Abstract
High-elevation species are predicted to have larger elevational ranges compared with species of lower elevations. The reasoning is that temperature variability is greater at higher elevation, selecting for wider niche breadth and more plastic genotypes. We used macroevolutionary comparisons involving 90 Brassicaceae species of the central Alps to test for associations among median elevation of occurrence, elevational range size and thermal variability over space and time on the one hand, and their associations with performance breadth or trait plasticity on the other hand. Performance breadth and trait plasticity were estimated by raising replicate plants per species under three temperature treatments (mild, recurrent frost, recurrent heat). Against prediction, we found that mid-elevation species had the largest elevational ranges, and their ranges were associated with increased spatial thermal variability. Nevertheless, variability in the thermal regime was positively associated neither with niche breadth nor with plasticity. Evidence for adaptive constraints was limited to a trade-off between acclimation-based increases in frost and heat resistance, and phylogenetic niche conservatism for median elevation of occurrence and temporal thermal variability. Results suggest that large elevational range size is associated with divergent adaptation within species, but not with more niche breadth or trait plasticity.
This article is part of the theme issue ‘Species’ ranges in the face of changing environments (part I)’.
Keywords: climate-variability hypothesis, elevational gradient, generalist-specialist trade-off, niche breadth, Rapoport's rule, thermal plasticity
1. Introduction
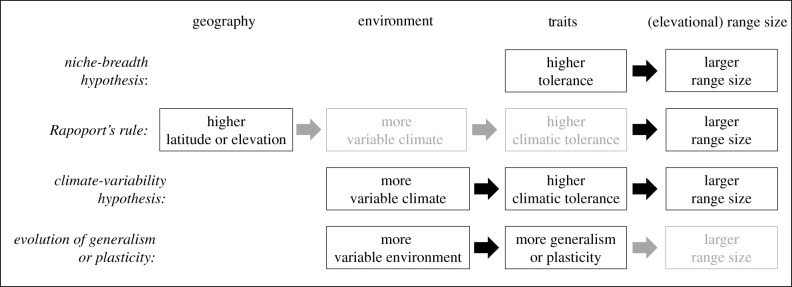
Ecologists and evolutionary biologists have been working on the causes of range limits for at least the last 200 years (e.g. [1]), while they have devoted less attention to the causes of varying range sizes (e.g. [2]). Understanding why some species are spatially restricted while others are common is important e.g. for understanding biodiversity and for conservation. Several hypotheses for variation in range size have been proposed, from variation in niche breadth among species to phylogenetic non-independence and sampling artefacts (summarized in [2]). In the last decade, the niche-breadth hypothesis has attracted increasing support [3]. In short, the hypothesis posits that highly tolerant species are expected to have larger geographical ranges ([4]; figure 1). However, further empirical studies are needed, to understand the evolutionary constraints of niche breadth (e.g. generalist–specialist trade-off) and how environmental heterogeneity shapes the evolution of range size [5], especially in the context of climatic gradients such as latitude or, as studied here, elevation.
Figure 1.
Summary of the different hypotheses associating geography, environmental variability and traits with elevational range size. Arrows and boxes in black indicate explicit links made by models, unlike those indicated in grey. References are given in the main text.
The niche-breadth hypothesis builds on the concept of the ecological niche, defined as the abiotic and biotic conditions needed by a species to maintain viable populations [6]. The niche breadth of a species is often depicted as the width of the performance curves along gradients of environmental conditions [5]. If greater niche breadth is associated with larger range sizes, it is taken as support for the niche-breadth hypothesis [4]. The reasoning is that tolerance to a wider range of environmental conditions allows a species to live in more types of habitats, and range sizes are, therefore, large. Applied to elevational gradients that are generally associated with a decline in temperature of 0.5 K per 100 m increase in elevation [7], the prediction is that species capable of maintaining viable populations over a wide temperature range have larger elevational ranges. There are many studies supporting the niche breadth–range size association (e.g. [8,9]). In a meta-analysis including 63 studies, Slatyer et al. [3] identified positive relationships between several types of variables depicting tolerance, diet and habitat, and range size. However, fewer than 10% of these studies were on physiological tolerance, even though most species' geographical and elevational ranges can be predicted well by climate [10].
Other hypotheses on the causes of variation in range size focus—indirectly or directly—on the role of climate (summarized in figure 1). Rapoport's rule predicts increasing range size with latitude [11]. Stevens [12] proposed an explanatory mechanism for this rule termed the climate-variability hypothesis: With increasing latitude, the climate becomes more variable, and climatic variability selects for broader climatic tolerance that results in larger ranges. Rapoport's rule was predicted to be valid also for elevation [13] and to be caused by the mechanism of climate variability. Indeed, apart from a systematic decline in temperature with increasing elevation, elevational gradients are positively associated with increasing temperature variability over time, within days and across seasons [7,14,15]. Thermal variability also occurs over space, as nearby microclimatic pockets may differ considerably in temperature at high elevation; differences in temperature of up to 15 K were observed between neighbouring locations that differed in their exposure [7,14–16]. Therefore, according to the climate-variability hypothesis, selection at higher elevation should favour broader climatic tolerance in species, and species of higher elevations should, therefore, have larger ranges. Rapoport's rule on latitude has been tested directly (e.g. [17,18]); however, the majority of tests have been indirect, by associating latitude with thermal sensitivity (e.g. [19–21]) or physiological tolerance (e.g. [22–24]). Rapoport's rule on elevation has rarely been tested so far (but see [25,26]).
Both the niche-breadth hypothesis and the climate-variability hypothesis are related to hypotheses on the evolution of generalism versus specialism and adaptive phenotypic plasticity. Plasticity has long been suggested to be associated with larger range sizes [27] and to be favoured in temporally or spatially heterogeneous environments [28–33]. However, no study that we are aware of has tested for a positive link between phenotypic plasticity and elevational range size, and evidence for a more general link between plasticity, climate and geographical range size is overall mixed. On the one hand, empirical studies generally support a reduction in plasticity (generalism) in species with greater thermal specialization (e.g. [34–36]), and others corroborate a positive association among plasticity, niche breadth, and climate (e.g. [37–40]). On the other hand, studies that tried to link plasticity with distribution or commonness produced mainly inconclusive or negative results (e.g. [24,41–45]). Hence, the relationship between plasticity or environmental generalism and distribution remains unresolved, particularly for elevational gradients.
Here, we studied the extent to which elevational range size, niche breadth and plasticity in functional traits were associated with spatial and temporal thermal variability along an elevational gradient. Furthermore, we tested for evolutionary constraints including trade-offs and phylogenetic inertia. The study involved 90 Brassicaceae species from the European Alps, having an elevational range width varying between 12 and 1300 m. Plants were raised under three temperature treatments: control conditions, and the regular occurrence of either frost or heat. Performance estimates were used to quantify the thermal niche breadth, and values of ten eco-morphological and -physiological traits were used to calculate a thermal plasticity index, based on the simplified relative distance plasticity index (RDPIS in [46]). The following questions were addressed: (i) What is the relationship between median elevation of occurrence, elevational range size and spatial and temporal thermal variability? (ii) Are increased spatial and temporal variability in the thermal regime associated with increased breadth of thermal performance or trait plasticity in response to temperature? (iii) Is trait plasticity positively related with thermal performance breadth, and is there a trade-off between coping with frost and heat? (iv) How phylogenetically constrained are range size, thermal niche breadth and trait plasticity?
2. Material and methods
(a) . Species choice and sampling
One hundred taxa belonging to the Brassicaceae family and naturally occurring from the colline to the alpine life zone in the Swiss Alps (or Jura) were selected (electronic supplementary material, S1). Seeds of each species were collected from March to September during the years 2015–2017 at two different sites in Switzerland, located at least 50 km apart from each other and preferentially in different biogeographic regions. The sites were at the most common elevation for each species based on data reported in an online national database (InfoFlora, infoflora.ch). For endangered species inscribed in the Red List 2002 [47], authorization for sampling was obtained from the respective cantonal authority.
(b) . Descriptors of elevational range size
Elevational range size was depicted as variation in the elevation of occurrences of each species. Occurrence data from InfoFlora had previously been validated and thinned [48]. The elevation of each occurrence point of a species was extrapolated from a national digital elevation model with a resolution of 100 m (DEM100; based on the dataset from [49]) with ‘extract’ {raster} [50]. Median elevation of occurrences of a species and the interquartile range were calculated with the basic ‘IQR’ function, the latter depicting elevational range size (IQR_Elev).
Climatic variability was captured by two measures: the spatial temperature variability (Spatial_Var) and the temporal temperature variability (Temporal_Var). For each occurrence point, the minimum, average and maximum monthly temperatures were extracted from DEM100. Monthly records were first merged with ‘stack’ {utils}. Spatial_Var was calculated as the interquartile range of the monthly mean temperature over the two months preceding peak flowering (centre month of the flowering period reported for Switzerland [51]), depicting spatial variability in temperature during a phase of vegetative growth over the entire distribution of a species. Temporal_Var was defined as the difference between the mean maximum and the mean minimum temperature of the two months before peak flowering, depicting temporal variability within the growing season. Data management and summary statistics were performed with the packages included in the {tidyverse} suite [52].
(c) . Sowing, growing conditions and treatments
(i) . Design
The experimental design involved the raising of 100 taxa, each represented by two populations and three seed families (i.e. seeds from different mother plants in the field) per population, i.e. six seed families per species. The experiment was split into six blocks, with a different seed family per species in each block, and each block contained the three temperature treatments (regular frost, mild, regular heat). The final design resulted in 1800 individuals (100 taxa × 6 families, each in a different block × 3 treatments = 1800 individuals). At the stage of trait measuring, only 90 taxa had replicate plants in all treatments, because seeds of some species did not germinate well, and some plants died during treatment. Seeds of each seed family were selected haphazardly and incubated in 500 µl of gibberellic acid solution (500 ppm, Merck KGeA, Dornstadt, Germany) for one week in the dark and cold (4°C constant; Climecab 1400 Kälte 3000 AG, Landquart, Switzerland). Gibberellic acid was used to achieve synchronous germination. Seeds were sown in multipot-trays (0.06 l, 54 pots per tray with Ø 4.4 cm each, BK Qualipot; gvz-rossat, Otelfingen, Switzerland) and transferred to growth chambers (MobyLux GroBanks, CLF Plant Climatics, Wertingen, Germany). All growth chambers were located inside a PlantMaster (CLF Plant Climatics) with controlled temperature and humidity. Trays were kept at 15/18°C night/day, 75% relative humidity, light : dark 8 : 16 h at 150 µmol m−2 s−1 of light (fluorescent white lamps and red-LED). After three weeks, excess seedlings were used to fill pots without any germination. In week 4, germinated plants were moved back into the Climecabs and subjected to the temperature treatments.
(ii) . Treatments
Plants were subjected to three temperature treatments: ‘Frost’ (F), ‘Mild/control’ (M) and ‘Heat’ (H). Frost: 20°C (day), then −2°C for 1 h (−4.8 K h−1; night), back to 20°C (+7.3 K h−1; night), 20°C (day). Mild: 20°C constant. Heat: 20°C (day), then 40°C for 1 h (+5.5 K h−1; day), back to 20°C (−8.3 K h−1; day), 20°C (night). We selected extreme temperatures based on records in the field during the vegetation period [7,53,54], while for the Mild treatment, we used a standard temperature. All treatments were conducted at a daily cycle of 12 : 12 h light : dark, 300 µmol m−2 s−1 of light (LED white lamp) and 75% relative humidity. Plants were kept under these conditions until the ninth week after sowing.
(d) . Performance and trait plasticity
(i) . Thermal performance breadth
Plant performance was defined as the product of the number of leaves (NLEA) and their asymptotic size in millimetres (derived from a three-parameter logistic growth curve based on weekly measuring of the two longest leaves; ASYM). Then for each species–treatment combination (i.e. Frost, Mild and Heat), the mean across replicate plants was calculated. Thermal niche breadth was defined as the mean performance across treatments, divided by the standard deviation (the inverse of the coefficient of variation). Higher values reflected wider niche breadth (i.e. similar values of performance across environments), while lower values indicated more specialization.
(ii) . Trait plasticity (RDPIS)
Ten traits representing aspects of growth (except NLEA and ASYM, which represented performance), leaf morphology and thermal resistance were used to calculate trait plasticity using the simplified RDPIS (model 16 in [46]). The traits were: initial growth rate, maximum growth rate, time to fastest growth, leaf area, leaf dissection index, specific leaf area, leaf dry matter content, leaf thickness and heat and frost resistance based on electrolyte leakage (for more details see electronic supplementary material, S2). For each trait–species–treatment combination, we calculated the mean trait value. Plasticity was calculated as the absolute difference between the trait values of the different thermal environments (FH: Frost–Heat, MF: Mild–Frost, MH: Mild–Heat). Values were then divided by the sum of the values within the paired treatments, resulting in a plasticity index ranging from 0 (no plasticity) to 1 (maximal plasticity).
| 2.1 |
(e) . Statistical analysis
(i) . Relationships between elevation of occurrence, range size and thermal variability
All analyses were performed with the software R v. 4.0.2 [55], and figures were produced with {ggplot2}. An initial analysis on the relationships between environmental descriptors was performed using generalized linear mixed models based on Markov Chain Monte Carlo techniques with the ‘brm’ function of the package {brms} [56]. For each pair of variables (i.e. IQR_Elev or Spatial_Var or Temporal_Var on Median elevation, IQR_Elev on Spatial_Var or Temporal_Var and Spatial_Var on Temporal_Var), two models were fitted: a linear (y Approx. I + x) and a quadratic one (y Approx. I + x + x2). For each model, the LOO (leave-one-out cross-validation) was calculated with the ‘add_criterion’ function of {brms}, and then the model fit was compared based on the expected log pointwise predictive density (i.e. ELPD) with the ‘loo_compare’ function. For the better model, the Bayesian version of the R2 function ‘bayes_R2’ was also computed (robust argument = TRUE). Furthermore, since environmental variables were all positive, response variables were modelled assuming a lognormal distribution.
In these and further analyses, we accounted for non-independence of data among species by considering the phylogeny. The phylogeny had been produced based on several dozen chloroplast genes [48]. It was a random effect in the models, even though it changed neither the significance of fixed predictors nor what the best supported model was. The phylogeny was pruned to species included in this study with the function ‘treedata’ of package {GEIGER} [57]. The final matrix was obtained with function ‘vcv’ {ape} [58] and specified within the ‘cov_ranef’ setting in brm. Analyses were performed at the level of species, because some variables could only be estimated at this resolution (e.g. elevational range size), and because some species had generally low germination and several missing plants across the blocks, which prevented analyses at the level of the seed family/block.
Significance of Bayesian mixed-effects models was based on 90% credible intervals (90CI), since they are more stable for Bayesian statistics [59]. In addition, the probability of direction was calculated with the function ‘p_direction’ {bayestestR} [60], which is correlated with the two-sided p-value. Values were drawn from four independent parallel chains, with burn-in, number of iterations, thinning interval, maximal tree-depth and adaptive delta being adjusted for each model to have an effective sampling size (ESS) of at least 1000. Calculations were performed at sciCORE (http://scicore.unibas.ch), the scientific computing centre of the University of Basel.
(ii) . Relationships between thermal variability, thermal performance breadth and trait plasticity
The relationship of thermal performance breadth or trait plasticity and spatial and temporal thermal variability was tested using again Bayesian generalized linear mixed-effects models analysed with the ‘brm’ function. Performance breadth was modelled assuming a lognormal distribution. Since RDPIS had values between 0 and 1 (in our dataset min = 0, max = 0.5), we modelled it assuming a true beta distribution after data transformation: [61]. Transformation was necessary to include the zero values, which were assumed not to come from a different process but to reflect an approximation issue. Transformed data ranged from 0.00012 to 0.5. The crossed random effects were species relatedness, and thermal environment and trait (only included in the analyses of RDPIS′). Predictions of the Bayesian models were visualized with ‘conditional_effects'.
Similarly, we tested for a positive relationship between trait plasticity and performance breath. In a first model, RDPIS′ (square-root transformed, centred to a mean of 0 and scaled to unit standard deviation) was the dependent variable and thermal performance breadth was the independent variable. To test for an association between plasticity to frost and plasticity to heat, frost plasticity was modelled as the dependent variable (assuming a beta distribution) and heat plasticity (square-root transformed) was the independent variable. Because of the special behaviour of thermal resistance compared with the rest of the plant traits and its relevance in a thermo-biological context, a model predicting RDPIS′frost resistance dependent on RDPIS′heat resistance (square-root transformed) was also run. Predictors were centred to a mean of 0 and scaled to unit standard deviation with the base function ‘scale’.
(iii) . Phylogenetic signals
Analysis on phylogenetic signals was performed using the ‘phyloSignal’ function in {phylosignal} [62]. Three different measures of phylogenetic conservatism were tested: Moran's I, Bloomberg's K and Pagel's λ. A Moran's I of 0 means that species resemble each other as predicted under Brownian motion (BM); if I < 0, species resemble each other less than predicted under BM, and if I > 0, related species are more similar. Bloomberg's K = 0 indicates no phylogenetic signal, K = 1 suggests that trait distribution follows BM, and K > 1 indicates stronger similarity among closely related species. Similarly, Pagel's λ values range between 0, implying no phylogenetic signal, and 1, when a trait evolves under BM. p-Values were calculated based on a total of 10 000 permutations. To test for phylogenetic autocorrelations, we produced correlograms based on Moran's I using the function ‘phyloCorrelogram’ {phylosignal}. The 95% confidence interval (CI) of the correlation was assessed using 1000 bootstrap replicates. CI curves not overlapping with 0 were considered to indicate significant phylogenetic autocorrelation.
3. Results
(a) . Relationships between elevation of occurrence, range size and thermal variability
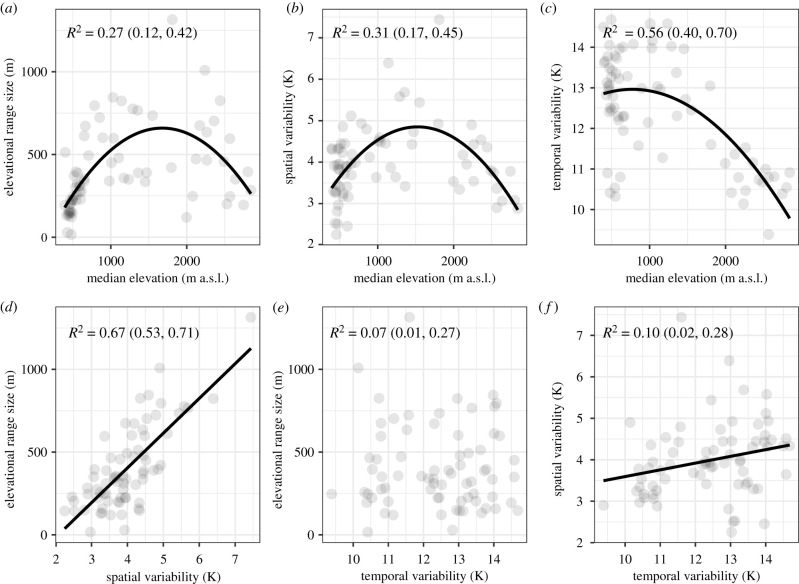
All pairs of environmental variables except one shared a best supported relationship that was either linear or quadratic (electronic supplementary material, S3, table S3.1; figure 2). The best-supported relationship between range size and median elevation of occurrence was quadratic, with low- and high-elevation species having smaller elevational ranges compared with mid-elevation species (figure 2a). Similarly, spatial thermal variability showed a curvilinear relationship with elevation, with low- and high-elevation species experiencing less spatial temperature variability across their ranges (figure 2b), possibly driven by their smaller elevational ranges and the positive, linear relationship between elevational range size and spatial temperature variability across the range (figure 2d). The relationship between temporal thermal variability and elevation was also negative-quadratic, with high temporal thermal variability (but considerable variation) at low elevation and a drop only beyond intermediate elevation, suggesting that alpine species were less subject to temperature change between minima and maxima during the growing season (figure 2c). Spatial and temporal thermal variability were weakly positively correlated (figure 2f), while neither a linear nor a quadratic relationship between range size and temporal thermal variability could be detected (figure 2e), unless temporal variability was defined as seasonality or as a difference (max − min) over the entire distribution; then a positive linear relationship was supported (electronic supplementary material, table S3.2 and figure S3.1). Results did not change qualitatively when an outlier species was excluded (Arabis alpina, IQR or Spat_Var > mean + 5 × standard deviation). In summary, low-elevation species generally occupy smaller ranges, are exposed to less spatial temperature variability across their ranges but experience more temperature variability across the growing season. High-elevation species also generally occupy small ranges, are exposed to less spatial temperature variability across their ranges, and experience less temporal temperature variability prior to peak flowering. By contrast, the montane/sub-alpine species (i.e. growing at 1000–2000 m a.s.l.) have the largest ranges and their exposure to spatial but also temporal temperature variability is consistently high.
Figure 2.
Relationships among environmental variables. For all pairs of variables, the better-supported relationship is indicated, either linear (solid line) or quadratic (solid curve), or neither of them was found significant. R2 is the median Bayesian R-squared, with the 90% CI in brackets. Each dot represents a species.
(b) . Relationships between thermal variability, thermal performance breadth and trait plasticity
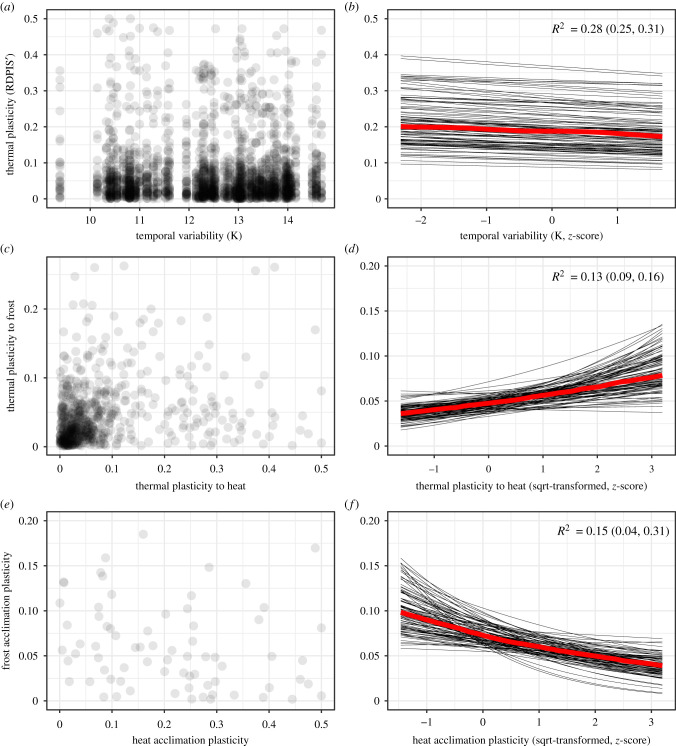
Next, we tested for a linear relationship of thermal performance breadth or plasticity with spatial and temporal thermal variability (table 1 and figure 3). Performance breadth was significantly associated neither with spatial thermal variability nor with temporal thermal variability, and results did not change if an outlier species (Petrocallis pyrenaica) was excluded. Also the relationship between RDPIS′ and spatial thermal variability was non-significant, yet that between RDPIS' and temporal thermal variability was significant, but negative (figure 3a,b).
Table 1.
Results of mixed-effects models on the relationship between thermal performance breadth or thermal plasticity (RDPIS′) and spatial and temporal thermal variability. Values for fixed effects represent posterior means and those for random effects variances. 90% credible intervals (90CI) are reported for the fixed and random effects. Significance is given when the 90CI does not overlap with 0, and by the probability of direction (pd, %). pd greater than 97.5% is *p < 0.05, and pd greater than 99.95% is ***p < 0.001. Significant predictors are shown in italic. The random effect of species depicts the phylogenetic relatedness.
| effects | thermal performance breadth | pd | thermal plasticity (RDPIS′) | pd |
|---|---|---|---|---|
| fixed | ||||
| intercept | 1.369 [1.183, 1.547] | 100*** | −1.503 [−2.170, −0.834] | 99.99*** |
| spatial variability | 0.012 [−0.103, 0.123] | 56.46 | 0.022 [−0.009, 0.057] | 86.76 |
| temporal variability | 0.061 [−0.056, 0.179] | 80.1 | −0.050 [−0.091, −0.011] | 98.26* |
| random | ||||
| species | 0.061 [0, 0.222] | 0.072 [0.037, 0.107] | ||
| thermal environment | — | 0.804 [0.255, 1.298] | ||
| trait | — | 0.583 [0.367, 0.862] | ||
| R2 | 0.054 [0.007, 0.237] | 0.278 [0.247, 0.309] | ||
Figure 3.
Relationships between thermal plasticity (RDPIS′) and temporal thermal variability (a,b), between plasticity to frost and plasticity to heat across traits (c,d), and between (acclimation) plasticity of frost resistance in response to frost and of heat resistance in response to heat (e,f). On the left, estimates of species are shown, in (a) for each environment–trait combination. On the right, model predictions (z-scores) are shown, with the thin black lines representing draws of 100 runs of model prediction. Thicker, red lines connect median values. (Online version in colour.)
Trait plasticity in response to experimental temperature was negatively related with thermal performance breadth when the outlier species P. pyrenaica was included (electronic supplementary material, table S3.3) and unrelated with thermal performance breadth when P. pyrenaica was excluded. The result suggests no positive link between plasticity assessed mainly on leaf-based traits and performance breadth across thermal regimes. Thermal plasticity in response to frost covaried positively with thermal plasticity in response to heat (figure 3c,d; electronic supplementary material, table S3.3). However, plasticity in frost resistance in response to frost exposure (during growth) and plasticity in heat resistance in response to heat exposure were negatively related (figure 3e,f; electronic supplementary material, table S3.3). As resistance generally increased under the regular occurrence of either frost or heat during plant growth, plasticity here actually reflected acclimation.
(c) . Phylogenetic signals
The phylogenetic signal of environmental variables and thermal performance breadth is summarized in table 2. Median elevation showed a moderate phylogenetic effect (with a positive autocorrelation going back 10 Myr), while elevational range size did not show an enhanced effect of species relatedness. Also, spatial thermal variability over the area of occurrence showed no enhanced imprint of species relatedness. However, temporal thermal variability across the range again showed a moderate phylogenetic effect (with an autocorrelation going back 20 Myr; electronic supplementary material, S4). Finally, thermal performance breadth revealed no phylogenetic signal. A trend for a phylogenetic signal was observed for plasticity in a few traits in some thermal environments (leaf dissection indexMild–Frost, not supported by K, and leaf thermal resistanceMild–Heat not supported by λ; electronic supplementary material, S4).
Table 2.
Indices of phylogenetic signal for variables depicting the geography of distribution, the thermal environment and thermal performance. p-values are based on 10 000 permutations: (.), p < 0.1; ***p < 0.001. Indices significantly different from 0 are shown in italic. A short key to the interpretation of indices: constrained evolution relative to Brownian motion if Moran's I (max. range: −1; +1) >0, Bloomberg's K (0; >1) >1, and Pagel's λ (0; 1) ≈0.5. For full details see electronic supplementary material, S4.
| geography |
thermal environment |
thermal performance |
|||
|---|---|---|---|---|---|
| median elevation | elevational range size | spatial variability | temporal variability | performance breadth | |
| I | 0.173*** | −0.017 | −0.034 | 0.138*** | −0.018 |
| K | 0.347*** | 0.103 | 0.127 | 0.330*** | 0.161 |
| λ | 0.795 (.) | 0.000 | 0.000 | 0.532*** | 0.000 |
4. Discussion
Past conceptual and empirical studies in ecology, biogeography and evolution have led to some general predictions about positive associations between elevation, environmental variability, traits such as niche breadth or plasticity and range size (summarized in figure 1). We tested them in a phylogenetic context on 90 Brassicaceae species of the central Alps and revealed mixed support. Contrary to expectations, it was not species with high-elevation distributions that had generally large ranges, but those of mid elevations (figure 2a). Furthermore, mid-elevation species occurred over areas with more spatial thermal variability (figure 2b). Spatial and temporal thermal variability during the growing season of plants was then tested for associations with niche breadth or trait plasticity in response to thermal treatments. We found no significant positive relationships between spatial or temporal thermal variability and thermal performance breadth or trait plasticity (table 1). On the contrary, temporal thermal variability during the growing season, which was lowest in high-elevation species, was negatively associated with trait plasticity (figure 3a,b). We discuss these results and others on potential evolutionary limits in the context of elevational distribution and the evolution of generalism versus specialism.
(a) . Elevation of occurrence, range size and thermal variability
Based on Rapoport's rule applied to elevational gradients, larger ranges are expected at high elevation, where climatic variability should be higher (figure 1). Our results did not support these predictions (electronic supplementary material, table S3.1). Instead, the relationship between median elevation of species occurrence and elevational range size was not linear but had the peak of large range size for mid-elevation species (figure 2a). Similarly, spatial and temporal variability in the thermal regime experienced by plants during the growing season peaked in the range of mid-elevation species (figure 2b,c). However, for temporal thermal variability the peak was far to the left, and temporal thermal variability was only consistently low for high-elevation species (figure 2c). Overall, and against predictions by Rapoport's rule for elevation, high-elevation species had smaller elevational range sizes, and both spatial and temporal thermal variability during the growing season were consistently the lowest where these species occurred.
To our knowledge, few studies have directly tested Rapoport's rule applied to elevational gradients, and most were done on animals (e.g. [25,63,64], but see [26,65]). Authors reported conflicting results showing either a positive relationship between elevation and range size [63–66], no significant relationship, or a negative one ([25,26,64,67] in temperate species). Interestingly, a study on plants and snails in the Caucasus showed that the shape of the relationship depended on the method used [26]. Species range sizes were derived from field observations of species occurrences along a gradient from 600 to 2200 m a.s.l., in steps of 200 m. When means of elevational range sizes of all species occurring in the same elevational band were regressed against elevation, elevational range size decreased with increasing elevation. By contrast, when means of elevational range sizes of all species with a midpoint in the same elevational band were regressed against elevation, range size peaked at mid elevation (around 1500 m a.s.l.). This study also provided no support for Rapoport's rule on elevation.
Generally in line with our results, previous studies that explicitly tested for an association between median or mean elevation of species occurrence and spatial or temporal thermal variability at the sites where a species occurred found a negative relationship between the two (e.g. [25,26]). Furthermore, a climatological review among mountain systems found that with increasing elevation, daily variability did not increase systematically [68], contrary to what was stated in other contributions [15]. However, several studies that tested for a relationship between temporal climate variability and elevational range size, independent of the elevation of occurrences, found support for a positive link, despite the use of different ways of assessing thermal variability (e.g. [25,63,69]). In our study, we found no support for a positive link between temporal thermal variability during the growing season and range size (electronic supplementary material, table S3.1; figure 2e). However, the relationship was positive when temporal thermal variability was measured over the entire elevational range (and not based on individual occurrences; electronic supplementary material, table S3.2). We think that our approach of considering temporal thermal variability during the growing season at occurrence sites (even if they are weather station data or interpolated data) is more meaningful. It depicts what individual plants experience, during a time of the year when they are non-dormant, an approach that has also been suggested by others (e.g. [70,71]). What our approach, however, did not consider is microclimatic variability across space and time. Nevertheless, our results and those of others suggest that Rapoport's rule for elevation seems unable to depict patterns in nature.
(b) . Thermal variability, thermal performance breadth and trait plasticity
Species occupying environments with higher spatial and/or temporal heterogeneity were predicted to have greater physiological flexibility (e.g. [28,29,72,73]) and be adapted to cope with a wider range of conditions (e.g. [30]; climate-variability hypothesis [12]; Rapoport's rule for elevation [13]). Our results indicate that thermal performance breadth and trait plasticity in response to thermal conditions were not related or were negatively related with spatial and temporal thermal variability (table 1 and figure 3a,b). It could be argued that our assessment of performance or plasticity was incomplete, but we think that with depicting performance by plant size and leaf numbers, and with profiling plastic responses based on growth, leaf morphology and leaf thermal resistance, we worked with a fair representation of both. Furthermore, these results are in line with the few other studies that tested for a positive association between breadth of tolerance and elevational range size; those studies found only mixed support (e.g. [71,74,75]). Note that here we assessed performance breadth and thermal plasticity typically for replicate plants of two populations per species, and those populations came from around the centre of the elevational range. Given this important detail of our study, we lean that it is apparently not an inherent feature of a species, its populations and most of its genotypes, to show thermal performance breadth or high trait plasticity and therefore to cover a wider range of thermal conditions across its species range.
Based on these results, it seems more likely that a wide elevational range is achieved by divergent climate adaptation. The hypothesis also makes sense in the light of pollen and seed dispersal mechanisms in Brassicaceae. Most species are pollinated by insects and seeds disperse by gravity, with the likely effect of gene flow being limited over space. Limited gene flow is predicted to facilitate local adaptation in a spatially heterogeneous environment [76]. Furthermore, temporal variability during the growing season may be predictable such that fixed adaptation is more likely to evolve than adaptive plasticity. The result on the negative relationship between temporal thermal variability and thermal plasticity indirectly supports the role of divergent local adaptation in contributing to large range size. Mid-elevation species occurred where temporal thermal variability during the growing season was consistently high, though species growing under such conditions were associated with lower thermal plasticity. The question remains why some species are more likely to occupy larger ranges presumably owing to more divergent adaptation, and why mid-elevation species seem to do better in this regard in our study system.
(c) . Constraints and evolutionary conservatism
Based on classical assumptions and recent evidence, generalist species (with a wider niche breadth) are expected to exhibit larger plastic responses (e.g. [77,78]). By contrast, our analysis suggested that phenotypic plasticity was not associated with performance breadth across thermal environments (electronic supplementary material, table S3.3). As Brassicaceae are likely to have a warm-climate origin and colonized cooler areas or higher elevations relatively recently [79], the observation may reflect a situation where thermal performance breadth or thermal plasticity has not yet reached the optimum. However, though rarely, low plasticity related to generalism finds support in the recent literature. Comparisons between low- versus high-elevation species detected negative relationships between niche breadth and plasticity across different abiotic factors (nutrients, light and moisture) ([43,80]). These results appear to support the specialization hypothesis [81], which posits that specialization to a specific environment should be associated with an increase in plasticity. Our result of no association between niche breadth and plasticity points to a scenario of complex joint evolution.
It is well known that phenotypic plasticity, when adaptive, helps organisms to withstand stressful conditions [27,82]. However, this ability may be costly or limited by internal factors (e.g. genetic trade-offs; e.g. [73,83]). Here, we found that higher plasticity in response to regular heat was associated with higher plasticity to regular frost (electronic supplementary material, table S3.3; figure 3c,d). Results imply that a species is either generally responsive to frost and heat, or not that much. Similar results have been reported in animals [84]. For thermal resistance, the relationship was, however, negative; acclimation (plasticity) in frost resistance and acclimation in heat resistance traded off against each other (electronic supplementary material, table S3.3; figure 3e,f). As acclimation is important in achieving higher resistance to thermal extremes, this trade-off is likely to be an important limiting factor affecting species distribution.
The extent to which aspects of the physical environment or traits differ between related species can provide information about how labile their evolution is. While range size and spatial thermal variability did not reveal evidence for phylogenetic conservatisms, median elevation of species occurrence and temporal thermal variability revealed a moderate phylogenetic effect (table 2). Furthermore, trait plasticity was generally weakly constrained by shared history (electronic supplementary material, S4). In fact, phylogenetic signal for plasticity expressed by the ten ecophysiological traits measured in the three pairs of environments was rarely significant, and for none of the traits was it supported by all three indices. Studies addressing phylogenetic signals for plasticity are scarce. In a recent study on amphibians, Relyea et al. [85] explored phylogenetic conservatism in plasticity in life-history traits and found only few traits (17% of 30) with such a signature. Strong phylogenetic signals were rarely observed, providing evidence consistent with the idea that plasticity is evolutionarily labile [86], since species exhibit distinct plasticity profiles despite a common evolutionary history. The lack of a trade-off between plasticity in response to frost and to heat, and the lack of support for phylogenetic niche conservatism in performance breath across thermal regimes reinforce this view. Further macroevolutionary studies are needed to clarify the role of plasticity in being associated with elevational distribution and, by extension, with thermal heterogeneity across elevational gradients.
5. Conclusion
Several hypotheses in ecology, biogeography and evolution link environmental heterogeneity with performance breadth or plasticity, and some with species range size. Based on our results on Brassicaceae over an elevational gradient, several of them were not supported. Our data did not support Rapoport's rule for elevation, as no evidence for a positive link between elevation of occurrence and elevational range size was found. Also the climate-variability hypothesis—making mechanistic links for Rapoport's rule—was not supported: neither spatial nor temporal thermal variability increased with elevation, and species occupying areas with more thermal variability had neither wider performance breadth nor higher thermal plasticity. Based on our results and those of others, we suggest that it may be commonly mid-elevation species that have the largest elevational ranges. These larger ranges are likely associated with more (realized) spatial but not more temporal thermal variability. Furthermore, we hypothesize that large elevational range size is often accomplished by more divergent climate adaptation within species.
Acknowledgements
We thank Lea Bona, Camilla Jenny, Adrian Möhl, Ramon Müller, Jens Paulsen, Florian Schreier and Daniel Slodowicz for collecting seeds in the field. We thank Georg Armbruster, Olivier Bachmann, Kay Lucek, Michela Meier, Jens Paulsen, Antoine Perrier, Susanna Riedl and Darío Sánchez-Castro for help with data collection and for fruitful discussions. We thank Theofania-Sotiria Patsiou for sharing species occurrence data, and all the field botanists who contributed to the collection of occurrence data from the InfoFlora database.
Data accessibility
All relevant data are within the paper and its electronic supplementary material, S1–S5 [87]. Trait values are deposited at try-db.org.
Authors' contributions
A.M. and Y.W. conceived the study and conducted the field work. A.M. executed the experimental work, ran the statistical analyses and wrote the first draft of the manuscript. Y.W. contributed to writing. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We were supported by the Fondation Pierre Mercier pour la Science, Pully, Switzerland, and by the University of Basel.
References
- 1.von Humboldt A, Bonpland A, Kunth KS. 1805. Voyage de Humboldt et Bonpland. Paris, France: F. Schoell. [Google Scholar]
- 2.Gaston KJ. 2003. The structure and dynamics of geographic ranges. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Slatyer RA, Hirst M, Sexton JP. 2013. Niche breadth predicts geographical range size: a general ecological pattern. Ecol. Lett. 16, 1104-1114. ( 10.1111/ele.12140) [DOI] [PubMed] [Google Scholar]
- 4.Brown JH. 1984. On the relationship between abundance and distribution of species. Am. Nat. 124, 255-279. ( 10.1086/284267) [DOI] [Google Scholar]
- 5.Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA. 2017. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 48, 183-206. ( 10.1146/annurev-ecolsys-110316-023003) [DOI] [Google Scholar]
- 6.Hutchinson GE. 1957. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415-427. ( 10.1101/SQB.1957.022.01.039) [DOI] [Google Scholar]
- 7.Körner C. 2021. Alpine plant life. Cham, Switzerland: Springer Nature. [Google Scholar]
- 8.Gaston K, Blackburn T. 2008. Pattern and process in macroecology. New York, NY: John Wiley & Sons. [Google Scholar]
- 9.Trakimas G, Whittaker RJ, Borregaard MK. 2016. Do biological traits drive geographical patterns in European amphibians? Glob. Ecol. Biogeogr. 25, 1228-1238. ( 10.1111/geb.12479) [DOI] [Google Scholar]
- 10.Lee-Yaw JA, Kharouba HM, Bontrager M, Mahony C, Csergő AM, Noreen AME, Li Q, Schuster R, Angert AL. 2016. A synthesis of transplant experiments and ecological niche models suggests that range limits are often niche limits. Ecol. Lett. 19, 710-722. ( 10.1111/ele.12604) [DOI] [PubMed] [Google Scholar]
- 11.Rapoport EH. 1982. Areography: geographical strategies of species. Oxford, UK: Pergamon Press. [Google Scholar]
- 12.Stevens GC. 1989. The latitudinal gradient in geographical range: how so many species coexist in the Tropics. Am. Nat. 133, 240-256. ( 10.1086/284913) [DOI] [Google Scholar]
- 13.Stevens GC. 1992. The elevational gradient in altitudinal range: an extension of Rapoport's latitudinal rule to altitude. Am. Nat. 140, 893-911. ( 10.1086/285447) [DOI] [PubMed] [Google Scholar]
- 14.Larcher W. 2012. Bioclimatic temperatures in the high Alps. In Plants in alpine regions: cell physiology of adaption and survival strategies (ed. Lütz C), pp. 21-27. Vienna, Austria: Springer. [Google Scholar]
- 15.Jackson LS, Forster PM. 2010. An empirical study of geographic and seasonal variations in diurnal temperature range. J. Clim. 23, 3205-3221. ( 10.1175/2010JCLI3215.1) [DOI] [Google Scholar]
- 16.Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353-362. ( 10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- 17.Pither J. 2003. Climate tolerance and interspecific variation in geographic range size. Proc. R. Soc. Lond. B 270, 475-481. ( 10.1098/rspb.2002.2275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu F, Groen TA, Wang T, Skidmore AK, Huang J, Ma K. 2017. Climatic niche breadth can explain variation in geographical range size of alpine and subalpine plants. Int. J. Geogr. Inf. Sci. 31, 190-212. ( 10.1080/13658816.2016.1195502) [DOI] [Google Scholar]
- 19.van Berkum FH. 1988. Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. Am. Nat. 132, 327-343. ( 10.1086/284856) [DOI] [Google Scholar]
- 20.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739-745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823-1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calosi P, Bilton DT, Spicer JI, Atfield A. 2008. Thermal tolerance and geographical range size in the Agabus brunneus group of European diving beetles (Coleoptera: Dytiscidae). J. Biogeogr. 35, 295-305. [Google Scholar]
- 23.Sheth SN, Angert AL. 2014. The evolution of environmental tolerance and range size: a comparison of geographically restricted and widespread Mimulus. Evolution 68, 2917-2931. ( 10.1111/evo.12494) [DOI] [PubMed] [Google Scholar]
- 24.De Araujo LI, Karsten M, Terblanche JS. 2019. Exploring thermal flight responses as predictors of flight ability and geographic range size in Drosophila. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 236, 110532. ( 10.1016/j.cbpa.2019.110532) [DOI] [PubMed] [Google Scholar]
- 25.Beck J, Liedtke HC, Widler S, Altermatt F, Loader SP, Hagmann R, Lang S, Fiedler K. 2016. Patterns or mechanisms? Bergmann's and Rapoport's rule in moths along an elevational gradient. Community Ecol. 17, 137-148. ( 10.1556/168.2016.17.2.2) [DOI] [Google Scholar]
- 26.Mumladze L, Asanidze Z, Walther F, Hausdorf B. 2017. Beyond elevation: testing the climatic variability hypothesis vs. Rapoport's rule in vascular plant and snail species in the Caucasus. Biol. J. Linn. Soc. 121, 753-763. ( 10.1093/biolinnean/blx027) [DOI] [Google Scholar]
- 27.Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115-155. ( 10.1016/S0065-2660(08)60048-6) [DOI] [Google Scholar]
- 28.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505-522. ( 10.1111/j.1558-5646.1985.tb00391.x) [DOI] [PubMed] [Google Scholar]
- 29.Alpert P, Simms EL. 2002. The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evol. Ecol. 16, 285-297. ( 10.1023/A:1019684612767) [DOI] [Google Scholar]
- 30.Lande R. 2014. Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J. Evol. Biol. 27, 866-875. ( 10.1111/jeb.12360) [DOI] [PubMed] [Google Scholar]
- 31.Gabriel W, Lynch M. 1992. The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 5, 41-59. ( 10.1046/j.1420-9101.1992.5010041.x) [DOI] [Google Scholar]
- 32.Gomulkiewicz R, Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390-411. ( 10.1111/j.1558-5646.1992.tb02047.x) [DOI] [PubMed] [Google Scholar]
- 33.Gabriel W, Luttbeg B, Sih A, Tollrian R. 2005. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 166, 339-353. ( 10.1086/432558) [DOI] [PubMed] [Google Scholar]
- 34.Kelly MW, Grosberg RK, Sanford E. 2013. Trade-offs, geography, and limits to thermal adaptation in a tide pool copepod. Am. Nat. 181, 846-854. ( 10.1086/670336) [DOI] [PubMed] [Google Scholar]
- 35.van Heerwaarden B, Kellermann V, Sgrò CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947-1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 36.Gilbert AL, Miles DB. 2019. Antagonistic responses of exposure to sublethal temperatures: adaptive phenotypic plasticity coincides with a reduction in organismal performance. Am. Nat. 194, 344-355. ( 10.1086/704208) [DOI] [PubMed] [Google Scholar]
- 37.Molina-Montenegro MA, Naya DE. 2012. Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species. PLoS ONE 7, e47620. ( 10.1371/journal.pone.0047620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonino MF, Moreno Azócar DL, Schulte JA, Abdala CS, Cruz FB. 2015. Thermal sensitivity of cold climate lizards and the importance of distributional ranges. Zoology 118, 281-290. ( 10.1016/j.zool.2015.03.001) [DOI] [PubMed] [Google Scholar]
- 39.Lovell JT, McKay JK. 2015. Ecological genetics of range size variation in Boechera spp. (Brassicaceae). Ecol. Evol. 5, 4962-4975. ( 10.1002/ece3.1746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aspinwall MJ, et al. 2019. Range size and growth temperature influence Eucalyptus species responses to an experimental heatwave. Glob. Change Biol. 25, 1665-1684. ( 10.1111/gcb.14590) [DOI] [PubMed] [Google Scholar]
- 41.Pohlman CL, Nicotra AB, Murray BR. 2005. Geographic range size, seedling ecophysiology and phenotypic plasticity in Australian Acacia species. J. Biogeogr. 32, 341-351. ( 10.1111/j.1365-2699.2004.01181.x) [DOI] [Google Scholar]
- 42.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80-S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 43.Dostal P, Fischer M, Chytry M, Prati D. 2017. No evidence for larger leaf trait plasticity in ecological generalists compared to specialists. J. Biogeogr. 44, 511-521. ( 10.1111/jbi.12881) [DOI] [Google Scholar]
- 44.Hirst MJ, Griffin PC, Sexton JP, Hoffmann AA. 2017. Testing the niche-breadth–range size hypothesis: habitat specialization vs. performance in Australian alpine daisies. Ecology 98, 2708-2724. ( 10.1002/ecy.1964) [DOI] [PubMed] [Google Scholar]
- 45.Mitchell RM, Wright JP, Ames GM. 2017. Intraspecific variability improves environmental matching, but does not increase ecological breadth along a wet-to-dry ecotone. Oikos 126, 988-995. ( 10.1111/oik.04001) [DOI] [Google Scholar]
- 46.Valladares F, Sanchez-Gomez D, Zavala MA. 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 94, 1103-1116. ( 10.1111/j.1365-2745.2006.01176.x) [DOI] [Google Scholar]
- 47.Moser D, Gygax A, Bäumier B, Wyler N, Palese R. 2002. Rote Liste der gefährdeten Farn- und Blütenpflanzen der Schweiz [The Red List of threatened ferns and flowering plants of Switzerland]. Bern, Switzerland: BUWAL. [In German.] [Google Scholar]
- 48.Patsiou TS, Walden N, Willi Y. 2021. What drives species’ distributions along elevational gradients? Macroecological and evolutionary insights from Brassicaceae of the central Alps. Global Ecol. Biogeogr. 30, 1030-1042. ( 10.1111/geb.13280) [DOI] [Google Scholar]
- 49.Zimmermann NE, Kienast F. 1999. Predictive mapping of alpine grasslands in Switzerland: species versus community approach. J. Veg. Sci. 10, 469-482. ( 10.2307/3237182) [DOI] [Google Scholar]
- 50.Hijmans, RJ, Van Etten J.. 2016. raster: Geographic data analysis and modeling. R package version, 2.8. See http://CRAN.R-project.org/package=raster.
- 51.Lauber K, Wagner G, Gygax A, Eggenberg S, Michel A. 1998. Flora helvetica. Bern, Switzerland: P. Haupt. [Google Scholar]
- 52.Wickham H, et al. 2019. Welcome to the tidyverse. J. Open Source Softw. 4, 1686. ( 10.21105/joss.01686) [DOI] [Google Scholar]
- 53.Larcher W, Wagner J. 1976. Temperaturgrenzen der CO2-Aufnahme und Temperaturresistenz der Blätter von Gebirgspflanzen im vegetationsaktiven Zustand [Temperature limits of CO2 uptake and temperature resistance of leaves of mountain plants in the vegetatively active state]. Oecol. Plant. 11, 361-374. [In German.] [Google Scholar]
- 54.Sutinen ML, Arora R, Wisniewski M, Ashworth E, Strimbeck R, Palta J. 2001. Mechanisms of frost survival and freeze-damage in nature. In Conifer cold hardiness (eds Bigras FJ, Colombo SJ), pp. 89-120. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 55.R Core Team. 2014. R, a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 56.Bürkner PC. 2017. Advanced Bayesian multilevel modeling with the R package brms. arXiv, 1705.11123 (stat.CO).
- 57.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129-131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 58.Paradis E, Schliep K. 2018. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526-528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 59.Kruschke J. 2014. Doing Bayesian data analysis. Amsterdam, The Netherlands: Academic Press, Elsevier. [Google Scholar]
- 60.Makowski D, Ben-Shachar MS, Lüdecke D.. 2019. bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 4, 1541. ( 10.21105/joss.01541) [DOI] [Google Scholar]
- 61.Smithson M, Verkuilen J. 2006. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11, 54-71. ( 10.1037/1082-989X.11.1.54) [DOI] [PubMed] [Google Scholar]
- 62.Keck F, Rimet F, Bouchez A, Franc A. 2016. phylosignal: An R package to measure, test, explore the phylogenetic signal. Ecol. Evol. 6, 2774-2780. ( 10.1002/ece3.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pintor AFV, Schwarzkopf L, Krockenberger AK. 2015. Rapoport's rule: do climatic variability gradients shape range extent? Ecol. Monogr. 85, 643-659. ( 10.1890/14-1510.1) [DOI] [Google Scholar]
- 64.Böhm M, Kemp R, Williams R, Davidson AD, Garcia A, McMillan KM, Bramhall HR, Collen B. 2017. Rapoport's rule and determinants of species range size in snakes. Divers. Distrib. 23, 1472-1481. ( 10.1111/ddi.12632) [DOI] [Google Scholar]
- 65.Feng J, Hu X, Wang J, Wang Y. 2016. Support for the elevational Rapoport's rule among seed plants in Nepal depends on biogeographical affinities and boundary effects. Ecol. Evol. 6, 7246-7252. ( 10.1002/ece3.2473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogwu MC, Takahashi K, Dong K, Song HK, Moroenyane I, Waldman B, Adams JM. 2019. Fungal elevational Rapoport pattern from a high mountain in Japan. Scient. Rep. 9, 6570. ( 10.1038/s41598-019-43025-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCain CM, Knight KB. 2013. Elevational Rapoport's rule is not pervasive on mountains. Glob. Ecol. Biogeogr. 22, 750-759. ( 10.1111/geb.12014) [DOI] [Google Scholar]
- 68.Linacre E. 1982. The effect of altitude on the daily range of temperature. J. Climatol. 2, 375-382. ( 10.1002/joc.3370020407) [DOI] [Google Scholar]
- 69.Chan WP, Chen IC, Colwell RK, Liu WC, Huang C, Shen SF. 2016. Seasonal and daily climate variation have opposite effects on species elevational range size. Science 351, 1437-1439. ( 10.1126/science.aab4119) [DOI] [PubMed] [Google Scholar]
- 70.Kingsolver JG, Watt WB. 1983. Thermoregulatory strategies in Colias butterflies: thermal stress and the limits to adaptation in temporally varying environments. Am. Nat. 121, 32-55. ( 10.1086/284038) [DOI] [Google Scholar]
- 71.Sheldon KS, Tewksbury JJ. 2014. The impact of seasonality in temperature on thermal tolerance and elevational range size. Ecology 95, 2134-2143. ( 10.1890/13-1703.1) [DOI] [PubMed] [Google Scholar]
- 72.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233-249. ( 10.1086/282487) [DOI] [Google Scholar]
- 73.Gilchrist GW. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252-270. ( 10.1086/285797) [DOI] [Google Scholar]
- 74.Van Damme R, Bauwens D, Castilla AM, Verheyen RF. 1989. Altitudinal variation of the thermal biology and running performance in the lizard Podarcis tiliguerta. Oecologia 80, 516-524. ( 10.1007/BF00380076) [DOI] [PubMed] [Google Scholar]
- 75.Shah AA, et al. 2017. Climate variability predicts thermal limits of aquatic insects across elevation and latitude. Funct. Ecol. 31, 2118-2127. ( 10.1111/1365-2435.12906) [DOI] [Google Scholar]
- 76.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183-189. ( 10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 77.Svanbäck R, Schluter D. 2012. Niche specialization influences adaptive phenotypic plasticity in the threespine stickleback. Am. Nat. 180, 50-59. ( 10.1086/666000) [DOI] [PubMed] [Google Scholar]
- 78.Wang SP, Althoff DM. 2019. Phenotypic plasticity facilitates initial colonization of a novel environment. Evolution 73, 303-316. ( 10.1111/evo.13676) [DOI] [PubMed] [Google Scholar]
- 79.Franzke A, Lysak MA, Al-Shehbaz IA, Koch MA, Mummenhoff K. 2011. Cabbage family affairs: the evolutionary history of Brassicaceae. Trends Plant Sci. 16, 108-116. ( 10.1016/j.tplants.2010.11.005) [DOI] [PubMed] [Google Scholar]
- 80.Dostal P, Fischer M, Prati D. 2016. Phenotypic plasticity is a negative, though weak, predictor of the commonness of 105 grassland species. Glob. Ecol. Biogeogr. 25, 464-474. ( 10.1111/geb.12429) [DOI] [Google Scholar]
- 81.Taylor DR, Aarssen LW. 1988. An interpretation of phenotypic plasticity in Agropyron repens (Graminae). Am. J. Bot. 75, 401-413. ( 10.1002/j.1537-2197.1988.tb13454.x) [DOI] [Google Scholar]
- 82.Levitt J. 1980. Chilling, freezing, high temperature stresses. Responses of plants to environmental stresses, vol. 1. New York, NY: Academic Press. [Google Scholar]
- 83.van Tienderen PH. 1991. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45, 1317-1331. ( 10.1111/j.1558-5646.1991.tb02638.x) [DOI] [PubMed] [Google Scholar]
- 84.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61-66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 85.Relyea RA, et al. 2018. Phylogenetic patterns of trait and trait plasticity evolution: insights from amphibian embryos. Evolution 72, 663-678. ( 10.1111/evo.13428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blomberg SP, Garland Z, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717-745. ( 10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 87.Maccagni A, Willi Y. 2022. Niche breadth and elevational range size: a comparative study on Middle-European Brassicaceae species. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Maccagni A, Willi Y. 2022. Niche breadth and elevational range size: a comparative study on Middle-European Brassicaceae species. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
All relevant data are within the paper and its electronic supplementary material, S1–S5 [87]. Trait values are deposited at try-db.org.