Abstract
Acetamiprid is a pesticide active substance with insecticidal action currently under the third renewal (AIR3) of the Commission implementing regulation (EU) No 844/2012. Following concerns that this substance may pose high risks to humans and the environment, the French authorities asked the Commission to restrict its uses under Article 69 of Regulation (EC) No 1107/2009. To support this request, competent Authorities from France cited a series of literature papers investigating its hazards and/or exposure to humans and the environment. Consequently, the EFSA PPR Panel was mandated to advise on the likelihood that body of evidence would constitute proof of serious risks to humans or the environment. Therefore, the EFSA PPR Panel evaluated the likelihood of these studies indicating new or higher hazards and exposure to humans and the environment compared to previous EU assessments.A stepwise methodology was designed, including: (i) the initial screening; (ii) the data extraction and critical appraisal based on the principles of OHAT/NTP; (iii) the weight of evidence, including consideration of the previous EU assessments; (iv) the uncertainty analysis, followed, whenever relevant, by an expert knowledge elicitation process. For human health, no conclusive evidence of higher hazards compared to previous assessment was found for genotoxicity, developmental toxicity, neurotoxicity including developmental neurotoxicity and immunotoxicity. However, due to the lack of adequate assessment of the current data set, the PPR Panel recommends conducting an assessment of endocrine disrupting properties for acetamiprid in line with EFSA/ECHA guidance document for the identification of endocrine disruptors. For environment, no conclusive, robust evidence of higher hazards compared to the previous assessment was found for birds, aquatic organisms, bees and soil organisms. However, the potential of high inter‐species sensitivity of birds and bees towards acetamiprid requires further consideration.
Keywords: acetamiprid, neonicotinoids, insecticides, endocrine disruption, uncertainty analysis, expert knowledge elicitation, environmental risk assessment, birds, aquatic organisms, bees, soil organisms
Short abstract
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2022.7030/full
Summary
Acetamiprid is a neonicotinoid insecticide currently under the third renewal (AIR3) of the Commission implementing regulation (EU) No 844/2012.
In November 2020, French Authorities asked the Commission to prohibit the sale and use of acetamiprid and flupyradifurone under Article 69 of Regulation (EC) No 1107/2009, in the light of potential concerns that these substances may pose high risks to humans and the environment. The French Authorities included in their notification scientific evidence to support this request, including references to published peer‐reviewed studies. According to France, these studies indicate that, for acetamiprid and flupyradifurone, the approval criteria, referred to in Article 4 of Regulation (EC) No 1107/2009, are no longer fulfilled.
In addition, in June 2020, the Dutch Authorities (hereafter referred to as CTGB) notified the Commission, under Article 56 of Regulation (EC) No 1107/2009, of new information on flupyradifurone on the wild bee species Megachile rotundata. This notification is also referred to in the French notification on flupyradifurone.
Consequently, the EFSA PPR Panel was mandated to advise on the likelihood that body of evidence would constitute proof of serious risks to humans or the environment. Specifically, the EFSA PPR Panel evaluated the new studies aiming to quantify the likelihood of them indicating new or higher hazards and exposure to humans and the environment compared to previous EU assessments.
A total of 40 studies were referenced, which underwent an initial screening process based on pre‐defined criteria. Upon screening, 24 studies were deemed relevant to the hazard assessment of acetamiprid for humans (n = 10) or the environment (n = 14). Among these, five references aimed to mechanistically explore differences in tolerance across bee species towards nicotinic acetylcholine receptor (nAChR) competitive modulators. These references were not entirely focused on acetamiprid or flupyradifurone, but were, nonetheless, retained in the assessment as supportive, read‐across information.
All references retained after the screening underwent a full data extraction process, following which each measured endpoint was critically appraised following the principles of the Office of Health Assessment and Translation (OHAT)‐NTP risk of bias (RoB) assessment tool (NTP, 2019). For this purpose, ad hoc critical appraisal tools (CATs) were designed for the human health and environmental part, consisting of a series of questions aimed to quantify the relevance, reliability and precision of the assessments. For this purpose, each question was answered using a multiple‐level scoring system. Upon appraisal, all endpoints and lines of evidence were summarised using heatmaps, where the overall classification of studies (i.e. the risk of bias (RoB)) was calculated using pre‐defined algorithms. Specifically, in these calculations key questions for the assessment were given higher weight than others.
For the human health assessment, this step was followed by the assessment of uncertainties related to hazard identification (Step 1) and characterisation (Step 2). This was achieved by using a stepwise, hierarchical approach and a set of predefined factors/domains and related guiding questions tailored by lines of evidence. In a third step, experts were asked to compare the available evidence with the EU assessments by EFSA and ECHA. Where deemed necessary, this step was followed by an expert knowledge elicitation (EKE) process.
For the environment part, following appraisal similar data (i.e. assessment endpoints) were further collated into lines of evidence, where an additional indicator, the internal consistency, quantified how well these endpoints mapped together. Finally, the WG was asked to quantify (i) the likelihood of each line of evidence indicating higher hazards than the EU assessment and (ii) the uncertainty around this judgement.
The following key conclusions were drawn by the PPR Panel.
For human health, no conclusive evidence of higher hazards of acetamiprid compared to previous assessment was found for genotoxicity, developmental toxicity, neurotoxicity including developmental neurotoxicity and immunotoxicity. The following recommendations were given by the PPR Panel: (i) for genotoxicity, developmental toxicity, neurotoxicity including developmental neurotoxicity and immunotoxicity assessment endpoint categories, the newly submitted evidence did not change the current conclusion from EFSA and ECHA on acetamiprid and recommends that no further actions should be taken; (ii) for endocrine disruption assessment endpoint category, the assessment of endocrine disrupting properties for acetamiprid should be conducted in line with EFSA/ECHA guidance document for the identification of endocrine disruptors under Regulations (EU) No 528/2012 and (EC) No 1107/2009 (ECHA/EFSA, 2018).
For the environment, no conclusive, robust evidence of higher hazards compared to the previous assessment was found for birds, aquatic organisms, honey bees and soil organisms. However, the potential of high inter‐species sensitivity of birds and bees towards acetamiprid may require further consideration. Therefore, the following recommendations were given by the PPR Panel: (i) for birds, that the reproductive hazard to Passeriformes from long‐term exposure to acetamiprid is explicitly investigated and addressed in the risk assessment; (ii) for aquatic organisms, that data gaps identified in the previous peer review (EFSA, 2016) concerning the sensitivity of Naididae (worms) are explicitly addressed; (ii) for bees, that the potentially higher sensitivity of M. rotundata to acetamiprid compared to other bee species is investigated and that – when data become available – an appropriate specific risk assessment for the intended uses is performed; (iv) For soil organisms, that the hazard to earthworms under standard conditions is clarified.
Finally, while acknowledging the purpose of this mandate, the PPR panel considered that the elective selection of evidence may constitute an intrinsic bias to the assessment and, hence, to the conclusions reported above for both human health and the environment. Therefore, the PPR Panel recommends that systematic review approaches should be used in the future.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Acetamiprid is an active substance covered by the third batch of the renewal program for pesticides (‘AIR3’) in accordance with commission implementing regulation (EU) No 844/2012. The active substance was first approved by Commission Directive 2004/99/EC and its approval was renewed by Commission Implementing Regulation (EU) 2018/113. A potential next renewal process needs to be initiated by 28 February 2031 at the latest.
Flupyradifurone is a novel butenolide insecticide, first approved as an active substance for use in plant protection products by Commission Implementing Regulation (EU) 2015/2084. To maintain the approval, a renewal process for this active substance needs to be initiated by interested applicants by 9 December 2022 at the latest.
On 30 November 2020, the French Authorities asked the Commission, under Article 69 of Regulation (EC) No 1107/2009, to prohibit the sale and use of these substances, taking into account the serious risks to health or the environment that their use may pose. Scientific evidence to support this request, including references to published peer‐reviewed studies, were provided by France and the Netherlands.
By means of the mandate received on March 2021 from the European Commission, for flupyradifurone and acetamiprid, as foreseen in Article 69 of Regulation (EC) No 1107/2009, and for flupyradifurone under Article 56 of Regulation (EC) No 1107/2009 too, the Commission requested the EFSA PPR Panel to assess and explain whether:
based on the new information notified by France and the Netherlands and considering any other information available to the Panel from the recent evaluations by EFSA (2015), including weight of evidence considerations, there are indications of a serious risk to human or animal health or the environment from the use of flupyradifurone;
based on the new information notified by France and considering any other information available to the Panel from the recent evaluations by EFSA (2016), and ECHA,1 including weight of evidence considerations, there are indications of a serious risk to human or animal health or the environment from the use of acetamiprid.
1.2. Interpretation of the Terms of Reference
In line with the Terms of Reference (ToR), this EFSA statement aimed to assess the additional information provided by the French and Dutch competent authorities for the hazard identification and characterisation of pesticide active substance acetamiprid. For the environmental part, the assessment is extended to the exposure characterisation i.e. whether new routes of exposure to non‐target organisms are identified and whether these are covered by the ones previously assessed.
This additional evidence complements the available one included in the latest evaluations conducted by EFSA1 and ECHA3 to assess the impact on risk assessment.
In the human health part, it was first identified the toxicological assessment endpoints of interest in the areas of developmental toxicity, endocrine toxicity, neurotoxicity including developmental neurotoxicity (DNT), immunotoxicity and genotoxicity.
In the environmental part, the working group (WG) first identified reliable tier 1 endpoints for most groups of non‐target organisms from the previous peer review evaluation (summarised in the relevant EFSA conclusions). Any higher tier study available in the previous peer review was also considered, together with a mapping of the route of exposure/exposure scenarios previously deemed relevant for the risk assessment. In addition, situations where a high risk was concluded on the basis of the previous evaluations will be transparently reported in this statement.
For the studies newly submitted by France and the Netherlands, in both parts (i.e. human health and environment), an endpoint‐specific weight of the evidence (WoE) was performed. Eventually, this culminated in an expert opinion on hazard identification and characterisation and impact on risk assessment, to support the decision making with regard to the application of Article 69 of Regulation (EC) No 1107/2009.
It should be pointed out that this statement is not based on a systematic review of all published and available information for the endpoints assessed, therefore, it is not excluded that additional work will be necessary outside the remit of this mandate.
|
Working definitions What is measured in experimental studies and the results of such measurements are often generically referred to as ‘endpoints’. Other terms are also used, e.g. ‘outcome’, ‘response’, etc. In order to make some clarity, working definitions are proposed here. These definitions should be interpreted as specific for this protocol. Similar, but slightly different definitions of the same terminology are reported elsewhere (e.g. U.S. EPA, 2003). This is not an attempt to overrule such existing definitions, but rather to make operative concepts that are relevant for the present project, and to ensure consistency between the assessment of human health and the environment. Assessment endpoint: a parameter which is monitored and/or measured in one experiment. This may have a continuous, discrete, or dichotomic nature. Different assessment endpoints may be grouped in families of assessment endpoints when they refer to a common process (e.g. reproduction, development, DNA damage, apoptosis, oxidative stress, etc.) Measured endpoint: the results of the measurements of the assessment endpoint. Depending on the nature of the endpoint, this may be expressed with a classification (e.g. positive/negative; present/absent) or with a quantification of an effect level by using a certain metric, often in comparison to a negative control. In some cases, the measured endpoint expresses the link between the effect level and the level of exposure triggering such effect. |
2. Human health
2.1. Data and methodologies
2.1.1. Data
In support of the request to prohibit the sale and use of acetamiprid in accordance with Article 69 of regulation (EC) No 1107/2009, the French and the Dutch authorities provided scientific evidence, including studies published in the open literature, on the potential serious risks that acetamiprid may pose to human health and to environment.
For the evaluation of the human health data, all 10 references mentioned in the mandate were screened for relevance for the human health risk assessment. After screening, three lines of evidence were identified: in vitro, in vivo experimental data, and human observational data; toxicological assessment endpoint categories (developmental toxicity, endocrine toxicity, neurotoxicity including DNT, immunotoxicity and genotoxicity) were therefore identified along these three lines of evidence.
For acetamiprid, three studies for the in vitro line of evidence were available; these studies focused on the following toxicology assessment endpoints categories: genotoxicity (RefID 7 – Senyildiz et al., 2018), developmental toxicity (RefID 9 – Gomez et al., 2020) and neurotoxicity (2018; RefID 30, Çamlica et al., 2019). The study from Çamlica et al. (2019) was considered in the context of the human health evaluation even though the study was conducted on the sciatic nerve of Rana ridibunda.
Five in vivo experimental studies were notified. The assessment endpoints categories identified were neurotoxicity (RefID 4 – Terayama et al., 2016), endocrine disruption (RefID 2 – Kong et al., 2017 and RefID 5 – Terayama et al., 2018), immunotoxicity (RefID 3 – Marzouki et al., 2017) and DNT (RefID 6 – Kagawa and Nagao, 2018).
Two human observational studies were also notified (Marfo et al., 2015 and Ichikawa et al., 2019). However, only the paper by Marfo et al. (2015, RefID 1) was considered further in the assessment. In this case, the selected toxicological assessment endpoint category was neurotoxicity; this was done for practical reasons as indeed this study is mainly assessing the association between acetamiprid urinary metabolite and neonicotinoids related symptoms, including neurotoxicity. It was agreed to consider the paper by Ichikawa et al. (2019) out of the scope for the current evaluation because the study was mainly focussed on analytical method validation and further applied to a case series of very low birth weight infants to determine exposure to neonicotinoids instead of adverse outcomes, therefore not in line with the ToRs.
2.1.2. Methodologies
Concerning the human health part, a pre‐defined protocol was developed based on EFSA (2020) and reported in Annex A. The protocol includes both the problem formulation and the methodology planned for the assessment. Below only a brief summary of the methodology is reported for the sake of completeness. In addition, in Section 2.1.3, deviations from the original plan as described in the protocol are reported. The following steps were performed as part of the assessment: investigation of the internal validity using critical appraisal tools (CATs) (Risk of Bias assessment); extraction of the relevant evidence; and data synthesis including uncertainty analysis.
2.1.2.1. Critical appraisal of the evidence internal validity (risk of bias)
Risk of bias (RoB) for the in vivo and for human observational studies (HOS) was appraised using customised versions of the Office of Health Assessment and Translation (OHAT)‐NTP RoB assessment tool (NTP, 2019). For in vitro studies, the tool used in the monograph on PFOS and PFAS (NTP, 2016) was adopted and adapted to fit the context of this assessment. CATs were defined upfront and are described in the protocol (Annex A). Overall, the OHAT/NTP tool outlines 11 questions, grouped in six bias domains (selection, confounding, performance, attrition/exclusion, detection, and selective reporting) and one ‘other sources of bias’. Table 1 shows the questions and domains appraised for the in vivo, in vitro and human lines of evidence with the agreed Key Questions for this specific assessment.
Table 1.
Questions and domains appraised for the in vivo, in vitro and human lines of evidence with the agreed Key Questions for this specific assessment endpoints
| Selection Bias | In vitro | In vivo | Human |
|---|---|---|---|
| Was administered dose or exposure level adequately randomised? | YES | YES | – |
| Was allocation to study groups adequately concealed? | – | YES | YES |
| Did selection of study participants result in appropriate comparison groups? | – | – | YES |
| Confounding Bias | |||
| Did the study design or analysis account for important confounding and modifying variables? | – | – | Key Q |
| Performance Bias | |||
| Were experimental conditions identical across study groups? | YES | YES | – |
| Were the research personnel (cell maintenance and cell dosing ) blinded to the study group during the study? | YES | YES | – |
| Attrition/exclusion | |||
| Were the measured endpoint data complete without attrition or exclusion from analysis? | YES | YES | YES |
| Detection bias | |||
| Can we be confident in the exposure characterization? | Key Q | Key Q | Key Q |
| Can we be confident in the assessment of the results? | Key Q | Key Q | Key Q |
| Selective reporting | |||
| Were all measured endpoints reported? | YES | YES | YES |
| Other bias | |||
| Were there other potential threats to internal validity? | Key Q (cytotoxicity) | Systemic Toxicity | Statistics |
| Were there other potential threats to internal validity? | Replicates | – | – |
The evidence was appraised by at least two independent reviewers from the WG and EFSA staff using a 4‐level scale. Answers were summarised at the level of individual studies and an algorithm was used to combine the answers to the appraisal question and to allocate the studies to the different classes: low (class 1), moderate (class 2) or high (class 3) RoB. Different weight was given to Key Questions as they are related to elements of the studies considered having a greater impact on the bias. Discrepancies in rating between assessors were solved through discussion to reach the final recorded RoB rating for each question.
Eventually, the results of the appraisal were narratively reported in Annex B and graphically displayed in a heatmap (Annex C). The results were also contextualised in the uncertainty analysis step.
2.1.2.2. Data extraction
Data were collected (i.e. extracted) from the provided studies by one EFSA staff and validated by another. A predefined form that comprises data on the characteristics of the study (study design, funding source, test system, species, ethnicity), the concentration/dose/exposure characteristics, the endpoints assessed and methods for measuring them, and the results was used to extract data at individual study level. The data model for extraction was tailored for each study type (i.e. in vitro, in vivo) and was provided (see Annex D). Due to the specific nature of data, no model was created to extract information from HOS. The data, uncertainties and limitations of these studies were assessed using expert knowledge and reported in a written report included under Section 2.2.1 (see protocol deviations n. 1 and 2 in Section 2.1.3).
It should be noted that the endpoint category included in the uncertainty analysis was selected a priori, based on the endpoints measured and reported in the different studies (see Section 2.1.1 Data), while, the specific endpoints were selected as part of the appraisal step and not after the data extraction. However, the impact of this temporal sequence was very limited and only few endpoints were merged and split during the data extraction and uncertainty analysis (see Section Critical Appraisal Results 2.2.2).
2.1.2.3. Uncertainty analysis and expert knowledge elicitation
The uncertainty analysis was performed within each line of evidence (for in vitro and in vivo studies) and hierarchical level (i.e. assessment endpoint category and specific assessment endpoints) to support conclusions on hazard identification and hazard characterisation. The final purpose was to assess the impact of the additional evidence provided by the French and Dutch Authorities on the current assessments done by EFSA and ECHA for acetamiprid in 2016 and 2017, respectively. A stepwise approach was used.
Differently from what was initially planned in the protocol, one additional question (Q3) was added to better reflect the aim of the assessment (see Section 2.1.3 protocol deviation n. 3). Moreover, the names of the active substances were no longer reported in Q1 and Q2. This is because there were many uncertainties in relation to the exposure characterisation (RoB class 3 for the majority of the studies) and therefore exposure reliability was considered a relevant uncertainty.
In steps 1 and 2, the uncertainties related to hazard identification (Step 1) and characterisation (Step 2) were analysed. The uncertainty analysis was performed using a predefined list of factors/domains and related guiding questions tailored by lines of evidence. The factors/domains were assessed in two ways. First, potential explanations for the identified heterogeneity in the results (if any) were assessed. If inconsistencies could not be justified by any factor/domain, the unexplained inconsistencies were treated as a source of uncertainty. Second, the same factors/domains were appraised for adequateness in the body of evidence in relation to the specific endpoint/endpoint category/adverse outcome. Factors/domains considered not adequate were retained as sources of uncertainty. A detailed list of factors/domains by line of evidence is provided in Annex E (hereafter referred to as uncertainty tables). For both steps (assessment of the inconsistencies and of the potential sources of uncertainty), the judgement was achieved answering to specific ‘guiding questions’ related to each domain and line of evidence. Synthetic answers (Yes/No/Not Relevant) and a narrative explanation for the rationale of the assessment were provided by EFSA Staff and checked by the WG.
The assessment was performed using a stepwise approach starting from the lower hierarchical levels and progressed at the higher levels (e.g. conclusions on the assessment endpoint category were based on those achieved for the specific assessment endpoints). Progression of the assessment towards a higher level (e.g. assessment endpoint category – endocrine disruption) was carried out also if at the lower level (i.e. specific assessment endpoint) the measured endpoint was not affected in dose or concentration response relationship. This approach was taken to allow drawing conclusions on all the assessment endpoints categories identified in the scientific evidence provided by the French and the Dutch authorities.
Based on the answers to the ‘guiding questions’ a judgement was made on:
specific endpoint being associated/affected in a dose/concentration‐response relationship in the evaluated study (Q1 in Table 2).
minimum dose/concentration at which the assessment endpoint is perturbed in the study evaluated (Q2 in Table 2).
Table 2.
Assessment questions for the uncertainty analysis on hazard identification and characterisation
| Line of evidence | Question 1. Hazard identification | Question 2. Hazard characterisation | Answer |
|---|---|---|---|
| In vitro experimental studies | Is the measured endpoint affected in a concentration‐response relationship in the evaluated study? | What is the lowest concentration at which exposure affects the endpoint? | (Q1. Yes/No + Q2. Lowest concentration/dose) + summary of the uncertainties for the assessment endpoint category |
| In vivo experimental studies | Is the measured endpoint affected in a dose–response relationship in the evaluated study? | What is the lowest dose at which exposure affects the endpoint? |
In step 3, experts were asked to assess the contribution of the available evidence on the conclusions currently reached by EFSA and ECHA for acetamiprid (Q3 in Table 3).
Table 3.
Assessment questions for assessing the contribution of the available evidence on the conclusions currently reached by EFSA and ECHA for acetamiprid
| Line of evidence | Question 3 | Answer |
|---|---|---|
| In vitro experimental studies | Is the available evidence able to modify the conclusions currently reached by ECHA and EFSA for acetamiprid? | Yes/No + Recommendation on the assessed endpoint (including EKE where necessary) |
| In vivo experimental studies | Is the available evidence able to modify the conclusions currently reached by ECHA and EFSA for acetamiprid? |
Where necessary, in line with the recommendation from the experts, Step 3, was followed by an expert knowledge elicitation (EKE) process (EFSA, 2014). If the experts’ recommendation did not include an EKE, the process ended here. This was the case when all the available evidence in the updated data set (including the new evidence and the evidence already available in the EFSA/ECHA conclusion) was already sufficient to conclude without the support of the EKE for the WoE analysis. The purpose of the EKE, when conducted, was to express the uncertainty using a quantitative WoE approach to address Q3. In this case, the uncertainty was quantified as probability (i.e. very low, low, moderate, and high).
A customised version of the OHAT approach (NTP, 2015) was used to integrate the available evidence and to rate the certainty in a causal and positive association between exposure and a given toxicological endpoint category. The adaptation affected several aspects of the original approach: (1) the original concept of ‘confidence in the evidence’ was replaced by the concept of ‘certainty in a causal and positive association’; (2) the initial certainty in the association was not attributed automatically according to the study design but assessed on the basis of whether the 4 OHAT criteria (controlled exposure, exposure assessment, endpoints individually measured, comparison group used) were overall actually met by the available studies; (3) the verbal description of the level of certainty (classified as high, moderate, low and very low) was translated into probability ranges; (4) two criteria for decreasing the level of certainty were dropped, notably the imprecision (since the rationale for its assessment was unclear) and lack of biological plausibility (as biological plausibility by definition is assumed to be met for all the endpoints). A summary of the adaptations introduced in the OHAT approach for evidence integration for hazard identification is shown in Figure 1.
Figure 1.
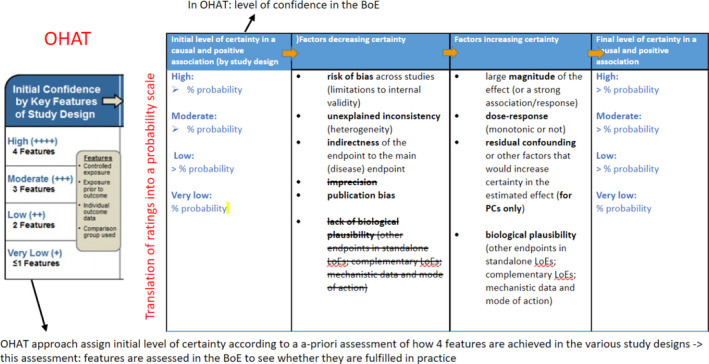
Adaptations to the OHAT approach introduced in this assessment
The conclusion on a causal and positive association was drawn by considering the strengths and the weaknesses in a collection of human, animal and in vitro studies that constitute the body of evidence for a specific health outcome (see protocol deviation n.4 in Section 2.1.3). The following steps were therefore taken.
First, available evidence on a given toxicological assessment endpoint category was grouped by study design for each design‐group. The following key features were assessed: (1) controlled exposure (whether the exposure to the substance is experimentally controlled), (2) exposure assessment (whether exposure occurred concurrent2 with aggravation/amplification of an existing condition), (3) individual measured endpoints (whether each single endpoint measured in the study was reported as individual raw data or as average of the measure) and (4) comparison group used (whether an appropriate control group was included in the study). Then each group of studies received an initial rating based on whether these features were met or not. The rating reflects the certainty that the findings support the conclusion of a causal association between exposure to a substance and the effect, with the latter showing an increase with increasing exposure (positive association).
Second, the initial rating was downgraded for factors that decrease certainty in the positive and causal association (e.g. risk of bias, unexplained inconsistency, indirectness or lack of applicability, and publication bias) and upgraded for factors that increase this certainty (e.g. large magnitude of effect, existence of a dose response, consistency across study designs/populations/animal models or species, and consideration of residual confounding that is expected to bias the effect towards the null). The reasons for downgrading (or upgrading) certainty were based on expert judgement using agreed weighted factors and were not fixed a priori.
At the end, the final ratings of the certainty were translated into a probability scale to reduce ambiguity in the interpretation. The outcome is reported in the Annex F.
2.1.3. Deviations from the protocol
Because of the nature and features of the HOS, the data extraction was performed and reported as a written expert report (Section 2.2.4).
Likewise, for HOS the uncertainty analysis was performed and reported as a written expert report (Section 2.2.4).
For in vivo and in vitro studies, differently from what was initially planned in the protocol, one additional question (Q3) was added. The name of the active substance is no longer reported in Q1 and Q2 to better reflect the uncertainties in exposure (please, refer to Section 2.1.2.3 for more details).
The Roulette method proposed in the protocol was not applied. A customised version of the OHAT approach (NTP, 2015) was used instead to integrate the available evidence and to rate the certainty in a causal and positive association between exposure and health outcomes. This protocol deviation also accounted for lack of a quantitative estimation of the uncertainties as was planned for the Roulette method.
2.2. Assessment
2.2.1. Data from the latest evaluations by EFSA and ECHA
Acetamiprid was first approved as an active substance for use in plant protection products by Commission Directive 2004/99/EC. Its approval was renewed for a period of 15 years by Commission Implementing Regulation (EU) 2018/113 and a potential next renewal process needs to be initiated by 28 February 2031 at the latest.
Acetamiprid has been also assessed under another European Regulatory Framework(s) Regulation (EU) No 528/2012 of the European Parliament and of the Council 22 May 2012 concerning the making available on the market and use of biocidal products (BPRs) (ECHA, 2017, 3). No additional concerns were identified in this parallel assessment.
A harmonised classification is also available and on May 2020 a Risk Assessment Committee (RAC) opinion on the harmonised classification and labelling for acetamiprid was adopted (RAC, 2020). Although no new studies were submitted, RAC considered the reductions in pup body weight, postnatal survival and delayed male rat pubertal attainment observed in a DNT study sufficient for classification as Reproductive toxicity category 2, H361d for adverse effects on development (CLH, 2020; RAC, 2020).
The following is a summary of the peer review conducted by EFSA (2016) for the toxicological assessment endpoints categories identified in the newly provided scientific evidence (i.e. developmental toxicity, endocrine toxicity, neurotoxicity including DNT, immunotoxicity and genotoxicity).
Genotoxicity
Acetamiprid was tested in both in vitro and in vivo tests to assess the genotoxicity potential. In vitro the active substance did not induce gene mutation in the Ames test and in the mammalian cell study (CHO/HPRT) and was inactive to induce DNA damage in the unscheduled DNA synthesis (UDS) test with rat liver cells. However, acetamiprid was positive in a chromosomal aberration assay in CHO cells, with and without metabolic activation.
Chromosomal aberrations were not confirmed in the in vivo studies where acetamiprid was found to not induce DNA damage in the UDS test with rat liver cells in vivo, and to not induce any significant increase of micronucleated bone marrow polychromatic erythrocytes in the micronucleus test conducted in the rat bone marrow. No evidence of bone marrow toxicity (i.e. polychromatic erythrocyte/normochromatic erythrocyte (PCE/NCE)) was reported; however, tremors and mortalities were observed after dosing in the study indicating that higher doses were not feasible.
It was concluded that Acetamiprid is unlikely to be genotoxic in vivo.
Developmental toxicity
Developmental toxicity data were available for both rats and rabbits. No‐observed‐adverse‐effect‐level (NOAEL) for developmental toxicity in rats was 16 mg/kg body weight (bw) per day based on shortening of the 13th rib. No adverse effects were observed in rabbits and the NOAEL was set at 30 mg/kg bw per day (highest dose tested).
Neurotoxicity including developmental neurotoxicity
Acute and repeated neurotoxicity studies were available in rats. The NOAEL (neurotoxicity) derived from acute studies was 10 mg/kg bw, based on behavioural changes and reduced locomotor activity. The NOAEL (neurotoxicity) derived from repeated dose studies was 118 mg/kg bw per day (highest dose tested), based on the lack of neurotoxicity evidence.
Additional studies, including delayed neurotoxicity in hens and DNT in rats were also available. No delayed neurotoxic potential was observed in hens treated at the determined LD50 value of 129 mg/kg bw. A NOAEL of 2.5 mg/kg bw per day was derived from the DNT studies in rats (based on reduced auditory startle response).
Endocrine toxicity
According to the EFSA conclusion, acetamiprid was proposed to be classified as carcinogenic category 2 but not as toxic for reproduction category 2, in accordance with the provisions of Regulation (EC) No 1272/2008. Conditions concerning human health for the consideration of endocrine disrupting properties in humans were not met according to the data and knowledge available when the assessment was conducted.
However, in the latest EFSA conclusion (EFSA, 2016), an endocrine assessment based on criteria for the identification of substances having endocrine disrupting properties, as outline in Commission Regulation (EU) No 2018/605 and implemented in ECHA/EFSA guidance (2018), was not available.
Immunotoxicity
No immunotoxic potential was demonstrated in the 4‐week studies conducted in rats and mice. NOAELs of 62.9 mg/kg bw per day in rats, and 128 mg/kg bw per day in mice were therefore derived based on absence of effects at the highest dose tested.
2.2.2. Critical appraisal results
For acetamiprid (ACE), the results of the appraisal were narratively reported in Annex B and graphically displayed in a heatmap (Annex C). A summary of the results is, however, included in the following lines and graphically displayed in Figures 1, 2–3.
Figure 2.
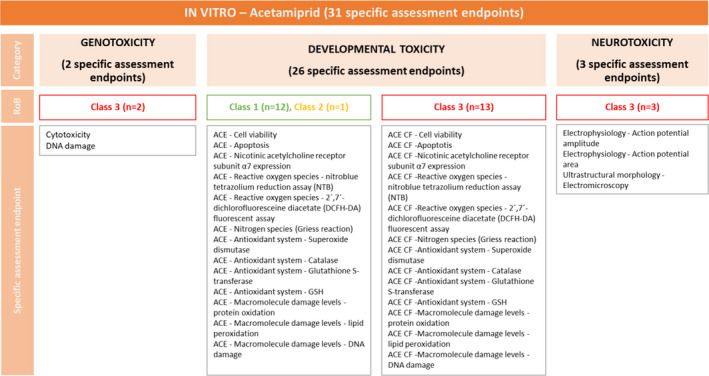
Summary of the RoB conducted for the in vitro lines of evidence. The results were reported per assessment endpoint categories (i.e. genotoxicity, developmental toxicity and neurotoxicity) and per specific assessment endpoint. Green: class 1 risk of bias; Orange: Class 2 risk of bias; Red: class 3 risk of bias
Figure 3.
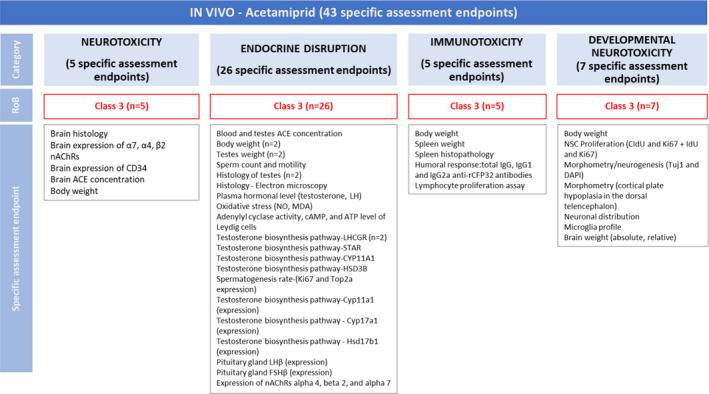
Summary of the RoB conducted for the in vivo lines of evidence. The results were reported per assessment endpoint categories (i.e. neurotoxicity, endocrine disruption, immunotoxicity and developmental neurotoxicity) and per specific assessment endpoint. Red: class 3 risk of bias
All the toxicological assessment endpoints were used for the evidence synthesis in line with the ToRs of the current mandate. It is important to point out that the specific endpoints were identified at the level of appraisal of the evidence and not after the data extraction. The impact of this on the overall results was very limited implying the merging of few specific endpoints during the uncertainty analysis phase. Specifically:
-
–
ACE reactive oxygen species – nitroblue tetrazolium reduction assay (NTB) and ACE reactive oxygen species – 2´,7´‐dichlorofluoresceine diacetate (DCFH‐DA) fluorescent assay were appraised as individua‐specific assessment endpoint and then merged under ‘reactive oxygen species’ in the uncertainty analysis table;
-
–
ACE – antioxidant system – superoxide dismutase, ACE – antioxidant system – catalase, ACE – Antioxidant system – glutathione S‐transferase and ACE – antioxidant system – GSH were appraised as individual‐specific assessment endpoints and then merged under ‘antioxidant system’ in the uncertainty analysis table;
-
–
ACE – macromolecule damage levels – protein oxidation, ACE – macromolecule damage levels – lipid peroxidation and ACE – macromolecule damage levels – DNA damage was appraised as individual‐specific assessment endpoints and then merged under ‘macromolecule damage level’ in the uncertainty analysis table.
-
–
ACE CF reactive oxygen species – NTB and ACE CF reactive oxygen species –DCFH‐DA fluorescent assay were appraised as individual‐specific assessment endpoint and then merged under ‘reactive oxygen species’ in the uncertainty analysis table;
-
–
ACE CF – antioxidant system – superoxide dismutase, ACE CF – antioxidant system – catalase, ACE CF – antioxidant system – glutathione S‐transferase and ACE CF – antioxidant system – GSH were appraised as individual‐specific assessment endpoints and then merged under ‘antioxidant system’ in the uncertainty analysis table;
-
–
ACE CF – macromolecule damage levels – protein oxidation, ACE CF – macromolecule damage levels – lipid peroxidation and ACE – macromolecule damage levels – DNA damage were appraised as individual specific assessment endpoints and then merged under ‘macromolecule damage level’ in the uncertainty analysis table.
For HOS, the risk of bias assessment was performed only for one of the two available studies (Marfo et al., 2015) (Figure 4). The paper by Ichikawa et al. (2019) was considered out of the scope for the current evaluation because the study was focused mainly on analytical method validation, and further applied to a number of very low birth weight infants to determine exposure to neonicotinoids instead of adverse outcomes, therefore it is not in line with the ToRs.
Figure 4.
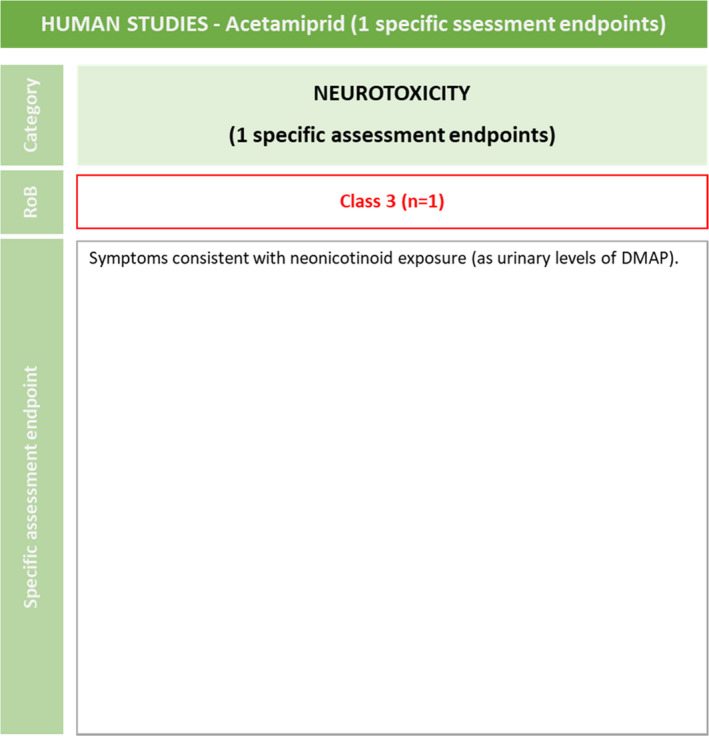
Summary of the RoB conducted for the human lines of evidence. The results were reported per assessment endpoint categories (i.e. neurotoxicity) and per specific assessment endpoint. Red: class 3 risk of bias
2.2.3. Outcome of the uncertainty analysis and of the expert knowledge elicitation
The uncertainty analysis table used to perform this evaluation includes information on the studies reported in the EFSA and ECHA conclusions (EFSA, 2016; ECHA, 2017). The analysis also provides a comparative assessment of the new data vs. the critical NOAEL on the same toxicological assessment endpoint category used in the regulatory process of EFSA and ECHA for hazard identification and characterisation. In addition, a conclusive position on the impact of the new submitted studies on the latest evaluations (EFSA/ECHA conclusions; EFSA, 2016; ECHA, 2017) is also reported, which includes a recommendation on further steps necessary to fulfil the ToRs (Annex E).
Annex E also includes an uncertainty analysis for general toxicity endpoints for in vivo studies (i.e. body weight and blood and testes ACE concentration) and endpoints used to define cytotoxicity and or to establish the maximum concentration tested in the cell assay (i.e. cytotoxicity and cell viability). These endpoints were not further considered in the assessment and were included in the uncertainty analysis as complementary evidence to define how specific the observed effects for the toxicological assessment endpoints category were.
For the toxicological assessment endpoint category genotoxicity, following detailed assessment of the available evidence and uncertainties, it was concluded that moving to the EKE was not necessary. The available evidence is not able to modify the conclusion reached by EFSA and ECHA. The details of the uncertainty analysis showed that there was a statistically significant increase in DNA damage at the maximum concentration, in one out of two test systems used in the study. It was noted that this paper was classified as class 3 for the risk of bias, because of lack of information on stability and solubility of the test item used in the experiment. The maximum concentration was selected on cytotoxicity data that seems to be acceptable. Nevertheless, the positive effect on DNA damage (assessment endpoint of interest) was only observed at maximum concentration tested in only one of the two test systems used (neuroblastoma cell line) and no biological replicates are reported in the paper. The test systems are immortalised human derived cell lines and the sensitivity versus other non‐immortalised cell line such as primary cells, is unknown. The overall WoE indicates that the probability of acetamiprid being genotoxic according to the evidence provided in this paper is low. Based on this evaluation, and considering the available database on genotoxicity evaluated by EFSA (2016) and ECHA (2017), it was concluded that there is not sufficient evidence to move to the EKE for the endpoint category genotoxicity and the current assessments provided by EFSA (2016) and ECHA (2017) were considered still valid.
For the toxicological endpoint category developmental toxicity, the detailed assessment of the available evidence and uncertainties allowed to conclude that moving to the EKE was not necessary. The available evidence is not able to modify the conclusion reached by EFSA and ECHA. The details of the uncertainty analysis showed that the in vitro study on developmental toxicity was using human derived trophoblast as a test system and the observed effects were consistent with an induction of apoptosis and oxidative stress. It was acknowledged that this test system may be helpful to elucidate key mechanisms underlining placental development and function. In the context of developmental and reproduction assessment endpoints, changes in in vitro toxicity on trophoblasts should be viewed as potential early key events for which a relationship to the adverse outcome is uncertain. The endpoints measured (i.e. apoptosis and oxidative stress) are mainly referring to endpoints of cytotoxicity rather than functional assessment endpoints that can be translated to measurable adverse outcome(s). Therefore, their extrapolation to in vivo assessment endpoints remains uncertain (e.g. it is not known what endpoint of reproductive or developmental toxicity might be affected in vivo). The overall WoE from this in vitro study indicates that in this test system, acetamiprid has the potential to induce oxidative stress and may cause an increase in apoptosis. However, the postulated link between oxidative stress and increase in apoptosis is speculative and essentiality studies were not conducted, although the biological plausibility cannot be excluded. It was concluded that in the absence of an in vivo reproductive and developmental toxicity adverse outcome in the available data set for acetamiprid, the use of these data do not support a causative link between oxidative stress in trophoblast and reproductive, developmental toxicity and remains inconclusive. Indeed, acetamiprid is classified as reproductive category 2 based on effects observed in pups body weight, postnatal survival and delayed male rat pubertal attainment observed in a DNT study (CLH, 2020; RAC, 2020) for which a causal relationship with placentation cannot be established.
For the toxicological endpoint category neurotoxicity, a detailed assessment of the available evidence and uncertainties led to the conclusion that moving to the EKE was not necessary. The available evidence is not able to modify the conclusion reached by EFSA and ECHA. In the study by Çamlica et al. (2019), the effect of acetamiprid on nerve conduction and morphology of the sciatic nerve of the frog (Rana ridibunda) was investigated. The data indicated that the test item immediately suppressed action potential/nerve conduction from the lowest tested concentration of 1 µM and this was associated with an immediate ultrastructural change in the myelin sheet morphology. There is a lot of uncertainty on the biological plausibility of such effect due to the lack of a more physiological dose response and the immediate occurrence of the morphological changes which was considered to be unlikely biologically plausible. The vehicle was not reported and indeed this represents a very relevant RoB for the interpretation of the results. Without this information, a biological artefact, potentially associated with the solvent effect of the vehicle cannot be excluded. In the in vivo study (Terayama et al., 2016), the effects of acetamiprid were investigated in mouse. Because the active substance was found both in control(s) animals’ group as well as in groups receiving acetamiprid by drinking water, this led to uncertainty in the quality of the paper. In addition, most of the measured endpoints were not specific for neurotoxicity (i.e. brain expression of α7, α4β2 nAChRs, brain expression of CD34), were not affected (i.e. CD34) or a biological plausible relationship to an endpoint for neurotoxicity cannot be established with the presented outcomes (i.e. expression of nAChR). Moreover, there is uncertainty in the amount of the administered dose because the test of concentration in the drinking water was not done and the amount of water drank by the animals was not reported. Overall, it was concluded that both papers were of insufficient quality to trigger any additional assessment and that the biological plausibility of the result is questionable.
For the toxicological endpoint category DNT, following detailed assessment of the available evidence and uncertainties, it was concluded that moving to the EKE was not necessary. The available evidence does not allow to modify the conclusion reached by EFSA and ECHA. The DNT endpoints measured in the paper were mainly of morphological nature; the authors claimed hypoplasia of cortical plate in the dorsal telencephalon as related to a decrease in neurogenesis and migration defect affecting immature neurons. The paper also includes additional morphological endpoint (e.g. activation of specific microglia population). Several uncertainties were identified and were associated with the interpretation of the resulting outcomes: (1) RoB class 3 (e.g. methodological approach); (2) no quantitative morphometry in terms of linear measurements; (3) lack of data reporting (i.e. brain weight and body weight); (4) lack of a positive control and historical control data (HCD) making the interpretation of the biological significance/plausibility very difficult.
For the toxicological endpoint category immunotoxicity, it was concluded, following a detailed assessment of the available evidence and uncertainties, that moving to the EKE was not necessary. The available evidence does not allow to modify the conclusion reached by EFSA and ECHA. Several uncertainties were identified in the evidence submitted. These uncertainties were limiting the interpretation of the results: (1) only one dose was tested (5 mg/kg bw per day); (2) high RoB (class 3).
For the toxicological endpoint category endocrine disruption, it was concluded, following detailed assessment of the available evidence and uncertainties, that there was evidence to move to the next step, and that an EKE for the question ‘Is the available evidence able to modify the conclusions currently reached by ECHA and EFSA?’ was necessary. It was noted that an ED assessment in line with the EFSA/ECHA guidance document for the identification of endocrine disruptors under Regulations (EU) No 528/20122 and (EC) No 1107/2009 (ECHA/EFSA, 2018) was not available in the evaluations by EFSA (2016) and ECHA (2017). Therefore, it was concluded that an EKE was needed to assess the impact of the new submitted studies on the current EFSA and ECHA conclusions. Two in vivo studies in animals provided relevant data (Kong et al., 2017; Terayama et al., 2018). The analysis of the data for the assessment endpoint category endocrine disruption showed that there is uncertainty on how the systemic toxicity in these two studies was evaluated and its impact on endocrine function was not assessed. The overall experts’ judgement was that the main observed effects, i.e. decrease in testosterone concentration in blood, histological changes in the testes and changes in the pathways of testosterone biosynthesis, are probably secondary to an oxidative damage at mitochondrial level. With the high uncertainty associated with the histopathological evaluation (i.e. a summary table indicating the number of animals affected and the severity scores of the findings was not presented; the description of the results were based on selected pictures; the method of fixation was considered suboptimal and the authors mentioned that staging of the seminiferous epithelium was evaluated but was not reported) of the testes and the lack of hormonal measurements in mice (Terayama et al., 2018), there is uncertainty on whether the evidence provided in the paper could be assessed as adverse and how to link them to an endocrine mode of action. There is evidence in the two studies of modulation of protein levels and gene expression related to testosterone synthesis. In the rat study (Kong et al., 2017), there was evidence of an increase in circulating levels of luteinising hormone (LH) with a lower circulating level of testosterone, possibly indicating disruption of the hypothalamus–pituitary–gonadal (HPG) axis. However, the histological assessment was considered not representative of a testosterone‐mediated effect. In the mice study (Terayama et al., 2018), the effect was inconsistent, because there was no measurement of the circulating hormones, but there was evidence of a decrease in the testosterone synthesis (through measurement of the steroidogenic enzymatic processes). This evidence was not associated with an increase in transcript for LH‐β and for Follicle Stimulating Hormone subunit Beta FSH‐β in the pituitary gland. Therefore, there is uncertainty on how at these doses the effect is of sufficient magnitude to disrupt the HPG axis. Terayama et al. (2018) expressed the doses as mg/day per mouse. Recalculation by mg/kg bw per day, resulted in a low dose of around 104–86.6 mg/kg bw per day and a high dose of 864–720 mg/kg per day. These doses were calculated based on an average mouse body weight of 25–30 g/mouse.
There is an additional uncertainty due to a possible cross‐contamination in Terayama et al. (2018) study as the active substance was also detected in untreated as well as in vehicle control tissues (the test item was measured in the testes and blood) and the exposure characterisation was categorised as class 3 RoB due to lack of information on solubility and stability of the test item.
Based on the uncertainty analysis described above, only for the toxicological assessment endpoint category endocrine disruption an EKE was performed. As described in the methods Section 2.1.2, a customised version of the OHAT approach (NTP, 2015) was used to integrate the available evidence and to rate the certainty in a causal and positive association between exposure and the assessment endpoint category endocrine disruption.
The outcome of this initial rating analysis is depicted in Table 4. It was concluded that the certainty that the findings in the studies accurately supports the conclusion of a causal association between exposure to acetamiprid and the positive effect with the latter increasing as exposure raised (positive association) is very low.
Table 4.
Initial rating of the certainty in a positive causal association between the exposure to a substance and the effect
| Study design | Controlled exposure | Exposure assessment | Individual measured endpoint | Comparison group used | Initial certainty rating |
|---|---|---|---|---|---|
| Experimental animal | For the two papers from literature, the exposure was measured in testes and blood. For the data set included in RAR (a) the exposure is likely controlled | For the two papers from literature, increased levels of acetamiprid in blood and testes are statistically significant at high dose only; however, effects were observed at the lower doses tested. For the data set, the assumption is that since the studies were conducted at the MTD, the exposure was maximised | Data expressed as average and raw data not available for the two literature studies. For the data set in RAR, the raw data were available and peer reviewed | The appropriateness of the vehicle control group in Terayama et al. (2018) is questionable: there is evidence of contamination of the vehicle control and untreated group with the active substance (with higher/equivalent levels in both plasma and testes as the low dose treatment group). For the data set included in the RAR, the data were peer reviewed and a comparison group (control group) is included in the study | Very low |
| Rate | Likely | May be or may not | May be or may not | May be or may not |
RAR: Renewal Assessment Report.
In a following step, the initial rating was downgraded for factors that decrease certainty in the causal association between the positive effect and exposure to acetamiprid (i.e. risk of bias, unexplained inconsistency, indirectness or lack of applicability and publication bias) and upgraded for factors that increase this certainty (i.e. large magnitude of effect, dose response, consistency across study designs/animal models or species, and consideration of residual confounding that might have biased the results towards the null). First, weight factors were applied using expert judgement to consider the relative importance of each of the 8 factors (see Annex F and Figure 5). For the factors decreasing the certainty in the positive and causal association, a weight factor of 1 was agreed to be attributed to indirectness, a weight factor of 2 to risk of bias and unexplained inconsistencies and a factor of 3 to publication bias. For the factors increasing the certainty a weight factor of 1 was agreed to be attributed to residual confounding and a weight factor of 2 to large magnitude, dose response and consistency across study design/animal models.
Figure 5.
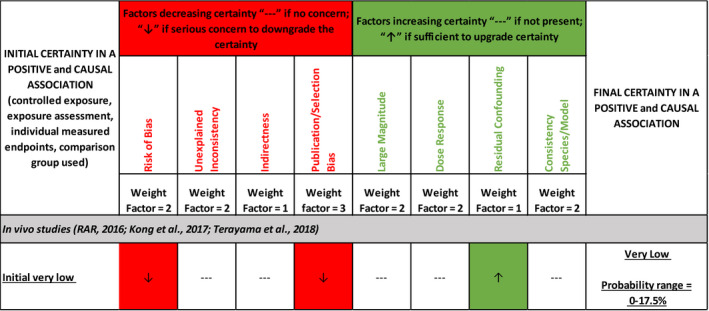
Final certainty rating in the causal association between exposure to a substance and the positive effect
In the next step for each of the 8 factors, it was considered which of them did actually affect the certainty in the positive effect and the causal association. It was concluded that the risk of bias and publication bias were factors that decreased this certainty and that residual confounding was a factor that increased certainty in the causal relation between exposure to acetamiprid and the positive effect. The factors unexplained inconsistency, indirectness, large magnitude of effect, dose response and consistency across study designs/animal models or species were considered not to affect the final certainty rating (see Annex F) (see Figure 5). Therefore, starting from ‘very low’, the final rating was assessed as ‘very low’ since the outcome of the evaluation was to decrease certainty of four levels (decreasing five levels, increasing 1 level) that was impossible being the ranking already the lowest. At the end, the final rating of the certainty was translated into a probability scale, and it was concluded that the probability that the available body of evidence supports conclusion of a causal and positive association between the exposure to acetamiprid and an effect on endocrine disruption ranges between 0% and 17.5% (see Figure 5).
Based on the outcome of the EKE for the endpoint category endocrine disruption, taking into account the literature studies provided by France and the Netherlands and also considering the evaluations by EFSA (2016) and ECHA (2017), it was concluded that the certainty in a causal association between the greater exposure to acetamiprid and the larger endocrine disruption effect was considered ranging between 0% and 17.5% probability (i.e. very low). As recommended in the Guidance on Communication of Uncertainty (EFSA, 2019) the initial and final grading of the certainty has been expressed quantitatively, notably using approximate probabilities.
2.2.4. Human observational studies (HOS)
As described above in Section 2.1.2, data extraction and uncertainty analyses for human evidence were performed using expert knowledge instead of using OHAT methodology due to the nature of the papers assessed. The outcome is reported below.
The study of Marfo et al. (2015) collected spot urine samples from patients attending a clinical study in Japan over a period of 3 years. Patients’ aged from 4 to 87 years old and they all lived close to agricultural fields. Patients were categorised into 3 groups: typical symptomatic group (TSG, n = 19), atypical symptomatic group (ASG, n = 16), and non‐symptomatic group (NSG, n = 50). Typical symptoms were defined as having two objective symptoms (recent memory loss and finger tremor), and more than five of six subjective symptoms (headache, general fatigue, palpitation/chest pain, abdominal pain, muscle pain/weakness/spasm and cough). N‐Desmethyl‐acetamiprid (DMAP) and six neonicotinoids were quantified in urine by liquid chromatography–tandem mass spectrometry (LC–MS/MS). The frequency of detection of DMAP was highest for the TSG group (47.4%), followed by ASG (12.5%) and NSG (6.0%) with maximum concentrations being 6, 4.4 and 2.2 mg/L, respectively. Detection of DMAP was associated with the prevalence of symptoms (ORTSG vs NSG 14; 95% CI 3.5–57), which led authors to conclude that urinary DMAP could be used as a biomarker for environmental exposure to acetamiprid (Marfo et al., 2015).
The assessment of this study identified several uncertainties and limitations. Most of symptoms reported were non‐specific (e.g. headache, fatigue, abdominal pain, cough, palpitations) and may be related to clinical conditions other than neonicotinoid exposure. Common symptoms of acute intoxication include nausea, vomiting, abdominal pain, dizziness, hypertension, tachycardia, eye irritation, dermatitis and oral mucosal lesions. Neurological symptoms consist of fatigue, headache, agitation, fasciculations, seizures, disorientation, drowsiness, decreased muscle tone and coma (Selvam and Srinivasan, 2019; Costas‐Ferreira and Faro, 2021). Furthermore, symptoms specific of stimulation of nicotinic acetylcholine receptors in the autonomic nervous system (e.g. tachycardia, hypertension, diaphoresis, and mydriasis) were not considered by Marfo et al. (2015). DMAP was not detected in 52.6% of TSG and in 87.5% of ASG, which supports that the observed symptoms are not specifically related to neonicotinoids exposure. Furthermore, DMAP was detected in 6% of the NSG, who lacked clinical symptoms. Thus, no causality can be inferred from detection of DMAP in urine and the presence of symptoms.
Despite data for age, gender, food and tea intake being available, these covariates were not included as potential predictors in the logistic regression analysis. An unadjusted binary logistic regression analysis was presented instead. Tea beverages were most frequently consumed in 8 TSG cases; however, other potential co‐exposures (e.g. contaminants present in tea such as heavy metals) might have a role in the TSG and this was not controlled in the study. The reduced sample size and the use of a unique spot urine sample limit drawing robust conclusions.
The maximum DMAP concentration found in this study was 6 ng/mL, which is within the range reported by Ospina et al. (2019) for the NHANES study, and among the 75th and 95th percentiles reported by of Wang et al. (2020), and lower than the maximum concentration reported in these two studies (34.7 and 64.9 ng/mL, respectively).
Although the study from Ichikawa et al. (2019), was excluded from the current evaluation, it was assessed by expert(s) and is reported here for transparency. This study developed an analytical method for seven neonicotinoids and the metabolite DMAP in human urine using LC–ESI/MS/MS. This method was then applied to 65 very low birth weight (VLBW, 500–1500 g) infants of gestational age 23–32 weeks admitted to the neonatal intensive care unit of a Japanese Hospital over 2 years. Urine samples were collected in the first 2 days (PND 1–2) and at day 14 (PND 14) after birth. DMAP was detected in 14 urine samples collected at birth (24.6%, median level 0.048 ppb) and in 7 samples from postnatal day 14 (11.9%, median level 0.09 ppb). The urinary DMAP detection rate and level was significantly higher in infants belonging to the group where the gestational age was shorter, namely small for the gestational age (SGA) than in the infants belonging to the group with an appropriate for the gestational age (AGA). However, DMAP levels showed no correlation with infant physique indexes (length, height, and head circumference SD scores).
A number of uncertainties and limitations were identified in this study. Urine samples (PND 1–2) were collected at the same time than the outcome assessment, which precludes any causal inference. Also, there is no information on in utero exposure, which might potentially impact on neonatal anthropometry and gestational age. The units expressed in text and tables for DMAP and neonicotinoids (ppb) are in disagreement with those expressed for the method validation (ng/mL). The non‐parametric Wilcoxon rank sum test was used to compare DMAP concentrations between PND 1–2 and PND 14, as well as between SGA and AGA; however, the study reports significant differences by means despite data did not fit a normal distribution. Median and interquartile range should have been used instead. If so, median levels would have increased from 0.05 at PND 1–2 to 0.09 at PND 14, which contrarily to the interpretation given in the study would indicate postnatal exposure to neonicotinoids. Conversely, no difference in median levels between SGA and AGA was observed. The relative low sample size, taken from a unique hospital from Japan, limits the external validity of the study and represents an important limitation.
According to this study, acetamiprid is an insecticide commonly used in Japan for fruits, vegetables, tea leaves, rice paddies, turf, ornamental flowers, and pine trees. Around 10% total radioactive residue (TRR) of DMAP metabolite has been found in edible crop parts (JMPR, 2005), meaning that the same proportion of the parent compound can be ingested in the form of DMAP instead of acetamiprid. It is not clear whether this metabolite is active or not, but according to authors, oral LD50 of DMAP is 1,843 mg/kg whereas that of acetamiprid is 146 mg/kg.
2.3. Conclusion for human health part
In line with the ToRs, the contribution of the additional information notified by France and the Netherlands on the latest evaluations on acetamiprid conducted by EFSA and by ECHA was assessed in the current statement. This was carried out using a defined methodology with a probabilistic quantification of the certainty on a causal and positive association between the exposure to the substance and the health outcome.
For genotoxicity, developmental toxicity, neurotoxicity including DNT and immunotoxicity assessment endpoint categories, it was concluded, following a detailed assessment of the available evidence and uncertainties, that moving to the EKE was not necessary and the available evidence does not modify the conclusion reached by EFSA and ECHA. Therefore, for these toxicological assessment endpoint categories, the conclusions issued by EFSA (2016) and ECHA (2017) are still considered valid.
For the endocrine disruption category, based on the uncertainties related to the newly submitted studies (Kong et al., 2017; Terayama et al., 2018) and despite the poor quality of the data (high risk of bias), an assessment in line with the current criteria defined in Commission Regulation (EU) No 2018/605 and implemented in the EFSA/ECHA guidance for the identification of endocrine disruptors (ECHA/EFSA Guidance, 2018), is lacking also in the current conclusions by EFSA (2016). Therefore, an EKE for the endpoint category ED was performed.
Indeed, in the newly submitted studies, there is evidence of modulation of protein synthesis and gene expression associated with the pathway of testosterone synthesis. In rats, there is also evidence of an increase in the circulating levels of LH with a decrease in testosterone. However, the same was not reported for mice where the effects on the HPG axis seem to be less consistent. In mice, although a decrease in testosterone secretion (by measurements of the expression of the enzymes involved in the process) is likely, circulating hormones were not measured and no effect on the expression levels of LH‐β and FSH‐β in pituitary glands were observed. For both mice and rats, the quality of the histological assessment was considered insufficient and this added further uncertainty on adversity definition in the study and how to link adversity to an endocrine mode of action. Furthermore, the study of Kong et al. (2017) attributed their findings to oxidative stress but not to an endocrine mode of action.
Although no concern for endocrine disruption was reported in the latest EFSA/ECHA evaluations, the assessment was not performed in line with the current EFSA/ECHA guidance for the identification of substances having endocrine disruption properties. Despite the poor quality of the evidence assessed, the PPR Panel recognises that determination of LH and testosterone concentrations in plasma, mRNA transcript of genes encoding enzymes involved in the testosterone biosynthesis pathway as well as genes encoding LH and FSH in the pituitary gland along with a robust histological examination of seminiferous tubules may provide potentially valuable mechanistic information.
Based on the available body of evidence, the PPR Panel concludes that the probability of a causal and positive association between exposure to the acetamiprid and an endocrine disruption health outcome was considered ranging between 0% and 17.5% probability (i.e. very low). However, a concern on endocrine disruption for acetamiprid could not be completely excluded (probability greater than 0) based on the current assessment and additional information provided by France and the Netherlands.
2.4. Recommendation for human health part
For genotoxicity, developmental toxicity, neurotoxicity including DNT and immunotoxicity assessment endpoint categories:
-
–
The current assessment was made on selected scientific evidence notified by French and the Netherlands authorities. The PPR Panel concludes that the newly submitted evidence does not change the current conclusion from EFSA and ECHA on acetamiprid and recommends that no further actions should be taken.
For endocrine disruption assessment endpoint category:
-
–
The PPR Panel recommends conducting an assessment of endocrine disrupting properties for acetamiprid in line with EFSA/ECHA guidance document for the identification of endocrine disruptors under Regulations (EU) No 528/20122 and (EC) No 1107/2009 (ECHA/EFSA, 2018).
The PPR Panel recommends that elective selection of evidence, as it was done for this mandate, should be avoided and that a systematic review approach should be instead applied in the future.
3. Environment
3.1. Data
In support of the request to prohibit the sale and use of acetamiprid in accordance with Article 69 of regulation (EC) No 1107/2009, the French authorities provided scientific evidence, including published studies, on the potential serious risks that acetamiprid may pose to human health and to the environment. The mandate received from the EU Commission included also an assessment of the substance flupyradifurone, for which data were submitted by the French and the Dutch authorities.
For the evaluation of the environmental data, all 40 references mentioned in the mandate were screened for relevance for the environmental risk assessment. After a first screening (see Section 3.3.1), information on acetamiprid was available for four groups of non‐target organisms, namely: birds, aquatic organisms, soil organisms, and bees.
3.2. Methodology
Concerning the environmental part, the full methodology used for the assessment is reported in the protocol (Annex A). Below only a brief summary of the methodology is reported for the sake of completeness.
3.2.1. Screening
All documents submitted by France and the Netherlands underwent a screening phase, to identify whether each document reports potentially useful information for the environmental risk assessment. Papers were considered relevant if they contained:
data potentially informing the assessment/quantification of hazard and/or exposure for acetamiprid and flupyradifurone; In this case papers were also classified on the basis of the type of experiments reported (e.g. laboratory, field effect, field exposure) and on the basis of the non‐target group investigated.
mechanistic data that support the explanation of the difference in tolerance between bee species, not necessarily related to acetamiprid and flupyradifurone. The focus of the available papers was mostly on the activity of specific enzymes belonging to the superfamily of cytochromes P450 (CYPs). Some of these enzymes are known to play a role in the phase I detoxification pathways, and thus the presence/absence of some specific enzymes may drive the difference in experimental sensitivity. None of the assessment endpoints measured in these experiments can be used as input in any existing risk assessment model. Nonetheless, it is considered that these experiments may contribute to increase the mechanistic understanding behind the toxicity of some insecticides towards bees, and they may also be used as lines of evidence to aid the extrapolation of toxicity information from one species to another.
3.2.2. Data extraction
The data extraction process was performed differently for hazard/exposure experiments and mechanistic experiments.
Particularly for hazard data, the measured endpoints which can inform the environmental risk assessment for both flupyradifurone and acetamiprid were extracted using a structured data model. This step was implemented in the web‐based tool DistillerSR. Extraction was performed by one reviewer, followed by a thorough check by another reviewer (quality check). Extraction data models were tailored to the different study typologies, and in particular they were different for laboratory and field studies.
For mechanistic data, the extraction was not performed following the same systematic structure used for hazard/exposure studies. The data extraction was on the contrary performed in a more narrative way, also due to the difficulties in finding a common structure for summarising the findings of very diverse experiment types.
3.2.3. Critical appraisal of the evidence (risk of bias and precision)
In this step of the process, the risk of internal and external bias (RoB) and (im)precision was assessed separately in relation to each assessment endpoint.
Internal bias refers to any error in the conduct of the study that results in a conclusion which is different from the truth we are interested in. The method for measuring an assessment endpoint not being reliable/accurate is an example for a source of internal bias in the studies relevant to this assessment. This term is often referred to as the intrinsic reliability of the assessment endpoint.
External bias affects the extent to which the study results are generalisable to the assessment question, e.g. when the study settings are not representative of the reference population/conditions/landscape settings. This term is often referred to as the relevance of the assessment endpoint.
The third aspect next to internal and external bias that was assessed concerns the possible imprecision of the studies included in the assessment, which is related to random error and indicates the ability of a study to provide similar results when repeated under the same conditions. These aspects are mainly related to the sample size of the studies, which may not be large enough for providing a precise estimate of the assessment endpoint, resulting in an imprecise measured endpoint. Similarly, precision of the measured endpoint may depend on the number and the selection of the tested exposure levels.
For hazard/exposure experiments, internal and the external validity (or risk of internal and external bias) and (im)precision were appraised for each individual study using different CATs. A 4‐level rating was used for internal and external validity, in line with the OHAT/NTP tool for RoB assessment (NTP, 2015) and the human health assessment. Assessment of precision only used a 2‐level scale as previous experiences (e.g. EFSA et al., 2020) demonstrated that establishing thresholds for intermediate categories can be extremely challenging for this part of the appraisal.
After a preliminary screening of the studies to be assessed, CATs were developed for different study typologies, which include:
-
–
Laboratory studies investigating effects on bees
-
–
Laboratory studies investigating effects on aquatic organisms
-
–
Laboratory studies investigating effects on soil organisms
-
–
Field studies investigating potential effects on bees
-
–
Field studies providing information on exposure to bees (only external and internal validity).
A single study investigating effects of acetamiprid on birds was also available. For this, no specific CAT has been developed, and the study was assessed following the principles included in the other CATs and elements of the standard OECD test guidelines for birds (e.g. OECD TG 206; OECD, 1984). The tools were translated in a digital form using DistillerSR. Appraisal for the only bird study was done outside of this tool. For each study, the appraisal was independently performed at assessment endpoint level by two reviewers. In agreement with the protocol, any disagreement was first discussed among the two reviewers and, if no solution was possible, the issue was discussed by the whole WG.
For each of the CATs, key questions and non‐key questions were identified in order to assess internal and external validity and precision. Key and non‐key questions were combined into a single scoring method, classifying each assessment endpoint from each study into a different class (from class 1 to class 3) reflecting the risk of bias.
Questions were considered key when a probably high (PH) RoB or a definitely high (DH) RoB would immediately cause the assessment endpoint not to achieve the highest class. Key questions have also a higher weight in determining whether the assessment endpoint can achieve a class 2. Classification of questions in key and non‐key was largely based on validity criteria from the most relevant OECD test guidelines, but it was also complemented by expert judgement and it considered the objectives highlighted in the most relevant guidance documents for the risk assessment.
It should be highlighted that a high risk of bias for key criteria did not translate in the dismissal of the assessment endpoint. All endpoints were considered in a final WoE (see Section 3.2.5), whether they were considered critical or not. This was done to provide a more transparent and comprehensive picture of the available information.
For mechanistic experiments, the appraisal was performed in a more narrative way. Since none of the assessment endpoints contained in those experiments will be directly used to quantify the hazard and/or the exposure, the need for classifying those into a specific ‘risk of bias level’ was deemed limited. Thus, while criteria guiding such appraisal were defined a priori (see Annex A) these were uniquely used as guiding principles, and no explicit categorisation of the risk of bias was performed. In this case the appraisal was done by one reviewer and later checked by a second reviewer.
3.2.4. Calibration
The full process involving screening, data extraction and appraisal for hazard/exposure experiments underwent a calibration exercise involving a limited number of documents (n = 3). This was used to check the status of alignment among reviewers and to identify critical aspects that needed further clarifications and better definitions in order to avoid different interpretations of the same criteria.
3.2.5. Weight of evidence and uncertainty analysis
This part of the methodology was not fully detailed in the protocol, as this required an approach tailored to the available data, whose knowledge was limited before the full extraction and appraisal.
Initial assessment
The outcome of the critical appraisal was summarised using heatmaps. This data visualisation tool allowed to quantitatively synthetise precision, external and internal validity relative to each appraisal question (Appendix A). Additionally, the overall classification of precision, external and internal validity for each endpoint was first calculated using the algorithm described in Annex A, and then summarised using the same data visualisation tool described above (see figures under Section 3.3.2, as an example). The latter heatmaps were used to inform the evaluation of the available evidence, primarily, as a screening tool to identify the scores of reliability, relevance and precision. Additionally, heatmaps were used to support the identification and grouping of similarly relevant endpoints. These groups later defined the lines of evidence used in the final assessment.
Identification of the lines of evidence and comparison with previous endpoints
Given the heterogeneity of study designs and complexity of data, the identification of the lines of evidence required a certain degree of expert judgement and, therefore, could not be fully standardised across studies. Nonetheless, a significant effort was made to harmonise the approaches used across non‐target organisms and study types. To facilitate the synthesis of endpoints, results of the data extraction were arranged by study typology, exposure regime and assessment endpoint type. Then, the resulting endpoint groups were graphically plotted using standard data visualisation tools (Wickham, 2016; R Core Team, 2021). A limitation of this approach is that data visualisation tools are intrinsically limited by the number of aesthetics which can be assigned to given variables. Therefore, a careful choice of the type of data aggregation was required on a case‐by‐case. This is particularly relevant, since studies were heterogeneously designed and not standardised. However, this should not be considered a major limitation, given that data visualisation was used as a tool for‐, and not the outcome of the WoE. Additionally, because of the nature and heterogeneity of data, and consequent to the data extraction process, standard research synthesis methods (e.g. meta‐analytical approaches) were not deemed practical.
Plots were standardised in the following aspects:
The x‐axis (continuous) represented the exposure level
The y‐axis (factor) identified specific combinations of study and experiment ID
The aesthetics (i.e. dot size, shape and colour) were assigned to the most relevant combination of grouping variables for a given line of evidence (i.e. species; exposure route; internal validity; effect level and assessment endpoint type)
Where plots were arranged in multiple panels, the latter were used to display and sort endpoints by external validity or assessment endpoint type
Whenever the exposure regime used in the studies under assessment was comparable (or could be approximated) to the standard regimes used across studies of the EU assessment, the relevant EU agreed endpoints were also plotted in the same graphs as vertical dashed lines.
Only comparable exposure units were used in a single line of evidence. Whenever possible, concentrations were converted accordingly. Whenever conversion to the same exposure units used in risk assessment was not possible, endpoints were discarded. Indeed, harmonising exposure units to those used in the EU risk assessment was considered key to this mandate.
Our methodology did not exclude any data a priori but, rather, gave higher consideration to endpoints characterised by the highest scores of relevance, validity and precision. For this purpose, heatmaps were used as screening tool to identify – and therefore, focus on – those endpoints characterised by the highest validity and precision. Endpoints with the lowest score of internal validity were given low weight in the final assessment, but were still described, summarised and discussed in each line of evidence.
Weighing the evidence and the uncertainty by expert judgement
Upon assessment, different lines of evidence were collated in individual tables following a categorisation by study type and assessment endpoint group. These tables summarised the WoE and uncertainty analysis with a structured approach. For this purpose, the strength of each line of evidence was defined by its overall scores of validity and precision. Additionally, a new, 3‐level (i.e. low; moderate and high) quality score named ‘internal consistency’ was introduced. The purpose of this indicator was to quantify the coherence across endpoints characterising each line of evidence. Finally, a 3‐level (i.e. low; moderate and high) judgement was assigned to the potential of each line of evidence to indicate a higher hazard compared to the data considered in the previous peer review (EFSA, 2016). Paired to this judgement, a threefold qualitative indicator of the uncertainty of such judgement was introduced indicating the level of certainty of the assessment. Specifically, the uncertainty – whose quantification required a certain degree of expert judgement – was defined as the link between external validity, internal validity, precision and internal consistency. Additionally, a text column was used to further justify the rationale behind the judgement.
Mechanistic studies
As reported in Annex A, a series of ad hoc criteria were developed for the data extraction and appraisal of mechanistic studies. Briefly, because of the different nature of the mechanistic data, it was decided to extract and appraise the endpoints with a descriptive approach and not to assign quantitative (validity and precision) indicators to each endpoint. Particularly, the data extraction was initially performed narratively, along with the appraisal. Nonetheless, upon later reconsideration, an additional schematic and more structured data extraction was deemed useful to collate the different lines of evidence (Annex G).
It should be noted the references including mechanistic data also included description of experiments with standard laboratory designs, which were considered directly and highly relevant to the scope of this mandate (e.g. Hayward et al., 2019; RefID 32). These endpoints underwent a full, separate assessment, using the CATs developed for bee laboratory studies.
The resulting mechanistic endpoints were collated into a single WoE and uncertainty analysis (i.e. including consideration of both acetamiprid and flupyradifurone), which – similar to the appraisal – were done in a more descriptive way than other designs. The reason behind this choice is that a considerable proportion of mechanistic data were not specifically linked to any pesticide (i.e. phylogenetic studies and expression profiling). Furthermore, other endpoints were used as read‐across information (i.e. linked to substances other than acetamiprid and flupyradifurone, but still indirectly informative of their assessment). Consequently, the proportion of mechanistic data specifically linked to either acetamiprid or flupyradifurone was low. Therefore, the same evaluation of the mechanistic experiments was reported in both the statements.
3.2.6. Deviations from the protocol
CATs for hazard/exposure studies
Some modifications of the CATs were considered necessary after the evaluation process started. These were needed as the original formulations of the different risk of bias categories for some criteria and for specific situations not tested in the calibration exercise, resulted in contradictory interpretations between the reviewers. These deviations were transparently reported in yellow‐highlighted cells directly in the protocol description in Annex A.
Weight of evidence and uncertainty analysis for hazard data and mechanistic studies
The methodology for the WoE and the uncertainty analysis was not fully detailed in the protocol. Hence, the methodology outlined in Section 3.2.5 is considered a deviation from the original plan.
3.3. Assessment
3.3.1. Results of the screening step
For acetamiprid, hazard data were available for four groups of non‐target organisms (birds, aquatic organisms, bees, soil organisms). For bees, mechanistic data were also available. Relevant exposure data were on the contrary not available.
Apart from the 10 references considered in the human health assessment, there were other references which reported environmental data, which, nonetheless, were not considered relevant for the present assessment.
Traynor et al. (2016; RefID 12) measured residues from live in‐hive bees, stored pollen, and wax in migratory colonies over time and compared exposure to colony health. However, no residues of acetamiprid were reported. Thus, the study cannot inform the exposure assessment for the active substance under investigation.
Çamlica et al., 2019; RefID 30) reported an in‐vitro experiment on the effects of acetamiprid on the sciatic nerve of Rana ridibunda. The study does not provide endpoints that can be used in the context of the environmental risk assessment of pesticides. The study was nevertheless considered in the context of the human health evaluation.
O’Neill and O’Neill (2011; RefID 35) analysed the pollen load composition and size in Megachile rotundata. The study does not provide any direct information about exposure to any of the two substances considered in this mandate. In principle, if information on the uses of these substances were defined, pollen preferences might be qualitatively used to predict the relevance of the exposure to these two substances in conditions comparable to the ones of the study. The study was carried out in Montana (US) in an area characterised by alfalfa monoculture, which is therefore not so relevant for EU. The predominant pollen types both by count and by volume were alfalfa, mustard, and sweet clover. The landscape was dominated by alfalfa, so it is not surprising that this was dominant in the pollen loads. High abundance of mustard confirms attractiveness of brassica flowers. The proportion of crop/non‐crop flowers in the area is not known, so it is difficult to extrapolate these findings to other contexts. However, the authors do mention that ‘The relative densities […] other flowering plants at the same site was assessed in an earlier study, in which we showed that the proportion of pollen types extracted from females correlated with the relative density of different plant species within 50 m of nest boxes (O’Neill et al., 2004)’. Hence, this study as such does not provide specific information that allows dismissing foraging on crops in general nor on crops other than alfalfa and Brassicaceae. Overall, the paper does not provide usable exposure information for the risk assessment.
Sinu and Bronstein (2018; RefID 36) reported foraging preferences of leafcutter bees regarding leaf discs used as nesting materials. This source of exposure, while possibly relevant, is not considered in the current risk assessment scheme. Preference for nesting materials may be completely different compared to preference for pollen and nectar foraging, which is the main route of exposure currently considered. In addition, the study reports about investigations carried out mainly in non‐agricultural crop (most were in urban areas) and hence the relevance of the findings for agricultural areas are disputable.
Of the 40 references available, 13 (2 for human health and 11 for the environment) reported data for flupyradifurone (EFSA PPR Panel, 2021) and are therefore not further considered in this statement. For the present statement 14 references were further considered for the environment.
3.3.2. Birds
3.3.2.1. Data from previous peer review
In the previous peer review (EFSA, 2016) for acetamiprid, acute data on birds were available for three different species, showing a considerable variability in sensitivity. LD50s varied from 5.7 mg a.s./kg bw for Poephilia guttata to 98 and > 100 mg a.s./kg bw for Anas platyrhyncos and Colinus virginianus.
The risk assessment was performed using the geometric mean of the three available acute LD50s (38.2 mg a.s./kg bw). Nonetheless, EFSA later identified a data gap for further risk assessment refinements, to ensure the most sensitive species would be protected.
Indeed, the interspecies difference in sensitivity was confirmed by two short‐term studies, not considered for the risk assessment. The 8‐days LD50 (5 days exposure) for Poephilia guttata was 14 mg a.s./kg bw per day, while it was > 856 mg a.s./kg bw per day for Anas platyrhyncos.
Data on reproductive long‐term toxicity were available uniquely for Anas platyrhyncos, with a NOAEL of 9.5 mg a.s./kg bw per day.
All available data from the previous peer review is summarised in Table 5.
Table 5.
Summary of bird endpoints from the previous peer review (EFSA, 2016)
| Species | Test item | Test type | Endpoint |
|---|---|---|---|
| Anas platyrhyncos | Acetamiprid | Acute | LD50 = 98 mg a.s./kg bw |
| Colinus virginianus | Acetamiprid | Acute | LD50 > 100 mg a.s./kg bw |
| Poephilia guttata | Acetamiprid | Acute | LD50 = 5.7 mg a.s./kg bw |
| Anas platyrhyncos | Acetamiprid | Short‐term | LD50 > 856 mg a.s./kg bw per day |
| Poephilia guttata | Acetamiprid | Short‐term | LD50 = 14 mg a.s./kg bw per day |
| Acetamiprid | Long‐term | NOAEL = 9.5 mg a.s./kg bw per day |
LD50: lethal dose, median; NOAEL: no‐observed‐adverse‐effect‐level.
3.3.2.2. Outline of the submitted studies
Among the studies object of the present mandate, only one focussed on adverse effects on birds (Humann‐Guilleminot et al., 2019; RefID 27), specifically on wild‐captured Passer domesticus. The experiment presented a non‐standard design, where birds were exposed for 19 days with an oral (gavage) dose every 3 days. The experiment measured several assessment endpoints for the male individuals, some related to growth, other to reproduction (i.e. sperm quality), other more related to subindividual biomarkers measured in bird sperm. Only one dose was tested, i.e. 7.125 µg a.s./bird, which is equivalent to 28.5 µg a.s./kg bw (= 0.0285 mg a.s./kg bw). This is the total dose which was administered over 19 days.
3.3.2.3. Hazard characterisation and evaluation of the newly available data
Since only one experiment on birds was available in the data set, a specific CAT was not developed (see Annex A). Nevertheless, similar criteria used for assessing other laboratory studies on other non‐target organism groups were used to appraise the bird study as well, together with the principles derived from the OECD TG 206 (OECD, 1984).
No significant effects were seen at the only tested dose for the growth‐related assessment endpoint (i.e. body weight). Similarly, no significant effects of the treatment were recorded for sperm biomarkers related to its oxidative status. Thus, for all these assessment endpoints, the only tested dose was considered as a NOEAL.
Several assessment endpoints were related to sperm quality and thus indirectly to reproduction. No significant effect of the treatment was recorded for the majority of those (e.g. sperm velocity, longevity, mobility, ejaculate size). The only affected parameter was sperm density, which was reduced by about 40% on average.
A moderate risk of bias for external validity (class 2, see Figure 6) was assigned to the growth‐related assessment endpoint (i.e. body weight) and to reproduction endpoints, except for bio‐markers (class 3, high risk of bias). Hence, the measured endpoints can hardly be used as benchmark for the risk assessment, mainly due to the non‐standard nature of the study design.
Figure 6.
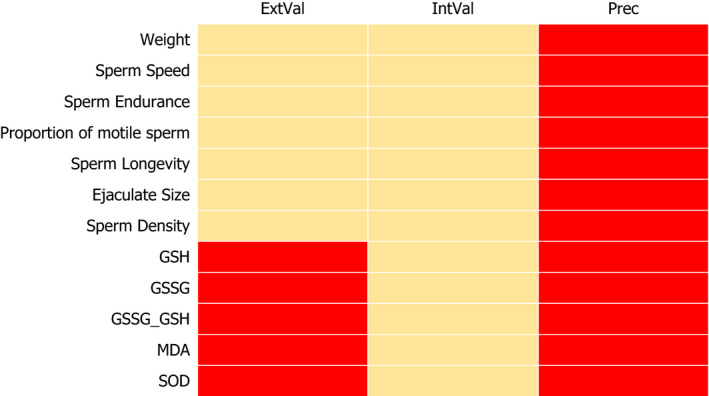
Summary of the appraisal done on the assessment endpoints for laboratory experiments with birds. The outcome takes into account the risk of bias and the precision for several criteria combined with a pre‐defined algorithm (see Annex A). Green indicates low risk of bias or high precision (class 1), yellow moderate risk of bias (class 2 for external and internal validity), while red indicates high risk of bias (class 3) or low precision (class 2)
A moderate risk of bias (class 2, see Figure 5) was also assigned to internal validity to all assessment endpoints. Finally, a low precision (Figure 5) was also assigned, mainly due to the NOEAL/lowest observed adverse effect level (LOAEL) based on a single tested dose only. For the detailed appraisal of this reference, please refer to Annex H.
3.3.2.4. Comparison of new data with previous hazard characterisation
The only effect seen in the study is related to sperm density, whose LOAEL is 28.5 µg a.s./kg bw. It must be noted that this is the total dose given to the birds over 19 days. Generally, for the reproductive risk assessment a daily dose is used. In this case, such daily dose is not straightforward, as birds were given the treatment once every 3 days. Each single dose was 4.07 µg a.s./kg bw. This value is 1000 times lower than the endpoint previously used for risk assessment.
It is unclear how sperm density may relate to population‐level effects, which is the focus of the reproductive risk assessment. In addition, either NOAEL or benchmark dose (BMD) values are generally used for the risk assessment of birds, while in this case, the only available measured endpoint is a LOAEL, which is obtained from a non‐standard study presenting also issues of internal validity. Hence, the use of the derived LOAEL for sperm density directly in the risk assessment is discouraged. However, the available evidence suggests that difference between species for acetamiprid toxicity should be considered further.
In fact, while the reproductive‐related assessment endpoints are not comparable between the submitted non‐standard experiment (Humann‐Guilleminot et al., 2019; RefID 27) and the standard one previously used for the risk assessment, there is indication of a potentially higher chronic sensitivity of Passer domesticus (Passeriformes) with respect to Anas platyrhyncos (Anseriformes). It is acknowledged that the new data are not conclusive by themselves in providing an inter‐species comparison for chronic data. However, evidence of potential differences between the two orders were already recorded for both acute toxicity and short‐term toxicity (Poephilia guttata also belongs to Passeriformes, see Table 5). Hence, this aspect should be further evaluated in an updated risk assessment.
3.3.2.5. Weight of evidence and uncertainty analysis
The analysis reported in Table 6 addresses the question whether the new studies could potentially indicate a higher hazard in comparison to the data used in the previous peer‐reviewed risk assessment (EFSA, 2016).
Table 6.
Weight of evidence and uncertainty analysis of the available prolonged exposure laboratory data for birds. EV = external validity; IV = internal validity; Prec = precision; IC = internal consistency
| Assessment endpoint group | RefID|exp | Strength of the line of evidence | Potential to indicate a higher hazard compared to EFSA (2015) | |
|---|---|---|---|---|
| Judgement | Rationale | |||
| Reproduction | 27|1 |
EV: moderate RoB IV: moderate RoB Prec: low IC: NA |
High with low certainty |
The data are informative for the risk assessment, but their relevance is limited by the fact that the experiment design is non‐standard and because the assessment endpoints are not immediately linked to a reproductive output and thus do not translatable into a population level effect. All available endpoints present a low precision and a moderate risk of bias for internal validity. Since only one experiment is available, the internal consistency of the line of evidence cannot be assessed. Sperm density was significantly reduced at a dose which is considerably lower than the endpoint previously used for risk assessment, despite an exposure which was considerably shorter than the one used in standard testing. Within this line of evidence, it must be considered that the reproductive endpoint used in the previous peer review is related to mallard duck. Evidence of potential differences between Passeriformes and Anseriformes (with the former being substantially more sensitive than the latter) were already recorded for both acute toxicity and short‐term toxicity from the dossier data |
| Growth | 27|1 |
EV: moderate RoB IV: moderate RoB Prec: low IC: NA |
Low with low certainty |
The data are informative for the risk assessment, but their relevance is limited by the fact that the experiment design is non‐standard. All available endpoints present a low precision, and a moderate risk of bias for internal validity. Since only one experiment is available, the internal consistency of the line of evidence cannot be assessed. No significant effects on growth assessment endpoints (body weight) were recorded in the only available experiment |
| Subindividual alteration | 27|1 |
EV: high RoB IV: moderate RoB Prec: low IC: NA |
Low with low certainty |
The data are not relevant for the risk assessment, as the biological meaning of the monitored assessment endpoint is not fully clear even at the individual level, thus their relevance at the colony level is, for the time being, considered very low. All available endpoints present a low precision, and a moderate risk of bias for internal validity. Since only one experiment is available, the internal consistency of the line of evidence cannot be assessed. No significant effects on subindividual alterations were recorded in the only available experiment |
3.3.2.6. Conclusion for birds
The newly available data cannot be directly used in the risk assessment, but they seem to be consistent with an interspecies difference in the sensitivity to acetamiprid, with Passeriformes showing higher sensitivity. This aspect was already flagged in the previous EFSA conclusion (EFSA, 2016) where a data gap was identified with reference to the acute data. With the newly available information, the issue extended to chronic data, as the chronic risk assessment was previously performed with an endpoint derived for mallard duck, which is likely not to be protective for Passeriformes. A more systematic analysis of the literature would be needed to exclude bias.
While the present analysis does not refer to any specific use, it is highlighted that a high acute and chronic risk to insectivorous birds was already concluded in EFSA (2016) for the use on pome fruit.
3.3.3. Aquatic organisms
3.3.3.1. Data from previous peer review
In an acute test on Cyprinodon variegatus the LC50 was 100 mg active ingredient a.i./L under flow‐through conditions. Two further fish species showed a lower sensitivity towards acetamiprid with LC50 > 100 mg/L in a static or flow‐through test. In a flow‐through conditions test on Pimephales promelas the lowest value from available EC10 (effect concentration, 10%) and NOEC (no observed effect concentration) values was NOEC = 9.4 mg a.i./L, based on effects on hatchability observed in an early life stage test. In an amphibian metamorphosis assay with Xenopus laevis, NOEC = 2.6 mg/L based on effects on growth and weight.
In an acute test on Daphnia magna the EC50 (effect concentration, median) was 49.8 mg a.i./L under static conditions. Two insect species (Chironomus riparius and Simulium latigonium) had lower endpoints than three crustaceans, resulting in a geomean of EC50 = 0.0085 mg/L for aquatic insects and geomean EC50 = 0.069 mg a.i./L for crustaceans. The lowest endpoint listed is for the blackfly larvae Simulium latigonium with EC50 = 0.0037 mg a.i./L in a static study. In a reproduction test on D. magna the EC10 was 2.96 mg to be specified above in the text a.i./L, based on effects on reproduction under semi‐static conditions. For Chironomus riparius, EC10 = 0.000235 mg a.i./L based on effects on emergence in a static study. More data regarding the acute and chronic toxicity to species belong to Naididae were considered necessary.
In a static test on Anabaena flos‐aquae the EC50 was > 1.3 mg a.i./L and for Lemna gibba EC50 > 1 mg a.i./L based on fronds number.
Aquatic invertebrates were the most critical group with the lowest first tier endpoint for Simulium latigonium with EC50 = 0.0037 mg a.i./L. In an outdoor mesocosm study (two applications with an interval of 14 days) ETO‐RAC = 0.00037 mg a.i./L was derived with a safety factor of 3 from a NOEC of 0.0011 mg/L based on class 2 effects on Asellus aquaticus. Class 5B effects were observed on Cloeon sp. at the next concentration of 0.0026 mg a.i./L. Only class 1 effects were observed at 0.0005 mg a.i./L. A summary of the endpoints derived in the previous peer review in EFSA (2016) is presented below (Table 7).
Table 7.
Summary of endpoints for aquatic organisms from the previous peer review (EFSA, 2016)
| Group | Species | Test item | Time‐scale | Endpoint |
|---|---|---|---|---|
| Fish | Cyprinodon variegatus | Acetamiprid | Acute (4 days) | LC50 = 100 mg/L |
| Pimehales promelas | Acetamiprid | Chronic (35 days) | NOEC = 9.4 mg/L | |
| Aquatic invertebrates | Simulium latigonium | Acetamiprid | Acute (4 days) | EC50 = 0.0037 mg/L |
| Chironomus riparius | Acetamiprid 20% SP | Chronic (28 days) | EC10 = 0.000235 mg/L | |
| Algae | Anabaena flos‐aquae | Acetamiprid | (5 days) | EC50 > 1.3 mg/L |
| Higher plants | Lemna gibba | Acetamiprid | (14 days) | EC50 > 1.0 mg/L |
LC50: lethal concentration, median.
3.3.3.2. Outline of the submitted studies
Two references reporting experiments on aquatic organisms were submitted. One acute study (Demirci and Güngördü, 2020; RefID 28) consisted of three experiments and evaluated lethal (experiment 1) and biochemical effects of a SP formulation (20% ai) on the non‐target eastern mosquitofish Gambusia holbrooki after 24‐ (experiment 2) and 96‐h (experiment 3) exposure with a renewal of the solution every 24 h. Changes in biomarkers response could only be measured after 24‐ and not 96‐h exposure. The other chronic study (Cossi et al., 2020; RefID 16) evaluated the lethal and sublethal toxicity of the technical active substance (experiment 1) and a WP formulation (75% ai, experiment 3) after 14 days of exposure on the non‐target freshwater gastropod Biomphalaria straminea as an indicator species. The exposure was semi‐static over 14 days with a renewal of solution after 48 h. Effects on reproduction were measured after 30 days (experiments 2 and 4), resulting in four experiments altogether. The toxicity of acetamiprid on the gastropod species was mainly related to effects on detoxification and oxidative metabolism responses.
3.3.3.3. Hazard characterisation and evaluation of the newly available data
Acute data (standard assessment endpoints)
In an acute semi‐static study with the eastern mosquitofish (Demirci and Güngördü, 2020; RefID 28) over 96 h the LC50 was calculated as 42.2 mg a.i./L. The external and internal validity for the survival endpoints were considered to have a low risk of bias (class 1, see Figure 7). The precision was considered low as only three concentrations were tested without a range finding study.
Figure 7.
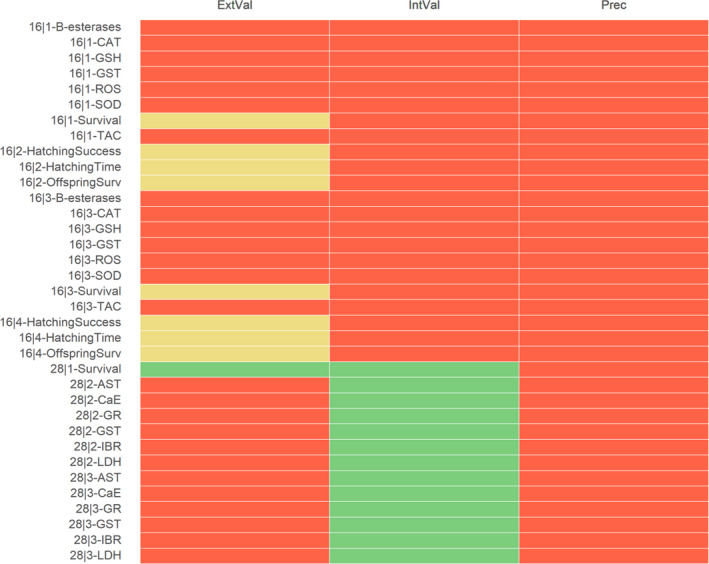
Summary of the appraisal done on the assessment endpoints for laboratory experiments with aquatic organisms. The outcome takes into account the risk of bias and the precision for several criteria combined with a pre‐defined algorithm (see Annex A). Green indicates low risk of bias or high precision (class 1), yellow moderate risk of bias (class 2 for external and internal validity), while red indicates high risk of bias (class 3) or low precision (class 2)
Chronic data (standard assessment endpoints)
No effects on survival and reproduction were observed after 14 days exposure up to 1.5 mg a.i./L on the gastropod Biomphalaria straminea (Cossi et al., 2020; RefID 16). No difference in toxicity was observed between the technical ai and the WP formulation. Assessment endpoints were hatching success, hatching time and offspring survival. The chronic endpoints based on survival and reproduction (NOEC = 1.5 mg a.s./L) are considered to have a low risk of bias (class 1) with regard to external validity and are considered useful for the comparison. However, the internal validity is low as the exposure was not measured and no effects on survival and reproduction were observed. Also, the precision in the study with the gastropods is assessed as being low as only two concentrations were tested, and no effects were observed.
Subindividual alteration (non‐standard acute and chronic assessment endpoints)
In the acute study with the eastern mosquitofish effects (Demirci and Güngördü, 2020; RefID 28) the SP formulation effects on subindividual endpoints were observed below the LC50. Effects on GST (glutathione S‐transferase) and IBR (integrated biomarker response) occurred at the lowest observed effect concentration (LOEC) = 21.1 mg/L after 24‐h exposure, but not after 96‐h. No effects on AST (aspartate aminotransferase), CaE (carboxylesterase) and GR (glutathione reductase) were observed up to NOEC = 21.1 mg/L. The most sensitive subindividual endpoint was LDH (lactate dehydrogenase) with effects at 8.44 mg/L after 24‐h exposure, but not after 96‐h.
In the chronic study with the gastropod Biomphalaria straminea (Cossi et al., 2020; RefID 16), effects on B‐esterase, GST, GSH (glutathione) and SOD (superoxide dismutase) were measured after exposure to the WP formulation at LOEC = 0.15 mg a.i./L. Effects on CAT (catalase), ROS (reactive oxygen species) and TAC (total antioxidant capacity) were only observed at LOEC = 1.5 mg a.i./L. After 14‐d exposure to the technical ai effects on GST and SOD were observed at LOEC = 0.15 mg/L, and effects on B‐esterase and ROS were observed at LOEC = 1.5 mg/L. No effects on CAT, GST and TAC were observed up to NOEC = 1.5 mg a.i./L after exposure to the technical ai.
The subindividual endpoints are not considered suitable for a comparison with the previous evaluation, as those endpoints are not part of the standard evaluation procedure and can therefore not be related to the attribute to protect. The external validity was therefore class 3. As the internal validity was class 1, all measured endpoints in the acute experiment may be used as supportive information. However, as the exposure was not measured in the chronic study the internal validity is class 3. As only two concentrations were tested, the precision is assessed as low for both experiments.
3.3.3.4. Comparison of new data with previous hazard characterisation
Although the LC50 for eastern mosquitofish is lower than the previously reported acute endpoints for fish (Figure 8), the newly submitted studies did not provide an endpoint which increases the concern due to the toxicity of acetamiprid as the aquatic risk assessment is triggered by aquatic invertebrates.
Figure 8.
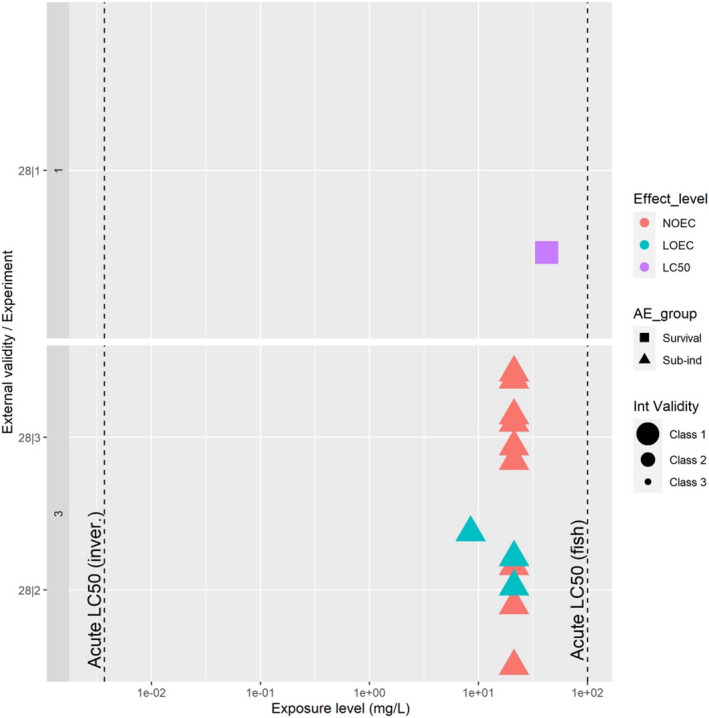
Summary plot of the acute aquatic data available for acetamiprid. Each line on the y‐axis represents an experiment within a reference (e.g. XX|Y indicate experiment Y within reference XX), organised by external validity class (class 1 representing low risk of bias). Colours identify the type of measured endpoint (effect level), shapes the assessment endpoint group, and size of the markers identify the internal validity class (class 1 representing low risk of bias). Vertical dashed lines highlight the fish acute endpoints available in the EU peer review (EFSA, 2016). The chronic endpoint for survival and reproduction for the snail was imprecise and higher than previous endpoints for aquatic invertebrates (see Figure 9). Subindividual alterations were also observed at concentrations higher than chronic endpoints validated in the previous peer review
Figure 9.
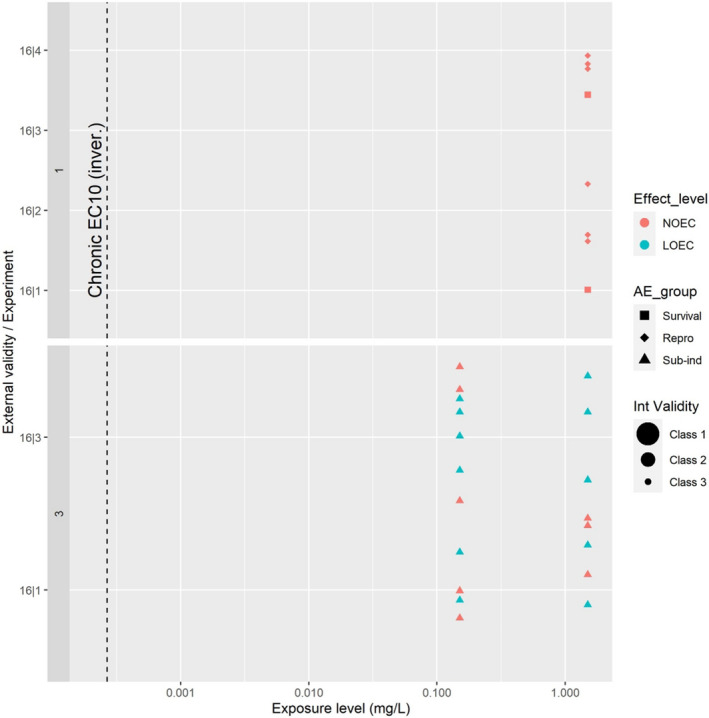
Summary plot of the chronic aquatic data available for acetamiprid. Each line on the y‐axis represents an experiment within a reference (e.g. XX|Y indicate experiment Y within reference XX), organised by external validity class (class 1 representing low risk of bias). Colours identify the type of measured endpoint (effect level), shapes the assessment endpoint group, and size of the markers identify the internal validity class (class 1 representing low risk of bias). Vertical dashed lines highlight the endpoints available in the EU peer review (EFSA, 2016). Weight of evidence and uncertainty analysis
3.3.3.5. Weight of evidence and uncertainty analysis
The analysis reported in Table 8 addresses the question whether the new studies could potentially indicate a higher hazard in comparison to the data used in the previous peer‐reviewed risk assessment (EFSA, 2016).
Table 8.
Weight of evidence and uncertainty analysis of the available laboratory data for aquatic organisms. EV = external validity; IV = internal validity; Prec = precision; IC = internal consistency
| Assessment endpoint group | RefID|exp | Strength of the line of evidence | Potential to indicate a higher hazard compared to EFSA (2016) | |
|---|---|---|---|---|
| Judgement | Rationale | |||
| Survival |
acute 28|1 |
EV: low RoB IV: low RoB Prec: low IC: NA |
High with a high certainty for fish Low with a high certainty for aquatic organisms as a whole |
The data are relevant for the risk assessment of aquatic organisms, but as the aquatic risk assessment is driven by aquatic invertebrates the overall potential of the new study with fish to indicate a higher hazard is low. Overall, the data seem robust. Since only one study with this species is available, the internal consistency of the line of evidence cannot be assessed |
|
chronic 16|1 16|3 |
EV: moderate RoB IV: high RoB Prec: low IC: NA |
Low with a low certainty | The data are relevant for the risk assessment of aquatic organisms, but as the previous endpoint for aquatic invertebrates is lower than the tested concentrations the potential of the new study with snails to indicate a higher hazard is low. Overall, the data seem not robust as the concentrations were not measured. Since only one study with this species is available, the internal consistency of the line of evidence cannot be assessed | |
|
Reproduction |
chronic 16|4 |
EV: moderate RoB IV: high RoB Prec: low IC: NA |
Low with a low certainty | The data are relevant for the risk assessment of aquatic organisms, but as the previous endpoint for aquatic invertebrates is lower than the tested concentrations the potential of the new study with snails to indicate a higher hazard is low. Overall, the data seem not robust as the concentrations were not measured. Since only one study with this species is available, the internal consistency of the line of evidence cannot be assessed |
| Subindividual |
Acute and chronic 28|2 28|3 16|1 16|3 |
EV: high RoB IV: low to high RoB Prec: low IC: NA |
Low with a moderate certainty | The data are of limited relevance for the risk assessment, due to the impossibility to link subindividual effects to effects at the population level. The potential to indicate a higher hazard is low as the tested concentrations were higher than the previous endpoint triggering the aquatic risk assessment. Overall, the data seem not robust as the concentrations were not measured. Since only one study with this species is available, the internal consistency of the line of evidence cannot be assessed |
3.3.3.6. Conclusion for aquatic organisms
The two newly submitted studies with aquatic organisms (fish and snail) do not alter the previous aquatic hazard assessment as all endpoints (lethal, reproduction and subindividual) were higher than the previous endpoint driving the risk assessment, which is based on aquatic invertebrates.
No studies were submitted addressing the data gap from the previous peer review (EFSA, 2016) with regard to the uncertainties in sensitivity of species belonging to Naididae (worms).
3.3.4. Bees
3.3.4.1. Data from previous peer review
Data covered in the previous peer review of the risk assessment of acetamiprid (EFSA, 2016) included laboratory acute contact and oral toxicity studies on the honey bee Apis mellifera and an acute contact toxicity study on the bumble bee Bombus terrestris using the formulation EXP 60707A/Acetamiprid 20 SG/Mospilan 20 SG (Table 9). There was also one acute oral study with B. terrestris but variable number of bees consumed the sugar solution and there was no clear dose response, so it was considered questionable. The acute oral LD50 (48 h) for honey bees was 8.85 μg a.s./bee. The acute contact LD50 (48 h) was for honey bees 9.26 μg a.s./bee and > 100 μg a.s./bee for bumble bees. There was also one laboratory chronic oral toxicity study on honey bees over 10 days and a honey bee larval study using technical grade acetamiprid (Table 9). The chronic oral median lethal daily dose (LDD50) was 11.7 μg a.s./bee per day and the larval LD50 and LD10 were 21.7 and 1.3 μg a.s./larvae per developmental period, respectively.
Table 9.
Summary of tier‐1 endpoints for bees from the previous peer review (EFSA, 2016)
| Species | Test item | Test type | Endpoint |
|---|---|---|---|
| Apis mellifera | EXP 60707A | Acute oral | LD50 = 8.85 μg a.s./bee |
| EXP 60707A | Acute contact | LD50 = 9.26 μg a.s./bee | |
| Acetamiprid | Chronic oral | LDD50 = 11.7 μg a.s./bee per day | |
| Acetamiprid | Repeated exposure larvae | LD10 = 1.3 μg a.s./larva per dev. period | |
| Bombus terrestris | EXP 60707A | Acute contact | LD50 > 100 μg a.s./bee |
LD50: lethal dose, median.
These studies were, according to the risk assessment report, evaluated based on EFSA (2013), since the guidance at the time (EC 2002) did not cover chronic toxicity for honeybee adults or their brood, which should be provided along with the acute toxicity for honeybee adults following Regulation (EU) No 283/2013. A low risk was concluded for honey bee acute, chronic and larval exposure as well as for bumble bee acute contact exposure for uses on potato and post flower application in pome fruit. There were not enough data to conduct a complete risk assessment for bumble bees or solitary bees. The same was true for metabolites for honey bees, even if a low risk was expected based on their non‐insecticidal activity.
In addition to the laboratory studies, there were a number of semi‐field and field studies focusing on honey bees. Application was done during full flowering (phacelia or oilseed rape) and bee activity, usually once with 50–120 g a.s./ha. The semi‐field and fields studies were however considered to not be reliable or robust enough to include in the risk assessment because of short observation duration, no confirmation of exposure level and inclusion of few fields and colonies. There were notes on occasional and often transient effects on adult mortality and flight activity and brood development, but no clear patterns emerged so it was concluded that these studies could not be used to inform the risks for honey bees. It was highlighted that other uses than the representative may result in higher exposure and that there was not sufficient information to conclude on chronic effects on adults or brood development.
A systematic literature review was conducted and literature that was relevant and given a Klimisch score 1–2 was included, resulting in the inclusion of 11 relevant and reliable publications and an additional relevant publication added by the rapporteur member state (RMS). The included literature indicated observable sublethal effects at 0.1 μg a.s./bee, but it was noted that it was unclear how this related to honey bee colony survival and development. The literature review outcome also indicated that bumble bees may be more sensitive than honey bees.
3.3.4.2. Outline of the submitted studies
Hazard experiments
Among the submitted references, four reported on acetamiprid and all of them on the honey bee A. mellifera. These were El Hassani et al. (2014; RefID 11), Shi et al. (2020a,b; RefID 15 and RefID 17 respectively) and Mazi et al. (2020; RefID 18). Studies included both adult (all four studies) and larval stages (Shi et al., 2020, b; RefID 17) of honey bees, both contact (El Hassani et al., 2014; RefID 11, Shi et al., 2020a; RefID 15 and Mazi et al., 2020; RefID 18) and oral (El Hassani et al., 2014; RefID 11 and Mazi et al., 2020; RefID 18) exposure and acute (El Hassani et al., 2014; RefID 11, Shi et al., 2020a; RefID 15 and Mazi et al., 2020; RefID 18) as well as prolonged exposure (Shi et al., 2020b; RefID 17). Three studies were conducted under laboratory conditions and one on free foraging bees exposed under controlled conditions (Shi et al., 2020a; RefID 15). There were no studies focusing on bumble bees or solitary bees and no semi‐field or field studies using application of acetamiprid on plants as the exposure route.
El Hassani et al. (2014; RefID 11) measured several behavioural assessment endpoints (locomotion and proboscis extension response, PER) after acute oral (exp. 1) or contact (exp. 2) exposure to technical grade acetamiprid at three different doses (0.1, 0.5 and 1 µg a.s./bee).
Shi et al. (2020a; RefID 15) measured survival and several behavioural endpoints related to foraging after acute contact exposure to a commercial formulation with acetamiprid using three exposure levels (0.5, 1.0 and 2.0 µg a.s./bee). Endpoint measurements were based on free‐flying honey bees monitored using radiofrequency identification (RFID), which is not part of the standard chronic contact toxicity test (OECD 214; OECD, 1998). Observation for survival was much longer than in the standard test, covering the lifespan of the bees up to over 40 days (compared to the at most 96 h in the standard test). However, there is no reporting of the mortality within the first 24 h.
Shi et al. (2020b; RefID 17) used an elaborate exposure scheme of repeated larval exposure followed by prolonged oral exposure of the adult stage at two concentrations of acetamiprid as a commercial formulation in the sugar solution diet (5 and 25 µg a.s./mL diet). The four daily doses at the larval stage align with the guidance on the standard repeated exposure larval toxicity test (OECD 239; OECD, 2016) but was started 1 day earlier. Exposure in the adult stage was longer than in the standard chronic exposure test (14 days instead of 10 days (OECD 245; OECD, 2017)) and consumption of the diet was not recorded. Assessment endpoints included survival, reproduction, growth and subindividual aspects, with the latter focusing on gene expression.
Mazi et al. (2020; RefID 18) performed rather standard contact (experiment 1) and oral (experiment 2) acute assays using acetamiprid in the formulation OPTIMAL, where only survival was measured. Observation was shorter than in standard tests (24 h instead of 48 h).
Overall, there were 27 endpoints extracted on survival (survival, lifespan), reproduction (capped cells, emergence rate), growth (individual larvae and adult weight), behaviour (foraging behaviour, locomotion, number of foraging trips, onset of foraging, and proboscis extension reflex (PER) reflecting memory and olfactory learning) and subindividual alteration (AChE, detoxification, immune and memory gene expression), all on honey bees. Nine of the 27 endpoints (all for Shi et al. (2020b; RefID 17)) that were related to adult prolonged oral exposure could not be considered since the exposure was expressed as acetamiprid concentration in the sugar solution diet and there was no information on the consumption that would be needed to calculate exposure dose. Additionally, all the 14 endpoints from the same study that were related to larvae exposure were also expressed as a concentration in sugar solution (Shi et al., 2020b; RefID 17). An attempt was made to consider these by recalculating the LD10 reported in the RAR to a LC10.
Mechanistic experiments
A series of studies included lethal and subindividual assessments aimed to investigate the genetic and molecular basis of the inter‐species sensitivity of bees towards nicotinic acetylcholine receptor (nAChR) competitive modulators, including neonicotinoids and the butenolide insecticide flupyradifurone.
These studies included standard toxicity experiments, which were mainly used as ground base to further explore the molecular basis of bee sensitivity to neonicotinoid exposure. Because of this reason, these were identified (and are hereby referred to as) mechanistic studies.
Below is the list of these studies:
-
–
RefID: 31 – Beadle et al. (2019)
-
–
RefID: 32 – Hayward et al. (2019)
-
–
RefID: 33 – Johnson et al. (2018)
-
–
RefID: 34 – Manjon et al. (2018)
-
–
RefID: 37 – Troczka et al. (2019)
Across the studies listed above, Johnson et al. (2018; RefID 33) looked at the phylogeny of cytochrome P450s in 10 bee species, to search for footprints of eusociality in phytochemical detoxification. As such, and because not specifically focusing on (nAChR) competitive modulators, this reference was deemed outside the scope of this mandate and was therefore excluded from the WoE.
Despite not necessarily focusing on acetamiprid and flupyradifurone, all other references were deemed informative of the assessment of acetamiprid and flupyradifurone. Indeed, upon more careful evaluation, it became apparent that mechanistic studies could have been used as supportive (i.e. read‐across) evidence on the mode of action and metabolisation of the pesticides under assessment. Additionally, they may be used as lines of evidence to aid the extrapolation of toxicity information from one species to another.
For evaluation purpose, the mechanistic experiments were allocated to one of the following categories: i) bee survival; ii) phylogenetic analyses (including consideration of genome assembly); iii) pharmacokinetics (i.e. pesticide uptake upon topical exposure); iv) receptor binding studies; v) pesticide metabolism; vi) gene expression profiling; vii) survival of recombinant Drosophila melanogaster.
Across experiment categories, a total of 79 endpoints were extracted, which are briefly listed below:
Sixteen survival endpoints characterised the effects of thiacloprid and imidacloprid, alone or in combination with a P450 inhibitor, on Apis mellifera (n = 4), Osmia bicornis (n = 4), Megachile rotundata (n = 2) and Bombus terrestris (n = 6).
The phylogeny of P450 genes was investigated across 4 studies. As previously mentioned, one additional reference including a phylogenetic analysis was not deemed directly relevant to this assessment (Johnson et al., 2018; RefID 33).
Two pharmacokinetic studies investigated the speed of cuticular penetration of radiolabelled 14C‐imidacloprid and 14C‐thiacloprid in Osmia bicornis.
Ten endpoints provided information on receptor (radioligand) binding affinity of imidacloprid (n = 4), thiacloprid (n = 4) and flupyradifurone (n = 2) in Osmia bicornis (n = 2), Megachile rotundata (n = 3), Apis mellifera (n = 3) and Bombus terrestris (n = 2).
Seventeen metabolism endpoints provided information on the ability of microsomal preparation (7) or cell lines (10) expressing P450s from Osmia bicornis (n = 3), Megachile rotundata (n = 2) and Apis mellifera (n = 2) to metabolise thiacloprid (n = 6), imidacloprid (n = 4), flupyradifurone (n = 1), acetamiprid (n = 4), tau fluvalinate (n = 1) and nicotine (n = 1).
Seven expression profiling endpoints provided information on the whole‐body (n = 1) or tissue‐specific (n = 6) expression of P450 genes involved in the neonicotinoids detoxification in Osmia bicornis (n = 3), Megachile rotundata (n = 2) and Apis mellifera (n = 2).
Sixteen survival endpoints investigated if and how the functional, in vivo expression of key recombinant P450 genes in Drosophila melanogaster induced increased tolerance to imidacloprid (n = 7), thiacloprid (n = 8) and acetamiprid (n = 1).
3.3.4.3. Hazard characterisation and evaluation of the newly available data
Acute oral exposure experiments with adult honey bees
Mazi et al. (2020; RefID 18) reported the LC50 after acute oral exposure of honey bees to acetamiprid in a formulation. The LC50 and reported consumption of the sugar solution diet was used to calculate the LD50 at 24 h as 0.0470 µg a.s./bee. Survival is directly relevant for the honey bee risk assessment, but the study was conducted in Cameroon leading to uncertainty on the subspecies used and the observation duration was only 24 h compared to the recommended 48 h. This resulted in moderate risk of bias for external validity (Figure 10). Internal validity was set to class 3 (the lowest) because of issues with the study performance including poor reporting on age and health status of the bees, sugar syrup consumption and control mortality. Even if replication and number of doses appeared to follow guidelines, the doses were too high with the lowest yielding over 40% mortality and the three highest 100% mortality after 24 h.
Figure 10.
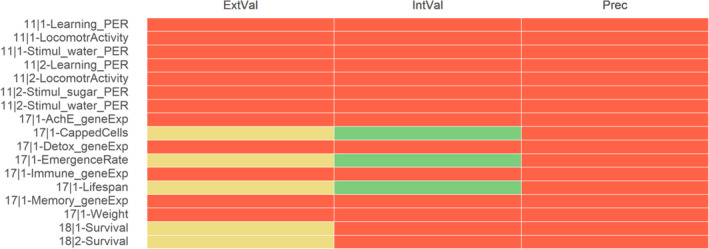
Summary of the appraisal done on the assessment endpoints for laboratory experiments with bees. The outcome takes into account the risk of bias and the precision for several criteria combined with a pre‐defined algorithm (see Annex A). Green indicates low risk of bias or high precision (class 1), yellow moderate risk of bias (class 2 for external and internal validity), while red indicates high risk of bias (class 3) or low precision (class 2)
In the acute oral experiment (experiment 1) by El Hassani et al. (2014; RefID 11) locomotor activity was not impacted at any of the three tested doses, hence the NOED was set to 1 µg a.s./bee, i.e. the highest tested. Similarly, no significant effects were recorded in the response (PER) to water (NOED = 1 µg a.s./bee). PER was also used to evaluate learning and memory to an olfactory response to sugar stimulation. Oral exposure to acetamiprid did not impair learning, but the response after 48 h (memory) was decreased in the lowest treatment level (0.1 µg a.s./bee). This dose was thus selected as a lowest observed effect dose (LOED). However, it is interesting to note that the effect on memory at 0.1 µg a.s./bee was uniquely observed after 48 h, but not after 24 h. In addition, no negative effect on learning was recorded when bees were exposed to higher doses (0.5 and 1 µg a.s./bee), thus highlighting a picture which is not fully consistent. All assessment endpoints in this experiment are related to individual behaviour and are thus not so informative for effects at the colony level. Hence, they were all assigned class 3 for external validity (Figure 10). In addition, all assessment endpoints in this experiment were assigned to the lowest class (class 3) for internal validity, due to several issues in the experimental procedure and especially in the reporting. Finally, all assessment endpoints were considered to have low precision, due to the low number of tested doses and the low replication.
The LD50s in Mazi et al. (2020; RefID 18) for acute oral exposure were below the NOED for the behavioural endpoints in Hassani et al. (2014; RefID 11), which is unexpected but may be explained by weaknesses in the study performance indicated by consistently high risk of bias for internal validity and low precision (Figure 10). The internal validity for all endpoints in the two studies (El Hassani et al., 2014; RefID 11; Mazi et al., 2020; RefID 18), whether related to survival or behaviour, were assigned to class 3 (i.e. the lowest class) which indicates a high risk of bias.
Acute contact exposure experiments with adult honey bees
Mazi et al. (2020; RefID 18) also reported the LC50 after acute contact exposure of honey bees to acetamiprid in a formulation. Similarly to the acute oral test, the reported LC50 and application of 1 µg/bee was used to calculate the LD50 at 24 h as 0.00526 µg a.s./bee. The considerations were the same as for the oral exposure for external and internal validity and precision, yielding moderate risk of bias for external validity, high risk of bias for internal validity and low precision (Figure 10).
In the acute contact experiment (experiment 2) by El Hassani et al. (2014; RefID 11) locomotor activity was significantly decreased at 0.1 µg a.s./bee (LOED) and at 0.5 µg a.s./bee. However, no significant decrease compared to the control was seen in the highest treatment dose (1 µg a.s./bee), thus highlighting a lack of a clear dose response. The author reported no significant effects for PER, when this was used to evaluate learning and memory to an olfactory response to sugar stimulation (NOED = 1 µg a.s./bee). However, the reporting for the memory is not straightforward in the paper: the relevant figure reports significant differences between the response (PER) to a range of solutions with increasing sugar concentrations before and after the exposure to acetamiprid. In all cases (including the control) the response was lower in the ‘post’ phase, except for the highest treatment, for which the response was equivalent in the ‘pre’ and ‘post’ exposure phase. Hence, for the present analysis, the NOED was set to 0.5 µg a.s./bee, noting a high uncertainty. Finally, the response (PER) to water stimulation increased significantly in all treatment levels (LOED = 0.1 µg a.s./bee) in a dose‐related manner. Similarly to the oral experiment, all assessment endpoints included in this experiment were assigned class 3 (high risk of bias) for both external and internal validity, and were considered to have low precision (Figure 10).
Similarly to the oral exposure, the LD50s for acute contact exposure in Mazi et al. (2020; RefID 18) were below the NOED for the behavioural endpoints in Hassani et al. (2014; RefID 11). All endpoints in the two studies (El Hassani et al., 2014; RefID 11; Mazi et al., 2020; RefID 18) were, however, flagged for high risk of bias for internal validity (class 3) which may explain the inconsistencies in ordering of lethal and sublethal impacts.
There was also a third acute contact exposure study (Shi et al., 2020a; RefID 15) where three exposure levels were used (0.5, 1.0 and 2.0 µg a.s./bee) and endpoints were monitored for free‐flying honey bees using RFID. Mortality was not reported for the first 24 h, but for the bees that survived to the second day the NOED was determined to 1.0 µg a.s./bee and the LOED to 2.0 µg a.s./bee (the highest tested dose) based on lifespan estimates for free‐flying bees using RFID technique. The lifespan was reduced from 17.2 (range: 16.2–18.2) to 12.9 (11.7–14.0) days when comparing the control and the LOED. Survival was rated as class 3 for external validity (i.e. the lowest class) because even if the endpoint is relevant for the risk assessment, the study was conducted in China and the study area was not described so it is unclear if the conditions are representative of the EU (Figure 11). Internal validity was rated as class 1 (i.e. the highest class), as there was clear reporting of the methods used even if there are no standards for this type of study. Precision was determined to be high, because even if no power analysis was conducted significant deviations from the control could be determined at the highest exposure level. For the behavioural endpoints foraging start day, number of foraging trips and days without foraging, the NOED and LOED followed those for lifespan. Internal and external validity and precision also followed that for lifespan, indicating high risk of bias for external validity (class 3), but low risk of bias (class 1) for internal validity and precision (Figure 11).
Figure 11.
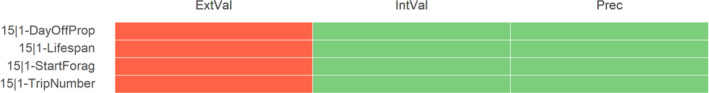
Summary of the appraisal done on the assessment endpoints for field effect experiments with bees. The outcome takes into account the risk of bias and the precision for several criteria combined with a pre‐defined algorithm (see Annex A). Green indicates low risk of bias or high precision (class 1), yellow moderate risk of bias (class 2 for external and internal validity), while red indicates high risk of bias (class 3) or low precision (class 2)
Prolonged oral exposure experiment with adult honey bees
In addition to the two acute oral exposure studies, there was also a prolonged oral exposure study with honey bees, that was preceded by a larval repeated exposure test (Shi et al., 2020b; RefID 17). Two exposure levels were used (5 and 25 µg a.s./mL diet). LOEC for adult lifespan was determined to 25 µg a.s./mL diet (the highest tested dose), with a reduction from 13.6 to 12.3 days when compared to control. There were also non‐standard endpoints on growth (weight of individual adults) and subindividual endpoints related to gene expression. The LOEC for adult weight was 5 µg a.s./mL diet. There was no consistent pattern in the gene expression endpoints, with LOEC and NOEC usually determined at the highest exposure level (25 µg a.s./mL diet). Lifespan is directly informative for the risk assessment but since the exposure duration was longer than the standard chronic oral exposure test, the endpoint was assigned class 2 external validity while internal validity was set to class 1 and precision to low (Figure 10). External and internal validity was rated class 3 and precision to be low for both individual weight and gene expressions since it is unclear how these relate to colony strength and because there were study performance issues relating to not measuring diet consumption, analytically confirming exposure level and using only two exposure levels which means that a full dose response cannot be determined.
Prolonged honey bee larval exposure experiment
The prolonged oral toxicity study with adult honey bees was preceded by a larval repeated exposure test (Shi et al., 2020b; RefID 17). In both life stages, two exposure levels were used (5 and 25 µg a.s./mL diet). In the larval repeated exposure part of the study, the LOEC for the reproduction related endpoint emergence rate was 5 µg a.s./mL diet (i.e. the lowest tested dose) and the NOEC for proportion of capped brood cells was 5 µg a.s./mL diet. The LOEC for emergence rate was lower than the LOEC for the other endpoints, which were usually determined to the highest exposure level. There were also non‐standard endpoints on growth (weight of individual larvae) and subindividual endpoints related to gene expression. The NOEC for larval weight was 25 µg a.s./mL diet. The internal validity in the honey bee larval study ranged from low to high risk of bias (class 1–3), with low risk of bias for the most relevant endpoints on survival and reproduction (Figure 11). It should be noted that, in the present context and from a colony perspective, the number of capped brood and emergence rate were classified as ‘reproduction’ assessment endpoints. Nevertheless, they are also informative of ‘survival’ from a larval perspective. Precision was classified as low for all endpoints.
3.3.4.4. Comparison of new data with previous hazard characterisation
Acute exposure experiments with adult honey bees
Acute exposure‐related endpoints for honey bee adults were available from three studies (El Hassani et al., 2014; RefID 11, Shi et al., 2020a; RefID 15 and Mazi et al., 2020; RefID 18) including oral and contact exposure and endpoints related to survival and behaviour.
For the acute exposure to honey bee adults, considering both oral and contact exposure, all endpoints for both survival and behaviour from El Hassani et al. (2014; RefID 11) or Mazi et al. (2020; RefID 18) are below the LD50 from the 2016 peer review (Figure 12), which means that new concerns may be triggered. Negative impacts started to occur at doses about an order of magnitude below the oral LD50 previously determined in the risk assessment. However, 50% effect on survival was below the NOED for behaviour which would not be expected. With acute contact exposure, the NOEC was determined to 1 µg a.s./bee in one study (free foraging bees) compared to the LD50 of 0.05 µg a.s./bee in the other (laboratory) study, pointing towards low internal consistency. In addition to the LD50s being below the LOED/NOED for behavioural endpoints and the lack of internal consistency among the two studies, all of the endpoints have class 3 internal validity (the lowest), which means that these outcomes should be interpreted with caution.
Figure 12.
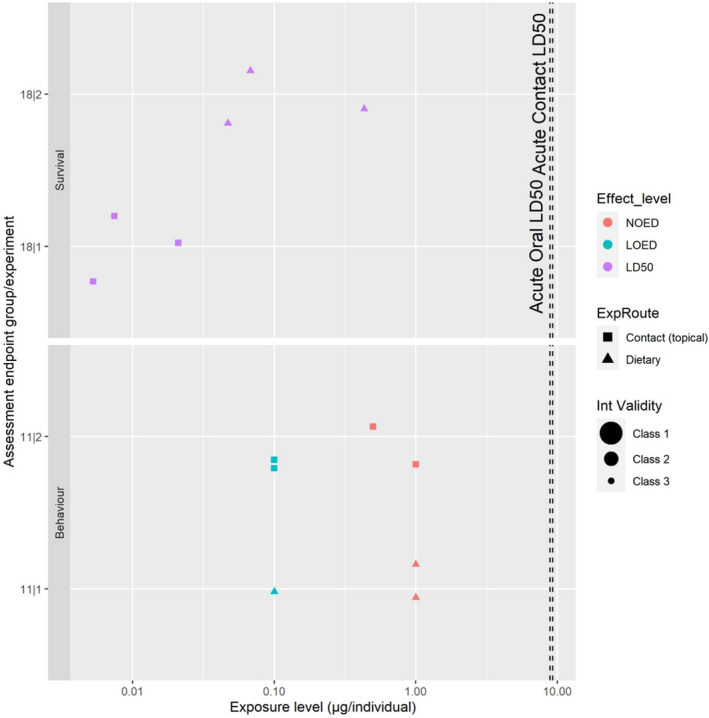
Summary plot of the available data on honey bee adults acutely exposed to acetamiprid under laboratory conditions. Each line on the y‐axis represents an experiment within a reference (e.g. XX|Y indicate experiment Y within reference XX), organised by assessment endpoint group (survival, behavioural). Colours identify the type of measured endpoint (effect level), shapes the route of exposure (contact, dietary), and size of the markers identify the internal validity class (class 1 representing low risk of bias). Vertical dashed lines highlight the endpoints available in the EU peer review (EFSA, 2016)
Behavioural endpoints were not included in the first tier part of the RAR and thus not considered in the previous peer review. The NOED and LOED for behavioural endpoints are in the newly submitted literature 0.1–2 µg a.s./bee, which is in line with the literature review outcome in the RAR noting that sublethal effects may be observable at 0.1 µg a.s./bee. The relation of the behavioural endpoints to colony strength is unclear and weight together with the consistent picture of exposure level for sublethal endpoints it is unlikely that the submitted studies would trigger new concerns.
Prolonged exposure experiments with honey bee larvae and adults
There was one study providing information on survival, reproduction, growth and subindividual aspects after repeated exposure in the larval stage and prolonged exposure in the adult stage of honey bees (Shi et al., 2020b; RefID 17).
A challenge in comparing endpoints from Shi et al. (2020b; RefID 17) to that of the RAR was that in Shi et al. (2020b; RefID 17), endpoints were expressed as a concentration in the diet while those in the RAR were expressed as a dose.
For adult chronic exposure, the 10 days LDD50 was determined to 11.7 μg a.s./bee per day in the RAR, while the new literature provided a LOEC of 25 µg a.s./mL diet leading to a 10% reduction of survival compared to control.
For the honey bee larval endpoints in the RAR, LD10 (lethal dose, 10%; 1.3 µg a.s./larva) was recalculated to a LC10 (lethal concentration 10%; 8.4 µg a.s./mL diet) according to the following. The density of the diet is unknown, but the total amount of the solution is known (155 µL/larva). Hence the endpoint has been recalculated as 1.3 [µg a.s./larva]/155 [µL diet/larva] × 1,000, resulting into 8.4 µg a.s./mL diet.
The repeated exposure to honey bee larvae indicated a higher LOEC for emergence rate, considered as reproductive endpoints but also related to larval and pupal survival, compared to the LC10 from the previous peer review, which may be a reason for concern. However, the reduction in emergence rate was very small at the highest exposure levels (97.0 ± 0.11%, mean ± standard error) compared to the control (98.6 ± 0.52) and it is unlikely that this small difference is relevant from a biological point of view (EFSA Scientific Committee, 2011) and in an ecological context.
The LOEC for the non‐standard endpoints related to subindividual aspects and larval weight were variable and mainly above the RAR LC10 and these endpoints are usually not considered in risk assessment (Figure 13).
Figure 13.
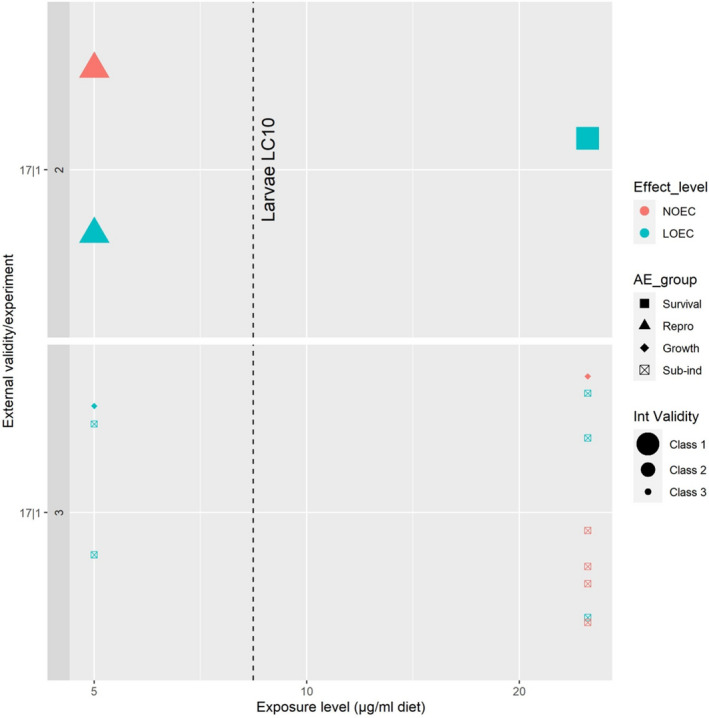
Summary plot of the honey bee larval and adult data available for prolonged exposure to acetamiprid under laboratory conditions. Each line on the y‐axis represents an experiment within a reference (e.g. XX|Y indicate experiment Y within reference XX), organised by external validity class (class 1 representing low risk of bias). Colours identify the type of measured endpoint (effect level), shapes the assessment endpoint group, and size of the markers identify the internal validity class (class 1 representing low risk of bias). The vertical dashed line highlights the endpoints available in the EU peer review (EFSA, 2016)
3.3.4.5. Weight of evidence and uncertainty analysis
The analysis reported in Table 10 addresses the question whether the new studies could potentially indicate a higher hazard in comparison to the data used in the previous peer‐reviewed risk assessment (EFSA, 2016).
Table 10.
Weight of evidence and uncertainty analysis of the available laboratory and field data for bees. EV = external validity; IV = internal validity; Prec = precision; IC = internal consistency
| Assessment endpoint group | Exposure RefID|exp | Strength of the line of evidence | Potential to indicate a higher hazard compared to EFSA (2016) | |
|---|---|---|---|---|
| Judgement | Rationale | |||
| Survival |
Acute oral 18|2 |
EV: moderate RoB IV: high RoB Prec: low IC: NA |
Low with low certainty | The endpoint is relevant for the risk assessment of honey bees, but the relevance is reduced by the study location (Cameroon) and observation time (24 instead of 48 h). In addition, the robustness of data is questionable because of high risk of bias for internal validity and low precision. Negative impacts started to occur at doses about an order of magnitude below the oral LD50 previously used in the risk assessment. However, 50% effect on survival was below the NOED for behaviour which would not be expected. Taken together, concerns on the data quality are weighed against concerns triggered by the lower LD50, resulting in a low indication of new concerns with a low certainty due to the study performance issues |
|
Acute contact 15|1 18|1 |
EV: moderate to high RoB IV: low to high RoB Prec: high IC: low |
Moderate with low certainty | The endpoint is directly relevant for the risk assessment for honey bees but relevance is reduced due to study location and observation duration and issues with internal consistency. The NOEC of 1 µg a.s./bee in one study (free foraging bees) vs the LD50 of 0.05 µg a.s./bee in the other (laboratory) study results in a low internal consistency | |
|
Prolonged oral 17|1 |
EV: moderate RoB IV: low RoB Prec: low IC: NA |
Low with moderate certainty | Survival is directly relevant for the honey bee risk assessment and the adult LOEC was higher than the previous larval LC10, thus there are probably low potential for new concerns. The adult chronic endpoint from the RAR cannot be directly compared to the adult LOEC in the new study since the lack of measured consumption precludes the recalculation between concentration and dose. The internal validity indicates low risk of bias, but the precision is low leading to a moderate certainty for the judgement | |
| Reproduction |
Prolonged oral 17|1 |
EV: moderate RoB IV: low RoB Prec: low IC: NA |
Low with moderate certainty | Reproduction, here capped brood cells and adult emergence rate from pupae, are indirectly relevant for the honey bee risk assessment and can be indirectly linked to colony size. The LOEC for emergence rate was determined on the basis of statistical testing. This is slightly below the previous LC10, but the observed effect size (< 2%) is so small that it is not considered biologically and ecologically relevant, also as the emergence rate in the treatment was 97%. The internal validity indicates low risk of bias, but the precision is low leading to a moderate certainty for the judgement |
| Growth |
Prolonged oral 17|1 |
EV: high RoB IV: high RoB Prec: low IC: NA |
Low with low certainty | Larval weight cannot be easily related to colony strength which means that the endpoint is less relevant for the risk assessment. Exposure in relation to larval growth was expressed as a concentration in the diet, which means that the endpoint is not directly comparable to the dose in the RAR. However, when recalculating that LD10 to an LC10, the indication is that the NOED for larval weight is lower. The adult weight cannot be related to the previous peer review since the exposure is expressed as a concentration in the diet and consumption is not measured or reported. The adult LOEC (5 µg a.s./mL diet) was however lower than the larval NOEC (25 µg a.s./mL diet). Internal validity was low as was the precision, which indicates that the endpoints should be interpreted with caution |
| Behaviour |
Acute oral 11|1 |
EV: high RoB IV: high RoB Prec: low IC: NA |
Low with low certainty | Behavioural endpoints were not included in the first tier part of the RAR and thus not considered in the previous peer review. The NOED and LOED for behavioural endpoints are in the new literature 0.1–1 µg a.s./bee, which is in line with the literature review outcome in the RAR noting that sublethal effects may be observable at 0.1 µg a.s./bee. The relation of the behavioural endpoints to colony strength is unclear and weight together it is unlikely that the submitted studies would trigger new hazard concerns. In addition, there is a high risk of bias for internal validity as well as low precision |
|
Acute contact 11|2 15|1 |
EV: high RoB IV: low to high RoB Prec: low and high IC: moderate |
Low with moderate certainty | Behavioural endpoints were not included in the first tier part of the RAR and thus not considered in the previous peer review. The NOED and LOED for behavioural endpoints are in the new literature 0.1–2 µg a.s./bee, which is consistent and also in line with the literature review outcome in the RAR noting that sublethal effects may be observable at 0.1 µg a.s./bee. The relation of the behavioural endpoints to colony strength is unclear and weight together it is unlikely that the submitted studies would trigger new hazard concerns. Additionally, internal validity and precision varied | |
| Subindividual |
Prolonged oral 17|1 |
EV: high RoB IV: high RoB Prec: low IC: NA |
Low with low certainty | Behavioural endpoints were not included in the first tier part of the RAR and thus not considered in the previous peer review. The data on gene expression have limited relevance for the risk assessment and are characterised by a high risk of bias and low precision |
3.3.4.6. Mechanistic studies on bees
Survival
Along with the data discussed under Section 3.3.4.2, this WoE includes endpoints from three references (i.e. Bayer, 2017a,b,c, RefID 1001, 1002, 1003) characterising lethal hazards of flupyradifurone (i.e. formulated as flupyradifurone 200 g/L SL4) to Megachile rotundata, Osmia cornuta and Osmia bicornis. These endpoints were further compared to EFSA (2015):
-
–
Honey bee 72‐h contact LD50 = 15.7 µg/bee.
-
–
Bumble bee 48‐h contact LD50 > 100 µg/bee.
This data set provided important information on the inter‐species sensitivity of bees towards nAChR competitive modulators alone or interactively with P450 inhibitors.
Individual substances:
These data confirm previous evidence that nAChR competitive modulators are not equally toxic to bees (Figure 14), with N‐cyanoamidine (i.e. thiacloprid) and butenolide (i.e. flupyradifurone) compounds being less lethally toxic than N‐nitroguanidine (i.e. imidacloprid) to Apis mellifera, Bombus terrestris and Osmia spp. An additional, key information is that such difference was not observed in Megachile rotundata, which, instead, appeared similarly and highly sensitive to imidacloprid, thiacloprid and flupyradifurone. Additionally, Megachile rotundata appeared more sensitive than Apis mellifera to flupyradifurone and thiacloprid by 2 and 3 orders of magnitudes, respectively (Figure 15). This finding is of particular relevance, given that the sensitivity of Megachile rotundata towards flupyradifurone would not be covered by the standard assessment factor of 10 applied to honey bee endpoints, and considered protective of other bee species (EFSA, 2013). Additionally, although not directly informative of this assessment, the results observed for thiacloprid were consistent with what was observed for flupyradifurone, hence, further supporting the mechanistic basis of the high sensitivity of Megachile rotundata towards neonicotinoids and butenolides.
Figure 14.
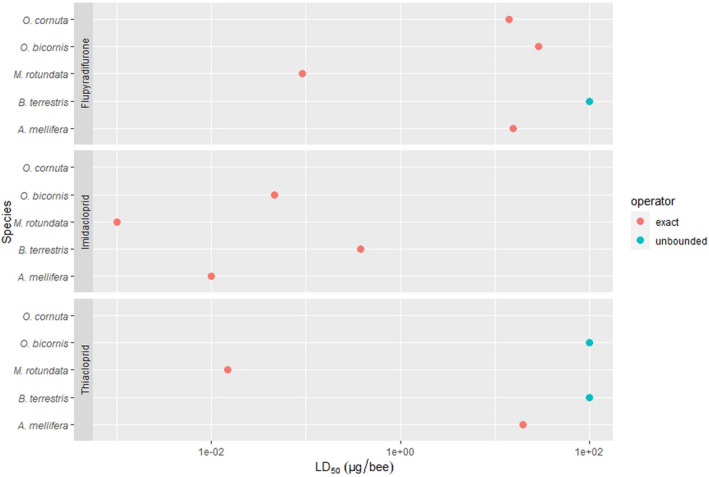
The acute toxicity of flupyradifurone, imidacloprid and thiacloprid (top to bottom) to Apis mellifera, Bombus terrestris, Megachile rotundata, Osmia bicornis and Osmia cornuta. Bee species were listed on the y‐axis, while the acute contact LD50 values were plotted as dots against the x‐axis. Unbounded (i.e. higher than) and exact values were colour coded as specified in the plot legend
Figure 15.
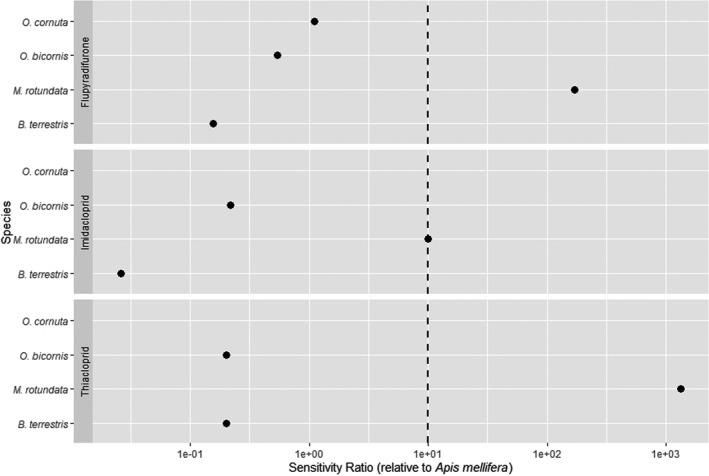
The sensitivity of bees to flupyradifurone, imidacloprid and thiacloprid (top to bottom). Bee species were listed on the y‐axis, while the sensitivity ratio (i.e. calculated as the honey bee LD50 divided by the LD50 of other bee species) was reported on the x‐axis (base‐10 log scale). The dashed vertical line represents the sensitivity ratio = 10, used as default safety factor by EFSA (2013). Values on the right of the dashed line indicate higher sensitivity than what covered by previous assessments. The comparison is based on the bee 72‐h contact LD50 = 15.7 µg a.s./bee (EFSA, 2015) from the formulation endpoint, however, the endpoint from the active substance study was higher
A number of considerations relative to assessment of survival endpoints for individual substances were made, including the following:
-
i
Survival endpoints for M. rotundata were available for flupyradifurone, but not acetamiprid. Consequently, it could not be excluded that M. rotundata would be similarly sensitive to these two compounds. Indeed, there is indication that acetamiprid is metabolised by the same subfamily of CYP genes proven to metabolise neonicotinoids such as thiacloprid in, e.g. A. mellifera (see metabolism section below). Therefore, as M. rotundata lacks this subfamily of P450s (see phylogeny section below), it may also be more sensitive to acetamiprid than other bee species
-
ii
Risk assessment schemes routinely rely on toxicity data on few bee species. Moreover, a limited proportion of species has been tested in pesticide toxicity bioassays by non‐regulatory research. Consequently, our knowledge of the inter‐species sensitivity to pesticide may be considerably biased and potentially incomplete. Therefore, other bee species may show similar patterns of sensitivity as M. rotundata
-
iii
Similarly, with our knowledge of pesticide metabolism by bees being limited, it cannot be excluded that M. rotundata might be more sensitive to other pesticides too.
-
Interactions with P450 inhibitors:
In addition to the survival experiments above, a series of toxicity studies were produced to explore the interaction of imidacloprid and thiacloprid with bee P450 enzymes (Figure 16).
-
Figure 16.
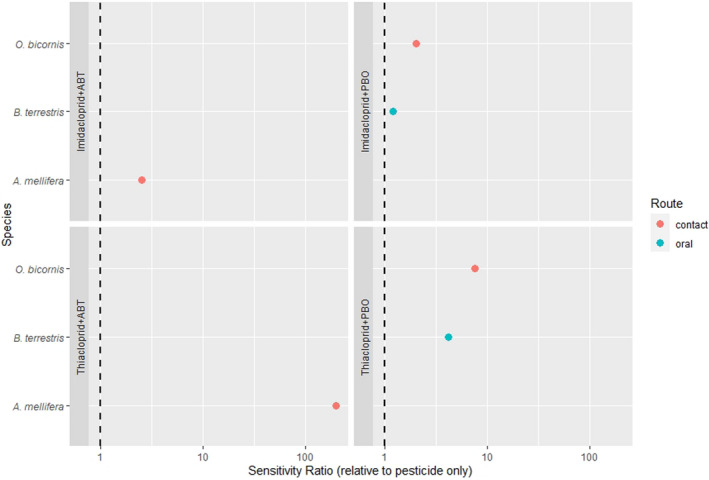
The interactive toxicity of imidacloprid (top) and thiacloprid (bottom) with the P450 inhibitors Piperonyl butoxide (PBO – right) and 1‐aminobenzotriazole (ABT – left). The bee species were listed on the y‐axis, while the sensitivity ratio (i.e. the toxicity ratio of the pesticide alone/pesticide + synergist) was reported on the x‐axis (base‐10 log scale). Data points (dots) were colour‐coded by route of exposure, as specified in the plot legend. The dashed vertical lines represent the sensitivity ratio = 1, indicating no interactive toxicity. Data on the right side of the dashed line indicate higher sensitivity induced by the P450 inhibitor
Apis mellifera became about 200 times more sensitive to thiacloprid, but only 2.7 times more sensitive to imidacloprid, upon pre‐treatment with a P450 inhibitor. Bombus terrestris became 4.16 times more sensitive to thiacloprid, and 1.19 times more sensitive to imidacloprid, upon pre‐treatment with a P450 inhibitor. Osmia bicornis became > 7.5 times more sensitive to thiacloprid, and 2 times more sensitive to imidacloprid, upon pre‐treatment with a P450 inhibitor. Overall, this body of evidence suggests that the tolerance of bees to thiacloprid, but not imidacloprid is downregulated by a P450 inhibitor. In other words, the higher tolerance towards thiacloprid may be linked to one or more members of the cytochrome P450 superfamily. This does not seem to be the case of imidacloprid (at least not at the same extent), which is, indeed, more lethally toxic than thiacloprid.
However, this assessment was not specific to acetamiprid or flupyradifurone, and it is not fully clear if extrapolating such evidence across substances would be fully justified. Additionally, the differences in the sensitivity ratios for the two tested substances was not very consistent across bee species and did not clearly match the n‐fold difference in the toxicity between imidacloprid and thiacloprid shown in (Figure 14). Moreover, the route of exposure was not entirely consistent across experiments.
Phylogenetic analyses
Building on the results observed in vivo, further experiments including phylogeny studies were carried out, to test the hypothesis that the difference in sensitivity across bee species towards nAChR competitive modulators was driven by differences in their ability to produce cytochrome P450s, which are known to be a key route of xenobiotic detoxification in bees, as well as insects in general. Therefore, phylogenetic studies aimed to explore potential differences in the CYPome (i.e. the genes encoding for P450s).
Therefore, phylogeny of bee P450s was explored across three species in 4 studies, each including a phylogenetic analysis. These data showed that:
-
iv
The CYP9Q subfamily, which has a primary role in neonicotinoid detoxification, is shared by both Apis mellifera and Bombus terrestris, with the second having 6 genes (CYP9P1, CYP9P2, CYP9R1, CYP9Q4, CYP9Q5 and CYP6) clustering with honeybee CYP9s.
-
v
The genome of O. bicornis lacks the CYP9Q subfamily, but, instead, has the CYP9BU subfamily
-
vi
However, M. rotundata did not have the CYP9Q gene family or closely related genes.
Altogether these data were deemed informative, although uncertainties were identified concerning the methodological approaches (i.e. mainly related to the use of potentially fragmented or non‐optimal genome assemblies by today’s standards), which were further discussed in Annex G. These methodological limitations may have influenced the outcome of the phylogenetic analysis, although it is difficult to predict how much weight these choices actually had. In principle, it cannot be excluded that the use of suboptimal, relatively fragmented genome assemblies might have had a negative impact on the detection of specific CYPs. Nonetheless, based on the information presented across mechanistic studies, it appears that differences exist in the distribution and phylogeny of CYP, with M. rotundata lacking a family of P450s proven to metabolise neonicotinoids in other species.
Pharmacokinetics
A possible explanation of the differences in sensitivity observed across substances and species might be the speed of cuticular penetration. Therefore, to explore the role of uptake rate on pesticide sensitivity, cuticular penetration was studied using radiolabelled 14C‐imidacloprid and 14C‐thiacloprid in Osmia bicornis. No difference in the absorption of the two compounds was observed, suggesting that cuticular penetration did not explain the higher sensitivity of O. bicornis towards imidacloprid.
Similar to other assessments, these endpoints are not specific to acetamiprid or flupyradifurone, and it is not fully clear if extrapolating such evidence across substances and bee species would be fully justified.
Receptor binding
Another factor potentially driving differences in toxicity across species and substances is the interaction at the molecular target site. Specifically, higher binding affinity at the nAChR may exacerbate toxic effects. Therefore, across the mechanistic studies, 10 endpoints provided information on receptor (radioligand) binding affinity (Figure 17) of imidacloprid (n = 4), thiacloprid (n = 4) and flupyradifurone (n = 2) in Osmia bicornis (n = 2), Megachile rotundata (n = 3), Apis mellifera (n = 3) and Bombus terrestris (n = 2). Results showed that imidacloprid, thiacloprid and flupyradifurone reversibly bound bee nAChRs with nanomolar affinity. The resulting half maximal inhibitory concentrations (IC50s) differed across substances and tested species. However, such differences were within a 10‐fold range, which suggest that receptor binding might not be a primary factor in determining either of the inter‐species sensitivity or the differential toxicity of different nAChR modulators.
Figure 17.
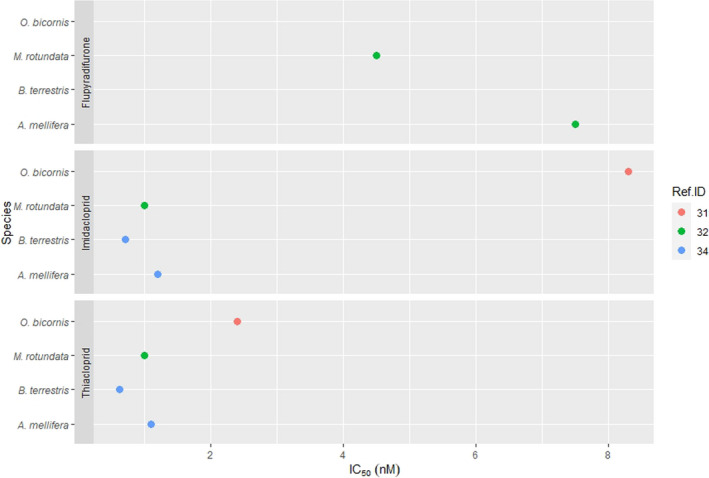
Radioligand binding examined by displacement of tritiated imidacloprid by unlabelled imidacloprid, thiacloprid and flupyradifurone. Dots represent the half maximal inhibitory concentration IC50 (nM). Lower IC50 values indicate higher binding affinity
Metabolism
Seventeen metabolism endpoints provided information on the ability of microsomal preparation (7) or cell lines (10) expressing P450s from Osmia bicornis (n = 3), Megachile rotundata (n = 2) and Apis mellifera (n = 2) to metabolise thiacloprid (n = 6), imidacloprid (n = 4), flupyradifurone (n = 1), acetamiprid (n = 4) tau fluvalinate (n = 1) and nicotine (n = 1). Altogether, this body of evidence functionally confirmed the primary role of CYP9Q (or closely related) subfamilies in the metabolism of nAChR modulators (i.e. acetamiprid, imidacloprid and thiacloprid).
A brief outline of the main findings across studies is given below,
-
vii
CYP9Q1‐5 (honey bee, bumble bee) significantly metabolised acetamiprid, with CYP9Q2‐3 (honey bee) resulting in the highest level of metabolisation.
-
viii
Across 27 honey bee recombinant P450s, CYP9Q3 showed the highest level of imidacloprid and thiacloprid metabolisation. The overall activity against thiacloprid was higher than that of imidacloprid.
-
ix
Among 5 bumble bee candidate P450s, CYP9Q4 and CYP9Q5 metabolised thiacloprid and imidacloprid. In general, thiacloprid was metabolised more efficiently than imidacloprid by these CYPs. However, recombinant CYP9Q6 was later shown to metabolise thiacloprid and imidacloprid more efficiently than CYP9Q4 CYP9Q5 with no clear difference across substances.
-
x
O. bicornis CYP9BU1‐2 showed more efficient metabolic activity against thiacloprid, than imidacloprid.
-
xi
Microsomal preparations from M. rotundata did not show metabolic activity flupyradifurone, thiacloprid, imidacloprid or tau fluvalinate, but significantly metabolised the naturally occurrent xenobiotic nicotine.
This body of evidence appears as a functional validation of phylogenetic analyses, highlighting that the difference in sensitivity between highly toxic neonicotinoids and less acutely lethal substances might be at least partially explained by different metabolisation efficiency. Indeed, across species, thiacloprid metabolism appeared more efficient than imidacloprid. However, this does not seem to be the case of M. rotundata, which was shown to be unable to metabolise flupyradifurone, thiacloprid and imidacloprid. This latter finding seems as a plausible functional validation of the hypothesis that the lack of CYP9Q genes drives higher sensitivity towards thiacloprid and flupyradifurone.
Although representing a robust body of evidence, the pesticide metabolism experiments were not performed comprehensively across substances and bee species. Specifically, data for acetamiprid and flupyradifurone are scarce comparatively to the other tested substances. Particularly the ability of M. rotundata to metabolise acetamiprid is unknown. Additionally, most studies used cell lines recombinantly expressing target genes to test the metabolic rate. It is unclear if these data can be considered fully representative of the in vivo metabolic response in bees.
Expression profiling
A series of studies focussing on the expression of P450 candidate genes showed that i) candidate gene expression is not upregulated by the exposure to acetamiprid and thiacloprid; ii) candidate genes are mainly expressed in the brain, midgut and malpighian tubules.
Survival of transgenic flies
Sixteen survival endpoints investigated if and how the functional, in vivo expression of key P450 genes (O. bicornis, A. mellifera and B. terrestris) induced increased tolerance to imidacloprid (n = 7), thiacloprid (n = 8) and acetamiprid (n = 1) (Figure 18). Overall, although not consistently across transgenes, recombinant expression of candidate bee genes induced slight to moderate tolerance to thiacloprid in D. melanogaster. However, this did not seem to be clearly the case of imidacloprid.
Figure 18.
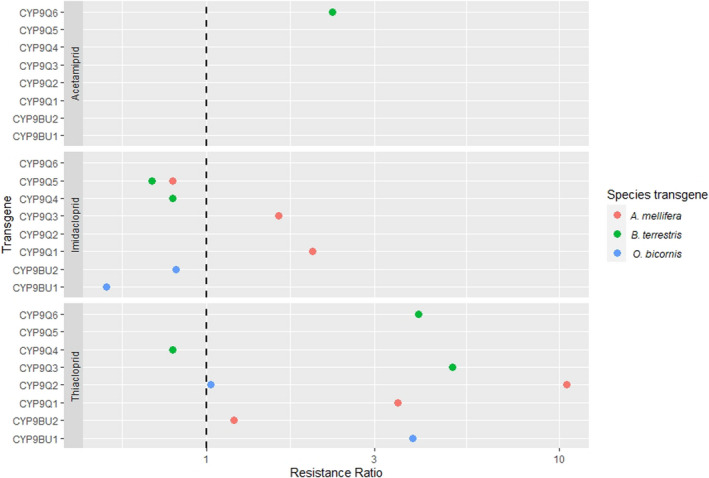
The Resistance Ratio (RR) calculated as the ratio of the LC50s of flies expressing the transgene to the LC50s of flies not expressing the transgene (x = log scale). Values at the right of the dashed line indicate higher pesticide tolerance in transgenic flies
Flies expressing bee CYP transgenes CYP9Q2‐3 conferred slightly higher (less than twofold) tolerance to imidacloprid in transgenic flies.
Flies expressing bee CYP transgenes (i.e. CYP9BU1 and CYP9Q2, 3, 4, 6) gained higher tolerance towards thiacloprid. However, CYP9BU2, CYP9Q1 and CYP9Q5 did not confer resistance to transgenic flies.
Data for acetamiprid were comparatively scarce, with only one transgene (i.e. CYP9Q6) conferring slight (2.3‐fold) tolerance to transgenic flies.
This body of evidence was produced by testing transgenic flies expressing bee P450s. Considering that the model species is a dipteran, there is uncertainty on how accurately it may represent the responses across bee species. Moreover, it is unclear whether the ‘basal’ response of a non‐transgenic fly line represents a true control for the functional characterisation of P450s.
Similarly, it may be argued that the functional characterisation of P450s, as presented across studies, might not fully map to the in vivo toxicity in bees. As an example, Megachile rotundata was found to be more than 1000 times sensitive to thiacloprid than Apis mellifera. Such difference could be partially explained by the lower bodyweight of the former species. Additionally, it was justified in Hayward et al. (2019; RefID 32) that the higher sensitivity of Megachile rotundata than Apis mellifera is related to the lack of CYP9Q genes. However, in Manjon et al. (2018 ref. ID 34), CYPQ1‐3 – which were found to be the most active in neonicotinoid metabolisation – only caused a 1–10.5‐fold increase in tolerance to thiacloprid.
3.3.4.7. Conclusion for bees
Overall, there are no major indications of new concerns for honey bees based on the studies submitted by France and the Netherlands in comparison to the data already included in the RAR and considered in the previous peer review. There are however two points to note. First, there are survival endpoints from one study that indicate considerably lower doses for 50% mortality than in the previous data. However, there are issues with internal consistency and validity as well as precision, which overall lead to a judgement of low potential to trigger new concerns with a low level of certainty. Overall, it was considered that the acute lethal effects of acetamiprid on honey bees were reliably addressed with the existing dossier data. Similarly, there is a reproduction related endpoint after prolonged larval exposure that indicates a lower LOEC compared to the one estimated based on the LD10 from the RAR. However, the effect size related to this endpoint is so small (< 2%) that it is not considered ecologically relevant at the colony level. Second, both the previous, except for one study on B. terrestris, and the new data are solely on the honey bee A. mellifera. There are new mechanistic studies highlighting that there may be other bee species that are considerably more sensitive to the N‐cyanoamidine neonicotinoid insecticides and similar substances, in line with the indication of the literature review included in the RAR.
This means that gaps identified in the EFSA (2016) peer review remain. These included insufficient information for performing risk assessments for bumble bees and solitary bees as well as considering uses other than the ones specified. No new studies were submitted covering exposure, so also this aspect was not improved by the newly submitted data.
3.3.5. Soil organisms
3.3.5.1. Data from previous peer review
For soil organisms, most data available in the database for the assessment of the active substance acetamiprid were derived in tests with formulations and not the active.
Only acute tests indicating the LC50 for earthworms were performed with acetamiprid (see Table 11). The LC50 varied between 1.52 and 9.00 mg acetamiprid/ kg soil.
Table 11.
Summary of tier‐1 endpoints for soil organisms from the previous peer review (EFSA, 2016)
| Species | Test item | Test type | Endpoint |
|---|---|---|---|
| Eisenia fetida | Acetamiprid | Acute (14 d) | LC50 = 9.00 mg/kg |
| Eisenia fetida | Acetamiprid | Acute (14 d) | LC50 = 1.52 mg/kg |
| Eisenia fetida | EXP 60707 A | Acute (14 d) | LC50 = 3.66 mg/kg |
| Folsomia candida | Acetamiprid 20 SG | Chronic (28 d) |
Reproduction NOEC = 0.27 mg/kg EC10 = 0.47 mg/kg |
|
Mortality NOEC = 0.49 mg/kg EC10 = 0.82 mg/kg | |||
| Hypoaspis aculeifer | Acetamiprid 20 SG | Chronic (14 d) |
Mortality and Reproduction NOEC = 180 mg/kg EC10 = 50.8 mg/kg |
| Earthworm community | Acetamiprid 20 SG | Field study (365 d) | NOEAR = 80 g/ha |
Remarkably, no chronic endpoint was available for earthworms exposed to either acetamiprid or a formulation for the peer review in 2016 (the submitted study was not acceptable).
A field study with the earthworm community of a field in Germany did not detect impacts on earthworm species abundance or biomass 1 year after application of up to 80 g acetamiprid/ha applied as Acetamiprid 20 SG. The biomass of juvenile Lumbricus terrestris and of total Allolobophora longa were significantly reduced at the first and second sampling date, respectively. There was for both species a tendency to lower biomasses with increasing application rates, but the differences to the control plots was not statistically significant at the end of the study. An analysis of the statistical power of the study or on the minimum detectable differences (MDD) for the different endpoints at the respective sampling date was not reported.
Regarding soil mesofauna, tests with Folsomia candida and Hypoaspis aculeifer were performed with the formulation Acetamiprid 20 SG. The endpoints for the collembolan were a NOEC = 0.27 and an EC10 = 0.47 mg acetamiprid/kg soil. The mite H. aculeifer was far less sensitive to acetamiprid compared to collembola, showing a NOEC = 180 mg a.s./kg soil and an EC10 = 50.80 mg a.s./kg soil. No higher tier data were available for soil mesofauna.
3.3.5.2. Outline of the submitted studies
Two references were submitted in the remit of this mandate.
One reference (Renaud et al., 2018; RefID 13) presents the results of standard reproduction tests with the soil organisms Folsomia candida (Collembola, experiment 1), Eisenia andrei (earthworm; experiment 2) and Enchytraeus crypticus (Enchytraeidae; experiment 3) exposed to an acetamiprid formulation (Epik 200 SG) in standard artificial soil with 5% organic matter as peat.
Another reference (Saggioro et al., 2019; RefID 14) reports on the outcomes of different experiments with the earthworm Eisenia andrei. One set‐up was an acute test with the earthworms exposed via contact to contaminated moist filter paper for up to 72 h (experiment 1). A second experiment (experiment 2) investigated the avoidance behaviour of E. andrei exposed to control and contaminated natural tropical sandy soil (Latosol with low organic matter content). The same soil was used in a third experiment (experiment 3) examining the reproductive output of E. andrei over 45 days exposure. Next to the reported standardised endpoints, Saggioro et al. (2019; RefID 14) measured endpoints at the suborganism level in the acute and the reproduction experiment.
3.3.5.3. Hazard characterisation and evaluation of the newly available data
Renaud et al. (2018) determined the chronic EC10 and EC50 for reproduction of the three tested species. Additionally, the LC50 were calculated (see Table 12).
Table 12.
Summary of the most relevant measured endpoints for soil organisms exposed to acetamiprid or acetamiprid formulations submitted with new studies in the framework of this statement
| Species | Test item | Timescale | Assessment endpoint group | Measured endpoint | Reference |
|---|---|---|---|---|---|
| Folsomia candida | Epik 20 SG | Chronic (28 days) | Reproduction | EC10 = 0.20 mg/kg | Renaud et al. (2018) (RefID 13) |
| EC50 = 0.29 mg/kg | |||||
| Survival | LC50 = 0.35 mg/kg | ||||
| Eisenia andrei | Epik 20 SG | Chronic (56 days) | Reproduction | EC10 = 0.22 mg/kg | |
| EC50 = 0.32 mg/kg | |||||
| Survival | LC50 = 0.85 mg/kg | ||||
| Enchytraeus crypticus | Epik 20 SG | Chronic (28 days) | Reproduction | EC10 = 0.15 mg/kg | |
| EC50 = 1.97 mg/kg | |||||
| Survival | LC50 = 17.49 mg/kg | ||||
| Eisenia andrei | Acetamiprid | Chronic (45 days) | Reproduction | NOEC = 0.01 mg/kg | Saggioro et al. (2019) (RefID 14) |
| Reproduction | Surrogate EC50 * = 0.05 mg/kg |
LC50: lethal concentration, median.
48% effect on No. cocoon.
The study of Renaud et al. (2018; RefID 13) was assigned with class 1 external and internal validity for all endpoints (i.e. low risk of bias). Low precision was identified in some of the experiments: those with F. candida could have benefitted of lower test concentrations, since 25% mortality was recorded at the lowest tested level.
Saggioro et al. (2019; RefID 14) determined a NOEC for reproduction for E. andrei at 0.01 mg acetamiprid technical/kg soil (cocoons per individual and hatchlings per cocoon, see Table 12). Avoidance was observed at 0.5 mg a.s./kg soil.
Biomarker responses reported in the work by Saggioro et al. (2019; RefID 14) were mostly not constant over time, with initial elicitations (e.g. shifts in immune defence cells, oxidative stress biomarker) but later levelling. In the chronic studies, NOEC for biomarker responses were often determined at the same concentration ranges as the reproductive endpoints. Two exceptions are the measurement of the glutathione S‐transferase (GST) enzyme in the acute test (LOEC = 1.6 × 10−5 mg a.s./kg soils, lowest tested concentration), which was constant over 72 h and was similarly inhibited at all tested concentrations; and the measurement of glutathione enzyme levels in the chronic study (NOEC = 0.001 mg a.s./kg soil, increase at higher concentrations but no dose response pattern).
The external validity was rated class 3 (i.e. high risk of bias, see Figure 19) for the endpoints derived in the acute contact filter paper test (experiment 1), since the test conditions were according to guideline, but the test design is not aligned with the current state of science anymore. Also, the biomarker endpoints could not be directly related to the attribute to protect (class 3). The risk of bias for both external and internal validity was low (class 1) for the assessment endpoints measuring the weight changes in the earthworms (no impact detected), the number of produced cocoons and the mean hatchlings per cocoon. However, there are minor reporting shortcomings regarding the set‐up of the stock solutions. Also, the test conditions differ slightly form those required in the OECD guidelines as the test temperature is higher than requested (25°C ± 2) and the natural soil used is a Latosol with low organic matter content. These conditions are appropriate for the tested soil organisms but reflect typical tropical conditions. Avoidance response was assigned a moderate risk of bias (class 2) for external and a low risk of bias (class 1) for internal validity. All endpoints determined in the study of Saggioro et al. (2019; RefID 14) were assigned a low precision because of the limited number of concentrations tested (4 plus control; 3 in the avoidance experiment, see Figure 19).
Figure 19.
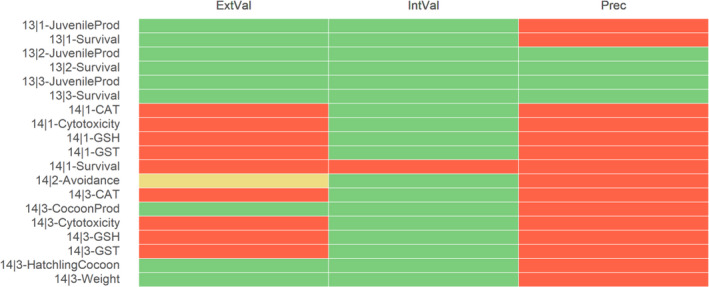
Summary of the appraisal done on the assessment endpoints for laboratory experiments with soil organisms. The outcome takes into account the risk of bias and the precision for several criteria combined with a pre‐defined algorithm (see Annex A). Green indicates low risk of bias or high precision (class 1), yellow moderate risk of bias (class 2 for external and internal validity), while red indicates high risk of bias (class 3) or low precision (class 2)
3.3.5.4. Comparison of new data with previous hazard characterisation
When comparing the new endpoints with the ones available from the peer review of the active substance acetamiprid from 2016, it can be seen that the newly submitted studies provide chronic endpoints for earthworms, which were not available before.
The lowest endpoint for earthworms regarding effects of acetamiprid on reproduction, hence relevant for the risk assessment, is the NOEC = 0.01 mg acetamiprid/kg soil (see Figure 20) observed in the study of Saggioro et al. (2019; RefID 14), which is one order of magnitude lower than the previously reported endpoints for soil organisms. In the study of Renaud et al. (2018; RefID 13), the EC10 for earthworm reproduction is 0.22 mg a.s./kg soil, derived from testing a formulation. In the same paper, the EC10 for E. crypticus of 0.15 mg a.s./kg soil is the only datapoint available for this group of soil organisms. It was, however, not possible to determine a 95% confidence interval for this value. While the respective was not explicitly reported in the paper from Renaud et al. (2018; RefID 13), this can be worked out from the plots available therein. On such basis, a NOEC = 1.3 mg a.s./kg soil is derived (n.b. not part of the data extraction, thus not shown in Figure 20) which is higher than the available EC10 endpoint for E. andrei.
Figure 20.
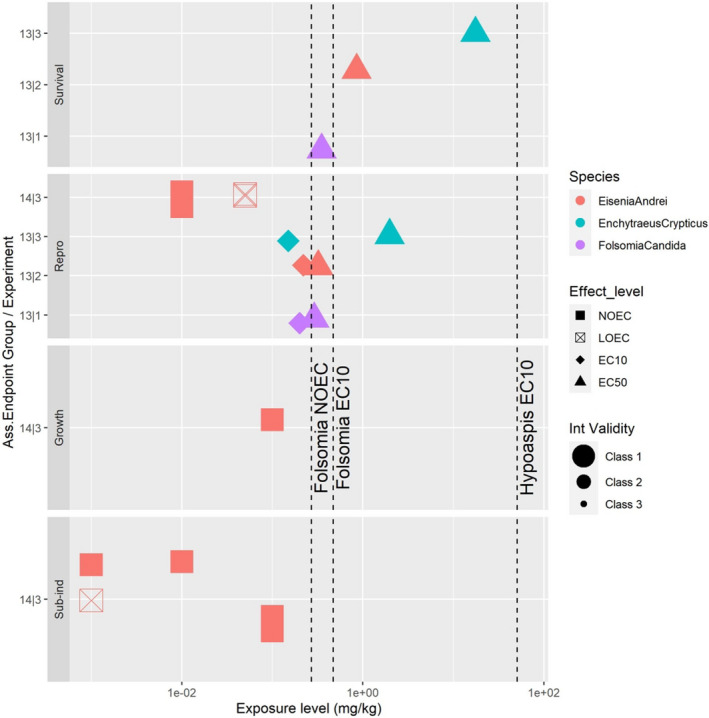
Summary plot of the chronic data on soil organisms available for acetamiprid. Each line on the y‐axis represents an experiment within a reference (e.g. XX|Y indicate experiment Y within reference XX), organised by assessment endpoint group. Colours identify the tested species, shapes the type of measured endpoint (effect level), and size of the markers identify the internal validity class (class 1 representing low risk of bias). Vertical dashed lines highlight the endpoints available in the EU peer review (EFSA, 2016)
For collembola, the endpoints determined in the study of Renaud et al. (2018; RefID 13) are comparable but slightly lower than those previously available (EC10 = 0.22 vs. 0.27 mg a.s/kg soil from EFSA, 2016). This endpoint should be further considered in an updated risk assessment.
3.3.5.5. Weight of evidence and uncertainty analysis
The analysis reported in Table 13 addresses the question whether the data delivered with the new studies could potentially indicate a higher hazard for soil organisms in comparison to the previous risk assessment.
Table 13.
Weight of evidence and uncertainty analysis of the available laboratory data for soil organisms. EV = external validity; IV = internal validity; Prec = precision; IC = internal consistency
| Assessment endpoint group | RefID|exp | Strength of the line of evidence | Potential to indicate a higher hazard compared to EFSA (2016) | |
|---|---|---|---|---|
| Judgement | Rationale | |||
| Oligochaeta | ||||
| Survival |
Eisenia andrei 13|2 |
EV: low RoB IV: low RoB Prec: high IC: NA |
Low with high certainty |
The data are of low/moderate relevance for the hazard characterisation for oligochaeta exposed to acetamiprid. They indicate that survival is affected at slightly higher concentration than reproductive endpoints. However, survival is not considered a sensitive endpoint in hazard characterisation for soil organisms anymore, since the assessment is based on the data for the reproductive output |
| Reproduction |
Eisenia andrei 13|2 14|3 |
EV: low RoB IV: low RoB Prec: low to high IC: low |
Moderate with moderate certainty |
The data are relevant for the hazard characterisation, and provide the missing standard chronic endpoints for earthworms and additional endpoints for other oligochaeta exposed to acetamiprid (products). The submitted data deliver the lowest endpoint compared to previously available ones. Internal consistency is low because experiments in different studies deliver endpoints for reproduction diverging by nearly one order of magnitude. These differences foot possibly on testing acetamiprid technical vs a formulation and one test setting more relevant for tropical environments (RefID 14). A field study was available indicating low risks to earthworms at 80 g acetamiprid/ha. Hence, while some data indicate a potential concern, there are indications of low effects that were observed in the field. However, if an updated lower tier risk assessment would flag issues, than the available field study should be re‐assessed according to the current state of science and in light of the new data |
|
Enchy‐traeus crypticus 13| 3 |
EV: low RoB IV: low RoB Prec: high IC: NA |
|||
| Behavioural endpoints |
Eisenia andrei 14|2 |
EV: moderate RoB IV: low RoB Prec: low IC: NA |
Low with high certainty |
The data are of limited relevance for the risk assessment, due to the difficulties to link behavioural responses to effects at population level. The onset of effects was observed at slightly higher concentration compared to reproduction endpoints |
| Suborganisms endpoints |
Eisenia andrei 14|1 14|3 |
EV: high RoB IV: low RoB Prec: low IC: NA |
Low with high certainty |
The data are of low relevance for the risk assessment, due to the difficulties to link suborganism responses to effects at population level. The onset of effects was observed at slightly lower concentration compared to reproduction endpoints. The external validity for these studies is low |
| Soil Arthropods (Mesofauna) | ||||
| Survival |
Folsomia candida 13|1 |
EV: low RoB IV: low RoB Prec: low IC: NA |
Low with high certainty |
The data are of low/moderate relevance for the hazard characterisation for soil mesofauna exposed to acetamiprid. They indicate that survival is affected at slightly higher concentration than reproductive endpoints. However, survival is not considered a sensitive endpoint in hazard characterisation anymore, since the assessment is based on the data for the reproductive output |
| Reproduction |
Folsomia candida 13|1 |
EV: Low RoB IV: low RoB Prec: low IC: high |
Low with high certainty | The data confirm the hazard characterisation as assessed in EFSA (2016). Endpoints derived in the submitted studies are very close to the previously available ones |
3.3.5.6. Conclusion for soil organisms
The new data made available with the submitted studies do not indicate major concerns regarding the hazard characterisation for soil organisms exposed to acetamiprid as performed in the previous assessment from EFSA (2016). Particularly, the new data on soil mesofauna are very close to the endpoints that were available for the peer review. However, the submitted new data describing the sensitivity of earthworms towards acetamiprid diverge by one order of magnitude. Given that the two submitted studies investigate both the response of Eisenia andrei but under different experimental set‐ups (acetamiprid vs. formulation with acetamiprid; standard vs natural soil; slightly diverging temperatures), it is recommended to clarify the response of Eisenia fetida/andrei under standard conditions, with the aid of initial measurements of chemical concentrations in soils for the verification of exposure and the evaluation of all available data according to the current state of science. This would include the results of the available field study with earthworms, which was evaluated for the peer review and indicated acceptable risks for the assessed intended rates in 2016.
4. Conclusions
The newly available data for birds cannot be directly used in the risk assessment, but they confirm a considerable inter‐species difference in the sensitivity to acetamiprid, with Passeriformes showing higher sensitivity. This aspect was already flagged in the previous EFSA conclusion (EFSA, 2016) where a data gap was identified with reference to the acute data. With the newly available information, the issue extended to chronic data, as the chronic risk assessment was previously performed with an endpoint derived for mallard duck, which is likely not to be protective for Passeriformes.
The two newly submitted studies with aquatic organisms (fish and snail) do not alter the previous aquatic risk assessment as all endpoints (lethal, reproduction and subindividual) were higher than the previous endpoint triggering the risk assessment based on aquatic invertebrates.
The four relevant submitted studies on bees do not trigger major concerns for honey bees in relation to the previous peer review, if combined considering measured endpoint levels and validity, precision and ecological relevance of the new material. The hazard characterisation for honey bees does not need any further action. The data gaps on bumble bees and solitary bees remain from the previous EFSA (2016) conclusion and are reinforced by new mechanistic studies that highlight higher sensitivity of a non‐Apis species to N‐cyanoamidine neonicotinoids and similar insecticidal substances.
The new data made available with the submitted studies do not indicate major concerns regarding the hazard characterisation for soil organisms exposed to acetamiprid as performed in the previous assessment from EFSA (2016). The data describing the sensitivity of earthworms towards acetamiprid diverge though by one order of magnitude, indicating that the response of Eisenia fetida/andrei to acetamiprid under standard conditions should be clarified, including the evaluation of all available data according to the current state of science.
5. Recommendations
The PPR Panel recommends that elective selection of evidence should be avoided and that a systematic evidence‐based approach should be applied instead, in order to avoid bias.
For birds, the PPR Panel recommends addressing explicitly the reproductive hazard to Passeriformes due to long‐term exposure to acetamiprid (by generating and/or collecting appropriate data) and to update the risk assessment accordingly.
For aquatic organisms, no studies were submitted addressing the data gap from the previous peer review (EFSA, 2016) with regard to the uncertainties in sensitivity of species belonging to Naididae (worms). The PPR Panel recommends that this is addressed by generating and/or collecting appropriate data and by updating the risk assessment accordingly.
For bees, the PPR Panel recommends addressing the potential concerns linked to a potentially higher sensitivity of M. rotundata. When data become available, it is recommended that an appropriate specific risk assessment for the intended uses is performed.
For soil organisms, the PPR Panel recommends clarifying the hazard to earthworms under standard conditions.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- AIR
Annex I Renewal
- AST
aspartate aminotransferase
- b.w.
body weight
- BMD
benchmark dose
- BPR
biocidal products
- CaE
carboxylesterase
- CAT
catalase
- CAT
critical appraisal tool
- DCFH‐DA
2´,7´‐dichlorofluoresceine diacetate
- DH RoB
risk of bias definitely high
- DL RoB
risk of bias definitely low
- DNT
developmental neurotoxicity
- EC10
effect concentration, 10%
- EC50
effect concentration, 50%
- EKE
expert knowledge elicitation
- GR
glutathione reductase
- GSH
glutathione
- GST
glutathione S‐transferase
- HCD
historical control data
- HOS
human observational studies
- HPG
hypothalamus–pituitary–gonadal
- IBR
integrated biomarker response
- LC50
lethal concentration, median
- LD50
lethal dose, median
- LDH
lactate dehydrogenase
- LOEC
lowest observed effect concentration
- LDD50
lethal daily dose, median
- LH
luteinising hormone
- LOED
lowest observed effect dose
- LC10
lethal concentration, 10%
- LD10
lethal dose, 10%
- nAChRs
nicotinic acetylcholine receptors
- NCE
normochromatic erythrocytes
- NOAEL
no‐observed‐adverse‐effect‐level
- NOEC
no observed effect concentration
- NTB
nitroblue tetrazolium reduction assay
- OHAT/NTP
The Office of Health Assessment and Translation/National Toxicology Programme
- PCE
polychromatic erythrocyte
- PFAS
perfluoroalkyl substances
- PFOS
perfluorooctane sulfonate, perfluorooctane sulfonic acid
- PH RoB
risk of bias probably high
- PL RoB
risk of bias probably low
- RAR
Renewal Assessment Report
- RoB
risk of bias
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TAC
total antioxidant capacity
- UDS
UDS unscheduled DNA synthesis
- US EPA
United States Environmental Protection Agency
Appendix A – Detailed results of the appraisal phase for hazard experiments (environment)
The following figures are a graphical representation of the appraisal exercise performed on the literature studies considered eligible according to the criteria listed in the protocol (see Annex A).
Results are presented for each assessment question. For every figure the strings on the left identify the *RefID|ExperimentID|Endpoint*, or in other words, the identifiers for: the reference, the experiment within the reference (where multiple experimental units were identified in the same reference) and the assessment endpoint investigated.
The acronyms for the single criteria are explained by means of tables for each assessment question. The colours used to fill each cell of the matrix represent the risk of bias or precision, in accordance with the following legend.
| Definitely low risk of bias/high precision | |
| Probably low risk of bias | |
| Probably high risk of bias | |
| Definitely high risk of bias/low precision | |
| Criterion not applicable |
A.1 Aquatic organisms
Table A.1 Outline of the appraisal questions for aquatic organisms
| Section | Acronym | Question |
|---|---|---|
| External validity | Q1_EV | How confident are we that the assessment endpoint can be used to inform the risk assessment of aquatic organisms? |
| Q2_EV | Are the test organisms exposed to either flupyradifurone or acetamiprid in isolation (without any other active substances)? | |
| Q3_EV | Are the tested organisms relevant for Europe? | |
| Q4_EV | Is the duration of the exposure and observation in line with the standard testing? | |
| Internal validity | Q1_IV | Is the origin of the tested organism trustable? |
| Q2_IV | Is the age and sex of the tested organisms known and appropriate? | |
| Q3_IV | Were the test organisms properly acclimatised to the study setup before the exposure started? | |
| Q4_IV | Are the test organisms healthy and stress‐free at the start of the experiment? | |
| Q5_IV | Is the methodology used (including the experimental setup) for measuring the assessment endpoint reliable? | |
| Q6_IV | Is the negative (blank) control performing adequately? | |
| Q7_IV | If a solvent is used, is the effect of the solvent appropriately accounted for? | |
| Q8_IV |
Are the test conditions appropriate? This includes relevant OECD validity criteria concerning optimal tests conditions for the tested species (e.g. oxygen saturation). |
|
| Q9_IV | Is the test item clearly identified and characterised? | |
| Q10_IV | Is exposure characterised by analytical measurements and is it maintained during the test duration? | |
| Q11_IV | Was a clear dose response observed in the study? | |
| Q12_IV | Is the derivation of the measured endpoint(s) performed with sound statistical methods? | |
| Precision | Q1_PR | Are the sample size and replication appropriate? |
| Q2_PR | Is the number of tested concentrations/doses appropriate? | |
| Q3_PR | Is doses selection (including the space between them) appropriate? |
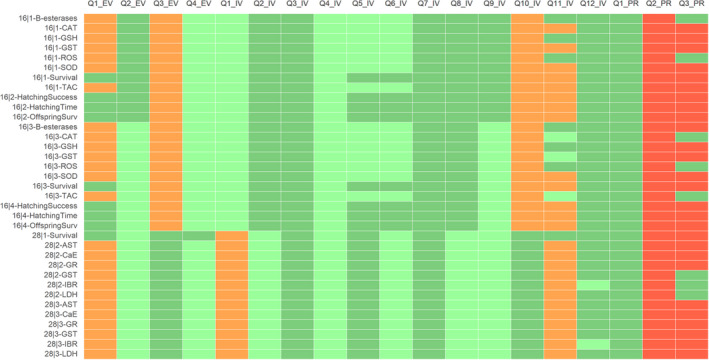
Figure A.1 The heatmap summarising the outcome of the appraisal of experiments with aquatic organisms
A.2 Bees (laboratory experiments)
Table A.2 Outline of the appraisal questions for bee laboratory experiments
| Section | Acronym | Question |
|---|---|---|
| External validity | Q1_EV | How confident are we that the assessment endpoint can be used to inform the risk assessment of bees? |
| Q2_EV | Are the test organisms exposed to either flupyradifurone or acetamiprid in isolation (without any other active substances)? | |
| Q3_EV | Are the tested organisms relevant for Europe? | |
| Q4_EV | Is the duration of the exposure and observation in line with the standard testing? | |
| Internal validity | Q1_IV | Is the origin of the tested organism trustable? |
| Q2_IV | Is the age and sex of the tested organisms known and appropriate? | |
| Q3_IV |
Were the test organisms properly acclimatised to the study setup before the exposure started? For acute studies this is generally not a problem |
|
| Q4_IV | Are the test organisms healthy and stress‐free at the start of the experiment? | |
| Q5_IV | Is the methodology used (including the experimental setup) for measuring the assessment endpoint reliable? | |
| Q6_IV | Is the negative (blank) control performing adequately? | |
| Q7_IV | Is the system sensitive enough? | |
| Q8_IV | If a solvent is used, is the effect of the solvent appropriately accounted for? | |
| Q9_IV | Are the test conditions appropriate? | |
| Q10_IV | Is the test item clearly identified and characterised? | |
| Q11_IV | Is exposure characterised by analytical measurements (residues or confirmed dose)? | |
| Q12_IV |
Is exposure underpinned by appropriate measurements/estimation of test item consumption? (only relevant for oral exposure) |
|
| Q13_IV | Was a clear dose response observed in the study? | |
| Q14_IV | Is the derivation of the measured endpoint(s) performed with sound statistical methods? | |
| Precision | Q1_PR | Are the sample size and replication appropriate? |
| Q2_PR | Is the number of tested concentrations/doses appropriate? | |
| Q3_PR | Is doses selection (including the space between them) appropriate? |
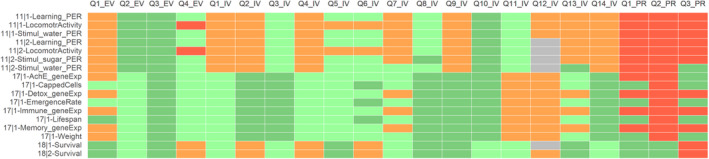
Figure A.2 The heatmap summarising the outcome of the appraisal of bee laboratory experiments
A.3 Soil organisms
Table A.3 Outline of the appraisal questions for soil organisms
| Section | Acronym | Question |
|---|---|---|
| External validity | Q1_EV | How confident are we that the assessment endpoint can be used to inform the risk assessment of bees? |
| Q2_EV | Are the test organisms exposed to either flupyradifurone or acetamiprid in isolation (without any other active substances)? | |
| Q3_EV | Are the tested organisms relevant for Europe? | |
| Q4_EV | Is the duration of the exposure and observation in line with the standard testing? | |
| Internal validity | Q1_IV | Is the origin of the tested organism trustable? |
| Q2_IV | Is the age and sex of the tested organisms known and appropriate? | |
| Q3_IV | Were the test organisms properly acclimatised to the study setup before the exposure started? | |
| Q4_IV | Are the test organisms healthy and stress‐free at the start of the experiment? | |
| Q5_IV | Is the methodology used (including the experimental setup) for measuring the assessment endpoint reliable? | |
| Q6_IV | Is the negative (blank) control performing adequately? | |
| Q7_IV | Is the system sensitive enough? | |
| Q8_IV | If a solvent is used, is the effect of the solvent appropriately accounted for? | |
| Q9_IV | Are the test conditions appropriate? | |
| Q10_IV | Is the test item clearly identified and characterised? | |
| Q11_IV | Is exposure characterised by analytical measurements and is it maintained during the test duration? | |
| Q12_IV | Was a clear dose response observed in the study? | |
| Q13_IV | Is the derivation of the measured endpoint(s) performed with sound statistical methods? | |
| Precision | Q1_PR | Are the sample size and replication appropriate? |
| Q2_PR | Is the number of tested concentrations/doses appropriate? | |
| Q3_PR | Is doses selection (including the space between them) appropriate? |
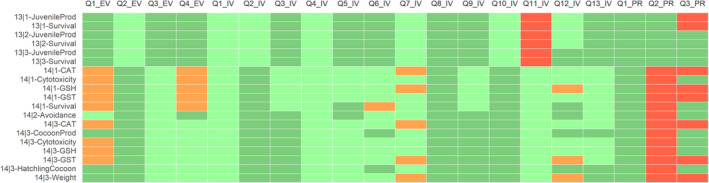
Figure A.3 The heatmap summarising the outcome of the appraisal of experiments with soil organisms
A.4 Bees (field effect experiments)
Table A.4 Outline of the appraisal questions for field effect experiments with bees
| Section | Acronym | Question |
|---|---|---|
| External validity | Q1_EV | How confident are we that the assessment endpoint can be used to inform the risk assessment of bees? |
| Q2_EV | Are the test organisms exposed to either flupyradifurone or acetamiprid in isolation (without any other active substances)? | |
| Q3_EV | Are the tested organisms relevant for Europe? | |
| Q4_EV | Is the study location representative of any EU biogeographical region? | |
| Q5_EV | Is the study setting representative of an EU agricultural landscape? | |
| Q6_EV | Do the experimental conditions represent a reasonable worst‐case for both exposure and possible triggering of the effects? | |
| Internal validity | Q1_IV | Is the origin of the tested organism trustable? |
| Q2_IV |
Were the test organisms properly acclimatised to the study setup before the exposure started? |
|
| Q3_IV | Are the test organisms healthy and stress‐free at the start of the experiment? | |
| Q4_IV | Is the methodology used (including the experimental setup) for measuring the assessment endpoint reliable? | |
| Q5_IV | Is the negative control free from contamination and performing adequately? | |
| Q6_IV | Are the treatments and exposure levels well characterised? | |
| Q7_IV | Is the test item clearly identified and characterised? | |
| Q8_IV | Is the duration of the test appropriate to characterise the assessment endpoint? | |
| Q9_IV | Is the presence of other stressors checked and accounted for? | |
| Q10_IV | Is the derivation of the measured endpoint(s) performed with sound statistical methods? | |
| Precision | Q1_PR | Are the sample size and replication appropriate? |

Figure A.4 The heatmap summarising the outcome of the appraisal of field effect experiments with bees
Annex A – For the assessment of the data concerning the active substances acetamiprid and flupyradifurone
Annex B – Outcome RoB acetamiprid for human health
Annex C – RoB HeatMap acetamiprid for human health
Annex D – Data extraction acetamiprid for human health
Annex E – Uncertainty analysis table acetamiprid for human health
Annex F – Acetamiprid EKE for human health
Annex G – Data extraction, appraisal, and weight of evidence concerning mechanistic studies with bees
Annex H – Detailed results of the appraisal for bird experiments
Annex I – Detailed results of the appraisal for experiments with aquatic organisms
Annex J – Detailed results of the appraisal for laboratory experiments with bees
Annex K – Detailed results of the appraisal for field experiments with bees
Annex L – Detailed results of the appraisal for experiments with soil organisms
Supporting information
For the assessment of the data concerning the active substances acetamiprid and flupyradifurone
Outcome RoB acetamiprid for human health
RoB HeatMap acetamiprid for human health
Data extraction acetamiprid for human health
Uncertainty analysis table acetamiprid for human health
Acetamiprid EKE for human health
Data extraction, appraisal, and weight of evidence concerning mechanistic studies with bees
Detailed results of the appraisal for bird experiments
Detailed results of the appraisal for experiments with aquatic organisms
Detailed results of the appraisal for laboratory experiments with bees
Detailed results of the appraisal for field experiments with bees
Detailed results of the appraisal for experiments with soil organisms
Suggested citation: EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernandez Jerez A, Adriaanse P, Berny P, Coja T, Duquesne S, Focks A, Marinovich M, Millet M, Pelkonen O, Pieper S, Tiktak A, Topping C, Widenfalk A, Wilks M, Wolterink G, Rundlöf M, Ippolito A, Linguadoca A, Martino L, Panzarea M, Terron A and Aldrich A, 2022. Statement on the active substance acetamiprid. EFSA Journal 2022;20(1):7031, 71 pp. 10.2903/j.efsa.2022.7031
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00160
Panel members: Paulien Adriaanse, Annette Aldrich, Philippe Berny, Tamara Coja Sabine Duquesne, Andreas Focks, Antonio Hernandez‐Jerez, Marina Marinovich, Maurice Millet, Olavi Pelkonen, Silvia Pieper, Aaldrik Tiktak, Christopher Topping, Anneli Widenfalk, Martin Wilks and Gerrit Wolterink.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel on Plant Protection Products and their Residues wishes to thank Emilio Benfenati and Thomas J. Colgan for their support to the WG as hearing experts.
Adopted: 29 November 2021
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2022.7030/full
Notes
NOTE: concurrent refer to the analytical determination of the a.s. in the plasma and in the testes, which was performed on the same day of sampling for the assessment of toxicological endpoints (for the two literature studies).
Competent Authority Report on Acetamiprid Product type 18 Insecticides, acaricides and products to control others arthropods. Available at this link: https://echa.europa.eu/fr/information‐on‐chemicals/biocidal‐active‐substances/‐/disas/factsheet/1235/PT18
BYI 02960 SL 200 G = BYI 02960 SL 200 g/L = Flupyradifurone SL 200 G = Sivanto.
References
- Bayer , 2017a. Flupyradifurone SL 200: acute contact toxicity test with the Solitary Bee Osmia bicornis in the Laboratory (non‐GLP study). Unpublished report.
- Bayer , 2017b. Flupyradifurone SL 200: acute contact toxicity test with the Solitary Bee Osmia cornuta in the laboratory (non‐GLP study). Unpublished report.
- Bayer , 2017c. Acute contact toxicity test with Flupyradifurone SL 200 with the solitary Leafcutter Bee (Megachile rotundata L.) in the laboratory (non‐GLP study). Unpublished report.
- Beadle K, Singh KS, Troczka BJ, Randall E, Zaworra M, Zimmer CT, Hayward A, Reid R, Kor L, Kohler M, Buer B, Nelson DR, Williamson MS, Davies TGE, Field LM, Nauen R and Bass C, 2019. Genomic insights into neonicotinoid sensitivity in the solitary bee Osmia bicornis . PLoS Genetics, 15, e1007903. 10.1371/journal.pgen.1007903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çamlica Y, Bediz SC, Comelekoglu U and Yilmaz SN, 2019. Toxic effect of acetamiprid on Rana ridibunda sciatic nerve (electrophysiological and histopathological potential). Drug and Chemical Toxicology, 42, 264–269. 10.1080/01480545.2018.1442475 [DOI] [PubMed] [Google Scholar]
- CLH , 2020. CLH report proposal for harmonised classification and labelling for acetamiprid. Available online: https://echa.europa.eu/documents/10162/2c6b3db4‐7884‐b0c9‐3109‐677ebd3d5b01 and https://echa.europa.eu/documents/10162/0e50b786‐1aa5‐ec33‐7caf‐54403a450e40
- Cossi PF, Herbert LT, Yusseppone MS, Perez AF and Kristoff G, 2020. Toxicity evaluation of the active ingredient acetamiprid and a commercial formulation (Assail(R) 70) on the non‐target gastropod Biomphalaria straminea (Mollusca: Planorbidae). Ecotoxicology and Environmental Safety, 192, 110248. 10.1016/j.ecoenv.2020.110248 [DOI] [PubMed] [Google Scholar]
- Costas‐Ferreira C and Faro LRF, 2021. Neurotoxic effects of neonicotinoids on mammals: what is there beyond the activation of nicotinic acetylcholine receptors?‐A systematic review. International Journal of Molecular Sciences, 22, 8413. 10.3390/ijms22168413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci Ö and Güngördü A, 2020. Evaluation of the biochemical effects of an acetamiprid‐based insecticide on a non‐target species, Gambusia holbrooki . Water and Environment Journal, 34, 481–489. 10.1111/wej.12549 [DOI] [Google Scholar]
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) , with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA Biocidal Products Committee (BPC) , 2017. Opinion on the application for approval of the active substance acetamiprid for product type 18.
- European Food Safety Authority, 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) . 2014. Guidance on Expert knowledge elicitation in food and feed safety risk assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance flupyradifurone. EFSA Journal 2015;13(2):4020, 106 pp. 10.2903/j.efsa.2015.4020 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2016. Conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA Journal 2016;14(11):4610, 26 pp. 10.2903/j.efsa.2016.4610 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Hart A, Maxim L, Siegrist M, Von Goetz N, da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019. Guidance on communication of uncertainty in scientific assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Ippolito A, del Aguila M, Aiassa E, Guajardo IM, Neri FM, Alvarez F, Mosbach‐Schulz O and Szentes C, 2020. Review of the evidence on bee background mortality. EFSA Supporting Publications 2020:EN‐1880, 76 pp. 10.2903/sp.efsa.2020.EN-1880 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernández‐Jerez AF, Adriaanse P, Berny P, Coja T, Duquesne S, Focks A, Marinovich M, Millet M, Pelkonen OR, Pieper S, Tiktak A, Topping CJ, Widenfalk A, Wilks M, Wolterink G, Rundlöf M, Ippolito A, Linguadoca A, Martino L, Panzarea M, Terron A and Aldrich AP, 2021. Statement on the active substance flupyradifurone. EFSA Journal 2021;19(12):7031, 71 pp. 10.2903/j.efsa.2021.7031 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Statistical significance and biological relevance. EFSA Journal 2011;9(9):2372, 17 pp. 10.2903/j.efsa.2011.2372 [DOI] [Google Scholar]
- El Hassani AK, Dacher M, Gary V, Lamin M, Gauthier M and Armengaud C, 2004. Effets sublétaux de l’Acétamipride et du Thiamethoxam sur le comportement de l’abeille (Apis mellifera). Available from: https://www.researchgate.net/publication/255636607_Effets_subletaux_de_l%27Acetamipride_et_du_Thiamethoxam_sur_le_comportement_de_l%27abeille_Apis_mellifera#fullTextFileContent [Accessed: 6 January 2022].
- El Hassani AK, Dacher M, Gary V, Lamin M and Armengaud C, 2014. Effets sublétaux de l’Acétamipride et du Thiamethoxam sur le comportement de l’abeille (Apis mellifera).
- Gomez SD, Bustos PS, Sanchez VG, Ortega MG and Guinazu N, 2020. Trophoblast toxicity of the neonicotinoid insecticide acetamiprid and an acetamiprid‐based formulation. Toxicology, 431, 152363. 10.1016/j.tox.2020.152363. [DOI] [PubMed] [Google Scholar]
- Hayward A, Beadle K, Singh KS, Exeler N, Zaworra M, Almanza MT, Nikolakis A, Garside C, Glaubitz J, Bass C and Nauen R, 2019. The leafcutter bee, Megachile rotundata, is more sensitive to N‐cyanoamidine neonicotinoid and butenolide insecticides than other managed bees. Nature Ecology & Evolution, 3, 1521–1524. 10.1038/s41559-019-1011-2 [DOI] [PubMed] [Google Scholar]
- Humann‐Guilleminot S, Tassin de Montaigu C, Sire J, Grunig S, Gning O, Glauser G, Vallat A and Helfenstein F, 2019. A sublethal dose of the neonicotinoid insecticide acetamiprid reduces sperm density in a songbird. Environmental Research, 177, 108589. 10.1016/j.envres.2019.108589 [DOI] [PubMed] [Google Scholar]
- Ichikawa G, Kuribayashi R, Ikenaka Y, Ichise T, Nakayama SMM, Ishizuka M, Taira K, Fujioka K, Sairenchi T, Kobashi G, Bonmatin JM and Yoshihara S, 2019. LC‐ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS One, 14, e0219208. 10.1371/journal.pone.0219208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JMPR (Joint FAO/WHO Meeting on Pesticide Residues), 2005. Acetamiprid (246). Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report11/Acetamiprid.pdf
- Johnson RM, Harpur BA, Dogantzis KA, Zayed A and Berenbaum MR, 2018. Genomic footprint of evolution of eusociality in bees: floral food use and CYPome “blooms”. Insectes Sociaux, 65, 445–454. 10.1007/s00040-018-0631-x [DOI] [Google Scholar]
- Kagawa N and Nagao T, 2018. Neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to acetamiprid. Journal of Applied Toxicology, 38, 1521–1528. 10.1002/jat.3692 [DOI] [PubMed] [Google Scholar]
- Kong D, Zhang J, Hou X, Zhang S, Tan J, Chen Y, Yang W, Zeng J, Han Y, Liu X, Xu D and Cai R, 2017. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells. Biology of Reproduction, 96, 254–265. 10.1095/biolreprod.116.139550 [DOI] [PubMed] [Google Scholar]
- Manjon C, Troczka BJ, Zaworra M, Beadle K, Randall E, Hertlein G, Singh KS, Zimmer CT, Homem RA, Lueke B, Reid R, Kor L, Kohler M, Benting J, Williamson MS, Davies TGE, Field LM, Bass C and Nauen R, 2018. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Current Biology, 28, 1137–1143.e1135. 10.1016/j.cub.2018.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfo JT, Fujioka K, Ikenaka Y, Nakayama SM, Mizukawa H, Aoyama Y, Ishizuka M and Taira K, 2015. Relationship between urinary N‐desmethyl‐acetamiprid and typical symptoms including neurological findings: a prevalence case‐control study. PLoS One, 10, e0142172. 10.1371/journal.pone.0142172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouki S, Bini Dhouib I, Benabdessalem C, Rekik R, Doghri R, Maroueni A, Bellasfar Z, Fazaa S, Bettaieb J, Barbouche MR and Ben Ahmed M, 2017. Specific immune responses in mice following subchronic exposure to acetamiprid. Life Sciences, 188, 10–16. 10.1016/j.lfs.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Mazi S, Vroumsia T, Yahangar M‐N, Malla M and Zroumba D, 2020. Determination of acute lethal doses of acetamiprid and cypermethrin for the Native Bee Apis mellifera (Hymenoptera: Apidae) in Cameroon. Open Journal of Ecology, 10, 404–417. 10.4236/oje.2020.107026 [DOI] [Google Scholar]
- NTP , 2015. OHAT risk of bias rating tool for human and animal studies. Office of Health Assessment and Translation, National Toxicology Program, 2015. [Google Scholar]
- NTP , 2016. Monograph on immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS). National Toxicological Program, Research Triangle Park. [Google Scholar]
- NTP , 2019. Office of Health Assessment and Translation (OHAT) –NTP , 2019. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. [Google Scholar]
- O’Neill KM, O’Neill RP, Blodgett S and Fultz JE, 2004. Variation in Megachile rotundata pollen load composition in relation to flower diversity (Hymenoptera: Megachilidae). Journal of the Kansas Entomological Society, 77 pp. 619–625. [Google Scholar]
- O’Neill RP and O’Neill KM, 2011. Pollen load composition and size in the leafcutting bee Megachile rotundata (Hymenoptera: Megachilidae). Apidologie, 42, 223–233. 10.1051/apido/2010057 [DOI] [Google Scholar]
- OECD , 1984. Test No. 206: avian reproduction test, OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris. 10.1787/9789264070028-en [DOI] [Google Scholar]
- OECD , 1998. Test No. 214: honeybees, acute contact toxicity test, OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris. 10.1787/9789264070189-en [DOI] [Google Scholar]
- OECD , 2016. Guidance Document on Honey Bee (Apis mellifera) larval toxicity test, repeated exposure. series on testing and assessment No. 239 series on testing and assessment No. 239. ENV/JM/MONO(2016)34.
- OECD , 2017. Test No. 245: Honey Bee (Apis Mellifera L.), chronic oral toxicity test (10‐day feeding), OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris. 10.1787/9789264284081-en [DOI] [Google Scholar]
- Ospina M, Wong LY, Baker SE, Serafim AB, Morales‐Agudelo P and Calafat AM, 2019. Exposure to neonicotinoid insecticides in the U.S. general population: data from the 2015–2016 National Health and Nutrition Examination Survey. Environmental Research, 176, 108555. 10.1016/j.envres.2019.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R‐project.org/ [Google Scholar]
- RAC , 2020. Committee for Risk Assessment RAC Opinion proposing harmonised classification and labelling at EU level of acetamiprid (ISO); (1E)‐N‐[(6chloropyridin‐3‐yl)methyl]‐N'‐cyano‐N‐methylethanimidamide;(E)‐N1‐[(6‐chloro‐3‐pyridyl)methyl]‐N2‐cyano‐N1‐methylacetamidine. Available online: https://echa.europa.eu/documents/10162/f6c14e84‐8e85‐1e71‐2f34‐76c1f39cf10d
- Renaud M, Akeju T, Natal‐da‐Luz T, Leston S, Rosa J, Ramos F, Sousa JP and Azevedo‐Pereira H, 2018. Effects of the neonicotinoids acetamiprid and thiacloprid in their commercial formulations on soil fauna. Chemosphere, 194, 85–93. 10.1016/j.chemosphere.2017.11.102 [DOI] [PubMed] [Google Scholar]
- Saggioro EM, do Espírito Santo DG, Sales Júnior SF, Hauser‐Davis RA and Correia FV, 2019. Lethal and sublethal effects of acetamiprid on Eisenia andrei: behavior, reproduction, cytotoxicity and oxidative stress. Ecotoxicology and Environmental Safety, 183, 109572. 10.1016/j.ecoenv.2019.109572 [DOI] [PubMed] [Google Scholar]
- Selvam V and Srinivasan S, 2019. Neonicotinoid poisoning and management. Indian Journal of Critical Care Medicine, 23(suppl 4), S260–S262. 10.5005/jp-journals-10071-23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyildiz M, Kilinc A and Ozden S, 2018. Investigation of the genotoxic and cytotoxic effects of widely used neonicotinoid insecticides in HepG2 and SH‐SY5Y cells. Toxicology and Industrial Health, 34, 375–383. 10.1177/0748233718762609 [DOI] [PubMed] [Google Scholar]
- Shi J, Yang H, Yu L, Liao C, Liu Y, Jin M, Yan W and Wu XB, 2020a. Sublethal acetamiprid doses negatively affect the lifespans and foraging behaviors of honey bee (Apis mellifera L.) workers. Science of the Total Environment, 738, 139924. 10.1016/j.scitotenv.2020.139924 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang R, Pei Y, Liao C and Wu X, 2020b. Exposure to acetamiprid influences the development and survival ability of worker bees (Apis mellifera L.) from larvae to adults. Environmental Pollution, 266, 115345. 10.1016/j.envpol.2020.115345 [DOI] [PubMed] [Google Scholar]
- Sinu PA and Bronstein JL, 2018. Foraging preferences of leafcutter bees in three contrasting geographical zones. Diversity and Distributions, 24, 621–628. 10.1111/ddi.12709 [DOI] [Google Scholar]
- Terayama H, Endo H, Tsukamoto H, Matsumoto K, Umezu M, Kanazawa T, Ito M, Sato T, Naito M, Kawakami S, Fujino Y, Tatemichi M and Sakabe K, 2016. Acetamiprid accumulates in different amounts in murine brain regions. Int J Environ Res Public Health, 13, 937. 10.3390/ijerph13100937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama H, Qu N, Endo H, Ito M, Tsukamoto H, Umemoto K, Kawakami S, Fujino Y, Tatemichi M and Sakabe K, 2018. Effect of acetamiprid on the immature murine testes. International Journal of Environmental Health Research, 28, 683–696. 10.1080/09603123.2018.1504897 [DOI] [PubMed] [Google Scholar]
- Traynor KS, Pettis JS, Tarpy DR, Mullin CA, Frazier JL, Frazier M and vanEngelsdorp D, 2016. In‐hive pesticide exposome: assessing risks to migratory honey bees from in‐hive pesticide contamination in the Eastern United States. Scientific Reports, 6, 33207. 10.1038/srep33207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troczka BJ, Homem RA, Reid R, Beadle K, Kohler M, Zaworra M, Field LM, Williamson MS, Nauen R, Bass C and Davies TGE, 2019. Identification and functional characterisation of a novel N‐cyanoamidine neonicotinoid metabolising cytochrome P450, CYP9Q6, from the buff‐tailed bumblebee Bombus terrestris . Insect Biochemistry and Molecular Biology, 111, 103171. 10.1016/j.ibmb.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA , 2003. Generic Ecological Assessment Endpoints (GEAEs) for Ecological Risk Assessment, Washington, DC. USA: Risk Assessment Forum. EPA/630/P‐02/004B
- Wang H, Yang D, Fang H, Han M, Tang C, Wu J, Chen Y and Jiang Q, 2020. Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: a biomonitoring‐based study. Environment International, 143, 105918. 10.1016/j.envint.2020.105918 [DOI] [PubMed] [Google Scholar]
- Wickham H, 2016. ggplot2: elegant graphics for data analysis. Springer‐Verlag, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For the assessment of the data concerning the active substances acetamiprid and flupyradifurone
Outcome RoB acetamiprid for human health
RoB HeatMap acetamiprid for human health
Data extraction acetamiprid for human health
Uncertainty analysis table acetamiprid for human health
Acetamiprid EKE for human health
Data extraction, appraisal, and weight of evidence concerning mechanistic studies with bees
Detailed results of the appraisal for bird experiments
Detailed results of the appraisal for experiments with aquatic organisms
Detailed results of the appraisal for laboratory experiments with bees
Detailed results of the appraisal for field experiments with bees
Detailed results of the appraisal for experiments with soil organisms


