Abstract
A newborn girl presenting with respiratory distress soon after birth was found to have a neck mass and required transfer to a paediatric intensive care unit with neonatal expertise. She subsequently underwent endoscopic airway assessment with microlaryngoscopy and bronchoscopy proceeding to open excision of the lesion in the right thyroid lobe on day thirteen of life, resulting in resolution of airway compromise and complete pathological clearance. The baby was discharged 10 days after surgery. Histology confirmed a thyroid teratoma. At 12 months, the child was thriving with no evidence of recurrence. This case illustrates a rare but serious diagnosis that, if not managed in a timely manner, can lead to significant morbidity and mortality.
Keywords: otolaryngology / ENT, paediatric surgery, paediatric intensive care
Background
Thyroid teratomas are a rare subset of thyroid tumours.1 2 Teratomas are germ-cell tumours and tend to display features of all three germ cell layers; ectoderm, mesoderm and endoderm leading to a heterogeneous tissue composition. The large majority of teratomas are found in the gonads or sacrococcygeal region,3 as the gonads represent the normal destination of germ-cell migration and because totipotent cells of the primitive streak of the early embryo persist at these sites after the fourth week of gestation.4 Rarely, they are found in other regions such as the head and neck, retroperitoneum and mediastinum.3 5 Teratomas of the head and neck are typically found in infants and are usually benign.6 Many patients present with symptoms of respiratory distress, contributing to a historically high mortality rate.7 8 Uncommonly, they are seen in adults and confer a poor prognosis.9 10 The aim of this report is to present a case of a neonate presenting with airway compromise shortly after birth secondary to a thyroid teratoma.
Case presentation
A female Caucasian infant was born by normal vaginal delivery at 37+1 weeks in a district general hospital, with no concerns noted on routine antenatal scans. One hour following delivery a ‘choking episode’ was observed and intravenous antibiotic treatment was initiated for presumed aspiration pneumonia along with intermittent positive pressure ventilation, followed by continuous positive airway pressure (CPAP), to maintain oxygen saturations.
Two days after delivery, she developed increased work of breathing, increasing FiO2 requirements and became acidotic (pH 7.21). Fibreoptic laryngoscopy showed ‘gross epiglottic collapse’ and orotracheal intubation was performed with an age appropriate size 3.5 endotracheal tube, but the procedure was difficult requiring video laryngoscopy, cricoid pressure and bougie, facilitated by lateral airway manipulation. Her clinical condition improved, acidosis resolved and she was transferred to a paediatric intensive care unit with neonatal expertise at a tertiary referral centre.
Investigations
Further clinical examination demonstrated a soft central neck mass anterior to the trachea. A cervical ultrasound scan (USS) showed a 5.2×3.1×4.5 cm focal lower neck mass with cystic and solid components, calcification and vascularity. The mass was predominantly right sided but crossed the midline, overlying the larynx and upper trachea. The mass was not separate from the thyroid and the suspicion of a teratoma was raised.
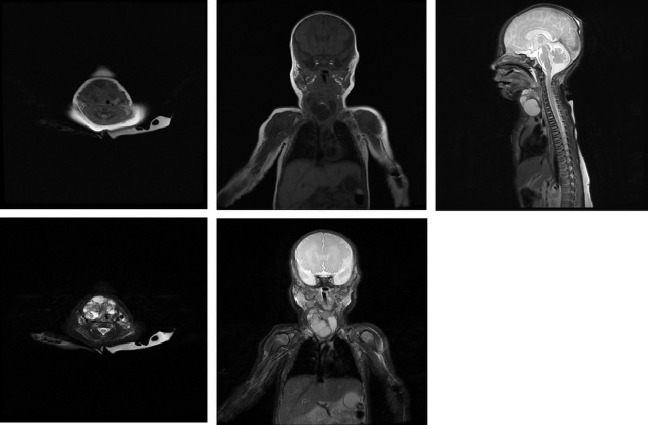
MRI of the neck and chest with contrast on day 4 of life showed a multiloculated cystic lesion with some solid components and calcifications (figure 1) extending from the level of the hyoid to the left brachiocephalic vein. There was displacement of the trachea and right neck vessels. There was no evidence of lymphadenopathy or metastatic spread to bone, lung, spine or mediastinum. Radiological imaging favoured the differential of a cystic teratoma rather than lymphatic or vascular malformation owing to the heterogeneous nature and calcifications, considered as pathognomonic.11 Baseline serum tumour markers obtained at day 6 of life were beta-human chorionic gonadotropin <1 U/L (normal range 0–2) and alpha-fetoprotein (αFP) 41 813 KU/L (normal range 50 000–150 000). Note that αFP levels tend to be elevated in newborns and drop considerably over the first few weeks of life, as was the case here. Persistently high αFP levels would lead to a suspicion of possible malignancy.12 13
Figure 1.
Preoperative MRI with contrast demonstrating the cervical mass in axial, coronal and sagittal section.
On the fifth day of life, she was successfully extubated. However, she remained CPAP dependent with increasing oxygen requirements and there were clinical and radiological (repeat USS) concerns over increase in size of the cervical mass on day 10 of life.
Treatment
Following a multidisciplinary discussion, a decision was made to perform an endoscopic airway assessment with microlaryngoscopy and bronchoscopy (MLB) proceeding to open excision of the lesion on day thirteen of life. MLB demonstrated partial obstruction of the trachea from the level of the subglottis to mid trachea secondary to extrinsic compression by the cervical mass (figure 2). There was no evidence of mucosal infiltration and the distal trachea was normal. Open surgical approach through a midline transverse anterior cervical incision, revealed a large discrete tumour arising from the right thyroid lobe without local infiltration. The left thyroid lobe and isthmus were normal and there were no enlarged lymph nodes found. The right thyroid lobe was excised (figure 3). Intraoperative recurrent laryngeal nerve monitoring (inomed Neurocare, London) confirmed preservation of function of both the vagus and right recurrent laryngeal nerves. Repeat MLB after excision and extubation under endoscopic guidance demonstrated resolution of airway compression (figure 4) and normal vocal cord function.
Figure 2.
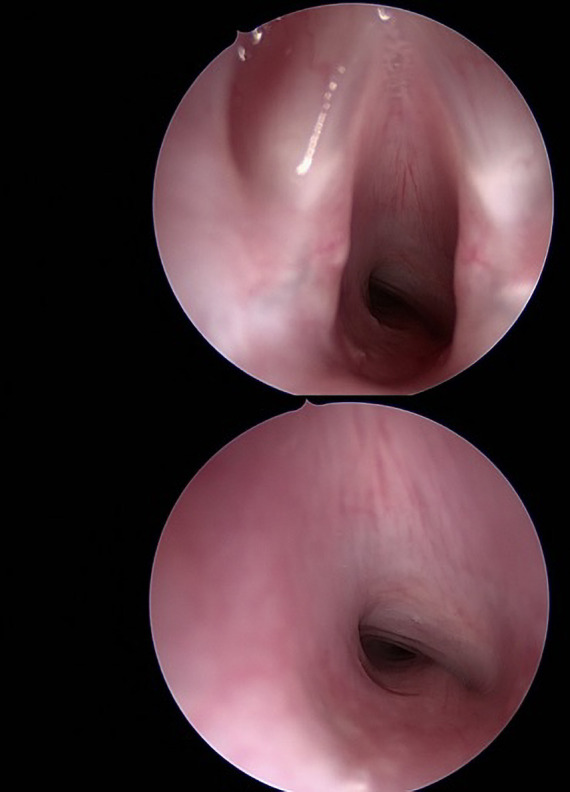
Two intraoperative endoscopic images taken at MLB prior to excision of the cervical mass, at glottic level and in subglottis, showing partial airway obstruction secondary to extrinsic tracheal compression extending from subglottis to mid tracheal level. MLB, microlaryngoscopy and bronchoscopy.
Figure 3.
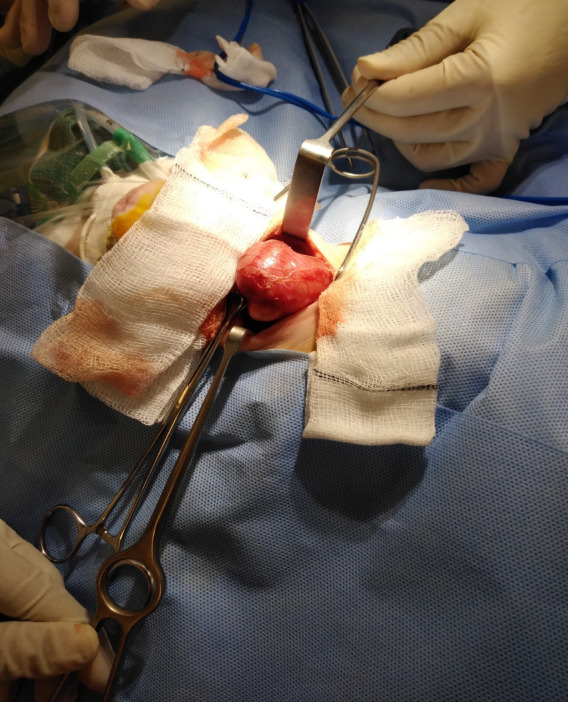
Intraoperative photograph demonstrating the right thyroid mass delivered through the surgical incision prior to excision.
Figure 4.
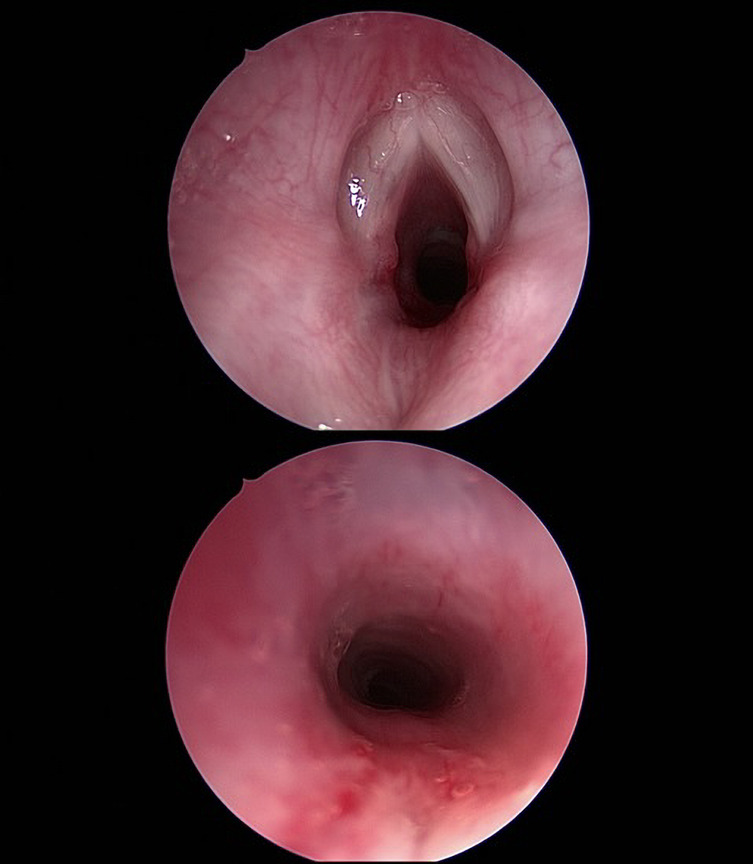
Two intraoperative endoscopic images taken at MLB after excision of the cervical mass, showing resolution of airway obstruction. MLB, microlaryngoscopy and bronchoscopy.
After surgery, the patient required CPAP for 4 days followed by one further day of nasal high flow therapy on the high-dependency unit. The patient was treated with a short course of dexamethasone and a 7-day course of co-amoxiclav (amoxicillin and clavulanic acid). On day six after surgery, when she was off high flow therapy for 24 hours and tolerating oral feeds, she was stepped down to the ward. She was discharged 10 days after surgery.
Outcome and follow-up
Histology confirmed a completely excised mature teratoma within the right thyroid lobe, measuring 4.5×4.3×1.5 cm, with neuroepithelial, cartilage, adipose, bowel, squamous, respiratory, epithelial, osseous and choroid plexus tissues. No dedifferentiated, carcinomatous or sarcomatous elements were seen.
Postoperative outpatient follow-up was continued under both otolaryngology and endocrine teams. Clinically, at 5 weeks after surgery, the patient was thriving with no airway or feeding difficulties. There remained no signs of recurrence at over 12 months after surgery.
At 3 weeks postoperatively the serum αFP had decreased to 365 KU/L (normal range 1500–2500). Serum thyroid function tests performed at 3, 7 and 17 weeks after surgery remained normal.
Discussion
Thyroid teratomas tend to be equally distributed among genders.7 In the largest case series of thirty patients to date, the median age of presentation was newborn.7 Patients present with an anterior or lateral cervical lump, although this can be subtle especially in the newborn period, as was the case here. Many patients present with symptoms of respiratory distress, contributing to a historically high mortality rate.7 8 In addition to mass effect on the airway,8 14 compression of the pharynx and oesophagus may affect feeding in infants. In utero, this may cause polyhydramnios and require amniocentesis.1 5 15
With the advent of prenatal imaging, thyroid teratomas may be diagnosed in utero.16–18 Preoperative imaging in the form of ultrasound sonography, CT and MRI show characteristic features such as mixed cystic and solid constitution, calcific deposits and prominent vascularity.19
The intrinsic totipotency of germ-cells results in a diverse and heterogeneous histological composition usually including cells from all three germ cell lineages. This is a hallmark of a teratoma. The tumours commonly have a fibrous capsule or pseudocapsule. The histologically ‘immature’ tumours devoid of thyroid tissue, are generally malignant in adults, but benign in infants.7 19 In infants, outcomes are good irrespective of tumour maturity.7 12 19 In this case, histology confirmed a mature teratoma arising from the thyroid with no evidence of malignancy and complete surgical excision.
There has been debate over the definition of a thyroid teratoma.8 20 21 It is generally accepted that a cervical teratoma may be classed as a thyroid teratoma if it contains thyroid tissue, is connected to the thyroid gland, or replaces the thyroid gland entirely.7 19 In this patient, the mature teratoma was confirmed to be within the thyroid capsule on microscopy.
Surgical removal of the teratoma is the optimal management for thyroid teratomas.3 This can be done electively, often in the early neonatal period. If diagnosed before birth and if there are concerns regarding airway compromise at delivery, an ex utero intrapartum treatment procedure involving multidisciplinary approach is performed where the fetus is delivered by elective caesarean section,14 15 the airway secured and ventilation established while still receiving placental support.15 In this case, routine antenatal imaging had not revealed a tumour.
In the unlikely event of a malignant thyroid teratoma in an infant, adjuvant chemotherapy may be used with serial monitoring of αFP levels.13 αFP levels seem to be highest in immature teratomas and the normalisation of αFP levels suggests successful treatment.12 13
There has only been one other published case of thyroid teratoma in a child in the recent literature in the last 3 years, demonstrating the rarity of this diagnosis.22 This case study described the management of a 2-year-old child who presented with a slowly enlarging cervical lump over the preceding 14 months. The lesion was completely surgically resected and histological characterisation was similar—a heterogeneous multicystic lesion with calcifications and tissues from all three germ cell layers. This case, however, presented at a later age with a slower growing mass and no evidence of airway compromise, which typically represents the main cause of morbidity and mortality in these lesions.7 8 22 Our case demonstrated the requirement of urgent management including early stabilisation, transfer to a tertiary referral centre with neonatal expertise, multidisciplinary management, and early surgery with perioperative endoscopic airway assessment.
Patient’s perspective.
When I found out that I was pregnant I was 8 weeks. As I was a smoker I was told that I would be having more scans than usual but then I developed gestational diabetes. I had stopped smoking at this stage. At the twenty weeks scan I was told I was having a girl and I was delighted. Each scan was all good with no concerns noted.
At thirty-eight weeks in our local district hospital, I gave birth to what looked like a very blue baby girl. She was given to me and I laid her on my chest, I started to have my concerns about her being so blue, but I was reassured by the midwife that babies get mucus, and this would probably go when she had a feed. We were told to gently pat her back to see if this would help release it. The nurse finished stitching me up and then went and got a bottle to do a feed. She was handed to the nurse and she started to orally feed her. The nurse gently told us not to panic but was going to press the red button to get assistance as she had gone all floppy. There must have been around ten people in the room with us, most of them around my baby. I was very tearful as I did not understand what was happening but all the time, I was being reassured that everything would be ok.
I fell asleep and when I woke, I was told that she had to go onto the neonatal ward as she needed a little oxygen to help her breath. My heart sank when I saw her having to have help with her breathing, I was able to touch her tiny hand and it was at this point I cried like I had never cried before.
A day later I noticed a lump appear on her throat while I sat and watched her in the incubator and I raised my concerns, she was still on the oxygen. We were told that she would be going by ambulance to the nearest Specialist hospital she could not cope with her breathing without oxygen. We followed by car and she was put on to the paediatric intensive care unit. Lots of tubes and lots of bleep noises is what we saw and heard when looking at my little girl.
She was then put onto CPAP as it was clear she could not cope with the oxygen alone.
The growth on her neck was getting bigger each day and as I looked at her, I did wonder a few times if she would ever pull through. Sometimes we could cuddle her and sometimes she was too poorly to hold.
The doctors were amazing, always talking to us and talking us through what they have done to make her comfortable. We asked lots of questions and they always had time to answer. They said that it looked like she has a teratoma which is rare in babies and they would like to operate to try and remove it as it was getting bigger each day.
On the day of her operation, we kissed her and told her everything would be ok and that we would see her later in the day. At this point our concerns were huge and worrying. It was the longest day of my life, having to wait.
After she came back onto the ward (HDU), she still required CPAP for a further 4 days and then she started to take oral feeds. Ten days after surgery she could go home just before Christmas!
It was indeed a teratoma and we were shown photos of the x rays taken on her throat before the operation.
Since her operation we have been back to the Specialist hospital to have check-ups on her thyroid, and everything is normal and not growing back. She is now thriving (has a fine set of lungs) and makes lots of noise, something we thought she might not have with the teratoma and where it was.
Without the knowledge of the doctors and nurses at the Specialist hospital my baby would not have survived. All the time we were told things, talked them through and reassured. Our greatest thanks go out to these people.
Learning points.
In a neonate with airway obstruction a thorough examination of the cervical region should be performed—thyroid teratomas are very rare tumours with a wide range of differential diagnoses and so pose a challenge for management. They often present with airway obstruction and a cervical mass, which can be subtle in the newborn.
Radiological imaging, including ultrasound scan and MRI, is essential to guide the diagnosis and management of teratomas, but each modality has limitations.
This case highlighted the importance of early surgery, particularly if there is associated airway compromise, oxygen requirement or increasing dependency on non-invasive ventilation.
The combined use of endoscopic airway assessment with microlaryngoscopy and bronchoscopy before and after excision and an open neck approach with the use of a nerve monitor enabled optimal and complete excision of the tumour, without nerve damage or recurrence in the short term.
Such cases should be managed in a tertiary referral centre with a neonatal intensive care unit, and with multidisciplinary care involving experienced otolaryngology, neonatal, endocrine and anaesthetic teams.
Footnotes
Contributors: SLG and SB were the surgeons who performed the case. SB and SLG conceived the idea of writing up this case for submission. JR reviewed the case, compiled the information, performed the literature review for the case and drafted the article. SB and SLG critically revised the case report. All authors gave final approval of the final version of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.Bergé SJ, von Lindern JJ, Appel T, et al. Diagnosis and management of cervical teratomas. Br J Oral Maxillofac Surg 2004;42:41–5. 10.1016/S0266-4356(03)00174-8 [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti A, Shashidhar TB, Naglot S, et al. Head and neck teratomas in children: a case series. Indian J Otolaryngol Head Neck Surg 2011;63:193–7. 10.1007/s12070-011-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper D, Lack EE, Lack D, Tapper EE. Teratomas in infancy and childhood. A 54-year experience at the children's Hospital medical center. Ann Surg 1983;198:398–409. 10.1097/00000658-198309000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon HM, Byeon S-J, Hwang J-Y, et al. Sacrococcygeal teratomas in newborns: a comprehensive review for the radiologists. Acta Radiol 2018;59:236–46. 10.1177/0284185117710680 [DOI] [PubMed] [Google Scholar]
- 5.Martino F, Avila LF, Encinas JL, et al. Teratomas of the neck and mediastinum in children. Pediatr Surg Int 2006;22:627–34. 10.1007/s00383-006-1724-6 [DOI] [PubMed] [Google Scholar]
- 6.Lack EE. Extragonadal germ cell tumors of the head and neck region: review of 16 cases. Hum Pathol 1985;16:56–64. 10.1016/S0046-8177(85)80214-8 [DOI] [PubMed] [Google Scholar]
- 7.Thompson LDR, Rosai J, Heffess CS. A clinicopathologic study of 30 cases. Cancer 2000;88:1149–57. [PubMed] [Google Scholar]
- 8.Jordan RB, Gauderer MW. Cervical teratomas: an analysis. literature review and proposed classification. J Pediatr Surg 1988;23:583–91. 10.1016/S0022-3468(88)80373-7 [DOI] [PubMed] [Google Scholar]
- 9.Ain KB. Unusual types of thyroid cancer. Rev Endocr Metab Disord 2000;1:225–31. 10.1023/A:1010039317050 [DOI] [PubMed] [Google Scholar]
- 10.Kimler SC, Muth WF. Primary malignant teratoma of the thyroid: case report and literature review of cervical teratomas in adults. Cancer 1978;42:311–7. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Weerakody Y, Deng F, et al. Congenital cervical teratoma. Available: https://radiopaedia.org/articles/congenital-cervical-teratoma?lang=gb [Accessed 29 Nov 2020].
- 12.Dharmarajan H, Rouillard-Bazinet N, Chandy BM. Mature and immature pediatric head and neck teratomas: a 15-year review at a large tertiary center. Int J Pediatr Otorhinolaryngol 2018;105:43–7. 10.1016/j.ijporl.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 13.Muscatello L, Giudice M, Feltri M. Malignant cervical teratoma: report of a case in a newborn. Eur Arch Otorhinolaryngol 2005;262:899–904. 10.1007/s00405-005-0917-2 [DOI] [PubMed] [Google Scholar]
- 14.Hullett BJ, Shine NP, Chambers NA. Airway management of three cases of congenital cervical teratoma. Paediatr Anaesth 2006;16:794–8. 10.1111/j.1460-9592.2006.01859.x [DOI] [PubMed] [Google Scholar]
- 15.Laje P, Johnson MP, Howell LJ, et al. Ex utero intrapartum treatment in the management of giant cervical teratomas. J Pediatr Surg 2012;47:1208–16. 10.1016/j.jpedsurg.2012.03.027 [DOI] [PubMed] [Google Scholar]
- 16.Schoenfeld A, Edelstein T, Joel-Cohen SJ. Prenatal ultrasonic diagnosis of fetal teratoma of the neck. Br J Radiol 1978;51:742–4. 10.1259/0007-1285-51-609-742 [DOI] [PubMed] [Google Scholar]
- 17.Rahbar R, Vogel A, Myers LB, et al. Fetal surgery in otolaryngology: a new era in the diagnosis and management of fetal airway obstruction because of advances in prenatal imaging. Arch Otolaryngol Head Neck Surg 2005;131:393–8. 10.1001/archotol.131.5.393 [DOI] [PubMed] [Google Scholar]
- 18.Tsuda H, Matsumoto M, Yamamoto K, et al. Usefulness of ultrasonography and magnetic resonance imaging for prenatal diagnosis of fetal teratoma of the neck. J Clin Ultrasound 1996;24:217–9. [DOI] [PubMed] [Google Scholar]
- 19.Riedlinger WFJ, Lack EE, Robson CD, et al. Primary thyroid teratomas in children: a report of 11 cases with a proposal of criteria for their diagnosis. Am J Surg Pathol 2005;29:700–6. 10.1097/01.pas.0000151934.18636.d5 [DOI] [PubMed] [Google Scholar]
- 20.Bale GF. Teratoma of the neck in the region of the thyroid gland; a review of the literature and report of 4 cases. Am J Pathol 1950;26:565–79. [PMC free article] [PubMed] [Google Scholar]
- 21.Vujanić GM, Harach HR, Minić P, et al. Thyroid/cervical teratomas in children: immunohistochemical studies for specific thyroid epithelial cell markers. Pediatr Pathol 1994;14:369–75. 10.3109/15513819409024265 [DOI] [PubMed] [Google Scholar]
- 22.Lv Z, Bai X, Sheng Q, et al. A case report of a giant mature teratoma of the thyroid gland in a young girl. Medicine 2019;98:e14703. 10.1097/MD.0000000000014703 [DOI] [PMC free article] [PubMed] [Google Scholar]