Abstract
Background
The visual system could be included in the diagnostic criteria for multiple sclerosis (MS) to demonstrate dissemination in space (DIS) and dissemination in time (DIT).
Objective
To investigate the diagnostic value of retinal asymmetry in MS.
Methods
A prospective, longitudinal study in individuals with MS (n=151) and healthy controls (n=27). Optical coherence tomography (OCT) was performed at 0, 2 and 4 years. Macular ganglion cell and inner plexiform layer (mGCIPL) thickness was determined as well as measures for retinal asymmetry: the inter-eye percentage difference (IEPD) and inter-eye absolute difference (IEAD). Receiver operator characteristics curves were plotted and the area under the curve (AUC) was calculated for group comparisons of the mGCIPL, IEPD, IEAD and atrophy rates.
Results
The diagnostic accuracy of both the IEPD and IEAD for differentiating bilateral and unilateral MS optic neuritis was high and stable over time (AUCs 0.88–0.93). The IEPD slightly outperformed the IEAD. Atrophy rates showed low discriminatory abilities for differentiating MS from controls (AUC 0.49–0.58).
Conclusion
The inter-eye differences of the mGCIPL have value for demonstration of DIS but in individuals with longstanding MS not for DIT. This may be considered as a test to detect DIS in future diagnostic criteria. Validation in a large prospective study in people presenting with symptoms suggestive of MS is required.
Keywords: multiple sclerosis, neuroophthalmology
Introduction
The potential role for retinal optical coherence tomography (OCT) for diagnostic criteria for multiple sclerosis (MS) had been identified.1 The contribution of a paraclinical test to the diagnostic criteria has evolved to permit for substitution for dissemination in space (DIS) and dissemination in time (DIT) of MS disease pathology.2–5 There is mounting evidence that OCT permits for highly sensitive, accurate and reproducible detection of retinal atrophy as a result of optic neuritis.6 7 Recently, a simple measure for retinal asymmetry has been identified.8–14 This measure gives a single numeric value. The inter-eye difference (IED) is calculated from the inner retinal layer thicknesses of the right and left eye. The IED can be expressed as a dimensionless percentage value (IEPD) or as an absolute value (IEAD) in µm. Several studies have now provided evidence that these measures for retinal asymmetry permit to reliably identify a previous episode of MS-associated optic neuritis (MSON). The diagnostic accuracy is high in differentiating these patients from healthy controls and from patients without a previous episode of MSON (non-MSON).12 13 This suggests that the OCT may qualify as a useful assessment tool to detect a fifth central nervous system location for DIS.12 14 To date retinal asymmetry has only ever been applied in cross-sectional data sets. It is not known how well measures of retinal asymmetry perform over time. As a potential diagnostic test for MS it is relevant to note that these measures of retinal asymmetry also identify individuals with MS who never had MSON according to the contemporary definition.6 9 It is understood that MS lesions affecting the posterior visual pathway trigger retrograde trans-synaptic axonal degeneration which reaches the retina, thereby causing retinal asymmetry.15–17 While encouraging, these findings only encourage a role of OCT as a substitute for DIS in MS diagnostics. The value of OCT for potential substitution for DIT is not known. Therefore the present prospective longitudinal study investigated the diagnostic value of retinal asymmetry for DIS and DIT.
Methods
Written informed consent was obtained from all subjects before study inclusion.
Study design and participants
For this prospective study, patients suffering from MS and healthy control (HC) subjects were enrolled from the Amsterdam UMC. All subjects underwent clinical and OCT assessments at baseline, 2 years and 4 years. The diagnosis of MS and MSON was made according to consensus criteria.5 18 This consisted of a detailed history-taking (pain worsening on eye movements, timing of visual loss and patterns of recovery) and review of medical records. Patients were subdivided into relapsing–remitting, secondary progressive and primary progressive MS.19 Clinical assessments included physical and cognitive disability scales and visual function as reported before for this cohort.12 20 21
Optical coherence tomography
Spectral domain OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) was performed and OCT quality assessment was based on validated consensus criteria, as previously described.22–24 The thickness of the macular ganglion cell and inner plexiform layer (mGCIPL) and parapapillary nerve fibre layer was determined. The inter-eye differences of the mGCIPL were calculated as described previously.12 13 For each analysis we used both the inter-eye percentage difference (IEPD, a dimensionless metric) and inter-eye absolute difference (IEAD, in μm) from the ETDRS grid. The atrophy rates were calculated by dividing the GCIPL difference between two time points by the follow-up time in years (annualised atrophy rates).
Statistical analysis
Analyses followed EQUATOR (Enhancing the QUAlity and Transparency Of Health Research) reporting guidelines and were consistent with the Strengthening the Reporting of Observational Studies in Epidemiology outline for cohort studies (https://www.equator-network.org/reporting-guidelines/). SAS V.9.4m7 (SAS Institute) was used for all analyses. Data distribution was checked by the Shapiro-Wilk test and visually. Normally distributed data were presented as mean and SD. The median and IQR were shown for non-Gaussian data. Categorical data were presented as number (percentage). Statistical analyses of the OCT data and reporting followed consensus guidelines.25 The accuracy of diagnostic performance was tested using receiver operator characteristics (ROC) curve analyses. Areas under the curves where compared by a non-parametric approach.26 Single time point IEPD and IEAD were defined as DIS. Longitudinal changes were used for definition of DIT. First, DIT was analysed for the mean annual atrophy rate. Next, DIT was calculated as the change of the IEPD/IEAD over time. Statistical comparison of ROC curves area under the curve (AUC) and 95% CI for the incremental contribution of each measure was performed using the Wald test for the entire cohort.27 Test results were considered to be statistically significant for alpha <0.05. Sensitivity and specificity values were based on a 5% cut-off for the IEPD and a 4 µm cut-off for the IEAD.12 13
Results
In total 151 patients with MS and 27 HCs were included. The demographic and clinical characteristic are summarised in table 1. Patients had a mean disease duration of 20.8 (±6.5) years and 98 (65%) showed a relapsing–remitting disease course. Eighty patients (53%) had experienced a clinically identified episode of MSON, of which 30 bilaterally. Characteristic of the MSON groups are shown in table 1.
Table 1.
Subject characteristics (baseline)
| Patients with MS | |||||
| Healthy controls (n=27) |
All (n=151) |
Non-MSON (n=71) |
Unilateral MSON (n=50) |
Bilateral MSON (n=30) |
|
| Sex (N, female) | 12 (44) | 99 (66) | 45 (63) | 34 (68) | 20 (67) |
| Age (years) | 52.2±5.4 | 53.8±9.6 | 56.1±9.0 | 51.3±9.2 | 52.2±10.6 |
| Disease duration (years) | N/A | 20.8±6.5 | 20.3±6.5 | 20.4±6.5 | 22.9±6.1 |
| EDSS score | N/A | 3.5 (3.0–5.0) | 3.5 (3.0–6.0) | 3.5 (2.5–5.0) | 4.0 (3.0–4.5) |
| Disease course | |||||
| Relapsing–remitting (N) | N/A | 98 (65) | 42 (59) | 36 (72) | 20 (67) |
| Secondary progressive (N) | N/A | 34 (23) | 11 (32) | 14 (28) | 9 (30) |
| Primary progressive (N) | N/A | 19 (13) | 18 (25) | 0 (0) | 1 (3) |
| Current DMT use (N) | N/A | 44 (29) | 16 (23) | 19 (38) | 9 (30) |
| mGCIPL mean ODS (μm)* | 92.1±6.2 | 77.2±14.0 | 82.6±12.6 | 72.8±12.4 | 70.7±15.4 |
Data are shown as mean±SD, median (IQR) or N (%).
*Baseline GCIPL ODS values available for 24 healthy controls and 118 patients with MS.
DMT, disease modifying treatment; EDSS, Expanded Disability Status Scale; mGCIPL, macular ganglion cell and inner plexiform layer; MS, multiple sclerosis; MSON, multiple sclerosis-associated optic neuritis; N, number; N/A, not applicable; ODS, left and right eye.
Inter-eye differences
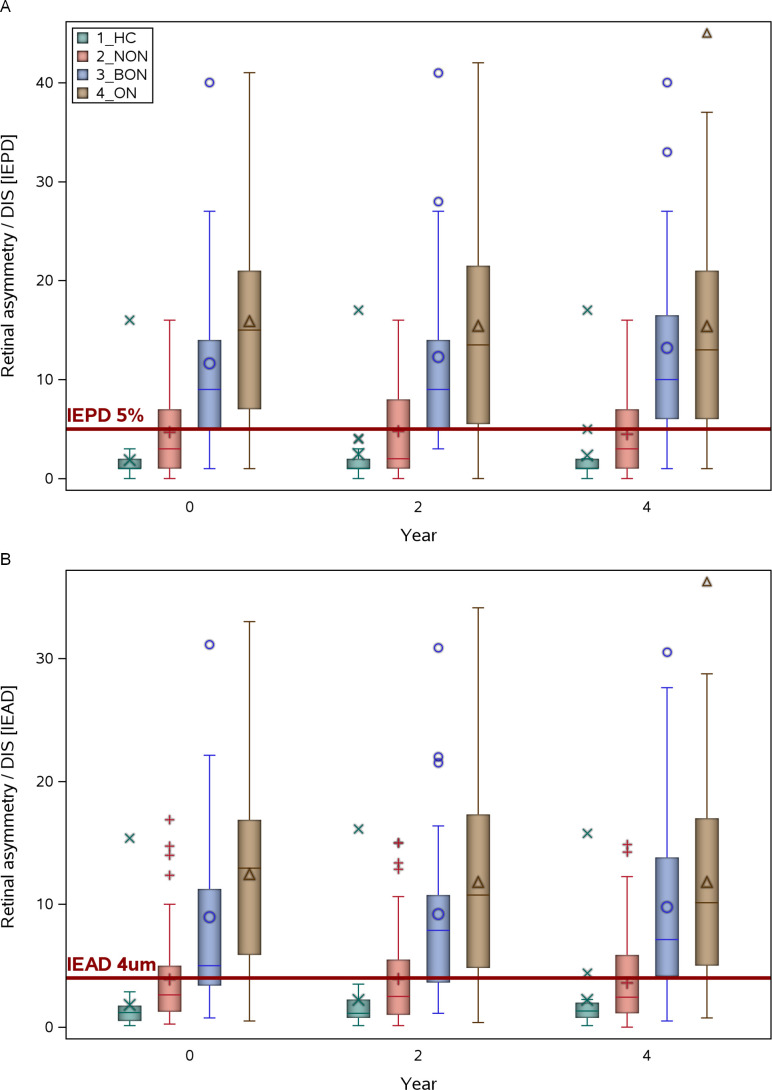
The IEPD and IEAD values of the HC and MS subgroups are visualised in figure 1. Because the percentage male/female subjects differed between patients with MS and HCs (table 1, p=0.037), the associations between sex and IEPD/IEAD values was tested in both groups. There was no significant association. Likewise, there was no significant effect of current disease modifying treatment use on IEPD/IEAD values. The diagnostic performance of the IEPD and IEAD at different time points is shown in online supplemental table 1 and the ROC curves are visualised in online supplemental figure 1. The diagnostic accuracy differentiating MSON from HCs was high, with good ROC curve AUCs (0.88–0.93). Overall, the IEPD slightly outperformed the IEAD in differentiating MS and MSON from HC, although this difference was not statistically different (comparison of area under the ROC curve, online supplemental table 1). AUC values of both IEPD and IEAD did not change significantly over time. Furthermore, the diagnostic accuracy for bilateral and unilateral MSON was similar. As expected, diagnostic performance for differentiating non-MSON from HC was lower than for differentiating MSON from HC. The AUC value for differentiating the whole MS group from HC was around 0.8 at every time point.
Figure 1.
Box-and-Whisker plots of (A) the inter-eye percentage differences (IEPD) and (B) the inter-eye absolute difference (IEAD) at baseline, year 2 and year 4. The red line indicates the 5% cut-off for the IEPD and the 4 µm cut-off of the IEAD. BON, bilateral MS associated optic neuritis; HC, healthy controls; MS, multiple sclerosis; NON, no MS associated optic neuritis; ON, unilateral MS associated optic neuritis. The median (bold horizontal line), 25–75 percentiles (box), 5–95 percentiles (whiskers), mean (symbol in the box) and outliers (symbols outside the box) are shown.
jnnp-2021-327468supp001.pdf (895.7KB, pdf)
Atrophy rate and change of inter-eye differences
No significant associations between sex and atrophy rates were found for both patients with MS and HCs. Furthermore, no effect of current use of disease modifying treatment on atrophy rate was found in this cohort. The atrophy rates of the mGCIPL at the different time points for the MS and HC group are visualised in online supplemental figure 2A. The mean annualised atrophy rate in patients with MS from baseline to year 2 was 0.26 (±0.57), from year 2 to year 4 −0.38 (±0.82) and from baseline to year 4 −0.31 (±0.47). This was not significantly different from HCs (the atrophy rate of individual subjects and of the MSON subgroups is visualised in online supplemental figures 3 and 4, respectively). The diagnostic performance of the atrophy rates of the mGCIPL for differentiating patients with MS from HCs is shown in online supplemental table 2 and the ROC curves are visualised in online supplemental figure 1. The atrophy rate from baseline to year 2 showed an AUC of 0.59, from year 2 to year 4 an AUC of 0.66 and from baseline to year 4 an AUC of 0.52. Because of this low discriminatory ability, no relevant diagnostic cut-off for the atrophy rate could be determined. Similar to the atrophy rate, the IEPD and IEAD did not substantially change over time, both for patients with MS and HCs (online supplemental figure 2 B+C, and online supplemental figures 5 and 6 for individual subjects).
Discussion
The main finding of this study was that the inter-eye difference of the mGCIPL has a high accuracy in diagnosing patients with MS with both unilateral and bilateral optic neuritis over time. The IEPD showed a slightly higher accuracy than the IEAD. The atrophy rate of mGCIPL over a 4-year period showed a low discriminatory value for differentiating patients with MS from HCs in this group of patients with longstanding disease.
Our results on the diagnostic accuracy of inter-eye differences are in line with previous literature.8–10 12 In online supplemental table 3, sensitivity and specificity of optimised cut-off of the inter-eye differences of the mGCIPL in these studies are summarised. Both diagnostic accuracy and the optimised cut-off value varied depending on the groups compared in the study. Highest accuracy (sensitivity 100% and specificity 98%) was found when a group of patients with unilateral optic neuritis (not specifically due to MS) was compared with HCs.8 When the reference is a diseased group, sensitivity and specificity drop,9 10 to respectively 67.3% and 67.4% when comparing MSON to non-MSON.9 Our longitudinal result confirm the previous cross-sectional finding12 that the inter-eye differences also have a high diagnostic accuracy for bilateral MSON, besides for unilateral MSON.
In our study and previous studies.10 14 the diagnostic accuracy was higher for the IEPD than the IEAD. One advantage of the IEPD is that it is a dimensionless measure, with might be helpful to for pooling data from different devices and different segmentation software. This however has to be confirmed in future studies which investigate different devices in parallel. The IEPD is probably not sensitive in differentiating MSON from other types of optic neuritis. Therefore it could be useful as an additional paraclinical test in the diagnosis of MS.
The diagnostic accuracy of the inter-eye differences for differentiating non-MSON from HCs was moderate to good (AUC 0.66–0.73). The 5% cut-off for the IEPD resulted in sensitivity values between 41% and 49%. This suggests that at least part of this group has either suffered from a subclinical optic neuritis or asymmetrical pathology further along the visual pathway, resulting in retrograde (trans-synaptic) degeneration. A limitation of the present study is that we cannot make conclusion about the aetiology of this asymmetry. Studies in earlier phases of the disease with frequent radiological and OCT follow-up would be useful in evaluating the chain of events leading to (asymmetrical) retinal atrophy. Future development of automated region of interest analysis are eagerly awaited as they permit for a more detailed comparison of hemispheric asymmetry in MS lesion distribution. Presently this is still a very labour and time consuming manual approach.28
The atrophy rate of the mGCIPL was similar in our MS group and the HC group. One reason for this could be the relatively long disease duration of the patients with MS. Previous studies have shown that thinning of the retinal layers occurs most rapidly during the early stages of diseases.29 Potentially, in the larger part of our group a plateau effect occurred, which is a limitation of the present study. Extending the current approach to a MS group early in the disease could be useful. Related to this, the target population for using inter-eye differences for DIS would be patients suspected of (relapsing–remitting) MS. This differs from our population with a longer disease duration and more frequently a progressive disease type. Another limitation of our study is the relatively low number of HCs. We would be hesitant to extrapolate from present study to longitudinal atrophy rates across all MS subtypes, as we neither have the cohort size nor did we apply mixed-effect modelling. Based on the present data we cannot recommend OCT to substitute for DIS. Finally, we did not investigate the effect of the number of clinical episodes of MSON on mGCIPL or IEPD/IEAD. Such studies should also include functional outcome measures, as structural and functional damage after one or more episodes of optic neuritis might not be completely congruent.30 31
In conclusion, the inter-eye differences of the mGCIPL are robust measures over time for diagnosing a previous episode of MSON. This provides support for OCT as an additional para-clinical test for a fifth central nervous system location for DIS.
Footnotes
Contributors: JNB - Data analysis and interpretation, writing & revision of manuscript BMJU - Data interpretation, critique & revision of manuscript AP - Data analysis and interpretation, writing & revision of manuscript
Funding: This study was funded by the Dutch MS Research Foundation, grant nr. 18-1027.
Competing interests: JNB is supported by the Dutch MS Research Foundation, grant nr. 18-1027. BMJU has received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. AP reports personal fees from Novartis, Heidelberg Engineering, Zeiss, grants from Novartis, outside the submitted work; and AP is part of the steering committee of the OCTiMS study which is sponsored by Novartis. AP is part of the steering committee of Angio-OCT which is sponsored by Zeiss. He does not receive honorary as part of these activities. The NIHR BRC at Moorfields Eye Hospital supported AP.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Medical Ethical Committees of the Amsterdam UMC, the Netherlands, and is in accordance with the 1964 Declaration of Helsinki.
References
- 1. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 2. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31. 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 3. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–7. 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 2005;58:840–6. 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 5. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017;16:797–812. 10.1016/S1474-4422(17)30278-8 [DOI] [PubMed] [Google Scholar]
- 7. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010;9:921–32. 10.1016/S1474-4422(10)70168-X [DOI] [PubMed] [Google Scholar]
- 8. Behbehani R, Ali A, Al-Omairah H, et al. Optimization of spectral domain optical coherence tomography and visual evoked potentials to identify unilateral optic neuritis. Mult Scler Relat Disord 2020;41:101988. 10.1016/j.msard.2020.101988 [DOI] [PubMed] [Google Scholar]
- 9. Davion J-B, Lopes R, Drumez Élodie, et al. Asymptomatic optic nerve lesions: an underestimated cause of silent retinal atrophy in MS. Neurology 2020;94:e2468–78. 10.1212/WNL.0000000000009504 [DOI] [PubMed] [Google Scholar]
- 10. Outteryck O, Lopes R, Drumez Élodie, et al. Optical coherence tomography for detection of asymptomatic optic nerve lesions in clinically isolated syndrome. Neurology 2020;95:e733–44. 10.1212/WNL.0000000000009832 [DOI] [PubMed] [Google Scholar]
- 11. Villoslada P, Sanchez-Dalmau B, Galetta S. Optical coherence tomography: a useful tool for identifying subclinical optic neuropathy in diagnosing multiple sclerosis. Neurology 2020;95:239–40. 10.1212/WNL.0000000000009840 [DOI] [PubMed] [Google Scholar]
- 12. Coric D, Balk LJ, Uitdehaag BMJ, et al. Diagnostic accuracy of optical coherence tomography inter-eye percentage difference for optic neuritis in multiple sclerosis. Eur J Neurol 2017;24:1479–84. 10.1111/ene.13443 [DOI] [PubMed] [Google Scholar]
- 13. Nolan RC, Galetta SL, Frohman TC, et al. Optimal Intereye difference thresholds in retinal nerve fiber layer thickness for predicting a unilateral optic nerve lesion in multiple sclerosis. J Neuroophthalmol 2018;38:451–8. 10.1097/WNO.0000000000000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petzold A, Chua SYL, Khawaja AP, et al. Retinal asymmetry in multiple sclerosis. Brain 2021;144:224–35. 10.1093/brain/awaa361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 2015;78:801–13. 10.1002/ana.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balk LJ, Steenwijk MD, Tewarie P, et al. Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:419–24. 10.1136/jnnp-2014-308189 [DOI] [PubMed] [Google Scholar]
- 17. Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, et al. Trans-Synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 2014;75:98–107. 10.1002/ana.24030 [DOI] [PubMed] [Google Scholar]
- 18. Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 2014;10:447–58. 10.1038/nrneurol.2014.108 [DOI] [PubMed] [Google Scholar]
- 19. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis Society (USA) Advisory Committee on clinical trials of new agents in multiple sclerosis. Neurology 1996;46:907–11. 10.1212/wnl.46.4.907 [DOI] [PubMed] [Google Scholar]
- 20. Nij Bijvank JA, van Rijn LJ, Balk LJ, et al. Diagnosing and quantifying a common deficit in multiple sclerosis: internuclear ophthalmoplegia. Neurology 2019;92:e2299–308. 10.1212/WNL.0000000000007499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eijlers AJC, Meijer KA, Wassenaar TM, et al. Increased Default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 2017;88:952–60. 10.1212/WNL.0000000000003689 [DOI] [PubMed] [Google Scholar]
- 22. Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012;7:e34823. 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler 2015;21:163–70. 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 24. Coric D, Balk LJ, Verrijp M, et al. Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler 2018;24:158–66. 10.1177/1352458517694090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016;86:2303–9. 10.1212/WNL.0000000000002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 27. Seshan VE, Gönen M, Begg CB. Comparing ROC curves derived from regression models. Stat Med 2013;32:1483–93. 10.1002/sim.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Vries-Knoppert WA, Baaijen JC, Petzold A. Patterns of retrograde axonal degeneration in the visual system. Brain 2019;142:2775–86. 10.1093/brain/awz221 [DOI] [PubMed] [Google Scholar]
- 29. Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 2016;263:1323–31. 10.1007/s00415-016-8127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meneguette NS, Almeida KMFR, Figueiredo MTJdeO, et al. Optic neuritis in Asian type opticospinal multiple sclerosis (OSMS-ON) in a non-Asian population: a functional-structural paradox. Mult Scler Relat Disord 2021;56:103260. 10.1016/j.msard.2021.103260 [DOI] [PubMed] [Google Scholar]
- 31. Oertel FC, Specovius S, Zimmermann HG, et al. Retinal optical coherence tomography in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2021;8. 10.1212/NXI.0000000000001068. [Epub ahead of print: 15 09 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-327468supp001.pdf (895.7KB, pdf)