Abstract
Li-Fraumeni syndrome (LFS) is a rare autosomal dominant cancer predisposition syndrome with exceptionally high lifetime cancer risks, caused primarily by germline TP53 variants. Early-onset breast cancer is the most common cancer in women with LFS. Associations between female reproductive factors and breast cancer risk have been widely studied in the general population and BRCA1/2 mutation-carriers, but not in LFS. We evaluated whether reproductive factors are associated with breast cancer in LFS. Questionnaire data was collected on 152 women with confirmed germline TP53 variants enrolled in the National Cancer Institute’s LFS study ( NCT01443468), of which 85 had breast cancer, confirmed by pathology/medical reports. Fisher’s exact test and Cox Proportional Hazards were used to calculate the effect of reproductive factors on breast cancer risk. Lifetime breastfeeding for at least 7 months was associated with lower breast cancer risk (hazard ratio [HR] 0.57, p=0.05). Parity did not independently change breast cancer risk (HR 1.08, p=0.8), but suggested an increased risk with older age at first livebirth (HR 2.14, p=0.05). Age at menarche (HR 1.09, p=0.24) and use of oral contraceptives (OCP) (HR 0.88; p= 0.7) did not significantly affect breast cancer risk. In this first study of reproductive factors and breast cancer in women with LFS, breastfeeding was observed to be protective against breast cancer risk, especially with at least 7 months lifetime breastfeeding. Older age at first livebirth was suggested to slightly increase breast cancer risk. Larger prospective studies of reproductive factors are warranted in women with LFS before making definitive clinical recommendations.
Background:
Li-Fraumeni syndrome (LFS) is a rare inherited cancer predisposition syndrome with very high lifetime risks of developing multiple cancer types, beginning in childhood.(1) Early-onset/pre-menopausal breast cancer, bone and soft tissue sarcomas, brain tumors, and adrenocortical carcinoma are ‘core’ LFS cancers in affected families. Individuals with LFS are also at a high risk of developing multiple primary cancers during their lifetimes, with over 50% of those diagnosed with a primary cancer going on to develop a subsequent primary malignancy.(2, 3) First described in 1969, classic LFS is diagnosed based on a personal history of early-onset sarcoma and a specific cancer family history pattern based on age-at-onset and cancer type.(4) Less stringent classifications have been used more recently to guide clinical genetic testing for LFS.(5, 6) Pathogenic germline variants in TP53, inherited in an autosomal dominant pattern, are the only known genetic cause for LFS and have been estimated to account for between 60–80% of families with classic LFS. (7, 8)
The lifetime risk of cancer in people with LFS has been previously reported to be nearly 100% by age 60 years in women and 73% in men,(9) with an overall cumulative incidence of 50% by age 40 years.(10) We previously reported a cumulative cancer incidence of 50% by age 31 in females and age 46 years in males with LFS.(11) Age-at-onset and cancer type can be highly variable within families carrying the same mutation, suggesting that other genetic and non-genetic factors modify the inherited risk.(12) Additionally, a recent study of cancer in over 2000 carriers of pathogenic germline TP53 variants reported incomplete penetrance with about 80% of individuals developing cancer by the age of 80 years.(12)
This sex difference of cancer incidence in LFS is mainly driven by the exceptionally high risk of early-onset breast cancer in women, which can reach 49% by age 60 years, with a median age at diagnosis of 32 years.(11) In the general population, female reproductive factors including early parity and longer duration of breastfeeding independently lower the overall risk of breast cancer. While this reduction in breast cancer risk is postulated to be associated with lifetime exposure to ovarian hormones, which influences the number of cumulative ovulatory cycles and differentiation of breast lobules,(13) large meta-analyses have not shown significant change in breast cancer risk by menopausal status and the underlying biological mechanisms of hormonal breast carcinogenesis are not fully elucidated.(14–16) Female reproductive factors in the setting of heritable breast cancer due to pathogenic variants in BRCA1/2 have been extensively studied, and breastfeeding has been consistently observed to be protective, with up to a 32% reduction in breast cancer risk in BRCA1 women who breastfed for at least 12 months.(17)
There are scarce data on potential non-genetic cancer risk modifiers of breast cancer in LFS. In this study, we evaluated the association of female reproductive factors and breast cancer risk in women with LFS.
METHODS
Study Participants
This retrospective observational study consisted of participants enrolled in the National Cancer Institute’s (NCI) Institutional Review Board approved LFS Study (11-C-0255, ClinicalTrails.gov; Identifier NCT01443468; www.lfs.cancer.gov)(3) between 2011 and 2016. Written informed consent was obtained from all participants. Detailed family history and individual information questionnaires (IIQs) were completed. The IIQ includes self-reported data on the individual’s demographics, medical and surgical history, and all cancer diagnoses. Females also reported on reproductive factors such as age at menarche, number of pregnancies, childbirth and breastfeeding, fertility experiences and use of oral contraceptives (OCP). Adult participants who self-identified as female, completed an IIQ, and had a known pathogenic/likely pathogenic germline TP53 variant were included in this analysis. We confirmed breast cancer diagnoses and hormone receptor status (estrogen receptor [ER], progesterone receptor [PR], and HER2/neu) through the evaluation of pathology reports, surgical operative notes, consultation reports, and/or medical provider notes. Breast sarcomas and malignant phyllodes tumors were excluded from this analysis. Parity was defined as having reported at least one live-birth. Breastfeeding was defined as the total number of months of lifetime breastfeeding reported. Germline genetic testing reports were examined to confirm the presence of a known germline TP53 variant.
Statistical Analysis
Odds ratios (OR) on contingency tables of breast cancer status vs. dichotomized cumulative breastfeeding duration (e.g., fewer than 12 months, over 12 months) were calculated using Fisher’s Exact Test. Cox Proportional Hazards models were used to calculate the effect of hormonal factors on breast cancer risk. Hazard rates were derived by comparing women based on similar dichotomized breastfeeding duration (e.g., fewer than 3 months, over 3 months) at each monthly timepoint. Parity and number of live births were treated as time-varying covariates, and participants were censored at date of death, end of study, mastectomy, or study drop-out. As mastectomy and breast cancer diagnosis may occur concurrently or within nearly the same timeframe in women with TP53 mutations, women who had a mastectomy (unilateral or bilateral) prior to a cancer diagnosis were censored at the time of mastectomy if it occurred more than one year prior to their diagnosis and excluded from the analyses. Women who reported unilateral or bilateral mastectomy after breast cancer diagnosis were included. Survival curves were visualized using Kaplan-Meier curves and non-parametric graphical representations accounting for time-varying covariate status.(18) All analyses were performed using statistical software R version 3.4,(19) using survival package version 2.38.(20)
Role of the funding source
The funding source for this study had no role in study design, data collection, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
This study evaluated reproductive factors in 152 women with a pathogenic or likely pathogenic germline variant in TP53 (Supplemental Table 1). Eighty-five women (56%) developed at least one breast cancer and 13 (15%) of these women had bilateral synchronous breast cancers. The median age at first breast cancer diagnosis was 32 years (range 20–54 years). Six of these women were post-menopausal at diagnosis. Twenty of the 85 women (23%) were diagnosed with a subsequent primary breast cancer at a median age of 40 years (range 29–63 years), two of whom also developed a third primary breast cancer. Of the 64 first breast cancers with available hormone receptor status data, 60% were ER/PR+ and of the 48 cancers with available HER2/neu status, 57% were HER2/neu+, compared with a prior report of hormone status in women with germline TP53 variants, that showed 76% ER+/67%PR+ and 65% HER2/neu+ breast cancers.(11) Of the 85 women with breast cancer, only one woman reported a risk-reducing bilateral oophorectomy after her breast cancer diagnosis. None of the 67 breast cancer-free women had undergone mastectomy at the time of IIQ completion. None of the women reported significant problems with menstrual cycles or infertility. Fifteen of the 85 women (18%) had a prior cancer diagnosis before developing breast cancer. Of these 15, four reported receiving radiation therapy for their previous cancer. One of the four women developed a subsequent breast cancer in the field of prior radiation therapy, 28 years after her radiation treatment. Two women reported having received chemotherapy prior to breast cancer diagnosis, and one woman received prior immunotherapy.
In parous women, breastfeeding for any length of time was associated with reduced breast cancer risk. The strongest association occurred with lifetime breastfeeding for at least 7 months (hazard ratio [HR] 0.57, 95% confidence interval [CI] 0.33–1.00, p=0.05). This association of reduced breast cancer risk was consistent through at least 12 months of lifetime breastfeeding (HR 0.49, 95% CI 0.26–0.89, p=0.02) (Table 1, Figure 1). Breast cancer risk was reduced with each month of breastfeeding with the log OR decreasing by 0.19 per month in logistic regression models. The protective effect of lifetime breastfeeding for at least 12 months remained consistent after controlling for age of the participant and age at first breast cancer diagnosis (HR 0.48, 95% CI 0.26–0.90, p=0.02). There was no statistically significant difference in the ER/PR status of the breast cancers between women who breastfed for less than or at least 12 months (OR 0.57 for ER+ breast cancer, 95% CI 0.1–3.1, p=0.48). Similarly, there was no difference in the age at cancer diagnosis between women who breastfed for less than or at least 12 months (HR 0.7, 95% CI 0.41–1.2, p=0.23).
Table 1.
Risk of breast cancer associated with each cumulative month of lifetime breastfeeding in women with Li-Fraumeni syndrome due to known germline TP53 variants. Analysis performed only in women who reported at least one live birth.
| Lifetime breastfeeding reported (months) |
Number of women |
Number of women with breast cancer |
Hazard Ratio (vs. < months) |
95% CI | p-value |
|---|---|---|---|---|---|
| ≥ 1 | 67 | 43 | 0.97 | 0.48–1.97 | 0.94 |
| ≥ 2 | 63 | 37 | 0.84 | 0.45–1.57 | 0.59 |
| ≥ 3 | 59 | 34 | 0.73 | 0.41–1.33 | 0.3 |
| ≥ 4 | 57 | 32 | 0.69 | 0.39–1.23 | 0.21 |
| ≥ 5 | 53 | 30 | 0.70 | 0.40–1.24 | 0.22 |
| ≥ 6 | 53 | 30 | 0.70 | 0.40–1.24 | 0.22 |
| ≥ 7 | 48 | 26 | 0.57 | 0.33–1.0 | 0.05 |
| ≥ 8 | 47 | 25 | 0.56 | 0.32–0.99 | 0.04 |
| ≥ 9 | 42 | 22 | 0.55 | 0.31–0.97 | 0.04 |
| ≥ 10 | 39 | 21 | 0.61 | 0.34–1.08 | 0.09 |
| ≥ 11 | 35 | 17 | 0.49 | 0.26–0.89 | 0.02 |
| ≥ 12 | 35 | 17 | 0.49 | 0.26–0.89 | 0.02 |
CI; Confidence intervals. Hazard ratios calculated in comparison to women who breastfed less than the “lifetime breastfeeding months” reported.
Figure 1. Breast cancer risk by cumulative breastfeeding duration among women with Li-Fraumeni syndrome due to known germline TP53 variants who had at least one live birth.
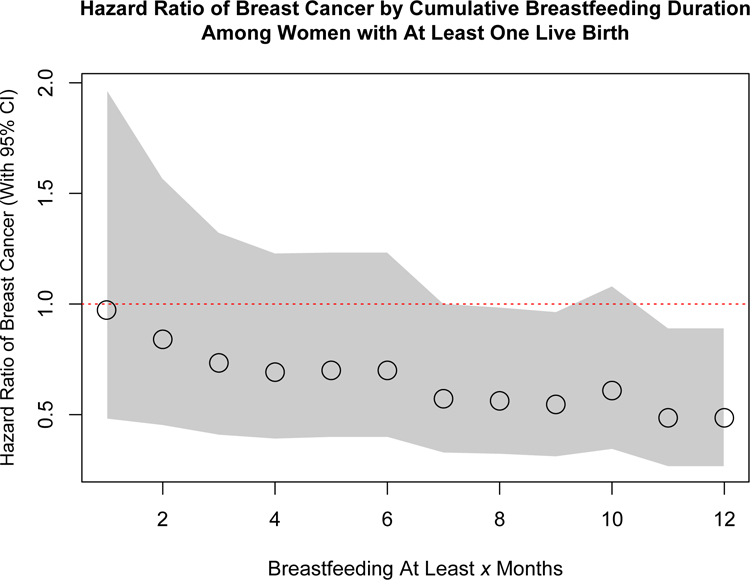
Red dotted line denotes a hazard ratio of 1.0. The circles denote the hazard ratio and the grey area encompasses the corresponding 95% confidence intervals for each added month of cumulative breastfeeding.
There was no observed evidence of a difference in breast cancer risk between nulliparous and parous women with LFS, when parity was independently evaluated as a time-varying covariate, and after adjusting for age at first live birth (HR 1.08, 95% CI 0.65–1.78, p=0.8) (Table 2, Figure 2). When stratifying the data to account for women who had not developed breast cancer and were alive at the age of 40 years, there was no evidence that parity affected breast cancer risk after age 40 years (Table 2). The number of live births in parous women was not associated with significant change in breast cancer risk. However, there was a borderline statistically significant excess risk among women who had their first live birth after age 30 years (HR 2.14 95% CI 0.99–4.6, p=0.05) (Figure 3, Table 2).
Table 2.
Parity as an independent risk factor for breast cancer in women with Li-Fraumeni syndrome due to known germline TP53 variants
| Parameter | Number of women without breast cancer |
Number of women with breast cancer |
Hazard Ratio |
95%CI | p-value |
|---|---|---|---|---|---|
| Effect of parity on breast cancer risk (all women) | |||||
| Nulliparous | 34 | 33 | - | ||
| Parous | 33 | 52 | 1.07 | (0.66, 1.77) | 0.8 |
| Effect of parity on breast cancer risk stratified by age at study entry | |||||
| ≤ 40 years | |||||
| Nulliparous | 43 | 30 | - | ||
| Parous | 43 | 34 | 1. 12 | (0.66,1.91) | 0.7 |
| >40 years | |||||
| Nulliparous | 4 | 3 | - | ||
| Parous | 21 | 11 | 0.85 | (0.24,3.07) | 0.8 |
| Number of live births | |||||
| Nulliparous | 37 | 30 | - | ||
| 1 | 5 | 13 | 1.23 | (0.69,2.19) | 0.5 |
| 2 | 16 | 32 | 1.13 | (0.61,2.09) | 0.7 |
| 3 | 5 | 6 | 0.82 | (0.31,2.20) | 0.7 |
| 4+ | 5 | 3 | 0.29 | (0.04,2.12) | 0.23 |
| Per live birth | 0.93 | (0.76,1.14) | 0.5 | ||
| Age at first live birth (years) | |||||
| Nulliparous | 33 | 33 | - | ||
| <25 | 23 | 16 | 0.71 | (0.38,1.41) | 0.29 |
| 25–29 | 7 | 21 | 1.44 | (0.83,2.97) | 0.26 |
| ≥30 | 5 | 14 | 2.14 | (1.1,5.24) | 0.05 |
Parous= at least one liveborn child
Figure 2. Parity as an independent risk factor for breast cancer in women with Li-Fraumeni syndrome due to known germline TP53 variants.
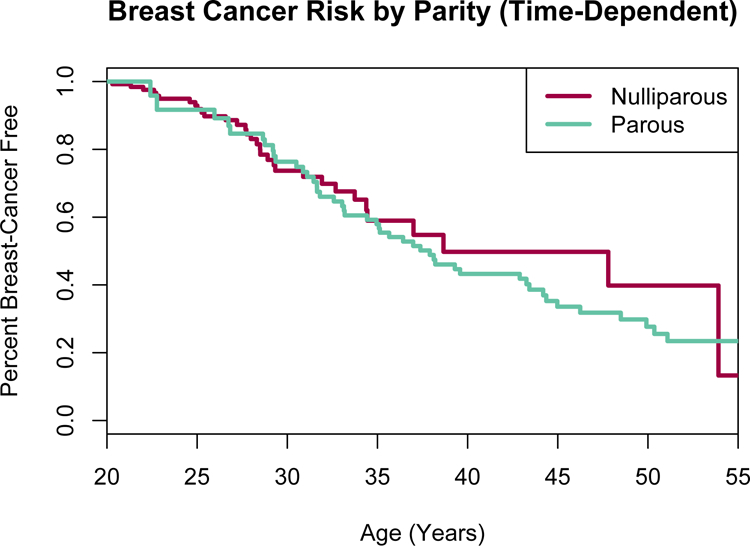
Parous = at least one liveborn child. Parity analyzed as a time-dependent covariate, meaning anytime a woman had a liveborn child, she automatically crossed over to the “parous” group.
Figure 3. Occurrence of breast cancer stratified by age at first livebirth in women with Li-Fraumeni syndrome due to known germline TP53 variants.
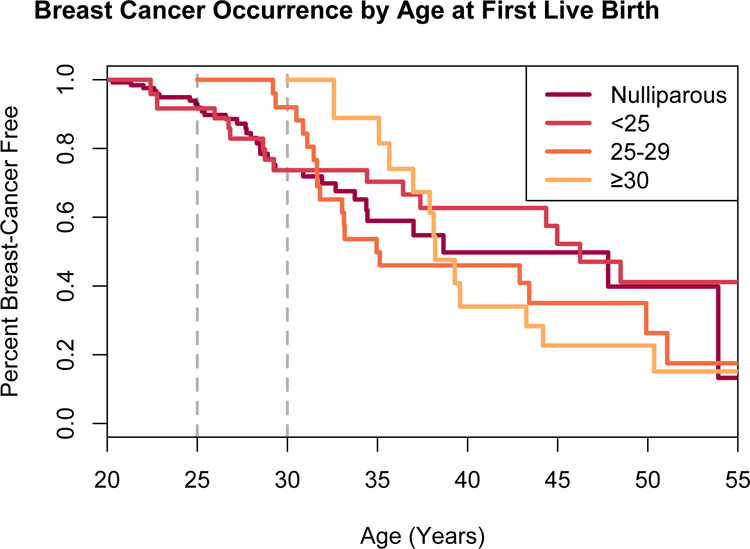
Y-axis is the probability a woman is breast cancer-free, based on age at first live birth in years as a time-dependent covariate.
Breast cancer risk was not associated with self-reported OCP use in women with LFS (OR 2.06; 95% CI 0.79–5.6; p=0.12; age-adjusted analysis conferred a HR=0.89; 95% CI 0.44–1.78; p= 0.7). Assuming continuous use of OCPs for the reported duration, the risk of breast cancer was suggested to slightly increase with increasing duration of OCP use (HR 1.07; 95% CI 1.02–1.12; p=0.01).
The overall median age at menarche for women in this study was 12 years (range 9–18 years). Median age at menarche was not different among women with or without breast cancer by study entry (median age at menarche in women with breast cancer =13 years, range 9–17 years; median age in women without breast cancer = 12 years, range 10–18 years; p>0.05). Our data show that younger age at menarche did not significantly affect breast cancer risk of women with LFS (OR 1.09, 95% CI 0.94–1.27, p=0.24) (Figure 4).
Figure 4. Breast cancer occurrence by age at menarche in women with Li-Fraumeni syndrome due to known germline TP53 variants.
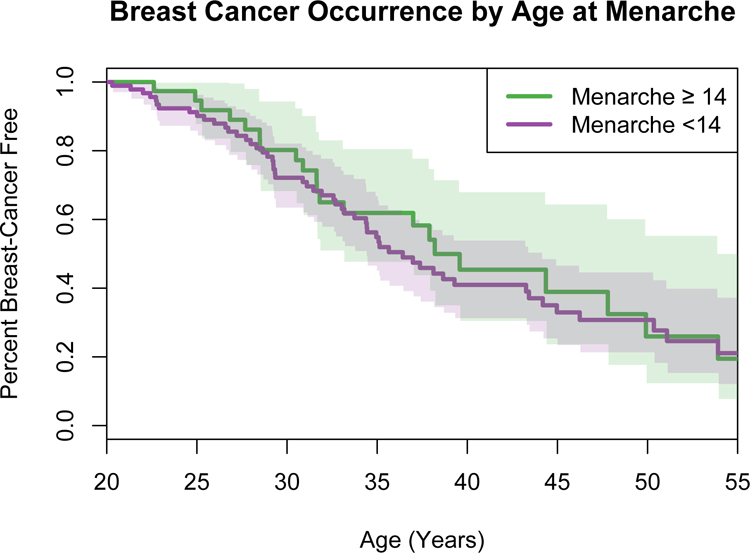
Y-axis depicts the probability a woman is breast cancer-free by age in years on the x-axis. Colored areas show the corresponding 95% confidence intervals. Green line = age at menarche at least 14 years or older (n=39), purple line = age at menarche under 14 years (n=104). Age at menarche was not available for nine women.
LFS is considered a radiation-sensitive syndrome, and genotoxic therapy such as chemotherapy and/or radiation therapy has been shown to increase the risk of development of subsequent primary malignancies in a mouse model of LFS.(21) Therefore, we performed the same statistical analyses excluding the five women who received genotoxic therapy prior to their breast cancer diagnosis. In this evaluation of 81 women, the associations between breastfeeding, parity, age at first livebirth, OCP use and age at menarche were robust and consistent with the results reported above (data not shown).
Discussion
Female reproductive factors are associated with the risk of breast cancer in the general population and among carriers of pathogenic germline variants in BRCA1/2.(15, 22–25) However, this association has not yet been explored in germline TP53 mutation carriers. We report a protective effect of breastfeeding on breast cancer risk in TP53 mutation-carriers, with breastfeeding of at least 7 months conferring a 43% risk reduction. This effect of breastfeeding is consistent with findings in the general population and among BRCA1/2 mutation-carriers. In the general population, large-scale pooled epidemiological analyses have reported that breast cancer risk decreased by over 4% for every 12 months of breastfeeding.(13) Evaluations of BRCA1/2 cohorts have shown consistently that breastfeeding is protective in BRCA1 carriers, with up to a 32% reduction in breast cancer in women who breastfed for 12 months.(17, 26)
Studies of parity and breast cancer risk in both the general population and in women with pathogenic germline BRCA1/2 variants have had variable results. Some have reported a protective effect of younger age at first childbirth and higher risk with first live birth at older ages.(25, 27) However, other studies in women with BRCA1/2 mutations vs non-carriers showed no difference in breast cancer risk by mean age at first live birth; or between parous and nulliparous women.(23) Additional studies of BRCA1/2-associated breast cancer stratified outcomes of full-term pregnancies or looked independently at age at first full-term pregnancy and number of children.(23, 25, 27) We defined parity as a pregnancy resulting in reported live birth, but were unable to capture other outcomes of full-term pregnancies such as stillbirth or late fetal loss. Our analyses did not find that parity was associated with breast cancer risk in LFS, however our data suggest that women with age of live birth after age 30 years may have an increased breast cancer risk, regardless of how long they breastfed.
In the general population, OCP use has been associated with a slightly higher breast cancer risk that appears to decrease after cessation of OCPs.(28, 29) Reports in BRCA1/2 cohorts have shown slightly conflicting results between studies and depending on the particular gene, with some data showing increasing duration of OCP being associated with increased breast cancer risk.(26, 27, 30) Our data did not identify an association between OCPs use and breast cancer risk in LFS. However, it must be noted that only 25 of the 154 women in the study reported never having used OCP, making our comparison group limited by sample size.
In this LFS cohort, age at menarche was not associated with alteration in breast cancer risk in contrast to the slightly higher risk of breast cancer with younger age at menarche in the general population.(27, 31, 32) The age of 14 years was chosen as a statistical cut-off based on global estimates of average age at menarche. The lack of significant association between menarchal age and breast cancer could be due to the earlier age at onset of breast cancer in LFS (median age at diagnosis of 32 years in LFS and 62 years in the general population), with fewer cumulative ovulatory cycles and reproductive hormone exposure prior to breast cancer diagnosis, compared to the women in the general population and those with BRCA1/2 predisposition.
The effect of genotoxic cancer therapy on subsequent development of malignancy in LFS has not been quantified in humans but mouse models show that chemotherapy and radiation therapy increase the risk of subsequent cancers.(21) The exclusion of women who received genotoxic therapy prior to breast cancer diagnosis did not significantly change the effect of each reproductive factor on breast cancer risk, suggesting that these factors may impact breast cancer risk independent of prior therapy in these women. However, the understanding of the potential impact cancer therapy has on subsequent malignancies and breast cancer in women with LFS is an important factor to consider in future larger cohort studies of LFS.
The relatively small sample size is a limitation of this study. However, an important strength of our study was the detailed clinical data, which permitted a comprehensive evaluation of the reproductive factors in breast cancer in LFS. Since our data is self-reported, there is a potential for survival bias in women who report their prior cancer history, and we acknowledge the critical need for continued follow-up of women with LFS who have not yet developed breast cancer. Breastfeeding was reported as lifetime duration, without detailed stratification of breastfeeding per childbirth for women with more than one liveborn child. Of the 86 women in our study who developed breast cancer, 15 (17%) had a previous cancer diagnosis. However, none of these women reported medical/surgical menopause or fertility concerns prior to breast cancer diagnosis.
It is important to consider the medical benefits of breastfeeding in the context of cancer screening and prevention in women with LFS. The American Academy of Pediatrics and World Health Organizations, among other expert consensus, strongly recommend breastfeeding for its medical and emotional benefits in infants and mothers, such as decreased post-partum bleeding in the mother and decreased occurrence of infections and immune-mediated disorders in the infant.(33) In LFS, where the median age of breast cancer development is 32–33 years, (11, 12) and breast cancer accounts for over a quarter of the cancer diagnoses,(12) balancing a woman’s reproductive choices such as breastfeeding against the high risk of early-onset breast cancer is an important discussion. Women with LFS who continue to have breast tissue through their reproductive years have the additional burden of screening with annual breast MRI +/−mammography.(34) Further consideration of cancer screening recommendations during pregnancy and lactation are important to discuss on a continuum in women with LFS.
In conclusion, we report a statistically significant protective effect of breastfeeding on breast cancer risk in LFS. Parity and OCP use were not seen to be independent risk factors for breast cancer. While we acknowledge that these results require replication in a larger sample of women with LFS due to pathogenic germline TP53 variants, our data provide critical information to build future studies of breast cancer and hormonal carcinogenesis in LFS. If confirmed, our results suggest a main effect of breastfeeding among the reproductive risk factors for breast cancer risk in LFS. This may inform clinical and reproductive decision-making in women with LFS, specifically those who are weighing their reproductive options and established benefits of breastfeeding against that of prophylactic risk-reducing mastectomies.
Supplementary Material
Highlights.
Early-onset breast cancer is the most frequent cancer in Li-Fraumeni syndrome (LFS)
Breastfeeding reduces breast cancer risk in women with LFS
Breast cancer risk reduced most significantly with at least 7 months breastfeeding
Parity, age at menarche, oral contraceptive use do not affect breast cancer risk
Older age at first livebirth may slightly increase breast cancer risk in LFS
Acknowledgements
We thank the participants for taking the time to contribute to this study. Janet Bracci, RN, Kathy Nichols, RN and Nicole Dupree-Battle, MPH, Westat Inc. provided excellent study support. This work was supported by the intramural research program of the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors of this manuscript have no conflicts of interest to declare.
Declaration of interests
All the authors have no conflicts of interest to declare.
References
- 1.Schneider K, Zelley K, Nichols KE, Garber J. Li-Fraumeni Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)) Seattle (WA)1993. [Google Scholar]
- 2.Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009;27(8):1250–6. [DOI] [PubMed] [Google Scholar]
- 3.Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016;122(23):3673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li FP, Fraumeni JF Jr., Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res 1988;48(18):5358–62. [PubMed] [Google Scholar]
- 5.Birch JM, Hartley AL, Tricker KJ, Prosser J, Condie A, Kelsey AM, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994;54(5):1298–304. [PubMed] [Google Scholar]
- 6.Eeles RA. Germline mutations in the TP53 gene. Cancer Surv 1995;25:101–24. [PubMed] [Google Scholar]
- 7.Malkin D Li-fraumeni syndrome. Genes Cancer 2011;2(4):475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guha T, Malkin D. Inherited TP53 Mutations and the Li-Fraumeni Syndrome. Cold Spring Harb Perspect Med 2017;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chompret A, Brugieres L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000;82(12):1932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustbader ED, Williams WR, Bondy ML, Strom S, Strong LC. Segregation analysis of cancer in families of childhood soft-tissue-sarcoma patients. Am J Hum Genet 1992;51(2):344–56. [PMC free article] [PubMed] [Google Scholar]
- 11.Masciari S, Dillon DA, Rath M, Robson M, Weitzel JN, Balmana J, et al. Breast cancer phenotype in women with TP53 germline mutations: a Li-Fraumeni syndrome consortium effort. Breast Cancer Res Treat 2012;133(3):1125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amadou A, Waddington Achatz MI, Hainaut P. Revisiting tumor patterns and penetrance in germline TP53 mutation carriers: temporal phases of Li-Fraumeni syndrome. Curr Opin Oncol 2018;30(1):23–9. [DOI] [PubMed] [Google Scholar]
- 13.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol 2001;2(3):133–40. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Group on Hormonal Factors in Breast C. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360(9328):187–95. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein L Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia 2002;7(1):3–15. [DOI] [PubMed] [Google Scholar]
- 16.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature 1983;303(5920):767–70. [DOI] [PubMed] [Google Scholar]
- 17.Kotsopoulos J, Lubinski J, Salmena L, Lynch HT, Kim-Sing C, Foulkes WD, et al. Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res 2012;14(2):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 1984;3(1):35–44. [DOI] [PubMed] [Google Scholar]
- 19.Team RC. R: A Language and Environment for Statistical Computing 2017.
- 20.Therneau TMG, Patricia M. Modelling Survival Data: Extending the Cox Model Dietz KG M; Krickeberg K; Samet J; Tsiatis A, editor. New York: Springer; 2000. [Google Scholar]
- 21.Kasper E, Angot E, Colasse E, Nicol L, Sabourin JC, Adriouch S, et al. Contribution of genotoxic anticancer treatments to the development of multiple primary tumours in the context of germline TP53 mutations. Eur J Cancer 2018;101:254–62. [DOI] [PubMed] [Google Scholar]
- 22.Gronwald J, Byrski T, Huzarski T, Cybulski C, Sun P, Tulman A, et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat 2006;95(2):105–9. [DOI] [PubMed] [Google Scholar]
- 23.Andrieu N, Goldgar DE, Easton DF, Rookus M, Brohet R, Antoniou AC, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer Inst 2006;98(8):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotsopoulos J, Gronwald J, Lynch HT, Eisen A, Neuhausen SL, Tung N, et al. Age at first full-term birth and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2018. [DOI] [PubMed]
- 25.Evans DG, Harkness EF, Howel S, Woodward ER, Howell A, Lalloo F. Young age at first pregnancy does protect against early onset breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2018;167(3):779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toss A, Grandi G, Cagnacci A, Marcheselli L, Pavesi S, De Matteis E, et al. The impact of reproductive life on breast cancer risk in women with family history or BRCA mutation. Oncotarget 2017;8(6):9144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park B, Hopper JL, Win AK, Dowty JG, Sung HK, Ahn C, et al. Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget 2017;8(60):102110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collaborative Group on Hormonal Factors in Breast C. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996;347(9017):1713–27. [DOI] [PubMed] [Google Scholar]
- 29.Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017;377(23):2228–39. [DOI] [PubMed] [Google Scholar]
- 30.Narod SA. Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer 2002;2(2):113–23. [DOI] [PubMed] [Google Scholar]
- 31.Kotsopoulos J, Lubinski J, Lynch HT, Neuhausen SL, Ghadirian P, Isaacs C, et al. Age at menarche and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Causes Control 2005;16(6):667–74. [DOI] [PubMed] [Google Scholar]
- 32.Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13(11):1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med 2012;7(5):323–4. [DOI] [PubMed] [Google Scholar]
- 34.Mai PL, Khincha PP, Loud JT, DeCastro RM, Bremer RC, Peters JA, et al. Prevalence of Cancer at Baseline Screening in the National Cancer Institute Li-Fraumeni Syndrome Cohort. JAMA Oncol 2017;3(12):1640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


