Graphical Abstract
INTRODUCTION
The unique properties of the entangled, antisymmetric nuclear spin state of dihydrogen, para-hydrogen (pH2), has intrigued physicists, chemists, and other scientists for almost a century. pH2 was used as a model system in the early days of quantum mechanics1 and is used for fueling rockets as well as combustion-free cars today. In the 1980s, pH2 was discovered as a convenient and potent source of spin order, allowing the enhancement of the signals of magnetic resonance (MR) by several orders of magnitude.2–4 In the advent of hyperpolarized (HP) contrast agents (CA) for biomedical MR imaging (MRI) that followed, pH2-based hyperpolarization methods played an important role in the acquisition of the first HP 13C in vivo images (Fig. 1).5,6
Figure 1.
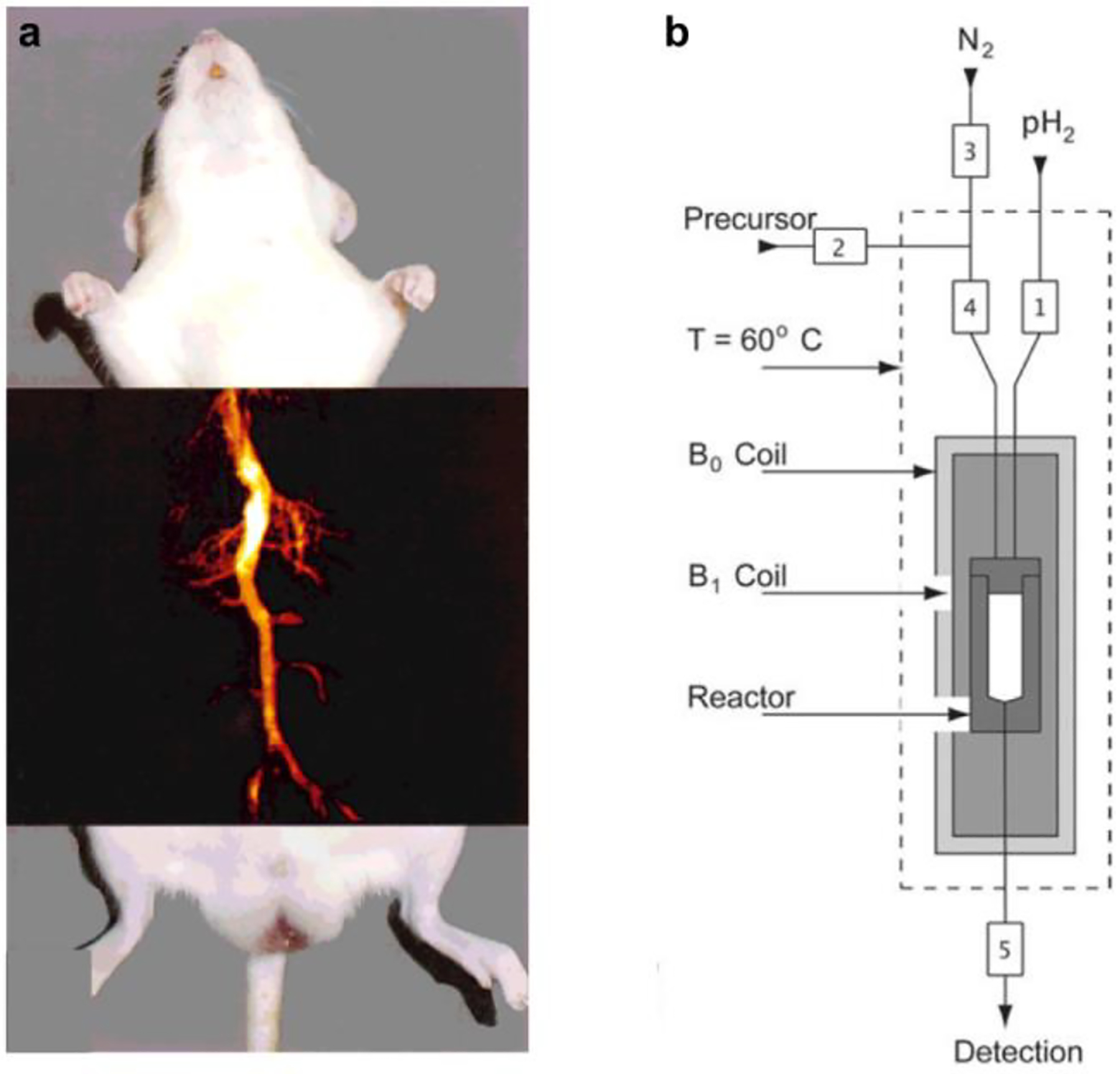
First hyperpolarized 13C in vivo MRI ever published (a). The contrast agent, hydroxyethyl [1-13C]propionate-d3 (HEP), was produced using a prototype commercial millitesla polarizer (Amersham Biosciences, healthcare company), similar to the one shown schematically in (b). Broadly speaking, the polarizers consisted of a unit handling the fluids, the actual hyperpolarization (spin-order transfer), and the coordination of the entire process (numbers 1 – 5 represent valves). Images reproduced from Parahydrogen-Induced Polarization in Imaging: Subsecond 13C Angiography, Golman, K.; Axelsson, O.; Johannesson, H.; Mansson, S.; Olofsson, C.; Petersson, J. S. Magn. Reson. Med., Vol. 46, Issue 1 (ref 5). Copyright 2001 Wiley (a), and reprinted by permission from Springer: Magn Reson Mater Phy, PASADENA Hyperpolarization of 13C Biomolecules: Equipment Design and Installation, Hövener, J.-B.; Chekmenev, E. Y.; Harris, K. C.; Perman, W. H.; Robertson, L. W.; Ross, B. D.; Bhattacharya, P., Vol. 22 (ref 13). Copyright Springer 2009. (b).
Ever since, pH2 has proven to be highly valuable for analytical investigations and fundamental research, e.g., in analytical and catalytic chemistry or in the physics of singlet spin states.7–10
pH2 is produced fast and stored easily. As one of four (nuclear) spin states of dihydrogen, 25 % of H2 at room temperature are pH2, while the other 75 % are ortho-Hydrogen (oH2, following the Boltzmann distribution). At lower temperatures, however, the para-fraction becomes more and more enriched until at approx. 25 K, 100 % pH2 is obtained. While the para-enrichment is fast using appropriate catalyst, pH2 can be stored for hours to days at room temperature without significant loss if the catalyst is absent. These unique properties make pH2 a spin order that can be produced easily, stored conveniently (in a pressurized bottle) and used on demand, e.g., for producing hyperpolarized CAs or observing chemical reactions with enhanced sensitivity.
When it comes to clinical applications, however, dissolution dynamic nuclear polarization (dDNP) evolved faster than pH2–based HP methods.11,12 The reasons for this may be that a) at first, only few biologically relevant CAs were available (because of limited chemistry at that time), b) that mastering the complex process of pH2 induced polarization (PHIP) was not straight forward (involving quantum mechanics, chemical reactions, and magnetic resonance tailored to each individual CA) and that c), no pH2-polarizers were commercially available, while there were at least three different generations for dDNP.
While pH2 is easily produced in large quantities, at low cost and with a shelf life of days (depending on the storing conditions), there are some hurdles to overcome before a CA is ready for administration. For one, the pH2 spin order per se is MR invisible (total spin of 0!) and well hidden inside the dihydrogen molecule. To obtain a hyperpolarized CA from pH2, typically, the following steps have to be taken: a), bringing pH2 and the target into contact (by catalytic addition or reversible exchange); b), to transfer or transform the pH2-derived spin order into a desired form; and c), the purification and quality assurance (QA) prior to an in vivo application. These steps usually involve a chemical reaction at elevated temperatures, pressures, sometimes in aggressive media or extreme pH – synchronized with quantum mechanical spin order transfer (SOT) mediated by evolution at constant or varying magnetic fields and radiofrequency (RF) pulses. To realize this process, various devices have been ingeniously devised; however, a single, unified design has not yet emerged. The lack of such a device may be attributed to the fact that the power and versatility of pH2 has resulted in quite a few different methods – the magnetic fields alone vary by a factor of 109 (from nanotesla to tesla). Even today, pH2 hyperpolarization methods keep evolving at a fast pace; among the ground-breaking advances, SABRE,14 gases,15,16 continuos HP,17–19 PHIP-SAH,20 RASER,21–23 precipitation,24 and relay methods25,26 are only examples from the last decade. These methods require specific experimental conditions and ultimately dedicated instrumentation, which differs from method to method.
For a standardized, clinical application of a specific contrast agent, however, a consolidated setup is required, that can be certified and approved, provides reliable polarization and quality assurance. In this respect, much can be learned from SEOP27 and DNP11,12 with respect to polarizers and regulatory approval.
Here, we review the different instrumentations for pH2 hyperpolarization, with an emphasis on biomedical application. To keep this review concise, we focus on setups for hydrogenative pH2-based hyperpolarization alone and the most recent literature (~last 5 years); still, we refer to pioneering and game-changing developments whenever appropriate, and when other methods (SABRE, DNP, SEOP, etc.) show similar instrumentational aspects. Dedicated reviews on SABRE-related instrumentation, spin-order transfer, and pH2 production are expected to be published elsewhere.
ANALYSIS OF PH2 POLARIZERS
The requirements for a biomedical polarizer may be defined as to i) provide a clean, ii) aqueous solution of iii) highly polarized, iv) appropriately concentrated agents at v) physiological conditions that is preferably produced in a iv) good-manufacturing process (GMP). To make such a contrast agent, the role of hardware may be categorized as follows:
making the pH2 spin order available to the target molecule (hydrogenation);
transferring the pH2-derived spin order into the desired spin hyperpolarization (typically longitudinal X-nuclear magnetization);
purification of the solution and assuring quality.
We will elaborate on these steps in the following.
Parahydrogen addition
Before the addition, the lifetime of gaseous28–30 or dissolved31–33 pH2 is typically long (days or many minutes, respectively). However, once bound to the catalyst34 or the precursor,9,35–39 the lifetime of the spin order is drastically reduced because the added protons, referred to as I1 and I2, interact with the environment and the rest of the (now larger) spin system. Thus, the hydrogenation should be conducted as fast as possible to reduce relaxation losses. The hydrogenation kinetics are typically affected by a multitude of coupled reaction parameters, like temperature, pH2-availability, solvent, catalyst, precursor molecule or pH value. While the catalyst is needed for the addition, it may cause relaxation at the same time and needs to be removed before an injection if it is harmful.
The starting point for the SOT, i.e. the density matrix after the hydrogenation, is strongly dependent on the coupling between I1 and I2. It is determined by the molecular structure and the external static magnetic field B0. If the spins are “strongly coupled” (that is, if their mutual J-coupling JI1I2 is much larger than the difference of their Larmor precession frequencies δv (δv « JI1I2)), the eigenstates of the two-spin system are essentially the singlet-triplet (S-T) basis states. In this case, the “singlet spin order” I1∙I2 = I1xI2x + I1yI2y + I1zI2z (also known as J-order) is usually the starting point for the SOT. In the opposite case (δv » JI1I2), the spins are referred to as weakly coupled. Then, I1ZI2Z spin order is typically the starting point for SOT after the off-diagonal elements of the density matrix were averaged away during the hydrogenation.40 However, I1∙I2 order can be preserved in weakly coupled systems by applying sufficiently strong 1H decoupling,41,42 or can be encountered in effectively instantaneous reactions as in photo-PHIP experiments,43,44 where reactions with pH2 happen within a few microseconds. The exact form of the spin order in the intermediate coupling regime, including the effect from singlet-triplet (S-T) mixing at the hydrogenation catalysts, was described by Bowers, Natterer and others.39,45–48 Such S-T mixing takes place in high and low fields and is one of the main reasons for reduced hyperpolarization yield.
In so-called PASADENA experiments (pH2 and synthesis allows dramatically enhanced nuclear alignment), hydrogenation and SOT (or direct proton detection) take place at the same magnetic field.3 Another experimental scheme often applied is referred to as ALTADENA (adiabatic longitudinal transport after dissociation engenders net alignment).49 The latter typically features hydrogenation at lower fields (with S-T eigenbasis) followed by an adiabatic (slow) increase of B0 into the weakly-coupled regime.50 This results in population of either the |αβ〉 or the |βα〉 state of the high-field Zeeman eigenbasis, where α is the spin up, and β is the spin down state in the combined spin angular momentum state of both nuclei.
Spin-order transfer
As the literature on SOT may fill an entire review article alone,51 we are focusing on the parts relevant for the instrumentation only. The available spin order after hydrogenation, the spin system, its eigenstates and its interactions determine the most-effective SOT strategy. While the molecular spin system as such is usually fixed (with workarounds, e.g. PHIP-SAH,20 PHIP-X26), the interactions can be tailored to some extent by varying the (static) external magnetic field (BSOT) or by applying B1 fields over a period of time.52 Likewise, the spin system can be affected, to some degree, by isotope labeling and reaction parameters such as temperature and pH. Deuteration is a convenient way to reduce relaxation and simplify the spin system, e.g., to an effective 3-spin-½ system in RF-pulsed SOT.42,53,54
Roughly speaking, a SOT can be achieved by:
Evolution at one static field (sometimes referred to as “spontaneous” transfer),
Evolution at different fields – magnetic field cycling (MFC-SOT)20,24,55,56,
Evolution plus specific manipulations by RF pulses (RF-SOT)9,40,42,51,57–64.
Understanding the SOT requires profound knowledge of the underlying quantum mechanics. While analytical equations can be derived for simple systems, numerical simulations are usually required to determine the optimal parameters of more complex or realistic systems. These simulations can be implemented in any programming environment capable of matrix algebra. Using or building on existing (open source) packages may be convenient.65–68
Realizing the different variants of SOT usually requires a magnet (superconducting, resistive, permanent; sometimes shims), sometimes a multi-layer mu-metal shield (to reach fields BSOT in the nanotesla or microtesla range), and an NMR unit to excite and receive MR signal.
Purification and Quality Assurance
While the addition of pH2 is performed by catalysis at sometimes harsh conditions, a physiological solution devoid of the catalyst is needed for in vivo administration. Various approaches have been described to achieve this goal, including:
Filtering homogeneous catalysts,69
Using immobilized catalysts that remain in the polarizer,70–72
Heterogeneous catalysts that facilitate filtration,18,71,73–78
Different agents will generally require individual approaches as their chemical properties vary significantly. A QA module similar to that used for DNP will have to encompass (at least) purity, pH, a low bioburden and temperature; no such device has emerged yet. For all approaches, time is of the essence, as precious signal enhancement is rapidly lost once the agent is hyperpolarized.
Components and capabilities of a polarizer
Orchestrating these steps requires a dedicated unit, often referred to as a “polarizer”. Some have been described, with varying goals, properties, and capabilities, e.g., SOT schemes, pressures, temperatures, in situ detection, automation, dosing, etc.13,24,36,38,42,55,83–90 For convenience, such a polarizer may be separated in different units, some of which were described in literature:
A fluid unit, handling the gases and liquids. It is typically composed of electromagnetic, manual, or pneumatic valves and tubes, borrowed, e.g., from high performance liquid chromatography (HPLC), as well as a custom-made reaction chamber or a (high-pressure) NMR tube. Inert materials, pressure and temperature resistance are important factors, as is the option to clean or sterilize.91
An NMR unit, taking care of the SOT either by applying a constant magnetic field, a defined field cycle, or RF pulses. Low-field detection facilitates flip angle calibration and quality assurance.91–93
A control unit: a software controlling a digital-to-analog converter (DAC) and an analog-to-digital converter (ADC), e.g., for switching valves, B0 control, temperature readings, NMR. These needs have been addressed by using the hardware of the NMR / MRI,87,88,94 by PC-controlled digital-acquisition boards (DAQ) with85,86 and without13,42,55,83,89,95 an additional NMR spectrometer. As a minimum, the control software will have to accommodate easy access to the (sometimes overlapping) timings of each step in the polarization procedure, and may extend to acquiring NMR signal in situ to facilitate calibrations and improve polarization (e.g., Paravision, LabVIEW,85–87,92,96 MATLAB38,95).
REVIEW OF PUBLISHED INSTRUMENTS
This section comprises a brief description of instrumentation to produce pH2, setups for producing HP solutions, HP of gases, purification methods and translation. The section on setups to produce HP liquids is structured by the magnetic field BSOT, where the SOT takes place.
Parahydrogen generators
As a manifestation of the generalized Pauli exclusion principle, hydrogen molecules exist as two different nuclear spin isomers: the triplet spin states, called orthohydrogen (oH2), and the singlet spin state, called pH2. The energy separation between the ground states of pH2 and oH2 is 170.6 K83. Hence, in thermal equilibrium at low temperatures (below 25 K), almost all hydrogen molecules are in the singlet state, i.e nearly pure pH2. Note that the temperature determines the achievable pH2-fraction fpH2.
The production of pH2 is straightforward: letting hydrogen gas flow over an ortho-para conversion catalyst at low temperatures results in enriched pH2. Common conversion catalysts are granular materials with high surface area, such as Fe(OH)O,28,29,97 nickel sulfate,98 or chromic oxide (CrO3) supported on silica gel.99 Activated charcoal has been used as an ortho-para conversion catalyst in early works, but the efficiency appears to be lower than for the ferromagnetic oxide materials, especially at high flow rates. The catalytic effect originates from (ferro- or para-) magnetic properties, which induce highly inhomogeneous local magnetic fields that accelerate ortho-para conversion.36
The published pH2 generator designs offer different properties with respect to cost, pH2-fraction, pressure, flow rate and ease of use. Aside from commercial products (Bruker BPHG 90, XeUS Technologies LTD, HyperSpin Scientific UG, Advanced Research Systems, IDB Budzylek), many designs for home-built generators have arisen over the last decades. In particular, three primary coolant technologies dominate: single-stage or dual-stage closed-cycle Helium cryostats operating at 13.5 to 40 K,29,36,100–104 liquid helium dewars at 14 to 30 K98,99,105,106 and liquid nitrogen dewars at 77 K.18,97,107–111 Consequently, a dual stage or liquid-helium-based pH2 generator can practically reach enrichments approaching 100% and a liquid-nitrogen-based pH2 generator enrichments of up to 52 %, respectively. In a liquid N2 generator pumped below ambient pressure, the enrichment can be increased by lowering the temperature further, e.g., to 63 K at 21 mbar allowing a pH2 fraction of ≈ 65 %.112
The quantification of the pH2 enrichment can be done optically (e.g., Raman), or by NMR spectroscopy. Raman spectroscopy is about 500 times faster than NMR and does not require a reference sample since it detects both oH2 and pH2 directly.110 However, NMR spectroscopy is generally the quantification method of choice since it is already available in the MR labs where PHIP experiments are carried out. The details of quantification have been reviewed thoroughly previously.113
SOT at tesla fields
SOT conditions.
In PASADENA experiments, the hydrogenation reaction, observation, and SOT take place at the high magnetic field of an NMR or MRI system (≈ Tesla). If pH2 is added at chemically nonequivalent sites, I1 and I2 typically become weakly coupled (Figure 2a). The strongly coupled case is typically only present, if δv ≈ 0.39,114
Figure 2. PHIP setups for SOT at constant magnetic fields > 1 T,
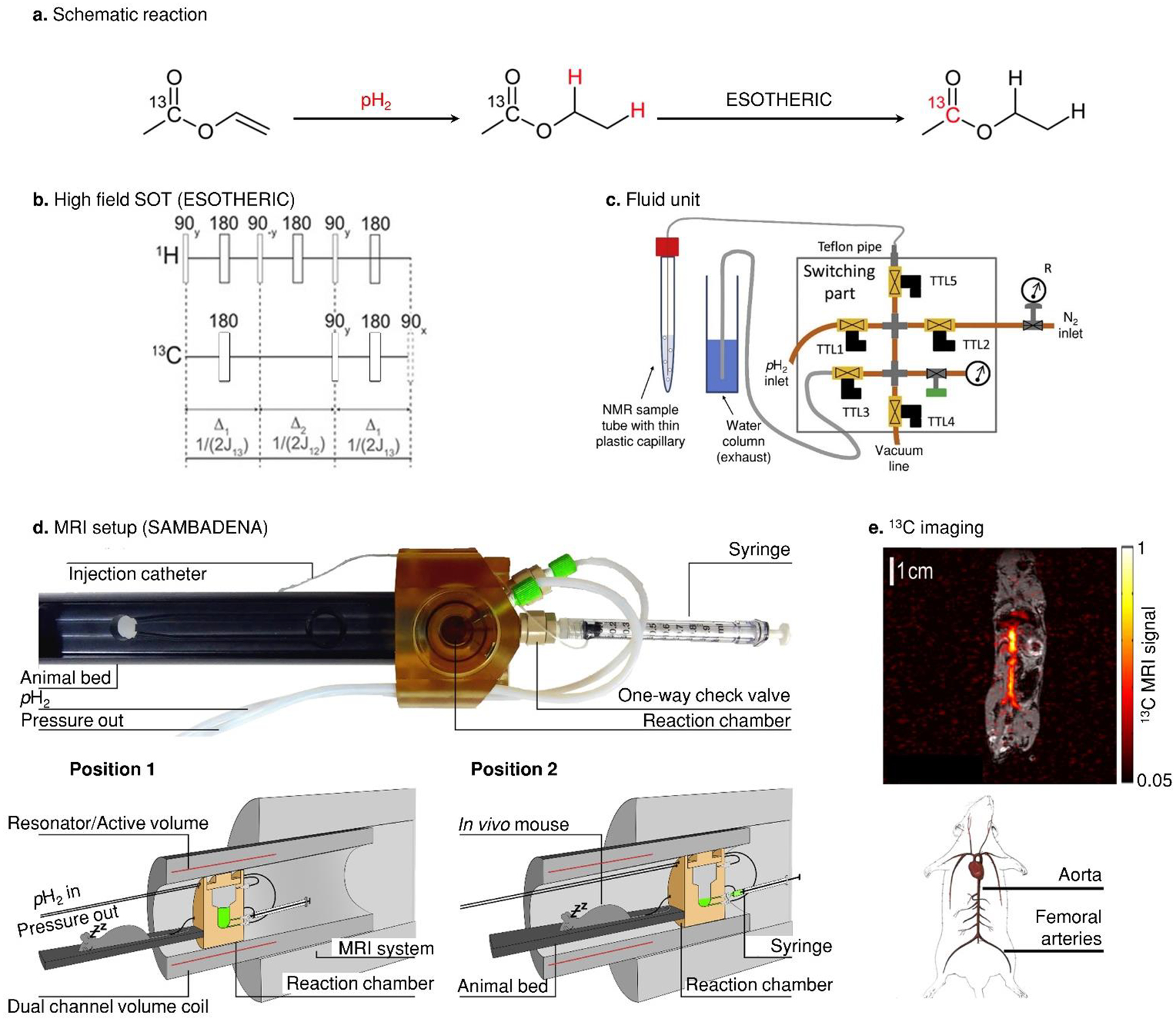
where spin systems are typically in the weak-coupling regime. Schematic view of the hyperpolarization process at high field, where ethyl acetate is polarized by adding pH2 (a) and spin order transfer with ESOTHERIC (b) or SLIC. The fluidics are usually handled by a unit consisting of switchable magnetic valves and flow regulation for guiding gases or to apply vacuum to a tube or reactor (c). For MRI systems, dedicated reaction chambers were combined with animal beds allowing animal monitoring, anesthesia, life support and fast administration of the HP contrast agent (d). As an example, sub-second in vivo 13C angiography of a mouse was demonstrated only seconds after the polarization (e), while compatible temperature, pressure and sterility was assured. Figure 2a reproduced from Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by Utilizing Para-Hydrogen, Korchak, S.; Yang, S.; Mamone, S.; Glöggler, S., ChemistryOpen, Vol. 7, Issue 5 (ref 62). Copyright 2018 Wiley. Figure 2b reproduced from Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A Para-Hydrogen Approach, Korchak, S.; Mamone, S.; Glöggler, S. ChemistryOpen, Vol. 7, Issue 9 (ref 127). Copyright 2018 Wiley. Figure 2c reprinted from J. Magn. Reson., Vol. 285 (Supplement C), Kiryutin, A. S.; Sauer, G.; Hadjiali, S.; Yurkovskaya, A. V.; Breitzke, H.; Buntkowsky, G. A Highly Versatile Automatized Setup for Quantitative Measurements of PHIP Enhancements, pp. 26 – 36 (ref 94). Copyright 2017, with permission from Elsevier. Figures 2d and 2e reproduced from In Vivo 13C-MRI Using SAMBADENA, Schmidt, A. B.; Berner, S.; Braig, M.; Zimmermann, M.; Hennig, J.; Elverfeldt, D. von; Hövener, J.-B. PLOS ONE, Vol. 13, Issue 7 (ref 90). Copyright 2018 Public Library of Science.
For the former case, PHIP 1H signal can be observed as anti-phase peaks by a simple 45° excitation. Alternatively, spin order can be converted into in-phase magnetization using RF pulse sequences115–117 which also enables detection in inhomogeneous B0 fields.118,119 However, the first in-phase PHIP 1H spectra were obtained by Pravica et al. by field cycling (ALTADENA)49 – an approach that has also enabled polarization transfer to 19F.50
Several techniques have been published to convert I1zI2z into observable magnetization of X-nuclei (often 13C). Here, most RF-SOT sequences are adaptations of the insensitive nuclei enhanced by polarization transfer (INEPT) sequence that considers initial I1zI2z spin order, Figure 2b.59,120 After the early work from Haake, Natterer and Bargon, who introduced the pH2 INEPT+ (phINEPT+) sequence,60 l-PHINEPT+,51 selective excitation of polarization using PASADENA (SEPP) INEPT,121 selective-90 (s90) phINEPT54,122, and efficient SOT to heteronuclei via relayed INEPT chains (ESOTHERIC, Fig. 2b)62,82 sequences were suggested. Note that depending on the spin system, and neglecting relaxation, all RF-SOT schemes can yield ~100 % 13C-polarization, in principle, except phINEPT+, which has a theoretical maximum of ~50 % because of the initial 45° 1H pulse.38,123 If the initial spin order is I1∙I2, different SOT schemes are required, e.g. Goldman’s sequence42 for succinate obtained by reacting a fumarate precursor molecule.39,48 Additional RF-SOT techniques for strongly-coupled protons will be introduced in the next section (SOT at millitesla fields). Adiabatic passages through level anti crossings (also referred to as avoided crossings) were shown to be efficient for the transfer of singlet pH2 spin order to 13C polarization.124–126
Published setups:
Various setups for performing PHIP in NMR94,122 and MRI systems have been described.38,90 Such implementations offer the advantage that parts of the sophisticated MR machines, including NMR excitation and acquisition and highly homogeneous field over a large volume, can be employed in the hyperpolarization process. Typically, the NMR or MRI system offers TTL outputs that allow control of the fluid path directly from within the pulse program.
To perform the hyperpolarization in an NMR system, Kiryutin et al. introduced a setup consisting of several valves for supplying gases or liquids, e.g., a cleaving agent to convert a precursor into the desired metabolite.94 Using standard 5 mm or 10 mm NMR tubes for hydrogenation, SOT, detection, and purification, the system can be implemented in all standard and benchtop NMR spectrometers and has been done so by many labs (Figure 2c).62,119,122,127,128 For in vivo imaging, the HP contrast agent has to be transferred to an imaging system.
Performing hyperpolarization within the bore of an MRI system allows in vivo imaging only seconds after the hydrogenation (SAMBADENA). Here, the hydrogenation takes place in a reactor enclosed in a dual tune, transmit-receive 1H/13C volume coil, equipped with a local receive coil, for RF-SOT and in vivo MRI.38 The reactor was mounted on the animal bed (with animal warming, vital sign monitoring, anesthesia) such that the contrast agent was delivered directly into a syringe for injection at high field, Figure 2e.90 In vivo imaging of a sterile solution of hydroxyethyl [1-13C]propionate-d3 within 15 s after hyperpolarization was demonstrated (Figure 2f), although no purification (i.e. catalyst filtering) was performed.90
Published agents:
High 13C polarizations for highly concentrated molecules have been demonstrated with phINEPT+ and ESOTHERIC sequences and SAMBADENA.38,119,127 Promising PHIP agents that were produced at high field with polarization above 10% are tetrafluoropropyl [1-13C]propionate-d3 (TFPP),129 [1-13C]succinate-d2,39,48 and [2-13C]pyruvate.130 The latter was achieved using PHIP-SAH, ESOTHERIC and subsequent cleavage of the side arm of cinnamyl [2-13C]pyruvate. Remarkable polarizations were also achieved for other promising PHIP-SAH molecules, namely ethyl [1-13C]acetate119,127 and cinnamyl [1-13C]pyruvate.62,82
Challenges:
The main challenges of in situ polarizer setups such as SAMBADENA are due to the limited space within the bore of the MRI or NMR magnet: accommodating production, purification, QA, and the administration within a small volume is not an easy task. RF-SOT sequences - theoretically - achieve ≈ 100 % 13C-polarization, but only in fully 2H-labeled molecules as additional J-couplings will interfere (neglecting relaxation).38,54,122,127,128 In NMR spectrometers, radiation damping can disturb the SOT, while the limited RF power is a serious concern in MRI setups119,131–133 because of the large excitation bandwidth needed.134 Moreover, translational motion of the molecules in the inhomogeneous field of the relatively large reaction chamber in an MRI during SOT can reduce the polarization.135
SOT at millitesla fields
SOT conditions:
The characteristic feature of SOT in the millitesla range is the strongly-coupled regime (δv « JI1I2). Typically, the pairwise pH2 addition takes place under proton decoupling at BSOT, in part to reduce relaxation, but also to preserve the I1∙I2 spin order (Figure 3f and Figure 3h). After the decoupling, RF-SOT sequences are used to transfer the spin order to a 13C nucleus. A wide range of efficient pulse sequences have been reported51,57,58 to accomplish polarization transfer in millitesla field range including the pioneering sequence developed by Goldman and co-workers (Figure 3e).42,83
Figure 3. Setups and examples for PHIP at millitesla fields:
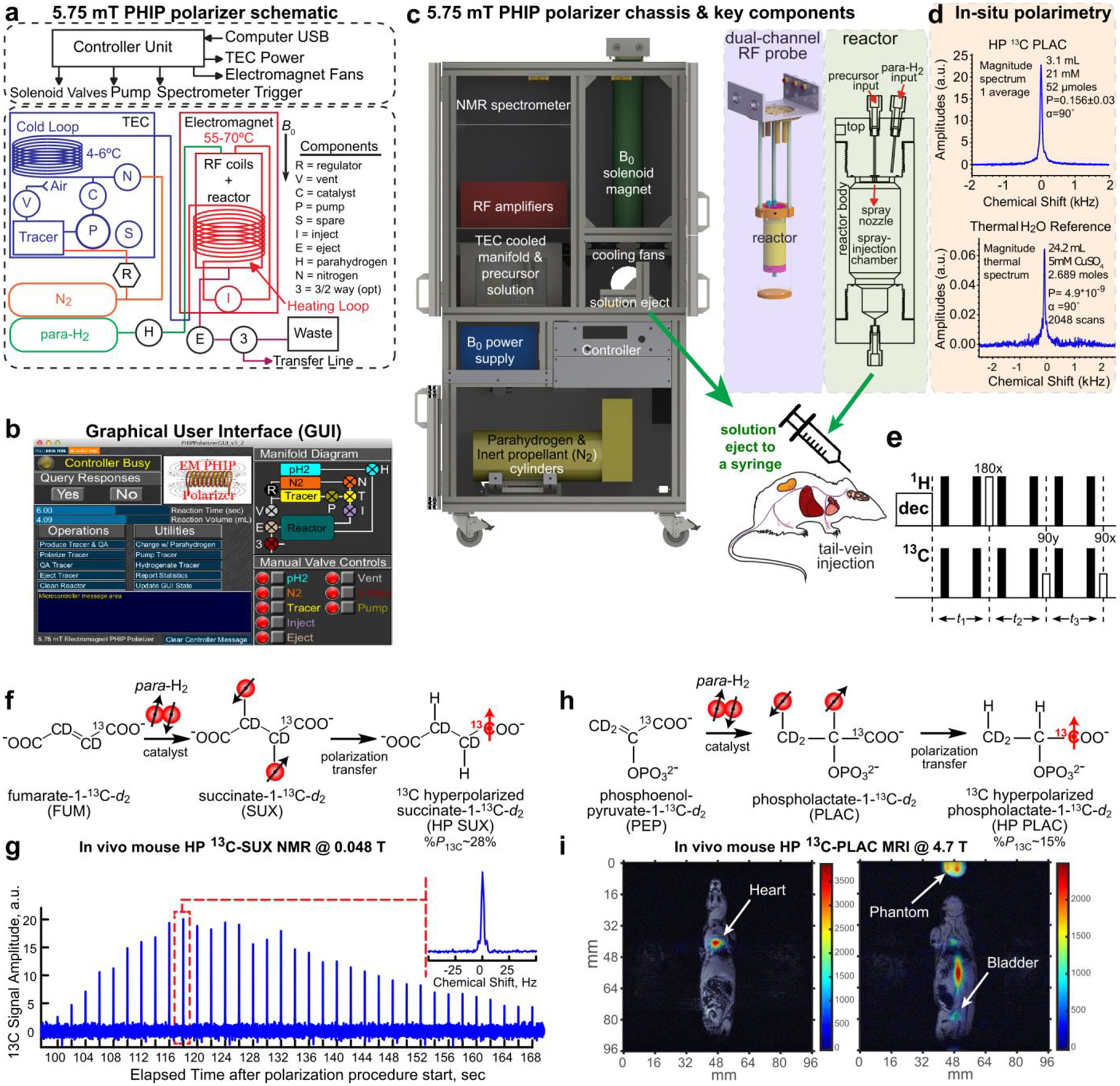
Overall schematic (a), graphical user interface (b) and rendering (c) of a 5.75 mT automated, pre-clinical, open-source PHIP polarizer. A dual-channel, 1H/13C RF coil was placed inside a B0 solenoid electromagnet (c, left), and a spray-injection reactor was nested inside the coil. In situ NMR detection at ~62 kHz enabled RF calibration and quantification of 13C polarization with respect to thermally polarized water (d). During the hydrogenation reaction of a few seconds, 1H-decoupling was applied to preserve I·S spin order, and before the polarization was transferred to 13C using the sequence proposed by Goldman et al. (e) – note, that there are only three ‘effective’ pulses (white) used while the others (gray) are for refocusing of the Zeeman evolution only. Reaction scheme (f) and in vivo 13C-NMR (g) of contrast agent SUX detected at low magnetic fields (~0.048 T). Reaction scheme (h) and in vivo 13C MRI imaging (i) of contrast agent PLAC at 4.7 T: 13C-MRI (color) was acquired approximately 5 – 10 s after the injection of ~0.2 mL, ~30 mM hyperpolarized PLAC into the tail vein of a healthy nude mouse (prior tumor implantation) and overlaid on representative 1H images (gray scale, 13C: GRE, 3 × 3 mm2 in-plane resolution, 6 mm slice thickness, FOV = 96 × 96 mm2). Figures 3a–d, 3h and 3i reproduced from Coffey, A. M.; Shchepin, R. V.; Truong, M. L.; Wilkens, K.; Pham, W.; Chekmenev, E. Y. Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem. 2016, 88, 8279–8288 (ref. 86). Copyright 2016 American Chemical Society. Copyright 2006, with permission from Elsevier. Figures 3f and 3g reprinted from J. Magn. Reson., Vol. 281 (Supplement C), Coffey, A. M.; Feldman, M. A.; Shchepin, R. V.; Barskiy, D. A.; Truong, M. L.; Pham, W.; Chekmenev, E. Y. High-Resolution Hyperpolarized in Vivo Metabolic 13C Spectroscopy at Low Magnetic Field (48.7mT) Following Murine Tail-Vein Injection, pp. 246 – 252 (ref 147). Copyright 2017, with permission from Elsevier.
Published setups:
As the (13C) hyperpolarization can persist for several minutes, the contrast agent can be transferred from the polarizer to the detector without overwhelming loss, e.g., for polarimetry (i.e., measuring the degree of induced polarization) or ultimate application (i.e., in vivo imaging). As a result, several millitesla setups with the main static magnetic field ranging from 1.7 mT13,89 to 50 mT were set up and reported.85
The use of the low magnetic field offers certain advantages: (1) the field can be generated by inexpensive electromagnets, resulting in an overall cost-efficient, portable setup;92 (2) susceptibility effects are low, and B0 inhomogeneities can be compensated for by magnet design,92 shims, and sufficiently strong RF pulses. Utilizing an electromagnet has allowed SOT over large reaction volumes – up to 100 mL were reported13 – allowing the production of clinical-scale doses of 13C-hyperpolarized contrast agents.83
These translational advantages were likely decisive for Amersham Biosciences, a healthcare company, to choose this approach for the first commercial prototype – in vivo feasibility studies using the Amersham polarizer have been reported extensively.42,69,83,136 The overall design of the Amersham polarizer was never reported in detail,42,83,84,137 but several closely related PHIP millitesla polarizer designs were subsequently reported.13,36,85–87,89,91–93,96
As described above, the lifetime of the pH2-derived 1H spin order is of the order of seconds and hence, for effective 13C HP, the hydrogenation needs to be completed quickly.36,138 In practice, fast hydrogenation is achieved through the use of a reactor pressurized to ca. 10 bar with pH2 followed by a spray injection of a hot stream of precursor solution into the chamber.13,89 For biomedical applications, the reaction is performed in aqueous media employing water-soluble PHIP catalyst at elevated temperature (70–95 °C).13,36,86,89,137 As a result, the entire bolus of the precursor molecule can be reacted quickly (in a few seconds), i.e., on a time scale that is faster or similar to the decay of the I1∙I2 spin order.
The experimental hardware for the hyperpolarization process is relatively similar among reported millitesla PHIP polarizers (Fig. 1). By definition, such system employs a B0 magnet operating in the millitesla range with typical field strength of 2 – 9 mT13,36,86,89 although the use of higher field (48 mT) permanent magnets have been reported.85 A large-volume (ca. 300 mL) dual-channel RF probe is placed inside the magnet to deliver 1H and 13C RF pulses for the SOT sequence. The high-pressure reactor is nested inside the dual-channel RF coil – Figure 3c shows an example of such an electromagnet-based polarizer. A millitesla-PHIP polarizer is typically connected to (or contains) cylinders of compressed ultrahigh-purity (>99.999% or 5.0) pH2 and inert propellant gas (N2 or Ar). A series of high-pressure valves and tubes form a manifold (Fig. 3a) to fill the reaction chamber with pH2.139 This step is followed by the injection of hot precursor solution into the chamber through a nozzle using an inert propellant gas. Various setups were designed to inject a defined amount of the precursor solution into the reaction chamber, and to eject the HP contrast agent into a receiver ready for transfer and in vivo MRI. Although specialized heaters were employed to control the reaction temperature, the design shown in Figure 3c employs heating generated by a >100 W electromagnet.
Several approaches were developed to orchestrate the interplay of actuating multiple valves and playing out the RF-SOT, usually including software and a hardware interface (LabVIEW-,13,36,95,96 Arduino-,86 Matlab,95 and NMR spectrometer based87). A typical graphical user interface (GUI) allows setting various parameters (reaction time, precursor dose), choosing the SOT sequence, actuating individual valves, and executing various polarization or maintenance routines and sometimes performing NMR (Figure 3b, open-source Arduino-based controller software).86 All published controller designs have their own merits.
To achieve high and reliable polarization through robust device operation, the accurate and precise application of the RF-SOT is critical.51 Optimal RF-SOT performance requires calibrated B1 power and transmission resonance frequencies for both RF channels in particular. Performing these calibrations in situ by detecting NMR signal in the reaction chamber using a transmit-receive polarizer design drastically facilitates these procedures. In situ calibrations, however, are not necessarily needed to produce the HP agents, because an external (i.e., ex situ) NMR spectrometer, MRI scanner or a low-field polarimetry station may be employed for the calibrations,89 and for probing the achieved 13C polarization level.69 This so-called transmit-only design is less complex and typically result in a lower device cost as no extra NMR receive hardware is needed. The transmit RF pulses can be generated using simple waveform generators (e.g., NI, Austin, TX, USA), consumer-grade RF amplifiers (WRAT, Onkyo, Osaka, Japan), and untuned RF coils with low Q factor, mitigating radiation damping issues noted in the above section.
The hyperpolarizers employing transmit-receive design were realized by using a commercial, low-frequency, dual-channel NMR spectrometer and dual tune transmit-receive coil (e.g., Kea2, Magritek).86,87 In another approach, a geometrically decoupled, single or dual tuned receive coil was added to the untuned transmit coil, using the same low-cost waveform generators, amplifiers and ADC/DAC hardware as for the transmit-only design described above.92,95
These transmit-receive designs allow using the signal of thermally polarized water to calibrate B0 and B1.86,87,92,95,140 As the polarization of HP samples is independent of the detection field, a high signal-to-noise ratio (SNR) spectrum was readily obtained in in-situ low-field polarimetry as shown for the HP contrast agent [1-13C]phospholactate-d2 (PLAC) at ~62 kHz (Figure 3d).
While transmit-receive designs are more complex and can be more expensive (by ca. $30,000 for the Kea2), calibration is relatively straightforward, and good reliability and reproducibility of the hyperpolarization yield is achieved.
The efficiency of SOT can be improved further by reducing the complexity of the spin system. For example, deuteration was used to simplify HEP, SUC and PLAC to an effective 3-spin-1/2 system (two nascent protons and one 13C nucleus), which increased the final 13C polarization for PLAC to 15% versus 1% for the fully protonated variant, Figure 3h.53,141 While deuterium (and phosphorus-31) nuclei possess a spin, they are not excited by the RF and hence not effectively involved in the SOT.42 In favorable cases, P13C of more than 20 % was achieved with millitesla polarizers,36,42,137 e.g. P13C = 28% for [1-13C]succinate-d2 (SUX, Figure 3f,). Despite the enormous signal enhancement provided by hyperpolarization, metabolic imaging with HP contrast agents usually results in low SNR images. Thus, it is not surprising that only deuterated precursors with high P13C were translated to in vivo studies using millitesla PHIP polarizers so far.13,69,83,86,89,142–148
To date, millitesla-polarizers were employed for in vivo 13C MRI or MRS with SUX147,149, SUX esters145, HEP,5,69,150 and TFPP,143 and PLAC86, Figures 3g,i. Thorough reviews covering the in vivo applications can be found elsewhere.113,151
Two interesting developments, which broaden the scope of polarizable molecules drastically, are PHIP-SAH20 and PHIP-X.26 The experimental realizations of these techniques will be described in more detail below, but the millitesla polarizers described here appear to be well positioned for these emerging protocols.
Challenges:
The millitesla PHIP polarizers have been shown to be successful devices for the efficient production of HP 13C contrast agents in aqueous media with high P13C exceeding 25% for in vivo applications. Using PHIP-SAH molecules may dramatically enlarge the pool of agents, e.g., to 13C-labeled pyruvate. Despite this success, however, all in vivo translated precursors so far require deuterated substrates in addition to 13C labeling. While deuteration offers the benefit of a prolonged polarization lifetime, it also increases the cost and the complexity of synthesis of the precursors, which is a clear drawback compared to MFC-SOT. No millitesla polarizer incorporating a purification unit was presented so far.
SOT at ultralow fields (ULF)
SOT conditions:
At ultra-low fields (micro- or nanotesla), the frequency differences between heteronuclei and protons (γ1H-γX) are reduced to be in the order of the J-couplings or below. At such low fields, a spin bath between all spin-spin coupled nuclei is effectively established through which polarization can easily propagate spontaneously. In other words, if a part of a nuclear spin network is initialized in the singlet state via pH2, and the Zeeman interactions are negligible compared to the J-couplings, then the polarization spreads through the network.152,153 The beauty of this “spontaneous” polarization transfer approach is that it is general and does not require specialized pulse sequences that are highly dependent on the specific spin system under study. Note that this concept was realized early on, when the so-called ALTADENA approach was established,49 where pH2 was introduced into the spin system through hydrogenation and polarization “flows” to other 1H spins at millitesla fields or below,154 as mentioned in the section above and is theoretically analysed in the following section. However, to polarize a spin-1/2 heteronucleus, such as 13C, 15N, 31P, etc., ultra-low microtesla fields are required to strongly couple the protons of the spin system with the target X-nucleus to allow spontaneous polarization transfer. Over the last decade, a wide variety of experiments have been demonstrated taking advantage of this heteronuclear spin bath at microtesla fields, including hydrogenative20,124,155–160 and non-hydrogenative PHIP.161–164 Newest approaches have used pulse sequences at microtesla fields,125,165–167 which can focus polarization transfer in a more targeted way to specific nuclei, but become highly dependent on the specific spin system under study.
Published setups:
While dedicated setups to produce diagnostically relevant contrast agent have not yet emerged, some interesting setups were described that allow exploiting the unique properties at these fields. Among these are (a) the distribution of the polarization across an entire molecule and different coherences,106 (b), the unique sensors to detect signals in the Hz – kHz range,168–170 and (c) the identification of molecules by their J-couplings rather than their chemical shift.171–173
To establish magnetic fields below the Earth’s field, entering nanotesla to microtesla field regimes, typically mu-metal shields are used.168,174 Inside such shielding, well-defined magnetic fields are commonly generated with conventional resistive coils in Helmholtz, solenoid or other configurations driven by low noise current sources.
To acquire HP spectra (typically from small molecules in solution), a variety of ULF MR setups have been designed, Figure 4. The detection sensitivity for conventional RF coils, as used for high field MR experiments, decreases with frequency.175 Therefore, at ultralow fields different magnetic field detectors such as atomic magnetometers (Figure 4a),152,153,169,174,176,177 or superconducting quantum interference devices (SQUIDs) (Figure 4b)168,178 may be more sensitive than inductive detection. Such sensors are even sensitive enough to perform magnetoencephalography,179 but can also be used to detect nuclear spins. They can operate as broad band detectors (SQUIDs: DC – ≈1 GHz, atomic magnetometers: DC – ≈1 kHz), where no matching and tuning is required and the MR signal of different nuclei such as 13C, 15N, 19F and 1H can be detected simultaneously.106 More recently, fluorescent nitrogen vacancy (NV) centers in diamonds (Figure 4c) have also been used to detect PHIP signal. While the NV centers typically have less absolute magnetic field sensitivity, the advantage is that they can be brought in direct contact with the solution (unlike SQUIDS or atomic magnetometers).170,180 These can also be used for magnetometry at zero field.181 The linewidth of the detected MR signals for ULF setups can be less than 1 Hz down to tens of mHz,171 so that J-couplings can be easily resolved. Narrow linewidths are obtained since field inhomogeneities decrease with the field strength, which enables chemically specific, high resolution J-coupling spectroscopy.
Figure 4. Setups and examples suitable for PHIP at ULF.
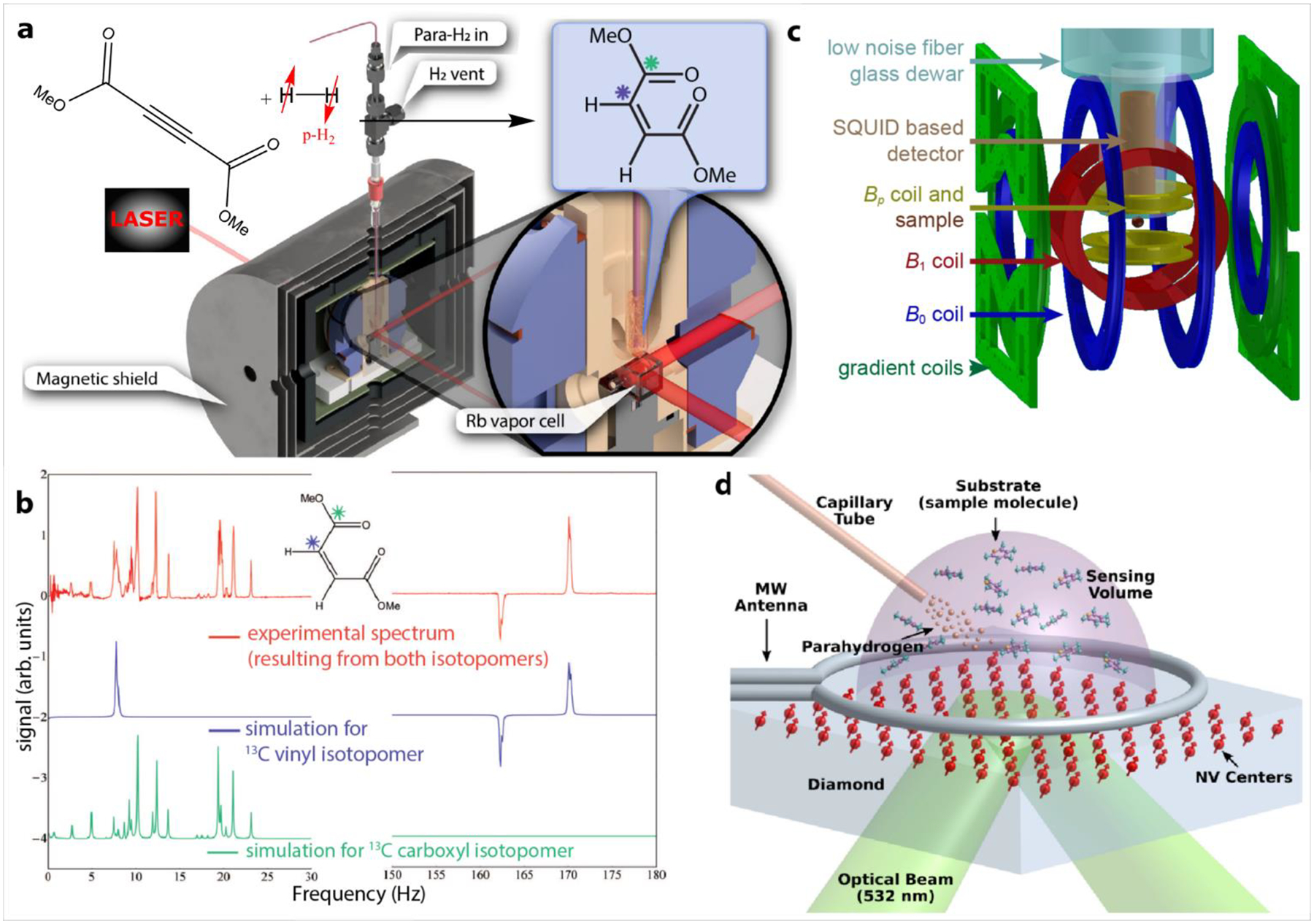
(a) atomic magnetometer system based on a Rb vapor cell. (b) PHIP enhanced zero field NMR spectrum of dimethyl maleate acquired with a Rb vapor magnetometer. (c) SQUID based system. (d) optically probed nitrogen-vacancy (NV)-NMR spectrometer. Figure 4a reproduced from Towards Large-Scale Steady-State Enhanced Nuclear Magnetization with in Situ Detection, Blanchard, J. W.; Ripka, B.; Suslick, B. A.; Gelevski, D.; Wu, T.; Münnemann, K.; Barskiy, D. A.; Budker, D.Magn. Reson. Chem., Vol. -, Issue - (ref 169). Copyright 2021 Wiley. Figure 4b reprinted from Parahydrogen-Induced Polarization at Zero Magnetic Field, Butler, M. C.; Kervern, G.; Theis, T.; Ledbetter, M. P.; Ganssle, P. J.; Blanchard, J. W.; Budker, D.; Pines, A., J. Chem. Phys., 2013, Vol. 138, Issue 23 (ref 152), with permission of AIP Publishing. Figure 4c reprinted from Mutual Benefit Achieved by Combining Ultralow-Field Magnetic Resonance and Hyperpolarizing Techniques,Buckenmaier, K.; Rudolph, M.; Fehling, P.; Steffen, T.; Back, C.; Bernard, R.; Pohmann, R.; Bernarding, J.; Kleiner, R.; Koelle, D.; Plaumann, M.; Scheffler, K., J. Rev. Sci. Instrum., 2018, Vol. 89, Issue 12 (ref 168), with permission of AIP Publishing. Figure 4d reproduced from Micron-Scale NV-NMR Spectroscopy with Signal Amplification by Reversible Exchange, Arunkumar, N.; Bucher, D. B.; Turner, M. J.; TomHon, P.; Glenn, D.; Lehmkuhl, S.; Lukin, M. D.; Park, H.; Rosen, M. S.; Theis, T.; Walsworth, R. L., PRX Quantum, Vol. 2, Issue 1 (ref 170). Copyright 2021 American Physical Society.
Challenges:
Generating and detecting HP signals at ULF offers unique insights into the investigated spin systems and provides NMR and MRI without requiring superconducting magnets. However, a disadvantage of all ULF detection methods remains, which is the frequency-dependent noise of the sensors (SQUIDs ≈ 1 fT/Hz−1/2, atomic magnetometers ≈ 10 fT/Hz−1/2). Accordingly, there is an ongoing quest to decrease the noise level below the sample noise. Moreover, medically viable polarizers or sensors that take advantage of PHIP at ULF have not emerged yet. The aspiration remains that the described discoveries and techniques will soon be translated into broad application.
Magnetic field cycling (MFC)
SOT conditions:
MFC is another method that is widely used for the polarization transfer from I1∙I2 spin order to heteronuclei. In contrast to the previous sections, which dealt with SOT at a constant magnetic field B0, MFC exploits varying the magnetic field from values close to geomagnetic field (30–50 μT) to nearly zero field, and a few T. MFC-SOT was the first method to produce net 1H magnetization49 and used to produce the first 13C in vivo MRI in 2001.5 The method is not to be confused with the fast field cycling relaxometry where the magnetic field is varied to investigate relaxation or spin-state evolution at a low magnetic field in order to gain information about physical or chemical properties of the system under study.182 For PHIP, a MFC process does not explicitly require in situ signal detection, although some setups do this.88
To give an idea of the effect of MFC on the spin state populations in PHIP-polarized molecules, we consider a three-spin system formed by two pH2 protons (H and H’) and a heteronucleus (X). The spin states can be conveniently described using the singlet-triplet-Zeeman basis. The eight basis states are:
where the first letter refers to the proton spin state, and the second refers to the heteronuclear spin state.
Upon pH2 addition to a precursor at geomagnetic fields the states |S0α〉 and |S0β〉 are populated almost equally. At these “higher” fields, the 1H and heteronuclei are weakly coupled and no heteronuclear magnetization is obtained, because to a good approximation only the |T0α〉-|S0α〉 states and the |T0β〉-|S0β〉 states are mixed. However, when the magnetic field is low enough such that the difference in proton and heteronuclear Larmor frequencies approximately matches the J-coupling frequencies, the states with equal angular momentum projection along the field axis (z): |T+β〉, |T0α〉, |S0α〉, and |T−α〉, |T0β〉, |S0β〉 are mixed. This leads to level anticrossings (LACs) between the relevant states, and by varying the magnetic field either rapidly (diabatic passage) or slowly (adiabatic passage), it is possible to transfer spin state populations between the eigenstates.183 The spin state energies and populations are shown in Figure 5a, and the relevant Hamiltonians are reported in ref.156.
Figure 5. Diagrams and Setups for PHIP by MFC.
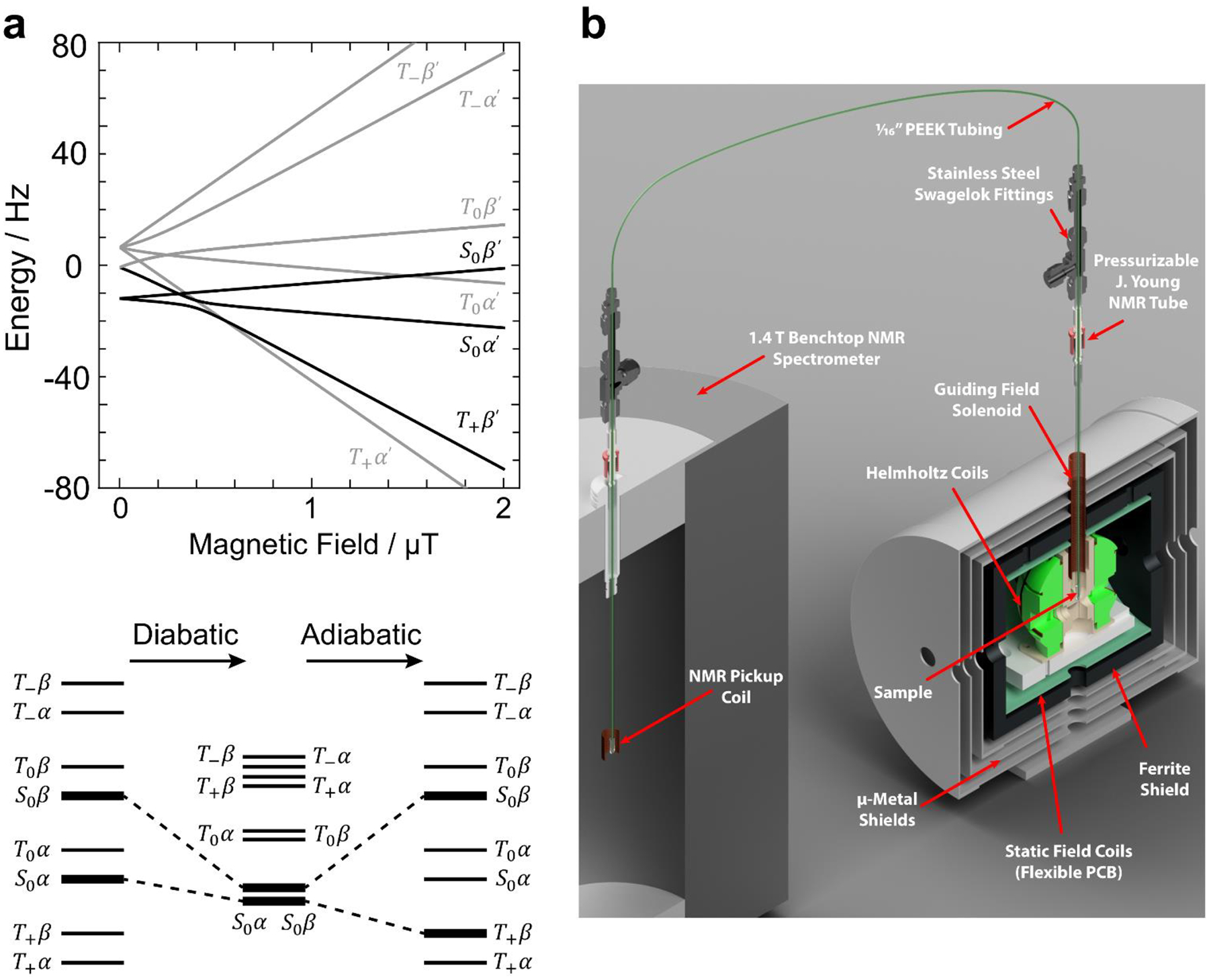
(a) Energies of the eigenstates of [1-13C]fumarate, plotted as a function of magnetic field strength, with states relevant to MFC-SOT highlighted in black (top). In an ideal MFC, the pH2-derived singlet order I1·I2 is converted into heteronuclear magnetization by first diabatic passage to near-zero-field, followed by adiabatic passage back to microtesla field (bottom). The diabatic passage preserves the state of the system with the protons in a singlet state (i.e., |S0α〉 and |S0β〉), but during the subsequent adiabatic return to high field the state |S0α〉 evolves into |T+β〉, meaning some degree of proton singlet order is lost, but heteronuclear polarization in the β state is gained. (b) Illustration of a polarizer where a sample is polarized by MFC or field sweeping and transferred to an NMR system for detection. Figure reprinted from Polarization Transfer via Field Sweeping in Parahydrogen-Enhanced Nuclear Magnetic Resonance, Eills, J.; Blanchard, J. W.; Wu, T.; Bengs, C.; Hollenbach, J.; Budker, D.; Levitt, M. H., J. Chem. Phys., 2019, Vol. 150, Issue 17 (ref 156), with permission of AIP Publishing.
In the most common example of MFC, the hydrogenation reaction using pH2 is carried out in the laboratory field (usually tens of microtesla), which is high enough to prevent leakage of proton spin order onto heteronuclear spins, but low enough that proton chemical shift differences, which would lead to loss of spin order, are suppressed, i.e., the protons remain strongly coupled. The field is then diabatically (rapidly) reduced to near-zero field, and then adiabatically (slowly) increased to the tesla range, such that the spin state populations follow the eigenstates as the field passes through the LACs. Overall, this process leads to I1∙I2 order being transformed into both proton and heteronuclear magnetization. For biomedical applications, it is the heteronuclear Zeeman order that is of interest, although we note that the protons are also polarized by this process, Figure 5a.
Published setups:
The most basic MFC experiment is a hydrogenation in a few millitesla followed by transfer to a few tesla for detection.49 To transfer polarization to X-nuclei, a mu-metal chamber can be used to reach near-zero field conditions (nanotesla). The two passages can be achieved manually, by dropping the sample after hydrogenation into the shield (diabatic passage) and lifting it out slowly (adiabatic passage).5 In more sophisticated setups, the speed of passages is controlled by means of an electromagnet inside the mu-metal shield.157,184 The use of coils allows more-precise control over the magnetic fields and has become a routine approach for MFC. A schematic of an experimental apparatus used for MFC is shown in Figure 5b.
The approach was first introduced experimentally by K. Golman et al. in 2001.5,55 After pairwise addition of pH2, the sample was (rapidly) dropped into a three-layer mu-metal shield at < 100 nT and then slowly lifted at approximately 10 cm/s rate. Using this method, the authors generated 4 % 13C polarization on [1-13C]maleic acid dimethyl ester. A more thorough theoretical explanation of the technique and description of the setup followed,55,137 now including coils inside the magnetic shield to provide time-dependent variable fields. The magnetic field was initially held at 100 μT as the hydrogenated sample was inserted into the shield, then diabatically reduced to 30 nT in about 1 ms, and then exponentially ramped back to 100 μT on the order of seconds. This approach, in combination with a spray-injection chamber for the hydrogenation with pH2, led to 13C polarization on hydroxyethyl-propionate of ≈21 %. In later work, this experimental apparatus was used to produce HP 13C contrast agents for coronary angiography imaging in pigs.150
There is merit to the approach of shuttling samples in and out of a magnetic shield by hand in the simplicity and low experimental requirements, and this approach was employed to hyperpolarize different heteronuclei such as 13C,185 15N,186 and 129Si.187 A few years later, this approach was employed to hyperpolarize the 13C spins in a number of molecules, including pyruvate and acetate, by means of PHIP-SAH.20,56 A detailed description of a coil-based magnetic field cycling setup was provided by Shchepin et al. where they studied the dependence of 13C polarization in [1-13C]ethyl acetate on the minimum field used during the MFC.184 They demonstrated that the MFC should reach fields below 1 μT for efficient 13C polarization. Using this optimized coil-based approach, the 13C polarization of [1-13C]pyruvate generated via side-arm hydrogenation was improved from 2.3 %20,56 to 8.3 %,157 and the method was also applied for hyperpolarizing [1-13C]fumarate (FUM).24,188
In FUM, derived from hydrogenation of ADC (acetylene dicarboxylic acid), an AA’X spin system is formed.189 Because of its simplicity, the system is useful for studying and optimizing MFC methods. It was on this molecular system that a MFC variant known as magnetic field sweeping was tested.156 In this method, rather than a diabatic field reduction followed by an adiabatic increase, the magnetic field is inverted adiabatically, i.e., passing through zero field. The field sweeping method was then applied to hyperpolarize vinyl-acetate, and the effect of the magnetic field sweep step size and rate was investigated.88 The authors found that for MFCs using a field range of a few microtesla, a sweep rate of 50 steps/μT/s was sufficient, with higher values leading to no improvement. Magnetic field sweeping does not need rapid field changes, and hence seems more amenable for application to liquids under continuous flow. Its lower efficiency with respect to field cycling,156 is likely due to the deleterious effects of transverse magnetic fields when passing through the zero-field point, and hence in future work an MFC approach was used for generating HP FUM.24,188
This molecular system was also used to study the benefits of constant-adiabaticity methods for MFC and field sweeping.190 Under the constant-adiabaticity constraint, the magnetic field variations are slow at the LAC field, but the field can be varied more rapidly away from this key feature. This is particularly useful for spin systems that relax rapidly during the MFC process.
Challenges:
More complicated molecular systems can present some pitfall to the application of MFC, in particular when quadrupolar nuclei such as 2H191 or 14N186 are J-coupled to the pH2–derived protons. The strong-coupling condition that is reached at nearly zero field brings these heteronuclei in contact with the spin order of pH2 and quadrupolar relaxation can work as a hyperpolarization sink.
Another caveat to be considered is that exposure to magnetic fields magnetizes the mu-metal, and so the magnetic shielding should be periodically degaussed to ensure the near-zero field condition is met inside it. In cases where the magnetic shield is near a high-field magnet, it is common to use active shimming with coils inside the shield to achieve the near-zero field condition.
PHIP OF GASES
While low sensitivity of NMR often is an issue, it is particularly severe for gases as their densities at ambient pressure are ca. 1000-fold lower compared to liquids. PHIP has been applied to produce HP molecules in the gas phase15,16,192 - mostly gases, but also vapors of volatile liquids and even solids. One approach is to use a solution of a metal complex in a homogeneous hydrogenation with subsequent transfer of the target species to gas phase. For instance, aqueous stoichiometric hydrogenation of norbornadiene resulted in the release of water-insoluble hyperpolarized norbornene to the gas phase.193 Bubbling of a mixture of pH2 with propylene (propyne, etc.) through a solution of a rhodium or iridium complex was used to produce polarized gases.194 Another approach relies on the use of solid catalysts to produce PHIP in heterogeneous hydrogenations (HET-PHIP). By bubbling pH2 through a suitable volatile liquid, pH2 is saturated with its vapor, and this gaseous mixture is then supplied to a cell with a solid catalyst for hydrogenation. Often, unsaturated gases premixed with pH2 are used in HET-PHIP experiments.
Published setups:
Published examples include hydrogenation of vinyl acetate vapors over a Rh/TiO2 catalyst with subsequent dissolution and hydrolysis of ethyl acetate to hyperpolarized ethanol and acetate,195 and hydrogenation of vinylethyl ether to hyperpolarized diethyl ether, a known inhalable anesthetic.196 Unsaturated gases are simply premixed with pH2 and supplied to a catalytic reactor to yield a continuous stream of hyperpolarized gas.15,16,192,197
The experiments with gases can be performed under PASADENA or ALTADENA condition. For propane (H3C-CH2-CH3) produced upon hydrogenation of propylene (H2C=CH-CH3), in PASADENA experiments, a pair of enhanced antiphase multiplets is observed with an admixture of in-phase contributions of opposite sign for the two signals. In ALTADENA experiments with hydrogenation at the Earth’s field, where all 1H spins in a product molecule are strongly coupled, transfer of the gas to the NMR probe results in the observation of polarization for all coupled 1H nuclei. For propyne hydrogenation to propylene, it is possible to estimate the stereoselectivity of the hydrogenation by fitting the experimental spectra to ALTADENA numerical simulations, which would be otherwise impossible for this reaction.198 The adiabatic condition of low-to-high field transfer is seldom met fully with gases as T1 times are short, and slow transfer leads to major polarization losses. Short relaxation times in the gas phase are due to spin-rotation interaction, which also makes the T1 of heteronuclei shorter than for protons. As a result, polarization transfer from 1H to heteronuclei is impractical even though its feasibility has been demonstrated.199
PASADENA experiments with gases are particularly easy to implement – the substrate gas and pH2 (and, if required, a diluent gas, e.g., N2) can be premixed in a gas cylinder or supplied by combining the outputs of mass flow controllers, and the hydrogenation reaction performed in an NMR tube containing a solid catalyst, its suspension in a liquid, or a solution of a suitable transition metal complex. ALTADENA experiments provide a much broader flexibility in experimental conditions, Figure 6a. A reactor can be as simple as a temperature-controlled section of stainless steel or copper tubing or a quartz U-tube containing solid catalyst powder which is held in place by glass wool plugs. It is important to monitor the reactor bed temperature as hydrogenation is exothermic and run-away heating effects are possible. The gas is supplied via gas lines from a gas cylinder to the reactor and then from the reactor to the NMR tube and eventually towards the exhaust; all connections should be gas-tight to avoid gas leaks, and all components should withstand the required gas pressures. Commercially available flow NMR probes, popularized with the advent of liquid chromatography (LC) NMR, are well suited for acquiring the spectra on aliquots of the flowing hyperpolarized gases. However, gas flow rates through the smaller coil volumes of these probes need to be reduced accordingly to avoid residence-time line-broadening. Such effects can also be averted with an interrupted-flow system where the gas flow is allowed to bypass the probe during NMR acquisition while not perturbing the steady-state of the reactor bed. This is also a useful way to isolate a gas sample in the probe to acquire thermally equilibrated spectra. A provision for heating the reactor and catalyst pretreatment in a stream of H2 or gas mixtures is useful. A somewhat more sophisticated setup was designed200 for a controlled clinical-scale (>300 mL in 2 s) batch production of HP propane (Figure 6c), which makes provisions for efficient preheating of reactants and subsequent dissipation of heat produced in the highly exothermic hydrogenation reaction, as well as for operation at elevated pressures (~ 8 bar).
Figure 6. Setups for PHIP of gases.
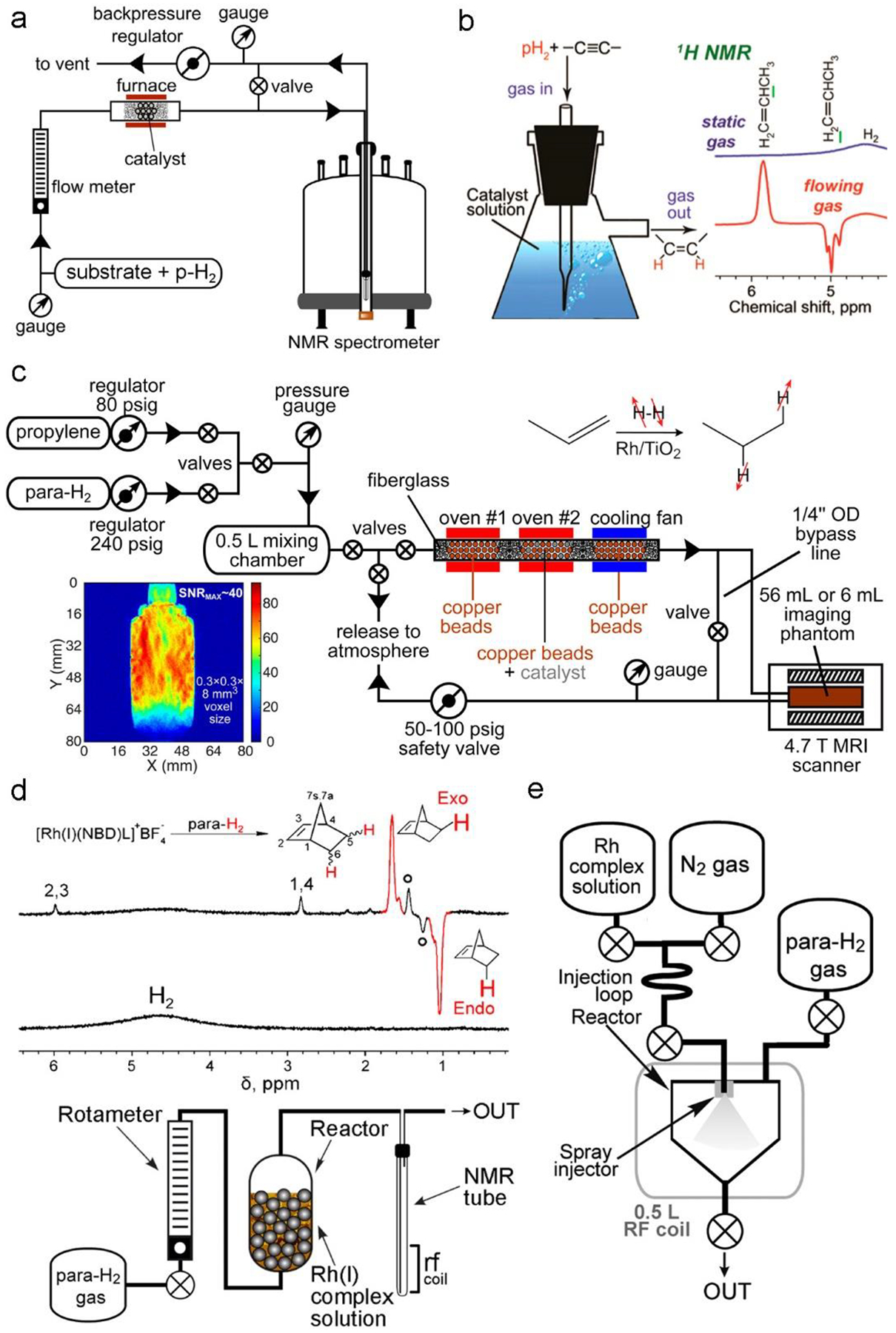
a) Experimental setup employed for producing hyperpolarized gases or vapors via heterogeneous hydrogenation of unsaturated precursors premixed with pH2. b) Schematics of an experimental setup (left) for biphasic hydrogenation of gases upon bubbling their mixture with pH2 through a homogeneous solution of metal complex catalyst, with the hyperpolarized product continuously escaping into the gas phase. The spectra shown as an example (right) are for biphasic hydrogenation of propyne to propylene. c) Schematics of a more advanced experimental setup used for a rapid batch-mode production and MRI detection of hyperpolarized propane gas. The insets show the reaction scheme (top-right) and a gradient echo 2D MR image of ~200 mL of hyperpolarized propane gas in an ~56 mL container acquired at 4.7 T (bottom-left). d) The diagram of the experimental setup used to produce hyperpolarized vapor of norbornene by bubbling pH2 through an aqueous solution of a Rh(I) complex possessing a norbornadiene ligand (bottom), the reaction scheme (top), and the resulting gas-phase 1H NMR spectra (middle). Open circles label the signals of norbornane. Plastic spheres in the reactor were used to reduce its volume. e) The diagram of the experimental setup used for injecting Rh(I) complex solution into a 56-mL volume containing pH2 at ≈7 bar pressure for subsequent in situ 1H NMR spectroscopy of hyperpolarized norbornene at 47.5 mT. Figure 6a reproduced from Heterogeneous Parahydrogen-Induced Polarization of Diethyl Ether for Magnetic Resonance Imaging Applications, Salnikov, O. G.; Svyatova, A.; Kovtunova, L. M.; Chukanov, N. V.; Bukhtiyarov, V. I.; Kovtunov, K. V.; Chekmenev, E. Y.; Koptyug, I. V. Angew. Chem. Int. Ed. Engl., Vol. 27, Issue 4 (ref 196). Copyright 2021 Wiley. Figure 6b reproduced from Kovtunov, K. V.; Zhivonitko, V. V.; Skovpin, I. V.; Barskiy, D. A.; Salnikov, O. G.; Koptyug, I. V. Toward Continuous Production of Catalyst-Free Hyperpolarized Fluids Based on Biphasic and Heterogeneous Hydrogenations with Parahydrogen. J. Phys. Chem. C 2013, 117 (44), 22887–22893 (ref. 194). Copyright 2013 American Chemical Society. Figure 6c reproduced from Salnikov, O. G.; Nikolaou, P.; Ariyasingha, N. M.; Kovtunov, K. V.; Koptyug, I. V.; Chekmenev, E. Y. Clinical-Scale Batch-Mode Production of Hyperpolarized Propane Gas for MRI. Anal. Chem. 2019, 91 (7), 4741–4746 (ref. 200). Copyright 2019 American Chemical Society. Figures 6d and 6e reproduced from Kovtunov, K. V.; Barskiy, D. A.; Shchepin, R. V.; Coffey, A. M.; Waddell, K. W.; Koptyug, I. V.; Chekmenev, E. Y. Demonstration of Heterogeneous Parahydrogen Induced Polarization Using Hyperpolarized Agent Migration from Dissolved Rh(I) Complex to Gas Phase. Anal. Chem. 2014, 86 (13), 6192–6196 (ref. 193). Copyright 2014 American Chemical Society.
HP norbornene vapor was produced from an aqueous solution of Rh(I) complex incorporating a norbornadiene ligand by either bubbling pH2 through it (for high-field NMR; Figure 6d) or by spraying it into a chamber pressurized with pH2 (for NMR at 47.5 mT; Figure 6e).193 Gas-liquid biphasic hydrogenations employed a dissolved catalyst, and gaseous reactants were bubbled through the solution, Figure 6b. The reaction product returned to the gas phase and retained a significant level of hyperpolarization, providing a complete separation of the hyperpolarized substance from the catalyst.194
Prepolarized propane was used in many studies to image voids in various objects201–203 including microfluidic devices.204,205 Imaging of HP reaction products formed in an operating model catalytic reactor facilitates mapping of their spatial distribution within the catalyst bed.206–209
Challenges:
The main challenge with gases is the short T1 time; for propane it is below 1 s at 9.4 T and 1 bar, but depends strongly on pressure and gas mixture composition.210 T1 times tend to be longer for shorter molecular rotational correlation times, i.e., for larger and/or heavier molecules, higher gas pressures, or gases in small pores. The T1 values of small gas molecules generally increase upon dissolution in liquids. For example, T1 ≈ 1 ms for H2 gas at 1 bar but it increases by ca. 1000-fold upon dissolution in methanol-d4. Condensation of hyperpolarized diethyl ether vapor was also shown to prolong hyperpolarization lifetime.196 Furthermore, because of the strong coupling for protons at low fields, they can exhibit properties of long-lived spin states (LLSS).211 For instance, measurements at 0.05 T for propane gas gave TLLSS/T1 ≈ 3.1 at 3 – 7.6 bar, with T1 ≈ 4 s and TLLSS ≈13 s at 7.6 bar.212 Similar trends were reported for diethyl ether vapor.213 The spin-lock induced crossing (SLIC) pulse sequence can be conveniently used to convert LLSS to an observable signal.212,213 Another challenge with heterogeneous hydrogenation is that it is outperformed by its homogeneous counterpart in the polarization levels achieved due to low selectivity to pairwise H2 addition, often P1H = 1 – 3 % or even lower. Values of P1H = 7 – 10 %203,214–216 or even 60 %217 have been reported using heterogeneous catalysis, but in most cases accompanied by low levels of catalytic conversion.
PURIFICATION
As soon as the development of PHIP as a hyperpolarization method for metabolic MRI began,5,137 it became apparent that purification of the reaction solution would be necessary for preclinical and clinical in vivo applications. In this regard, PHIP presents some challenges. As outlined above, the hyperpolarization often requires rather non-physiological conditions (organic solvents, pH value, temperature) and high concentrations of organometallic catalysts.5,13,14,20,24,89,127,128,137,149 Moreover, the hydrogenation reaction may be incomplete or lead to side products.24 Hence, purifying the polarized solution is important and - keeping in mind the short lifetime of liquid-state hyperpolarization - needs to be performed as quickly as possible, ideally within a few tens of seconds. Many purification strategies exist in chemistry already and some were adapted to PHIP as described below.
Catalyst scavenging
One way to remove the catalyst is metal scavenging. In this approach, the scavenger is either used in a filtration column or mixed with the reaction solution and subsequently filtered out.81,114,137,218 A commercially available metal scavenger (QuadraPure TU) was employed to clean a solution of HP fumarate prior to in vivo MRI.114 Near complete removal of ruthenium was achieved by slowly passing the solution through the scavenger in about a minute, but a reduction of concentration from ~7 g/L to 100 mg/L was reached within seconds.114 Similar scavenging approaches have been introduced by Kidd et al.218 and Barskiy et al.81 to remove an iridium-based SABRE catalyst.
A shortcoming of metal scavenging is that although the catalyst is removed, unreacted starting materials and side products may remain. Nevertheless, it was found to be a useful step for reducing the remaining metal contamination further in already purified solutions.81
Liquid-liquid phase separation:
Liquid-liquid (LL) phase extraction was found to provide near catalyst-free aqueous solutions of PHIP contrast agents. This method relies on the following steps: a) hydrogenation of a labile, lipophilic precursor of the target substrate in a hydrophobic organic solvent (e.g. chloroform); b) SOT; c) hydrolysis of the hyperpolarized product by means of fast reaction with an aqueous base - for instance, carboxylate sodium salts (hydrophilic) can be obtained by hydrolysis of the corresponding esters (lipophilic); d) extraction of the aqueous phase that contains the hydrophilic product, Figure 7.79 To obtain a biocompatible pH, an acidic buffer was added.56,80,82,219 The technique was shown to work well with PHIP-SAH20 and has provided aqueous solution of hyperpolarized sodium [1-13C]pyruvate and acetate, e.g., by hydrogenating their propargylic or vinyl esters.
Figure 7.
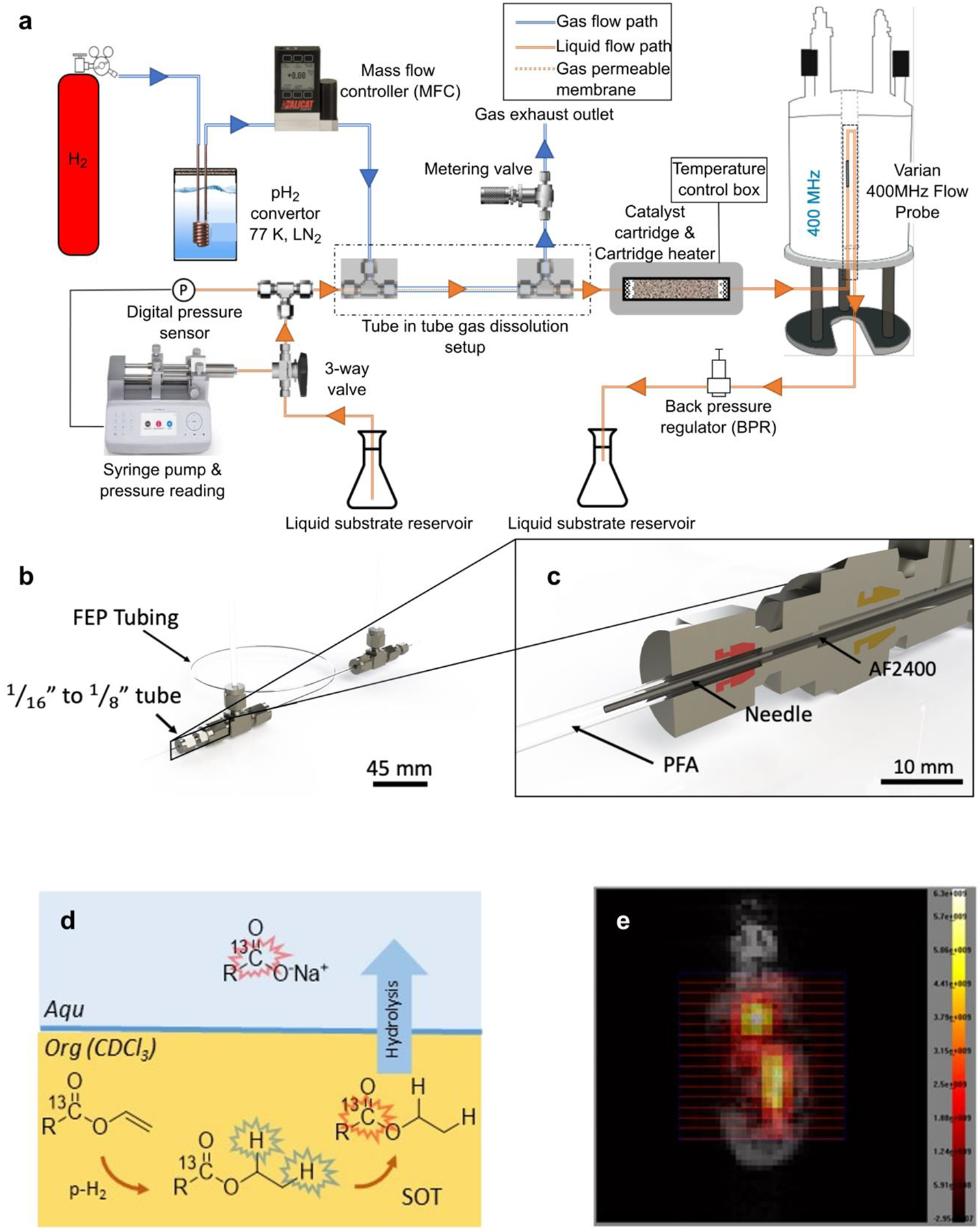
Upper panel: Continuous-flow liquid-state ALTADENA HET-PHIP polarizer. a) The system incorporated a packed-bed heterogeneous reactor containing a slid Rh/TiO2 nano-rod catalyst and a tube-in-tube membrane system for bubble-free pH2 dissolution. The liquid is drawn into the syringe from the left liquid reservoir and the 3-way valve is then changed to allow the liquid to flow through the tube-in-tube and then on to the heated catalyst cartridge. b) Rendering of the tube-in-tube device. C) Close up of the liquid inlet port showing the PFA (clear) tubing, 316 stainless steel needle (grey), and AF2400 membrane ‘inner tube’ (black). Figure reproduced from Toward Continuous-Flow Hyperpolarisation of Metabolites via Heterogenous Catalysis, Side-Arm-Hydrogenation, and Membrane Dissolution of Parahydrogen, Hale, W. G.; Zhao, T. Y.; Choi, D.; Ferrer, M.-J.; Song, B.; Zhao, H.; Hagelin-Weaver, H. E.; Bowers, C. R. ChemPhysChem, Vol. 22, Issue 9 (ref 18). Copyright 2021 Wiley. Lower panel: PHIP-SAH LL-separation (left) and HP in vivo 13C-MRI (right). The scheme on the left shows the LL separation of a carboxylate sodium salt in the water phase from its ester in the organic phase. HP [1-13C]pyruvate obtained using this method has been applied in vivo for metabolic studies (right): 13C-CSI image of [1-13C]pyruvate overlaid with an anatomical image (1H-RARE) of a healthy mouse (image acquired at 3T).
The LL phase extraction can be performed in any solvent-resistant container, e.g., in NMR tubes or a dedicated reactor. Its efficiency depends on the level of dispersion of small droplets of the organic solution into the aqueous phase, as hydrolysis likely occurs at the LL interface. As the dispersion should happen as fast as possible, injecting heated and pressurized base solution into the organic phase was suggested (a video showing the procedure can be found in ref.220). Instead of using pure hydrophobic chloroform,221 mixtures containing some (few %) hydrophilic solvents (i.e., ethanol, methanol) or toluene have been suggested82,220 to further improve the LL mixing and hydrolysis, as well as bubbling N2 gas through the solution.82 Ultimately, thanks to the instability of the LL mixture, the two phases separate within a few seconds.
Notably, this approach was used for the first in cellulo and in vivo metabolic studies with PHIP-polarized pyruvate,80,219,221 after a first demonstration of hyperpolarized [1-13C]succinate (from maleic anhydride).79 Losses of polarization during hydrolysis and phase transfer of the ester derivatives have been observed and could not be explained by T1 relaxation alone.157 Instead, the effect has been attributed to the presence of paramagnetic impurities derived from catalyst degradation in the organic phase and a beneficial effect on hyperpolarization has been obtained through the addition of a radical scavenger (sodium ascorbate) to the aqueous base. The concentration of the metal (rhodium) in the aqueous phase has been determined to be 30 μM.222
Solvents such as methanol, ethanol, and acetone mix well with water and are transferred to the aqueous phase during phase extraction. Therefore, their application in the hyperpolarization of substrates for biological use (in cells and in vivo) must be considered carefully, due to their toxicity, especially for methanol. Nevertheless, it must be mentioned that, even when pure hydrophobic solvents (chloroform and toluene) were used, their dissolution and ultimately the concentration in the aqueous phase is non-negligible and a cytotoxicity effect has been observed.219 In order to solve this issue, filtration of the aqueous solution through a lipophilic resin (Tenax TA, Porous Polymer Adsorbent, 60–80 mesh, Supelco) was recently shown to lead to a reduction of the concentration of these solvents well below the concentration recommended by Environmental Protection Agencies (EPA).69,220
Heterogeneous Catalysts
In contrast to homogeneous catalysis, heterogeneous catalysis affords straightforward separation of the solution-state hyperpolarized hydrogenation adducts from the solid catalyst due to the insolubility of the latter. Moreover, heterogeneous catalysis is inherently compatible with continuous-flow production of HP gases and liquids. These advantages were already recognized in the initial demonstration of HET-PHIP73 and continue to drive the development of HET-PHIP catalysts and reactor systems.
In ref.73 silica and a polymer were functionalized with phosphine groups to chelate the Rh complex (e.g. Wilkinson’s catalyst). The linkage proved to be resilient, but the catalysts still suffered from low stability, possible leaching into solution upon oxidation of the phosphine moieties, reduction during the reaction, and metal complex dimerization.192 A recent article described an alternative linkage scheme, where a silica supported polymer incorporating pyridyl groups was used to tether Wilkinson’s catalyst.223 This catalyst showed better stability and resistance to leaching. Modest signal enhancements of up to 200 were reported for the hydrogenation of styrene in acetone-d6.
In the quest to realize efficient, stable and robust HET-PHIP catalysts, metal-oxide-supported nanoparticle catalysts consisting of Pt, Pd, Rh, Ru, Ir as well as bimetallic compositions (e.g. Pd-In)224 and intermetallic nanoparticles (e.g. PtSn, Pt3Sn)214 with varying shapes, sizes, and support materials have been explored.192 Most of the published solution-state HET-PHIP studies were performed using a batch reactor configuration,214,224–227 where hydrogen is bubbled through a heated NMR tube containing the insoluble solid catalyst and the unsaturated substrate in solution. For larger catalyst particles (millimeter size), settling out of the catalyst after cessation of bubbling can occur within seconds. Under the relatively mild conditions of solution-state hydrogenation, supported metal nanoparticles were found to resist leaching. For example, after hydrogenation of 2-hydroxyethyl acrylate over 25 mg of Pt3Sn intermetallic nanoparticles in 2 mL D2O at 120 °C and 5.7 bar, Pt and Sn levels in the decanted solution were found to be well below 100 ppb (by mass).214 Transfer of hyperpolarization to heteronuclei in aqueous media for biologically relevant compounds has been addressed as well.228
As noted above, MRI of HP 13C pyruvate provides a means for detection of abnormal metabolism in malignant tumors and other pathologies. There is evidence, however, that administration of a continuous stream of the HP pyruvate over longer periods is preferable to a single large bolus for some applications.229 Production of purified continuous-flow streams of HP pyruvate by either dissolution DNP or PHIP is challenging and has not yet been demonstrated.
Hale et al. recently presented a novel apparatus that allowed continuous production of hyperpolarized allyl acetate by hydrogenation of propargyl acetate with pH2, Figure 7. The apparatus incorporated a packed-bed catalytic reactor, side-arm hydrogenation, and pH2 membrane dissolution.18 The polarizer continuously achieved a conversion of 30 % and 1H signal enhancements up to 300 (relative to thermal equilibrium at 9.4 Tesla) were shown to be feasible. However, the polarization transfer to 13C, side-arm cleavage, and transfer to the aqueous phase have yet to be addressed.
Challenges for heterogeneous catalysis include lower product concentrations and lower polarizations compared to homogeneous approaches. To solve these shortcomings, emphasis is made on improved flow reactor design as well as the rational design of catalysts to obtain higher pairwise selectivity without sacrificing yield.
Precipitation
Very recently, a scheme to purify HP molecules based on precipitation was introduced.24,230 HP fumarate was generated in an aqueous solution using a trans-selective hydrogenation catalyst. After hydrogenation and polarization transfer, the reaction solution contained the ruthenium-based catalyst, unreacted starting material, and side products in addition to the desired HP fumarate salt. To purify the solution, acid and non-polarized fumarate were added to the solution so that fumaric acid precipitated out of the solution almost immediately.
Because fumarate precipitated very efficiently and the catalyst, starting material, and side products remained mostly dissolved, separation was easily achieved by decanting the solution. Subsequently, the solid, HP fumaric acid was re-dissolved in aqueous solution. It was important to keep the solid fumarate (from precipitation to re-dissolution) at a sufficiently high magnetic field to avoid fast relaxation in the solid state. The remaining metal concentration was found to be 16 μM after a washing procedure.24 While this method worked well for fumarate, the generality of this approach remains an open question, and further research towards this promising approach is certainly warranted.
OTHER INTERESTING DEVELOPMENTS
PHIP-on-a-chip:
An emerging research area that is mainly pursued at high magnetic fields is the integration of PHIP into lab-on-a-chip devices.18,231–234 The advantage offered by working at a microfluidic scale (i.e., with sample volumes on the order of microliters) is in the much higher degree of experimental control that can be leveraged, in terms of sample contact time with pH2, molecular diffusion, temperature and pressure. The other important benefit of microfluidic implementation of PHIP experiments is in the short transport paths (and hence time) between the point of the PHIP process occurring and signal detection. This is particularly important when nuclear spin relaxation times are short.
RASER:
Radio amplification by stimulated emission (RASER) using PHIP was recently discovered21,235 in PASADENA and ALTADENA conditions.22,236 RASER emission was detected at low (millitesla)235 and high fields (tesla) and reproduced by simulations.22 Although the RASER effect was first observed using other HP techniques,237–239 PHIP has the advantage that the polarization can be continuously refreshed by a constant supply of pH2 as long as substrate is not depleted. While a biomedical application of RASER was not described, newly emerged applications included observation of NMR spectra with very narrow signals,22,235 background-free proton NMR spectroscopy,236 and polarization transfer to other molecules via intermolecular dipole-dipole interaction.23
PHIP-X:
pH2 hyperpolarization relayed via chemical exchange (PHIP-X)26 facilitates polarization of molecules that undergo suitable proton exchange like glucose.25,240–242 First, pH2 is added to an intermediary molecule by homogeneous catalysis, where the polarization is then transferred to an exchanging proton. Secondly, the polarization is incorporated by the target molecule by exchange of a polarized proton from the reacted intermediary molecule. Experimentally, this process was implemented by placing a high-pressure reaction chamber in millitesla fields. Upon hydrogenation, the sample was transferred to high field NMR for detection. If necessary, the target molecule can be added after the hydrogenation to avoid interference with the hydrogenation step. Both millitesla and MFC setups appear to be well suited to host this process, which involves SOT in the intermediary and target molecule, for which the spin physics has yet to be fully elucidated.
TOWARDS CLINICAL APPLICATION
The results described above impressively demonstrate the power, versatility, and maturity of pH2-based hyperpolarization approaches and instrumentation. Indeed, HP [1-13C]pyruvate produced by hydrogenative PHIP has been recently employed to detect the response of the heart to altered metabolism in real time.80
While preclinical imaging with PHIP agents was demonstrated in more than 15 papers since 2001, this method has not been translated to human imaging yet.5,13,36,69,80,86,87,90,114,142–146,150
Still, we may have reached a tipping point, as all ingredients for producing clean, aqueous solutions of interesting (not only biocompatible) agents were described in the literature. As we speak, work continues at multiple locations to make studies with PHIP-polarized agents a reality.
Let us assume that all relevant technical hurdles were to be addressed, and that there was a device that produces a clean and pure solution or solid of highly polarized contrast agent with a fast and relevant function in vivo. Still, there will be regulatory aspects to meet before human studies may commence. Here, much can be learned from the path taken so impressively by the DNP community.
Contrast agents are considered drugs by most regulatory bodies. As no injectable HP contrast agents are approved as drugs (yet), the guidelines for the application and evaluation of non-approved drugs apply. On the other hand, propane (also known as E944) is approved for unlimited use in foods and is already regulated by FDA. The above regulatory requirements may vary between countries, but are likely to include
GMP manufacturing of ingredients by vendor or in-house – pH2, catalyst, solvents, precursors
ISO 5 clean bench (or higher depending on regulations) preparation of ingredients on site – mixing precursor solution, pH2
Polarization and rapid QC – measure sample concentration, temperature, pH and polarization; residual catalyst concentration, perform sterile filter integrity test.
These steps would be followed by transfer, administration by an MD or qualified person, and imaging. However, prior to injection the hyperpolarized CA should be passed through a 0.2 μm pore size filter (the integrity of the filter would need to be tested in accordance with manufacturer regulations to ensure sterility). Given the limited lifetime of hyperpolarization, the QC needs to be rapid (< 30 s) and is recommended to be performed in parallel with the sample transfer and filter integrity test to minimize the time to injection. The transfer distance will be determined by the lifetime, and long-lived samples may be transported between sites. Attempts for individual treatment of patients on a small scale may require less stringent regulations. Safety and dose escalation trials would be followed by efficacy tests.
CONCLUSIONS
pH2-based hyperpolarization has come a long way since its inception in the 1980s, and the first demonstration of hyperpolarized in vivo 13C-MRI in 2001. For hyperpolarizing small molecules in solution for in vivo MRI application, dDNP is currently the state-of-the-art methodology, but PHIP is beginning to emerge as a viable alternative. Arguably, there is no hyperpolarization technique that is more flexible and that offers more variants than PHIP. In addition, PHIP is inexpensive, (often) easy to implement, fast and scalable. Consequently, many applications have emerged, ranging from basic physics to chemistry and biomedicine. The latter, however, has not advanced as fast as, e.g., with dDNP.
The main advantages of PHIP lie in the inherent low cost, ease of implementation, rate of hyperpolarized sample turnover, and scalability. These advantages stem mainly from the chemical nature of the technique; hyperpolarization is delivered via chemical reactions rather than requiring cryogenic cooling of the to-be-polarized substance and/or irradiation with high power microwaves, as is the case for dDNP. The chemical basis of the process unfortunately carries some drawbacks: (1) the method is limited to polarizing molecules that can be generated through hydrogenation reactions; (2) the HP solutions are contaminated with other chemicals from the reaction, and; (3) polarization levels are often only modest since the nuclear spins relax during the hyperpolarization process.
All three drawbacks are being overcome through state-of-the-art research, much of which is covered in this review: Many biologically and diagnostically promising agents are available by direct hydrogenation (SUX, FUM, TFPP, PLAC) and variants such as PHIP-SAH and PHIP-X (pyruvate, acetate, glucose, lactate). Physicochemical methods for purifying the HP solutions through phase separation have been shown for a few PHIP-polarized metabolites, and scavenging can be used to remove the catalyst from solutions. Advances in instrumentation for hydrogenation, polarization transfer, and sample transport, have helped to improve the resulting polarization levels in PHIP-polarized molecules. PHIP seems to be a promising hyperpolarization method for preclinical MRI and in vitro studies where experiment turnover is high but requirements for high polarization and solution sterility/nontoxicity are lower.
Nonetheless, it remains to be seen whether PHIP has a future role to play in clinical imaging. To date no device is commercially available – not even for in vitro and preclinical in vivo studies. Over the years, some initiatives were and still are active (Amersham, Stelar, Spindynamics, Bruker, XEUS Technologies LTD, NVision), and numerous patents were filed. The technical challenges towards clinical applications are accompanied by regulatory aspects, which must be met for a use in human. Here, the contrast agents do not only need to be pure and highly polarized, but also produced effectively and compliant to GMP. The reactive nature of PHIP may pose a challenge, too. Ideally, agents must have a long enough lifetime for testing and transferring after polarization.
Although the focus of this review has largely been on producing PHIP-polarized biomolecules for in vivo applications, there are many more uses of PHIP. Examples include studying chemical reaction mechanisms and as a source of signal enhancement for high-resolution NMR. Advances in instrumentation have underpinned much of the method development made in recent years, and will continue to pave the way into the future. Even if PHIP (or hyperpolarization in general) would eventually turn out to be ineligible for clinics, many more applications are lurking behind the corner – the marriage of pH2 and MR are far from over yet, and the best has yet to come.
Figure 8.
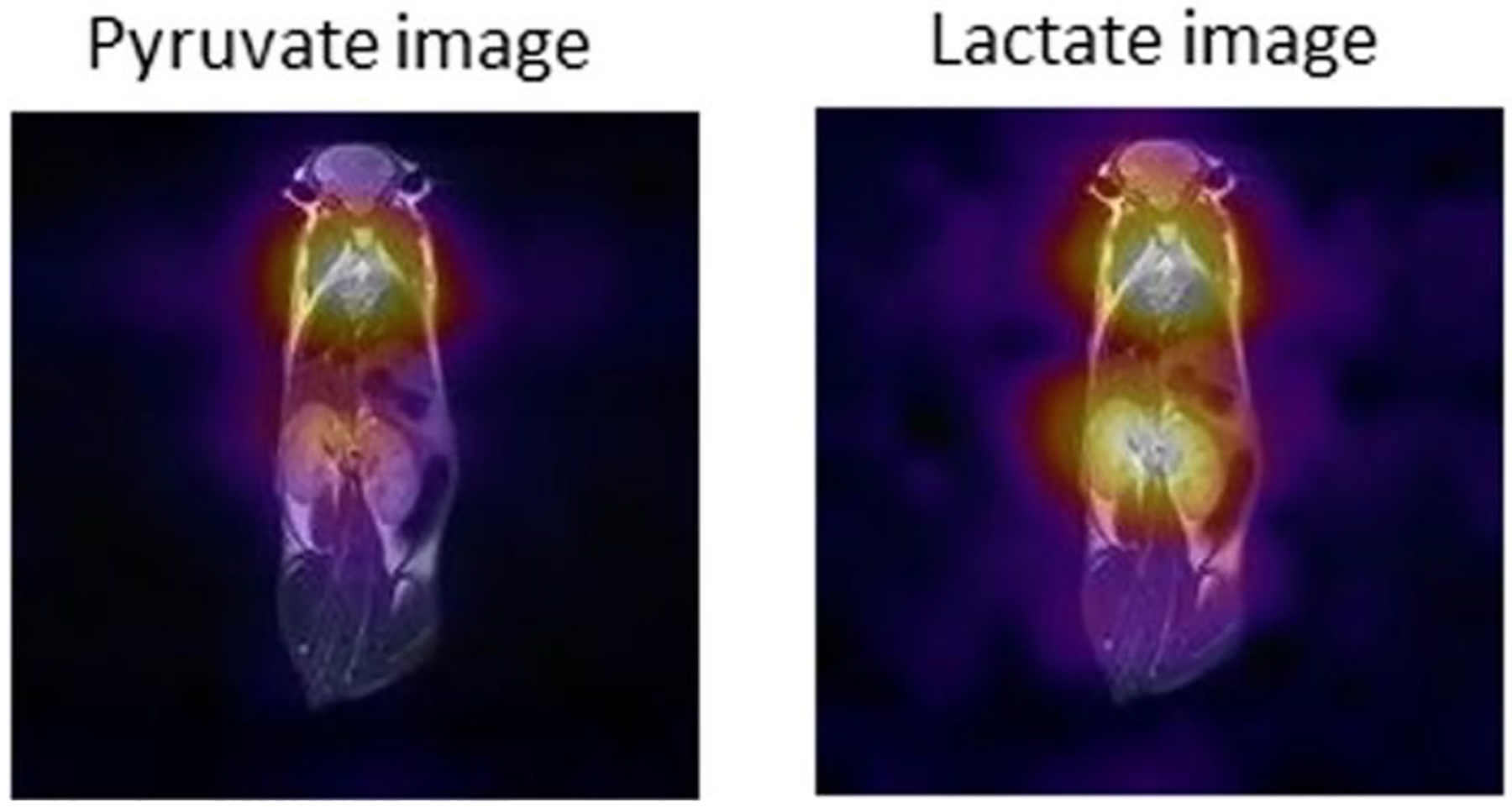
13C-chemical shift imaging reporting of pyruvate and lactate distribution acquired on living mice, obtained upon the injection of a dose of [1-13C] pyruvate hyperpolarized using pH2. The spatial localization of each metabolite is shown upon overlapping the 13C-CSI results to the anatomical proton image (T2 weighted fast spin echo image). Each metabolite map is scaled individually and is displayed on a fire color scale so that the region of highest metabolite signal appears white and the lowest appears black. Reprinted by permission from Nature: Sci. Rep., The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time, Cavallari, E.; Carrera, C.; Sorge, M.; Bonne, G.; Muchir, A.; Aime, S.; Reineri, F., Sci. Rep., Vol. 8, Issue 1 (ref 80). Copyright Nature 2018.
ACKNOWLEDGMENT
We acknowledge funding from German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (01ZX1915C), German Cancer Consortium (DKTK), DFG (SCHM 3694/1-1, HO-4602/2-2, HO-4602/3, GRK2154-2019, EXC2167, FOR5042, SFB1479, TRR287), Kiel University and the Faculty of Medicine, Research Commission of the University Medical Center Freiburg (SCHM2146-20). MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053), National Science Foundation (NSF) grants CHE-2108306, CBET-1933723, and the National High Magnetic Field Laboratory, which is supported by the NSF Cooperative Agreement No. DMR-1644779* and the State of Florida. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No. 766402. E.Y.C thanks NSF CHE-1904780, DOD CDMRP W81XWH-20-10576, NIH NHLBI R21 HL154032. TT acknowledges support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number NIH R01EB029829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. IVK acknowledges the Russian Ministry of Science and Higher Education (contract 075-15-2021-580) for financial support. University of Torino was funded by the EU H2020 FETOPEN under the grant agreement No. 858149 (Alternatives to Gd). JWG acknowledges support from the National Institutes of Health (P41EB013598).
Footnotes
EYC has a stake of ownership in XeUS Technologies LTD.
SK is employed at NVision Imaging GMBH Ulm, Germany.
TT holds stock in Vizma Life Sciences LLC (VLS) and is President of VLS. The terms of this arrangement have been reviewed and approved by NC State University in accordance with its policy on objectivity in research.
XeUS Technologies LTD, NVision Imaging GMBH, and VLS are developing products related to the research being reported.
All other authors declare no competing interests.
REFERENCES
- (1).Born M; Heisenberg W The Quantum Theory of Molecules. Ann Phys-Berl. Ann Phys-Berl 1924, 74 (9), 1–31. [Google Scholar]
- (2).Bowers CR; Weitekamp DP Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett 1986, 57 (21), 2645–2648. 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- (3).Bowers CR; Weitekamp DP Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc 1987, 109 (18), 5541–5542. [Google Scholar]
- (4).Eisenschmid TC; Kirss RU; Deutsch PP; Hommeltoft SI; Eisenberg R; Bargon J; Lawler RG; Balch AL Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc 1987, 109 (26), 8089–8091. [Google Scholar]
- (5).Golman K; Axelsson O; Johannesson H; Mansson S; Olofsson C; Petersson JS Parahydrogen-Induced Polarization in Imaging: Subsecond 13C Angiography. Magn. Reson. Med 2001, 46 (1), 1–5. [DOI] [PubMed] [Google Scholar]
- (6).Johansson E; Olsson L. e.; Månsson S; Petersson J. s.; Golman K; Ståhlberg F; Wirestam R Perfusion Assessment with Bolus Differentiation: A Technique Applicable to Hyperpolarized Tracers. Magn. Reson. Med 2004, 52 (5), 1043–1051. 10.1002/mrm.20247. [DOI] [PubMed] [Google Scholar]
- (7).Bargon J; Kandels J; Kating P Nuclear Magnetic Resonance Studies of Homogeneous Catalysis Using Parahydrogen: Analysis of Nuclear Singlet–Triplet Mixing as a Diagnostic Tool to Characterize Intermediates. J. Chem. Phys 1993, 98 (8), 6150–6153. 10.1063/1.464853. [DOI] [Google Scholar]
- (8).Messerle BA; Sleigh CJ; Partridge MG; Duckett SB Structure and Dynamics in Metal Phosphine Complexes Using Advanced NMR Studies with Para-Hydrogen Induced Polarisation. J. Chem. Soc. Dalton Trans 1999, No. 9, 1429–1436. 10.1039/A809948K. [DOI] [Google Scholar]
- (9).Eills J; Stevanato G; Bengs C; Glöggler S; Elliott SJ; Alonso-Valdesueiro J; Pileio G; Levitt MH Singlet Order Conversion and Parahydrogen-Induced Hyperpolarization of 13C Nuclei in near-Equivalent Spin Systems. J. Magn. Reson 2017, 274, 163–172. 10.1016/j.jmr.2016.11.010. [DOI] [PubMed] [Google Scholar]
- (10).Duckett SB; Wood NJ Parahydrogen-Based NMR Methods as a Mechanistic Probe in Inorganic Chemistry. Coord. Chem. Rev 2008, 252 (21), 2278–2291. 10.1016/j.ccr.2008.01.028. [DOI] [Google Scholar]
- (11).Nelson SJ; Kurhanewicz J; Vigneron DB; Larson PEZ; Harzstark AL; Ferrone M; van Criekinge M; Chang JW; Bok R; Park I; Reed G; Carvajal L; Small EJ; Munster P; Weinberg VK; Ardenkjaer-Larsen JH; Chen AP; Hurd RE; Odegardstuen L-I; Robb FJ; Tropp J; Murray JA Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1–13C]Pyruvate. Sci. Transl. Med 2013, 5 (198), 108. 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ardenkjær-Larsen JH; Bowen S; Petersen JR; Rybalko O; Vinding MS; Ullisch M; Nielsen N. Chr. Cryogen-free Dissolution Dynamic Nuclear Polarization Polarizer Operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med 2019, 81 (3), 2184–2194. 10.1002/mrm.27537. [DOI] [PubMed] [Google Scholar]
- (13).Hövener J-B; Chekmenev EY; Harris KC; Perman WH; Robertson LW; Ross BD; Bhattacharya P PASADENA Hyperpolarization of 13C Biomolecules: Equipment Design and Installation. Magn Reson Mater Phy 2009, 22, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Adams RW; Aguilar JA; Atkinson KD; Cowley MJ; Elliott PIP; Duckett SB; Green GGR; Khazal IG; López-Serrano J; Williamson DC Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323 (5922), 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- (15).Barskiy DA; Coffey AM; Nikolaou P; Mikhaylov DM; Goodson BM; Branca RT; Lu GJ; Shapiro MG; Telkki V-V; Zhivonitko VV; Koptyug IV; Salnikov OG; Kovtunov KV; Bukhtiyarov VI; Rosen MS; Barlow MJ; Safavi S; Hall IP; Schröder L; Chekmenev EY NMR Hyperpolarization Techniques of Gases. Chem. – Eur. J 2017, 23 (4), 725–751. 10.1002/chem.201603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kovtunov KV; Koptyug IV; Fekete M; Duckett SB; Theis T; Joalland B; Chekmenev EY Parahydrogen-Induced Hyperpolarization of Gases. Angew. Chem. Int. Ed 2020, 59 (41), 17788–17797. 10.1002/anie.201915306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hövener J-B; Schwaderlapp N; Lickert T; Duckett SB; Mewis RE; Highton LAR; Kenny SM; Green GGR; Leibfritz D; Korvink JG; Hennig J; von Elverfeldt D A Hyperpolarized Equilibrium for Magnetic Resonance. Nat. Commun 2013, 4, 2946. 10.1038/ncomms3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hale WG; Zhao TY; Choi D; Ferrer M-J; Song B; Zhao H; Hagelin-Weaver HE; Bowers CR Toward Continuous-Flow Hyperpolarisation of Metabolites via Heterogenous Catalysis, Side-Arm-Hydrogenation, and Membrane Dissolution of Parahydrogen. ChemPhysChem 2021, 22 (9), 822–827. 10.1002/cphc.202100119. [DOI] [PubMed] [Google Scholar]
- (19).Eshuis N; Aspers RLEG; van Weerdenburg BJA; Feiters MC; Rutjes FPJT; Wijmenga SS; Tessari M 2D NMR Trace Analysis by Continuous Hyperpolarization at High Magnetic Field. Angew. Chem. Int. Ed 2015, 54 (48), 14527–14530. 10.1002/anie.201507831. [DOI] [PubMed] [Google Scholar]
- (20).Reineri F; Boi T; Aime S ParaHydrogen Induced Polarization of 13C Carboxylate Resonance in Acetate and Pyruvate. Nat. Commun 2015, 6, 5858. 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- (21).Appelt S; Kentner A; Lehmkuhl S; Blümich B From LASER Physics to the Para-Hydrogen Pumped RASER. Prog. Nucl. Magn. Reson. Spectrosc 2019, 114–115, 1–32. 10.1016/j.pnmrs.2019.05.003. [DOI] [PubMed] [Google Scholar]
- (22).Pravdivtsev AN; Sönnichsen FD; Hövener J-B Continuous Radio Amplification by Stimulated Emission of Radiation Using Parahydrogen Induced Polarization (PHIP-RASER) at 14 Tesla. ChemPhysChem 2020, 21 (7), 667–672. 10.1002/cphc.201901056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Korchak S; Kaltschnee L; Dervisoglu R; Andreas L; Griesinger C; Glöggler S Spontaneous Enhancement of Magnetic Resonance Signals Using a RASER. Angew. Chem. Int. Ed 2021, 60 (38), 20984–20990. 10.1002/anie.202108306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Knecht S; Blanchard JW; Barskiy D; Cavallari E; Dagys L; Dyke EV; Tsukanov M; Bliemel B; Münnemann K; Aime S; Reineri F; Levitt MH; Buntkowsky G; Pines A; Blümler P; Budker D; Eills J Rapid Hyperpolarization and Purification of the Metabolite Fumarate in Aqueous Solution. Proc. Natl. Acad. Sci 2021, 118 (13). 10.1073/pnas.2025383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Roy SS; Appleby KM; Fear EJ; Duckett SB SABRE-Relay: A Versatile Route to Hyperpolarization. J. Phys. Chem. Lett 2018, 9 (5), 1112–1117. 10.1021/acs.jpclett.7b03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Them K; Ellermann F; Pravdivtsev AN; Salnikov OG; Skovpin IV; Koptyug IV; Herges R; Hövener J-B Parahydrogen-Induced Polarization Relayed via Proton Exchange. J. Am. Chem. Soc 2021, 143 (34), 13694–13700. 10.1021/jacs.1c05254. [DOI] [PubMed] [Google Scholar]
- (27).Marshall H; Stewart NJ; Chan H-F; Rao M; Norquay G; Wild JM In Vivo Methods and Applications of Xenon-129 Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc 2021, 122, 42–62. 10.1016/j.pnmrs.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hoevener J-B; Bär S; Leupold J; Jenne K; Leibfritz D; Hennig J; Duckett SB; von Elverfeldt D A Continuous-Flow, High-Throughput, High-Pressure Parahydrogen Converter for Hyperpolarization in a Clinical Setting. NMR Biomed. 2013, 26, 124–131. [DOI] [PubMed] [Google Scholar]
- (29).Feng B; Coffey AM; Colon RD; Chekmenev EY; Waddell KW A Pulsed Injection Parahydrogen Generator and Techniques for Quantifying Enrichment. J. Magn. Reson 2012, 214 (Supplement C), 258–262. 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wagner S Conversion Rate of Para-Hydrogen to Ortho-Hydrogen by Oxygen: Implications for PHIP Gas Storage and Utilization. Magn. Reson. Mater. Phys. Biol. Med 2014, 27 (3), 195–199. 10.1007/s10334-013-0399-y. [DOI] [PubMed] [Google Scholar]
- (31).Aroulanda C; Starovoytova L; Canet D Longitudinal Nuclear Spin Relaxation of Ortho- and Para-Hydrogen Dissolved in Organic Solvents. J Phys Chem A 2007, 111 (42), 10615–10624. [DOI] [PubMed] [Google Scholar]
- (32).Buntkowsky G; Walaszek B; Adamczyk A; Xu Y; Limbach H-H; Chaudret B Mechanism of Nuclear Spin Initiated Para -H 2 to Ortho -H 2 Conversion. Phys. Chem. Chem. Phys 2006, 8 (16), 1929–1935. 10.1039/B601594H. [DOI] [PubMed] [Google Scholar]
- (33).Schmidt AB; Wörner J; Pravdivtsev A; Knecht S; Scherer H; Weber S; Hennig J; von Elverfeldt D; Hövener J-B Lifetime of Parahydrogen in Aqueous Solutions and Human Blood. ChemPhysChem 2019, 20 (19), 2408–2412. 10.1002/cphc.201900670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kiryutin AS; Sauer G; Yurkovskaya AV; Limbach H-H; Ivanov KL; Buntkowsky G Parahydrogen Allows Ultrasensitive Indirect NMR Detection of Catalytic Hydrogen Complexes. J. Phys. Chem. C 2017, 121 (18), 9879–9888. 10.1021/acs.jpcc.7b01056. [DOI] [Google Scholar]
- (35).Freeman R; Wittekoek S; Ernst RR High-Resolution NMR Study of Relaxation Mechanisms in a Two-Spin System. J. Chem. Phys 1970, 52 (3), 1529–1544. 10.1063/1.1673164. [DOI] [Google Scholar]
- (36).Kadlecek S; Vahdat V; Nakayama T; Ng D; Emami K; Rizi R A Simple and Low-Cost Device for Generating Hyperpolarized Contrast Agents Using Parahydrogen. NMR Biomed. 2011, 24 (8), 933–942. 10.1002/nbm.1757. [DOI] [PubMed] [Google Scholar]
- (37).Pravdivtsev AN; Ivanov KL; Kaptein R; Yurkovskaya AV Theoretical Study of Dipolar Relaxation of Coupled Nuclear Spins at Variable Magnetic Field. Appl. Magn. Reson 2013, 44 (1), 23–39. 10.1007/s00723-012-0404-z. [DOI] [Google Scholar]
- (38).Schmidt AB; Berner S; Schimpf W; Müller C; Lickert T; Schwaderlapp N; Knecht S; Skinner JG; Dost A; Rovedo P; Hennig J; von Elverfeldt D; Hövener J-B Liquid-State Carbon-13 Hyperpolarization Generated in an MRI System for Fast Imaging. Nat. Commun 2017, 8, 14535. 10.1038/ncomms14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Berner S; Schmidt AB; Zimmermann M; Pravdivtsev AN; Glöggler S; Hennig J; von Elverfeldt D; Hövener J-B SAMBADENA Hyperpolarization of 13C-Succinate in an MRI: Singlet-Triplet Mixing Causes Polarization Loss. ChemistryOpen 2019, 8 (6), 728–736. 10.1002/open.201900139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Natterer J; Bargon J Parahydrogen Induced Polarization. Prog. Nucl. Magn. Reson. Spectrosc 1997, 31 (4), 293–315. 10.1016/S0079-6565(97)00007-1. [DOI] [Google Scholar]
- (41).Nasibulov EA; Pravdivtsev AN; Yurkovskaya AV; Lukzen NN; Vieth H-M; Ivanov KL Analysis of Nutation Patterns in Fourier-Transform NMR of Non-Thermally Polarized Multispin Systems. Z. Für Phys. Chem 2013, 227 (6–7), 929–953. 10.1524/zpch.2013.0397. [DOI] [Google Scholar]
- (42).Goldman M; Johannesson H Conversion of a Proton Pair Para Order into 13C Polarization by Rf Irradiation, for Use in MRI. Comptes Rendus Phys. 2005, 6, 575–581. [Google Scholar]
- (43).Torres O; Procacci B; Halse ME; Adams RW; Blazina D; Duckett SB; Eguillor B; Green RA; Perutz RN; Williamson DC Photochemical Pump and NMR Probe: Chemically Created NMR Coherence on a Microsecond Time Scale. J. Am. Chem. Soc 2014, 136 (28), 10124–10131. 10.1021/ja504732u. [DOI] [PubMed] [Google Scholar]
- (44).N. Pravdivtsev A; V. Yurkovskaya A; A. Petrov P; Vieth H-M Coherent Evolution of Singlet Spin States in PHOTO-PHIP and M2S Experiments. Phys. Chem. Chem. Phys 2017, 19 (38), 25961–25969. 10.1039/C7CP04122E. [DOI] [PubMed] [Google Scholar]
- (45).Bowers CR Sensitivity Enhancement Utilizing Parahydrogen. In eMagRes; American Cancer Society, 2007. 10.1002/9780470034590.emrstm0489. [DOI] [Google Scholar]
- (46).Bowers CR Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. phd, California Institute of Technology, 1991. 10.7907/R5TV-Z220. [DOI] [Google Scholar]
- (47).Natterer J; Schedletzky O; Barkemeyer J; Bargon J; Glaser SJ Investigating Catalytic Processes with Parahydrogen: Evolution of Zero-Quantum Coherence in AA′X Spin Systems. J. Magn. Reson 1998, 133 (1), 92–97. 10.1006/jmre.1998.1421. [DOI] [PubMed] [Google Scholar]
- (48).Berner S; Schmidt AB; Ellermann F; Korchak S; Chekmenev EY; Glöggler S; von Elverfeldt D; Hennig J; Hövener J-B High Field Parahydrogen Induced Polarization of Succinate and Phospholactate. Phys. Chem. Chem. Phys 2021, 23 (3), 2320–2330. 10.1039/D0CP06281B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Pravica MG; Weitekamp DP Net NMR Alignment by Adiabatic Transport of Parahydrogen Addition Products to High Magnetic Field. Chem. Phys. Lett 1988, 145 (4), 255–258. 10.1016/0009-2614(88)80002-2. [DOI] [Google Scholar]
- (50).Plaumann M; Bommerich U; Trantzschel T; Lego D; Dillenberger S; Sauer G; Bargon J; Buntkowsky G; Bernarding J Parahydrogen-Induced Polarization Transfer to 19F in Perfluorocarbons for 19F NMR Spectroscopy and MRI. Chem. – Eur. J 2013, 19 (20), 6334–6339. 10.1002/chem.201203455. [DOI] [PubMed] [Google Scholar]
- (51).Bär S; Lange T; Leibfritz D; Hennig J; von Elverfeldt D; Hoevener J-B On the Spin Order Transfer from Parahydrogen to Another Nucleus. J. Magn. Reson 2012, 225, 25–35. [DOI] [PubMed] [Google Scholar]
- (52).Svyatova A; Skovpin IV; Chukanov NV; Kovtunov KV; Chekmenev EY; Pravdivtsev AN; Hövener J-B; Koptyug IV 15N MRI of SLIC-SABRE Hyperpolarized 15N-Labelled Pyridine and Nicotinamide. Chem. – Eur. J 2019, 25 (36), 8465–8470. 10.1002/chem.201900430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Shchepin RV; Coffey AM; Waddell KW; Chekmenev EY Parahydrogen Induced Polarization of 1–13C-Phospholactate-D2 for Biomedical Imaging with >30,000,000-Fold NMR Signal Enhancement in Water. Anal. Chem 2014, 86 (12), 5601–5605. 10.1021/ac500952z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Schmidt AB; Brahms A; Ellermann F; Berner S; Hennig J; von Elverfeldt D; Herges R; Hövener J-B; Pravdivtsev AN Selectively Pulsed Spin Order Transfer Increases Parahydrogen-Induced NMR Amplification of Insensitive Nuclei and Makes Polarization Transfer More Robust. ArXiv210904799 Phys. 2021. [Google Scholar]
- (55).Jóhannesson H; Axelsson O; Karlsson M Transfer of Para-Hydrogen Spin Order into Polarization by Diabatic Field Cycling. Comptes Rendus Phys. 2004, 5 (3), 315–324. 10.1016/j.crhy.2004.02.001. [DOI] [Google Scholar]
- (56).Cavallari E; Carrera C; Boi T; Aime S; Reineri F Effects of Magnetic Field Cycle on the Polarization Transfer from Parahydrogen to Heteronuclei through Long-Range J-Couplings. J. Phys. Chem. B 2015, 119 (31), 10035–10041. 10.1021/acs.jpcb.5b06222. [DOI] [PubMed] [Google Scholar]
- (57).Kadlecek S; Emami K; Ishii M; Rizi R Optimal Transfer of Spin-Order between a Singlet Nuclear Pair and a Heteronucleus. J. Magn. Reson 2010, 205 (1), 9–13. 10.1016/j.jmr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Cai C; Coffey AM; Shchepin RV; Chekmenev EY; Waddell KW Efficient Transformation of Parahydrogen Spin Order into Heteronuclear Magnetization. J. Phys. Chem. B 2013, 117 (5), 1219–1224. 10.1021/jp3089462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Duckett SB; Newell CL; Eisenberg R More than INEPT: Parahydrogen and INEPT + Give Unprecedented Resonance Enhancement to Carbon-13 by Direct Proton Polarization Transfer. J. Am. Chem. Soc 1993, 115 (3), 1156–1157. 10.1021/ja00056a054. [DOI] [Google Scholar]
- (60).Haake M; Natterer J; Bargon J Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. J Am Chem Soc 1996, 118 (86), 88–91. [Google Scholar]
- (61).Stevanato G Alternating Delays Achieve Polarization Transfer (ADAPT) to Heteronuclei in PHIP Experiments. J. Magn. Reson 2017, 274, 148–162. 10.1016/j.jmr.2016.10.009. [DOI] [PubMed] [Google Scholar]
- (62).Korchak S; Yang S; Mamone S; Glöggler S Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by Utilizing Para-Hydrogen. ChemistryOpen 2018, 7 (5), 344–348. 10.1002/open.201800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Pravdivtsev AN; Kozinenko VP; Hövener J-B Only Para-Hydrogen Spectroscopy (OPSY) Revisited: In-Phase Spectra for Chemical Analysis and Imaging. J. Phys. Chem. A 2018, 122 (45), 8948–8956. 10.1021/acs.jpca.8b07459. [DOI] [PubMed] [Google Scholar]
- (64).Stevanato G; Eills J; Bengs C; Pileio G A Pulse Sequence for Singlet to Heteronuclear Magnetization Transfer: S2hM. J. Magn. Reson 2017, 277, 169–178. 10.1016/j.jmr.2017.03.002. [DOI] [PubMed] [Google Scholar]
- (65).Pravdivtsev AN; Hövener J-B Coherent Polarization Transfer in Chemically Exchanging Systems. Phys. Chem. Chem. Phys 2020, 22 (16), 8963–8972. 10.1039/C9CP06873B. [DOI] [PubMed] [Google Scholar]
- (66).Hogben HJ; Krzystyniak M; Charnock GTP; Hore PJ; Kuprov I Spinach – A Software Library for Simulation of Spin Dynamics in Large Spin Systems. J. Magn. Reson 2011, 208 (2), 179–194. 10.1016/j.jmr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- (67).Smith SA; Levante TO; Meier BH; Ernst RR Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. J. Magn. Reson. A 1994, 106 (1), 75–105. 10.1006/jmra.1994.1008. [DOI] [Google Scholar]
- (68).Bengs C; Levitt MH SpinDynamica: Symbolic and Numerical Magnetic Resonance in a Mathematica Environment. Magn. Reson. Chem 2018, 56 (6), 374–414. 10.1002/mrc.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Bhattacharya P; Harris K; Lin AP; Mansson M; Norton VA; Perman WH; Weitekamp DP; Ross BD Ultra-Fast Three Dimensional Imaging of Hyperpolarized 13C in Vivo. Magn. Reson. Mater. Phys. Biol. Med 2005, 18 (5), 245–256. 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- (70).Skovpin IV; Zhivonitko VV; Koptyug IV Parahydrogen-Induced Polarization in Heterogeneous Hydrogenations over Silica-Immobilized Rh Complexes. Appl. Magn. Reson 2011, 41 (2), 393–410. 10.1007/s00723-011-0255-z. [DOI] [Google Scholar]
- (71).Kovtunov KV; Zhivonitko VV; Corma A; Koptyug IV Parahydrogen-Induced Polarization in Heterogeneous Hydrogenations Catalyzed by an Immobilized Au(III) Complex. J. Phys. Chem. Lett 2010, 1 (11), 1705–1708. 10.1021/jz100391j. [DOI] [Google Scholar]
- (72).Abdulhussain S; Breitzke H; Ratajczyk T; Grünberg A; Srour M; Arnaut D; Weidler H; Kunz U; Kleebe HJ; Bommerich U; Bernarding J; Gutmann T; Buntkowsky G Synthesis, Solid-State NMR Characterization, and Application for Hydrogenation Reactions of a Novel Wilkinson’s-Type Immobilized Catalyst. Chem. – Eur. J 2014, 20 (4), 1159–1166. 10.1002/chem.201303020. [DOI] [PubMed] [Google Scholar]
- (73).Koptyug IV; Kovtunov KV; Burt SR; Anwar MS; Hilty C; Han S-I; Pines A; Sagdeev RZ Para-Hydrogen-Induced Polarization in Heterogeneous Hydrogenation Reactions. J. Am. Chem. Soc 2007, 129 (17), 5580–5586. 10.1021/ja068653o. [DOI] [PubMed] [Google Scholar]
- (74).Kovtunov KV; Beck IE; Bukhtiyarov VI; Koptyug IV Observation of Parahydrogen-Induced Polarization in Heterogeneous Hydrogenation on Supported Metal Catalysts. Angew. Chem. Int. Ed 2008, 47 (8), 1492–1495. 10.1002/anie.200704881. [DOI] [PubMed] [Google Scholar]
- (75).Glöggler S; Grunfeld AM; Ertas YN; McCormick J; Wagner S; Schleker PPM; Bouchard L-S A Nanoparticle Catalyst for Heterogeneous Phase Para-Hydrogen-Induced Polarization in Water. Angew. Chem. Int. Ed Engl 2015, 54 (8), 2452–2456. 10.1002/anie.201409027. [DOI] [PubMed] [Google Scholar]
- (76).Kovtunov V; A. Barskiy D; G. Salnikov O; V. Shchepin R; M. Coffey A; M. Kovtunova L; I. Bukhtiyarov V; V. Koptyug I; Y. Chekmenev E Toward Production of Pure 13 C Hyperpolarized Metabolites Using Heterogeneous Parahydrogen-Induced Polarization of Ethyl[1–13 C]Acetate. RSC Adv. 2016, 6 (74), 69728–69732. 10.1039/C6RA15808K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Kaltschnee L; Jagtap AP; McCormick J; Wagner S; Bouchard L-S; Utz M; Griesinger C; Glöggler S Hyperpolarization of Amino Acids in Water Utilizing Parahydrogen on a Rhodium Nanocatalyst. Chem. Weinh. Bergstr. Ger 2019, 25 (47), 11031–11035. 10.1002/chem.201902878. [DOI] [PubMed] [Google Scholar]
- (78).Jagtap AP; Kaltschnee L; Glöggler S Hyperpolarization of 15N-Pyridinium and 15N-Aniline Derivatives by Using Parahydrogen: New Opportunities to Store Nuclear Spin Polarization in Aqueous Media. Chem. Sci 2019, 10 (37), 8577–8582. 10.1039/c9sc02970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Reineri F; Viale A; Ellena S; Boi T; Daniele V; Gobetto R; Aime S Use of Labile Precursors for the Generation of Hyperpolarized Molecules from Hydrogenation with Parahydrogen and Aqueous-Phase Extraction. Angew. Chem. Int. Ed 2011, 50 (32), 7350–7353. 10.1002/anie.201101359. [DOI] [PubMed] [Google Scholar]
- (80).Cavallari E; Carrera C; Sorge M; Bonne G; Muchir A; Aime S; Reineri F The 13 C Hyperpolarized Pyruvate Generated by ParaHydrogen Detects the Response of the Heart to Altered Metabolism in Real Time. Sci. Rep 2018, 8 (1), 8366. 10.1038/s41598-018-26583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Barskiy DA; Ke LA; Li X; Stevenson V; Widarman N; Zhang H; Truxal A; Pines A Rapid Catalyst Capture Enables Metal-Free Para-Hydrogen-Based Hyperpolarized Contrast Agents. J. Phys. Chem. Lett 2018, 9 (11), 2721–2724. 10.1021/acs.jpclett.8b01007. [DOI] [PubMed] [Google Scholar]
- (82).Dagys L; Jagtap AP; Korchak S; Mamone S; Saul P; Levitt MH; Glöggler S Nuclear Hyperpolarization of (1–13C)-Pyruvate in Aqueous Solution by Proton-Relayed Side-Arm Hydrogenation. Analyst 2021, 146 (5), 1772–1778. 10.1039/D0AN02389B. [DOI] [PubMed] [Google Scholar]
- (83).Goldman M; Johannesson H; Axelsson O; Karlsson M Design and Implementation of 13C Hyper Polarization from Para-Hydrogen, for New MRI Contrast Agents. Comtes Rendus Chim. 2006, 9, 357–363. [Google Scholar]
- (84).Goldman M; Axelsson O; Johannesson H; Ardenkjaer-Larsen J Method and Apparatus for Producing Contrast Agents for Magnetic Resonance Imaging. US20060127313A1, June 15, 2006. [Google Scholar]
- (85).Waddell KW; Coffey AM; Chekmenev EY In Situ Detection of PHIP at 48 MT: Demonstration Using a Centrally Controlled Polarizer. J. Am. Chem. Soc 2011, 133 (1), 97–101. 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Coffey AM; Shchepin RV; Truong ML; Wilkens K; Pham W; Chekmenev EY Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem 2016, 88, 8279–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Coffey AM; Shchepin RV; Feng B; Colon RD; Wilkens K; Waddell KW; Chekmenev EY A Pulse Programmable Parahydrogen Polarizer Using a Tunable Electromagnet and Dual Channel NMR Spectrometer. J. Magn. Reson 2017, 284 (Supplement C), 115–124. 10.1016/j.jmr.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Joalland B; Schmidt AB; Kabir MSH; Chukanov NV; Kovtunov KV; Koptyug IV; Hennig J; Hövener J-B; Chekmenev EY Pulse-Programmable Magnetic Field Sweeping of Parahydrogen-Induced Polarization by Side Arm Hydrogenation. Anal. Chem 2020, 92 (1), 1340–1345. 10.1021/acs.analchem.9b04501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Hövener J-B; Chekmenev EY; Harris KC; Perman WH; Tran TT; Ross BD; Bhattacharya P Quality Assurance of PASADENA Hyperpolarization for 13C Biomolecules. Magn Reson Mater Phy 2009, 22, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Schmidt AB; Berner S; Braig M; Zimmermann M; Hennig J; von Elverfeldt D; Hövener J-B In Vivo 13C-MRI Using SAMBADENA. PLOS ONE 2018, 13 (7), e0200141. 10.1371/journal.pone.0200141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Agraz J; Grunfeld AM; Cunningham K; Pozos R; Li D; Wagner S PHIP Instrumentation Pinch Valve System for Sample Delivery, Process and Collection. Adv. Biomed. Sci. Eng 2014, 1 (1), 8–18. [Google Scholar]
- (92).Borowiak R; Schwaderlapp N; Huethe F; Lickert T; Fischer E; Baer S; Hennig J; Elverfeldt D. v.; Hoevener JB A Battery-Driven, Low-Field NMR Unit for Thermally and Hyperpolarized Samples. Magn. Reson. Mater. Phys. Biol. Med 2013, 26 (5), 491–499. 10.1007/s10334-013-0366-7. [DOI] [PubMed] [Google Scholar]
- (93).Coffey AM; Shchepin RV; Wilkens K; Waddell KW; Chekmenev EY A Large Volume Double Channel 1H–X RF Probe for Hyperpolarized Magnetic Resonance at 0.0475T. J. Magn. Reson 2012, 220, 94–101. 10.1016/j.jmr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Kiryutin AS; Sauer G; Hadjiali S; Yurkovskaya AV; Breitzke H; Buntkowsky G A Highly Versatile Automatized Setup for Quantitative Measurements of PHIP Enhancements. J. Magn. Reson 2017, 285 (Supplement C), 26–36. 10.1016/j.jmr.2017.10.007. [DOI] [PubMed] [Google Scholar]
- (95).Hövener J-B; Schwaderlapp N; Borowiak R; Lickert T; Duckett SB; Mewis RE; Adams RW; Burns MJ; Highton LAR; Green GGR; Olaru A; Hennig J; von Elverfeldt D Toward Biocompatible Nuclear Hyperpolarization Using Signal Amplification by Reversible Exchange: Quantitative in Situ Spectroscopy and High-Field Imaging. Anal. Chem 2014, 86 (3), 1767–1774. 10.1021/ac403653q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Agraz J; Grunfeld AM; Li D; Cunningham K; Willey C; Pozos R; Wagner S LabVIEW-Based Control Software for Para-Hydrogen Induced Polarization Instrumentation. Rev. Sci. Instrum 2014, 85 (044705). [DOI] [PubMed] [Google Scholar]
- (97).Ellermann F; Pravdivtsev A; Hövener J-B Open-Source, Partially 3D-Printed, High-Pressure (50-Bar) Liquid-Nitrogen-Cooled Parahydrogen Generator. Magn. Reson 2021, 2 (1), 49–62. 10.5194/mr-2-49-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Juarez AM; Cubric D; King GC A Compact Catalytic Converter for the Production of Para-Hydrogen. Meas. Sci. Technol 2002, 13 (5), N52–N55. https://doi.org/10/fc82nq. [Google Scholar]
- (99).Sullivan NS; Zhou D; Edwards CM Precise and Efficient in Situ Ortho — Para-Hydrogen Converter. Cryogenics 1990, 30 (8), 734–735. 10.1016/0011-2275(90)90240-D. [DOI] [Google Scholar]
- (100).Hövener J-B; Bär S; Leupold J; Jenne K; Leibfritz D; Hennig J; Duckett SB; von Elverfeldt D A Continuous-Flow, High-Throughput, High-Pressure Parahydrogen Converter for Hyperpolarization in a Clinical Setting. NMR Biomed. 2013, 26 (2), 124–131. 10.1002/nbm.2827. [DOI] [PubMed] [Google Scholar]
- (101).Meier B; Dumez J-N; Stevanato G; Hill-Cousins JT; Roy SS; Håkansson P; Mamone S; Brown RCD; Pileio G; Levitt MH Long-Lived Nuclear Spin States in Methyl Groups and Quantum-Rotor-Induced Polarization. J. Am. Chem. Soc 2013, 135 (50), 18746–18749. 10.1021/ja410432f. [DOI] [PubMed] [Google Scholar]
- (102).Birchall JR; Coffey AM; Goodson BM; Chekmenev EY High-Pressure Clinical-Scale 87% Parahydrogen Generator. Anal. Chem 2020, 92 (23), 15280–15284. 10.1021/acs.analchem.0c03358. [DOI] [PubMed] [Google Scholar]
- (103).Sundararajan K; Sankaran K; Ramanathan N; Gopi R Production and Characterization of Para-Hydrogen Gas for Matrix Isolation Infrared Spectroscopy. J. Mol. Struct 2016, 1117, 181–191. 10.1016/j.molstruc.2016.03.068. [DOI] [Google Scholar]
- (104).Nantogma S; Joalland B; Wilkens K; Chekmenev EY Clinical-Scale Production of Nearly Pure (>98.5%) Parahydrogen and Quantification by Benchtop NMR Spectroscopy. Anal. Chem 2021, 93 (7), 3594–3601. 10.1021/acs.analchem.0c05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Du Y; Zhou R; Ferrer M-J; Chen M; Graham J; Malphurs B; Labbe G; Huang W; Bowers CR An Inexpensive Apparatus for up to 97% Continuous-Flow Parahydrogen Enrichment Using Liquid Helium. J. Magn. Reson 2020, 321, 106869. 10.1016/j.jmr.2020.106869. [DOI] [PubMed] [Google Scholar]
- (106).Buckenmaier K; Scheffler K; Plaumann M; Fehling P; Bernarding J; Rudolph M; Back C; Koelle D; Kleiner R; Hövener J; Pravdivtsev AN Multiple Quantum Coherences Hyperpolarized at Ultra-Low Fields. Chemphyschem 2019, 20 (21), 2823–2829. 10.1002/cphc.201900757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Kiryutin AS; Pravdivtsev AN; Ivanov KL; Grishin YA; Vieth H-M; Yurkovskaya AV A Fast Field-Cycling Device for High-Resolution NMR: Design and Application to Spin Relaxation and Hyperpolarization Experiments. J. Magn. Reson 2016, 263, 79–91. 10.1016/j.jmr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- (108).Jeong K; Min S; Chae H; Namgoong SK Detecting Low Concentrations of Unsaturated C—C Bonds by Parahydrogen-Induced Polarization Using an Efficient Home-Built Parahydrogen Generator. Magn. Reson. Chem 2018, 56 (11), 1089–1093. 10.1002/mrc.4756. [DOI] [PubMed] [Google Scholar]
- (109).Gamliel A; Allouche-Arnon H; Nalbandian R; Barzilay CM; Gomori JM; Katz-Brull R An Apparatus for Production of Isotopically and Spin-Enriched Hydrogen for Induced Polarization Studies. Appl. Magn. Reson 2010, 39 (4), 329–345. 10.1007/s00723-010-0161-9. [DOI] [Google Scholar]
- (110).Parrott AJ; Dallin P; Andrews J; Richardson PM; Semenova O; Halse ME; Duckett SB; Nordon A Quantitative In Situ Monitoring of Parahydrogen Fraction Using Raman Spectroscopy. Appl. Spectrosc 2019, 73 (1), 88–97. 10.1177/0003702818798644. [DOI] [PubMed] [Google Scholar]
- (111).Chapman B; Joalland B; Meersman C; Ettedgui J; Swenson RE; Krishna MC; Nikolaou P; Kovtunov KV; Salnikov OG; Koptyug IV; Gemeinhardt ME; Goodson BM; Shchepin RV; Chekmenev EY Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem 2021, 93 (24), 8476–8483. 10.1021/acs.analchem.1c00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Mhaske Y; Sutter E; Mahoney C; Daley J; Whiting N 65% Parahydrogen from a Liquid Nitrogen Cooled Generator. In PERM; Online, 2021. [DOI] [PubMed] [Google Scholar]
- (113).Hövener J-B; Pravdivtsev AN; Kidd B; Bowers CR; Glöggler S; Kovtunov KV; Plaumann M; Katz-Brull R; Buckenmaier K; Jerschow A; Reineri F; Theis T; Shchepin RV; Wagner S; Bhattacharya P; Zacharias NM; Chekmenev EY Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed 2018, 57 (35), 11140–11162. 10.1002/anie.201711842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Stewart NJ; Nakano H; Sugai S; Tomohiro M; Kase Y; Uchio Y; Yamaguchi T; Matsuo Y; Naganuma T; Takeda N; Nishimura I; Hirata H; Hashimoto T; Matsumoto S Hyperpolarized 13C Magnetic Resonance Imaging of Fumarate Metabolism by Parahydrogen-Induced Polarization: A Proof-of-Concept in Vivo Study. ChemPhysChem 2021, 22 (10), 915–923. 10.1002/cphc.202001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Sengstschmid H; Freeman R; Barkemeyer J; Bargon J A New Excitation Sequence to Observe the PASADENA Effect. J. Magn. Reson. A 1996, 120 (2), 249–257. 10.1006/jmra.1996.0121. [DOI] [Google Scholar]
- (116).Kiryutin AS; Ivanov KL; Yurkovskaya AV; Vieth H-M; Lukzen NN Manipulating Spin Hyper-Polarization by Means of Adiabatic Switching of a Spin-Locking RF-Field. Phys. Chem. Chem. Phys 2013, 15 (34), 14248–14255. 10.1039/C3CP52061G. [DOI] [PubMed] [Google Scholar]
- (117).Pravdivtsev AN; Sönnichsen F; Hövener J-B OnlyParahydrogen SpectrosopY (OPSY) Pulse Sequences – One Does Not Fit All. J. Magn. Reson 2018, 297, 86–95. 10.1016/j.jmr.2018.10.006. [DOI] [PubMed] [Google Scholar]
- (118).Pravdivtsev AN; Ivanov KL; Yurkovskaya AV; Vieth H-M; Sagdeev RZ New Pulse Sequence for Robust Filtering of Hyperpolarized Multiplet Spin Order. Dokl. Phys. Chem 2015, 465 (1), 267–269. 10.1134/S0012501615110044. [DOI] [Google Scholar]
- (119).Korchak S; Emondts M; Mamone S; Blümich B; Glöggler S Production of Highly Concentrated and Hyperpolarized Metabolites within Seconds in High and Low Magnetic Fields. Phys. Chem. Chem. Phys 2019, 21 (41), 22849–22856. 10.1039/C9CP05227E. [DOI] [PubMed] [Google Scholar]
- (120).Sørensen OW; Ernst RR Elimination of Spectral Distortion in Polarization Transfer Experiments. Improvements and Comparison of Techniques. J. Magn. Reson. 1969 1983, 51 (3), 477–489. 10.1016/0022-2364(83)90300-1. [DOI] [Google Scholar]
- (121).Barkemeyer J; Bargon J; Sengstschmid H; Freeman R Heteronuclear Polarization Transfer Using Selective Pulses during Hydrogenation with Parahydrogen. J. Magn. Reson. A 1996, 120 (1), 129–132. 10.1006/jmra.1996.0109. [DOI] [Google Scholar]
- (122).Pravdivtsev AN; Ellermann F; Hövener J-B Selective Excitation Doubles the Transfer of Parahydrogen-Induced Polarization to Heteronuclei. Phys. Chem. Chem. Phys 2021, 23 (26), 14146–14150. 10.1039/D1CP01891D. [DOI] [PubMed] [Google Scholar]
- (123).Stewart NJ; Kumeta H; Tomohiro M; Hashimoto T; Hatae N; Matsumoto S Long-Range Heteronuclear J-Coupling Constants in Esters: Implications for 13C Metabolic MRI by Side-Arm Parahydrogen-Induced Polarization. J. Magn. Reson 2018, 296, 85–92. 10.1016/j.jmr.2018.08.009. [DOI] [PubMed] [Google Scholar]
- (124).Pravdivtsev AN; Yurkovskaya AV; Lukzen NN; Ivanov KL; Vieth H-M Highly Efficient Polarization of Spin-1/2 Insensitive NMR Nuclei by Adiabatic Passage through Level Anticrossings. J. Phys. Chem. Lett 2014, 5 (19), 3421–3426. 10.1021/jz501754j. [DOI] [PubMed] [Google Scholar]
- (125).Rodin BA; Kozinenko VP; Kiryutin AS; Yurkovskaya AV; Eills J; Ivanov KL Constant-Adiabaticity Pulse Schemes for Manipulating Singlet Order in 3-Spin Systems with Weak Magnetic Non-Equivalence. J. Magn. Reson 2021, 327, 106978. 10.1016/j.jmr.2021.106978. [DOI] [PubMed] [Google Scholar]
- (126).Kozinenko VP; Kiryutin AS; Yurkovskaya AV; Ivanov KL Polarization of Low-γ Nuclei by Transferring Spin Order of Parahydrogen at High Magnetic Fields. J. Magn. Reson 2019, 309, 106594. 10.1016/j.jmr.2019.106594. [DOI] [PubMed] [Google Scholar]
- (127).Korchak S; Mamone S; Glöggler S Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A Para-Hydrogen Approach. ChemistryOpen 2018, 7 (9), 672–676. 10.1002/open.201800086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Svyatova A; Kozinenko VP; Chukanov NV; Burueva DB; Chekmenev EY; Chen Y-W; Hwang DW; Kovtunov KV; Koptyug IV PHIP Hyperpolarized [1–13C]Pyruvate and [1–13C]Acetate Esters via PH-INEPT Polarization Transfer Monitored by 13C NMR and MRI. Sci. Rep 2021, 11 (1), 5646. 10.1038/s41598-021-85136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Schmidt AB Liquid-State Nuclear Hyperpolarization without a Polarizer : Synthesis amid the Magnet Bore Allows a Dramatically Enhanced Nuclear Alignment. PhD Thesis, Albert-Ludwigs-Universität, Freiburg im Breisgau, 2020. [Google Scholar]
- (130).Korchak S; Jagtap AP; Glöggler S Signal-Enhanced Real-Time Magnetic Resonance of Enzymatic Reactions at Millitesla Fields. Chem. Sci 2021, 12 (1), 314–319. 10.1039/D0SC04884D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Lippens G; Dhalluin C; Wieruszeski JM Use of a Water Flip-Back Pulse in the Homonuclear NOESY Experiment. J. Biomol. NMR 1995, 5 (3), 327–331. 10.1007/BF00211762. [DOI] [PubMed] [Google Scholar]
- (132).Sklenar V Suppression of Radiation Damping in Multidimensional NMR Experiments Using Magnetic Field Gradients. J. Magn. Reson. A 1995, 114 (1), 132–135. 10.1006/jmra.1995.1119. [DOI] [Google Scholar]
- (133).Price WS; Wälchli M NMR Diffusion Measurements of Strong Signals: The PGSE-Q-Switch Experiment. Magn. Reson. Chem 2002, 40 (13), S128–S132. 10.1002/mrc.1113. [DOI] [Google Scholar]
- (134).de Maissin H; Berner S; Ivantaev V; Hoevener J-B; Hennig J; von Elverfeldt D; Schmidt AB Dramatic Effect of Pulse Length and Bandwidth on the Efficiency of Pulsed Spin-Order-Transfer Sequences at High Field. In Proc. Intl. Soc. Mag. Reson. Med 29; Online, 2021; p 3805. [Google Scholar]
- (135).Ivantaev V; Berner S; de Maissin H; Hoevener J-B; Hennig J; von Elverfeldt D; Kiselev V; Schmidt AB Molecular Translation in Inhomogeneous Field Can Dramatically Reduce the Efficiency of Spin-Order Transfer at High Field. In Proc. Intl. Soc. Mag. Reson. Med 29; Online, 2021; p 3798. [Google Scholar]
- (136).Golman K; Olsson LE; Axelsson O; Månsson S; Karlsson M; Petersson JS Molecular Imaging Using Hyperpolarized 13C. Br. J. Radiol 2003, 76 (suppl_2), S118–S127. 10.1259/bjr/26631666. [DOI] [PubMed] [Google Scholar]
- (137).Goldman M; Jóhannesson H; Axelsson O; Karlsson M Hyperpolarization of 13C through Order Transfer from Parahydrogen: A New Contrast Agent for MRI. Magn. Reson. Imaging 2005, 23 (2), 153–157. 10.1016/j.mri.2004.11.031. [DOI] [PubMed] [Google Scholar]
- (138).Shchepin RV; Coffey AM; Waddell KW; Chekmenev EY Parahydrogen-Induced Polarization with a Rh-Based Monodentate Ligand in Water. J. Phys. Chem. Lett 2012, 3 (22), 3281–3285. 10.1021/jz301389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Goodson BM; Whiting N; Coffey AM; Nikolaou P; Shi F; Gust BM; Gemeinhardt ME; Shchepin RV; Skinner JG; Birchall JR; Barlow MJ; Chekmenev EY Hyperpolarization Methods for MRS. eMagRes 2015, 4 (4). 10.1002/9780470034590.emrstm1457. [DOI] [Google Scholar]
- (140).Schmidt AB; Andrews DL; Rohrbach A; Gohn-Kreuz C; Shatokhin VN; Kiselev VG; Hennig J; von Elverfeldt D; Hövener J-B Do Twisted Laser Beams Evoke Nuclear Hyperpolarization? J. Magn. Reson 2016, 268, 58–67. 10.1016/j.jmr.2016.04.015. [DOI] [PubMed] [Google Scholar]
- (141).Shchepin RV; Coffey AM; Waddell KW; Chekmenev EY PASADENA Hyperpolarized 13C Phospholactate. J. Am. Chem. Soc 2012, 134 (9), 3957–3960. 10.1021/ja210639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Bhattacharya P; Chekmenev EY; Perman WH; Harris KC; Lin AP; Norton VA; Tan CT; Ross BD; Weitekamp DP Towards Hyperpolarized 13C-Succinate Imaging of Brain Cancer. J. Magn. Reson 2007, 186 (1), 150–155. 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Bhattacharya P; Chekmenev EY; Reynolds WF; Wagner S; Zacharias N; Chan HR; Bünger R; Ross BD Parahydrogen-induced Polarization (PHIP) Hyperpolarized MR Receptor Imaging in Vivo: A Pilot Study of 13C Imaging of Atheroma in Mice. NMR Biomed. 2011, 24 (8), 1023–1028. 10.1002/nbm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Perman WH; Bhattacharya P; Leupold J; Lin AP; Harris KC; Norton VA; Hövener J-B; Ross BD Fast Volumetric Spatial-Spectral MR Imaging of Hyperpolarized 13C-Labeled Compounds Using Multiple Echo 3D BSSFP. Magn. Reson. Imaging 2010, 28 (4), 459–465. 10.1016/j.mri.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Zacharias NM; Chan HR; Sailasuta N; Ross BD; Bhattacharya P Real-Time Molecular Imaging of Tricarboxylic Acid Cycle Metabolism in Vivo by Hyperpolarized 1–13C Diethyl Succinate. J. Am. Chem. Soc 2012, 134 (2), 934–943. 10.1021/ja2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Zacharias NM; McCullough CR; Wagner S; Sailasuta N; Chan HR; Lee Y; Hu J; Perman WH; Henneberg C; Ross BD; Bhattacharya P Towards Real-Time Metabolic Profiling of Cancer with Hyperpolarized Succinate. J. Mol. Imaging Dyn 2016, 6 (1), 123. 10.4172/2155-9937.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Coffey AM; Feldman MA; Shchepin RV; Barskiy DA; Truong ML; Pham W; Chekmenev EY High-Resolution Hyperpolarized in Vivo Metabolic 13C Spectroscopy at Low Magnetic Field (48.7mT) Following Murine Tail-Vein Injection. J. Magn. Reson 2017, 281 (Supplement C), 246–252. 10.1016/j.jmr.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Salnikov OG; Shchepin RV; Chukanov NV; Jaigirdar L; Pham W; Kovtunov KV; Koptyug IV; Chekmenev EY Effects of Deuteration of 13C-Enriched Phospholactate on Efficiency of Parahydrogen-Induced Polarization by Magnetic Field Cycling. J. Phys. Chem. C 2018, 122 (43), 24740–24749. 10.1021/acs.jpcc.8b07365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Chekmenev EY; Hövener J; Norton VA; Harris K; Batchelder LS; Bhattacharya P; Ross BD; Weitekamp DP PASADENA Hyperpolarization of Succinic Acid for MRI and NMR Spectroscopy. J. Am. Chem. Soc 2008, 130 (13), 4212–4213. 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Olsson LE; Chai C-M; Axelsson O; Karlsson M; Golman K; Petersson JS MR Coronary Angiography in Pigs with Intraarterial Injections of a Hyperpolarized 13C Substance. Magn. Reson. Med 2006, 55 (4), 731–737. 10.1002/mrm.20847. [DOI] [PubMed] [Google Scholar]
- (151).Reineri F; Cavallari E; Carrera C; Aime S Hydrogenative-PHIP Polarized Metabolites for Biological Studies. Magn. Reson. Mater. Phys. Biol. Med 2021, 34 (1), 25–47. 10.1007/s10334-020-00904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Butler MC; Kervern G; Theis T; Ledbetter MP; Ganssle PJ; Blanchard JW; Budker D; Pines A Parahydrogen-Induced Polarization at Zero Magnetic Field. J. Chem. Phys 2013, 138 (23), 234201. 10.1063/1.4805062. [DOI] [PubMed] [Google Scholar]
- (153).Theis T; Ganssle P; Kervern G; Knappe S; Kitching J; Ledbetter MP; Budker D; Pines A Parahydrogen-Enhanced Zero-Field Nuclear Magnetic Resonance. Nat. Phys 2011, 7 (7), 571–575. 10.1038/nphys1986. [DOI] [Google Scholar]
- (154).Emondts M; P. Colell JF; Blümich B; M. Schleker PP Polarization Transfer Efficiency in PHIP Experiments. Phys. Chem. Chem. Phys 2017, 19 (33), 21933–21937. 10.1039/C7CP04296E. [DOI] [PubMed] [Google Scholar]
- (155).Pravdivtsev AN; Ivanov KL; Yurkovskaya AV; Vieth H-M; Sagdeev RZ Spontaneous Transfer of Para-Hydrogen Induced Polarization to 13C Spins in Symmetric Molecules1. Dokl. Phys. Chem 2015, 464 (2), 247–250. 10.1134/S0012501615100073. [DOI] [Google Scholar]
- (156).Eills J; Blanchard JW; Wu T; Bengs C; Hollenbach J; Budker D; Levitt MH Polarization Transfer via Field Sweeping in Parahydrogen-Enhanced Nuclear Magnetic Resonance. J. Chem. Phys 2019, 150 (17), 174202. 10.1063/1.5089486. [DOI] [PubMed] [Google Scholar]
- (157).Cavallari E; Carrera C; Aime S; Reineri F Studies to Enhance the Hyperpolarization Level in PHIP-SAH-Produced C13-Pyruvate. J. Magn. Reson 2018, 289, 12–17. 10.1016/j.jmr.2018.01.019. [DOI] [PubMed] [Google Scholar]
- (158).Kuhn LT; Bommerich U; Bargon J Transfer of Parahydrogen-Induced Hyperpolarization to 19F. J. Phys. Chem. A 2006, 110 (10), 3521–3526. 10.1021/jp056219n. [DOI] [PubMed] [Google Scholar]
- (159).Stephan M; Kohlmann O; Niessen HG; Eichhorn A; Bargon J 13C PHIP NMR Spectra and Polarization Transfer during the Homogeneous Hydrogenation of Alkynes with Parahydrogen. Magn. Reson. Chem 2002, 40 (2), 157–160. 10.1002/mrc.973. [DOI] [Google Scholar]
- (160).Jonischkeit T; Bommerich U; Stadler J; Woelk K; Niessen HG; Bargon J Generating Long-Lasting H1 and C13 Hyperpolarization in Small Molecules with Parahydrogen-Induced Polarization. J. Chem. Phys 2006, 124 (20), 201109. 10.1063/1.2209235. [DOI] [PubMed] [Google Scholar]
- (161).Theis T; Truong ML; Coffey AM; Shchepin RV; Waddell KW; Shi F; Goodson BM; Warren WS; Chekmenev EY Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc 2015, 137 (4), 1404–1407. 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Truong ML; Theis T; Coffey AM; Shchepin RV; Waddell KW; Shi F; Goodson BM; Warren WS; Chekmenev EY 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119 (16), 8786–8797. 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Pravdivtsev AN; Ivanov KL; Yurkovskaya AV; Petrov PA; Limbach H-H; Kaptein R; Vieth H-M Spin Polarization Transfer Mechanisms of SABRE: A Magnetic Field Dependent Study. J. Magn. Reson 2015, 261, 73–82. 10.1016/j.jmr.2015.10.006. [DOI] [PubMed] [Google Scholar]
- (164).Zhivonitko VV; Skovpin IV; Koptyug IV Strong 31P Nuclear Spin Hyperpolarization Produced via Reversible Chemical Interaction with Parahydrogen. Chem. Commun 2015, 51 (13), 2506–2509. 10.1039/C4CC08115C. [DOI] [PubMed] [Google Scholar]
- (165).Lindale JR; Eriksson SL; Tanner CPN; Zhou Z; Colell JFP; Zhang G; Bae J; Chekmenev EY; Theis T; Warren WS Unveiling Coherently Driven Hyperpolarization Dynamics in Signal Amplification by Reversible Exchange. Nat. Commun 2019, 10 (1), 395. 10.1038/s41467-019-08298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Pravdivtsev AN; Kempf N; Plaumann M; Bernarding J; Scheffler K; Hövener J-B; Buckenmaier K Coherent Evolution of Signal Amplification by Reversible Exchange in Two Alternating Fields (Alt-SABRE). ArXiv210709294 Phys. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Dagys L; Bengs C; Levitt MH Low-Frequency Excitation of Singlet–Triplet Transitions. Application to Nuclear Hyperpolarization. J. Chem. Phys 2021, 155 (15), 154201. 10.1063/5.0065863. [DOI] [PubMed] [Google Scholar]
- (168).Buckenmaier K; Rudolph M; Fehling P; Steffen T; Back C; Bernard R; Pohmann R; Bernarding J; Kleiner R; Koelle D; Plaumann M; Scheffler K Mutual Benefit Achieved by Combining Ultralow-Field Magnetic Resonance and Hyperpolarizing Techniques. Rev. Sci. Instrum 2018, 89 (12), 125103. 10.1063/1.5043369. [DOI] [PubMed] [Google Scholar]
- (169).Blanchard JW; Ripka B; Suslick BA; Gelevski D; Wu T; Münnemann K; Barskiy DA; Budker D Towards Large-Scale Steady-State Enhanced Nuclear Magnetization with in Situ Detection. Magn. Reson. Chem 2021, 1–8. 10.1002/mrc.5161. [DOI] [PubMed] [Google Scholar]
- (170).Arunkumar N; Bucher DB; Turner MJ; TomHon P; Glenn D; Lehmkuhl S; Lukin MD; Park H; Rosen MS; Theis T; Walsworth RL Micron-Scale NV-NMR Spectroscopy with Signal Amplification by Reversible Exchange. PRX Quantum 2021, 2 (1), 010305. 10.1103/PRXQuantum.2.010305. [DOI] [Google Scholar]
- (171).Blanchard JW; Ledbetter MP; Theis T; Butler MC; Budker D; Pines A High-Resolution Zero-Field NMR J-Spectroscopy of Aromatic Compounds. J. Am. Chem. Soc 2013, 135 (9), 3607–3612. 10.1021/ja312239v. [DOI] [PubMed] [Google Scholar]
- (172).Blanchard JW; Wu T; Eills J; Hu Y; Budker D Zero- to Ultralow-Field Nuclear Magnetic Resonance J-Spectroscopy with Commercial Atomic Magnetometers. J. Magn. Reson 2020, 314, 106723. 10.1016/j.jmr.2020.106723. [DOI] [PubMed] [Google Scholar]
- (173).Burueva DB; Eills J; Blanchard JW; Garcon A; Picazo-Frutos R; Kovtunov KV; Koptyug IV; Budker D Chemical Reaction Monitoring Using Zero-Field Nuclear Magnetic Resonance Enables Study of Heterogeneous Samples in Metal Containers. Angew. Chem. Int. Ed 2020, 59 (39), 17026–17032. 10.1002/anie.202006266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Tayler MCD; Theis T; Sjolander TF; Blanchard JW; Kentner A; Pustelny S; Pines A; Budker D Invited Review Article: Instrumentation for Nuclear Magnetic Resonance in Zero and Ultralow Magnetic Field. Rev. Sci. Instrum 2017, 88 (9), 091101. 10.1063/1.5003347. [DOI] [PubMed] [Google Scholar]
- (175).Myers W; Slichter D; Hatridge M; Busch S; Mößle M; McDermott R; Trabesinger A; Clarke J Calculated Signal-to-Noise Ratio of MRI Detected with SQUIDs and Faraday Detectors in Fields from 10μT to 1.5T. J. Magn. Reson 2007, 186 (2), 182–192. 10.1016/j.jmr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- (176).Theis T; Blanchard JW; Butler MC; Ledbetter MP; Budker D; Pines A Chemical Analysis Using J-Coupling Multiplets in Zero-Field NMR. Chem. Phys. Lett 2013, 580, 160–165. 10.1016/j.cplett.2013.06.042. [DOI] [PubMed] [Google Scholar]
- (177).Theis T; Ledbetter MP; Kervern G; Blanchard JW; Ganssle PJ; Butler MC; Shin HD; Budker D; Pines A Zero-Field NMR Enhanced by Parahydrogen in Reversible Exchange. J. Am. Chem. Soc 2012, 134 (9), 3987–3990. 10.1021/ja2112405. [DOI] [PubMed] [Google Scholar]
- (178).Lee S-J; Jeong K; Shim JH; Lee HJ; Min S; Chae H; Namgoong SK; Kim K SQUID-Based Ultralow-Field MRI of a Hyperpolarized Material Using Signal Amplification by Reversible Exchange. Sci. Rep 2019, 9 (1), 12422. 10.1038/s41598-019-48827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Vesanen PT; Nieminen JO; Zevenhoven KCJ; Dabek J; Parkkonen LT; Zhdanov AV; Luomahaara J; Hassel J; Penttilä J; Simola J; Ahonen AI; Mäkelä JP; Ilmoniemi RJ Hybrid Ultra-Low-Field MRI and Magnetoencephalography System Based on a Commercial Whole-Head Neuromagnetometer. Magn. Reson. Med 2013, 69 (6), 1795–1804. 10.1002/mrm.24413. [DOI] [PubMed] [Google Scholar]
- (180).Bucher DB; Glenn DR; Park H; Lukin MD; Walsworth RL Hyperpolarization-Enhanced NMR Spectroscopy with Femtomole Sensitivity Using Quantum Defects in Diamond. Phys. Rev. X 2020, 10 (2), 021053. 10.1103/PhysRevX.10.021053. [DOI] [Google Scholar]
- (181).Zheng H; Xu J; Iwata GZ; Lenz T; Michl J; Yavkin B; Nakamura K; Sumiya H; Ohshima T; Isoya J; Wrachtrup J; Wickenbrock A; Budker D Zero-Field Magnetometry Based on Nitrogen-Vacancy Ensembles in Diamond. Phys. Rev. Appl 2019, 11 (6), 064068. 10.1103/PhysRevApplied.11.064068. [DOI] [Google Scholar]
- (182).Kimmich R Chapter 1 Principle, Purpose and Pitfalls of Field-Cycling NMR Relaxometry. 2018, 1–41. 10.1039/9781788012966-00001. [DOI] [Google Scholar]
- (183).Ivanov KL; Pravdivtsev AN; Yurkovskaya AV; Vieth H-M; Kaptein R The Role of Level Anti-Crossings in Nuclear Spin Hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc 2014, 81, 1–36. 10.1016/j.pnmrs.2014.06.001. [DOI] [PubMed] [Google Scholar]
- (184).Shchepin RV; Barskiy DA; Coffey AM; Manzanera Esteve IV; Chekmenev EY Efficient Synthesis of Molecular Precursors for Para-Hydrogen-Induced Polarization of Ethyl Acetate-1–13C and Beyond. Angew. Chem. Int. Ed 2016, 55 (20), 6071–6074. 10.1002/anie.201600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Reineri F; Viale A; Dastrù W; Gobetto R; Aime S How to Design 13C Para-Hydrogen-Induced Polarization Experiments for MRI Applications. Contrast Media Mol. Imaging 2011, 6 (2), 77–84. 10.1002/cmmi.407. [DOI] [PubMed] [Google Scholar]
- (186).Reineri F; Viale A; Ellena S; Alberti D; Boi T; Giovenzana GB; Gobetto R; Premkumar SSD; Aime S 15N Magnetic Resonance Hyperpolarization via the Reaction of Parahydrogen with 15N-Propargylcholine. J. Am. Chem. Soc 2012, 134 (27), 11146–11152. 10.1021/ja209884h. [DOI] [PubMed] [Google Scholar]
- (187).Ellena S; Viale A; Gobetto R; Aime S Para-Hydrogen Induced Polarization of Si-29 NMR Resonances as a Potentially Useful Tool for Analytical Applications. Magn. Reson. Chem 2012, 50 (8), 529–533. 10.1002/mrc.3832. [DOI] [PubMed] [Google Scholar]
- (188).Eills J; Cavallari E; Carrera C; Budker D; Aime S; Reineri F Real-Time Nuclear Magnetic Resonance Detection of Fumarase Activity Using Parahydrogen-Hyperpolarized [1–13C]Fumarate. J. Am. Chem. Soc 2019, 141 (51), 20209–20214. 10.1021/jacs.9b10094. [DOI] [PubMed] [Google Scholar]
- (189).Ripka B; Eills J; Kouřilová H; Leutzsch M; H. Levitt M; Münnemann K Hyperpolarized Fumarate via Parahydrogen. Chem. Commun 2018, 54 (86), 12246–12249. 10.1039/C8CC06636A. [DOI] [PubMed] [Google Scholar]
- (190).Rodin A; Eills J; Picazo-Frutos R; F. Sheberstov K; Budker D; L. Ivanov K Constant-Adiabaticity Ultralow Magnetic Field Manipulations of Parahydrogen-Induced Polarization: Application to an AA’X Spin System. Phys. Chem. Chem. Phys 2021, 23 (12), 7125–7134. 10.1039/D0CP06581A. [DOI] [PubMed] [Google Scholar]
- (191).Reineri F; Santelia D; Gobetto R; Aime S Effect of Low and Zero Magnetic Field on the Hyperpolarization Lifetime in Parahydrogenated Perdeuterated Molecules. J. Magn. Reson. San Diego Calif 1997 2009, 200 (1), 15–20. 10.1016/j.jmr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- (192).Pokochueva EV; Burueva DB; Salnikov OG; Koptyug IV Heterogeneous Catalysis and Parahydrogen-Induced Polarization. ChemPhysChem 2021, 22 (14), 1421–1440. 10.1002/cphc.202100153. [DOI] [PubMed] [Google Scholar]
- (193).Kovtunov KV; Barskiy DA; Shchepin RV; Coffey AM; Waddell KW; Koptyug IV; Chekmenev EY Demonstration of Heterogeneous Parahydrogen Induced Polarization Using Hyperpolarized Agent Migration from Dissolved Rh(I) Complex to Gas Phase. Anal. Chem 2014, 86 (13), 6192–6196. 10.1021/ac5013859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Kovtunov KV; Zhivonitko VV; Skovpin IV; Barskiy DA; Salnikov OG; Koptyug IV Toward Continuous Production of Catalyst-Free Hyperpolarized Fluids Based on Biphasic and Heterogeneous Hydrogenations with Parahydrogen. J. Phys. Chem. C 2013, 117 (44), 22887–22893. 10.1021/jp407348r. [DOI] [Google Scholar]
- (195).Salnikov OG; Kovtunov KV; Koptyug IV Production of Catalyst-Free Hyperpolarised Ethanol Aqueous Solution via Heterogeneous Hydrogenation with Parahydrogen. Sci. Rep 2015, 5 (1), 13930. 10.1038/srep13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Salnikov OG; Svyatova A; Kovtunova LM; Chukanov NV; Bukhtiyarov VI; Kovtunov KV; Chekmenev EY; Koptyug IV Heterogeneous Parahydrogen-Induced Polarization of Diethyl Ether for Magnetic Resonance Imaging Applications. Chem. – Eur. J 2021, 27 (4), 1316–1322. 10.1002/chem.202003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Du Y; Behera R; Maligal-Ganesh RV; Chen M; Chekmenev EY; Huang W; Bowers CR Cyclopropane Hydrogenation vs Isomerization over Pt and Pt–Sn Intermetallic Nanoparticle Catalysts: A Parahydrogen Spin-Labeling Study. J. Phys. Chem. C 2020, 124 (15), 8304–8309. 10.1021/acs.jpcc.0c02493. [DOI] [Google Scholar]
- (198).Zhou R; Cheng W; Neal LM; Zhao EW; Ludden K; Hagelin-Weaver HE; Bowers CR Parahydrogen Enhanced NMR Reveals Correlations in Selective Hydrogenation of Triple Bonds over Supported Pt Catalyst. Phys. Chem. Chem. Phys 2015, 17 (39), 26121–26129. 10.1039/C5CP04223B. [DOI] [PubMed] [Google Scholar]
- (199).Burueva DB; Kozinenko VP; Sviyazov SV; Kovtunova LM; Bukhtiyarov VI; Chekmenev EY; Salnikov OG; Kovtunov KV; Koptyug IV Gas-Phase NMR of Hyperpolarized Propane with 1H-to-13C Polarization Transfer by PH-INEPT. Appl. Magn. Reson 2021. 10.1007/s00723-021-01377-4. [DOI] [Google Scholar]
- (200).Salnikov OG; Nikolaou P; Ariyasingha NM; Kovtunov KV; Koptyug IV; Chekmenev EY Clinical-Scale Batch-Mode Production of Hyperpolarized Propane Gas for MRI. Anal. Chem 2019, 91 (7), 4741–4746. 10.1021/acs.analchem.9b00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Kovtunov KV; Barskiy DA; Coffey AM; Truong ML; Salnikov OG; Khudorozhkov AK; Inozemtseva EA; Prosvirin IP; Bukhtiyarov VI; Waddell KW; Chekmenev EY; Koptyug IV High-Resolution 3D Proton MRI of Hyperpolarized Gas Enabled by Parahydrogen and Rh/TiO2 Heterogeneous Catalyst. Chem. Weinh. Bergstr. Ger 2014, 20 (37), 11636–11639. 10.1002/chem.201403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Salnikov OG; Kovtunov KV; Nikolaou P; Kovtunova LM; Bukhtiyarov VI; Koptyug IV; Chekmenev EY Heterogeneous Parahydrogen Pairwise Addition to Cyclopropane. Chemphyschem Eur. J. Chem. Phys. Phys. Chem 2018, 19 (20), 2621–2626. 10.1002/cphc.201800690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Burueva DB; Kovtunov KV; Bukhtiyarov AV; Barskiy DA; Prosvirin IP; Mashkovsky IS; Baeva GN; Bukhtiyarov VI; Stakheev A. Yu.; Koptyug IV Selective Single-Site Pd−In Hydrogenation Catalyst for Production of Enhanced Magnetic Resonance Signals Using Parahydrogen. Chem. – Eur. J 2018, 24 (11), 2547–2553. 10.1002/chem.201705644. [DOI] [PubMed] [Google Scholar]
- (204).Telkki V-V; Zhivonitko VV; Ahola S; Kovtunov KV; Jokisaari J; Koptyug IV Microfluidic Gas-Flow Imaging Utilizing Parahydrogen-Induced Polarization and Remote-Detection NMR. Angew. Chem. Int. Ed 2010, 49 (45), 8363–8366. 10.1002/anie.201002685. [DOI] [PubMed] [Google Scholar]
- (205).Telkki V-V; Zhivonitko VV Analysis of Remote Detection Travel Time Curves Measured from Microfluidic Channels. J. Magn. Reson 2011, 210 (2), 238–245. 10.1016/j.jmr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- (206).Bouchard L-S; Burt SR; Anwar MS; Kovtunov KV; Koptyug IV; Pines A NMR Imaging of Catalytic Hydrogenation in Microreactors with the Use of Para-Hydrogen. Science 2008, 319 (5862), 442–445. 10.1126/science.1151787. [DOI] [PubMed] [Google Scholar]
- (207).Zhivonitko VV; Telkki V-V; Koptyug IV Characterization of Microfluidic Gas Reactors Using Remote-Detection MRI and Parahydrogen-Induced Polarization. Angew. Chem 2012, 124 (32), 8178–8182. 10.1002/ange.201202967. [DOI] [PubMed] [Google Scholar]
- (208).Kovtunov KV; Lebedev D; Svyatova A; Pokochueva EV; Prosvirin IP; Gerasimov EY; Bukhtiyarov VI; Müller CR; Fedorov A; Koptyug IV Robust In Situ Magnetic Resonance Imaging of Heterogeneous Catalytic Hydrogenation with and without Hyperpolarization. ChemCatChem 2019, 11 (3), 969–973. 10.1002/cctc.201801820. [DOI] [Google Scholar]
- (209).Svyatova A; Kononenko S; V. Kovtunov K; Lebedev D; Yu. Gerasimov E; V. Bukhtiyarov A; P. Prosvirin I; I. Bukhtiyarov V; R. Müller C; Fedorov A; V. Koptyug I Spatially Resolved NMR Spectroscopy of Heterogeneous Gas Phase Hydrogenation of 1,3-Butadiene with Para Hydrogen. Catal. Sci. Technol 2020, 10 (1), 99–104. 10.1039/C9CY02100K. [DOI] [Google Scholar]
- (210).Barskiy DA; Kovtunov KV; Gerasimov EY; Phipps MA; Salnikov OG; Coffey AM; Kovtunova LM; Prosvirin IP; Bukhtiyarov VI; Koptyug IV; Chekmenev EY 2D Mapping of NMR Signal Enhancement and Relaxation for Heterogeneously Hyperpolarized Propane Gas. J. Phys. Chem. C 2017, 121 (18), 10038–10046. 10.1021/acs.jpcc.7b02506. [DOI] [Google Scholar]
- (211).Ariyasingha NM; Salnikov OG; Kovtunov KV; Kovtunova LM; Bukhtiyarov VI; Goodson BM; Rosen MS; Koptyug IV; Gelovani JG; Chekmenev EY Relaxation Dynamics of Nuclear Long-Lived Spin States in Propane and Propane-D6 Hyperpolarized by Parahydrogen. J. Phys. Chem. C 2019, 123 (18), 11734–11744. 10.1021/acs.jpcc.9b01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Barskiy DA; Salnikov OG; Romanov AS; Feldman MA; Coffey AM; Kovtunov KV; Koptyug IV; Chekmenev EY NMR Spin-Lock Induced Crossing (SLIC) Dispersion and Long-Lived Spin States of Gaseous Propane at Low Magnetic Field (0.05T). J. Magn. Reson 2017, 276, 78–85. 10.1016/j.jmr.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Ariyasingha NM; Joalland B; Younes HR; Salnikov OG; Chukanov NV; Kovtunov KV; Kovtunova LM; Bukhtiyarov VI; Koptyug IV; Gelovani JG; Chekmenev EY Parahydrogen-Induced Polarization of Diethyl Ether Anesthetic. Chem. – Eur. J 2020, 26 (60), 13621–13626. 10.1002/chem.202002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Zhao EW; Maligal-Ganesh R; Xiao C; Goh T-W; Qi Z; Pei Y; Hagelin-Weaver HE; Huang W; Bowers CR Silica-Encapsulated Pt-Sn Intermetallic Nanoparticles: A Robust Catalytic Platform for Parahydrogen-Induced Polarization of Gases and Liquids. Angew. Chem 2017, 129 (14), 3983–3987. 10.1002/ange.201701314. [DOI] [PubMed] [Google Scholar]
- (215).Zhao EW; Xin Y; Hagelin-Weaver HE; Bowers CR Semihydrogenation of Propyne over Cerium Oxide Nanorods, Nanocubes, and Nano-Octahedra: Facet-Dependent Parahydrogen-Induced Polarization. ChemCatChem 2016, 8 (13), 2197–2201. 10.1002/cctc.201600270. [DOI] [Google Scholar]
- (216).Barskiy DA; Salnikov OG; Kovtunov KV; Koptyug IV NMR Signal Enhancement for Hyperpolarized Fluids Continuously Generated in Hydrogenation Reactions with Parahydrogen. J. Phys. Chem. A 2015, 119 (6), 996–1006. 10.1021/jp510572d. [DOI] [PubMed] [Google Scholar]
- (217).Sharma R; Bouchard L-S Strongly Hyperpolarized Gas from Parahydrogen by Rational Design of Ligand-Capped Nanoparticles. Sci. Rep 2012, 2 (1), 277. 10.1038/srep00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Kidd BE; Gesiorski JL; Gemeinhardt ME; Shchepin RV; Kovtunov KV; Koptyug IV; Chekmenev EY; Goodson BM Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122 (29), 16848–16852. 10.1021/acs.jpcc.8b05758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Cavallari E; Carrera C; Di Matteo G; Bondar O; Aime S; Reineri F In-Vitro NMR Studies of Prostate Tumor Cell Metabolism by Means of Hyperpolarized [1–13C]Pyruvate Obtained Using the PHIP-SAH Method. Front. Oncol 2020, 10, 497. 10.3389/fonc.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Bondar O; Cavallari E; Carrera C; Aime S; Reineri F Effect of the Hydrogenation Solvent in the PHIP-SAH Hyperpolarization of [1–13 C]Pyruvate. ArXiv210801497 Phys. 2021. 10.13140/RG.2.2.13422.95046. [DOI] [Google Scholar]
- (221).Cavallari E; Carrera C; Aime S; Reineri F Metabolic Studies of Tumor Cells Using [1–13 C] Pyruvate Hyperpolarized by Means of PHIP-Side Arm Hydrogenation. Chemphyschem Eur. J. Chem. Phys. Phys. Chem 2019, 20 (2), 318–325. 10.1002/cphc.201800652. [DOI] [PubMed] [Google Scholar]
- (222).Cavallari E; Carrera C; Reineri F ParaHydrogen Hyperpolarized Substrates for Molecular Imaging Studies. Isr. J. Chem 2017, 57 (9), 833–842. 10.1002/ijch.201700030. [DOI] [Google Scholar]
- (223).Srour M; Hadjiali S; Brunnengräber K; Weidler H; Xu Y; Breitzke H; Gutmann T; Buntkowsky G A Novel Wilkinson’s Type Silica Supported Polymer Catalyst: Insights from Solid-State NMR and Hyperpolarization Techniques. J. Phys. Chem. C 2021, 125 (13), 7178–7187. 10.1021/acs.jpcc.1c00112. [DOI] [Google Scholar]
- (224).Pokochueva EV; Kovtunov KV; Salnikov OG; Gemeinhardt ME; Kovtunova LM; Bukhtiyarov VI; Chekmenev EY; Goodson BM; Koptyug IV Heterogeneous Hydrogenation of Phenylalkynes with Parahydrogen: Hyperpolarization, Reaction Selectivity, and Kinetics. Phys. Chem. Chem. Phys 2019, 21 (48), 26477–26482. 10.1039/C9CP02913C. [DOI] [PubMed] [Google Scholar]
- (225).Salnikov OG; Chukanov NV; Shchepin RV; Manzanera Esteve IV; Kovtunov KV; Koptyug IV; Chekmenev EY Parahydrogen-Induced Polarization of 1–13C-Acetates and 1–13C-Pyruvates Using Sidearm Hydrogenation of Vinyl, Allyl, and Propargyl Esters. J. Phys. Chem. C 2019, 123 (20), 12827–12840. 10.1021/acs.jpcc.9b02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).McCormick J; Grunfeld AM; Ertas YN; Biswas AN; Marsh KL; Wagner S; Glöggler S; Bouchard L-S Aqueous Ligand-Stabilized Palladium Nanoparticle Catalysts for Parahydrogen-Induced 13C Hyperpolarization. Anal. Chem 2017, 89 (13), 7190–7194. 10.1021/acs.analchem.7b01363. [DOI] [PubMed] [Google Scholar]
- (227).Pei Y; Chen M; Zhong X; Yunpu Zhao T; Ferrer M-J; V. Maligal-Ganesh R; Ma T; Zhang B; Qi Z; Zhou L; R. Bowers C; Liu C; Huang W Pairwise Semi-Hydrogenation of Alkyne to Cis -Alkene on Platinum-Tin Intermetallic Compounds. Nanoscale 2020, 12 (15), 8519–8524. 10.1039/D0NR00920B. [DOI] [PubMed] [Google Scholar]
- (228).McCormick J; Korchak S; Mamone S; Ertas Y; Liu Z; Verlinsky L; Wagner S; Gloeggler S; Bouchard L Over 12% Polarization and 20 Minute Lifetime of 15N on Choline Derivative Utilizing Parahydrogen and Rh Nanocatalyst in Water. Angew. Chem. Int. Ed 0 (ja). 10.1002/anie.201804185. [DOI] [PubMed] [Google Scholar]
- (229).Kadlecek S; Shaghaghi H; Siddiqui S; Profka H; Pourfathi M; Rizi R The Effect of Exogenous Substrate Concentrations on True and Apparent Metabolism of Hyperpolarized Pyruvate in the Isolated Perfused Lung. NMR Biomed. 2014, 27 (12), 1557–1570. 10.1002/nbm.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Eills J; Alonso-Valdesueiro J; Salazar Marcano DE; Ferreira da Silva J; Alom S; Rees GJ; Hanna JV; Carravetta M; Levitt MH Preservation of Nuclear Spin Order by Precipitation. ChemPhysChem 2018, 19 (1), 40–44. 10.1002/cphc.201701189. [DOI] [PubMed] [Google Scholar]
- (231).Eills J; Hale W; Sharma M; Rossetto M; Levitt MH; Utz M High-Resolution Nuclear Magnetic Resonance Spectroscopy with Picomole Sensitivity by Hyperpolarization on a Chip. J. Am. Chem. Soc 2019, 141 (25), 9955–9963. 10.1021/jacs.9b03507. [DOI] [PubMed] [Google Scholar]
- (232).Bordonali L; Nordin N; Fuhrer E; MacKinnon N; Korvink JG Parahydrogen Based NMR Hyperpolarisation Goes Micro: An Alveolus for Small Molecule Chemosensing. Lab. Chip 2019, 19 (3), 503–512. 10.1039/C8LC01259H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Ostrowska SJ; Rana A; Utz M Spatially Resolved Kinetic Model of Parahydrogen Induced Polarisation (PHIP) in a Microfluidic Chip. ChemPhysChem 2021, 22 (19), 2004–2013. 10.1002/cphc.202100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Eills J; Hale W; Utz M Synergies between Hyperpolarized NMR and Microfluidics: A Review. Prog. Nucl. Magn. Reson. Spectrosc 2021. 10.1016/j.pnmrs.2021.09.001. [DOI] [PubMed] [Google Scholar]
- (235).Suefke M; Lehmkuhl S; Liebisch A; Blümich B; Appelt S Para-Hydrogen Raser Delivers Sub-Millihertz Resolution in Nuclear Magnetic Resonance. Nat. Phys 2017, 13 (6), 568–572. 10.1038/nphys4076. [DOI] [Google Scholar]
- (236).Joalland B; Theis T; Appelt S; Chekmenev EY Background-Free Proton NMR Spectroscopy with Radiofrequency Amplification by Stimulated Emission Radiation. Angew. Chem. Int. Ed n/a (n/a). 10.1002/anie.202108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Gilles H; Monfort Y; Hamel J 3He Maser for Earth Magnetic Field Measurement. Rev. Sci. Instrum 2003, 74 (10), 4515–4520. 10.1063/1.1605494. [DOI] [Google Scholar]
- (238).Heil W; Gemmel C; Karpuk S; Sobolev Y; Tullney K; Allmendinger F; Schmidt U; Burghoff M; Kilian W; Knappe-Grüneberg S; Schnabel A; Seifert F; Trahms L Spin Clocks: Probing Fundamental Symmetries in Nature. Ann. Phys 2013, 525 (8–9), 539–549. 10.1002/andp.201300048. [DOI] [Google Scholar]
- (239).Chen H-Y; Lee Y; Bowen S; Hilty C Spontaneous Emission of NMR Signals in Hyperpolarized Proton Spin Systems. J. Magn. Reson. San Diego Calif 1997 2011, 208 (2), 204–209. 10.1016/j.jmr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- (240).Iali W; Rayner PJ; Duckett SB Using Parahydrogen to Hyperpolarize Amines, Amides, Carboxylic Acids, Alcohols, Phosphates, and Carbonates. Sci. Adv 2018, 4 (1), eaao6250. 10.1126/sciadv.aao6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).M. Richardson P; O. John R; J. Parrott A; J. Rayner P; Iali W; Nordon A; E. Halse M; B. Duckett S Quantification of Hyperpolarisation Efficiency in SABRE and SABRE-Relay Enhanced NMR Spectroscopy. Phys. Chem. Chem. Phys 2018, 20 (41), 26362–26371. 10.1039/C8CP05473H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Knecht S; Barskiy DA; Buntkowsky G; Ivanov KL Theoretical Description of Hyperpolarization Formation in the SABRE-Relay Method. J. Chem. Phys 2020, 153 (16), 164106. 10.1063/5.0023308. [DOI] [PubMed] [Google Scholar]


