Abstract
Control processes allow us to constrain the retrieval of semantic information from long-term memory so that it is appropriate for the task or context. Control demands are influenced by the strength of the target information itself and by the circumstances in which it is retrieved, with more control needed when relatively weak aspects of knowledge are required and after the sustained retrieval of related concepts. To investigate the neurocognitive basis of individual differences in these aspects of semantic control, we used resting-state fMRI to characterise the intrinsic connectivity of left ventrolateral prefrontal cortex (VLPFC), implicated in controlled retrieval, and examined associations on a paced serial semantic task, in which participants were asked to detect category members amongst distractors. This task manipulated both the strength of target associations and the requirement to sustain retrieval within a narrow semantic category over time. We found that individuals with stronger connectivity between VLPFC and medial prefrontal cortex within the default mode network (DMN) showed better retrieval of strong associations (which are thought to be recalled more automatically). Stronger connectivity between the same VLPFC seed and another DMN region in medial parietal cortex was associated with larger declines in retrieval over the course of the category. In contrast, participants with stronger connectivity between VLPFC and cognitive control regions within the ventral attention network (VAN) had better controlled retrieval of weak associations and were better able to sustain their comprehension throughout the category. These effects overlapped in left insular cortex within the VAN, indicating that a common pattern of connectivity is associated with different aspects of controlled semantic retrieval induced by both the structure of long-term knowledge and the sustained retrieval of related information.
Keywords: Cognitive control, Left ventrolateral prefrontal cortex, Controlled semantic retrieval, Recent memory retrieval, Ventral attention network, intrinsic connectivity
1. Introduction
The control of memory retrieval plays a critical role in shaping cognition to suit the circumstances (Badre et al., 2005; Badre and Wagner, 2007; Barredo et al., 2015; Jefferies, 2013; Lambon Ralph, Jefferies, Patterson, and Rogers, 2017; Nyberg et al., 2003; Wagner, 2002). Within semantic cognition, control processes that regulate conceptual retrieval are thought to support our ability to focus on features and associations that are currently relevant, even when these aspects of knowledge are not dominant in long-term memory (Jefferies, 2013; Thompson-Schill et al., 1997; Whitney et al., 2010; Zhang et al., 2020). A distributed left-lateralised semantic control network shows a stronger response when semantic retrieval must be constrained to suit the current goal or context (Badre et al., 2005; Badre and Wagner, 2007; Jackson, 2020; Noonan et al., 2013; Zhang et al., 2020), and inhibitory stimulation of this network disrupts controlled retrieval (Davey et al., 2015; Hoffman et al., 2010; Whitney et al., 2010; Whitney et al., 2012). Left inferior frontal gyrus (LIFG), within ventrolateral prefrontal cortex (VLPFC), is the most strongly and consistently activated site across different contrasts designed to tap semantic control (Badre et al., 2005; Jackson, 2020; Noonan et al., 2013; Zhang et al., 2020).
LIFG and other sites in the semantic control network (SCN) are partially overlapping with the multiple demand network (MDN), which is functionally defined by identifying regions that respond to executive demands across domains (Crittenden and Duncan, 2014; Duncan, 2010; Fedorenko et al., 2013) – however, SCN peaks in anterior ventral LIFG and posterior middle temporal gyrus lie outside MDN (Badre et al., 2005; Davey et al., 2016; Gao et al., 2021; Wang et al., 2020). Moreover, the SCN is highly left-lateralised (Gonzalez Alam, Karapanagiotidis, Smallwood, and Jefferies, 2019; Gonzalez Alam et al., 2021), while the MDN is bilateral (Duncan, 2010; Fedorenko et al., 2013). These networks might play distinct roles in cognitive control: a recent study found that anterior and ventral LIFG responded more strongly to manipulations of semantic control than verbal working memory demands, while the reverse pattern was seen in right dorsolateral prefrontal cortex; moreover, there was shared neural coding of control demands across tasks in MDN, while SCN regions coded for the difficulty of these verbal tasks in different ways (Gao et al., 2021). Furthermore, while MDN supports a diverse set of demanding cognitive tasks (Fedorenko et al., 2013), the component regions of this network are not functionally homogeneous (e.g., Crittenden et al., 2016; Dosenbach et al., 2008; Shenhav et al., 2013). This system overlaps with several resting-state networks (frontoparietal, dorsal attention and ventral attention networks, from a parcellation of intrinsic connectivity from 1000 brains; Yeo et al., 2011) and even during task performance, it can be divided into frontoparietal and cingulo-opercular subnetworks (Crittenden et al., 2016; Dosenbach et al., 2008). These components may have dissociable roles in the instantiation of current goals and the detection of relevant stimuli and responses (Han et al., 2019; Sadaghiani and D'Esposito, 2015; Sestieri et al., 2014; Wallis et al., 2015).
Within semantic cognition, control requirements are modulated by both the structure of long-term semantic memory and the context in which retrieval occurs (e.g., Canini et al., 2016; Davey et al., 2015; Teige et al., 2019; Wimber et al., 2008; Zhang et al., 2020). (i) The retrieval of weaker aspects of semantic knowledge elicits greater activation of SCN; for example, when participants are asked to retrieve semantic connections between weakly, as opposed to strongly, associated words (e.g., car and rust vs. car and road; Badre et al., 2005; Teige et al., 2019; Thompson-Schill et al., 1997; Wagner et al., 2001; Zempleni et al., 2007; Zhang et al., 2020). In line with this suggestion, the activation in LIFG is modulated by semantic distance, with parametric increases in the response of this region as the strength of semantic association decreases (Gao et al., 2021; Zhang et al., 2020). (ii) Recent or sustained retrieval of related representations can also increase the requirements for controlled retrieval (Anderson, 2003; MacLeod et al., 2003; Nathaniel et al., 2018; Runnqvist et al., 2012; Wimber et al., 2008). This can arise within tasks such as picture naming when semantically-related items are presented in quick succession, creating ‘blocking’ effects that reflect temporary inaccessibility of information (Nathaniel et al., 2018). This pattern may occur because retrieval involves suppressing semantic neighbours of targets, which then cannot be easily accessed; in addition, the earlier activation of related concepts may create competition that increases subsequent control demands (Jefferies et al., 2007; Schnur et al., 2006; Thompson et al., 2015). A similar pattern of increasing control demands following retrieval can also occur within episodic memory: the selective retrieval of a subset of previously encoded memories can lead to a decline in later retrieval performance (Anderson, 2003, 1994; Wimber et al., 2008; Wimber et al., 2009). Previous studies have shown the importance of left VLPFC in supporting efficient retrieval in these circumstances (Canini et al., 2016; Kuhl et al., 2008; Wimber et al., 2008).
While controlled retrieval demands can be influenced by both the strength of long-term representations and the recent or sustained retrieval of conceptually-related information, it is unclear whether individual differences in these aspects of controlled retrieval reflect variation in the same underlying neural mechanisms. When weaker aspects of knowledge are required by a task, stronger but irrelevant features and associations of the same concepts may need to be suppressed, and target information may need to be boosted (e.g., Badre and Wagner, 2007; Jefferies, 2013; Zhang et al., 2020). Similarly, when memory retrieval follows the earlier recall of semantically-related information, there may be competition from this previously-activated material (MacLeod et al., 2003; Raaijmakers and Jakab, 2013) and/or retrieval-induced forgetting, whereby earlier retrieval leads to the inhibition of related memory representations (Anderson et al., 1994; Murayama et al., 2014). Moreover, some paradigms require participants to maintain an appropriate attentional focus on specific semantic information, and this may become more difficult over time. A recent study by Nathaniel et al. (2018) used a ‘paced serial semantic task’ that required participants to sustain attention to a semantic target category and detect relevant items at a rapid pace: healthy participants were less efficient at retrieving weak than strong associations, and showed within-category declines in target detection suggesting that they had greater difficulty sustaining attention or overcoming competition and/or retrieval-induced forgetting towards the end of each category. There was a release from these effects as the category changed, suggesting that this pattern reflected control over semantic retrieval as opposed to general fatigue. Interestingly, semantic aphasia patients with deficits of controlled semantic retrieval following damage centred on LIFG showed greater impairment when retrieving weak versus strong associations in this paradigm, yet did not show declining retrieval as related trials were presented over an extended period; control demands that relate to the structure of long-term semantic memory as opposed to the context in which retrieval occurs may draw on distinct mechanisms, differentially impaired in this patient group, but this hypothesis has not yet been tested in healthy participants.
In the current study, we used functional neuroimaging to examine the neurocognitive mechanisms that contribute to individual differences in the paced serial semantic task used by Nathaniel et al. (2018). Participants were asked to decide if auditorily presented target words were related to a particular category label, specified at the start of each block. This paradigm allowed us to compare the retrieval of weak versus strong associations (providing a manipulation of control demands based on the long-term structure of knowledge) and within-category declines in performance (reflecting the ability to maintain retrieval over an extended period of time, as semantically-related items continue to be presented). We investigated individual differences in these two features of the task, linking these distinct aspects of performance to the intrinsic connectivity of left VLPFC, which is implicated in the controlled retrieval of both episodic and semantic memory (Noonan et al., 2013; Vatansever et al., 2021; Wimber et al., 2008). This allowed us to determine whether common or divergent patterns of connectivity from VLPFC relate to the recovery of weakly-related semantic information, and the ability to sustain retrieval even when many related concepts are presented.
2. Materials and methods
2.1. Participants
Eighty-one undergraduate and postgraduate students were recruited for this study (age range 18–25, mean age ± standard deviation = 19.92 ± 1.43, 22 males), with each participant completing both resting-state brain scanning and behavioural assessment outside the scanner. All were right-handed native English speakers, and had normal or corrected-to-normal vision. None of them had any history of neurological impairment, diagnosis of learning difficulty or psychiatric illness. All provided written informed consent prior to taking part and received a monetary reward for their participation. Three participants were removed due to chance-level performance under each experimental condition; the final sample therefore consisted of 78 participants. Ethical approval was obtained from the Research Ethics Committees of the Department of Psychology and York Neuroimaging Centre, University of York. All research was performed in accordance with the relevant guidelines/regulations.
2.2. Materials
Fifteen categories labels (e.g., Bakery) were selected from the task used by Nathaniel et al. (2018), with each category containing 60 items. 20 items were semantically related to the category, including 10 targets that were strongly related to the category label, such as “bun”, and 10 that were weakly related, such as “knife”, while the remaining 40 items were unrelated to the category (e.g., panda) - these were recycled items from other categories. Overall, 300 semantically related and 600 unrelated words were included in this study. We focussed our analyses on semantically related items, using a fully-factorial within-subject design with manipulations of (i) Strength of semantic association between the targets and category (Strong association vs. Weak association), and (ii) Within-category decline (detection of semantically-related items presented in the first half vs. the second half of each category, divided equally across strongly and weakly associated targets) to create four conditions, with each experimental condition including 75 semantically related targets. In this way, the paradigm manipulated control demands relating to both the structure of long-term memory (strength of association) and the context in which retrieval occurred (within-category decline).
Target words were selected using the Edinburgh Associative Thesaurus (EAT; Kiss et al., 1973), supplemented by a pilot study in which ratings were collected for the strength of association of each word with the category label. In this pilot, 16 participants were asked to use a 7-point Likert scale to judge association, and items were categorised as strongly related (> 5.5), weakly related (2.2–5.5) or unrelated (< 2.2; also see Nathaniel et al., 2018). In order to avoid any confounding effects from linguistic properties, the strong and weak targets in the first and second halves of each category were matched for frequency (CELEX database; Baayen et al., 1993), number of syllables and imageability in the N-Watch database (Davis, 2005; p > .1, see Table 1).
Table 1.
Linguistic properties of the Target words (Mean ± SD).
| Conditions | Frequency | Number of syllables | Imageability* |
|---|---|---|---|
| Strong association in the 1st half of category | 1.29 ± 0.58 | 1.77 ± 0.83 | 570.91 ± 66.72 |
| Strong association in the 2nd half of category | 1.21 ± 0.63 | 1.66 ± 0.67 | 571.06 ± 50.36 |
| Weak association in the 1st half of category | 1.26 ± 0.68 | 1.84 ± 0.80 | 566.77 ± 63.36 |
| Weak association in the 2nd half of category | 1.20 ± 0.61 | 1.68 ± 0.66 | 547.57 ± 77.92 |
Imageability ratings were only available for 226 targets of the 300 semantic related items.
2.3. Behavioural assessment
A paced serial semantic task was adopted from previous study (Nathaniel et al., 2018), in which participants were asked to judge whether the spoken words (e.g., Bun or Panda) were semantically associated to a thematic category or not (e.g., Bakery). The experiment was presented using E-Prime 2.0 (Psychology Software Tools, Sharpsburg, PA). The categories were presented in a blocked manner, with each category starting with its category name presented as a written word in the centre of the screen. Participants were required to press the space bar to start the presentation of stimuli when they were ready. The semantically related targets and unrelated items were presented auditorily at a fast rate of presentation (i.e., 1000 ms inter-stimulus interval). Participants were asked to press ‘1′ each time they heard a word that was related to the presented category, and not to press for unrelated words. During this period, the category names were remained visible throughout each category block to reduce demands on working memory. After the presentation of each category, participants pressed the space bar to start the presentation of the next category.
The items in each category were presented in a pseudo-random order to ensure an equal distribution of strong and weak targets, as well as unrelated items in the first and second half of each category (i.e., 5 strong and 5 weak associations, as well as 20 unrelated items, in both the first and last 30 items of each category). We presented more unrelated than related items to maximise individual differences related to the requirement to sustain attention to a specific category; this is likely to contribute to within-category decline effects in this paradigm, since difficulties in goal maintenance and sustained attention should be magnified by the requirement to respond at speed to rare targets. Although retrieval-induced forgetting and/or competition from previously-activated targets might also contribute to within-category decline in this paradigm, these effects would be reduced in magnitude by this aspect of the design. Each participant was presented with all 15 categories.
2.4. Neuroimaging data acquisition
Structural and functional data were acquired using a 3T GE HDx Excite Magnetic Resonance Imaging (MRI) scanner utilizing an eight-channel phased array head coil at the York Neuroimaging Centre, University of York. Structural MRI acquisition in all participants was based on a T1-weighted 3D fast spoiled gradient echo sequence (repetition time (TR) = 7.8 s, echo time (TE) = minimum full (i.e., minimum achievable TE with full echo acquisition, which is applied to improve signal-to-noise ratio (SNR); ∼3 ms), flip angle = 20°, matrix size = 256 × 256, 176 slices, voxel size = 1.13 × 1.13 × 1 mm).
A 9-minute resting-state fMRI scan was used, recorded using single-shot 2D gradient-echo-planar imaging (TR = 3 s, TE = minimum full (∼19 ms), flip angle = 90°, matrix size = 64 × 64, 60 slices, voxel size = 3 × 3 × 3 mm, 180 vol). During resting-state scanning, participants were instructed to focus on a fixation cross with their eyes open and to keep as still as possible, without thinking about anything in particular. The resting-state data were collected first, followed by the collection of behavioural task data outside the scanner, so that measures of intrinsic connectivity could not be influenced by task performance.
2.5. Neuroimaging data pre-processing
Pre-processing was performed using the CONN-fMRI functional connectivity toolbox, Version 18a (http://www.nitrc.org/projects/conn; Whitfield-Gabrieli and Nieto-Castanon, 2012), based on Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/). Structural images were segmented into gray matter, White matter and Cerebrospinal Fluid tissues and normalized to the Montréal Neurological Institute (MNI) space with the unified segmentation and normalization procedure (Ashburner and Friston, 2005). Functional volumes were slice-time (bottom-up, interleaved) and motion-corrected, skull-stripped and co-registered to the high-resolution structural image, spatially normalised to MNI space using the unified-segmentation algorithm, smoothed with an 8 mm FWHM Gaussian kernel.
Pre-processing steps automatically create three first-level covariates: a realignment covariate containing the six rigid-body parameters characterising the estimated subject motion for each participant, a scrubbing covariate containing the potential outliers scans for each participant (all outlier volumes were identified through the artefact detection algorithm included in CONN, with intermediate settings: scans for each participant were flagged as outliers based on scan-by-scan change in global signal above z = 5, subject motion threshold above 0.9 mm, differential motion and composite motion exceeding 97% percentile in the normative sample), and a covariate containing quality assurance (QA) parameters (i.e., the global signal change from one scan to another and the framewise displacement, a measure of how much the participant moved from one scan to another) for each participant. Realignment parameters, potential outlier scans, signal from white matter and cerebrospinal fluid masks, and the effect of rest (i.e., an automatically estimated trend representing potential ramping effects in the BOLD timeseries at the beginning of the session), were then included as nuisance parameters into the model in the denoising step of the CONN toolbox. Using the implemented anatomical component-based (CompCor) approach (Behzadi et al., 2007), all of these effects were removed within a general linear regression model to increase the signal to noise ratio in the functional images (Chai et al., 2012). Functional images were then band-passed filtered (.008–.09 Hz) to constrain analyses to low-frequency fluctuations. A linear detrending term was also applied, eliminating the need for global signal normalisation (Chai et al., 2012; Murphy et al., 2009). Global signal regression was not performed because CompCor can efficiently account for subject movement effects and other sources of noise in the BOLD signal (Behzadi et al., 2007; Muschelli et al., 2014).
2.6. ROI selection
We selected left VLPFC as our seed region, since this control site has been implicated in both the controlled retrieval of weak aspects of semantic knowledge (Badre et al., 2005; Noonan et al., 2010; Thompson-Schill et al., 1997), and in the capacity to maintain retrieval in circumstances in which earlier task performance has been shown to increase control demands (e.g., Canini et al., 2016; Kuhl et al., 2008; Wimber et al., 2008). The seed region (MNI coordinates: −48, 26, 20) fell within the frontoparietal network (FPN) as defined by Yeo et al. (2011) and corresponded to the peak activation in VLPFC during the successful recall of retrieval-impaired memories in Wimber et al. (2008) study. The seed region was close to the peak response in LIFG for controlled semantic cognition identified by meta-analyses of neuroimaging studies of semantic control (Jackson, 2020; Noonan et al., 2013) – which like our seed, was located within FPN. The seed was also close to a site in LIFG showing stronger responses to both weak semantic associations and weak episodic memories (Vatansever et al., 2021). We created this ROI by placing a binarised spherical masque with a radius of 3 mm, centred on the MNI coordinates in the selected site. This site fell within mid-IFG (pars triangularis) and bordered inferior frontal sulcus associated with domain-general cognitive control (e.g., Duncan, 2010; Fedorenko et al., 2013). The supplementary materials provide a parallel analysis of a site in anterior IFG that is specifically linked to semantic and not domain-general aspects of control (Badre et al., 2005; Barredo et al., 2016; Gao et al., 2021; Poldrack et al., 1999).
2.7. Resting-state fMRI analysis: seed-to-voxel whole-brain connectivity
The functional connectivity seed-to-voxel analysis was performed to explore associations between behavioural task performance and intrinsic connectivity of left VLPFC. In our first-level analysis, we computed Pearson's correlation coefficients between the residual BOLD time course (i.e., the BOLD time series after pre-processing and denoising steps) from the selected seed (i.e., the mean timeseries of the seed) and the time course of all the voxels in brain by applying bivariate correlation and hemodynamic response function (HRF) weighting, which offers additional protection against transient effects in the BOLD signal at the beginning of scanning. Then, correlation coefficients were converted to normally distributed scores using Fisher's transform to allow for second-level GLM analysis. For the second-level analysis, the explanatory variables (EVs) were entered into a GLM analysis, including the response sensitivity (i.e., d prime) score of each of the four experimental conditions (i.e., Strong association in the 1st half of category, Strong association in the 2nd half of category, Weak association in the 1st half of category, Weak association in the 2nd half of category). We used two-sided tests to determine significant clusters. We defined the following contrasts of interest for this seed to examine the main effects of semantic association (Strong vs. Weak) and within-category change (1st half vs. 2nd half of category). In addition, we also included all the contrasts across the experimental conditions (the 1st half vs. 2nd half of category for Strong association, 1st half vs. 2nd half of category for Weak association, Strong vs. Weak association for the 1st half of category, Strong vs. Weak association for the 2nd half of category). To threshold the group-level brain maps, we used a cluster-level inference based on permutation analyses (Bullmore et al., 1999) as implemented in CONN. Instead of relying on Random Field Theory assumptions about the cluster probability distribution, this method estimates the probability density function of each cluster size under the null hypothesis, using 1000 permutations of the original data to simulate this null hypothesis. Group-level analyses were thresholded at a “height” or “cluster-defining” threshold of p < .005 to define a series of non-overlapping clusters, and amongst this resulting suprathreshold map, only clusters with a cluster-size FWE corrected p < .05 (two-tailed t tests) were reported as significant. These group-level differences were examined using a GLM. All figures were created using BrainNet Viewer (http://www.nitrc.org/projects/bnv/; Xia et al., 2013).
Prior to data analysis, all behavioural variables were z-transformed and outliers more than 2.5 standard deviations above or below the mean were identified. These outlying values were imputed with the cut-off value (i.e., +/−2.5 standard deviations above or below the mean).
2.8. Data and code availability statement
Neuroimaging data at the group-level statistical t maps are openly available in Neurovault at https://neurovault.org/collections/9212/. Semantic material and script for the task are accessible in the Open Science Framework at https://osf.io/uyhra/. The conditions of our ethical approval do not permit public archiving of the raw data because participants did not provide sufficient consent. Researchers who wish to access the data should contact the Research Ethics and Governance Committee of the York Neuroimaging Centre, University of York, or the corresponding authors. Data will be released to researchers when this is possible under the terms of the GDPR (General Data Protection Regulation).
3. Results
3.1. Behavioural results
Response sensitivity (d’) was used as the main dependant measure, in line with Nathaniel et al. (2018). This measure accounts for response bias (i.e., the general tendency to respond yes or no; Stanislaw and Todorov, 1999), with higher d’ scores indicating better ability to correctly recognise targets and reject distractors. The stimuli were presented at a rapid fixed pace creating a deadline for each response; consequently, response time was not thought to be an appropriate metric. To examine whether there was any decline in performance across the testing session, we included across-category fatigue as a within-subject variable by breaking down the whole experiment into the first and second half. In this way, the experiment had a 2 × 2 × 2 design, allowing us to examine the factors of across-category fatigue (1st half vs. 2nd half of session), strength of association (strong vs. weak category members), and within-category decline (1st half vs. 2nd half of category). As there was an equal distribution of strong and weak targets in the first half and second half of each category, within-category changes in performance could be examined by computing d’ separately for strong and weak associations in the first and second half of each category.
A 2 (Across-category fatigue: 1st half vs. 2nd half of session) by 2 (Semantic association: Strong vs. Weak) by 2 (Within-category decline: 1st half vs. 2nd half of category) repeated-measures ANOVA showed that there was a main effect of semantic association, F(1,77) = 1256.85, p < .001, ηp2 = 0.94; weak associations were harder to detect than strong associations, consistent with our hypothesis that these items have higher controlled retrieval demands. The main effect of within-category decline was also significant, F(1,77) = 20.63, p < .001, ηp2 = 0.21, with response sensitivity higher for the first half of each category than the second half. Within-category decline in this paradigm might reflect difficulty sustaining attention to a particular semantic category and/or negative effects of the earlier retrieval of semantically-related items on later retrieval. The main effect of across-category change was not significant, F(1,77) = 0.29, p = .59, ηp2 = 0.004, demonstrating that changes in performance were specific to each category, and did not reflect general fatigue effects. The interaction between semantic association and within-category decline was also significant, F(1,77) = 18.84, p < .001, ηp2 = 0.20. Post-hoc t tests suggested that this decline in sensitivity within each category affected performance on the weakly related targets, F(1,77) = 33.00, p < .001, ηp2 = 0.30, more than the strongly related targets, F(1,77) = 3.00, p = .087, ηp2 = 0.04. In addition, there was a significant three-way interaction, F(1,77) = 12.9, p = .001, ηp2 = 0.14. Tests of simple effects revealed that, in the first half of the experimental session, the effect of within-category decline was only significant for weak associations (t(77) = 4.54, p < .001), and not for strong associations (t(77) = 0.92, p = .36). In the second half of the experimental session, this effect was significant for both strong (t(77) = 3.34, p = .001) and weak associations (t(77) = 3.36, p = .001). These results suggest that, for strongly related items, within-category change only occurs in the second half of the experimental session, while for weak associations this change is persistent over the whole course of the experimental session. The behavioural results are shown in Fig. 1A.
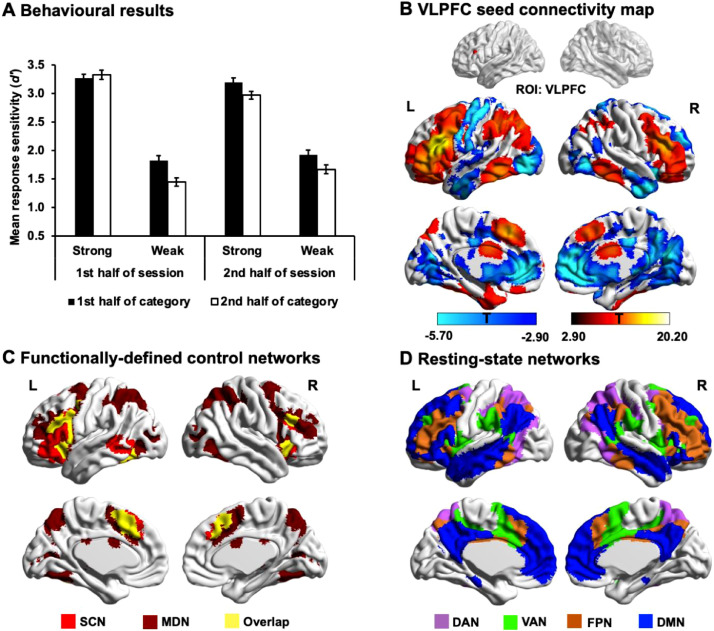
Fig. 1.
(A) Response sensitivity for the target words in each experimental condition (Strong association in the 1st half of category, Strong association in the 2nd half of category, Weak association in the 1st half of category, and Weak association in the 2nd half of category) for the first and second half of the testing session (across-category fatigue). Error bars represent the standard error. (B) The group-level patterns of relatively high functional connectivity (in red) and anti-correlated functional connectivity (in blue) from the VLPFC seed (MNI coordinates: −48, 26, 20) during resting-state fMRI (height threshold p < .005, cluster-size p-FWE < 0.05). (C) Strong functional connectivity from the VLPFC seed, shown in panel B, overlaps with regions implicated in cognitive control, within both the semantic control network (SCN; in red and yellow) from a formal meta-analysis of 925 peaks elicited by the manipulation of control demands (Jackson, 2020) and multiple demand network (MDN; in dark red and yellow) defined by the response to difficulty across a diverse set of demanding cognitive tasks (Fedorenko et al., 2013). The overlap between these two functionally-defined control networks is shown in yellow. (D) These functionally-defined control networks encompass several intrinsic large-scale networks, defined through a parcellation of 1000 resting-state fMRI datasets by Yeo et al. (2011). These networks include dorsal attention network (DAN; in purple), ventral attention network (VAN; in green), and frontoparietal network (FPN; in orange). Areas of anti-correlated functional connectivity from the VLPFC seed (shown in blue in panel B) largely overlap with regions of default mode network (DMN; in blue). L = Left hemisphere; R = Right hemisphere.
3.2. Resting-state functional connectivity
The VLPFC seed largely fell within FPN (i.e., for those voxels within this selected seed, 80% were within FPN and there was no overlap with DMN). This seed showed a pattern of strong connectivity with left prefrontal cortex, posterior temporal cortex/lateral temporal occipital cortex, intraparietal sulcus and anterior cingulate cortex/pre-supplementary motor area. The group-level intrinsic connectivity map of the VLPFC seed is shown in Fig. 1B. Areas of positive connectivity with this seed overlapped with regions implicated in cognitive control. These control networks included regions that respond to manipulations of semantic control demands from a formal meta-analysis of 925 peaks (see regions in red and yellow in Fig. 1C; Jackson, 2020), and key regions of MDN, defined by the response to difficulty across a diverse set of demanding cognitive tasks (see regions in yellow and dark red in Fig. 1C; Fedorenko et al., 2013). The regions within this functionally-defined MDN are not homogenous (e.g., Crittenden et al., 2016; Dosenbach et al., 2008; Gao et al., 2021) and it is located at the intersection of three large-scale cognitive control-relevant networks described by Yeo et al. (2011) in a 7-network parcellation of whole-brain functional connectivity, including frontoparietal network (FPN; regions in orange in Fig. 1D), ventral attention network (VAN; regions in green in Fig. 1D) and dorsal attention network (DAN; regions in purple in Fig. 1D). To better understand each identified connectivity pattern, we therefore focussed on the overlap of each map with the intrinsic connectivity networks defined by Yeo et al. (2011). We found that the thresholded positive connectivity map showed the greatest overlap with the FPN, while the negative connectivity map overlapped with DMN and visual regions (Table 3). These results confirm that the VLPFC seed forms a strong intrinsic functional network with regions associated with cognitive control, and shows weak connectivity to DMN regions associated with coherent conceptual representation (Davey et al., 2015; Lanzoni et al., 2020; Lau et al., 2013; Teige et al., 2019; Wang et al., 2020).
Table 3.
Overlap of resulting patterns from connectivity analysis of the VLPFC seed with large-scale intrinsic connectivity networks defined by Yeo et al. (2011)⁎.
| Effects | Visual | Somato-motor | DAN | VAN | Limbic | FPN | DMN |
|---|---|---|---|---|---|---|---|
| Group-level Positive connectivity | 2.5% | 0 | 18.1% | 4.8% | 11.3% | 45.2% | 18.1% |
| Group-level Negative connectivity | 44.8% | 5.5% | 0.7% | 1.9% | 5.7% | 1.8% | 39.6% |
| Low Control:Strong > Weak | 0 | 0 | 0 | 0 | 0 | 17.3% | 82.7% |
| High Control:Weak > Strong for 1st half of category | 0 | 11.5% | 6.5% | 79.5% | 0 | 2.3% | 0.2% |
| High Control:Weak > Strong for 2nd half of category | 0 | 0 | 26.4% | 0 | 0 | 35.3% | 38.3% |
| Low Control:1st > 2nd half of category for strong associations | 10.9% | 0 | 0 | 0 | 0 | 0 | 89.1% |
| High Control:2nd > 1st half of category for strong associations | 0 | 1.6% | 0 | 87.3% | 0 | 11.1% | 0 |
The percentage of voxels in the identified cluster that fell within the large-scale networks defined by Yeo et al. (2011) 7-network parcellation, disregarding voxels that did not fall within any of the Yeo networks.
Next, we explored whether individual differences in behavioural performance were associated with variation in this pattern of functional connectivity from VLPFC. We generated functional connectivity maps for each individual, and then analysed these spatial maps using a series of multiple regression analyses that included individual response sensitivity (i.e., d prime) in each condition (i.e., Strong association in the 1st half of category, Strong association in the 2nd half of category, Weak association in the 1st half of category, and Weak association in the 2nd half of category) as explanatory variables. The results for the identified patterns of VLPFC connectivity associated with behavioural performance are described below and summarised in Table 2; the overlap of each identified connectivity pattern with large-scale intrinsic connectivity networks defined by Yeo et al. (2011) are summarised in Table 3.
Table 2.
Peak coordinates resulting from connectivity analysis of the VLPFC seed.
| Effects | Connectivity | p-FWE | x | y | z | Voxels |
|---|---|---|---|---|---|---|
| Strong > Weak | Right frontal pole | .042 | 26 | 62 | 26 | 484 |
| Weak > Strong for 1st half of category | Right inferior frontal gyrus | .013 | 46 | 6 | 12 | 614 |
| Left insular cortex | .016 | −36 | 0 | 2 | 590 | |
| Weak > Strong for 2nd half of category | Left posterior parietal lobule | .048 | −32 | −72 | 36 | 470 |
| 1st > 2nd half of category for strong associations | Precuneus cortex | .043 | 6 | −48 | 10 | 482 |
| 2nd > 1st half of category for strong associations | Right supramarginal gyrus | .026 | 64 | −34 | 20 | 534 |
| Left insular cortex | .041 | −36 | 0 | 0 | 486 |
3.2.1. Semantic association strength (Controlled retrieval demands)
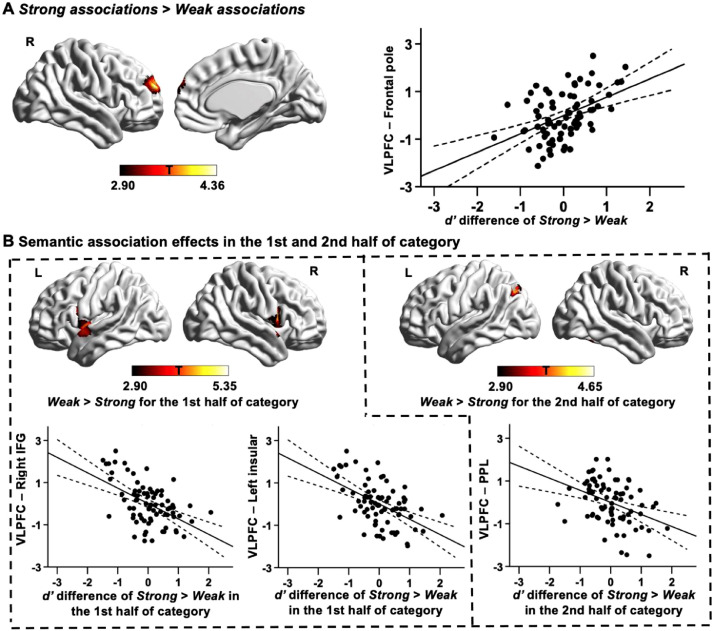
We found that VLPFC connectivity was related to individual differences in the effect of strength of association. Better performance on strong association trials relative to weak association trials was linked to stronger connectivity between the VLPFC seed region and right frontal pole (corrected cluster-size p-FWE value = 0.042; see Fig. 2A). The voxels in this cluster were strongly overlapping with DMN (Table 3). This pattern of results suggests that participants with stronger VLPFC-to-DMN connectivity had better semantic performance for items with lower controlled retrieval demands.
Fig. 2.
Functional connectivity of VLPFC linked to semantic association strength. (A) Regions of higher resting-state connectivity with the VLPFC seed were associated with better performance on strong associations relative to weak associations. The scatterplot shows the relationship between the average correlation with VLPFC (beta values) in the identified cluster and d’ differences for Strong > Weak associations. (B) Regions of higher resting-state connectivity with VLPFC seed associated with better performance on weak associations than strong associations in the 1st half (shown in the left column) and 2nd half of category (shown in the right column), respectively. The scatterplots show the relationship between the average correlation with VLPFC (beta values) in each identified cluster and d’ differences for Strong > Weak associations in the 1st half and 2nd half of category. The error lines on the scatterplots indicate the 95% confidence estimates of the mean. Each point describes one participant. All maps are cluster-corrected using a height threshold of p < .005 (cluster-size p-FWE < 0.05). PPL = Posterior parietal lobule; L = Left hemisphere; R = Right hemisphere.
There were also significant results when considering the effects of semantic association strength in the first half and second half of each category separately. In the first half of each category (when within-category decline was minimised), better performance on weak association trials relative to strong association trials was associated with stronger connectivity of VLPFC with left insula (corrected cluster-size p-FWE value = 0.016) and right inferior frontal gyrus (corrected cluster-size p-FWE value = 0.013; see the left column in Fig. 2B). These voxels were strongly overlapping with the VAN (Table 3).
For the effect of semantic association strength in the second half of each category, better performance on weak association trials compared to strong association trials was associated with stronger intrinsic connectivity between VLPFC and left posterior parietal lobule (corrected cluster-size p-FWE value = 0.048; see the right column in Fig. 2B). This cluster fell at the intersection of FPN, DAN and DMN, showing similar overlap with all three networks; see Table 3).
3.2.2. Within-category decline
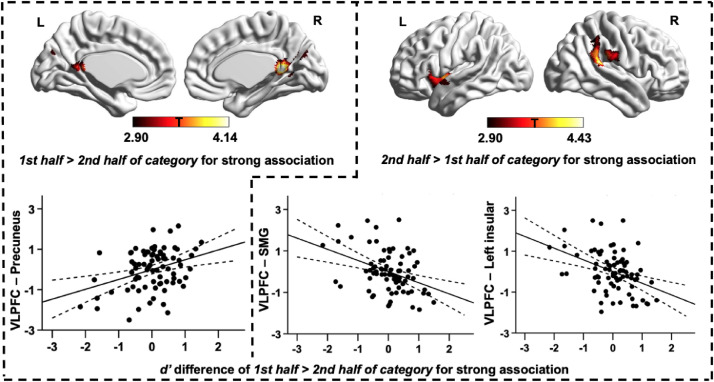
We found that individual differences in VLPFC connectivity were also related to within-category decreases in response sensitivity for strong associations. Better performance on the strong association trials in the first half of each category, relative to the second half (i.e., greater within-category decline), was associated with stronger connectivity between the VLPFC seed region and precuneus cortex (corrected cluster-size p-FWE value = 0.043; see the left column in Fig. 3). The majority of the voxels within this cluster fell within DMN as defined by Yeo et al. (2011); see Table 3. This pattern of results suggests that participants who showed higher levels of within-category decline for strong associations had stronger connectivity of VLPFC to DMN.
Fig. 3.
Functional connectivity of VLPFC linked to within-category effects. Regions of higher resting-state connectivity with the VLPFC seed that were associated with within-category decline effects – i.e., relatively good performance on strong associations in the first half of each category, compared with the second half, shown in the left column, and in second half relative to the first half of each category, shown in the right column. The scatterplots present the relationship between the average correlation with VLPFC (beta values) in each identified cluster and d’ differences. The error lines on the scatterplots indicate the 95% confidence estimates of the mean. Each point describes a single participant. All maps are cluster-corrected using a height threshold of p < .005 (cluster size p-FWE < 0.05). SMG = Supramarginal gyrus; L = Left hemisphere; R = Right hemisphere.
The opposite behavioural pattern – i.e., relatively good performance on the strong association trials in the second half of each category, given performance on the first half (i.e., reduced within-category decline) – was associated with stronger intrinsic connectivity between VLPFC and left insula (corrected cluster-size p-FWE value = 0.041) and right supramarginal gyrus (corrected cluster-size p-FWE value = 0.026; see the right column in Fig. 3). These clusters were highly overlapping with the VAN (Table 3). This pattern of results suggests that participants who were better able to overcome within-category decline had stronger connectivity of VLPFC to ventral attention regions.
There were no significant associations between connectivity of VLPFC and within-category decline for weak associations. To rule out the possibility that this was a Type 2 error, we used the clusters identified for strong associations, shown in Fig. 3, as masks and extracted functional connectivity for each participant. We examined the correlation between these functional connectivity values and effects of within-category decline (i.e., 1st half > 2nd half of category) for weakly associated items. There were no significant correlations (VLPFC – Right supramarginal gyrus: r = −0.02, p = .84; VLPFC – Left insula: r = 0.02, p = .89). Nevertheless, in a supplementary analysis using anterior IFG site as a seed, we found participants who were better able to overcome within-category decline for weak associations had stronger connectivity of anterior IFG to regions within VAN and DAN (see Figure S3 in Supplementary Materials).
3.2.3. Common neural mechanism for controlled semantic demand and within-category decline
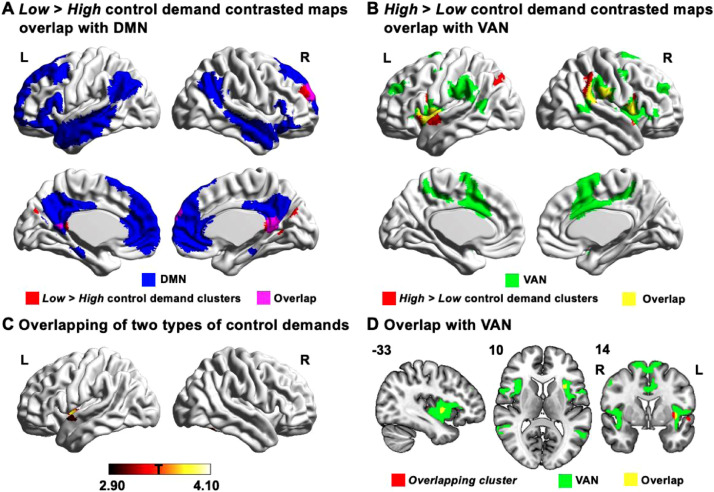
Our study set out to examine the neural mechanisms that support controlled semantic retrieval when this is required either due to the structure of long-term knowledge (i.e., the target is weakly associated) or because of the context in which retrieval occurs (i.e., the target is presented towards the end of the category, following sustained semantic attention and following the retrieval of other related concepts). Using an individual differences approach, we found that our VLPFC seed showed stronger intrinsic connectivity with regions of DMN (in blue in Fig. 4A) in participants who showed greater sensitivity to targets when control demands were relatively low (i.e., 1st half > 2nd half of category for Strong associations, and Strong > Weak associations; identified clusters in red and overlap with DMN in pink in Fig. 4A). Conversely, participants who showed good target detection when control demands were higher had stronger functional connectivity between the VLPFC seed and regions in VAN (in green in Fig. 4B) – this pattern was found for the 2nd half > 1st half of category for Strong associations, and Weak > Strong associations in 1st half of each category (identified clusters in red and overlap with VAN in yellow in Fig. 4B). There was also a cluster in posterior parietal lobule outside the VAN identified by the contrast of Weak > Strong associations in the 2nd half of each category.
Fig. 4.
Overlay of results in left insular cortex. (A) The clusters identified in the contrasts of Low > High control demand (in red) largely overlapped with DMN (in blue with overlap in pink). (B) The clusters identified in the contrasts of High > Low control demand (in red) largely overlapped with VAN (in green with overlap in yellow). (C) The left insular cortex which showed stronger connectivity with VLPFC seed in the contrast of 2nd half > 1st half of each category for Strong association largely overlapped with the left insular cortex cluster identified in the contrast of Weak > Strong for the 1st half of each category. (D) This overlapping cluster largely fell within VAN (in green with overlap in yellow). The number in the top left of the overlap map indicates the coordinate value of the corresponding plane. NB. The overlaps in panels A, B, and D were identified by overlaying our cluster-corrected maps with binarised Yeo et al. (2011) networks. In contrast, the conjunction effect in panel C was identified using the “easythresh” tool to identify voxels with t values > 2.90 in both connectivity maps. L = Left hemisphere; R = Right hemisphere.
In order to establish if there were common patterns of connectivity between the VLPFC seed and left insular cortex for contrasts relating to strength of association (Weak > Strong for the 1st half of each category) and within-category change (2nd half > 1st half of each category for Strong associations), we overlapped these two maps. Both effects were significant in left insular cortex (see Fig. 4C). This indicates that stronger functional coupling between VLPFC and left insular cortex is associated with both manipulations of controlled memory retrieval. Of the voxels within this overlapping cluster, 85.7% fell within VAN (in green with overlap in yellow in Fig. 4D), and 14.3% were within the somatomotor network, while there was no overlap with DMN.
4. Discussion
Cognitive control of memory is crucial both when retrieving weak associations, and after the sustained retrieval of semantically-related information. While VLPFC has been shown to contribute to controlled retrieval across a range of tasks and contexts (e.g., Badre and Wagner, 2007; Canini et al., 2016; Noonan et al., 2013; Wimber et al., 2008), it is still unclear whether individual differences across these varieties of control reflect variation within the same neurocognitive mechanisms. In the current study, we employed a paced serial semantic task that examined effects of both strength of association and within-category change, allowing us to establish if individual differences in the functional connectivity of VLPFC relate to these effects in the same way. For individuals with better performance on strong associations in the first half relative to the second half of each category (i.e., greater effects of within-category decline), VLPFC showed stronger connectivity with medial parietal cortex within DMN, and weaker connectivity with left insular cortex and right supramarginal gyrus, which largely fell within VAN. Similarly, for individuals with better retrieval of strong associations compared to weak associations, VLPFC showed greater connectivity with another DMN region in right frontal pole. For people with better retrieval of weak than strong associations, VLPFC showed stronger connectivity with both left insular cortex and right inferior frontal gyrus within VAN in the first half of each category, and greater connectivity with posterior parietal lobule in the second half of the category. Importantly, better controlled retrieval of weak associations and sustained performance towards the end of each category was associated with a common pattern of stronger intrinsic connectivity between VLPFC and left anterior insular cortex. This anterior insular site fell within VAN, and corresponded to a region of the MDN implicated in cognitive control across domains. Therefore, stronger functional coupling between VLPFC and VAN is associated with more controlled retrieval when task demands are determined by both the structure of long-term knowledge, and the context in which retrieval occurs. In contrast, participants with stronger connectivity of VLPFC and DMN regions show more efficient automatic semantic retrieval.
Left VLPFC is a key site for memory control (Badre and Wagner, 2007; Canini et al., 2016; Jackson, 2020; Kim, 2010; Noonan et al., 2013; Vatansever et al., 2021; Wimber et al., 2008, 2009), and anterior portions of this structure are implicated in semantic control but not in the control of cognition more widely (Badre et al., 2005; Barredo et al., 2016; Zhang et al., 2020). Partially distinct regions within left VLPFC are thought to participate within broader semantic control and multiple-demand networks (Badre and Wagner, 2007; Barredo et al., 2016; Poldrack et al., 1999; Snyder et al., 2007). The activation of regions within these networks increases for memory tasks with higher control demands (Badre et al., 2005; Canini et al., 2016; Davey et al., 2016; Wagner et al., 2001). VLPFC may also change its patterns of connectivity depending on task demands (Chiou et al., 2018): Canini et al. (2016) found stronger connectivity between VLPFC and dorsolateral prefrontal cortex for retrieval with greater semantic interference. The detection of weakly related semantic associations is thought to require greater control, since there are fewer shared semantic links with the target category and strongly-related yet interfering semantic features might also need to be inhibited to allow weak aspects of semantic knowledge to be brought to the fore (Noonan et al., 2010; Whitney et al., 2010; Zhang et al., 2020). Similar control processes may be needed following the sustained retrieval of semantically-related information, since participants may find it progressively more difficult to sustain attention to specific aspects of meaning (Nathaniel et al., 2018), and/or to detect goal-relevant concepts following the earlier presentation of semantically-related items which might then compete with later targets (e.g., Jefferies et al., 2007; Schnur et al., 2006), or give rise to retrieval-induced forgetting that accumulates for later targets (e.g., Anderson et al., 1994; Wimber et al., 2008). This may explain why similar patterns of connectivity from VLPFC to VAN were associated with these distinct manipulations of control demands in our study.
This pattern of stronger mid-LIFG connectivity to VAN was associated with greater resistance to within-category declines in memory performance – but only for strong associations, not for weak associations. Behaviourally, the effect of within-category decline was larger for weak associations but there is some evidence that strong and weak associations may be differently sensitive to distinct processes that are likely to contribute to within-category decline. Weak associations might be expected to be more vulnerable to declines in sustained semantic attention, since the goal-relevant features of these concepts are harder to retrieve (e.g., Badre et al., 2005; Gao et al., 2021; Wagner et al., 2001; Zhang et al., 2020). In contrast, strong associations show greater retrieval-induced interference (Anderson et al., 1994; Bäuml, 1998; Nathaniel et al., 2018), because strong associations accrue more inhibition from related items that are retrieved earlier. We found that stronger connectivity of mid-LIFG with anterior insula was associated with overcoming within-category declines in target detection for strong associations, i.e., even when other controlled retrieval demands were minimised. The same pattern of connectivity was also related to the detection of weak associations, even when there was minimal within-category decline, i.e., for the first half of the list. These results strengthen our conclusion that mid-LIFG to anterior insula connectivity supports multiple aspects of controlled retrieval. Moreover, although the intrinsic connectivity of mid-IFG was not linked to within-category change in performance for weak associations, this effect of within-category decline was found in a supplementary analysis examining the intrinsic connectivity of anterior IFG. While the patterns we observed for mid-LIFG and anterior LIFG both highlighted an important role of functional coupling with control and attention networks in more controlled aspects of semantic retrieval, there were also some differences in the results of these seeds which might relate to the functional distinction between these two sites in memory control (see Supplementary Materials; Badre et al., 2005; Barredo et al., 2016).
Our findings also add to a growing body of evidence that VAN plays a role in cognitive control over memory retrieval. Similar to our findings, a recent study also found that stronger connectivity between another semantic control site, posterior middle temporal gyrus, and left supramarginal gyrus within VAN was associated with the efficient retrieval of semantic associations (Gonzalez Alam et al., 2019). It has also been shown that VAN regions, for example in insular cortex, tend to functionally couple with frontoparietal association regions that support cognitive control (for a review see Uddin, 2015). VAN is thought to be important for the reorientation of attention during both exogenous attention shifts and internally-directed mental states; this network might interrupt and reset ongoing activity (Ahrens et al., 2019; Corbetta et al., 2008; Corbetta and Shulman, 2002; Kim, 2014; Turnbull et al., 2019). This network has also been argued to be important for maintaining task sets (Dosenbach et al., 2008). These findings give rise to at least two potential explanations of the common recruitment of VAN in both types of controlled semantic retrieval: (i) One is that VAN supports task-appropriate reallocation of attention to relevant memory representations in both situations. Weak associations might require attentional reorienting to focus on weak aspects of knowledge relevant to the current decision. For within-category declines in categorisation, the previous retrieval of related information might increase competition or cause the inhibition of target concepts, and to overcome these effects, attention may need to be directed towards previously suppressed and/or currently weakened representations. A recent study showed that intrinsic connectivity within VAN was associated with more fluent reading (Freedman et al., 2020), which might be related to the ability to efficiently reorient attention to internal semantic representations associated with the changing visual input in a similar way. (ii) Alternatively, VAN might aid the maintenance of ongoing task states. In our categorisation task paradigm, the auditory presentation of words was at a fast speed, requiring sustained attention to a stream of rapid semantic inputs over the course of the whole category. Reduced ability to implement an appropriate attentional set might disproportionally impact the detection of weak as opposed to strong targets, while difficulty maintaining this set would disrupt performance towards the second half of each category. Taken together, our findings suggest that both measures of controlled retrieval in our paradigm (strength of association and the requirement to sustain retrieval of semantically-related items) rely on interactions of VLPFC with VAN, consistent with the requirement in both cases to reorient internal attention to currently-relevant representations and/or to maintain the ongoing task set.
Our findings also reveal that better performance is associated with stronger functional coupling between VLPFC and regions within DMN when the control demands of the retrieval task are low. Previous studies have shown the importance of DMN in the heteromodal representation of semantic and episodic memories (Humphreys and Lambon Ralph, 2014; Margulies et al., 2016; Sestieri et al., 2011). This network is also thought to contribute to the efficient retrieval of strong associations and support states of semantic information integration that constrain ongoing semantic cognition (Davey et al., 2015; Humphreys and Lambon Ralph, 2014; Lanzoni et al., 2020; Lau et al., 2013; Teige et al., 2019; Wang et al., 2020) – presumably because in these circumstances, task-relevant patterns of retrieval can emerge relatively automatically from heteromodal representations in long-term memory in the absence of additional constraints from control networks. Greater functional coupling between VLPFC and DMN regions might allow more efficient retrieval of semantic knowledge, since individuals who showed stronger connectivity between DMN and control regions had better semantic performance in some studies (Evans et al., 2020; Krieger-Redwood et al., 2016). However, greater segregation between control networks and DMN has also been linked to better performance, particularly on tasks requiring a high degree of control (Mollo et al., 2016; Vatansever et al., 2017). While reduced coupling between cognitive control and DMN regions might relate to higher network integrity and consequently better cognitive performance, these networks also work together to underpin semantic cognition (Davey et al., 2016). Further research is needed to delineate exactly which kinds of tasks are benefitted by DMN-to-control connectivity patterns, and to understand whether all aspects of these networks show the same patterns.
Some limitations of our study should be acknowledged: first, the behavioural data did not show greater within-category decline for strong associations, although this pattern was observed in previous studies (Anderson et al., 1994; Bäuml, 1998; Nathaniel et al., 2018). While we are not able to fully explain the absence of this behavioural effect, the intrinsic connectivity of VLFPC with control regions was associated with resistance to within-category declines in categorisation for strong associations (as well as for weak associations using a more anterior VLFPC seed). Furthermore, our finding of stronger functional connectivity of VLPFC with VAN regions across different manipulations of controlled retrieval might suggest an important role for VAN in the maintenance of semantic task sets in a fast-paced paradigm. Future studies could investigate how the functional relevance of these patterns of connectivity varies as a function of the aspects of task design, for example, the presentation speed. Behaviourally, the effects of within-category decline in this paradigm are maximised by fast presentation speeds (Nathaniel et al., 2018), which is why this rate of presentation was selected for this investigation. Another limitation is that although our manipulations of strength of association and within-category decline were taken from the same behavioural paradigm, the underlying processes involved in these manipulations are not fully transparent. Future studies should better isolate specific aspects of control, such as the ability to maintain an attentional set, the capacity to overcome competition from semantically-related concepts and sensitivity to retrieval-induced interference. Future studies could also compare these aspects of mnemonic control with cognitive control processes beyond the domain of memory, since different aspects of control elicit somewhat different patterns of activation across VLPFC (Badre et al., 2005; Badre and Wagner, 2007).
In conclusion, we found that a common pattern of intrinsic connectivity from VLPFC predicted both the controlled semantic retrieval of weak associations and the ability to sustain categorisation even after the earlier retrieval of semantically-related items. Better retrieval when control demands were high was associated with greater functional coupling between VLPFC and other cognitive control regions, particularly within VAN. Stronger intrinsic connectivity between VLPFC and the same anterior insula region within the VAN was linked to better controlled retrieval, irrespective of whether the task demands reflected the structure of long-term knowledge or the recent retrieval of related information. In contrast, greater functional connectivity between VLPFC and DMN regions was associated with better retrieval when control demands were minimised. Consequently, individual differences in the intrinsic connectivity of VLPFC with DMN and VAN relates to the efficiency of more automatic and controlled aspects of memory retrieval, respectively.
Data and code availability statement
Neuroimaging data at the group-level statistical t maps are openly available in Neurovault at https://neurovault.org/collections/9212/. Semantic material and script for the task are accessible in the Open Science Framework at https://osf.io/uyhra/. The conditions of our ethical approval do not permit public archiving of the raw data because participants did not provide sufficient consent. Researchers who wish to access the data should contact the Research Ethics and Governance Committee of the York Neuroimaging Centre, University of York, or the corresponding authors. Data will be released to researchers when this is possible under the terms of the GDPR (General Data Protection Regulation).
Declaration of Competing Interest
The authors have declared that no competing interests exist.
Acknowledgements
This work was supported by the European Research Council (Project ID: 771863 – FLEXSEM to EJ).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118760.
Contributor Information
Meichao Zhang, Email: meichao.zhang@york.ac.uk.
Elizabeth Jefferies, Email: beth.jefferies@york.ac.uk.
Appendix. Supplementary materials
References
- Ahrens M.-.M., Veniero D., Freund I.M., Harvey M., Thut G. Both dorsal and ventral attention network nodes are implicated in exogenously driven visuospatial anticipation. Cortex. 2019;117:168–181. doi: 10.1016/j.cortex.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Anderson M.C. Rethinking interference theory: executive control and the mechanisms of forgetting. J. Mem. Lang. 2003;49(4):415–445. [Google Scholar]
- Anderson M.C., Bjork R.A., Bjork E.L. Remembering can cause forgetting: retrieval dynamics in long-term memory. J. Exper. Psychol. 1994;20(5):1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baayen R.H., Piepenbrock R., Van Rijn H. University of Pennsylvania; Philadelphia, PA: 1993. The CELEX Lexical Database (CD-ROM). Linguistic data Consortium. [Google Scholar]
- Badre D., Poldrack R.A., Paré-Blagoev E.J., Insler R.Z., Wagner A.D. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Barredo J., Öztekin I., Badre D. Ventral fronto-temporal pathway supporting cognitive control of episodic memory retrieval. Cereb. Cortex. 2015;25(4):1004–1019. doi: 10.1093/cercor/bht291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J., Verstynen T.D., Badre D. Organization of cortico-cortical pathways supporting memory retrieval across subregions of the left ventrolateral prefrontal cortex. J. Neurophysiol. 2016;116(3):920–937. doi: 10.1152/jn.00157.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäuml K.-h. Strong items get suppressed, weak items do not: the role of item strength in output interference. Psychon Bull Rev. 1998;5(3):459–463. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Canini M., Della Rosa P.A., Catricalà E., Strijkers K., Branzi F.M., Costa A., Abutalebi J. Semantic interference and its control: a functional neuroimaging and connectivity study. Hum. Brain Mapp. 2016;37(11):4179–4196. doi: 10.1002/hbm.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Castañón A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou R., Humphreys G.F., Jung J., Lambon Ralph M.A. Controlled semantic cognition relies upon dynamic and flexible interactions between the executive ‘semantic control’ and hub-and-spoke ‘semantic representation’ systems. Cortex. 2018;103:100–116. doi: 10.1016/j.cortex.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev.s Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crittenden B.M., Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb. Cortex. 2014;24(2):532–540. doi: 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden B.M., Mitchell D.J., Duncan J. Task encoding across the multiple demand cortex is consistent with a frontoparietal and cingulo-opercular dual networks distinction. J. Neurosci. 2016;36(23):6147–6155. doi: 10.1523/Jneurosci.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Cornelissen P.L., Thompson H.E., Sonkusare S., Hallam G., Smallwood J., Jefferies E. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J. Neurosci. 2015;35(46):15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Thompson H.E., Hallam G., Karapanagiotidis T., Murphy C., De Caso I.…Jefferies E. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165–177. doi: 10.1016/j.neuroimage.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.J. N-Watch: a program for deriving neighborhood size and other psycholinguistic statistics. Behav. Res. Methods. 2005;37(1):65–70. doi: 10.3758/bf03206399. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. (Regul. Ed.) 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. (Regul. Ed.) 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Evans M., Krieger-Redwood K., Gonzalez Alam T.R.d.j., Smallwood J., Jefferies E. Controlled semantic summation correlates with intrinsic connectivity between default mode and control networks. Cortex. 2020;129:356–375. doi: 10.1016/j.cortex.2020.04.032. [DOI] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl. Acad. Sci. 2013;110(41):16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L., Zivan M., Farah R., Horowitz-Kraus T. Greater functional connectivity within the cingulo-opercular and ventral attention networks is related to better fluent reading: a resting-state functional connectivity study. NeuroImage: Clin. 2020;26 doi: 10.1016/j.nicl.2020.102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Zheng L., Chiou R., Gouws A., Krieger-Redwood K., Wang X.…Jefferies E. Distinct and common neural coding of semantic and non-semantic control demands. Neuroimage. 2021 doi: 10.1016/j.neuroimage.2021.118230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Alam T.R.d.J., Karapanagiotidis T., Smallwood J., Jefferies E. Degrees of lateralisation in semantic cognition: evidence from intrinsic connectivity. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116089. [DOI] [PubMed] [Google Scholar]
- Gonzalez Alam T.R.d.J., Mckeown B.L., Gao Z., Bernhardt B., Vos de Wael R., Margulies D.S., Jefferies E. A tale of two gradients: differences between the left and right hemispheres predict semantic cognition. Brain Struct. Function. 2021:1–24. doi: 10.1007/s00429-021-02374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.W., Eaton H.P., Marois R. Functional fractionation of the cingulo-opercular network: alerting insula and updating cingulate. Cereb. Cortex. 2019;29(6):2624–2638. doi: 10.1093/cercor/bhy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P., Jefferies E., Lambon Ralph M.A. Ventrolateral prefrontal cortex plays an executive regulation role in comprehension of abstract words: convergent neuropsychological and repetitive TMS evidence. J. Neurosci. 2010;30(46):15450–15456. doi: 10.1523/JNEUROSCI.3783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.F., Lambon Ralph M.A. Fusion and fission of cognitive functions in the human parietal cortex. Cereb. Cortex. 2014;25(10):3547–3560. doi: 10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.L. The neural correlates of semantic control revisited. Neuroimage. 2020;224 doi: 10.1016/j.neuroimage.2020.117444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49(3):611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Jefferies E., Baker S.S., Doran M., Lambon Ralph M.A. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45(5):1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum Brain Mapp. 2014;35(5):2265–2284. doi: 10.1002/hbm.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G.R., Armstrong C., Milroy R., Piper J. The Computer and Literary Studies. 1973. An associative thesaurus of English and its computer analysis; pp. 153–165. [Google Scholar]
- Krieger-Redwood K., Jefferies E., Karapanagiotidis T., Seymour R., Nunes A., Ang J.W.A., Smallwood J. Down but not out in posterior cingulate cortex: deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage. 2016;141:366–377. doi: 10.1016/j.neuroimage.2016.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl B.A., Kahn I., Dudukovic N.M., Wagner A.D. Overcoming suppression in order to remember: contributions from anterior cingulate and ventrolateral prefrontal cortex. Cogn. Affect. Behav. Neurosci. 2008;8(2):211–221. doi: 10.3758/cabn.8.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Jefferies E., Patterson K., Rogers T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017;18(1):42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Lanzoni L., Ravasio D., Thompson H., Vatansever D., Margulies D., Smallwood J., Jefferies E. The role of default mode network in semantic cue integration. Neuroimage. 2020 doi: 10.1016/j.neuroimage.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E.F., Gramfort A., Hämäläinen M.S., Kuperberg G.R. Automatic semantic facilitation in anterior temporal cortex revealed through multimodal neuroimaging. J. Neurosci. 2013;33(43):17174–17181. doi: 10.1523/Jneurosci.1018-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod, C.M., Dodd, M.D., Sheard, E.D., Wilson, D.E., and Bibi, U. (2003). In opposition to inhibition.
- Margulies D.S., Ghosh S.S., Goulas A., Falkiewicz M., Huntenburg J.M., Langs G.…Petrides M. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. 2016;113(44):12574–12579. doi: 10.1073/pnas.1608282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo G., Karapanagiotidis T., Bernhardt B.C., Murphy C.E., Smallwood J., Jefferies E. An individual differences analysis of the neurocognitive architecture of the semantic system at rest. Brain Cogn. 2016;109:112–123. doi: 10.1016/j.bandc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K., Miyatsu T., Buchli D., Storm B.C. Forgetting as a consequence of retrieval: a meta-analytic review of retrieval-induced forgetting. Psychol. Bull. 2014;140(5):1383–1409. doi: 10.1037/a0037505. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J., Nebel M.B., Caffo B.S., Barber A.D., Pekar J.J., Mostofsky S.H. Reduction of motion-related artifacts in resting state fMRI using a CompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel U., Thompson H.E., Davies E., Arnold D., Hallam G., Stampacchia S.…Jefferies E. When comprehension elicits incomprehension: deterioration of semantic categorisation in the absence of stimulus repetition. Q. J. Exp. Psychol. 2018;71(9):1817–1843. doi: 10.1080/17470218.2017.1363793. [DOI] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Corbett F., Lambon Ralph M.A. Elucidating the nature of deregulated semantic cognition in semantic aphasia: evidence for the roles of prefrontal and temporo-parietal cortices. J. Cogn. Neurosci. 2010;22(7):1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Lambon Ralph M.A. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J. Cogn. Neurosci. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Nyberg L., Marklund P., Persson J., Cabeza R., Forkstam C., Petersson K.M., Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41(3):371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Wagner A.D., Prull M.W., Desmond J.E., Glover G.H., Gabrieli J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Raaijmakers J.G., Jakab E. Is forgetting caused by inhibition? Curr. Dir. Psychol. Sci. 2013;22(3):205–209. [Google Scholar]
- Runnqvist E., Strijkers K., Alario F.-.X., Costa A. Cumulative semantic interference is blind to language: implications for models of bilingual speech production. J. Mem Lang. 2012;66(4):850–869. [Google Scholar]
- Sadaghiani S., D'Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb. Cortex. 2015;25(9):2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur T.T., Schwartz M.F., Brecher A., Hodgson C. Semantic interference during blocked-cyclic naming: evidence from aphasia. J. Mem Lang. 2006;54(2):199–227. [Google Scholar]
- Sestieri C., Corbetta M., Romani G.L., Shulman G.L. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Corbetta M., Spadone S., Romani G.L., Shulman G.L. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J. Cogn. Neurosci. 2014;26(3):551–568. doi: 10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Feigenson K., Thompson-Schill S.L. Prefrontal cortical response to conflict during semantic and phonological tasks. J. Cogn. Neurosci. 2007;19(5):761–775. doi: 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Teige C., Cornelissen P.L., Mollo G., Gonzalez Alam T.R.d.J., McCarty K., Smallwood J., Jefferies E. Dissociations in semantic cognition: oscillatory evidence for opposing effects of semantic control and type of semantic relation in anterior and posterior temporal cortex. Cortex. 2019;120:308–325. doi: 10.1016/j.cortex.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S.L., D'Esposito M., Aguirre G.K., Farah M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl. Acad. Sci. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.E., Robson H., Lambon Ralph M.A., Jefferies E. Varieties of semantic ‘access’ deficit in Wernicke's aphasia and semantic aphasia. Brain. 2015;138(12):3776–3792. doi: 10.1093/brain/awv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A., Wang H., Murphy C., Ho N., Wang X., Sormaz M.…Smallwood J. Left dorsolateral prefrontal cortex involvement in context-dependent prioritisation of off-task thought. Nat. Commun. 2019;10(1):1–10. doi: 10.1038/s41467-019-11764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Bzdok D., Wang H.-.T., Mollo G., Sormaz M., Murphy C.…Jefferies E. Varieties of semantic cognition revealed through simultaneous decomposition of intrinsic brain connectivity and behaviour. Neuroimage. 2017;158:1–11. doi: 10.1016/j.neuroimage.2017.06.067. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Smallwood J., Jefferies E. Varying demands for cognitive control reveals shared neural processes supporting semantic and episodic memory retrieval. Nat Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-22443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D. Cognitive control and episodic memory. Neuropsychol. Memory. 2002;3:174–192. [Google Scholar]
- Wagner A.D., Paré-Blagoev E.J., Clark J., Poldrack R.A. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. https://www.ncbi.nlm.nih.gov/pubmed/11502262 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Wallis G., Stokes M., Cousijn H., Woolrich M., Nobre A.C. Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. J. Cogn. Neurosci. 2015;27(10):2019–2034. doi: 10.1162/jocn_a_00838. [DOI] [PubMed] [Google Scholar]
- Wang, X., Margulies, D.S., Smallwood, J., and Jefferies, E. (2020). A gradient from long-term memory to novel cognition: graded transitions through default mode and executive cortex. bioRxiv. [DOI] [PMC free article] [PubMed]
- Whitfield-Gabrieli S., Nieto-Castanon A.J.B.c. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitney C., Kirk M., O'sullivan J., Lambon Ralph M.A., Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb. Cortex. 2010;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C., Kirk M., O'Sullivan J., Lambon Ralph M.A., Jefferies E. Executive semantic processing is underpinned by a large-scale neural network: revealing the contribution of left prefrontal, posterior temporal, and parietal cortex to controlled retrieval and selection using TMS. J. Cogn. Neurosci. 2012;24(1):133–147. doi: 10.1162/jocn_a_00123. doi:DOI 10.1162/jocn_a_00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimber M., Bäuml K.-.H., Bergström Z., Markopoulos G., Heinze H.-.J., Richardson-Klavehn A. Neural markers of inhibition in human memory retrieval. J. Neurosci. 2008;28(50):13419–13427. doi: 10.1523/JNEUROSCI.1916-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimber M., Rutschmann R.M., Greenlee M.W., Bäuml K.-.H. Retrieval from episodic memory: neural mechanisms of interference resolution. J. Cogn. Neurosci. 2009;21(3):538–549. doi: 10.1162/jocn.2009.21043. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M.…Polimeni J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni M.-.Z., Renken R., Hoeks J.C., Hoogduin J.M., Stowe L.A. Semantic ambiguity processing in sentence context: evidence from event-related fMRI. Neuroimage. 2007;34(3):1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Zhang M., Varga D., Wang X., Krieger-Redwood K., Gouws A., Smallwood J., Jefferies E. Knowing what you need to know in advance: the neural processes underpinning flexible semantic retrieval of thematic and taxonomic relations. Neuroimage. 2020;224 doi: 10.1016/j.neuroimage.2020.117405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Neuroimaging data at the group-level statistical t maps are openly available in Neurovault at https://neurovault.org/collections/9212/. Semantic material and script for the task are accessible in the Open Science Framework at https://osf.io/uyhra/. The conditions of our ethical approval do not permit public archiving of the raw data because participants did not provide sufficient consent. Researchers who wish to access the data should contact the Research Ethics and Governance Committee of the York Neuroimaging Centre, University of York, or the corresponding authors. Data will be released to researchers when this is possible under the terms of the GDPR (General Data Protection Regulation).
Neuroimaging data at the group-level statistical t maps are openly available in Neurovault at https://neurovault.org/collections/9212/. Semantic material and script for the task are accessible in the Open Science Framework at https://osf.io/uyhra/. The conditions of our ethical approval do not permit public archiving of the raw data because participants did not provide sufficient consent. Researchers who wish to access the data should contact the Research Ethics and Governance Committee of the York Neuroimaging Centre, University of York, or the corresponding authors. Data will be released to researchers when this is possible under the terms of the GDPR (General Data Protection Regulation).