Abstract
Background
Lipoprotein(a) [Lp(a)] is associated with increased risk of myocardial infarction, although the mechanism for this observation remains uncertain.
Objectives
This study aims to investigate whether Lp(a) is associated with adverse plaque progression.
Methods
Lp(a) was measured in patients with advanced stable coronary artery disease undergoing coronary computed tomography angiography at baseline and 12 months to assess progression of total, calcific, noncalcific, and low-attenuation plaque (necrotic core) in particular. High Lp(a) was defined as Lp(a) ≥ 70 mg/dL. The relationship of Lp(a) with plaque progression was assessed using linear regression analysis, adjusting for body mass index, segment involvement score, and ASSIGN score (a Scottish cardiovascular risk score comprised of age, sex, smoking, blood pressure, total and high-density lipoprotein [HDL]–cholesterol, diabetes, rheumatoid arthritis, and deprivation index).
Results
A total of 191 patients (65.9 ± 8.3 years of age; 152 [80%] male) were included in the analysis, with median Lp(a) values of 100 (range: 82 to 115) mg/dL and 10 (range: 5 to 24) mg/dL in the high and low Lp(a) groups, respectively. At baseline, there was no difference in coronary artery disease severity or plaque burden. Patients with high Lp(a) showed accelerated progression of low-attenuation plaque compared with low Lp(a) patients (26.2 ± 88.4 mm3 vs −0.7 ± 50.1 mm3; P = 0.020). Multivariable linear regression analysis confirmed the relation between Lp(a) and low-attenuation plaque volume progression (β = 10.5% increase for each 50 mg/dL Lp(a), 95% CI: 0.7%-20.3%). There was no difference in total, calcific, and noncalcific plaque volume progression.
Conclusions
Among patients with advanced stable coronary artery disease, Lp(a) is associated with accelerated progression of coronary low-attenuation plaque (necrotic core). This may explain the association between Lp(a) and the high residual risk of myocardial infarction, providing support for Lp(a) as a treatment target in atherosclerosis.
Key Words: lipoprotein(a), coronary computed tomography angiography, low-attenuation plaque
Abbreviations and Acronyms: ASCVD, atherosclerotic cardiovascular disease; CCTA, coronary computed tomography angiography; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); OxPL, oxidized phospholipids
Central Illustration
Lipoprotein(a) (Lp[a]) has been widely recognized as a prevalent and independent cardiovascular risk factor. Post hoc analyses of recent clinical trials have substantiated Lp(a) as a potent marker of residual cardiovascular risk, even in patients receiving intensive lipid-lowering therapy (1). Current guidelines recommend measuring Lp(a) to identify patients with a high lifetime risk for atherosclerotic cardiovascular disease (ASCVD) (2). Although outcome studies of therapies dedicated to specific lowering of Lp(a) are ongoing (Assessing the impact of Lipoprotein(a) Lowering With TQJ230 on Major Cardiovascular Events in Patients With CVD [Lp(a) HORIZON]; NCT04023552), the exact mechanisms underlying the increased ASCVD-risk mediated by Lp(a) remain a matter of debate (3).
Structurally, Lp(a) is a low-density lipoprotein (LDL) to which apolipoprotein(a) is covalently bound; the latter carrying proinflammatory oxidized phospholipids (OxPLs) (4). The atherogenic mechanisms of this multifaceted particle are thought to include accumulation of the LDL component in atherosclerotic plaque, prothrombotic effects due to interference of the apolipoprotein(a) tail with plasminogen activation, as well as induction of a multilevel proinflammatory response mediated by OxPL-cargo (5). The prothrombotic and proinflammatory effects of Lp(a) have been suggested to promote plaque destabilization leading to plaque rupture and atherothrombotic events (6). Previous studies have shown an association between high serum levels of Lp(a) and high baseline atherosclerotic plaque volumes and the presence of adverse plaque features in patients with coronary artery disease (7,8). However, data on the interaction between Lp(a) and progression of coronary plaque volumes and composition in contemporary patients are lacking.
Advanced quantitative plaque assessments on coronary computed tomography angiography (CCTA) now allow the assessment of both calcific and noncalcific atherosclerotic plaque types facilitating the tracking of disease progression as well as changes in coronary plaque morphology with good reproducibility (9). Moreover, recent studies have shown a strong association between plaque composition determined by quantitative computed tomography (CT) analysis and clinical events (10,11). In particular, the burden of low-attenuation plaque (attenuation density <30 HU), which serves as a marker of necrotic core, provides powerful prediction of future myocardial infarction outperforming clinical risk scores, severity of luminal stenosis, and CT calcium scoring (12).
In this study, we assessed whether high concentrations of serum Lp(a) are associated with progression of adverse plaque phenotype in a cohort of patients with advanced stable coronary artery disease who were already using guideline-directed preventative therapies.
Methods
Study design
Participants were recruited as part of the DIAMOND (Dual Antiplatelet Therapy to Inhibit Coronary Atherosclerosis and Myocardial Injury in Patients With Necrotic High-Risk Coronary Plaque Disease) study, a double-blind, randomized, parallel-group, placebo-controlled trial conducted at a single center in Edinburgh, United Kingdom (13). The primary results of this study have previously been published and showed that dual antiplatelet therapy did not impact coronary atherosclerotic disease progression or adverse plaque phenotype. The study was approved by the local Institutional Review Board, the Scottish Research Ethics Committee (REC reference: 14/SS/0089), the Medicines and Healthcare products Regulatory Agency, and the United Kingdom Administration of Radiation Substances Advisory Committee. It was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent before any study procedures were initiated. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient selection
Patients with clinically stable multivessel coronary artery disease were recruited prospectively from the Edinburgh Heart Centre, United Kingdom, between March 2015 and March 2017. Patients were included if aged older than 40 years and had evidence of angiographically proven multivessel coronary artery disease, defined as at least 2 major epicardial vessels with any combination of either >50% luminal stenosis or previous revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery). Patients were excluded in the event of coronary revascularization within the preceding 3 months or acute coronary syndrome within the previous 12 months.
Laboratory measurements
Baseline serum and plasma samples were obtained at the time of recruitment and stored at −80°C until further use. Hematology, biochemistry, and lipid panels were determined according to standardized operating procedures in a core laboratory. Low-density-lipoprotein cholesterol was calculated using the Friedewald equation (14). Lp(a) was measured at a later time point from frozen serum samples, using a KIV2-independent immunoassay (Randox Laboratories).
Image acquisition
Baseline coronary CT scans were acquired on a hybrid positron emission tomography–CT scanner (64-multidetector Biograph mCT, Siemens Medical Systems) using a standardized study protocol. Before scanning, participants with a resting heart rate >65 beats/min were administered oral β-blockade (50 to 100 mg metoprolol) unless contraindicated. An electrocardiogram–gated breath-held noncontrast CT scan (tube voltage, 120 kV; tube current based on body habits) was performed for coronary CT calcium scoring and reconstructed in the axial plane with 3-mm slice width and 1.5-mm increment. Finally, an electrocardiogram-gated coronary CT angiogram (tube voltage 120 kV, tube current based on body habits) was performed in mid-diastole during held expiration following sublingual glyceryl trinitrate. Repeat CCTA and calcium scoring were performed using the same imaging protocol and on the same scanner after an interval of 12 months.
Image analysis
Coronary calcium score
Coronary calcium on noncontrast CT was quantified on both a per-participant and per-segment level by an experienced observer using dedicated software (Vitrea Advanced, Toshiba Systems). Calcification was quantified as calcium score (Agatston units [AU]). Calcium score was derived using the Agatston method (15). Coronary stents were excluded from the per-patient analysis by only including calcium proximal or distal to the border of the stented segment.
CCTA image analysis
CCTA segments and vessels were identified by landmarks such as bifurcations and side branches. Segments and vessels with stents were excluded from the analyses and an equal number of segments and vessels were assessed at baseline and follow-up. Segment-wise analysis was performed according to the 17-segment modified American Heart Association classification (15). Coronary atherosclerotic lesions were quantified for luminal stenosis severity by visual estimation. A segment involvement score was calculated as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of luminal stenosis within each segment (minimum = 0; maximum = 16) (16).
CT plaque analysis
Plaque measurements were performed using previously validated semiautomated software version 2.5 (AutoPlaque) (Figure 1) by a trained observer blinded to the patient’s clinical status (17). We have previously shown excellent reproducibility for these measurements (9).
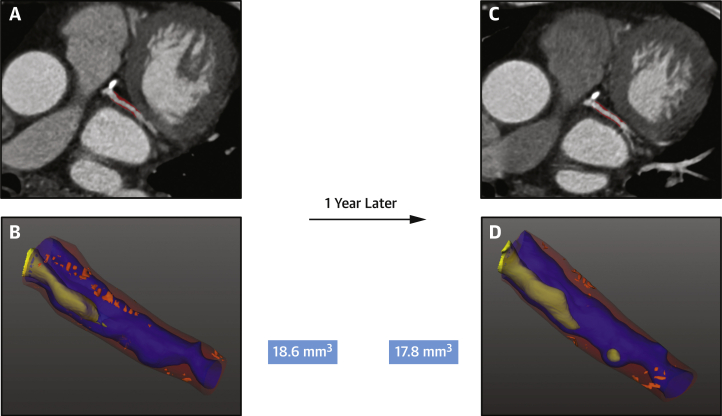
Figure 1.
Low-Attenuation Plaque Progression on CCTA in a Patient With Low Lp(a)
Coronary computed tomography angiography (CCTA) of a patient with low serum lipoprotein(a) [Lp(a)] concentration (9.2 mg/dL) showing atherosclerotic plaque in the left circumflex artery (A and C) with evidence of mixed plaque on automated plaque assessment (red overlay). Low-attenuation plaque is visualized in bright orange on the 3-dimensional reconstruction (B and D) and does not appear to progress on serial scanning (baseline volume 18.6 mm3, 1-year volume 17.8 mm3).
Coronary artery centerlines were extracted in a semiautomated fashion for each major artery and any tributary of >2-mm diameter with visually observed disease. A region of interest was placed in the aorta to define blood pool attenuation. Coronary artery segments were defined manually according to Society of Cardiovascular Computed Tomography guidance (18). All suitable vessel segments were manually identified and vessel wall and plaque constituents were automatically determined using scan-specific thresholds with manual adjustments made as required. Segments with coronary stents were excluded from the analysis.
Coronary atherosclerotic plaque volumes were measured for total plaque, calcific and noncalcific plaque, fibro-fatty plaque, and low-attenuation plaque as a marker of necrotic core. After delineating the lumen, the software automatically adjusted the cutoff points for different plaque types based on attenuation measured in the thoracic aorta. Plaque volumes (measured in mm3) for each plaque type were measured across all coronary segments and summed to generate the total plaque volume on a per-patient level. Baseline plaque burdens were calculated by dividing plaque volumes by the coronary vessel volume and multiplying by 100. Plaque progression was defined as the difference in plaque volumes between the baseline and the follow-up CCTA scan. We used plaque volume for this progression analysis as this is a less-derived measurement than plaque burden; therefore, it is more suitable for assessing changes in plaque status in individual patients over time.
Statistical analysis
Data are presented as mean ± SD for normally distributed variables, median with IQR for non-normally distributed variables, and number (percentage) for categorical variables. Individuals with serum Lp(a) concentrations >70 mg/dL were classified as having high Lp(a), based on the cutoff value for currently ongoing secondary prevention trials (NCT04023552). Between-group comparisons at baseline were assessed using a Student’s t-test for normally distributed data, Mann-Whitney U for non-normally distributed data, and chi-square or Fisher exact test for categorical data. To investigate the relationship between Lp(a) and plaque progression, we determined the change in plaque volumes from baseline to follow-up. The effect of Lp(a) on the percentage change in the plaque volume was assessed in univariable and in multivariable analysis accounting for body mass index, segment involving score (a prognostic marker of overall plaque burden), and the ASSIGN score (a Scottish cardiovascular risk score comprised of age, sex, smoking, blood pressure, total and high-density lipoprotein [HDL]–cholesterol, diabetes, rheumatoid arthritis, and deprivation index) which incorporates traditional cardiovascular risk factors and is calibrated to the Scottish population (19,20). Given that Lp(a) follows a highly skewed distribution in the general population, but is linearly associated with ASCVD, we standardized the effect size of Lp(a) to a 50-mg/dL increase as previously proposed (21). We constructed further univariable and multivariable models considering the effect of Lp(a) as a categorical variable (high Lp(a) ≥70 mg/dL vs low Lp(a) <70 mg/dL) on the absolute change in plaque volume. A 2-sided P < 0.05 was considered statistically significant. The statistical analyses were performed using RStudio software version 4.0.3 (R Foundation for Statistical Computing).
Results
Baseline characteristics
A total of 191 individuals in whom Lp(a) was measured and baseline CCTA performed were included in the present analysis. The mean age was 65.3 ± 8.3 years and 153 (79.7%) were male. Patients had advanced stable multivessel coronary atherosclerosis with 138 (71.5%) having a history of prior acute coronary syndrome, 148 (77.9%) having prior percutaneous coronary intervention, and 34 (17.9%) having prior coronary artery bypass graft surgery. At baseline, the use of preventative therapies was high: 180 patients (94.7%) were on statin therapy and 191 (100%) were on antiplatelet therapy. The median serum Lp(a) concentration was 15 mg/dL (IQR: 6 to 58 mg/dL) and the 80th percentile was 78 mg/dL. Across the whole cohort, the baseline total plaque volume was 1,378 mm3 (IQR: 1,048 to 1,994 mm3) and burden was 58% (IQR: 53% to 6%). Noncalcific plaque volume was 1,282 mm3 (IQR: 959 to 1,692 mm3) and burden was 54% (IQR: 50% to 58%). Calcific plaque volume was 95 mm3 (IQR: 37 to 224 mm3) and burden was 3.7% (IQR: 1.7% to 8.5%). Low-attenuation plaque volume was 87 mm3 (IQR: 48 to 168 mm3) and burden was 3.8% (IQR: 2.2% to 6.3%). Repeat CCTA after 12 months was available for 160 individuals (83.8%).
Clinical and imaging characteristics in high- and low-Lp(a) groups
There were no differences in body mass index, smoking status, diabetes mellitus, LDL-cholesterol, and serum creatinine concentrations between those with serum Lp(a) concentrations above or below 70 mg/dL (Table 1). Those with Lp(a) ≥70 mg/dL had higher HDL-cholesterol (1.27 ± 0.39 mmol/L vs 1.11 ± 0.28 mmol/L; P = 0.003), and lower systolic blood pressure (141 ± 18 mm Hg vs 148 ± 20 mm Hg; P = 0.035). The ASSIGN score was also higher in the group with lower Lp(a) concentrations (27.7% ± 16.0% vs 20.6% ± 10.1%; P = 0.006).
Table 1.
Patient Characteristics
| Lipoprotein(a) ≥70 mg/dL (n = 43) | Lipoprotein(a) <70 mg/dL (n = 148) | P Value | |
|---|---|---|---|
| Baseline | |||
| Lipoprotein(a), mg/dL | 100 (82–115) | 10 (5–24) | |
| Clinical | |||
| Age, y | 65.0 ± 7.1 | 65.3 ± 8.7 | 0.864 |
| Male | 31 (72.1) | 122 (82.2) | 0.217 |
| Body mass index, kg/m2 | 29.1 ± 5.0 | 30.0 ± 5.2 | 0.342 |
| Systolic blood pressure, mm Hg | 141 ± 18 | 148 ± 20 | 0.035 |
| Diastolic blood pressure, mm Hg | 78 ± 9 | 82 ± 11 | 0.033 |
| Active smoking | 5 (11.6) | 23 (15.6) | 0.682 |
| Diabetes mellitus | 5 (11.6) | 31 (21.1) | 0.242 |
| Total cholesterol, mmol/L | 4.27 ± 0.86 | 4.18 ± 1.00 | 0.545 |
| Low-density lipoprotein cholesterol, mmol/L | 2.36 ± 0.62 | 2.22 ± 0.81 | 0.270 |
| High-density lipoprotein cholesterol, mmol/L | 1.27 ± 0.39 | 1.11 ± 0.28 | 0.003 |
| Triglycerides, mmol/L | 1.20 (0.90–1.70) | 1.65 (1.20–2.30) | 0.002 |
| Creatinine, μmol/L | 77 ± 10 | 82 ± 17 | 0.066 |
| Statin use | 43 (100) | 137 (93.2) | 0.171 |
| Antiplatelet therapy | 40 (93) | 143 (97.3) | 0.399 |
| ASSIGN score | 20.6 (10.1) | 27.7 (16.0) | 0.006 |
| Acute coronary syndrome | 32 (74.4) | 105 (70.9) | 0.848 |
| Percutaneous coronary intervention | 36 (83.7) | 112 (76.2) | 0.402 |
| Coronary artery bypass graft | 9 (20.9) | 25 (17.0) | 0.716 |
| Imaging measurements | |||
| Vessel involvement | 0.396 | ||
| 0 | 3 (7.0) | 6 (4.1) | |
| 1 | 8 (18.6) | 43 (29.3) | |
| 2 | 20 (46.5) | 64 (43.5) | |
| 3 | 12 (27.9) | 30 (20.4) | |
| 4 | 0 (0) | 4 (2.7) | |
| Coronary segment involvement score | 5.1 (2.3) | 5.6 (2.3) | 0.192 |
| Coronary calcium score (Agatston units) | 378 (137–650) | 371 (101–894) | 0.902 |
| Total plaque burden, % | 57 (54–67) | 58 (53–66) | 0.531 |
| Calcific plaque burden, % | 3.6 (2.2–10.7) | 3.9 (1.5–8.5) | 0.644 |
| Noncalcific plaque burden, % | 53.7 (50.3–60.2) | 53.9 (49.6–58.0) | 0.584 |
| Low-density plaque burden, % | 3.7 (2.1–5.7) | 3.8 (2.3–6.5) | 0.848 |
| Fibro-fatty plaque burden, % | 20.5 (16.0–30.4) | 24.3 (16.6–28.8) | 0.578 |
| Total plaque volume, mm3 | 1,452 (1,043–1,963) | 1,351 (1,061–1,994) | 0.997 |
| Calcific plaque volume, mm3 | 98 (38–205) | 94 (32–231) | 0.857 |
| Noncalcific plaque volume, mm3 | 1,290 (959–1,668) | 1,282 (977–1,718) | 0.958 |
| Low-density plaque volume, mm3 | 87 (46–144) | 87.09 (50–174) | 0.742 |
| Fibro-fatty volume, mm3 | 471 (329–388) | 571 (313–836) | 0.864 |
Values are mean ± SD, median (IQR), or n (%). BoldP values <0.05 show statistically significant differences.
Coronary artery calcium scores on noncontrast CT were comparable between the high- and low-Lp(a) groups: 378 (IQR: 137 to 650) AU versus 371 (IQR: 101 to 894) AU (P = 0.902). On CCTA, there were no baseline differences between high- and low-Lp(a) groups in terms of total, calcific, noncalcific, and low-attenuation plaque volumes (Table 1). Respective plaque burdens were also similar between high- and low-Lp(a) groups (Table 1).
Accelerated progression of adverse plaque in high-Lp(a) patients
In the 160 individuals with repeat CCTA, we observed a mean increase of 83.0 ± 293.4 mm3 in total plaque volume, 4.4 ± 76.2 mm3 in calcific plaque volume, 78.6 ± 274.0 mm3 in noncalcific plaque volume, and 4.6 ± 56.7 mm3 in low-attenuation plaque volumes. Patients in the high-Lp(a) group showed a larger increase in low-attenuation plaque volumes compared to patients in the low-Lp(a) group (26.2 ± 88.4 mm3 vs -0.7 ± 50.1 mm3; P = 0.020) (Table 2, Figures 1 and 2). Patients in the high-Lp(a) group also showed a larger increase in fibro-fatty plaque volumes compared to patients in the low-Lp(a) group (55.0 ± 242.8 mm3 vs −25.0 ± 157.4 mm3; P = 0.020). In contrast, there was no difference in change in total plaque volume or change in calcific and noncalcific plaque volumes between the high- and low-Lp(a) groups.
Table 2.
Change in Coronary Plaque Volumes on Repeat CCTA in Patients With High and Low Lp(a)
| Lp(a) ≥70 mg/dL (n = 36) | Lp (a) <70 mg/dL (n = 125) | P Value | |
|---|---|---|---|
| Total plaque volume change | 128.2 ± 330.6 | 88.5 ± 312.2 | 0.508 |
| Calcific plaque volume change | 19.7 ± 69.7 | 1.0 ± 85.7 | 0.231 |
| Noncalcific plaque volume change | 108.5 ± 319.6 | 87.5 ± 288.6 | 0.708 |
| Low-density plaque volume change | 26.2 ± 88.4 | -0.7 ± 50.1 | 0.020 |
| Fibro-fatty volume change | 55.0 ± 242.8 | -25.0 ± 157.4 | 0.020 |
Values are mean ± SD of the absolute difference in plaque volume (mm3) between scan 1 and 2. Bold P values <0.05 show statistically significant differences.
CCTA = coronary computed tomography angiography; Lp(a) = lipoprotein(a).
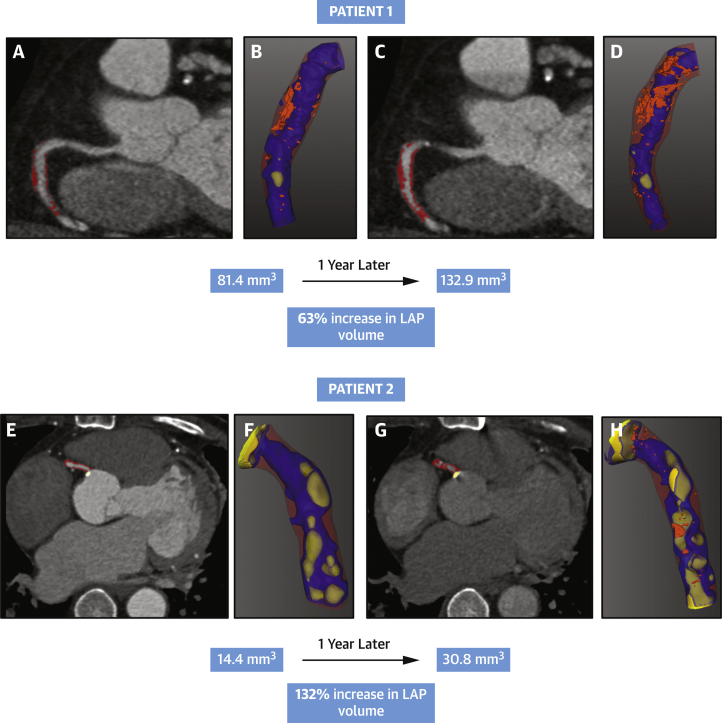
Figure 2.
Low-Attenuation Plaque Progression on CCTA in Patients With High Lp(a)
CCTA in 2 patients with high serum Lp(a) concentrations (82.2 and 152 mg/dL, respectively). In patient 1, atherosclerotic plaque in the mid-right coronary artery at baseline (A, noncalcific highlighted with red overlay) and after 1 year (C). Low-attenuation plaque is visualized in bright orange on the 3-dimensional reconstructions (B and D) showing progression from a volume of 81 mm3 to 133 mm3 1 year later. Similar representative images are seen in patient 2 with mixed atherosclerotic plaque in the mid-right coronary artery at baseline (E) and 1 year (G). Low-attenuation plaque progressed on serial scanning from a volume of 14.4 mm3(F) to 30.8 mm3 after 1 year (H). LAP = low-attenuation plaque; other abbreviations as in Figure 1.
Increasing Lp(a) is independently associated with progression of low-attenuation plaque
In univariable regression analysis, Lp(a) was associated with progression of low-attenuation plaque volume (β = 11.6% for each 50 mg/dL increase, 95% CI: 2.0% to 21.2%; P = 0.018), but not with progression of total, calcific, and noncalcific plaque volumes (Figure 3). The association between high Lp(a) and accelerated progression of low-attenuation plaque volume remained in multivariable linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score (β = 10.5% for each 50 mg/dL increase, 95% CI: 0.7% to 20.3%). Results were unchanged when considering high Lp(a) as a categorical variable (≥70 mg/dL) to evaluate its relation with absolute change in low-attenuation plaque volume (Table 3).
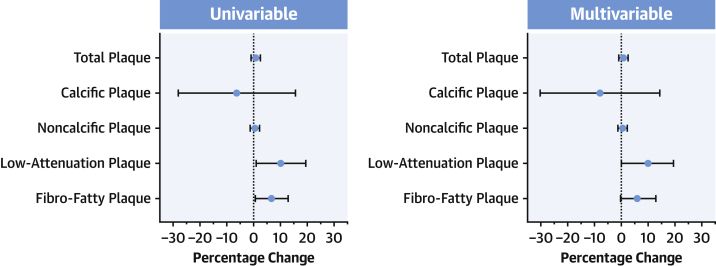
Figure 3.
Effect of Lp(a) on Plaque Progression
Data are depicted as betas with 95% CIs for the percentage change in plaque volume from baseline to follow-up CCTA, standardized for each 50 mg/dL increase in Lp(a). Lp(a) was associated with low-attenuation plaque progression in univariable (β = 10.2%, P = 0.031) and multivariable (β = 9.6%, P = 0.048) analyses, and with fibro-fatty plaque progression in univariable analysis (β = 6.7%, P = 0.034), showing a trend in multivariable analysis (β = 6.0%, P = 0.062). Abbreviations as in Figure 1.
Table 3.
High Lp(a) (≥70 mg/dL) and Absolute Change in Coronary Plaque Volumes on Repeat CCTA
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Beta (95% CI) | P Value | Beta (95% CI) | P Value | |
| Total plaque volume change | 50.1 (−65.4 to 165.6) | 0.393 | −23.2 (−140.4 to 94.1) | 0.697 |
| Calcific plaque volume change | 19.1 (−11.9 to 50.1) | 0.226 | −12.9 (−44.6 to 18.9) | 0.425 |
| Noncalcific plaque volume change | 31.0 (−76.8 to 138.8) | 0.571 | −10.3 (−120.2 to 99.6) | 0.853 |
| Low-attenuation plaque volume change | 27.9 (5.3 to 50.5) | 0.016 | 25.4 (2.5 to 48.2) | 0.030 |
| Fibro-fatty plaque volume change | 84.3 (17.6 to 150.9) | 0.014 | 73.8 (6.3 to 141.3) | 0.032 |
Values are betas with 95% CI for the absolute change in plaque volume (mm3) in patients with serum Lp(a) ≥70 mg/dL. Multivariable linear regression analysis is adjusted for body mass index, ASSIGN score, and segment involvement score. BoldP values <0.05 show statistically significant differences.
Abbreviations as in Table 2.
Results were also similar regarding fibro-fatty plaque progression. Higher concentrations of Lp(a) were associated with accelerated progression of fibro-fatty plaque in univariable regression (β = 7.0% for each 50 mg/dL increase, 95% CI: 0.9% to 13.2%), and showed a trend in multivariable regression (β = 6.2% for each 50 mg/dL increase, 95% CI: −0.1 to 12.4%) (Tables 3 and 4).
Table 4.
Increasing Lp(a) and Change in Coronary Plaque Volumes on Repeat CCTA
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Beta (95% CI) | P Value | Beta (95% CI) | P Value | |
| Total plaque volume change | 0.3 (−3.9 to 4.5) | 0.887 | 0.81 (−5.0 to 3.4) | 0.701 |
| Calcific plaque volume change | −9.6 (−40.6 to 21.4) | 0.542 | −12.75 (−44.5 to 19.0) | 0.428 |
| Noncalcific plaque volume change | 0.0 (−4.2 to 4.3) | 0.988 | −1.0 (−5.2 to 3.2) | 0.631 |
| Low-attenuation plaque volume change | 11.6 (2.0 to 21.2) | 0.018 | 10.5 (0.7 to 20.3) | 0.037 |
| Fibro-fatty plaque volume change | 7.0 (0.9 to 13.2) | 0.025 | 6.2 (−0.1 to 12.4) | 0.053 |
Values are betas with 95% CI for the percentage change in plaque volume, standardized for each 50 mg/dL increase in serum lipoprotein(a). Multivariable linear regression analysis is adjusted for body mass index, ASSIGN score and segment involvement score. BoldP values <0.05 show statistically significant differences.
Discussion
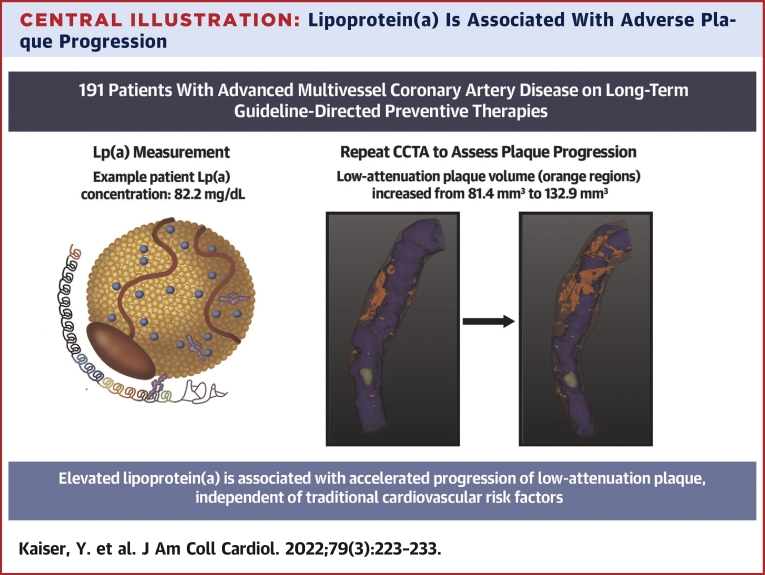
For the first time, we have used repeat CCTA to investigate the association between serum Lp(a) concentrations and progression of coronary plaque volume and phenotype in patients with advanced multivessel coronary atherosclerosis. Patients with high serum Lp(a) concentrations (≥70 mg/dL) showed accelerated progression of low-attenuation coronary plaque volume after 12 months (Central Illustration). Conversely, we found no difference in the progression of more stable plaque phenotypes between the high- and low-Lp(a) groups. These findings provide a mechanistic explanation for the association between high Lp(a) and the residual risk of myocardial infarction in patients already established on secondary prevention.
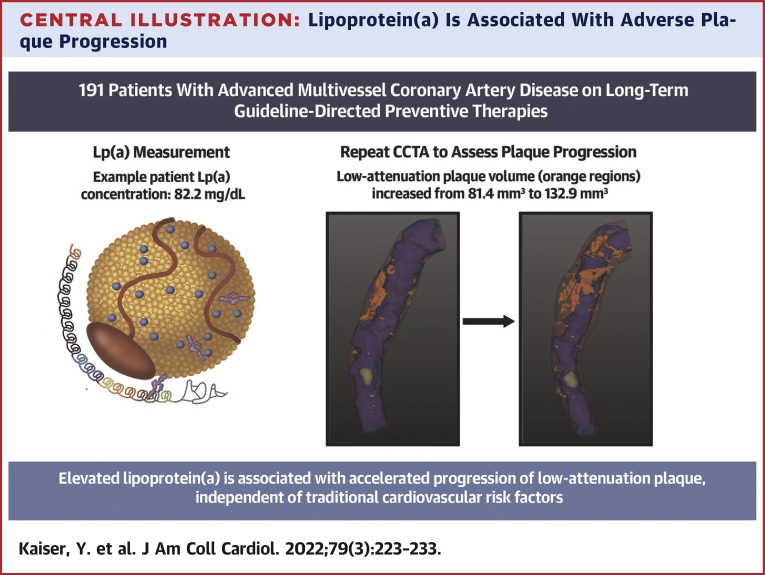
Central Illustration.
Lipoprotein(a) Is Associated With Adverse Plaque Progression
Patients with advanced multivessel coronary artery disease with elevated Lp(a) concentrations (≥70 mg/dL) showed accelerated progression of low-attenuation plaque. An example patient with elevated Lp(a) (82.2 mg/dL) showed marked progression of low-attenuation plaque volume (visualized in orange): increased from 81.4 to 132.9 mm3 after 1-year follow-up. CCTA = coronary computed tomography angiography; Lp(a) = lipoprotein(a).
CCTA and semiautomated quantitative analysis techniques now allow for the noninvasive assessment of different plaque types and their progression over time. Adverse plaque phenotype, including a large necrotic core, inflammation, microcalcification, and a thin fibrous cap, are associated with an increased propensity to rupture and therefore with an increased risk of myocardial infarction (20). Low-attenuation plaque on CCTA is a quantitative marker of necrotic core and provides prognostic information for cardiovascular outcomes (22). Indeed, in the SCOT-HEART (Scottish Computed Tomography of the Heart) trial, the burden of low-attenuation plaque was the most powerful predictor of fatal or nonfatal myocardial infarction, outperforming cardiovascular risk scores, assessments of plaque lumen stenosis, CT calcium scoring, and the burden of all other different plaque subtypes (23). Similarly, powerful prognostic value was also reported in the recent ICONIC (Incident Coronary Syndromes Identified by Computed Tomography) study, a large prospective multinational registry of patients undergoing CCTA (11). In the current study, we found that Lp(a) was independently associated with accelerated progression of low-attenuation plaque in patients with comparable disease severity at baseline. These findings suggest that in established coronary artery disease, Lp(a) drives ASCVD-risk by promoting progression of vulnerable plaque phenotypes, providing a potential mechanistic explanation for the association between Lp(a) and clinical atherothrombotic events. This association appears robust and independent of baseline disease severity, cardiovascular risk factors, and plaque burdens. Moreover, this progression in low-attenuation plaque phenotype was observed despite the near universal prescription of statins and other preventative medications in this cohort. Therefore, our data provide further support for Lp(a) as a novel risk marker of residual risk as well as reaffirming its potential as a treatment target in coronary atherosclerosis, particularly in the population investigated here: patients with high Lp(a) already established on preventative therapies. Randomized controlled trials are underway investigating whether Lp(a)-lowering therapy can improve cardiovascular outcomes in this patient population. Our data pave the way for mechanistic substudies to investigate whether any observed clinical benefit is due to a slowing in low-attenuation plaque progression with Lp(a) lowering.
What do these findings imply for the pathophysiological role of Lp(a) in coronary artery disease? As we did not observe a difference between high- and low-Lp(a) groups in the progression of total plaque volume or more stable subtypes of plaque, the sheer accumulation of Lp(a) particles in the atherosclerotic plaque does not appear to be the dominant mechanism. Instead, we observed that patients with high Lp(a) showed accelerated progression of low-attenuation plaque phenotype, a marker for necrotic cores. Lp(a) and its associated OxPL are known to trigger a proinflammatory response, resulting in cellular apoptosis and necrosis thereby contributing to accelerated necrotic core formation (24,25). Blocking of OxPL signaling using an antibody treatment reduced necrotic core formation by almost one-half its size in a murine model of atherosclerosis (26,27). Furthermore, human pathology studies show the increasing presence of Lp(a) and OxPL in coronary artery plaques as lesion severity progresses (26,28). Increased levels of circulating Lp(a) may therefore drive low-attenuation plaque progression predominantly via OxPL and its proinflammatory effects.
We did not observe any baseline difference in coronary plaque burdens between patients with high and low Lp(a) concentrations. This is an important strength because it allows us to assess the effect of Lp(a) on plaque progression independent of baseline plaque burden, which is a major determinant of disease progression. However, it is seemingly at odds with several previous intravascular ultrasound studies that have shown baseline differences in plaque burden with Lp(a) concentrations (7,8). These cohorts consisted primarily of low-risk individuals in a primary prevention setting, whereas we have focused on patients with established multivessel disease and previous coronary revascularization, the majority of whom had had a previous acute coronary syndrome. These inclusion criteria are likely to have caused a selection pressure in our cohort resulting in the recruitment of patients who all have a high plaque burden and a high burden of adverse plaque irrespective of their Lp(a) concentrations.
Study limitations
Our study includes one of the largest number of consecutive, prospectively enrolled patient cohorts to undergo repeat CCTA for the assessment of coronary plaque progression. However, we recognize that this is a single-center study comprising a largely Caucasian male population. Although we have previously shown the reproducibility of CCTA plaque assessments and repeat CCTA has been used in smaller randomized trials such as the EVAPORATE (Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients With Elevated Triglycerides on Statin Therapy) study, we acknowledge the need to confirm our findings in larger patient populations and cannot rule out smaller effects of Lp(a) on the progression of other coronary plaque subtypes (29,30). We have also focused on patients with advanced established coronary artery already established on secondary prevention, a common group in whom Lp(a) lowering is likely to prove the maximal benefit, and which is being investigated in the ongoing phase 3 Lp(a)-lowering Lp(a)HORIZON study (NCT04023552). Future studies across multiple centers using different scanners and enrolling more diverse populations with less advanced coronary artery disease are needed to explore the generalizability of our findings. Finally, quantification of plaque subtype can be a time-intensive process and the practical application of plaque quantification may be improved by further automation.
Conclusions
High concentrations of serum Lp(a) are associated with accelerated progression of low-attenuation plaque (necrotic core) in patients with advanced multivessel coronary artery disease despite receiving guideline-based preventative therapies. This provides a potential mechanistic explanation for the association between Lp(a) and the residual risk of myocardial infarction and supports Lp(a) as a novel treatment target in atherosclerosis. Randomized controlled trials of Lp(a) lowering are now required to assess whether this reduces both low-attenuation plaque progression and cardiovascular events.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with advanced coronary artery disease, elevated serum Lp(a) is associated with accelerated progression of the low-attenuation necrotic core of atherosclerotic plaques.
TRANSLATIONAL OUTLOOK: Randomized trials are required to evaluate whether Lp(a) lowering reduces low-attenuation plaque progression and prevents ischemic events.
Funding Support and Author Disclosures
Drs Kaiser and Stroes were supported by the Netherlands Heart Foundation CVON 2017-20: generating the best evidence-based pharmaceutical targets for atherosclerosis [GENIUS II]). Dr Dey is supported by the National Institute of Health/National Heart, Lung, and Blood Institute grants (1R01HL148787-01A1 and 1R01HL151266). Dr Dweek is supported by the British Heart Foundation (FS/14/78/31020) and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015 (15/JTA); has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence therapeutics. Dr Stroes has received research grants/support to his institution from Amgen, Sanofi, Resverlogix, and Athera; and has served as a consultant for Amgen, Sanofi, Esperion, Novartis, and Ionis Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Shah N.P., Pajidipati N.J., McGarrah R.W., et al. Lipoprotein (a): an update on a marker of residual risk and associated clinical manifestations. Am J Cardiol. 2020;126:94–102. doi: 10.1016/j.amjcard.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2019;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Boffa M.B., Koschinsky M.L. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16(5):305–318. doi: 10.1038/s41569-018-0153-2. [DOI] [PubMed] [Google Scholar]
- 4.Tsimikas S., Brilakis E.S., Miller E.R., et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/nejmoa043175. [DOI] [PubMed] [Google Scholar]
- 5.Gencer B., Kronenberg F., Stroes E.S., Mach F. Lipoprotein(a): the revenant. Eur Heart J. 2017;38(20):1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 6.Nestel P.J., Barnes E.H., Tonkin A.M., et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902–2908. doi: 10.1161/atvbaha.113.302479. [DOI] [PubMed] [Google Scholar]
- 7.Huded C.P., Shah N.P., Puri R., et al. Association of serum lipoprotein (a) levels and coronary atheroma volume by intravascular ultrasound. J Am Heart Assoc. 2020;9(23) doi: 10.1161/jaha.120.018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muramatsu Y., Minami Y., Kato A., et al. Lipoprotein (a) level is associated with plaque vulnerability in patients with coronary artery disease: an optical coherence tomography study. Int J Heart Vasc. 2019;24:100382. doi: 10.1016/j.ijcha.2019.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meah M.N., Singh T., Williams M.C., et al. Reproducibility of quantitative plaque measurement in advanced coronary artery disease. J Cardiovasc Comput. 2021;15(4):333–338. doi: 10.1016/j.jcct.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al’Aref S.J., Singh G., Choi J.W., et al. A boosted ensemble algorithm for determination of plaque stability in high-risk patients on coronary CTA. J Am Coll Cardiol Img. 2020;13(10):2162–2173. doi: 10.1016/j.jcmg.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Chang H.-J., Lin F.Y., Lee S.-E., et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511–2522. doi: 10.1016/j.jacc.2018.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams M.C., Moss A.J., Dweck M., et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. 2019;73(3):291–301. doi: 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss A.J., Dweck M.R., Doris M.K., et al. Ticagrelor to reduce myocardial injury in patients with high-risk coronary artery plaque. J Am Coll Cardiol Img. 2020;13(7):1549–1560. doi: 10.1016/j.jcmg.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 15.Austen W., Edwards J., Frye R., et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 16.Min J.K., Shaw L.J., Devereux R.B., et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 17.Dey D., Schepis T., Marwan M., Slomka P.J., Berman D.S., Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257(2):516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 18.Raff G.L., Abidov A., Achenbach S., et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput. 2009;3(2):122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Woodward M., Brindle P., Tunstall-Pedoe H., et al. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93(2):172. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dweck M.R., Maurovich-Horvat P., Leiner T., et al. Contemporary rationale for non-invasive imaging of adverse coronary plaque features to identify the vulnerable patient: a position paper from the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2020;21(11):1177–1183. doi: 10.1093/ehjci/jeaa201. [DOI] [PubMed] [Google Scholar]
- 21.Langsted A., Kamstrup P.R., Nordestgaard B.G. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40(33):2760–2770. doi: 10.1093/eurheartj/ehy902. [DOI] [PubMed] [Google Scholar]
- 22.Ferencik M., Mayrhofer T., Bittner D.O., et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3(2):144. doi: 10.1001/jamacardio.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams M.C., Kwiecinski J., Doris M., et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART) Circulation. 2020;141(18):1452–1462. doi: 10.1161/circulationaha.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Valk F.M., Bekkering S., Kroon J., et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–624. doi: 10.1161/circulationaha.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnitzler J.G., Hoogeveen R.M., Ali L., et al. Atherogenic lipoprotein(A) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Atherosclerosis. 2020;315:e10–e11. doi: 10.1016/j.atherosclerosis.2020.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dijk R.A., Kolodgie F., Ravandi A., et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53(12):2773–2790. doi: 10.1194/jlr.p030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Que X., Hung M.-Y., Yeang C., et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558(7709):301–306. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seimon T.A., Nadolski M.J., Liao X., et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2–dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budoff M.J., Bhatt D.L., Kinninger A., et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925–3932. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt D.L., Steg P.G., Miller M., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/nejmoa1812792. [DOI] [PubMed] [Google Scholar]