Abstract
To evaluate changes in Clostridioides difficile incidence rates for Maryland hospitals that participated in the Statewide Prevention and Reduction of C. difficile (SPARC) collaborative. Pre-post, difference-in-difference analysis of non-randomised intervention using four quarters of preintervention and six quarters of postintervention National Healthcare Safety Network data for SPARC hospitals (April 2017 to March 2020) and 10 quarters for control hospitals (October 2017 to March 2020). Mixed-effects negative binomial models were used to assess changes over time. Process evaluation using hospital intervention implementation plans, assessments and interviews with staff at eight SPARC hospitals. Maryland, USA. All Maryland acute care hospitals; 12 intervention and 36 control hospitals. Participation in SPARC, a public health–academic collaborative made available to Maryland hospitals, with staggered enrolment between June 2018 and August 2019. Hospitals with higher C. difficile rates were recruited via email and phone. SPARC included assessments, feedback reports and ongoing technical assistance. Primary outcomes were C. difficile incidence rate measured as the quarterly number of C. difficile infections per 10 000 patient-days (outcome measure) and SPARC intervention hospitals’ experiences participating in the collaborative (process measures). SPARC invited 13 hospitals to participate in the intervention, with 92% (n=12) participating. The 36 hospitals that did not participate served as control hospitals. SPARC hospitals were associated with 45% greater C. difficile reduction as compared with control hospitals (incidence rate ratio=0.55, 95% CI 0.35 to 0.88, p=0.012). Key SPARC activities, including access to trusted external experts, technical assistance, multidisciplinary collaboration, an accountability structure, peer-to-peer learning opportunities and educational resources, were associated with hospitals reporting positive experiences with SPARC. SPARC intervention hospitals experienced 45% greater reduction in C. difficile rates than control hospitals. A public health–academic collaborative might help reduce C. difficile and other hospital-acquired infections in individual hospitals and at state or regional levels.
Keywords: clinical practice guidelines, healthcare quality improvement, patient safety, quality improvement
Introduction
Clostridioides difficile infection, the most common hospital-acquired infection, results in approximately 12 000 deaths and $1 billion in attributable healthcare costs in the USA annually.1 In October 2016, the US Department of Health and Human Services announced a target 30% reduction of C. difficile rates in acute care hospitals nationwide by the end of 2020.2 By January 2018, Maryland’s state-wide C. difficile rate had only achieved an 8% reduction.3
The Maryland Department of Health leveraged Centers for Disease Control and Prevention (CDC) Epidemiology and Laboratory Capacity funding (5 NU50CK000506-02-00), developing a partnership with two academic institutions: the University of Maryland, Baltimore, and Johns Hopkins University, both designated CDC Prevention Epicenters for infection prevention research.4 Together, they created the Statewide Prevention and Reduction of C. difficile (SPARC) collaborative as a novel state public health–academic partnership to enhance hospital-based quality improvement (QI) efforts towards C. difficile reduction.5–10
The objectives of this QI report are to: describe SPARC; conduct a pre-post quantitative assessment of changes in C. difficile incidence rates for SPARC intervention versus control hospitals (outcome measure); and qualitatively assess hospital perspectives of the collaborative (process measures). This paper follows the Standards for Quality Improvement Reporting Excellence reporting guidelines for QI interventions.11
Methods
Intervention description
SPARC team
SPARC included public health experts from the Maryland Department of Health and C. difficile prevention subject matter experts from the University of Maryland, Baltimore, and Johns Hopkins University. NORC at the University of Chicago provided implementation support.
Development
The SPARC team conducted a literature review to understand the structure and frameworks of other successful hospital-acquired infection collaboratives, basing their SPARC approach on the CDC’s Targeted Assessment for Prevention (TAP) strategy for QI.12 The TAP strategy consists of three components: (1) ‘utilizing National Healthcare Safety Network (NHSN) reports to identify and target health care facilities with excess hospital-acquired C. difficile infections; (2) administering facility assessment tools to identify gaps in infection prevention; and (3) accessing infection prevention resources within TAP Implementation Guides to address those gaps’.12 Thus, identification of high-burden facilities and tailoring interventions to facility-specific gaps are defining attributes of this targeted SPARC strategy. Because CDC TAP assessment tools and implementation guides for C. difficile prevention were still in development when SPARC launched, the SPARC team created its own tools de novo.13–22 SPARC developed assessment tools and resources to support QI efforts focused on four domains: infection prevention, environmental cleaning, antimicrobial stewardship and diagnostic stewardship.
Collaborative structure
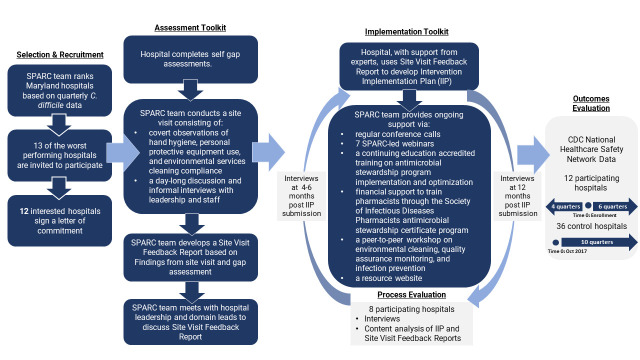
Key attributes of the SPARC collaborative were as follows (figure 1):
Figure 1.
Description of Statewide Prevention and Reduction of C. difficile (SPARC) intervention and evaluation approach. CDC, Centers for Disease Control and Prevention.
Selection and recruitment of the worst performing Maryland hospitals identified on the basis of trends in risk-adjusted NHSN C. difficile data over 2016–2017 (eight quarters).23
Hospital self-led and SPARC expert-led assessments: facilities first completed written self-evaluations followed by SPARC on-site, daylong assessments conducted by a total of 10–15 experts (~2–4 per domain).
Intervention selection: a smaller group of five to six experts worked with hospital leads on tailored intervention implementation plans based on assessment findings. The intervention implementation plans detailed each hospital’s intervention selection, areas of focus and who would lead each effort.
Technical assistance: the SPARC team provided support via regular check-ins (eg, monthly calls during the first months of implementation); hosting seven webinars on topics including environmental cleaning processes and tools, contact precautions and executive leadership engagement; an in-person meeting on best practices related to infection prevention and environment cleaning, an antimicrobial stewardship course training; and access to root cause analyses tools.
Evaluation, which is described further in the Evaluation of QI intervention section.
Twelve hospitals participated in SPARC, with staggered enrolment between June 2018 and August 2019.
Evaluation of QI intervention
This is a mixed-methods process and outcome evaluation using a pre-post, non-randomised design with control group to quantitatively assess changes in C. difficile rate and qualitative data to assess participants’ experiences (figure 1).
Measures
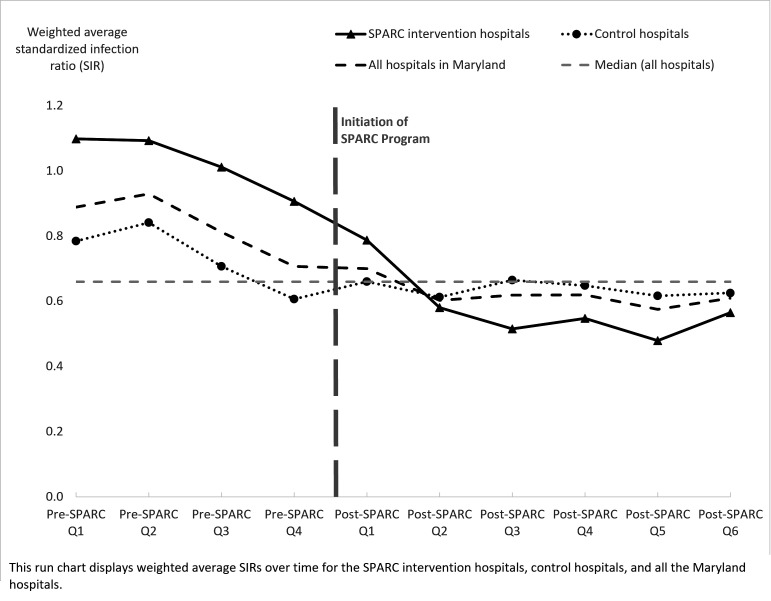
The primary outcome measure was C. difficile incidence rate measured as quarterly number of hospital-onset infections per 10 000 patient-days. We also generated a run chart17 to show the standardised infection ratio (SIR),24 which compares the actual number of hospital-acquired infections to the predicted number for both intervention and control hospitals (figure 2). State-wide and hospital-specific C. difficile incidence and SIRs were obtained from NHSN. NHSN produces a risk-adjusted SIR based on laboratory-identified C. difficile infections (positive C. difficile test results).23 25
Figure 2.
Clostridioides difficile standardised infection ratios for Statewide Prevention and Reduction of C. difficile (SPARC) intervention and control acute care hospitals.
The process evaluation was based on Steckler and Linnan’s framework, a methodology often used for public health interventions and research.26 Using a semistructured interview guide, we assessed the following process measures: (1) reach; extent of providers and frontline staff participation in intervention activities; (2) dose received; participation in any of the SPARC activities or resources; (3) fidelity; implementation progression as planned; (4) context; organisational and broader factors influencing implementation; and (5) implementation; experiences with, and extent of intervention, implementation.
Analysis
We included all Maryland acute care hospitals (12 interventions, 36 control hospitals) in the quantitative analysis. Fisher’s exact tests or Wilcoxon rank-sum tests were used to describe hospitals’ baseline characteristics. To assess the impact on C. difficile incidence, we used a difference-in-difference analytical approach comparing change from baseline to any time point between SPARC intervention and control hospitals. The unit of analysis was the hospital quarter. Intervention hospitals used four quarters of preintervention and six quarters of postintervention data from NHSN; specific preperiods and postperiods varied based on hospital enrolment date but ranged from April 2017 to March 2020. For control hospitals, we included 10 quarters of data from October 2017 to March 2020. We constructed mixed-effects negative binomial models regressing quarterly C. difficile counts on a dichotomous variable indicating intervention versus control group; dummy variables for time periods; interactions of the intervention group and time period dummy variables, and hospital-level random intercepts to account for the clustering of repeated measures from the same hospital, with an offset of patient-days. The regression model included the predicted C. difficile counts as a covariate, a measure generated by NHSN using negative binomial regression models that adjust for C. difficile test type, hospital type, hospital bed number, intensive care unit bed number, teaching status and C. difficile community-onset cases across hospitals.8 24 Incidence rate ratios (IRR) and 95% CIs are reported, and p<0.05 defined as statistical significance. Analyses were conducted using Stata V.15.1 (StataCorp, College Station, Texas, USA).
For qualitative analysis, we conducted content analysis of intervention implementation plans and site visit feedback reports from the self-led and expert-led assessments to inform interview guides. We conducted phone interviews from May 2019 to March 2020: at 4–6 months (round 1) and again at 12 months (round 2) after hospitals’ intervention implementation plan submission. We used purposive sampling to identify domain leads and a snowball technique, using participating domain leads’ suggestions, to recruit executive leadership and frontline staff (online supplemental appendix table 1). Outreach was conducted to 50 individuals in round 1 and 40 in round 2, sending at least two follow-up requests. Frontline staff were offered the option of a written response and small incentive. Semistructured interview guides, developed from site visit reports and intervention implementation plans, guided discussions. We interviewed all responsive individuals, and stopped recruiting additional participants when thematic saturation was reached. Interviews were recorded, transcribed and coded using NVivo (QSR International, Melbourne, Australia). The codebook was derived deductively from evaluation goals and the Steckler and Linnan process evaluation framework, and updated inductively based on emergent themes. Coders’ training was considered complete when inter-rater reliability met Cohen’s target of κ=0.7.27 Only salient and cross-cutting themes that were reported across multiple interviewee types and locations are reported.
bmjqs-2021-014014supp001.pdf (76.8KB, pdf)
Results
Hospital characteristics
Overall, 25% (12 of 48) of Maryland acute care hospitals and 92% (12 of 13) of invited hospitals participated in SPARC. The remaining 36 hospitals served as controls in this analysis. Compared with control hospitals, SPARC intervention hospitals had higher bed number, intensive care unit bed number, number of infection preventionist full-time equivalents and fewer surveillance hours per 100 beds (table 1). We interviewed 52 individuals from 8 of the 12 SPARC intervention hospitals. Response rates were 74% in round 1 and 38% in round 2 (see online supplemental appendix table 1).
Table 1.
Characteristics of SPARC intervention and control hospitals
| SPARC hospitals (n=12) |
Control hospitals (n=36) |
P value* | |||
| n | % | n | % | ||
| Hospital type | |||||
| General, acute care | 12 | 100 | 35 | 97 | 1.000 |
| Medical school affiliated | |||||
| No | 3 | 25 | 17 | 47 | 0.196 |
| Yes | 9 | 75 | 19 | 53 | |
| SPARC start time | |||||
| 2018 Quarter 2 | 1 | 8 | |||
| 2018 Quarter 4 | 7 | 59 | |||
| 2019 Quarter 1 | 3 | 25 | |||
| 2019 Quarter 3 | 1 | 8 | |||
| Median | IQR | Median | IQR | P value* | |
| Number of beds | 274 | (232–339) | 146 | (88–259) | 0.015 |
| Number of intensive care unit beds | 25 | (21–64) | 14 | (8–42) | 0.054 |
| Number of infection control practitioners per 100 beds | 1.1 | (0.7–1.2) | 1.0 | (0.8–1.6) | 0.505 |
| Hours for HAI surveillance per 100 beds per week | 12 | (8–16) | 20 | (16–32) | 0.005 |
| Hours for other infection control activities per 100 beds per week | 14 | (4–34) | 24 | (13–34) | 0.239 |
| Hours for IC activities per 100 beds (sum of the hours for both surveillance and other IC activities) |
26 | (15–45) | 42 | (30–63) | 0.030 |
*From Fisher’s exact tests or Wilcoxon rank-sum tests.
HAI, hospital-acquired infection; IC, infection control; SPARC, Statewide Prevention and Reduction of C. difficile.;
Impact on C. difficile rates (outcome)
Pre-SPARC and post-SPARC incidence rates were 7.6 and 3.1 per 10 000 patient-days, respectively, while rates for control hospitals were 5.0 and 3.7 per 10 000 patient-days, respectively. Pre-SPARC, both intervention and control hospital SIRs were decreasing with similar slopes (figure 2). However, in the first two quarters post-SPARC, intervention hospitals were associated with a greater SIR reduction compared with control hospitals. For the subsequent four post-SPARC quarters, intervention hospitals had similar or lower SIRs than control hospitals, which did not experience any further SIR reductions.
Adjusting for predicted C. difficile counts (based on NHSN C. difficile risk models) from the second quarter post-SPARC onwards, intervention hospitals had statistically greater IRR reduction compared with control hospitals (online supplemental appendix table 2). The IRR for the sixth quarter post-SPARC for intervention and control hospitals was 0.55 (95% CI 0.35 to 0.88, p=0.012), indicating intervention hospitals were associated with a 45% greater Clostridioides difficile Infection (CDI) reduction compared with control hospitals during the same period (online supplemental appendix table 2).
Maryland state-wide SIR for hospital-onset C. difficile decreased from 0.92 in 2017 to 0.8 in 2018 during initiation of SPARC, and to 0.61 in 2019 while SPARC was ongoing.
Process measures
Intervention implementation
Hospitals implemented a range of CDI improvement interventions aligning with SPARC CDI prevention domains. These interventions varied by hospital. While some hospitals identified existing infection prevention and environmental cleaning efforts, an infectious disease-trained pharmacist and/or dedicated antimicrobial stewardship staff, and good compliance with electronic health record testing guidelines as strengths, others included need for cleaning and disinfection process standardisation, staff resources for antimicrobial stewardship and adherence to hospital C. difficile testing guidance and ordering criteria under improvement opportunities. Table 2 describes the common strengths and opportunities identified in assessments by domain area and hospital-selected interventions within intervention implementation plans.
Table 2.
Preintervention strengths and opportunities and selected intervention approaches for SPARC intervention hospitals*
| Domain | Strengths (n=12, %) | Opportunities (n=12, %) | Selected interventions (n=11, %) |
| Infection prevention |
|
|
|
| Environmental cleaning |
|
|
|
| Antibiotic stewardship |
|
|
|
| Diagnostic stewardship |
|
|
|
Programme sources: site visit feedback reports for strengths and opportunities; intervention implementation plans for selected interventions.
*One participating hospital was missing an intervention implementation plan.
PPE, personal protective equipment; SPARC, Statewide Prevention and Reduction of C. difficile.
Interviewees reported that hospitals selected interventions that augmented existing efforts and resources. All hospitals reported providing some education or training activities. These activities were varied and included appropriate CDI testing, personal protective equipment protocols, the use of ultraviolet technology for room cleaning, reviewing and reporting trends in hospital CDI rates and correct utilisation of biomarkers (eg, procalcitonin) to guide antibiotic use. Interviewees identified data and leveraging use of electronic health records as instrumental to successful C. difficile tracking. They described their hospitals as implementing computerised clinical decision supports for C. difficile diagnostics including hard stop alerts, that is, requiring approval from a clinician before test processing or restricting certain classes of providers (eg, nurses) from inputting test orders, or as soft stops, which triggered best practice advisories that did not require an approval for the order to continue.
Lack of understanding of difference between colonisation and true C. difficile infection was identified in many participating hospitals as a common gap. Many interviewees posited that their high reported C. difficile rates pre-SPARC were driven by inappropriate testing, including testing of patients who were on laxatives and patients who had not had diarrhoea, defined by at least three loose or watery stools within 24 hours. Interviewees described their hospitals switching to two-step testing approaches under SPARC, that is, using toxin detection in combination with antigen tests or PCR. Many interviewees noted that two-step testing greatly reduced treatment of colonised patients. Interviewees posited that education and training on appropriate testing indications combined with these electronic health record-based alerts and two-step testing strategies led to early C. difficile reduction. Furthermore, changes to antibiotic and diagnostic stewardship guidelines, including changes to order sets, helped hospitals improve diagnostic practices, reduce unnecessary urine culturing and target inappropriate use of antibiotics like fluoroquinolones.
Reach
In site visit reports, the SPARC team noted a need for greater engagement of frontline staff, which many hospitals approached as part of their implementation approach. Interviewees noted that their providers and frontline staff were engaged in their selected intervention efforts, though there were some challenges. As implementation progressed, interviewees described education efforts as critical in promoting engagement and participation of physicians, other providers and frontline staff. Interviewees noted that active participation and engagement from executive leadership promoted a culture of CDI prevention and reduction within the hospitals and helped hospital leads gain more traction with internal staff. With implementation of new testing guidelines, some hospital leads described initial negative responses and pushback among some physicians, which were primarily resolved using physician champions to conduct peer-to-peer discussions.
Dose received
Twelve hospitals participated in SPARC, nine of which completed the full SPARC implementation planning process and developed intervention implementation plans (see online supplemental appendix table 3). All participating hospitals had staff representation in at least one webinar, in-person meeting or training. Interviewees reported positive experiences with SPARC, highlighting external experts, multidisciplinary collaboration, an accountability and tracking structure, peer-to-peer learning and educational resources as programme and collaborative structure elements that aided with their implementation of selected interventions (table 3). In addition, real-time evaluation allowed SPARC to conduct continuous improvements to collaborative efforts and technical assistance activities, such as integrating additional peer-to-peer and cross-hospital learning activities through conducting an in-person meeting, a gap highlighted in early interviews.
Table 3.
Perspectives on the SPARC collaborative from interviews with eight SPARC intervention hospitals
| Interviewees’ perspectives | Quote |
| SPARC provided | |
…access to external subject matter experts that lent credibility to and validated existing efforts, and increased awareness around reduction in Clostridioides difficile infection.
|
‘The respect of [Johns Hopkins University, University of Maryland, Baltimore], the state health department… we’re pulling evidence-based recommendations from other people, it wasn’t just something we were doing to make our numbers look right.’ (Infection control interviewee) |
…an organising structure for multidisciplinary collaboration.
|
‘[SPARC] involved many people and departments: eg, lab, environmental cleaning, nursing. It wasn’t just Infection Control… this became everybody’s problem.’ (Infection control director) |
…opportunities for peer-to-peer exchange across hospitals.
|
‘I think the SPARC-le day* was excellent; super helpful. It was… really great to have such a strong [environmental cleaning] team do the fluorescent gel demonstration.’ (Infection control director) |
…a structure for tracking progress and accountability.
|
‘[SPARC]… keeps us in line to focus on these interventions as a team. Now that we’re putting it down on paper, in black and white, it holds us accountable.’ (Infection control director) |
…preintervention site visits, which renewed momentum for C. difficile reduction and highlighted unknown issues.
|
‘We thought our staff and providers were doing a good job… when [SPARC] did observations on the unit, we found that we did have a little bit of a problem.’ (Nurse) |
Other SPARC resources (ie, webinars, trainings, tools) had mixed utility and limited reach.
|
‘The webinars were a helpful reinforcement tool for frontline staff who participate.’ (Infection control director) |
Once positive gains were achieved, engagement in SPARC decreased.
|
‘I don't have time to do this anymore. Big things† have come along and that’s what my focus is going to be.’ (Infection control interviewee) |
*SPARC-le was an in-person event focused on sharing best practices, challenges and lessons learnt in infection prevention and environmental cleaning to prevent C. difficile.
†Follow-up interviews coincided with the beginning of the COVID-19 pandemic, to which the respondent was referring.
SPARC, Statewide Prevention and Reduction of C. difficile.
Fidelity
In selecting interventions, hospitals identified those that were feasible given their hospital’s existing infrastructure, timeline, resources and available manpower. Interviewees reported identifying ‘low-hanging fruit’ for immediate focus, and then working on longer term approaches that may require time, education or culture change. Additionally, some interviewees reported choosing interventions based on which ones would have the greatest impact on their CDI rates. Some interviewees noted delays when implementing new interventions that were time intensive. Time is a valuable resource for hospital staff juggling multiple priorities. Some interventions like tracking and sharing data internally took longer to implement, including developing dashboards and reports on isolation and infection; ‘loose stool’ reports that compile all instances of patient loose stools that had been documented by clinicians in the electronic health record; lists of all tests ordered to be reviewed by the infection control team; reports on antibiotic prescriptions and appropriateness of these prescriptions; reports of who bypasses pop-ups and alerts; and push alerts for C. difficile positive cases for root cause analyses. When implemented, internal tracking and reporting helped hospitals assess their progress and identify areas of need for more targeted education efforts. Changes to electronic health records were longer term goals, in part because they required more intensive information technology support.
Context
Interviewees reported that staffing and resource constraints, combined with staffs’ competing priorities, made engagement difficult. Staff turnover, particularly in environmental cleaning, made maintaining consistency laborious. Electronic health record modifications and complex data extraction required significant expertise, creating administrative burden or delays. By offering expertise and guidance, SPARC partially alleviated these constraints, particularly for smaller hospitals with limited capacity. Optimising environmental cleaning and personal protective equipment usage required education, culture change and staff buy-in. Staff resistance to changes often was due to lack of education on testing guidelines, concerns about patient safety and desire for autonomy. Environmental cleaning staff also experienced difficulties following proper hand hygiene, personal protective equipment and room cleaning protocols when families and patients were in the room. Active participation and engagement from executive leadership made C. difficile prevention an institutional priority. This, coupled with peer-to-peer education (eg, staff champions) and multidisciplinary collaboration, led to increased staff awareness, buy-in and compliance with protocols.
Discussion
A state-wide QI collaborative allowed the state to reach the US Department of Health and Human Services’ 30% C. difficile reduction goal. SPARC intervention hospitals had 45% greater C. difficile reduction compared with control hospitals.28 SPARC enhanced hospitals’ in-house capacity and lent credibility to hospital C. difficile reduction efforts, particularly for engaging executive leadership. Self-led and expert-led assessments offered objective data on current practices and areas for improvement and allowed hospitals to tailor interventions. While prior efforts targeted more narrow domains,29 SPARC’s multifaceted interventions based on evidence-based approaches addressed multiple contributing factors leading to C. difficile infection. The collaborative was designed to be flexible and allowed hospitals to select interventions that they believed would work best within their context.
Site visits prompted a team-based structure across otherwise siloed staff. SPARC also offered peer-to-peer opportunities through webinars and in-person meetings. A prior systematic review of 12 QI collaboratives found that participants value peer-to-peer learning opportunities.8 SPARC also provided a framework for accountability through intervention implementation plans and monthly follow-up calls. As hospitals progressed and priorities shifted, continuous reporting was perceived as burdensome. Future collaboratives should be flexible in number of needed touchpoints.
Hospitals fostered sustainability by aligning with existing QI efforts and promoting changes to hospital-wide protocols, highlighting the importance of C. difficile reduction. Hospitals approached new efforts by establishing new norms; intervention efforts were ‘hard wired’ into hospital operations as updated diagnostic guidelines, documentation protocols or policies. This structure may have impact outside of CDI reduction, for example, general stewardship training for pharmacists. Furthermore, evaluating hospital efforts at multiple time points provided opportunities for the SPARC team to address in real-time cross-cutting challenges noted by the hospitals. SPARC team incorporated this feedback into decision-making on priority webinar topics and resource structures (eg, developing an in-person meeting to facilitate peer-to-peer exchange). However, due to the COVID-19 pandemic, some additional planned events and webinars around these common challenges did not come to fruition.
Strengths and limitations of the evaluation
While literature assessing C. difficile reduction uses only quantitative data,5 this evaluation’s mixed-methods approach enabled assessment of changes in hospitals’ C. difficile rates while providing qualitative context around implementation efforts. Multiple data collection points allowed SPARC to adjust efforts based on feedback to be more responsive to hospital needs. Furthermore, the evaluation included perspectives from a variety of roles, including those not directly involved with SPARC.
This evaluation has several limitations. As hospitals with the highest C. difficile rates were invited to participate, selection bias may impact the extent of reduction observed in SPARC intervention hospitals compared with control hospitals. Unlike other hospital-acquired infection collaboratives with mandated participation,30 intervention hospitals may have already been motivated to reduce C. difficile. However, using the TAP strategy, by focusing intensive reduction efforts where most needed, Maryland successfully reached (and surpassed) the 2020 state-wide target of 0.7 by December 2019. This approach has previously demonstrated significant decreases in C. difficile rate trends compared with a system-wide deployment.6 Given hospitals had existing hospital-acquired infection initiatives prior to SPARC, improvements in C. difficile reduction cannot be solely attributed to SPARC. However, statistical methods showed acceleration of C. difficile reductions post-SPARC, and a steeper decline compared with control hospitals in the same period.
A common limitation of bundled interventions in QI collaboratives is the inability to identify the contribution of each individual intervention, and was also true for SPARC. SPARC was intentionally flexible in collaborating with participating hospitals in selection of interventions that the hospital leads felt would work best within their hospitals. The effect of each individual intervention was less of a priority; the collaborative focused instead on equipping hospitals with access to evidence-based strategies and technical assistance support in implementing those strategies. While we did not collect data on the extent to which hospitals implemented each selected intervention within their intervention implementation plans, C. difficile diagnostic stewardship was commonly selected by participating hospitals and a reduction in testing as part of diagnostic stewardship interventions may have contributed to reduced CDI rates among participating hospitals.
Conclusion
The core of the federal antimicrobial resistance response31 is to support local and regional efforts. Supported by CDC funding and leveraging a strong public health–academic partnership,4 32 SPARC is a successful example of these efforts. SPARC helped participating hospitals and the state experience significant reductions in C. difficile rates. Future collaboratives should consider various key elements: providing hospitals access to independent, external experts; conducting preintervention assessments to understand hospitals’ strengths and needs; offering a menu of intervention options with flexibility to align with existing hospitals’ capacity and workflow; serving as an organising structure for multidisciplinary collaboration within and across hospitals, allowing peer-to-peer education on best practices; encouraging data collection, reporting and sharing to track progress and target education efforts for real-time process improvements; and maintaining flexibility in documentation requirements to minimise reporting burden. This collaborative is an example of a successful state-wide public health–academic partnership which could be replicated in other states and to other healthcare-associated infections beyond C. difficile.
Acknowledgments
The SPARC team would like to acknowledge all participating acute care hospitals in Maryland for their efforts as part of the collaborative and for participating in interviews for the qualitative assessment. The authors would also like to acknowledge Emily Sanders, Elizabeth Moriarty and Meagan Robichaud for their support with qualitative data collection efforts.
Footnotes
Twitter: @ClareRock1, @katesabol
Contributors: CR and SL accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. Coauthors Y-JH, RP, PSU, CR, SL and PD made substantial contributions to the design, analysis, interpretation of data and drafting of the manuscript. DB, JB, SC, KD, VF, ADH, EH, SK, LLM, AMM, DJM and RB made substantial contributions to the design and interpretation of data, and revised the manuscript critically for important intellectual content.
Funding: This collaborative was supported by the Centers for Disease Control and Prevention (CDC) Epidemiology and Laboratory Capacity (ELC) funding (CDC grant funding 5 NU50CK000506-02-00).
Disclaimer: The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: SC reports consulting fees from Basilea and Theravance for work on external infection adjudication committees. KC reports investigator-initiated study funds from Merck & Co and served as a speaker for GenMark Diagnostics. LLM reports research funds from Clorox.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This work was deemed a public health practice and QI project and did not require Institutional Review Board (IRB) approval per Maryland Department of Health guidelines. The NORC IRB granted a certificate of exemption as not human subjects research.
References
- 1. CDC . Antibiotic-Resistant germs: new threats. centers for disease control and prevention, 2021. Available: https://www.cdc.gov/drugresistance/biggest-threats.html [Accessed 29 Oct 2021].
- 2. Targets & Metrics | health.gov. Available: https://health.gov/our-work/health-care-quality/health-care-associated-infections/targets-metrics [Accessed 29 Oct 2021].
- 3. Current HAI progress report | HAI | CDC, 2021. Available: https://www.cdc.gov/hai/data/portal/progress-report.html [Accessed 29 Oct 2021].
- 4. Prevention Epicenters program | CDC, 2019. Available: https://www.cdc.gov/hai/epicenters/index.html [Accessed 29 Oct 2021].
- 5. Rizzo KR, Yi SH, Garcia EP, et al. Reduction in Clostridium difficile infection rates following a multifacility prevention initiative in Orange County, California: A controlled interrupted time series evaluation. Infect Control Hosp Epidemiol 2019;40:872–9. 10.1017/ice.2019.135 [DOI] [PubMed] [Google Scholar]
- 6. White KA, Soe MM, Osborn A, et al. Implementation of the Targeted Assessment for Prevention Strategy in a healthcare system to reduce Clostridioides difficile infection rates. Infect Control Hosp Epidemiol 2020;41:295–301. 10.1017/ice.2019.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. AHRQ . Implementing a State-Level quality improvement collaborative: a resource guide from the Medicaid network for evidence-based treatment (MEDNET). AHRQ Publication No. 14(15)-0064-EF, 2021. [Google Scholar]
- 8. Burton RA, Peters RA, Devers KJ. Perspectives on implementing quality improvement Collaboratives effectively: qualitative findings from the CHIPRA quality demonstration grant program. Jt Comm J Qual Patient Saf 2018;44:12–22. 10.1016/j.jcjq.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 9. Clostridioides Difficile . CDC Nebraska Infection Control Assessment & Promotion Program. Available: https://icap.nebraskamed.com/initiatives-2/clostridioides-difficile/ [Accessed 29 Oct 2021].
- 10. Clostridium difficile campaign | IDPH. Available: https://www.dph.illinois.gov/topics-services/prevention-wellness/patient-safety-quality/cdiff-campaign [Accessed 29 Oct 2021].
- 11. Squire | Squire 2.0 guidelines. Available: http://www.squire-statement.org/index.cfm?fuseaction=page.viewPage&pageID=471 [Accessed 29 Oct 2021].
- 12. The targeted assessment for prevention (TAP) strategy | HAI | CDC, 2021. Available: https://www.cdc.gov/hai/prevent/tap.html [Accessed 29 Oct 2021].
- 13. Blanco N, Robinson GL, Heil EL, et al. Impact of a C. difficile infection (CDI) reduction bundle and its components on CDI diagnosis and prevention. Am J Infect Control 2021;49:319–26. 10.1016/j.ajic.2020.10.020 [DOI] [PubMed] [Google Scholar]
- 14. Friedland AE, Brown S, Glick DR, et al. Use of computerized clinical decision support for diagnostic stewardship in Clostridioides difficile testing: an academic Hospital quasi-experimental study. J Gen Intern Med 2019;34:31–2. 10.1007/s11606-018-4659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco N, O'Hara LM, Robinson GL, et al. Health care worker perceptions toward computerized clinical decision support tools for Clostridium difficile infection reduction: a qualitative study at 2 hospitals. Am J Infect Control 2018;46:1160–6. 10.1016/j.ajic.2018.04.204 [DOI] [PubMed] [Google Scholar]
- 16. Rock C, Small BA, Hsu Y-J, et al. Evaluating accuracy of sampling strategies for fluorescent gel monitoring of patient room cleaning. Infect Control Hosp Epidemiol 2019;40:794–7. 10.1017/ice.2019.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizusawa M, Small BA, Hsu Y-J, et al. Prescriber behavior in Clostridioides difficile testing: a 3-Hospital diagnostic stewardship intervention. Clin Infect Dis 2019;69:2019–21. 10.1093/cid/ciz295 [DOI] [PubMed] [Google Scholar]
- 18. Rock C, Maragakis LL. Diagnostic stewardship for Clostridiodes difficile testing: from laxatives to diarrhea and beyond. Clin Infect Dis 2020;71:1479–80. 10.1093/cid/ciz982 [DOI] [PubMed] [Google Scholar]
- 19. Chiotos K, Rock C, Schweizer ML, et al. Current infection prevention and antibiotic stewardship program practices: a survey of the Society for healthcare epidemiology of America (SheA) research network (SRN). Infect Control Hosp Epidemiol 2019;40:1046–9. 10.1017/ice.2019.172 [DOI] [PubMed] [Google Scholar]
- 20. Rock C, Xie A, Andonian J, et al. Evaluation of environmental cleaning of patient rooms: impact of different fluorescent gel markers. Infect Control Hosp Epidemiol 2019;40:100–2. 10.1017/ice.2018.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Werzen A, Thom KA, Robinson GL, et al. Comparing brief, covert, directly observed hand hygiene compliance monitoring to standard methods: a multicenter cohort study. Am J Infect Control 2019;47:346–8. 10.1016/j.ajic.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fabre V, Markou T, Sick-Samuels A, et al. Impact of Case-Specific Education and Face-to-Face Feedback to Prescribers and Nurses in the Management of Hospitalized Patients With a Positive Clostridium difficile Test. Open Forum Infect Dis 2018;5:ofy226. 10.1093/ofid/ofy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dudeck MA, Weiner LM, Malpiedi PJ. Risk adjustment for healthcare Facility-Onset C. difficile and MRSA bacteremia Laboratory-identified event reporting in NHSN. 9. CDC, 2012. [Google Scholar]
- 24. The nhsn standardized infection ratio (SIR), 2021. Available: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-SIR-guide.pdf [Accessed 29 Oct 2021].
- 25. FAQs: Multidrug-Resistant Organism & Clostridioides difficile Infection (MDRO & CDI) | NHSN | CDC, 2021. Available: https://www.cdc.gov/nhsn/faqs/faq-mdro-cdi.html [Accessed 29 Oct 2021].
- 26. Steckler AB, Linnan L, Israel BA. Process evaluation for public health interventions and research. Wiley, 2014. [Google Scholar]
- 27. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276–82. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boly FJ, Reske KA, Kwon JH. The Role of Diagnostic Stewardship in Clostridioides difficile Testing: Challenges and Opportunities. Curr Infect Dis Rep 2020;22:7. 10.1007/s11908-020-0715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon SL, Plisko JD, Wittig SM, et al. Reducing environmental surface contamination in healthcare settings: a statewide collaborative. Am J Infect Control 2018;46:e71–3. 10.1016/j.ajic.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 30. Massachusetts coalition for the prevention of medical errors. Available: http://www.macoalition.org/cdiff-programs.shtml [Accessed 29 Oct 2021].
- 31. CDC . Antibiotic resistance is a national priority. centers for disease control and prevention, 2020. Available: https://www.cdc.gov/drugresistance/us-activities.html [Accessed 29 Oct 2021].
- 32. CDC - ELC Cooperative Agreement - DPEI - NCEZID, 2021. Available: https://www.cdc.gov/ncezid/dpei/epidemiology-laboratory-capacity.html [Accessed 29 Oct 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2021-014014supp001.pdf (76.8KB, pdf)
Data Availability Statement
No data are available.