Abstract
Specific-pathogen-free pigs were inoculated with one of two hepatitis E viruses (HEV) (one recovered from a pig and the other from a human) to study the relative pathogenesis of the two viruses in swine. Fifty-four pigs were randomly assigned to three groups. Seventeen pigs in group 1 served as uninoculated controls, 18 pigs in group 2 were intravenously inoculated with the swine HEV recovered from a pig in the United States, and 19 pigs in group 3 were intravenously inoculated with the US-2 strain of human HEV recovered from a hepatitis patient in the United States. Two to four pigs from each group were necropsied at 3, 7, 14, 20, 27, or 55 days postinoculation (DPI). Evidence of clinical disease or elevation of liver enzymes or bilirubin was not found in pigs from any of the three groups. Enlarged hepatic and mesenteric lymph nodes were observed in both HEV-inoculated groups. Multifocal lymphoplasmacytic hepatitis was observed in 9 of 17, 15 of 18, and 16 of 19 pigs in groups 1 to 3, respectively. Focal hepatocellular necrosis was observed in 5 of 17, 10 of 18, and 13 of 19 pigs in groups 1 to 3, respectively. Hepatitis lesions were very mild in group 1 pigs, mild to moderate in group 2 pigs, and moderate to severe in group 3 pigs. Hepatic inflammation and hepatocellular necrosis peaked in severity at 20 DPI and were still moderately severe at 55 DPI in the group inoculated with human HEV. Hepatitis lesions were absent or nearly resolved by 55 DPI in the swine-HEV-inoculated pigs. All HEV-inoculated pigs seroconverted to anti-HEV immunoglobulin G. HEV RNA was detected by reverse transcriptase PCR in feces, liver tissue, and bile of pigs in both HEV-inoculated groups from 3 to 27 DPI. Based on evaluation of microscopic lesions, the US-2 strain of human HEV induced more severe and persistent hepatic lesions in pigs than did swine HEV. Pig livers or cells from the livers of HEV-infected pigs may represent a risk for transmission of HEV from pigs to human xenograft recipients. Since HEV was shed in the feces of infected pigs, exposure to feces from infected pigs represents a risk for transmission of HEV, and pigs should be considered a reservoir for HEV.
Hepatitis E virus (HEV) is the leading cause of enterically transmitted non-A, non-B hepatitis in people in many developing countries (21, 28, 30). Transmission is thought to be primarily by the fecal-oral route, and waterborne epidemics are characteristic of hepatitis E (1, 28, 30). Clinical disease due to HEV infection is rarely diagnosed in industrialized countries, and most cases of HEV infection in industrialized countries occur in people who have traveled to regions where the disease is endemic (10, 21, 28, 30). Clinical cases occur predominantly in developing countries in Asia, Africa, and Mexico (1, 2, 28, 30). However, sporadic cases of acute hepatitis E in people in the United States and other industrialized countries have recently been reported (7, 8, 11, 18, 20, 22, 31, 32, 42). Hepatitis E generally affects young adults and usually is not fatal, although mortality rates of up to 20% have been reported for pregnant women (28, 30). In industrialized countries, where hepatitis E was thought to be nonendemic, anti-HEV antibodies have also been found in a significant proportion of healthy individuals (12, 16, 19, 21, 29, 33). Diagnosis of HEV infection is based on detection of the virus by reverse transcriptase PCR (RT-PCR) and/or detection of anti-HEV antibodies by serology. HEV was recently declassified from the Caliciviridae family and remains unclassified (14, 17, 27).
In 1997, a novel virus closely related to human HEV was discovered in pigs, characterized, and designated swine HEV (23). Subsequently, two strains of human HEV (US-1 and US-2) isolated from U.S. patients with acute hepatitis were characterized (7, 8, 31). The two U.S. strains of HEV share ≥97% amino acid identity with swine HEV in open reading frames 1 and 2 (ORF1 and ORF2, respectively) but are genetically distinct from other known strains of HEV worldwide. In Taiwan, Hsieh et al. (12) isolated another new strain of swine HEV from a pig. This Taiwanese strain of swine HEV shares 97.3% nucleotide sequence identity with a human strain of HEV isolated from a retired farmer in Taiwan but is distinct from the U.S. strain of swine HEV. More recently, Wang et al. (40) found that a Chinese strain (T1) of HEV recovered from a patient in mainland China is related to the Taiwanese swine HEV and human HEV strains reported by Hsieh et al. (12) and that they form a distinct genotype. Also in Taiwan, Wu et al. (41) identified yet another strain of swine HEV isolated from Taiwanese pigs. This strain of swine HEV shares 84 to 95% nucleotide sequence identity with Taiwanese human strains of HEV (41) which are related to the G9 and G20 Chinese strains of HEV (40).
The exact role of swine in the transmission of HEV among humans remains unclear. Serologic surveys done to date suggest that swine HEV is widespread in the midwestern U.S. swine population as well as in other regions of the world (5, 6, 12, 23, 26, 34, 41). Recently, we experimentally infected pigs with swine HEV (24) or with the US-2 strain of human HEV (25). The limited scope of that study did not allow us to define the temporal pathogenesis of infection of swine with these HEV strains, but clinical disease was not seen. In a reciprocal experiment, we experimentally infected nonhuman primates with the swine HEV (25) or the US-2 strain of human HEV (R. H. Purcell et al., unpublished data). The HEV-infected primates developed mild hepatitis and slightly elevated liver enzymes. The present comparative pathogenesis study was designed to gain a better understanding of the pathogenesis of HEV infection in pigs and to evaluate the usefulness of swine as an animal model of hepatitis E.
MATERIALS AND METHODS
Virus inocula.
The swine HEV used in this study was originally recovered from a pig in Illinois (23). An infectious pool of swine HEV was subsequently prepared as a 10% suspension of feces collected from a specific-pathogen-free (SPF) pig experimentally infected with swine HEV. This infectious pool was titrated in SPF pigs and was found to have a titer of 104.5 50% pig infectious doses (PID50) per ml (25). This standard infectious pool of swine HEV was used as one inoculum in this study. The US-2 strain of human HEV (kindly provided by Isa Mushahwar, Abbott Laboratories, North Chicago, Ill.) used in this study was originally recovered from a hepatitis patient in Tennessee and transmitted to cynomolgus monkeys (8, 31). An infectious pool of the US-2 strain of human HEV was prepared as a 10% suspension of feces collected from a rhesus monkey experimentally infected with the US-2 strain (Purcell et al., unpublished data). This infectious pool was titrated in rhesus monkeys and was found to have an infectious titer of 105 50% monkey infectious doses (MID50) per 0.5 ml (Purcell et al., unpublished data). This standard pool of the US-2 strain of human HEV was diluted to an infectious titer of 104.5 MID50/ml (equivalent to that of the swine-HEV inoculum) and used as the other inoculum in this study.
Animals.
Fifty-four crossbred, 3- to 4-week-old, SPF pigs (Sus scrofa domestica) were randomly assigned to three groups. All animals were confirmed to be negative for anti-HEV antibodies by an enzyme-linked immunosorbent assay (ELISA) (23, 26) prior to inoculation. Seventeen pigs in group 1 served as uninoculated controls. Eighteen pigs in group 2 were each intravenously (i.v.) inoculated with 104.5 PID50 of swine HEV. Nineteen pigs in group 3 were each i.v. inoculated with 104.5 MID50 of the US-2 strain of human HEV. Groups 1 and 2 were housed in a BL-2 facility, and group 3 was housed in a BL-3 facility. All pigs were allowed access to food and drinking water ad libitum.
Clinical signs and serum chemistry profiles.
Clinical signs and rectal temperature were recorded every 2 to 3 days through 55 days postinoculation (DPI). Blood was collected prior to inoculation and weekly thereafter for serum liver chemistry profiles (aspartate aminotransferase, gamma-glutamyl transferase, sorbital dehydrogenase, and bilirubin) on all pigs throughout the 55 days of the experiment. Feces and blood were also collected from all pigs at 0, 3, and 7 DPI and weekly thereafter through 55 DPI for detection of HEV RNA by RT-PCR and of anti-HEV immunoglobulin G (IgG) by ELISA. Fecal samples were collected directly from the rectum with a Dacron fiber tipped swab and then suspended in phosphate-buffered saline buffer and stored at −80°C until use.
Evaluation of gross and microscopic lesions.
Two to four randomly selected pigs from each group were necropsied at 3, 7, 14, 20, 27, or 55 DPI. Tissues examined grossly and collected for microscopic examination included brain, tonsil, mandibular salivary gland, lung, heart, stomach, liver, gall bladder, pancreas, duodenum, jejunum, ileum, colon, kidney, adrenal gland, urinary bladder, and skeletal muscle. One section each of the right lateral lobe, right medial lobe, left medial lobe, quadrate lobe, and caudate process of the liver was collected and processed for histologic examination. Two sections of the left lateral lobe were collected and examined. Tissues for histologic examination were fixed in 10% neutral buffered formalin, routinely processed, sectioned at a thickness of 6 μm, and stained with hematoxylin and eosin.
Liver sections were blindly examined microscopically and assigned a score for severity of lymphoplasmacytic hepatic lesions. Scores ranged from 0 to 4, where 0 is no inflammation, 1 is 1 to 2 focal lymphoplasmacytic infiltrates/10 hepatic lobules, 2 is 2 to 5 focal infiltrates/10 hepatic lobules, 3 is 6 to 10 focal infiltrates/10 hepatic lobules, and 4 is >10 focal infiltrates/10 hepatic lobules.
Detection of HEV RNA by RT-PCR.
RT-PCR was performed essentially as previously described (24, 25) to detect viral RNA in feces, serum, liver tissue, and bile samples. Total RNA was extracted from 100 μl of each sample (serum, 10% fecal suspension, 10% liver homogenate, or bile) with TriZol reagent (GIBCO/BRL, Gaithersburg, Md.). All samples were tested by a nested PCR with primers located in the putative capsid gene (ORF2) region (24, 25). The first-round PCR produced an expected fragment of 404 bp with the forward primer F1 (5′-AGCTCCTGTACCTGATGTTGACTC-3′) and the reverse primer R1 (5′-CTACAGAGCGCCAGCCTTGATTGC-3′). For the second-round PCR, the forward primer F2 (5′-GCTCACGTCATCTGTCGCTGCTGG-3′) and the reverse primer R2 (5′-GGGCTGAACCAAAATCCTGACATC-3′) produced an expected fragment of 266 bp. These two sets of primers were designed to amplify both swine HEV and the US-2 strain of human HEV (24, 25). Total RNA was reverse transcribed with the R1 reverse primer and SuperScript II reverse transcriptase (GIBCO/BRL) at 42°C for 1 h. The resulting cDNA was amplified by PCR with AmpliTaq Gold DNA polymerase. The PCR was carried out for 39 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1.5 min, followed by a final incubation at 72°C for 7 min. The second-round PCRs were performed with parameters similar to those described above with 10 μl of the first-round PCR mixture as the template. The amplified PCR products were examined by gel electrophoresis.
Detection of anti-HEV antibodies.
The standard ELISA for anti-HEV antibody in pigs was performed essentially as previously described (23, 24, 26). We used a purified 55-kDa truncated recombinant capsid protein derived from ORF2 of the human HEV strain Sar-55 as the antigen. Peroxidase-labeled goat anti-swine IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was used as the secondary antibody. All serum samples were tested in duplicate. Preimmune and hyperimmune (anti-HEV antibody-positive) swine sera were used as negative and positive controls, respectively.
Statistical analysis.
The presence or absence of hepatic lesions was analyzed by Fisher's exact test. Lymphoplasmacytic hepatitis lesion scores were analyzed by logistic regression. The lesion score was the dependent variable and, for the analysis, was considered to be an ordinal variable. Explanatory variables included infection group (nominal variable) and the DPI (ordinal variable). A P value of less than 0.05 was considered significant in all analyses.
RESULTS
Clinical evaluation.
Evidence of clinical disease or elevation of liver enzymes or bilirubin was not found in pigs from any of the groups (data not shown).
Gross and microscopic lesions.
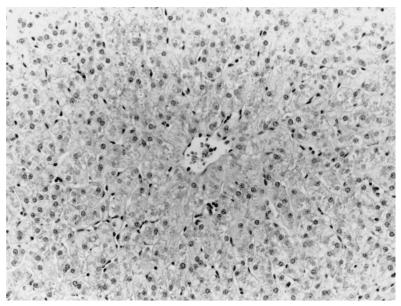
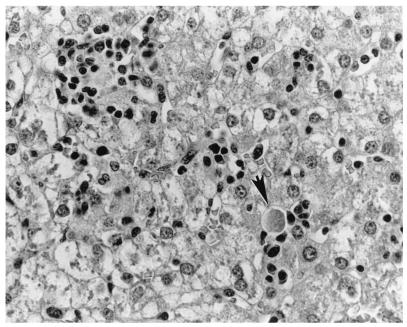
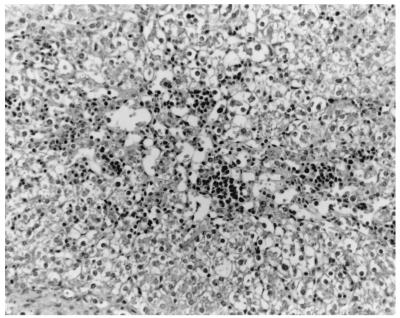
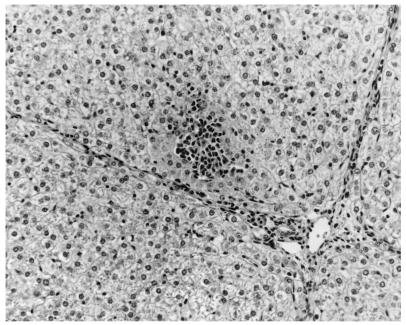
The only gross lesions observed were mildly to moderately enlarged hepatic and mesenteric lymph nodes from 7 to 55 DPI in the groups inoculated with swine or human HEV. Data on microscopic liver lesions are summarized in Tables 1 to 2 and Figures 1 to 4. Multifocal lymphoplasmacytic hepatitis was observed in 9 of 17 control pigs, 15 of 18 swine-HEV-inoculated pigs, and 16 of 19 human-HEV-inoculated pigs. The difference in the numbers of pigs with lymphoplasmacytic hepatitis was significant between the control and human-HEV-inoculated group (P = 0.047) but not between the control and swine-HEV-inoculated group (P = 0.057). Compared to those in controls, lymphoplasmacytic hepatic lesions were significantly more severe in pigs inoculated with human HEV (P = 0.005) or with swine HEV (P = 0.023) than in the controls. Although overall mean hepatitis scores were higher in the human-HEV-inoculated group (1.7) than in the swine-HEV-inoculated group (1.2), the differences were not significant. Focal hepatocellular necrosis was observed in 5 of 17, 10 of 18, and 13 of 19 of the pigs in groups 1 to 3, respectively. Hepatocellular necrosis was significantly (P = 0.022) more frequent in the human-HEV-inoculated group than in the controls. Although there were more pigs with hepatocellular necrosis in the swine-HEV-inoculated group (10 of 18 pigs) than in the control group (5 of 17 pigs), the differences were not significant (P = 0.111). In general, hepatic lesions were very mild in group 1 pigs (controls), mild to moderate in group 2 pigs (swine HEV), and moderate to severe in group 3 pigs (human HEV). Hepatic inflammation and hepatocellular necrosis peaked in severity at 20 DPI and were still moderately severe at 55 DPI in the group inoculated with human HEV (data not shown). Hepatocellular swelling and vacuolation (Fig. 3) were observed in both HEV-inoculated groups from 7 to 27 DPI. Hepatic lesions were absent or nearly resolved in the swine-HEV-inoculated pigs by 55 DPI. At each data point in time (3, 7, 14, 20, 27, and 55 DPI), the types and levels of severity of liver lesions within each infected group were similar whether the tissue sample was positive or negative for HEV nucleic acids by RT-PCR. For example, 3 of 3 pigs in the swine-HEV-inoculated group necropsied at 14 DPI had mild lymphoplasmacytic hepatitis, mild vacuolar swelling of hepatocytes, and low numbers of necrotic hepatocytes yet only 1 of 3 pigs was positive by RT-PCR for HEV in liver and 3 of 3 pigs were positive for HEV in bile. Lesions in other tissues were unremarkable.
TABLE 1.
Hepatitis lesions in control and HEV-inoculated pigs
| Lesion | Group | No. of pigs with lymphoplasmacytic hepatitis or hepatocellular necrosis lesions/no. of pigs examined on DPI:
|
Total no. of pigs with lesions/total no. of pigs examined | Mean score | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 20 | 27 | 55 | ||||
| Lymphoplasmacytic hepatitisa | Control | 1/3 (0.3) | 3/3 (1.0) | 2/3 (1.3) | 2/3 (0.7) | 0/3 (0) | 1/2 (0.5) | 9/17c | 0.6g |
| Swine HEV inoculated | 3/3 (1.3) | 3/3 (1.7) | 3/3 (1.0) | 3/3 (1.7) | 2/3 (0.7) | 1/3 (0.3) | 15/18d | 1.2h | |
| Human HEV inoculated | 1/3 (0.3) | 3/3 (1.0) | 3/3 (2.7) | 3/3 (3.3) | 2/3 (0.7) | 4/4 (2.3) | 16/19 | 1.7 | |
| Hepatocellular necrosisb | Control | 0/3 | 2/3 | 2/3 | 0/3 | 0/3 | 1/2 | 5/17e | |
| Swine HEV inoculated | 3/3 | 2/3 | 3/3 | 2/3 | 0/3 | 0/3 | 10/18f | ||
| Human HEV inoculated | 0/3 | 3/3 | 3/3 | 3/3 | 1/3 | 3/4 | 13/19 | ||
Lymphoplasmacytic hepatitis scores: 0 = no inflammation, 1 = 1 to 2 focal lymphoplasmacytic infiltrates/10 hepatic lobules, 2 = 3 to 5 focal infiltrates/10 hepatic lobules, 3 = 6 to 10 focal infiltrates/10 hepatic lobules, and 4 = >10 focal infiltrates/10 hepatic lobules.
Hepatocellular necrosis or apoptosis characterized by individual hepatocytes with eosinophilic cytoplasms and fragmented or absent nuclei.
P = 0.057 versus value obtained for swine-HEV-inoculated group and P = 0.047 versus value obtained for human-HEV-inoculated group.
P = 0.643 versus value obtained for human-HEV-inoculated group.
P = 0.111 versus value obtained for swine-HEV-inoculated group and P = 0.022 versus value obtained for human-HEV-inoculated group.
P = 0.320 versus value obtained for human-HEV-inoculated group.
P = 0.023 versus value obtained for swine-HEV-inoculated group and P = 0.005 versus human-HEV-inoculated group.
P = 0.927 versus value obtained for human-HEV-inoculated group.
TABLE 2.
HEV RNA in the feces, serum, liver, and bile of control and HEV-inoculated pigs
| Inoculum | Sample | No. of positive samples/total no. of samples tested on DPI:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 20 | 27 | 35 | 42 | 55 | ||
| None | ||||||||||
| Feces | 0/17 | NTa | NT | NT | NT | NT | NT | NT | 0/2 | |
| Serum | 0/17 | NT | NT | NT | NT | NT | NT | NT | 0/2 | |
| Liver | NT | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | NT | NT | 0/2 | |
| Bile | NT | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | NT | NT | 0/2 | |
| Swine HEV | ||||||||||
| Feces | 0/18 | NT | 15/15 | 12/12 | 4/9 | 1/6 | 0/3 | 0/3 | 0/3 | |
| Serum | 0/18 | NT | 9/15 | 8/12 | 1/9 | 0/6 | NT | NT | 0/3 | |
| Liver | NT | 2/3 | 3/3 | 1/3 | 1/3 | 0/3 | NT | NT | 0/3 | |
| Bile | NT | 3/3 | 3/3 | 3/3 | 2/3 | 1/3 | NT | NT | 0/3 | |
| Human HEV | ||||||||||
| Feces | 0/19 | NT | 16/16 | 13/13 | 9/10 | 3/7 | 2/4 | 0/4 | 0/4 | |
| Serum | 0/19 | NT | 15/16 | 13/13 | 5/10 | 0/7 | NT | NT | 0/4 | |
| Liver | NT | 3/3 | 3/3 | 3/3 | 2/3 | 2/3 | NT | NT | 0/4 | |
| Bile | NT | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | NT | NT | 0/4 | |
NT, samples not collected or tested on this DPI.
FIG. 1.
Liver of a sham-inoculated control pig (14 DPI) with rare lymphoplasmacytic infiltrates in hepatic sinusoids. Hematoxylin and eosin staining was performed.
FIG. 4.
Liver of the same pig as that shown in Fig. 3. Note the individual necrotic hepatocytes (arrow). Hematoxylin and eosin staining was performed.
FIG. 3.
Liver of a pig i.v. inoculated with HEV recovered from a human hepatitis patient in the U.S. There is severe lymphoplasmacytic and histiocytic hepatitis and severe vacuolar degeneration and swelling of hepatocytes at 14 DPI. Hematoxylin and eosin staining was performed.
Detection of HEV RNA.
Results of RT-PCR examination of feces, serum, bile, and liver specimens are summarized in Table 2. All pigs were negative for HEV RNA at 0 DPI, and control pigs remained negative throughout the experiment. HEV RNA was detected in the bile, liver, serum, and feces specimens of both swine-HEV- and human-HEV-inoculated pigs but not in those of control pigs (Table 2). HEV nucleic acids were detected by RT-PCR in the feces at 7 DPI in 100% (31 of 31 pigs) of the pigs inoculated with HEV (swine or human HEV). HEV RNA was detected in the bile of 100% (18 of 18 pigs) of the HEV-inoculated pigs (swine and human HEV) necropsied between 3 and 14 DPI. Similarly, HEV was detected in the livers of 100% (9 of 9 pigs) of the pigs inoculated with human HEV and necropsied between 3 and 14 DPI. Detection of HEV by RT-PCR in the livers of the swine-HEV-inoculated pigs was less successful. Swine HEV was detected in 67% (6 of 9) of the livers from the swine-HEV-inoculated pigs that were necropsied between 3 and 14 DPI; however, 100% of these pigs were positive for HEV nucleic acids in bile and feces. PCR products amplified from a selected bile sample of a swine-HEV-inoculated pig and from a selected bile sample from a human-HEV-inoculated pig were sequenced. Sequence analysis confirmed that the virus recovered from the inoculated pigs was the same virus as the virus in the inocula.
Serology.
All pigs were negative for anti-HEV antibodies prior to inoculation, and control pigs remained negative throughout the study. All pigs remained negative through 7 DPI. Anti-HEV antibody was detected in the sera of 1 of 14 pigs at 14 DPI, 5 of 11 pigs at 20 DPI, 5 of 8 pigs at 27 DPI, 4 of 5 pigs at 42 DPI, and 5 of 5 pigs at 55 DPI in the swine-HEV-inoculated group. Anti-HEV antibody was detected in the sera of 3 of 15 pigs at 14 DPI, 12 of 12 pigs at 20 DPI, 9 of 9 pigs at 27 DPI, 6 of 6 pigs at 42 DPI, and 6 of 6 pigs at 55 DPI in the human-HEV-inoculated group.
DISCUSSION
HEV infection in pigs appears to be widespread throughout the world. Antibodies to HEV are present in pigs in many industrialized countries, including the United States (23), Canada (26), Korea (26), Taiwan (12, 40), and Australia (5), and in pigs from countries where HEV is endemic such as Nepal (6), China (26), and Thailand (26). The high degree of genetic similarity between swine and human strains of HEV identified in the same geographic regions suggests that cross-species transmission may occur (8, 12, 25, 31, 41). Balayan et al. reported that Russian domestic swine could be experimentally infected with a central Asian strain of HEV isolated from a naturally infected patient (3). In that study, the infected swine developed jaundice (3). In the present study, we confirmed that SPF pigs are readily susceptible to infection with two strains of HEV recovered from a human and a pig, respectively. The US-2 strain of human HEV is known to be pathogenic in humans (7, 8, 31). However, in our study, experimental infection of pigs with either the U.S. human or U.S. swine strain of HEV failed to induce clinical disease in naïve growing pigs. Both strains of HEV replicated in growing pigs since the inoculated pigs seroconverted to anti-HEV antibody and HEV RNA was detected in the feces, bile, sera, and liver tissues of inoculated pigs. Therefore, pigs should be considered a reservoir for HEV and exposure to feces from infected pigs represents a risk for transmission of HEV to other pigs and possibly to other species, including humans.
In some control pigs, lymphoplasmacytic inflammation and rare focal necrotic hepatocytes were observed. Lesions characteristic of porcine circovirus infection (lymphoid depletion and granulomatous inflammation) were lacking in the lymphoid tissues and other organs. Porcine circovirus antigen was not detected by immunohistochemistry in liver sections of any of the control or HEV-inoculated pigs (data not shown). All control and HEV-inoculated pigs remained seronegative for porcine reproductive and respiratory syndrome virus and pseudorabies virus throughout the study (data not shown). The liver sections were blindly examined by a pathologist (P. G. Halbur) in the Iowa State University Veterinary Diagnostic Laboratory. Each year, thousands of pig livers from cases submitted for diagnosis of a variety of diseases (respiratory, enteric, central nervous system, etc.) are examined by pathologists at the Iowa State University Veterinary Diagnostic Laboratory. Based on this experience, mild lymphoplasmacytic infiltrates in hepatic sinusoids and the presence of rare necrotic or apoptotic hepatocytes are considered normal background changes for pig livers. The amount of inflammation and the number of necrotic hepatocytes in the HEV-inoculated pigs, especially those inoculated with human HEV, were above the normal threshold and considered to be evidence of mild viral hepatitis. Evaluation of microscopic lesions suggested that the US-2 strain of human HEV induced more severe and persistent hepatic lesions in pigs than did the swine HEV.
Overall, the liver lesions observed in the HEV-infected pigs were relatively mild. The exact sites of HEV replication are not known, but it is possible that HEV might replicate in tissues and organs other than the liver. Studies are under way to identify hepatic and possible extrahepatic sites of HEV replication by using immunohistochemistry, negative-strand RT-PCR, and in situ hybridization. The inoculation route used in this study was intravenous. However, fecal-oral exposure is thought to be the primary route of natural infection (28, 30). The infectious dose necessary for infection by the oral route is believed to be higher than that for infection by the intravenous route, based on a study of human HEV in nonhuman primates (35). The infectious titers of the standard swine and human HEV pools available may not be high enough to infect animals by the oral route. Nevertheless, additional research needs to be done to establish the oral route as the natural route of exposure and to study the pathogenesis of HEV infection in pigs following natural infection.
Since anti-HEV antibody is also detected in individuals from large U.S. cities (19, 33), where contact with pigs is uncommon, other sources or reservoirs of HEV are suspected. Recently, Karetnyi et al. (16) found that the anti-HEV antibody prevalence in Iowa patients with non-A, non-B, and non-C hepatitis (4.9%) and in field workers from the Iowa Department of Natural Resources (5.7%) was significantly higher than that in normal blood donors (2%; P < 0.05). This suggests that human populations with occupational exposure to wild animals may have increased risks of HEV infection. Anti-HEV antibody has been detected in many animal species, including monkeys, pigs, rodents, chickens, dogs, cows, sheep, and goats (6, 9, 12, 15, 21, 26, 34, 36, 37). In the United States, Kabrane-Lazizi et al. (15) found that about 44 to 90% of wild-caught rats from three U.S. states (Maryland, Hawaii, and Louisiana) were positive for anti-HEV antibody. Similarly, Favorov et al. (9) reported the detection of anti-HEV antibody among rodents trapped at multiple sites in the United States. The significance of HEV seropositivity in these animal species needs to be determined. The recent identification of numerous genetically distinct strains of HEV in several countries where hepatitis E is endemic (4, 13, 38, 39, 40) and nonendemic (8, 11, 12, 31, 32, 41, 42) has led to a hypothesis that these novel strains of human HEV may be of animal origin (22). The present study confirmed that one human-HEV strain (US-2 strain) was transmissible to pigs (25), although the clinical manifestations and course of disease were different from those observed in the single human reported to be infected with this strain (8, 18, 31).
There is increasing public health concern over the risk of inadvertent transmission of swine HEV from pig organs to human recipients during xenotransplantation (21). The data presented here indicated that pigs infected with HEV had microscopic evidence of liver damage. Therefore, pig livers or cells from the livers of HEV-infected pigs used in xenotransplantation may represent a risk for transmission of HEV from pigs to human xenograft recipients. Adequate screening of xenograft donor pigs for HEV infection is therefore recommended for xenotransplantation with pig organs.
In summary, the results from this study confirm that feces from HEV-infected pigs contain viable HEV, so swine feces may represent a risk for transmission of HEV. Our results suggest that swine may not be a perfect animal model for human HEV since there is a lack of clinical disease in infected pigs, at least with the strains of HEV we studied. However, the swine model will likely be useful to better understand some aspects of HEV pathogenesis and epidemiology. HEV should be considered along with porcine reproductive and respiratory syndrome virus, circovirus, and pseudorabies virus in the differential diagnosis of viral hepatitis in pigs.
FIG. 2.
Liver of a pig i.v. inoculated with HEV recovered from a U.S. pig. There is mild focal infiltration of lymphocytes, plasma cells, and macrophages and mild diffuse inflammation in hepatic sinusoids at 14 DPI. Hepatocytes are mildly swollen and vacuolated. Hematoxylin and eosin staining was performed.
ACKNOWLEDGMENTS
This study was supported by grants (to P.G.H.) from the National Pork Producers Council and the Iowa Livestock Health Advisory Council and by grants (to X.J.M.) from the National Institutes of Health (AI01653-01 and AI46505-01).
We thank Isa Mushahwar (Abbott Laboratories) for generously providing us with the US-2 strain of human HEV. We thank Doris Wong and Ron Engle for technical assistance with serological testing, Ryan Royer and Jeremy Bruna for animal care, and Prem Paul for technical advice and use of laboratory equipment. We also thank Brad Thacker for statistical analysis, G. Haqshenas for review of the manuscript, and Carles Rosell for review and consultation on liver histopathology.
REFERENCES
- 1.Arankalle V A, Chadha M S, Tsarev S A, Emerson S U, Risbud A R, Banerjee K, Purcell R H. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc Natl Acad Sci USA. 1994;91:3428–3432. doi: 10.1073/pnas.91.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arankalle V A, Tsarev S A, Chadha M S, Alling D W, Emerson S U, Banerjee K, Purcell R H. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 3.Balayan M S, Usmanov R K, Zamyatina D I, Karas F R. Brief report: experimental hepatitis E infection in domestic pigs. J Med Virol. 1990;32:58–59. doi: 10.1002/jmv.1890320110. [DOI] [PubMed] [Google Scholar]
- 4.Buisson Y, Grandadam M, Nicand E, Cheval P, Van Cuyck-Gandre H, Innis B, Rehel P, Coursaget P, Teyssou R, Tsarev S. Identification of a novel hepatitis E virus in Nigeria. J Gen Virol. 2000;81:903–909. doi: 10.1099/0022-1317-81-4-903. [DOI] [PubMed] [Google Scholar]
- 5.Chandler J D, Riddell M A, Li F, Love R J, Anderson D A. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet Microbiol. 1999;68:95–105. doi: 10.1016/s0378-1135(99)00065-6. [DOI] [PubMed] [Google Scholar]
- 6.Clayson E T, Innis B L, Myint K S, Narupiti S, Vaughn D W, Giri S, Ranabhat P, Shrestha M P. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am J Trop Med Hyg. 1995;53:228–232. doi: 10.4269/ajtmh.1995.53.228. [DOI] [PubMed] [Google Scholar]
- 7.De Groen P C. Hepatitis E in the United States: a case of “hog fever”? Mayo Clin Proc. 1997;72:1197–1198. doi: 10.1016/S0025-6196(11)63687-2. [DOI] [PubMed] [Google Scholar]
- 8.Erker J C, Desai S M, Schlauder G G, Dawson G J, Mushahwar I K. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J Gen Virol. 1999;80:681–690. doi: 10.1099/0022-1317-80-3-681. [DOI] [PubMed] [Google Scholar]
- 9.Favorov M O, Kosoy M Y, Tsarev S A, Childs J E, Margolis H S. Prevalence of antibody to hepatitis E virus among rodents in the United States. J Infect Dis. 2000;181:449–455. doi: 10.1086/315273. [DOI] [PubMed] [Google Scholar]
- 10.Herrera J L, Hill S, Shaw J, Fleenor M, Bader T, Wolfe M S. Hepatitis E among US travelers, 1989–1992. Morb Mortal Wkly Rep. 1993;42:1–4. [PubMed] [Google Scholar]
- 11.Hsieh S Y, Yang P Y, Ho Y P, Chu C M, Liaw Y F. Identification of a novel strain of hepatitis E virus responsible for sporadic acute hepatitis in Taiwan. J Med Virol. 1998;55:300–304. doi: 10.1002/(sici)1096-9071(199808)55:4<300::aid-jmv8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh S Y, Meng X J, Wu Y H, Liu S T, Tam A W, Lin D Y, Liaw Y F. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J Clin Microbiol. 1999;37:3828–3834. doi: 10.1128/jcm.37.12.3828-3834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang R, Nakazono N, Ishii K, Kawamata O, Kawaguchi R, Tsukada Y. Existing variations on the gene structure of hepatitis E virus strains from some regions of China. J Med Virol. 1995;47:303–308. doi: 10.1002/jmv.1890470403. [DOI] [PubMed] [Google Scholar]
- 14.Kabrane-Lazizi Y, Meng X J, Purcell R H, Emerson S U. Evidence that the genomic RNA of hepatitis E virus is capped. J Virol. 1999;73:8848–8850. doi: 10.1128/jvi.73.10.8848-8850.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabrane-Lazizi Y, Fine J B, Elm J, Glass G E, Higa H, Diwan A, Gibbs C J, Jr, Meng X J, Emerson S U, Purcell R H. Evidence for wide-spread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 16.Karetnyi Y V, Gilchrist M J, Naides S J. Hepatitis E virus infection prevalence among selected populations in Iowa. J Clin Virol. 1999;14:51–55. doi: 10.1016/s1386-6532(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 17.Koonin E V, Gorbalenya A E, Purdy M A, Rozanov M N, Reyes G R, Bradley D W. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc Natl Acad Sci USA. 1992;89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwo P Y, Schlauder G G, Carpenter H A, Murphy P J, Rosenblatt J E, Dawson G J, Mast E E, Krawczynski K, Balan V. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clin Proc. 1997;72:1133–1136. doi: 10.4065/72.12.1133. [DOI] [PubMed] [Google Scholar]
- 19.Mast E E, Kuramoto I K, Favorov M O, Schoening V R, Burkholder B T, Shapiro C N, Holland P V. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J Infect Dis. 1997;176:34–40. doi: 10.1086/514037. [DOI] [PubMed] [Google Scholar]
- 20.McCrudden R, O'Connell S, Farrant T, Beaton S, Iredale J P, Fine D. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut. 2000;46:732–733. doi: 10.1136/gut.46.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng X J. Zoonotic and xenozoonotic risks of hepatitis E virus. Infect Dis Rev. 2000;2:35–41. [Google Scholar]
- 22.Meng X J. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J Hepatol. 2000;33:842–845. doi: 10.1016/s0168-8278(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 23.Meng X J, Purcell R H, Halbur P G, Lehman J R, Webb D M, Tsareva T S, Haynes J S, Thacker B J, Emerson S U. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng X J, Halbur P G, Haynes J S, Tsareva T S, Bruna J D, Royer R L, Purcell R H, Emerson S U. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch Virol. 1998;143:1405–1415. doi: 10.1007/s007050050384. [DOI] [PubMed] [Google Scholar]
- 25.Meng X J, Halbur P G, Shapiro M S, Govindarajan S, Bruna J D, Mushahwar I K, Purcell R H, Emerson S U. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng X J, Dea S, Engle R E, Friendship R, Lyoo Y S, Sirinarumitr T, Urairong K, Wang D, Wong D, Yoo D, Zhang Y, Purcell R H, Emerson S U. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;58:297–302. [PubMed] [Google Scholar]
- 27.Pringle C. Minutes of the 27th International Committee on Taxonomy of Viruses Meeting. Arch Virol. 1998;143:1449–1459. [Google Scholar]
- 28.Purcell R H. Hepatitis E virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2831–2843. [Google Scholar]
- 29.Quiroga J A, Cotonat T, Castillo I, Carreno V. Hepatitis E virus seroprevalence in acute viral hepatitis in a developed country confirmed by a supplemental assay. J Med Virol. 1996;50:16–19. doi: 10.1002/(SICI)1096-9071(199609)50:1<16::AID-JMV4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Reyes G R. Overview of the epidemiology and biology of the hepatitis E virus. In: Willson R A, editor. Viral hepatitis. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 239–258. [Google Scholar]
- 31.Schlauder G G, Dawson G J, Erker J C, Kwo P Y, Knigge M F, Smalley D L, Rosenblatt J E, Desai S M, Mushahwar I K. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J Gen Virol. 1998;79:447–456. doi: 10.1099/0022-1317-79-3-447. [DOI] [PubMed] [Google Scholar]
- 32.Schlauder G G, Desai S M, Zanetti A R, Tassopoulos N C, Mushahwar I K. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J Med Virol. 1999;57:243–251. doi: 10.1002/(sici)1096-9071(199903)57:3<243::aid-jmv6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Thomas D L, Yarbough P O, Vlahov D, Tsarev S A, Nelson K E, Saah A J, Purcell R H. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244–1247. doi: 10.1128/jcm.35.5.1244-1247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tien N T, Clayson H T, Khiem H B, Sac P K, Corwin A L, Myint K S, Vaughn D W. Detection of immunoglobulin G to the hepatitis E virus among several animal species in Vietnam. Am J Trop Med Hyg. 1997;57:211. [Google Scholar]
- 35.Tsarev S A, Tsareva T S, Emerson S U, Yarbough P O, Legters L J, Moskal T, Purcell R H. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J Med Virol. 1994;43:135–142. doi: 10.1002/jmv.1890430207. [DOI] [PubMed] [Google Scholar]
- 36.Tsarev S A, Tsareva T S, Emerson S U, Rippy M K, Zack P, Shapiro M, Purcell R H. Experimental hepatitis E in pregnant rhesus monkeys: failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J Infect Dis. 1995;172:31–37. doi: 10.1093/infdis/172.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Tsarev S A, Shrestha M P, He J, Scott R M, Vaughn D W, Clayson E T, Gigliotti S, Longer C F, Innis B L. Naturally acquired hepatitis E virus (HEV) infection in Nepalese rodents. Am J Trop Med Hyg. 1998;59:242. [Google Scholar]
- 38.Van Cuyck-Gandre H, Zhang H Y, Tsarev S A, Warren R L, Caudill J D, Snellings N J, Begot L, Innis B L, Longer C F. Short report: phylogenetically distinct hepatitis E viruses in Pakistan. Am J Trop Med Hyg. 2000;62:187–189. doi: 10.4269/ajtmh.2000.62.187. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Ling R, Erker J C, Zhang H, Li H, Desai S, Mushahwar I K, Harrison T J. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J Gen Virol. 1999;80:169–177. doi: 10.1099/0022-1317-80-1-169. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Zhang H, Ling R, Li H, Harrison T J. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J Gen Virol. 2000;81:1675–1686. doi: 10.1099/0022-1317-81-7-1675. [DOI] [PubMed] [Google Scholar]
- 41.Wu J C, Chen C M, Chiang T Y, Sheen I J, Chen J Y, Tsai W H, Huang Y H, Lee S D. Clinical and epidemiological implications of swine hepatitis E virus infection. J Med Virol. 2000;60:166–171. [PubMed] [Google Scholar]
- 42.Zanetti A R, Schlauder G G, Romano L, Tanzi E, Fabris P, Dawson G J, Mushahwar I K. Identification of a novel variant of hepatitis E virus in Italy. J Med Virol. 1999;57:356–360. doi: 10.1002/(sici)1096-9071(199904)57:4<356::aid-jmv5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]