Abstract
Global correlations of range size and niche breadth, and their relationship to latitude, have long intrigued ecologists and biogeographers. Study of these patterns has given rise to a number of hypothesized ecological and evolutionary processes purported to shape biogeographic outcomes, including the climate variability hypothesis, oscillation hypothesis, ecological opportunity, competitive release and taxon cycles. Here, I introduce the alternative range shift–niche breadth hypothesis, which posits that broader niches and larger range sizes are jointly determined under eco-evolutionary processes unique to expanding ranges, which may or may not be adaptive, but which co-shape observed latitudinal gradients in niche breadth and range size during periods of widespread range expansion. I formulate this hypothesis in comparison against previous hypotheses, exploring how each relies on equilibrium versus non-equilibrium evolutionary processes, faces differing issues of definition and scale, and results in alternative predictions for comparative risk and resilience of global ecosystems. Such differences highlight that accurate understanding of process is critical when applying macroecological insight to biodiversity forecasting. Furthermore, past conceptual emphasis on a central role of local adaptation under equilibrium conditions may have obscured a ubiquitous role of non-equilibrium evolutionary processes for generating many important, regional and global macroecological patterns.
This article is part of the theme issue ‘Species’ ranges in the face of changing environments (part I)’.
Keywords: range shifts, evolution, biogeogrpahy, niche breadth
1. Introduction
Over the past several decades, evidence for contemporary range shifts has rapidly accumulated, with a majority of species rapidly spreading their geographical ranges upslope and to higher latitudes in response to warming climates [1,2]. The ecological and evolutionary consequences of such rapid, directional movement of species’ ranges, particularly at their leading edges, is a source of intense scientific scrutiny, as colonization of novel habitats by expanding populations can impact native communities, via disrupted patterns of competition and coexistence, and can in some cases lead to native extinctions within higher-latitude biotas [3–5]. Widespread range expansions are, therefore, expected to be a major source of ecosystem disruption, compounding direct effects of warming and fragmentation on biodiversity loss.
Rapid evolutionary change is often observed during range expansion, affecting traits relevant for colonization and local adaptation to novel niche space [6,7]. However, it can be difficult to reconcile such rapid niche shifts observed during range expansions with observations from stable-ranged species, which rarely expand niche or range limits under equilibrium conditions [8]. This observational discrepancy raises the question of whether the non-equilibrium demographic conditions experienced during range expansion increase the likelihood of or rate at which niche evolution can proceed. This knowledge gap is clearly important to address, because if, by virtue of rapid niche evolution, range-expanding species are better able to capitalize on novel conditions than species at equilibrium, this may compound the ecological impacts of expanding species [9,10]. Therefore, to understand and mitigate biodiversity turnover due to range-expanding species, it is critical to understand their associated non-equilibrium niche evolution processes, which can impact both the ongoing rate of the range expansion and its ecological consequences.
Interlinked with past and contemporary range expansions, several large-scale patterns of macroecological variation have classically been observed on regional and global scales: on average, geographical range sizes (resulting directly from range expansion) tend to increase with latitude (Rapoport's rule [11,12]), and to covary positively with niche breadth [13]. Owing to periods of glaciation and higher magnitudes of climate change at high latitudes, higher-latitude species are also often highly likely to have undergone more recent, post-glacial or contemporary, range expansions to achieve their contemporary range sizes [14], and high-latitude species further tend to exhibit broader ecological niches than their tropical counterparts [11,15–18].
Macroecological patterns, such as observed correlations among range size, niche breadth and latitude, are intriguing because they suggest that biodiversity responses are shaped by similar underlying processes in different times or places, and across disparate regions and taxa, and imply a certain predictability in the organization of global biotas. However, and unfortunately, recognition of pattern does not necessarily reveal the underlying process, and the reasons why niche breadth and range size tend to covary with each other and with latitude are not always well understood [19].
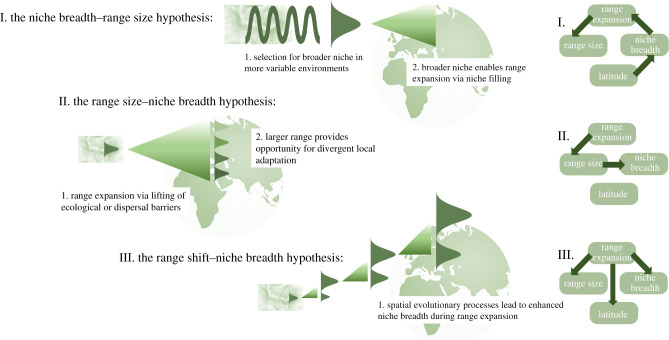
Two major and well-established hypotheses have been proposed to explain the mechanisms underpinning this general pattern (figure 1). First, the niche breadth–range size hypothesis (§3a; [11,16]) suggests that increased niche breadth initially adaptively evolves within a high-latitude population, and preadapts the associated lineage to tolerate a more diverse range of habitats across the landscape. Range expansion subsequently follows, under a niche-filling process. By contrast, the range size–niche breadth hypothesis (§3b; [20–22]) posits that niche breadth is instead likely to increase as a consequence, rather than as a cause, of range expansion, because populations of large-ranged (i.e. range-expanded) species have more opportunities to become divergently locally adapted. Thus, under the niche breadth–range size hypothesis, an adaptive evolutionary transition (achieving greater niche breadth within a population) facilitates an ecological consequence (range expansion). Under the range size–niche breadth hypothesis, however, an ecological transition (range expansion) facilitates an evolutionary consequence (divergent local adaptation and niche differentiation across the range, increasing the species-level niche). The order in which these transitions occur matters for our ability to predict whether ecological or evolutionary processes are more important for predisposing species to thrive or decline in the Anthropocene. However, both the niche breadth–range size and the range size–niche breadth hypotheses may ultimately oversimplify true underlying mechanisms, by treating ecological and evolutionary transitions as distinct steps in the overall biogeographic process. To address this over-simplification, I here propose the range shift–niche breadth hypothesis (§3c), which posits that niche breadth evolves under eco-evolutionary processes during range expansion, leading to broader niches in expanding populations. However, this process may or may not be adaptive or applicable to non-expanding species. I argue that better discrimination of these various biogeographic scenarios in real-world studies is needed in order to effectively apply macroecological insight to biodiversity predictions. I first review general mechanisms of niche evolution under equilibrium and non-equilibrium conditions, then outline major differences in how these processes underpin the various hypotheses, with implications for our ability to understand and predict future biodiversity responses.
Figure 1.
Schematic of mechanisms and direction of causation underpinning the three major hypotheses for macroecological correlations among range size, niche breadth and latitude. Bell curves represent locally evolved niches (i.e. individual or population level), and triangles represent range expansions. Ecological gradients are indicated via shading. The relevant scale of niche breadth expansion varies among hypotheses: in I, this must occur at the individual level (it must be heritable to spread); in II, niche breadth accrues at the species level; and in III, the population level is most relevant. The role of latitude also varies depending on the hypothesis: in I, latitude reflects environmental gradients that select locally for generalism versus specialism; in II, latitudinal patterns may emerge only indirectly, insofar as the grain of spatial environmental variation or species age varies with latitude; in III, latitude reflects net poleward range expansion of many species throughout the Holocene and Anthropocene. (Online version in colour.)
2. Niche evolution under equilibrium and non-equilibrium conditions
(a) . Considerations of definition and scale
Understanding the mechanisms underpinning macroecological patterns in niche breadth can be hindered by lack of precision in what is being studied [23–25]. Therefore, it is important to define the terms of study when making broad-scale comparisons, here starting with the niche, a notoriously difficult to define topic with a long history of debate [26,27]. In this paper, I consider niche breadth as the full range of conditions, along biotic or abiotic axes of environmental variation, that can be tolerated by a biological entity in either the short or long term. The niche breadth is in turn determined by a set of underlying traits that confer such tolerance. Here, I explore how this set of traits, and the emerging niche, evolve in equilibrium and non-equilibrium contexts, and contribute to macroecological patterns. For in-depth review of different niche definitions and associated traits, and further treatment of the niche in an evolutionary context, see [26,28].
Niche breadth can be evaluated as a property of any level of biological organization, but typically is estimated at the species, population or individual level [28]. For instance, a broad species-level niche may comprise multiple, various, locally and narrowly tolerant ecotypes, or may comprise a set of generalist populations, each of which exhibits broad and highly overlapping niches across the range. The population-level niche breadth, in turn, can be partitioned into among- versus within-individual variation, with further implications for evolvability and ecological response [29].
Finally, individual niche breadths may be conferred directly by traits that promote overall levels of plasticity or general tolerance (i.e. via changes in epigenetic machinery or generalized stress-resistance mechanisms [30]), or may be conferred indirectly by the accumulation of multiple, potentially physiologically unrelated traits that each affect fitness in a different aspect of the environment [13]. In the former case, we may expect that evolutionary forces including selection can act directly on individual niche breadth; however, in the latter case, niche breadth evolves indirectly as an emergent consequence of different episodes of evolution [31], although trade-offs may limit overall niche proliferation. These distinctions of scale and mechanism matter when exploring whether and how different evolutionary forces can impact niche breadth.
It is particularly salient to acknowledge issues of scale and mechanism when unravelling causal relationships between niche breadth, range size and latitude. Ecological and evolutionary processes associated with changes of range size or latitude are typically relevant at the population level and below, because range shifts are ultimately caused by local colonization, adaptation and extirpation processes. By contrast, niche breadth estimated at the species level may cumulatively result from influences of multiple factors across the species range, not all of which are relevant at the scale of the range-expanding or -contracting populations. Thus for determining the evolutionary and ecological drivers of range size, or to understand the processes by which niche breadth evolves, the niche must be defined appropriately to the scale of the process under consideration. However, limited data sometimes require researchers to generalize across taxonomic scales. While this may sometimes be acceptable, for example, if niches do not vary much among individuals within a population, or among populations across a species' range, if such assumptions are not met, which they often are not, then conclusions about underpinning processes may be flawed and true mechanisms obscured [23,25,32,33]. Below I highlight that niche equivalency across individuals, populations and species is a hallmark of the niche breadth–range size hypothesis, but this assumption is not met under the alternative explanations. This raises important implications about how data availability or interpolation may constrain mechanistic inference in macroecology.
(b) . Mechanisms of niche evolution
The niche breadth is traditionally considered to reflect patterns of selection imposed by local conditions, under an adaptation paradigm. For instance, theory and data suggest that when environments become more variable, the niche is selected to become broader [11,34]. Niche breadth can be further adaptively shaped by levels of intra- and interspecific competition, which mediate the strength and direction of selection on niche breadth, determine the manifestation of ecological trade-offs among niche axes or between generalists and specialists, and influence how the population-level niche breadth is partitioned within and among individuals [29,35]. Thus, under natural selection and within equilibrium populations or meta-populations, niche breadths are predicted to adaptively evolve depending on underlying local environmental variability and biotic context; the ability of populations to respond under such selection in turn depends on underlying genetic architecture and the processes shaping genetic variability for traits underpinning the niche.
Under such equilibrium conditions, however, range shifts rarely occur, and niche breadths rarely evolve enough to allow species to establish beyond their ancestral, equilibrium range [8,36]. This may be because the coevolution of niche breadth and range size requires the joint evolution of both dispersal and niche traits, which can be difficult to achieve under equilibrium conditions at the range edge [36,37]. Instead, under equilibrium conditions, and where not set by physical boundaries or strong evolutionary constraints, the range edge represents a threshold determined by an interaction between evolved dispersal rates and evolved population-level niche breadths, which is in turn determined by the steepness of the environmental gradient and effects of drift [36,37]: along a steep effective environmental gradient, individuals are generally selected to exhibit lower dispersal, in order to remain within their adapted conditions within the range. Reduced dispersal in turn reduces the number of colonists beyond the range, thus further reducing the input of genetic variation and associated opportunity for niche evolution, via strengthened effects of drift. Reduced opportunity to adapt beyond the range edge then reinforces selection against dispersal phenotypes. Eventually, feedback between low dispersal and lack of evolutionary niche expansion can cause increasing population fragmentation, erosion of genetic diversity and increased extinction risk [38].
However, a change in equilibrium conditions may lead to a reduction in the steepness of the environmental gradient (or otherwise remove dispersal barriers), reducing selection against dispersal and therefore promoting directional gene flow. Directional gene flow facilitates the input of genetic variation to locations at or beyond the former range edge, providing raw material for novel adaptation while counteracting effects of drift [39]. In addition, owing to associated demographic expansion, deleterious effects of gene flow on local maladaptation in expanding populations are unlikely to be as important as under equilibrium conditions, and adaptation can proceed despite swamping of maladapted alleles, provided that genetic variants that confer net positive absolute fitness are present in the colonized region [36]. Highly polygenic characteristics with genetic redundancy, which are, therefore, relatively immune to allelic turnover at individual loci, are furthermore likely to be most resistant to swamping [40]; dispersal and niche traits often exhibit these genetic characteristics, and are, therefore, particularly likely to rapidly evolve during range expansion.
Range expansion involves further non-equilibrium spatial genetic processes that can lead to adaptation to the range shift itself (see also §3c). First, during range expansion, colonizing individuals are already likely to self-select from among the highest genetic values for dispersal from the source populations. These dispersal-promoting genotypes assortatively mate at the range edge, further accelerating evolution of increased dispersal and colonization rates there under a ‘spatial sorting’ process that is well established both theoretically and empirically [6,41]. Second, low density in the (formerly unoccupied) new portion of the range will inherently favour genotypes that have high fitness at low density [42]. Thus, the spatial nature of the expansion will automatically select for high dispersing, drift-resistant genotypes at the vanguard. These combined processes act to both accelerate the rate of range expansion and slow the loss of beneficial alleles [42], providing the momentum needed for niche evolution to occur.
Thus, while range expansion may initially involve niche filling rather than niche expansion across space, the evolutionary dynamics of the range expansion itself create a situation in which genetic variation is enhanced through gene flow, and deleterious effects of drift and swamping are diminished; in this case, further adaptive evolution can occur beyond former niche limits as the expansion progresses. Range shifts and their concomitant processes of rapid demographic expansion and dispersal may, therefore, be required to overcome the typical selection for niche stasis at range edges under stable conditions. The potential role of spatial evolution during range expansions to generate macroecological gradients linking range size to niche breadth, however, may have previously gone underappreciated.
3. Ecological, evolutionary and eco-evolutionary processes behind macroecological patterns
(a) . The niche breadth–range size hypothesis
The niche breadth–range size hypothesis, as originally formulated, specifically invokes climatic niche breadth evolution as the key predictor of range size (figure 1). This hypothesis suggests that because mid- to higher-latitude locations are more temporally variable in climate than those in the tropics [43], high-latitude locations, therefore, select for individuals that tolerate the broad range of climatic conditions experienced throughout life [11,16,44]. The evolution of broadly tolerant genotypes in a particular location is hypothesized to subsequently facilitate the geographical spread of these generalist-adapted lineages across divergent climate regions in an ecological niche-filling process (figure 1). The niche breath–range size hypothesis, therefore, predicts strong, positive correlations between individual-, population- and species-level niche breadth, and thus that the niche breadth estimated at any of these biological scales is predictive of the species' range size.
In Stevens’ [11] original formulation of this hypothesis, latitudinal variation in climatic variability was considered the main driver of latitudinal gradients in niche breadth, and, therefore, colonization potential and range size (i.e. driving Rapoport's rule), as climate was then considered the factor most likely to limit species' ranges. This version of the hypothesis is often termed the climate variability hypothesis. However, a number of other environmental factors also exhibit latitudinal gradients, and may also select for increased generalism with latitude. For instance, latitudinal biodiversity gradients imply that dense, tropical communities may house more extreme resource specialists than can simpler, high-latitude communities, whereas potentially lower interspecific competition due to fewer species at higher latitudes may favour increased generalism [17,19]. In addition, shorter growing seasons might select for more relaxed discrimination rules, and thus broader resource or habitat niches at high latitudes, as longer search times become increasingly costly [45]. Increasingly broad diets or habitat requirements selected at high latitudes may, like broad climate niches, also facilitate range expansion [46,47], as biotic niche limits on species’ ranges are certainly well described [48]. Thus a more general formulation of the niche breadth–range size hypothesis suggests that any source of environmental variability could locally select for generalists predisposed to subsequent niche-filling across space. Brown [13] further argued that as niche breadth increases within a population across multiple ecological dimensions or axes, the overall local abundance and range size can each further increase; species that can tolerate the largest number of both biotic and abiotic conditions may achieve the highest local abundance in their optimal environments, as well as the greatest overall range sizes.
While the niche breadth–range size hypothesis is highly intuitive, some of the mechanisms remain obscure. For instance, increased ecological generalism within a lineage does not always, or perhaps even generally, correlate to its capacity for spatial spread [49,50], and there have been at least a few prominent counter-examples in which specialization predisposes lineages to range expansion [51,52]. Moreover, latitudinal variation in niche breadth does not always correspond to underlying environmental gradients [53,54]. Furthermore, niche breadth in one location often fails to predict niche breadth of other populations across the species' range, casting doubt on the role of this trait to determine a species’ range size via its spatial spread [32]. Finally, Ruggiero & Werenkraut [12] reviewed the evidence for links between latitude and range size; they concluded that while the overall relationship is robust, high variability in the strength of this pattern across taxa and regions implies that historical biogeographic processes may be important drivers of latitude–range size correlations, in contrast or in addition to impacts of latitudinal variation in selection on niche breadth.
(b) . The range size–niche breadth hypothesis
The range size–niche breadth hypothesis rests on the observation that range expansions, which may be triggered by either environmental change or removal of former dispersal limitations, lead to colonization of more geographically distant regions; and, owing to spatial autocorrelation of environments, larger ranges are increasingly likely to diverge in the local conditions to which populations must subsequently adapt (figure 1) [20,55]. Notably, the range size–niche breadth hypothesis often depends on multiple niche axes, with environmental change along one (previously limiting) niche axis providing opportunities to disperse beyond the ancestral range and subsequently adapt along other (non-limiting, adaptable) niche axes [20,35]. In other cases, a source of dispersal limitation is overcome to facilitate range expansion and, subsequently, a species-level niche expansion along a number of (non-limiting) dimensions [56]. In either case, recent range shifts to new regions provide opportunities for divergent niche adaptation across the range, contributing to enhanced species-level (but not necessarily individual or population) niche breadths.
One version of this hypothesis, the ecological opportunity hypothesis, suggests that any episode of environmental change that releases individuals from competition provides an opportunity for local adaptation to occur through competitive release [57]—such conditions are likely to be met following colonization of a new region, where individuals face both novel environments and release from former competitors. This process is further relevant for Wilson's concept of taxon cycles [22], which suggests that initial colonization (particularly of islands) may be followed by range expansion if the invading species can outcompete residents, resulting in increased range size and subsequent opportunity for spatial niche differentiation. Ultimately, the species' range will again become more fragmented and limited to a narrower overall range of conditions, under the influence of novel competition from new invaders. This process may contribute to incipient speciation within the older, competitively disadvantaged, and, therefore, fragmented, species ranges. Species with intermediate residence times in turn are predicted to exhibit the largest ranges and broadest set of geographically divergent niche adaptations, by virtue of the opportunity afforded by their time-limited competitive advantage.
A related idea is the oscillation hypothesis for diversification and specialization of terrestrial herbivores. This hypothesis suggests that as insects undergo (climate change-mediated) range expansion, they encounter and adapt to increasingly divergent hosts. Such resulting wide-ranged, divergently adapted species are then increasingly likely to undergo ecological speciation to produce daughter species that are each more specialized than the parent species, by virtue of their smaller ranges [21]. Under this model, the distribution of generalist and specialist species is driven by macroevolutionary dynamics of colonization and expansion followed by divergent local adaptation and finally speciation; evidence for this process has been found both empirically and in modelling [58].
The range size–niche breadth hypothesis does not directly make predictions about effects of latitude, and thus any latitudinal patterns in species-level niche breadth emerging from this process may reflect: more recent range expansion at high latitudes [14], relationships between latitude and species age [59], or effects of latitude on the spatial autocorrelation of limiting environments [60].
(c) . The range shift–niche breadth hypothesis
The range shift–niche breadth hypothesis is presented here as a new hypothesis for why range size, niche breadth and latitude may covary, and one that uniquely does not depend on an assumption that local adaptation shapes niche breadth, at either local or species scales. This hypothesis instead suggests that spatial evolutionary processes during range expansion drive the evolution of broader niches, while range expansion also directly contributes to larger range sizes and colonization of higher latitudes (figure 1) [25,33]. Under this hypothesis, populations at the leading edge experience some release from previous restrictions on dispersal, population growth or colonization. Evolutionary increases in rates of effective dispersal and demographic expansion at the vanguard of the range expansion then prime individuals to rapidly adapt to novel environmental conditions (see further explanation of this in §2b). Importantly, this process can increase the population- as well as species-level niche breadth of range-expanding lineages, according to the following mechanisms.
First, at the range expansion front, individuals and populations may express an adaptive evolutionary gain in niche breadth under similar spatial sorting processes as previously described for dispersal and drift resistance (§2b): individuals from the source populations happening to possess traits that confer broader niches (in terms of overall plasticity or tolerance, see §2a) will be more likely to successfully colonize new conditions beyond the former range, and, therefore, the expansion itself can select for broader niches at the vanguard. Broad niches increase in frequency both because they allow individuals to adaptively ‘anticipate’ novel conditions serially encountered along the expansion gradient and because of assortative mating of these individuals as they congregate at the range front [61]. Morevoer, macroevolutionary modelling suggests increased individual-level niche breadth is selected during colonization even when niche expansion is not important for fitness along the colonization gradient, as long as it increases dispersal rates via acceptance of novel habitats and thus promotes the rate of spread [62]. Thus, niche breadth may evolve under purely spatial, dispersal-promoting processes at range expansion fronts irrespective of whether it also improves adaptation to novel conditions. Subsequent empirical work has provided additional supportive evidence that ‘fast deciding’ individuals dominate recently colonized populations [45].
Second, individual or population-level niche breadth may increase indirectly within range-expanding populations as a consequence of the expansion. Expanding populations include alleles or individuals recently adapted to the novel conditions encountered, but, owing to directional gene flow and demographic expansion, also contain many more individuals possessing niche traits more adaptive in various parts of the core; for these potentially numerically dominant genotypes, the edge habitat may represent a sink or present conditions neutral to the genotype's impact on fitness [31]. Thus, while only the adapted genotypes contribute to long-term positive population growth at the edge, both adapted and mal- or non-adapted genotypes will be present and contribute to overall individual or population niche breadth observed there [63]. In the absence of strong selection against such ancestral adaptations travelling along the expansion front, it may take considerable time for these to break down under the impacts of selection or drift, especially in drift-resistant range edge populations and owing to weakened selection during demographic expansion. This mechanism is particularly relevant if overall niche breadth itself results from accumulation of diverse niche traits (§2a) that are not strongly affected by energetic or functional trade-offs among them [31,64].
When a range shift allows populations to expand poleward, such as during post-glacial and contemporary warming episodes, such leading-edge populations will primarily be in the poleward direction and this process will thus lead to clinal patterns of increased niche breadth and range size with latitude. Because niche breadths generated under this process entirely result from non-equilibrium spatial dynamics, rather than equilibrium processes of local adaptation, such mechanisms thus may often require new interpretations of the resulting ecological patterns [23,25,33]. For instance, Forister & Jenkins' neutral model of spatial niche evolution [62] intriguingly produces a landscape distribution of generalists coexisting with specialists in ratios that closely resemble real communities, implying that the role of fitness trade-offs in driving local distributions of generalists and specialists may have previously been overstated [65]. Moreover, widespread poleward range expansions have recently been shown to be very important in explaining and generating observed latitudinal gradients in thermal and resource niche breadth for insects [25,31,33,63], and biogeography emerges as a major predictor of latitudinal gradients in plant thermal niche breadth [66]; these empirical findings cast further doubt on a central role of local adaptation in niche macroecology. Non-equilibrium niche evolution during range expansion may also contribute to the observation that niche limits estimated in the range core tend to underpredict the position of poleward or high-elevation range limits, where recent climate-mediated range expansion is often most salient [23,67].
(d) . Importance of discriminating among hypotheses for predictive inference
Large-scale correlations between niche breadth, range size and latitude may be determined by anyone or more of the mechanisms outlined in §3a–c. However, it is important to discriminate these alternative processes, to improve the accuracy of predictive inferences based on presumed mechanisms derived from macroecological findings. For instance, the niche breadth–range size hypothesis suggests that high-latitude populations and species are nearly universally adapted to a broader range of conditions, and are, therefore, in general predicted to be more resilient to both environmental change and habitat loss than their tropical counterparts [53,68]. By contrast, the range size–niche breadth hypothesis suggests instead that a capacity for colonization and local adaptation, for instance via competitive ability, connectivity or genetic diversity, is much more important than in situ measures of ecological generalism in promoting range expansion and resilience to environmental changes [22]. Finally, the range shift–niche breadth hypothesis suggests that researchers need to dig deeper into eco-evolutionary dynamics to discover the underpinning drivers of interspecific variation in resilience and spread, and to provide accurate biodiversity forecasts. It furthermore suggests that predicted environmental responses of range-shifting species may be very different from those of species with stable or contracting ranges, irrespective of absolute latitude or underlying ecological conditions [25,33]. If recent range expansions rather than adaptive function explain broad tolerances at high latitudes or elevations, then it would be invalid to assume that high-latitude communities are more resilient; instead work should focus on discriminating the very different niche processes and limitations within species or lineages with different biogeographic histories and contemporary range shift trajectories.
In addition, as covered in §2a, ignorance of underlying ecological and evolutionary process can lead to niche assessments being made at the wrong scale, or inappropriately interpolated, which can then invalidate conclusions. Lack of precision on this can further impede comparability or synthesis among studies that purport to investigate the same process, but estimate the niche at different scales. Instead, if we are interested in ultimately understanding how biodiversity will respond to future climate change, we need to correctly identify the scale at which environmental change affects populations across the range—are impacts felt everywhere, or only in isolated environments?; are populations across the ranges simultaneously impacted or sequentially altered as environmental change progresses? These critical questions can only be answered by understanding how niche breadths are shaped and maintained, adaptively or non-adaptively, within and among populations increasingly impacted by non-equilibrium conditions due to climate change, habitat loss and novel interactions.
Additional data and synthesis work are needed to refine predictions and outcomes under each of the major hypotheses, and to determine the relative prevalence of each mechanism overall. Such studies may, for instance, compare macroecological patterns between range-expanding and stable-ranged species [25,33], employ theoretical or experimental approaches to distinguish alternative mechanisms [6,61,62,69] or evaluate the (changing) role of different microevolutionary forces on niche breadth, across species’ range expansion gradients [31,45,51,56]. Many of the available empirical studies of niche macroecology involve ectothermic species, with a historically strong focus on terrestrial insects, and typically only a single niche dimension is considered at a time. More work is needed to understand the taxonomic generality of processes in shaping macroecological patterns, and to determine how different dimensions of the niche might interact with each other and co-evolve through space and time. Moreover, better understanding of the genetic signatures of range expansion, and how these signatures then correspond to niche evolution and resilience [31], will enable improved and targeted management of individual species based on these general insights. Finally, while not a topic of this paper, eco-evolutionary dynamics of range contraction may also be important for niche evolution, and the importance of this for generating macroecological patterns deserves further investigation [61,69].
4. Conclusion
Macroecological patterns of niche breadth, latitude and range sizes present an ongoing puzzle of great interest for our understanding of ecological and evolutionary processes, and for our ability to confidently model and anticipate variation in species' responses to environmental changes. Classical explanations for why niche breadths and range sizes vary with latitude often (over-)rely on underlying gradients in selection that vary between the equator and the poles, and which are presumed to differentially select for generalism or specialism along them. However, such hypotheses rest on assumptions that populations are in equilibrium with their environments. The recent, dramatic and accelerating reorganization of biodiversity in the Anthropocene has highlighted that many if not most populations and species are in fact not in equilibrium states, opening the door to the proposition of alternative eco-evolutionary mechanisms of niche evolution that do not rely so heavily on adaptive explanations for meaningful biological patterns.
Spatial evolutionary processes, such as spatial sorting of dispersal and colonization syndromes, release from selection, spatial demographic expansion, directional gene flow and spatial asymmetries in drift, have increasingly been recognized for their special contribution to evolution at range edges. Even within static ranges, powerful non-equilibrium evolutionary processes may be ongoing [70], and are potentially not often enough invoked to explain major patterns of niche evolution and limitation. The potentially important role of these processes in determining geographical trait and genetic gradients may, therefore, have previously been overlooked. This review has particularly highlighted the special role of spatial evolutionary processes at expanding range margins to jointly impact niche and range limits, which in turn have been shown to shape major, macroecological gradients.
Acknowledgements
Thank you to the organizing committee of the CeMEB Evolution of Species Ranges symposium, University of Gothenberg, for the opportunity to contribute to the symposium and resulting special issue. Thanks also to Jake Alexander and the reviewers for valuable feedback.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Parmesan C, et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579-583. ( 10.1038/21181) [DOI] [Google Scholar]
- 2.Lenoir J, Bertrand R, Comte L, Bourgeaud L, Hattab T, Murienne J, Grenouillet G. 2020. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044-1059. ( 10.1038/s41559-020-1198-2) [DOI] [PubMed] [Google Scholar]
- 3.Fitt RNL, Lancaster LT. 2017. Range shifting species reduce phylogenetic diversity in high latitude communities via competition. J. Anim. Ecol. 86, 543-555. ( 10.1111/1365-2656.12655) [DOI] [PubMed] [Google Scholar]
- 4.Alexander JM, Diez JM, Levine JM. 2015. Novel competitors shape species' responses to climate change. Nature 525, 515-518. ( 10.1038/nature14952) [DOI] [PubMed] [Google Scholar]
- 5.Davey CM, Chamberlain DE, Newson SE, Noble DG, Johnston A. 2012. Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr. 21, 568-578. ( 10.1111/j.1466-8238.2011.00693.x) [DOI] [Google Scholar]
- 6.Miller TEX, et al. 2020. Eco-evolutionary dynamics of range expansion. Ecology 101, e03139. ( 10.1002/ecy.3139) [DOI] [PubMed] [Google Scholar]
- 7.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617-1627. ( 10.1890/09-0910.1) [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves AL, Samis KE, Eckert CG. 2014. Are species' range limits simply niche limits writ large? A review of transplant experiments beyond the range. Am. Nat. 183, 157-173. ( 10.1086/674525) [DOI] [PubMed] [Google Scholar]
- 9.Lancaster LT, Morrison G, Fitt RN. 2017. Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Phil. Trans. R. Soc. B 372, 20160046. ( 10.1098/rstb.2016.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart-Smith RD, Mellin C, Bates AE, Edgar GJ. 2021. Habitat loss and range shifts contribute to ecological generalization among reef fishes. Nat. Ecol. Evol. 5, 656-662. ( 10.1038/s41559-020-01342-7) [DOI] [PubMed] [Google Scholar]
- 11.Stevens GC. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240-256. ( 10.1086/284913) [DOI] [Google Scholar]
- 12.Ruggiero A, Werenkraut V. 2007. One-dimensional analyses of Rapoport's rule reviewed through meta-analysis. Glob. Ecol. Biogeogr. 16, 401-414. ( 10.1111/j.1466-8238.2006.00303.x) [DOI] [Google Scholar]
- 13.Brown JH. 1984. On the relationship between abundance and distribution of species. Am. Nat. 124, 255-279. ( 10.1086/284267) [DOI] [Google Scholar]
- 14.Hewitt GM. 1999. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 68, 87-112. ( 10.1111/j.1095-8312.1999.tb01160.x) [DOI] [Google Scholar]
- 15.Gaston KJ, et al. 2009. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595-612. ( 10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 16.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233-249. ( 10.1086/282487) [DOI] [Google Scholar]
- 17.Forister ML, et al. 2015. The global distribution of diet breadth in insect herbivores. Proc. Natl Acad. Sci. USA 112, 442-447. ( 10.1073/pnas.1423042112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B 267, 739-745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vázquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1-E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 20.Ackerly DD. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 164, S165-S184. ( 10.1086/368401) [DOI] [Google Scholar]
- 21.Janz N, Nylin S. 2005. The oscillation hypothesis of host-plant range and speciation. Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects (ed. K Tilmon), pp. 203-215. Oakland, CA: University of California Press. [Google Scholar]
- 22.Wilson EO. 1961. The nature of the taxon cycle in the Melanesian ant fauna. Am. Nat. 95, 169-193. ( 10.1086/282174) [DOI] [Google Scholar]
- 23.García-Robledo C, Kuprewicz EK, Staines CL, Erwin TL, Kress WJ. 2016. Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc. Nat. Acad. Sci. USA 113, 680-685. ( 10.1073/pnas.1507681113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger JR, Hou C, Hall CAS, Brown JH. 2021. Universal rules of life: metabolic rates, biological times and the equal fitness paradigm. Ecol. Lett. 24, 1262-1281. ( 10.1111/ele.13715) [DOI] [PubMed] [Google Scholar]
- 25.Lancaster LT. 2016. Widespread range expansions shape latitudinal variation in insect thermal limits. Nat. Clim. Change 6, 618-621. ( 10.1038/nclimate2945) [DOI] [Google Scholar]
- 26.Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA. 2017. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 48, 183-206. ( 10.1146/annurev-ecolsys-110316-023003) [DOI] [Google Scholar]
- 27.Kearney M. 2006. Habitat, environment and niche: what are we modelling? Oikos 115, 186-191. ( 10.1111/j.2006.0030-1299.14908.x) [DOI] [Google Scholar]
- 28.Carscadden KA, Emery NC, Arnillas CA, Cadotte MW. 2020. Niche breadth: causes and consequences for ecology, evolution, and conservation. Q. Rev. Biol. 95, 179-214. ( 10.1086/710388) [DOI] [Google Scholar]
- 29.Van Valen L. 1965. Morphological variation and the width of the ecological niche. Am. Nat. 99, 377-390. ( 10.1086/282379) [DOI] [Google Scholar]
- 30.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster LT, Dudaniec RY, Hansson B, Svensson EI. 2015. Latitudinal shift in thermal niche breadth results from thermal release during a climate-mediated range expansion. J. Biogeogr. 42, 1953-1963. ( 10.1111/jbi.12553) [DOI] [Google Scholar]
- 32.Herrando-Pérez S, Ferri-Yáñez F, Monasterio C, Beukema W, Gomes V, Belliure J, Chown SL, Vieites DR, Araújo MB. 2019. Intraspecific variation in lizard heat tolerance alters estimates of climate impact. J. Anim. Ecol. 88, 247-257. ( 10.1111/1365-2656.12914) [DOI] [PubMed] [Google Scholar]
- 33.Lancaster LT. 2020. Host use diversification during range shifts shapes global variation in lepidopteran dietary breadth. Nat. Ecol. Evol. 4, 963-969. ( 10.1038/s41559-020-1199-1) [DOI] [PubMed] [Google Scholar]
- 34.Levins R. 1968. Evolution in changing environments. Princeton, NJ: Princeton University Press. [Google Scholar]
- 35.Emery NC, Ackerly DD. 2014. Ecological release exposes genetically based niche variation. Ecol. Lett. 17, 1149-1157. ( 10.1111/ele.12321) [DOI] [PubMed] [Google Scholar]
- 36.Holt RD, Gomulkiewicz R. 1997. The evolution of species' niches: a population dynamics perspective. In Case studies in mathematical modeling: ecology, physiology, and cell biology (eds HG Othmer, FR Adler, MA Lewis, JC Dallon), pp. 25-50. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- 37.Polechová J, Barton NH. 2015. Limits to adaptation along environmental gradients. Proc. Natl Acad. Sci. USA 112, 6401-6406. ( 10.1073/pnas.1421515112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polechová J. 2018. Is the sky the limit? On the expansion threshold of a species’ range. PLoS Biol. 16, e2005372. ( 10.1371/journal.pbio.2005372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gompert Z, Springer A, Brady M, Chaturvedi S, Lucas LK. 2021. Genomic time-series data show that gene flow maintains high genetic diversity despite substantial genetic drift in a butterfly species. Mol. Ecol. 30, 4991-5008. ( 10.1111/mec.16111) [DOI] [PubMed] [Google Scholar]
- 40.Yeaman S. 2015. Local adaptation by alleles of small effect. Am. Nat. 186, S74-S89. ( 10.1086/682405) [DOI] [PubMed] [Google Scholar]
- 41.Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proc. Natl Acad. Sci. USA 108, 5708-5711. ( 10.1073/pnas.1018989108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erm P, Phillips BL. 2020. Evolution transforms pushed waves into pulled waves. Am. Nat. 195, E87-E99. ( 10.1086/707324) [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Dillon ME. 2014. Recent geographic convergence in diurnal and annual temperature cycling flattens global thermal profiles. Nat. Clim. Change 4, 988-992. ( 10.1038/nclimate2378) [DOI] [Google Scholar]
- 44.Gaston KJ, Chown SL. 1999. Why Rapoport's rule does not generalise. Oikos 84, 309-312. ( 10.2307/3546727) [DOI] [Google Scholar]
- 45.Martin Y, Titeux N, van Dyck H.. 2021. Range expansion, habitat use, and choosiness in a butterfly under climate change: marginality and tolerance of oviposition site selection. Ecol. Evol. 11, 2336-2345. ( 10.1002/ece3.7202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slatyer RA, Hirst M, Sexton JP. 2013. Niche breadth predicts geographical range size: a general ecological pattern. Ecol. Lett. 16, 1104-1114. ( 10.1111/ele.12140) [DOI] [PubMed] [Google Scholar]
- 47.Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K. 2009. Species traits explain recent range shifts of Finnish butterflies. Glob. Change Biol. 15, 732-743. ( 10.1111/j.1365-2486.2008.01789.x) [DOI] [Google Scholar]
- 48.Brown CD, Vellend M. 2014. Non-climatic constraints on upper elevational plant range expansion under climate change. Proc. R. Soc. B 281, 20141779. ( 10.1098/rspb.2014.1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLean SA, Beissinger SR. 2017. Species' traits as predictors of range shifts under contemporary climate change: a review and meta-analysis. Glob. Change Biol. 23, 4094-4105. ( 10.1111/gcb.13736) [DOI] [PubMed] [Google Scholar]
- 50.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677-689. ( 10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 51.Bridle JR, Buckley J, Bodsworth EJ, Thomas CD. 2014. Evolution on the move: specialization on widespread resources associated with rapid range expansion in response to climate change. Proc. R. Soc. B 281, 20131800. ( 10.1098/rspb.2013.1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richmond JQ, Ota H, Grismer LL, Fisher RN. 2021. Influence of niche breadth and position on the historical biogeography of seafaring scincid lizards. Biol. J. Linn. Soc. 132, 74-92. ( 10.1093/biolinnean/blaa172) [DOI] [Google Scholar]
- 53.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janion-Scheepers C, Phillips L, Sgrò CM, Duffy GA, Hallas R, Chown SL. 2018. Basal resistance enhances warming tolerance of alien over indigenous species across latitude. Proc. Natl Acad. Sci. USA 115, 145-150. ( 10.1073/pnas.1715598115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spence AR, Tingley MW. 2020. The challenge of novel abiotic conditions for species undergoing climate-induced range shifts. Ecography 43, 1571-1590. ( 10.1111/ecog.05170) [DOI] [Google Scholar]
- 56.van Boheemen LA, Hodgins KA.. 2020. Rapid repeatable phenotypic and genomic adaptation following multiple introductions. Mol. Ecol. 29, 4102-4117. ( 10.1111/mec.15429) [DOI] [PubMed] [Google Scholar]
- 57.Wellborn GA, Langerhans RB. 2015. Ecological opportunity and the adaptive diversification of lineages. Ecol. Evol. 5, 176-195. ( 10.1002/ece3.1347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braga MP, Araujo SBL, Agosta S, Brooks D, Hoberg E, Nylin S, Janz N, Boeger WA. 2018. Host use dynamics in a heterogeneous fitness landscape generates oscillations in host range and diversification. Evolution 72, 1773-1783. ( 10.1111/evo.13557) [DOI] [PubMed] [Google Scholar]
- 59.Paul JR, Tonsor SJ. 2008. Explaining geographic range size by species age: a test using neotropical piper species. In Tropical forest community ecology (eds Carson WP, Schnitzer SA), pp. 46-62. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 60.Saupe EE, Myers CE, Peterson AT, Soberón J, Singarayer J, Valdes P, Qiao H. 2019. Non-random latitudinal gradients in range size and niche breadth predicted by spatial patterns of climate. Glob. Ecol. Biogeogr. 28, 928-942. ( 10.1111/geb.12904) [DOI] [Google Scholar]
- 61.Schmid M, Dallo R, Guillaume F. 2019. Species' range dynamics affect the evolution of spatial variation in plasticity under environmental change. Am. Nat. 193, 798-813. ( 10.1086/703171) [DOI] [PubMed] [Google Scholar]
- 62.Forister ML, Jenkins SH. 2017. A neutral model for the evolution of diet breadth. Am. Nat. 190, E40-E54. ( 10.1086/692325) [DOI] [PubMed] [Google Scholar]
- 63.Singer MC, Parmesan C. 2021. Colonizations cause diversification of host preferences: a mechanism explaining increased generalization at range boundaries expanding under climate change. Glob. Change Biol. 27, 3505-3518. ( 10.1111/gcb.15656) [DOI] [PubMed] [Google Scholar]
- 64.Lancaster LT, Dudaniec R, Chauhan P, Wellenreuther M, Svensson EI, Hansson B. 2016. Gene expression under thermal stress varies across a geographic range expansion front. Mol. Ecol. 25, 1141-1156. ( 10.1111/mec.13548) [DOI] [PubMed] [Google Scholar]
- 65.Agosta SJ, Klemens JA. 2009. Resource specialization in a phytophagous insect: no evidence for genetically based performance trade-offs across hosts in the field or laboratory. J. Evol. Biol. 22, 907-912. ( 10.1111/j.1420-9101.2009.01694.x) [DOI] [PubMed] [Google Scholar]
- 66.Lancaster LT, Humphreys AM. 2020. Global variation in the thermal tolerances of plants. Proc. Natl Acad. Sci. USA 117, 13 580-13 587. ( 10.1073/pnas.1918162117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686-690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 68.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296-1297. ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 69.Hillaert J, Boeye J, Stoks R, Bonte D. 2015. The evolution of thermal performance can constrain dispersal during range shifting. J. Biol. Dyn. 9, 317-335. ( 10.1080/17513758.2015.1078503) [DOI] [PubMed] [Google Scholar]
- 70.Kling MM, Ackerly DD. 2021. Global wind patterns shape genetic differentiation, asymmetric gene flow, and genetic diversity in trees. Proc. Natl Acad. Sci. USA 118, e2017317118. ( 10.1073/pnas.2017317118) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.