologic diagnosis of respiratory syncytial virus infection, based on a synthetic peptide of the attachment protein G. J Immunol Methods. 1996;193:157–166. doi: 10.1016/0022-1759(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 14.Meyers G, Rumenapf T, Thiel H J. Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology. 1989;171:555–567. doi: 10.1016/0042-6822(89)90625-9. [DOI] [PubMed] [Google Scholar]
- 15.Moormann R J, Warmerdam P A, van der Meer B, Schaaper W M, Wensvoort G, Hulst M M. Molecular cloning and nucleotide sequence of hog cholera virus strain Brescia and mapping of the genomic region encoding envelope protein E1. Virology. 1990;177:184–198. doi: 10.1016/0042-6822(90)90472-4. [DOI] [PubMed] [Google Scholar]
- 16.Paton D J, Ibata G, Edwards S, Wensvoort G. An ELISA detecting antibody to conserved pestivirus epitopes. J Virol Methods. 1991;31:315–324. doi: 10.1016/0166-0934(91)90169-z. [DOI] [PubMed] [Google Scholar]
- 17.Paton D J, Simpson V, Done S H. Infection of pigs and cattle with bovine viral diarrhoea virus on a farm in England. Vet Rec. 1992;131:185–188. doi: 10.1136/vr.131.9.185. [DOI] [PubMed] [Google Scholar]
- 18.Pluimers F H, de Leeuw P W, Smak J A, Elbers A R, Stegeman J A. Classical swine fever in The Netherlands 1997–1998: a description of organisation and measures to eradicate the disease. Prev Vet Med. 1999;42:139–155. doi: 10.1016/s0167-5877(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 19.Ruggli N, Moser C, Mitchell D, Hofmann M, Tratschin J D. Baculovirus expression and affinity purification of protein E2 of classical swine fever virus strain Alfort/187. Virus Genes. 1995;10:115–126. doi: 10.1007/BF01702592. [DOI] [PubMed] [Google Scholar]
- 20.Rumenapf T, Stark R, Meyers G, Thiel H J. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol. 1991;65:589–597. doi: 10.1128/jvi.65.2.589-597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terpstra C, Bloemraad M, Gielkens A L. The neutralizing peroxidase-linked assay for detection of antibody against swine fever virus. Vet Microbiol. 1984;9:113–120. doi: 10.1016/0378-1135(84)90026-9. [DOI] [PubMed] [Google Scholar]
- 22.Terpstra C, Wensvoort G. Natural infections of pigs with bovine viral diarrhoea virus associated with signs resembling swine fever. Res Vet Sci. 1988;45:137–142. [PubMed] [Google Scholar]
- 23.Thiel H J, Stark R, Weiland E, Rumenapf T, Meyers G. Hog cholera virus: molecular composition of virions from a pestivirus J. Virol. 1991;65:4705–4712. doi: 10.1128/jvi.65.9.4705-4712.1991. . (Erratum, 66:612, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Oirschot J T. Diva vaccines that reduce virus transmission. J Biotechnol. 1999;73:195–205. doi: 10.1016/s0168-1656(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 25.van Zaane D, Hulst M M. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet Immunol Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 26.van Zijl M, Wensvoort G, de Kluyver E, Hulst M, van der Gulden H, Gielkens A, Berns A, Moormann R. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol. 1991;65:2761–2765. doi: 10.1128/jvi.65.5.2761-2765.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilcek S, Belak S. Genetic identification of pestivirus strain Frijters as a border disease virus from pigs. J Virol Methods. 1996;60:103–108. doi: 10.1016/0166-0934(96)02031-9. [DOI] [PubMed] [Google Scholar]