Abstract
SARS-CoV-2, the cause of COVID-19, has generated a global emergency. The endothelium is a target of SARS-CoV-2, generating endothelial dysfunction, an essential step for the development of cardiovascular complications. The number of endothelial progenitor cells acts as an indicator of vascular damage. However, its role in SARS-CoV-2 is unknown. The aim of this study was to quantify the number of endothelial colony forming cells (ECFCs) and assess for the first time if there is a significant increase after SARS-CoV-2 infection. This study also evaluates whether the number of ECFC is related to the presence of pulmonary embolism (PE), and if this increase correlates with any of the clinical parameters studied. A total of 63 subjects were recruited including 32 subjects 3-months after overcoming COVID-19 and 31 healthy controls. The results confirm the presence of vascular sequelae in post-COVID-19 patients, with an abnormal increase in the number of ECFCs in blood circulation compared to controls (2.81 ± 2.33 vs 1.23 ± 1.86, P = 0.001). There was no difference in ECFC production in COVID-19 who presented acute PE compared to those that did not (3.21 ± 2.49 vs 2.50 ± 2.23, P > 0.05). The appearance of ECFC colonies in COVID-19 patients was significantly related to male gender (P = 0.003), the presence of systemic hypertension (P = 0.01) and elevated hemoglobin levels (P = 0.02) at the time of ECFC isolation and lower PaO2 levels (P = 0.01) at admission. Whether these results indicate a prompt response of the patient to repair the damaged endothelium or reflect a postinfection injury that will persist in time is not known.
Abbreviations: 6MWT, 6-minute walk test; ACE-2, Angiotensin-converting enzyme 2; BMI, Body mass index; CRP, C-reactive protein; DD, Dimer-D; DLCO, Carbon monoxide diffusing capacity; DLP, Dyslipidemia; DM, Diabetes mellitus; ECFC, Endothelial colony forming cell; EPC, Endothelial progenitor cell; EPO, Erythropoietin; FEV, Forced expiratory volume; FIB, Fibrinogen; FVC, Forced vital capacity; Hb, Hemoglobin; HTA, Arterial hypertension; HTC, Hematocrit; LDH, Lactate dehydrogenase; Lym, Lymphocytes; MF, Maximum ferritin; PaO2, Partial pressure of oxygen; PBMC's, Peripheral blood mononuclear cells; PE, Pulmonary embolism; RV, Residual volume; TLC, Total lung capacity
At A Glance Commentary.
Poyatos P, et al.
Background
The endothelium is a target of SARS-CoV-2 infection causing severe endothelial damage. The number of endothelial progenitor cells acts as a biomarker of vascular damage.
Translational Significance
Our results show for the first time, an abnormal increase of endothelial progenitor cells in COVID-19 patients 3 months after SARS-CoV-2 infection compared to controls. There were no differences in endothelial progenitor cell production between post-COVID-19 patients who suffered an acute pulmonary embolism and those who did not. Patients with lower PaO2 levels at admission showed higher numbers of endothelial progenitor cells. These results confirm the presence of vascular sequelae in post-COVID-19 patients.
Alt-text: Unlabelled box
INTRODUCTION
Type 2 Coronavirus causing severe acute respiratory syndrome, SARS-CoV-2 and coronavirus disease 2019 (COVID-19) is an ongoing complex pandemic. The endothelium is one of the main targets of SARS-CoV-2 infection and it has been suggested that SARS-CoV-2 directly infects endothelial cells binding to the angiotensin-converting enzyme 2 (ACE-2) receptor and causes severe endothelial damage.1 Postmortem COVID-19 patient samples have shown the presence of intracellular SARS-CoV-2 viral structures within endothelial cells1 together with evidence of endothelial cell damage.
The endothelium is a dynamic organ involved in a wide range of vital functions. Endothelial dysfunction produces an unbalanced vascular homeostasis and represents a key step in the development of cardiovascular complications including coagulopathies and thromboembolisms.2 Recent studies showed that 30%–70% of COVID-19 patients admitted to intensive care units (ICUs) developed blood clots in the deep veins of legs or lungs. Wichmann et al2 reported that out of the total 174 of autopsies from COVID-19 patients performed, 33% of the patients showed massive pulmonary embolism (PE) with or without underlying deep vein thrombosis, despite the absence of a history of venous thromboembolism, indicating the possibility of an in situ pulmonary thrombosis.
Endothelial progenitor cell (EPC) number and function are shown to be important biomarkers of vascular injury for a wide range of diseases.3 Under pathological conditions, EPCs are thought to be mobilized from the bone marrow or from its niche in the vessel wall and recruited to sites of vascular injury with the aim to promote vascular regeneration.4 , 5 Kong et al5 demonstrated that EPC mobilization improved the repair of injured arteries by facilitating re-endothelialization. Similarly, Werner et al6 showed that vascular lesion and neointimal formation was moderated by bone marrow-derived progenitor cells. Recently, circulating bone marrow-derived CD34+CD31+CD146− EPCs has been shown to be increased in COVID-19 patients.7 However, the role of EPCs in SARS-CoV-2 is unknown.
EPCs considered to fulfil the criteria of a true EPC, have been named late outgrowth endothelial cells or endothelial colony forming cells (ECFCs).8 While the literature points out to the existence of more than one population of circulating EPCs, ECFC are the only ones with a robust clonogenic and proliferative potential, express endothelial markers, form tubule structures in vitro and support de novo angiogenesis when transplanted in vivo into immunodeficient mice.9 Additionally, ECFCs can be expanded in vitro and operate as a disease in vitro model to understand the disease pathology and to develop potential novel treatments.
It is not fully understood which factors trigger the release of ECFCs into the blood´s circulation. Previous reports have shown a significant increase of ECFCs 6-12h after coronary artery bypass surgery or burn injury, and 7 days after myocardial infarction.10 , 11 EPCs migrate in response to ischemia, to hypoxic sites following chemokine gradients, where they experience in situ differentiation and finally take part in the formation of new blood vessels.12 Under hypoxic conditions, there is an increase in the expression of EPC-attracting factors, induced by hypoxic-inducible factor-1, enhancing nitric oxide and erythropoietin (EPO) levels in the bone marrow, and mobilizing EPCs into circulation.13
The present study aims to evaluate for the first time, whether an increase of ECFCs is present after SARS-CoV-2 infection and whether the number of ECFCs differs from COVID-19 patients who suffered an acute PE during admission than the patients that did not.
MATERIALS AND METHODS
We recruited a total of n = 32 3 months post-COVID-19 patients and n = 31 healthy controls. N = 14 COVID-19 patients suffered PE during admission, detected by computed tomography (CT) examination. Post-COVID-19 patients included in the study were patients discharged from the Pulmonology Service with severe pneumonia and a diagnosis of COVID-19 by positive PCR. COVID-19 patients were classified based on whether they had a diagnosis of pulmonary embolism. Healthy control subjects were non-hospitalized and non-staff volunteers residing in our city and negative for SARS-CoV-2 infection. Healthy controls were confirmed negative for SARS-CoV-2 infection at the time of ECFC isolation by PCR and did not suffer any previous COVID-19 infection. ECFCs from all subjects were isolated during the months of March/April and October 2020 and were not vaccinated. Subject characteristics are described in Table 1 . The study was approved by the Clinical Research Ethic Committee from Hospital Universitari de Girona Dr. Josep Trueta (CEIm_COVID-Pneumo 2020.0099) in accordance with the Declaration of Helsinki. All subjects gave written informed consent.
Table 1.
Clinical and cellular parameters collected from COVID-19 patients and healthy controls.
Clinical characteristics of 3-months post-COVID-19 patients, with or w/o pulmonary embolism (PE) and healthy controls. Percentage of ECFC colonies, number of ECFC colonies and time for ECFC to appear in 3-months post-COVID-19 patients with or w/o PE and healthy controls, Mann-Whitney test, *p<0.05. Relationship between appearance of ECFC colonies and clinical characteristics in COVID-19 patients, Chi-square test, *p<0.05.
| Variables | Total COVID-19, n=32 | CL, n=31 | P-value (Total COVID-19 vs CL) | COVID-19 w/o PE, n=18 | COVID-19 with PE, n=14 | P-value (COVID-19 with PE vs w/o PE) |
|---|---|---|---|---|---|---|
| Age, years | 64,2 ± 13,6 | 58,6 ± 8,21 | ns | 67,5 ± 10,2 | 59,9 ± 16,5 | ns |
| Male sex n (%) | 27 (84,4%) | 20 (64,5%) | ns | 16 (88,9%) | 11 (78,6%) | ns |
| BMI (Kg/m2) | 27,5 ± 3,71 | 26,7 ± 4,78 | ns | 27,1 ± 3,10 | 27,9 ± 4,47 | ns |
| Smokers (%) | 1 (3,1%) | 1 (3,23%) | ns | 0 (0 %) | 1 (7,14%) | ns |
| No smokers (%) | 15 (46,9%) | 19 (31,3%) | ns | 5 (27,8 %) | 10 (71,4%) | ns |
| Ex-smokers (%) | 16 (50,0%) | 11 (35,5%) | ns | 13 (72,2%) | 3 (21,4%) | ns |
| HTA (%) | 17 (53,1%) | 7 (22,6%) | P<0.05 * | 8 (44,4%) | 9 (64,3%) | ns |
| DM (%) | 4 (12,5%) | 0 (0%) | P<0.05 * | 2 (11,1%) | 2 (14,3%) | ns |
| DLP (%) | 5 (15,6%) | 2 (6,45%) | ns | 3 (16,7%) | 2 (14,3%) | ns |
| FVC (%) | 98,5 ± 16,2 | 95,2 ± 13,8 | ns | 98,0 ± 18,5 | 98,8 ± 12,9 | ns |
| FEV1 (%) | 92,8 ± 27,9 | 100 ± 15,1 | ns | 90,3 ± 31,7 | 95,4 ± 21,8 | ns |
| FEV1/FVC (%) | 94,3 ± 22,5 | 81,0 ± 5,32 | P<0.0001**** | 92,9 ± 27,5 | 95,8 ± 14,1 | ns |
| TLC (L) | 109 ± 25,8 | ND | x | 113 ± 33,2 | 104 ± 13,9 | ns |
| RV (L) | 109 ± 25,9 | ND | x | 106 ± 20,7 | 114 ± 31,3 | ns |
| DLCO (%) | 74,3 ± 16,7 | ND | x | 72,9 ± 20,3 | 74,5 ± 12,3 | ns |
| 6MWT (m) | 364 ± 72,3 | ND | x | 343 ± 82,7 | 385 ± 55,5 | ns |
| Hb (g/dL) | 14,1 ± 1,80 | 14,4 ± 1,60 | ns | 13,7 ± 1,78 | 14,7 ± 1,77 | ns |
| HTC (%) | 43,2 ± 4,89 | 43,0 ± 3,92 | ns | 42,3 ± 5,20 | 44,6 ± 4,28 | ns |
| Lym (K/mcL) | 2,23 ± 0,87 | 1,94 ± 0,54 | ns | 2,33 ± 0,95 | 2,08 ± 0,74 | ns |
| LDH (mg/dL) | 193 ± 32,6 | 180 ± 18,4 | ns | 188 ± 34,6 | 201 ± 29,0 | ns |
| MF (ng/mL) | 153 ± 117 | 108 ± 93,9 | ns | 149 ± 105 | 161 ± 146 | ns |
| CRP (mg/dL) | 0,31 ± 0,58 | 0,10 ± 0,06 | ns | 0,42 ± 0,70 | 0,10 ± 0,05 | ns |
| Troponin (ng/L) | 17,94 ± 12,7 | 6,37 ± 3,65 | P<0.0001**** | 20,2 ± 12,7 | 14,2 ± 12,4 | ns |
| Positive DD (%) | 2 (6,25 %) | 1 (3,23 %) | ns | 2 (12,5 %) | 0 (0%) | ns |
| FIB (mg/dL) | 396 ± 76,3 | 410 ± 62,2 | ns | 408 ± 73,0 | 372 ± 82,4 | ns |
| Appearance of ECFC colonies (%) | 27 (84,4%) | 15 (48,4%) | P<0.01 ** | 14 (77,8%) | 13 (92,9%) | ns |
| Number of ECFC colonies | 2,81 ± 2,33 | 1,23 ± 1,86 | P<0.01** | 2,50 ± 2,23 | 3,21 ± 2,49 | ns |
| Time for ECFC to appear (days) | 10,9 ± 4,39 | 14,3 ± 4,76 | P<0.01** | 11,1 ± 3,16 | 10,7 ± 5,54 | ns |
Abbreviations and acronyms: Healthy control (CL); Pulmonary embolism (PE); Body mass index (BMI); Arterial hypertension (HTA); Diabetes mellitus (DM); Dyslipidemia (DLP); Forced vital capacity (FVC); Forced expiratory volume (FEV); Total lung capacity (TLC); Residual volume (RV); Carbon monoxide diffusing capacity (DLCO); Six minute walk test (6MWT); Hemoglobin (Hb); Hematocrit (HTC); Lymphocytes (Lym); Lactate Dehydrogenase (LDH); Maximum Ferritin (MF); C reactive protein (CRP); Dimer-D (DD); Fibrinogen (FIB); Endothelial colony-forming cells (ECFC).
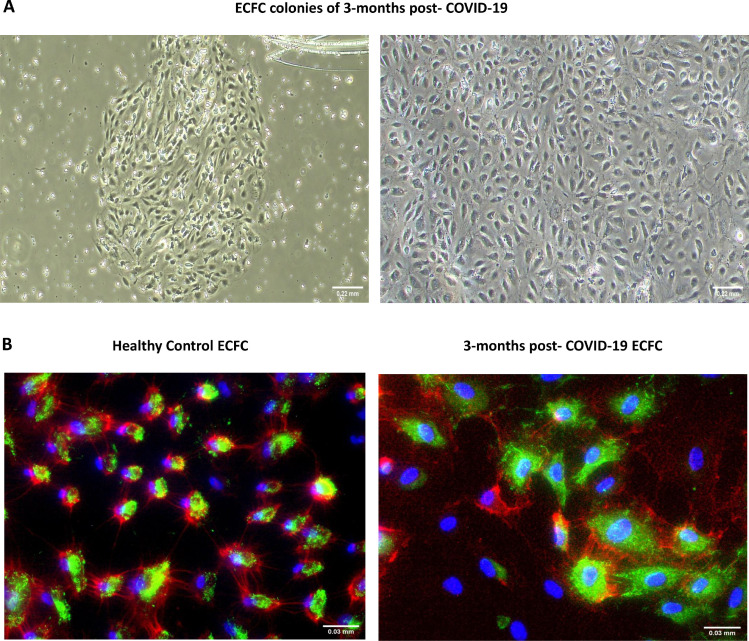
The isolation of ECFC from all subjects and immunofluorescence analysis were performed as previously described14 , 15 (Fig 1 , A and B). Briefly, peripheral blood mononuclear cells (PBMC's) were isolated by buoyant density centrifugation over Ficoll‐Paque Plus (GE Healthcare), resuspended in endothelial cell medium (ECM‐2 medium, ScienceCell, Research Laboratories) supplemented with 20% fetal bovine serum (FBS hyclone, Cytiva) and 1% penicillin-streptomycin (P/S, Lonza); and plated onto type‐1 rat‐tail collagen‐coated 6‐well tissue culture plates (BD Biosciences). Cells were incubated at 37°C, 5% CO2, and 95% relative humidity for 3–4 weeks.15 The medium was changed every 2 days until the appearance of ECFC colonies. Cells were expanded in ECM-2 culture medium supplemented with 10% FBS and were cryopreserved in 90% FBS with 10% DMSO.
Fig 1.
A, ECFC colonies of 3-months post-COVID-19 patients resembling typical cobblestone morphology appeared within 1-3 weeks of culture (4×). B, Immunofluorescence staining for CD31 (red), vWF (Green), nuclei (blue) of endothelial cells from healthy controls and post-COVID-19 patients (40×). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Colonies were counted after appearance, as an association of 2 or more individual cells together with the presence of a typical cobblestone endothelial morphology of ECFC. These colonies may appear on consecutive days and the number of colonies used in this study is the total number of colonies generated by a subject throughout their culture. The number of days it took for the first colony to appear was also quantified. All these parameters were evaluated between post-COVID-19 patients compared to healthy controls and between COVID-19 patients who suffered PE during admission than those that did not.
Statistical analyses were performed using GraphPad Prism 7 software, version 7.0e. Data are shown as mean ± SD. Pairwise comparisons were performed using t-student test or Mann-Whitney U test for non-normally distributed variables and Chi squared test in categorical variables. Pearson or Spearman rank correlation coefficient was used as a hypothesis test to study the dependence between 2 random variables. Statistical significance was assumed if P ≤ 0.05.
RESULTS AND DISCUSSION
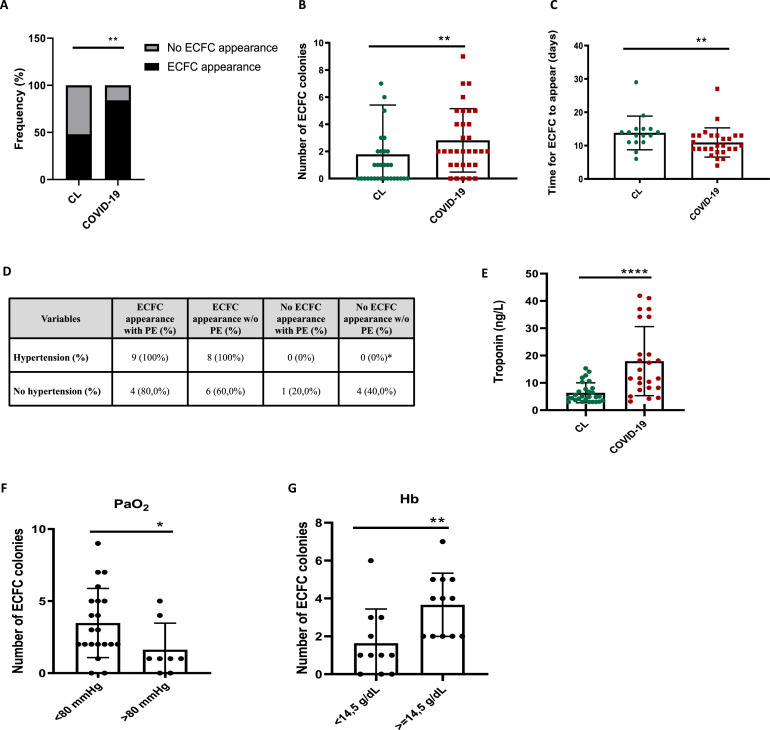
Our results showed a significant increase in ECFC production in 3 months post-COVID-19 patients compared to healthy subjects. Twenty-seven COVID-19 patients out of the total of 32 had ECFC colonies at a particularly high level of 84.4% compared to 48.4% of ECFC isolation in healthy subjects (Fig 2 , A; Table 1). The average number of colonies in post-COVID-19 patients was also higher compared to healthy subjects (Fig 2, B; Table 1) and the time needed for the colonies to appear also differed between patients and controls (Fig 2, C; Table 1). Additionally, there was no difference regarding the presence and the number of ECFCs between COVID-19 patients who suffered PE during admission than those that did not (Table 1).
Fig 2.
A–C, Percentage of appearance and no appearance of ECFC colonies, number and time for ECFC colonies to appear in healthy controls and 3 months post-COVID-19 patients, Mann-Whitney test **P < 0.01. D, Relationship between appearance of ECFC colonies and presence of hypertension in COVID-19 patients with or w/o PE, chi-square test, *P < 0.05 ECFC appearance vs no ECFC appearance w/o PE. E, Levels of troponin (ng/L) in healthy controls and post-COVID-19 patients at 3 months after SARS-CoV-2 infection. F and G, Number of ECFC colonies and levels of PaO2 (F) at admission *P < 0.05 and Hb (G) **P < 0.01 of 3 months post-COVID-19 patients, Mann-Whitney test. (Color version of figure is available online.)
In our series, the appearance of ECFC colonies in COVID-19 patients was significantly related to male gender (92.6%) and the presence of systemic hypertension (100%), both known risk factors for COVID-19 (Fig 2, D). In addition, although troponin levels were significantly higher in 3 months post-COVID-19 patients compared to healthy controls (Fig 2, E and Table 1), no correlation was found between troponin levels and the number or appearance of ECFC colonies. Interestingly, patients who had higher numbers of ECFC colonies presented levels of hemoglobin (Hb) above the median value (≥14.5 g/dL) at the time of ECFC isolation and higher hypoxemia with PaO2 levels below the median values (<80 mmHg) at admission (Fig 2, F–G). Bahlmann et al16 showed that EPO was able to enhance progenitor cell mobilization in both patients and healthy subjects and increase at a dose-dependent manner EPC angiogenic potential in vitro. Our study is the first to show that 3 months post-COVID-19 patients with greater amounts of Hb levels and more severe hypoxic conditions, were able to generate higher numbers of ECFCs when compared to patients with higher PaO2 levels. Whether these results indicate a counteractive or protective patient response to quickly restore the injured endothelium or it reflects greater vascular damage is not known and deserves further study.
Although there is literature about the importance of EPCs and their role in blood clot development and pulmonary embolism it remains largely understudied. Some observational studies hypothesize and support that EPCs would have a vascular protective role in venous thrombotic disease.17 Others demonstrated that circulating EPCs can accelerate thrombus recanalization by restoring damaged endothelium and enhancing neovascularization.18 , 19 Certainty, the renewal of the endothelial layer is crucial for the prevention of thrombus development or recurrency. In our series, there was no difference in the number of colonies generated between COVID-19 patients with or without PE and both subpopulations showed similar Hb levels. No significant differences were found between both populations in the clinical parameters analyzed (Table 1 and Supplementary Table 1). These results indicate that the rise of ECFC in COVID-19 is related to the infection itself and not to the development of PE.
EPCs are considered promising non-invasive surrogates providing insights on endothelial function status. Whether they can be used as biomarkers of endothelial damage in post-COVID-19 patients, and whether its monitoring could become a marker of long-term effects of coronavirus (long COVID) or response to therapy is still unknown.
This study has some limitations. Despite that our data shows a vascular dysfunction in post-COVID-19 patients does not indicate the ability of ECFC generation to predict better cardiopulmonary outcomes or reduce days of hospitalization. Additionally, larger cohorts and longitudinal studies are needed to evaluate whether this increase in ECFCs is transient until the patient's endothelium is repaired or becomes permanent in some individuals that could be associated with long-COVID effects. Another limitation of this study is that some conditions such as hypertension and diabetes are common among COVID-19 patients, and it could be difficult to interpret whether the elevated number of ECFCs in patients with COVID-19 was related to the presence of this underlying condition or as a consequence of the viral infection. Nevertheless, our data showed that the increase of ECFC in COVID-19 patients versus controls seems to be related to the infection rather than the presence of such co-morbidities (Supplementary Figs 1 and 2).
In summary, the results of our study identify for the first time, the presence of vascular sequelae in patients 3 months post-SARS-CoV-2 infection with an abnormally elevated ECFC number in the patient's peripheral blood circulation irrespective of whether they presented an acute PE or not. This data infers that the higher number of ECFC is related to the infection itself rather than subsequent PE episode.
Acknowledgments
Conflicts of interest: All the authors have read the journal's policy on conflicts of interest, declaring no conflicts of interest. All the authors have read the journal's authorship agreement.
This research was supported by the funding from a Miguel Servet grant from the Institute of Health Carlos III (CP17/00114), Spanish Society of Respiratory Medicine (SEPAR), Catalan Society of Pneumology (SOCAP), Menarini Laboratories and PI18/00960 from the Institute of Health Carlos III, Spain. P. Poyatos was a recipient of a Banco Santander-University of Girona grant (IFUdG2021). Cofunding was provided by the Fondo Europeo de Desarrollo Regional (FEDER); “Una manera de hacer Europa.”
Author contributions are as follows: P. Poyatos, N. Luque: Acquisition of information, isolation of ECFC from all patients and healthy controls, drafting of manuscript and critical revision. S. Eizaguirre, G. Sabater, L. Sebastián, I. F. Albesa: Patient and clinical parameters recruitment of all subjects included in the study. M. Peracaula: Acquisition of information and isolation of ECFC colonies. M. Boixadé: Technical support. R. Orriols: Critical revision of the manuscript. O. Tura-Ceide: Report conception and design, acquisition of information, drafting of manuscript and critical revision.
The authors acknowledge the Clinical laboratory from Parc Hospitalari Martí i Julià of Salt for their support, healthy volunteers for providing the samples and Ainhoa Garcia for technical assistance.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.trsl.2022.01.004.
Appendix. Supplementary materials
References
- 1.Pesaresi M, Pirani F, Tagliabracci A, et al. SARS-CoV-2 identification in lungs, heart and kidney specimens by transmission and scanning electron microscopy. Eur Rev Med Pharmacol Sci. 2020;24:5186–5188. doi: 10.26355/eurrev_202005_21217. [DOI] [PubMed] [Google Scholar]
- 2.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen S, McDonald SP, Coates PTH, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci. 2011;120:263–283. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- 4.Fujisawa T, Tura-Ceide O, Hunter A, et al. Endothelial Progenitor cells do not originate from the bone marrow. Circulation. 2019;140:1524–1526. doi: 10.1161/CIRCULATIONAHA.119.042351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong D, Melo LG, Gnecchi M, et al. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110:2039–2046. doi: 10.1161/01.CIR.0000143161.01901.BD. [DOI] [PubMed] [Google Scholar]
- 6.Werner N, Priller J, Laufs U, et al. Bone marrow–derived progenitor cells modulate vascular reendothelialization and neointimal formation. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.ATV.0000036417.43987.D8. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso P, Gidaro A, Gregato G, et al. Circulating endothelial progenitors are increased in COVID-19 patients and correlate with SARS-CoV-2 RNA in severe cases. J Thromb Haemost. 2020;18:2744–2750. doi: 10.1111/jth.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 9.Barclay GR, Tura O, Samuel K, et al. Systematic assessment in an animal model of the angiogenic potential of different human cell sources for therapeutic revascularization. Stem Cell Res Ther. 2012;3:23. doi: 10.1186/scrt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2 + AC133 + endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.RES.88.2.167. [DOI] [PubMed] [Google Scholar]
- 11.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher KA, Liu Z-J, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J Clin Invest. 2007;117:1249. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun R, Huang J, Sun B. Mobilization of endothelial progenitor cells in sepsis. Inflamm Res. 2019;69:1–9. doi: 10.1007/S00011-019-01299-9. [DOI] [PubMed] [Google Scholar]
- 14.Gallogly S, Fujisawa T, Hung JD, et al. Generation of a novel in vitro model to study endothelial dysfunction from atherothrombotic specimens. Cardiovasc Drugs Ther. 2021:1–10. doi: 10.1007/S10557-021-07151-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tura O, Skinner EM, Barclay R, et al. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells. 2013;31:338–348. doi: 10.1002/stem.1280. [DOI] [PubMed] [Google Scholar]
- 16.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 17.Bradbury C, Buckley T, Sun YZ, Rose P, Fitzmaurice D. Patients with high levels of circulating endothelial progenitor cells (EPC) following at least three months of anticoagulation for unprovoked venous thromboembolism (VTE) are at low risk of recurrent VTE—results from the ExACT randomised controlled trial. EClinicalMedicine. 2019;17:1–9. doi: 10.1016/j.eclinm.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li WD, Li XQ. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vascul Pharmacol. 2016;83:10–16. doi: 10.1016/j.vph.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Modarai B, Burnand KG, Sawyer B, Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. 2005;111:2645–2653. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.