Abstract
Understanding how environmental factors affect the thermal tolerance of species is crucial for predicting the impact of thermal stress on species abundance and distribution. To date, species' responses to thermal stress are typically assessed on laboratory-reared individuals and using coarse, low-resolution, climate data that may not reflect microhabitat dynamics at a relevant scale. Here, we examine the daily temporal variation in heat tolerance in a range of species in their natural environments across temperate and tropical Australia. Individuals were collected in their habitats throughout the day and tested for heat tolerance immediately thereafter, while local microclimates were recorded at the collection sites. We found high levels of plasticity in heat tolerance across all the tested species. Both short- and long-term variability of temperature and humidity affected plastic adjustments of heat tolerance within and across days, but with species differences. Our results reveal that plastic changes in heat tolerance occur rapidly at a daily scale and that environmental factors on a relatively short timescale are important drivers of the observed variation in thermal tolerance. Ignoring such fine-scale physiological processes in distribution models might obscure conclusions about species' range shifts with global climate change.
This article is part of the theme issue ‘Species’ ranges in the face of changing environments (part 1)’.
Keywords: insects, climate predictors, microhabitat environment, phenotypic plasticity, species distribution, thermal tolerance
1. Introduction
Temperature is an important abiotic environmental variable that can constrain the abundance and geographical distribution of species [1–4]. This can be either directly by temperatures exceeding physiological tolerance limits [5], or indirectly through interactions with other abiotic or biotic stressors [5,6]. Understanding how changing environmental conditions, such as increasing and less predictable temperatures, affect thermal performance and tolerances is thus crucial for predicting future species' range limits (e.g. [7,8]). This is highlighted by the current focus on modelling how future global warming scenarios will affect species abundance and distribution. This can be through the use of mechanistic species distribution models that incorporate physiological information on range-limiting processes, which has been suggested to provide robust predictions of future species distributions, and allows extrapolation beyond current climates [9,10].
However, fine-tuning such process-based models presents many challenges. A key challenge, which trait-based studies have attempted to answer for decades, is what physiological metrics serve as the best predictor of species' vulnerability to environmental change across species [10]. Commonly, species’ critical thermal limits have been used as proxies for species' vulnerability, as they define the space for the performance of vital physiological functions such as locomotion, growth and reproduction [4,11]. Studies have been successful in linking lower thermal limits to large-scale climate patterns, showing that cold tolerance increases with latitudinal change in climate [11–16], including the mean temperature of the coldest quarter, absolute monthly minimum and mean annual temperature for reptiles [8,14,15]. However, upper thermal limits vary less, or at negligible levels, across latitude [11–14,16,17], which makes upper thermal limits a weak predictor of species' vulnerabilities. This is despite the obvious role of heat tolerance in coping with increasing temperatures with climate change [4,18,19]. Likewise, empirical evidence suggests that these tolerance metrics may result in misleading conclusions on species' vulnerabilities to climate change (e.g. [20,21]). Efforts to explain this perplexity have suggested that species' thermoregulatory behaviour [22–25], low genetic variability for heat tolerance [15,17,26,27], physiological adjustments [28–31] and methodology [32–36] obscure the effectiveness of using heat tolerance as a predictor of range limits [35,37,38].
Studies examining how physiological adjustments impact on heat tolerance and the evolutionary and plastic capacity to alter heat tolerance have typically been executed in the laboratory, and on populations adapted to laboratory conditions (e.g. [37,39,40]). Further, common garden laboratory studies are limited to species that can be reared successfully in the laboratory, constraining the number of species studied, which have typically been model species of temperate and tropical origin (e.g. [10,38]). This can be problematic if those studies aim to predict future responses to climate change in the field, because laboratory conditions do not reflect natural variable and unpredictable temperatures, and also because responses shown by model species may not be representative [41,42]. In addition, very few studies have addressed the realized thermal niche across different temporal scales, which can lead to both underestimates and overestimates of acclimatization, e.g. upper thermal limits (but see [43,44]). Finally, thermoregulatory behaviour is unaccounted for when organisms are restricted under laboratory conditions. Thus, species have no opportunity to behaviourally shape their thermal environment, which will often alter responses to environmental stress [3,9].
When fine-tuning mechanistic models with the goal to provide robust predictions of future distributions, an issue is defining the scale of bioclimatic variables that best explain physiological information [45–47]. To date, species' responses to environmental change are typically assessed using coarse, interpolated and low-resolution climate data that is measured in the air, high above the ground level, and at distances spanning several km2 [45,48,49]. Such data ignore the climate-forcing processes that operate near the ground, and thermal heterogeneity across the environment [46,50], and studies report that the microclimate can deviate by up to 35°C [51] from air temperature. Thus, the conditions met by small organisms in the field bear little resemblance to the macroclimate [52]. Further, currently used climate data usually consist of long-term measures (monthly averages) and do not account for fine-scale spatio-temporal variability [25], thereby disregarding exposures to e.g. extreme conditions on the short time-scale (minutes, hours, days). Ignoring the frequency that organisms are exposed to stressful conditions may cause inaccurate predictions of species' ranges.
In order to provide field data on the ability to respond to daily fluctuations in temperature, and obtain climatic predictors of heat tolerance, we here examine the daily temporal variation in heat tolerance in seven insects collected in temperate Melbourne, Australia (Nysius caledoniae, Stenophyella macreta, Uroleucon sonchi, Hyperomyzus lactucae, Aphis nerii, Drosophila melanogaster, Psyllidae sp.) and in five species from tropical Cape Tribulation, Australia (Pseudopachybrachius guttus, Oecophylla smaragdina, Drosophila rubida, Scaptodrosophila novoguineensis, Cicadelliadae sp.). In order to do this, we collected individuals at four different time points on multiple days, and immediately tested for heat tolerance using an acute heat knockdown assay. Further, data on local microclimate (temperature and humidity) were recorded at the collection sites up to two weeks prior to testing to pinpoint microclimatic parameters on a temporal scale that could affect changes in thermal tolerance and plasticity for individual species across time. We observed marked and highly species-specific plasticity in heat tolerance, providing evidence that some species can change their heat knockdown time (HKDT) by up to 90% in a day relative to the lowest recorded daily knockdown time whereas others are much more constrained. Our data also showed that both means of temperature and humidity, as well as their variability experienced prior to organisms being tested, were useful for predicting heat tolerance. These results suggest that the input data typically used in mechanistic models will often not provide accurate measures of thermal robustness, because they fail to take into account local thermal conditions and the ability of many species to respond strongly to temperature variability on a daily scale.
2. Methods
(a) . Study regions and microhabitat climates
Insects were collected and tested for heat tolerance in temperate (Melbourne, Australia, latitude 37.8° S) and tropical (Cape Tribulation, Australia, latitude 16.1° S) locations, characterized by highly variable temperatures and humidity at the temperate location and more constant climatic conditions at the tropical location (figure 1). The specific sites, dates and times of collection of insects for thermal assessment are presented in electronic supplementary material, table S1. At each field site, the temperature and humidity were recorded every 5 min using Easylog USB data loggers (LASCAR Electronics, EL-USB-2+). The data loggers were placed in the shade at 20 cm above the soil surface. The microclimatic variables recorded at each study site were associated with the heat tolerance of each insect species collected at the specific sites at a given time point.
Figure 1.
Study sites used for insect collection and local temperature and humidity data. The insects were collected at the Daintree Research Observatory (latitude 16.1° S) in tropical Australia (green) from 3 June to 28 June 2019, and in Melbourne (latitude 37.8° S) in temperate Australia (orange) from 1 February to 2 June 2019. A period of the microhabitat temperature (°C) and air humidity (%RH) recorded 20 cm above the soil surface are displayed for each sampling site from each region. (Online version in colour.)
(b) . Field experiment
We used sweep nets to catch adult individuals of each species at four time points throughout the day; morning (ca 8.00), noon (ca. 12.00), afternoon (ca 16.00) and night (ca 20.00). This was done for 4–8 days for each species. The specific collection times depended on weather conditions and the abundance of the species, given that sufficient numbers were required for tolerance tests. Individuals were caught within a radius of 25 m from the microhabitat data loggers and near laboratory facilities. In the field, individuals were placed into 4 ml screw-cap glass vials (45 × 14.7 mm) held in the shade until a sufficient number of individuals had been collected. We used 15–20 individuals of each sex for every assay (for those species where we could differentiate between sexes; table 1). The individuals were sexed by eye in the field prior to the assays and the sexes were verified under a stereo microscope after the thermal assay had been completed. After field collection the vials were quickly transported to the laboratory facilities, and heat tolerance of individuals was determined (see next section), 30–90 min following collection in the field. Subsequently, individuals were stored in 90% ethanol and identified using identification keys [53,54], or by comparison against insect collections at La Trobe University in Melbourne and at the Daintree Rainforest Observatory, with guidance from experienced entomologists.
Table 1.
Overview of the species used for heat knockdown time assessment and their thermal performance. The species-specific knockdown temperatures, THKDT, used were based on the species' critical thermal maximum, CTmax (s.e.m., n). Superscript letters a–f denote significant differences in CTmax between species, based on Dunn's post hoc tests. The average knockdown time, HKDTavr (s.e.m. in seconds, n), measured on the total number of individuals tested across days and times are displayed. Plasticity is expressed as the mean of the daily coefficients of variation (CVs) recorded across test days, and the minimum and maximum CVs are provided in parentheses. The daily change in mean HKDT relative to the minimum (ΔHKDT) is displayed as an average across days and the lowest and highest recorded daily changes are provided in parentheses. Plasticity and ΔHKDT were only calculated when more than 3 days of measurements were available, and otherwise are indicated by ‘n.a.’. Sex is indicated as male (m), female (f) or not available (n.a.).
| region | order | species | CTmax (°C) | THKDT (°C) | HKDTavr (min : s) | plasticity (%CV) | ΔHKDT (%) | sex |
|---|---|---|---|---|---|---|---|---|
| temperate | Hemiptera | Stenophyella macreta | 51.1 (0.1, 30)f | 49.7 | 16 : 20 (22, 475) | 22.9 (14.2–29.1) | 23.1 (9.2–46.6) | m + f |
| temperate | Hemiptera | Nysius caledoniae | 49.9 (0.2, 20)ef | 48.5 | 11 : 49 (13, 1069) | 21.2 (14.1–35.5) | 17.6 (5.8–48.4) | m + f |
| tropical | Hymenoptera | Oecophylla smaragdina | 48.0 (0.1, 28)ef | 46.6 | 24 : 47 (31, 592) | 22.9 (18.3–25.2) | 14.6 (5.6–26.8) | n.a. |
| tropical | Hemiptera | Pseudopachybrachius guttus | 46.2 (0.1, 38)ce | 44.8 | 23 : 55 (43, 939) | 24.6 (21.8–30.8) | 14.5 (8.0–25.7) | m + f |
| tropical | Hemiptera | Cicadellidae sp. | 44.8 (0.2, 40)ac | 43.4 | 30 : 51 (101, 799) | 28.1 (18.9–41.0) | 32.9 (9.5–63.7) | m + f |
| temperate | Hemiptera | Psyllidae sp. | 44.2 (0.2, 15)abc | 42.8 | 11 : 49 (28, 349) | 31.6 (22.7–38.7) | 21.0 (11.8–39.9) | n.a. |
| temperate | Hemiptera | Aphis nerii | 43.9 (0.0, 28)ab | 42.5 | 12 : 13 (47, 137) | n.a. | n.a. | f |
| temperate | Hemiptera | Uroleucon sonchi | 43.8 (0.0, 24)ab | 42.4 | 10 : 23 (21, 382) | 22.9 (19.1–27.6) | 21.9 (8.7–37.4) | f |
| temperate | Hemiptera | Hyperomyzus lactucae | 43.3 (0.1, 19)ab | 41.9 | 14 : 01 (35, 253) | 27.2 (21.4–34.7) | 31.6 (15.4–47.7) | f |
| tropical | Diptera | Scaptodrosophila novoguineensis | 42.6 (0.1, 39)bd | 40.7 | 26 : 59 (78, 946) | 35.9 (32.2–43.2) | 33.4 (19.6–87.6) | m + f |
| temperate | Diptera | Drosophila melanogaster | 41.4 (0.1, 13)bd | 40.0 | 15 : 28 (49, 920) | 32.8 (22.5–46.2) | 35.7 (16.9–59.8) | m + f |
| tropical | Diptera | Drosophila rubida | 40.0 (0.1, 45)d | 38.6 | 26 : 22 (42, 954) | 41.9 (28.1–51.7) | 31.7 (6.4–90.3) | m + f |
(c) . Heat tolerance assessment
We used a static knockdown assay to assess heat tolerance across days and sampling times for each species. The temperature used for the heat knockdown assays depended on the species, and was initially determined by finding critical upper lethal limits (CTmax) on haphazardly collected individuals of each species using a dynamic ramping assay. The heat knockdown temperature used was set 1.4°C below the species-specific CTmax, and 1.9°C below CTmax for S. novoguineensis owing to immediate knockdown when the temperature was set only 1.4°C below CTmax (table 1 for CTmax and knockdown temperatures). For this initial work, field-collected individuals were placed into 4 ml screw-cap glass vials (45 × 14.7 mm) and submerged in a water bath at 25°C. The temperature was then increased gradually at a rate of 0.2°C min−1, and CTmax was scored as the temperature at which all movement ceased, i.e. individuals went into heat coma [55].
In the acute knockdown assays, the vials were submerged into a water bath heated to the species-specific test temperature. HKDT was then scored as the time it took for individuals to go into a heat-induced coma [55]. The knockdown temperatures used resulted in individuals going into a coma within 20–40 min, and thus there was a limited opportunity for hardening effects to develop during the tests.
(d) . Statistical analysis
The recorded climate data at each study location were summarized by daily mean temperature and daily coefficient of variation (CV).
Differences in CTmax between species were tested using a Kruskal–Wallis test followed by Dunn's post hoc test to clarify the observed differences. The p-values from Dunn's test were corrected using Holm adjustments. For each species, we used N-way ANOVAs to examine differences in HKDT according to the independent categorical variables ‘day’, ‘time of day’ and ‘sex’ (when relevant). In most cases, residuals of the models adhered to normal distributions; however, the HKDT data were transformed using the rank inverse transformation to ensure normal distribution for all species before ANOVAs were carried out. The p-values generated across the two-way or three-way ANOVAs were then corrected for multiple testing using Benjamini–Hochberg false discovery rate (FDR) adjustments. In addition, the changes in HKDT within days were visualized for each day, species and sex using polynomial regressions on raw HKDT data. Thus, polynomial curves were fitted when the regressions had a significant quadratic term, but not when only linear terms were significant. Regressions with no significant terms were not considered further.
Daily adjustments in HKDT were quantified for each species in two ways: (i) using %CV as an unbiased measure of variability, i.e. s.d./mean × 100. Within each day, %CV was calculated based on raw HKDTs for each sex (when relevant). The daily %CVs were then averaged across days. (ii) Calculating percentage change in ‘mean HKDT’ relative to the lowest mean within each day. Specifically, this was calculated as the difference between the maximal and the minimal mean HKDT recorded within a day, relative to the minimal knockdown time:
Finally, short-term (hardening) and long-term (acclimatization) effects of climate on adjustments in HKDT were examined by Pearson's correlations. First, the mean and variability (CV) of field temperature and humidity recordings were extracted at a range of time intervals. Short-term intervals consisted of climate measures extracted in rolling 1 h bins within the first 24 h preceding thermal assessment, while longer-term intervals consisted of 24 h bins in the period 14 days prior to the assessment of heat tolerance (as illustrated in electronic supplementary material, figure S1). Additionally, the climate variables were extracted in ‘windows’ by moving the past time boundary back in time, in either 1 h (short-term window) or 24 h (long-term window) intervals, thereby accumulating the time windows across which climate measures were extracted (electronic supplementary material, figure S1). Both ‘bins’ and ‘windows’ were used for the analyses to explore whether accumulated impacts, or short bursts of temperature change were more important for HKDT of species in their natural environments. Before correlating ‘mean HKDT’ with the short-term responses, potentially confounding effects of long-term acclimation responses on short-term correlations were controlled. We first regressed ‘mean HKDT’ on ‘test day’ for each species and extracted the residuals from the regressions. The residuals were then correlated with all short-term climate variables (less than 24 h prior to tests). The long-term responses were examined by correlating ‘mean HKDT’ with the extracted long-term bins or windows (1–14 days preceding tests). All analyses were carried out using the software R [56], and raw files for the analyses can be accessed in the electronic supplementary material.
3. Results
(a) . Contrasting climatic conditions at study locations
The temperate study sites were characterized by high-temperature variability both within days and between months (figure 1 and electronic supplementary material, table S2). During the field experiments, the daily mean temperature averaged across month dropped from 21.30°C in February to 13.10°C in May. The daily variability in temperature (CV) ranged from 0.18 in March to 0.27 in April. As expected, temperatures were more stable at the tropical sites (figure 1 and electronic supplementary material, table S2). The daily average temperature in June was 21.04–21.53°C, and the daily temperature variability was extremely low, with CVs of 0.04–0.05 across the study sites.
Relative humidity (%RH) was highly correlated with the temperature at the temperate sites (electronic supplementary material, figure S2), but not at the tropical sites. The daily mean RH ranged from 60.9 to 75.4% at the temperate sites, and daily CVs were in the range of 0.14–0.23. In the tropical study sites, RH was high at all times, with daily means of 86.9–90.5% and CVs of 0.03–0.05 across study sites.
(b) . Both tolerance and plasticity for tolerance varied within and between days for many species
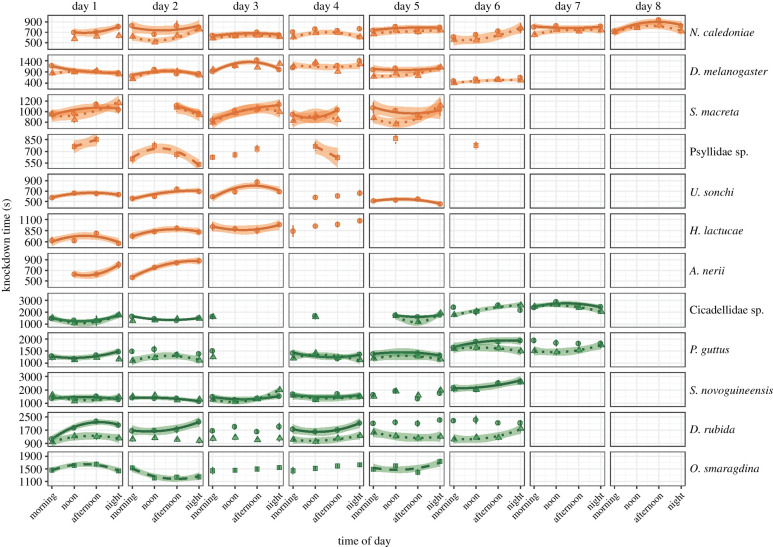
CTmax scores differed between the collected insect groups (Kruskal–Wallis H(11) = 314.71, p < 0.001, table 1), with the temperate species S. macreta being the most heat-tolerant, and the tropical species D. rubida being the least heat-tolerant. For all species, HKDT varied markedly within and across days (figure 2; electronic supplementary material, tables S3 and S4). Females had higher HKDTs than males in six of the seven species for which we had information on the sex (figure 2; electronic supplementary material, table S3). In addition, HKDT varied differently for the sexes between days, but not within days except for D. melanogaster and the Cicadellidae sp. The relationship between HKDT and the time of the day that individuals were tested was examined further by regression. In the majority of cases (71.2%), we observed a significant quadratic relation between ‘time of day’ and HKDT (74 out of 104 combinations of species and test days with more than one sampling time), and few cases (4.8%) had a linear shape. Overall, HKDT varied within days in 76% of the tested sex–species combinations; however, the direction in the change of HKDT varied across days.
Figure 2.
Mean knockdown times (HKDT) measured multiple times (1–4) daily and across days for each species from temperate locations (orange) and tropical locations (green). The knockdown temperatures are displayed for males (triangles) and females (circles) separately when sex was identified, and squares display species for which sex could not be determined. Bars are standard errors of the means. Significant relations between the HKDT and ‘time of day’ were visualized by polynomial or linear regressions with 95% confidence bands. Solid regression lines represent females, dotted lines are for males, and dashed lines are provided for species of unknown sex. (Online version in colour.)
In order to quantify variation in heat tolerance, we calculated the variability in HKDT compared with the mean HKDT value (%CV) for each day as a measure of plasticity, and the daily change in mean HKDT relative to the lowest mean HKDT (table 1). The mean CV ranged between 21.2 and 41.9%, with N. caledoniae and D. rubida representing the extremes. Plasticity levels varied notably across days for all species originating from both regions. The highest daily CVs were 51.7 and 46.2% for the tropical and temperate flies D. rubida and D. melanogaster, respectively, and the lowest daily CVs were 14.1 and 14.2% for the temperate N. caledoniae and S. macreta, followed by 18.3 and 18.9% for the tropical ant O. smaragdina and Cicadellidae sp., respectively.
The daily change in mean HKDT relative to the lowest mean for these species reflected the CVs. For instance, N. caledoniae had a relatively low average change in mean HKDT of 17.6% compared with that of D. rubida of 31.7% or D. melanogaster of 35.7%. Despite this, the daily change in HKDT for N. caledoniae was high on some days and reached a change of 48.4% in HKDT relative to the daily minimum. The maximum daily increase in HKDT was recorded for D. rubida, and reached 90.3% on one of the test days, followed by S. novoguineensis, for which the highest recorded daily change in HKDT was 87.6%.
(c) . Species-specific associations between microclimate variables and heat tolerance
The correlations between residuals of HKDT and short-term mean temperature bins were highly species- and time-specific; thus both the strength and direction of correlations varied in time for the different species (figure 3a). For most of the temperate species, the mean temperature experienced in the time prior to testing thermal tolerance was positively associated with HKDT for up to 10–12 h (figure 3). For example, the correlations of HKDT with mean temperature for D. melanogaster were strongest in the first 11 h, thereafter decreasing slightly. These short-term association patterns were less evident when using ‘time windows’ for extraction of climate variables (electronic supplementary material, figure S3A).
Figure 3.
Heatmap showing correlations between HKDT for each species and the mean and CV of microclimate temperature (a) and humidity (b) extracted in short-term (less than 24 h) and long-term (2–14 days) ‘time bins’. The species are grouped into origin from either temperate or tropical Australia. Grey boxes indicate that climate data were not available.
Long-term correlations between HKDT and mean temperature and humidity bins were more variable than short-term correlations, especially for temperate species. However, for several species of both temperate and tropical origin (e.g. D. melanogaster, U. sonchi, H. lactucae, Cicadellidae sp. and P. guttus), we found relatively strong positive correlations with long-term mean temperatures. Of these cases, H. lactucae, Cicadellidae sp. and P. guttus had no or very weak associations of HKDT with short-term temperature bins (figure 3a). Notably, long-term correlations for species of tropical origin were stronger and more directional compared with the short-term responses. An example is D. novoguineensis, a species for which long-term correlations between mean temperature and HKDT were mostly negative, but short-term associations were weaker and positive.
Comparison of temperature and humidity means revealed opposing directions of correlations for the temperate species (figure 3a,c). For example, HKDT of D. melanogaster was positively correlated with mean temperature but negatively correlated with mean humidity for both the short- and long-term bins. This pattern was not evident for most tropical species; for instance, long-term temperature and humidity means were both positively correlated with heat tolerance in P. guttus, or negatively correlated with heat tolerance in S. novoguineensis. The same correlations are apparent based on ‘time windows’ (electronic supplementary material, figure S3A,C).
Variability in temperature and humidity were only sporadically associated with HKDT in both the short and long term (less than 24 h) when assessing CV across 1 or 24 h bins, where the temperature does not vary much (figure 3b,d). Using accumulated CVs (time windows) for correlations with HKDT, in six of the species (50%) HKDT correlated with long-term mean temperature and humidity windows (electronic supplementary material, figure S3B,D). Temperature and humidity CVs were generally positively associated with HKDT in the late short-term windows, but the associations with variability in humidity were stronger for most species, e.g. the bugs N. caledoniae and S. macreta (10–16 h) (electronic supplementary material, figure S3D). Contrary to the climatic means, the CVs had the same direction of correlations for both temperature and humidity, in both the short and long term (electronic supplementary material, figure S3B,D).
4. Discussion
(a) . Extensive and species-specific plasticity
In the present study, we evaluated the temporal variation in heat tolerance of field-sampled individuals from different arthropod species from temperate and tropical Australia. We observed high levels of variability in heat tolerance for both tropical and temperate species (%CV, table 1). Further, some species, such as the tropical flies S. novoguineensis and D. rubida, occasionally increased their HKDT substantially relative to the lowest recorded HKDT within a day. Several studies have deemed tropical species especially vulnerable to increases in temperature as they are considered to be currently living at the edge of their thermal safety margins [3,4], and have limited capacity for adaptive and plastic responses to warm temperatures [11–14,16,17] and concomitant desiccation stress [57,58]. Our results suggest that many arthropods in nature have a high capacity to adjust their upper thermal tolerance to small alterations in the environment. Thus, the realized thermal acclimation likely plays an important role for species' ability to cope with fluctuating temperatures at a daily or monthly scale and the ability to induce plasticity is likely under strong selection.
The variability in thermal tolerance for the individual species observed within and across days may, however, not solely be related to climatic variation. Perhaps different genotypes are caught at different time points during the days and across sampling days, affecting thermal tolerances [59], although a recent field study on Orchesella cincta attributed monthly variation in heat tolerance to acclimation rather than genetic differences between animals [44]. Age [60–64], nutritional status [60,65–67] and mating status [68] are other factors known to affect heat tolerance in a range of insect species. These factors may differ in the individuals tested at different time points, which means that any differences in heat tolerance within and across days need to be interpreted cautiously.
Despite these issues, our data suggest that species have the potential for adjusting to new temperature and humidity regimes within a species' current range, and perhaps also to conditions outside their current distribution range. This could allow some species to exhibit larger range sizes in the future [69,70]. However, while the flexibility in heat tolerance detected within and across days in our study may be adaptive, some caution is required given that the fitness consequences of trait changes will be context-dependent [71], and that the same environmental changes might trigger adaptive plastic responses in some traits but trigger costly changes for others [72]. Moreover, recent studies have shown that non-lethal endpoints such as behavioural and reproductive traits might be impacted differently by temperature changes [20,21]. Male fertility thermal limits may often be lower than heat tolerance limits, and many species may, therefore, be exposed to temperatures closer to their upper thermal limits than currently presumed. Our assays also do not capture the effects of temperature plasticity on traits such as the ability to find food resources [73] or the ability to respond to more variable temperature conditions [44,74].
Finally, the acclimation potential of species may be much greater than observed in wild populations [75]. This may be particularly true for temperate species which are exposed to larger seasonal variation and unpredictable changes in temperatures when compared with tropical species. This could lead to underestimates of the actual acclimatization capacity of both tropical and temperate species in the present study as they have not been examined under the full range of temperatures that they potentially could endure. On the other hand, laboratory acclimation treatments are often inconsistent or insufficient to produce the maximal plastic responses for different species and exclude the option of species to behaviourally modify their thermal tolerance [75]. A combination of field and laboratory studies is needed to further investigate such issues on the same species.
(b) . Climatic conditions explaining heat tolerance
Optimizing process-based distribution models requires accurate understanding of the physiological metrics that serve as the best predictor of species’ vulnerability to environmental change across phylogenetically diverse species and geographical regions. In this study, we found large variation in heat tolerance across days and hours for all species, suggesting that the fine-scale spatio-temporal environment has a great impact on small invertebrates, and this might not be reflected in the macroclimate, which is typically used for modelling species' vulnerabilities. Defining the climate variables that best explain observed differences, and the spatio-temporal scale that these vary at, are thus central for understanding species' responses to increasingly warm and variable temperatures.
We found that short-term variation in the microhabitat mean temperature and humidity (variation 24 h prior to the assessment of heat tolerance) had a strong influence on some tropical and temperate species (figure 3a,c and electronic supplementary material, figure S3A,C). For most species, the short-term correlations between HKDT and mean habitat temperatures were positive, implying that heat hardening responses to shifts in microhabitat environmental temperatures take place in most organisms. This relationship was not evident for the bug species P. guttus, which had a negative correlation with temperature, thus being less heat-resistant at warm habitat temperatures. It is unclear whether this indicates a cost of warm temperatures and possibly other environmental factors on fitness components, or if resources are allocated for other vital physiological functions at high temperatures. In addition, the temporal scale at which the positive associations were observed differed markedly between species. Thus, for some, e.g. the Psyllidae sp., the mean temperature in the 2–3 h prior to testing was critical for heat tolerance, whereas for D. melanogaster, temperatures experienced up to 14 days prior to testing were typically positively associated with heat tolerance. For D. melanogaster, the life cycle is short (one to a few weeks depending on temperatures) [76], and it is well-known from laboratory studies that developmental temperature has a high impact on adult heat tolerance in insects (e.g. [77–79]). Thus, the strong correlations observed between long-term mean environmental temperatures and HKDT for D. melanogaster and several other species, might be explained by developmental or adult acclimation.
The impact of temperature on HKDT had opposing effects compared with the impact of humidity for some temperate species (figure 3a,c; electronic supplementary material, figure S3A,C). Thus, while recent exposure to high temperatures was associated with high HKDT, exposure to high humidity was associated with low HKDT. This pattern was likely caused by strong negative correlations between environmental temperature and humidity in species from the temperate locations (electronic supplementary material, figure S2), which complicates our ability to separate the effects of temperature and humidity. These variables were not correlated in the tropical locations, and the direction of correlations of temperature and humidity on HKDT did not oppose each other in tropical species (figure 3a,c). This suggests that both temperature and humidity are important climate variables for predicting tropical species' tolerances [57,80,81].
Finally, variability of temperature and humidity did not seem to affect thermal tolerances on a day to day basis (figure 3b,d), but CV measured over multiple days using ‘time windows’ for extraction of climate variables showed that climatic variability had an increasing significant association with HKDT on the long-term scale for several species. In accordance with this, a study that examined monthly differences in thermal tolerances in natural populations of the collembolan O. cincta found that diurnal range was the best predictor for HKDT and CTmax [44].
In conclusion, we found strong evidence for the importance of plasticity in insects for coping with variable thermal conditions in the field, and that climatic variables affecting heat tolerance were species-specific. While further investigations are needed, our results suggest that the evolution of plasticity is important to understand future responses of species to increasingly variable thermal environments. Obviously, studies like ours cannot be performed easily and on a large number of species throughout their range. However, our results suggest that microhabitat temperatures need to be considered in correlative and mechanistic species distribution modelling. Methods to obtain these climate data on fine temporal and spatial scales do exist [49,82–84]. Our results also indicate that trait information incorporated in mechanistic models should take into account the plasticity of these traits. For species of particular interest from a conservation or agricultural pest perspective, relevant data from the laboratory and ideally the field should be generated, including a consideration of populations from the edges of a species' distribution. Incorporating such information into models would improve the prediction of expected future species distributions. Standardized ways to measure the plasticity of relevant traits and to store data in an open-access database would facilitate this process.
Acknowledgements
We are grateful to Dr Peter Ridland at Bio21, Melbourne University, and Dr Mallik B. Malipatil at AgriBio, La Trobe University, for guidance on insect identification and searching for suitable sampling locations, and to Palle Duun Rohde, Aalborg University, for advice on the statistical analyses. We further thank the Staff at Melbourne Zoo, especially Thomas Meek for providing sampling permissions and access to the zoo backstage for collection of D. melanogaster.
Data accessibility
All data are presented in the main manuscript and electronic supplementary material. Data and raw files are provided in the electronic supplementary material [85].
Authors' contributions
S.B., T.N.K., A.A.H. and N.K.N. conceived the ideas and designed the methodology. M.S., S.B., T.N.K. and N.K.N. collected the data. M.Ø. and N.K.N. analysed the data. T.N.K. and N.K.N. led the writing of the manuscript, and all authors contributed to the drafts and gave final approval for publication. All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Competing interests
The authors declare no conflicts of interest.
Funding
This work was supported by a grant from the Danish Council for Independent Research (grant no. DFF-8021-00014B) to T.N.K and an Australian Research Council Discovery grant no. 120100916 to A.A.H. Travel grants were provided to N.K.N. from Otto Mønsted Fond, Knud Højgaards Fond and the Royal Danish Academy of Sciences and Letters.
References
- 1.Sinclair BJ, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372-1385. ( 10.1111/ele.12686) [DOI] [PubMed] [Google Scholar]
- 2.Bale JS, et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1-16. ( 10.1046/j.1365-2486.2002.00451.x) [DOI] [Google Scholar]
- 3.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610-5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale JS. 2002. Insects and low temperatures: from molecular biology to distributions and abundance. Phil. Trans. R. Soc. Lond. B 357, 849-862. ( 10.1098/rstb.2002.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araújo MB, Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743-753. ( 10.1111/j.1466-8238.2007.00359.x) [DOI] [Google Scholar]
- 7.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37-42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 8.Clusella-Trullas S, Blackburn TM, Chown SL. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738-751. ( 10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 9.Kearney M, Porter W. 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol. Lett. 12, 334-350. ( 10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- 10.Evans TG, Diamond SE, Kelly MW. 2015. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 3, cov056. ( 10.1093/conphys/cov056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overgaard J, Kearney MR, Hoffmann AA. 2014. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob. Change Biol. 20, 1738-1750. ( 10.1111/gcb.12521) [DOI] [PubMed] [Google Scholar]
- 12.Addo-bediako AA, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739-745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. Lond. B 278, 1823-1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Phil. Trans. R. Soc. B 374, 20180548. ( 10.1098/rstb.2018.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellermann V, Loeschcke V, Hoffmann AA, Kristensen TN, Fløjgaard C, David JR, Svenning JC, Overgaard J. 2012. Phylogenetic constraints in key functional traits behind species’ climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 66, 3377-3389. ( 10.1111/j.1558-5646.2012.01685.x) [DOI] [PubMed] [Google Scholar]
- 16.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80-S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 17.Kellermann V, Overgaard J, Hoffmann AA, Fljøgaard C, Svenning JC, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228-16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415-1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 19.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147. ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parratt SR, Walsh BS, Metelmann S, White N, Manser A, Bretman AJ, Hoffmann AA, Snook RR, Price TAR. 2021. Temperatures that sterilize males better match global species distributions than lethal temperatures. Nat. Clim. Change 11, 481-484. ( 10.1038/s41558-021-01047-0) [DOI] [Google Scholar]
- 21.van Heerwaarden B, Sgrò CM.. 2021. Male fertility thermal limits predict vulnerability to climate warming. Nat. Commun. 12, 2214. ( 10.1038/s41467-021-22546-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogert CM. 1949. Thermoregulation in reptiles; a factor in evolution. Evolution 3, 195-211. ( 10.1111/j.1558-5646.1949.tb00021.x) [DOI] [PubMed] [Google Scholar]
- 23.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835-3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huey RB, Tewksbury JJ. 2009. Can behavior douse the fire of climate warming? Proc. Natl Acad. Sci. USA 106, 3647-3648. ( 10.1073/pnas.0900934106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665-1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206-1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 27.Kelly MW, Sanford E, Grosberg RK. 2012. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349-356. ( 10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann AA, Chown SL, Clusella-Trullas S. 2013. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934-949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 29.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351-1364. ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 30.Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD. 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Phil. Trans. R. Soc. B 374, 20180174. ( 10.1098/rstb.2018.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MB, Shaw RG. 2001. Range shifts and adaptive responses to quaternary climate change. Science 292, 673-679. ( 10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann AA, Sørensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175-216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 33.Mitchell KA, Hoffmann AA. 2010. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694-700. ( 10.1111/j.1365-2435.2009.01666.x) [DOI] [Google Scholar]
- 34.Hoffmann AA, Dagher H, Hercus M, Berrigan D. 1997. Comparing different measures of heat resistance in selected lines of Drosophila melanogaster. J. Insect Physiol. 43, 393-405. ( 10.1016/S0022-1910(96)00108-4) [DOI] [PubMed] [Google Scholar]
- 35.Chown SL, Jumbam KR, Sørensen JG, Terblanche JS. 2009. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 23, 133-140. ( 10.1111/j.1365-2435.2008.01481.x) [DOI] [Google Scholar]
- 36.Jørgensen LB, Malte H, Ørsted M, Klahn NA, Overgaard J. 2021. A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Scient. Rep. 11, 12840. ( 10.1038/s41598-021-92004-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro PL, Camacho A, Navas CA. 2012. Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. PLoS ONE 7, 32083. ( 10.1371/journal.pone.0032083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezende EL, Tejedo M, Santos M. 2011. Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct. Ecol. 25, 111-121. ( 10.1111/j.1365-2435.2010.01778.x) [DOI] [Google Scholar]
- 39.Dahlgaard J, Loeschcke V, Michalak P, Justesen J. 1998. Induced thermotolerance and associated expression of the heat-shock protein Hsp70 in adult Drosophila melanogaster. Funct. Ecol. 12, 786-793. ( 10.1046/j.1365-2435.1998.00246.x) [DOI] [Google Scholar]
- 40.Bahrndorff S, Mariën J, Loeschcke V, Ellers J. 2009. Dynamics of heat-induced thermal stress resistance and hsp70 expression in the springtail, Orchesella cincta. Funct. Ecol. 23, 233-239. ( 10.1111/j.1365-2435.2009.01541.x) [DOI] [Google Scholar]
- 41.Manenti T, Sørensen JG, Moghadam NN, Loeschcke V. 2014. Predictability rather than amplitude of temperature fluctuations determines stress resistance in a natural population of Drosophila simulans. J. Evol. Biol. 27, 2113-2122. ( 10.1111/jeb.12463) [DOI] [PubMed] [Google Scholar]
- 42.Manenti T, Loeschcke V, Moghadam NN, Sørensen JG. 2015. Phenotypic plasticity is not affected by experimental evolution in constant, predictable or unpredictable fluctuating thermal environments. J. Evol. Biol. 28, 2078-2087. ( 10.1111/jeb.12735) [DOI] [PubMed] [Google Scholar]
- 43.Schou MF, Loeschcke V, Kristensen TN. 2015. Strong costs and benefits of winter acclimatization in Drosophila melanogaster. PLoS ONE 10, e0130307. ( 10.1371/journal.pone.0130307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen A, Alemu T, Alemneh T, Pertoldi C, Bahrndorff S. 2019. Thermal acclimation and adaptation across populations in a broadly distributed soil arthropod. Funct. Ecol. 33, 833-845. ( 10.1111/1365-2435.13291) [DOI] [Google Scholar]
- 45.Lenoir J, Hattab T, Pierre G. 2017. Climatic microrefugia under anthropogenic climate change: implications for species redistribution. Ecography 40, 253-266. ( 10.1111/ecog.02788) [DOI] [Google Scholar]
- 46.Lembrechts JJ, Lenoir J. 2020. Microclimatic conditions anywhere at any time! Glob. Change Biol. 26, 337-339. ( 10.1111/gcb.14942) [DOI] [PubMed] [Google Scholar]
- 47.Pincebourde S, Salle A. 2020. On the importance of getting fine-scale temperature records near any surface. Glob. Change Biol. 26, 6025-6027. ( 10.1111/gcb.15210) [DOI] [PubMed] [Google Scholar]
- 48.Potter KA, Woods HA, Pincebourde S. 2013. Microclimatic challenges in global change biology. Glob. Change Biol. 19, 2932-2939. ( 10.1111/gcb.12257) [DOI] [PubMed] [Google Scholar]
- 49.Kearney MR, Shamakhy A, Tingley R, Karoly DJ, Hoffmann AA, Briggs PR, Porter WP. 2014. Microclimate modelling at macro scales: a test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods Ecol. Evol. 5, 273-286. ( 10.1111/2041-210X.12148) [DOI] [Google Scholar]
- 50.Sears MW, Riddell EA, Rusch TW, Angilletta MJ. 2019. The world still is not flat: lessons learned from organismal interactions with environmental heterogeneity in terrestrial environments. Integr. Comp. Biol. 59, 1049-1058. ( 10.1093/icb/icz130) [DOI] [PubMed] [Google Scholar]
- 51.Kinzner MT, Kinzner MC, Kaufmann R, Hoffmann AA, Arthofer W, Schlick-Steiner BC, Steiner FM. 2019. Is temperature preference in the laboratory ecologically relevant for the field? The case of Drosophila nigrosparsa. Glob. Ecol. Conserv. 18, e00638. ( 10.1016/j.gecco.2019.e00638) [DOI] [Google Scholar]
- 52.Bahrndorff S, Loeschcke V, Pertoldi C, Beier C, Holmstrup M. 2009. The rapid cold hardening response of Collembola is influenced by thermal variability of the habitat. Funct. Ecol. 23, 340-347. ( 10.1111/j.1365-2435.2008.01503.x) [DOI] [Google Scholar]
- 53.Malipatil MB. 2010. Review and revision of Nysius Dallas of Australia and South West Pacific (Hemiptera: Heteroptera: Orsillidae). Zootaxa 44, 29-44. ( 10.11646/zootaxa.2410.1.2) [DOI] [Google Scholar]
- 54.Eyles AC, Malipatil MB. 2010. Nysius caledoniae Distant, 1920 (Hemiptera: Heteroptera: Orsillidae) a recent introduction into New Zealand, and keys to the species of Nysius, and genera of Orsillidae, in New Zealand. Zootaxa 1920, 45-52. ( 10.11646/zootaxa.2484.1.4) [DOI] [Google Scholar]
- 55.Overgaard J, Kristensen TN, Sørensen JG. 2012. Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS ONE 7, e32758. ( 10.1371/journal.pone.0032758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 57.Hoffmann AA, Hallas RJ, Dean JA, Schiffer M. 2003. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science 301, 100-102. ( 10.1126/science.1084296) [DOI] [PubMed] [Google Scholar]
- 58.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA.. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distribution. Science 325, 3-5. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 59.Fournier-Level A, Perry EO, Wang JA, Braun PT, Migneault A, Cooper MD, Metcalf CJE, Schmitt J. 2016. Predicting the evolutionary dynamics of seasonal adaptation to novel climates in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 113, E2812-E2821. ( 10.1073/PNAS.1517456113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chidawanyika F, Nyamukondiwa C, Strathie L, Fischer K. 2017. Effects of thermal regimes, starvation and age on heat tolerance of the parthenium beetle Zygogramma bicolorata (Coleoptera: Chrysomelidae) following dynamic and static protocols. PLoS ONE 12, e0169371. ( 10.1371/JOURNAL.PONE.0169371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowler K, Terblanche JS. 2008. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol. Rev. 83, 339-355. ( 10.1111/j.1469-185X.2008.00046.x) [DOI] [PubMed] [Google Scholar]
- 62.Slotsbo S, Schou MF, Kristensen TN, Loeschcke V, Sørensen JG. 2016. Reversibility of developmental heat and cold plasticity is asymmetric and has long-lasting consequences for adult thermal tolerance. J. Exp. Biol. 219, 2726-2732. ( 10.1242/JEB.143750) [DOI] [PubMed] [Google Scholar]
- 63.Kristensen TN, Loeschcke V, Tan Q, Pertoldi C, Mengel-From J. 2019. Sex and age specific reduction in stress resistance and mitochondrial DNA copy number in Drosophila melanogaster. Scient. Rep. 9, 12305. ( 10.1038/s41598-019-48752-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alemu T, Alemneh T, Pertoldi C, Ambelu A, Bahrndorff S. 2017. Costs and benefits of heat and cold hardening in a soil arthropod. Biol. J. Linn. Soc. 122, 765-773. ( 10.1093/biolinnean/blx092) [DOI] [Google Scholar]
- 65.Bujan J, Kaspari M. 2017. Nutrition modifies critical thermal maximum of a dominant canopy ant. J. Insect Physiol. 102, 1-6. ( 10.1016/J.JINSPHYS.2017.08.007) [DOI] [PubMed] [Google Scholar]
- 66.Manenti T, Cunha TR, Sørensen JG, Loeschcke V. 2018. How much starvation, desiccation and oxygen depletion can Drosophila melanogaster tolerate before its upper thermal limits are affected? J. Insect Physiol. 111, 1-7. ( 10.1016/J.JINSPHYS.2018.09.002) [DOI] [PubMed] [Google Scholar]
- 67.Andersen LH, Kristensen TN, Loeschcke V, Toft S, Mayntz D. 2010. Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. J. Insect Physiol. 56, 336-340. ( 10.1016/J.JINSPHYS.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 68.Xue Q, Sen Ma C. 2020. Aged virgin adults respond to extreme heat events with phenotypic plasticity in an invasive species, Drosophila suzukii. J. Insect Physiol. 121, 104016. ( 10.1016/J.JINSPHYS.2020.104016) [DOI] [PubMed] [Google Scholar]
- 69.Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 8, 461-467. ( 10.1111/j.1461-0248.2005.00739.x) [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann AA, Sgró CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479-485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 71.Bahrndorff S, Gertsen S, Pertoldi C, Kristensen TN. 2016. Investigating thermal acclimation effects before and after a cold shock in Drosophila melanogaster using behavioural assays. Biol. J. Linn. Soc. 117, 241-251. ( 10.1111/BIJ.12659) [DOI] [Google Scholar]
- 72.Hendry AP. 2016. Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 107, 25-41. ( 10.1093/JHERED/ESV060) [DOI] [PubMed] [Google Scholar]
- 73.Kristensen TN, Hoffmann A, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. 2008. Costs and benefits of cold acclimation in field-released Drosophila. Proc. Natl Acad. Sci. USA 105, 216-221. ( 10.1073/pnas.0708074105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Overgaard J, Sørensen JG. 2008. Rapid thermal adaptation during field temperature variations in Drosophila melanogaster. Cryobiology 56, 159-162. ( 10.1016/j.cryobiol.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 75.Pintor AF, Schwarzkopf L, Krockenberger AK. 2016. Extensive acclimation in ectotherms conceals interspecific variation in thermal tolerance limits. PLoS ONE 11, e0150408. ( 10.1371/journal.pone.0150408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernández-Moreno MA, Farr CL, Kaguni LS, Garesse R. 2007. Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol. Biol. 372, 33-49. ( 10.1007/978-1-59745-365-3_3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schou MF, Kristensen TN, Pedersen A, Karlsson G, Loeschcke V, Malmendal A. 2017. Metabolic and functional characterization of effects of developmental temperature in Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R211-R222. ( 10.1152/ajpregu.00268.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah AA, Funk WC, Ghalambor CK. 2017. Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr. Comp. Biol. 57, 977-987. ( 10.1093/icb/icx101) [DOI] [PubMed] [Google Scholar]
- 79.Terblanche JS, Chown SL. 2006. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). J. Exp. Biol. 209, 1064-1073. ( 10.1242/jeb.02129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angilletta MJ. 2009. Thermal acclimation. In Thermal adaptation: a theoretical and empirical synthesis, pp. 126-156. Oxford, UK: Oxford University Press. [Google Scholar]
- 81.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3-15. ( 10.3354/cr00879) [DOI] [Google Scholar]
- 82.Kearney MR, Gillingham PK, Bramer I, Duffy JP, Maclean IMD. 2019. A method for computing hourly, historical, terrain-corrected microclimate anywhere on earth. Methods Ecol. Evol. 11, 38-43. ( 10.1111/2041-210X.13330) [DOI] [Google Scholar]
- 83.Maclean IMD. 2019. Predicting future climate at high spatial and temporal resolution. Glob. Change Biol. 26, 1003-1011. ( 10.1111/gcb.14876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maclean IMD, et al. 2021. On the measurement of microclimate. Methods Ecol. Evol. 12, 1397-1410 ( 10.1111/2041-210x.13627) [DOI] [Google Scholar]
- 85.Noer NK, Ørsted M, Schiffer M, Hoffmann AA, Bahrndorff S, Kristensen TN. 2022. Into the wild—a field study on the evolutionary and ecological importance of thermal plasticity in ectotherms across temperate and tropical regions. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Noer NK, Ørsted M, Schiffer M, Hoffmann AA, Bahrndorff S, Kristensen TN. 2022. Into the wild—a field study on the evolutionary and ecological importance of thermal plasticity in ectotherms across temperate and tropical regions. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data are presented in the main manuscript and electronic supplementary material. Data and raw files are provided in the electronic supplementary material [85].