Abstract
While antimicrobial drug development has historically mitigated infectious diseases that are known, COVID-19 revealed a dearth of ‘in-advance’ therapeutics suitable for infections by pathogens that have not yet emerged. Such drugs must exhibit a property that is antithetical to the classical paradigm of antimicrobial development: the ability to treat infections by any pathogen. Characterisation of such ‘pan-pathogen’ antimicrobials requires consolidation of drug repositioning studies, a new and growing field of drug discovery. In this review, a previously-established system for evaluating repositioning studies is used to highlight 4 therapeutics which exhibit pan-pathogen properties, namely azithromycin, ivermectin, niclosamide, and nitazoxanide. Recognition of the pan-pathogen nature of these antimicrobials is the cornerstone of a novel paradigm of antimicrobial development that is not only anticipatory of pandemics and bioterrorist attacks, but cognisant of conserved anti-infective mechanisms within the host-pathogen interactome which are only now beginning to emerge. Ultimately, the discovery of pan-pathogen antimicrobials is concomitantly the discovery of a new class of antivirals, and begets significant implications for pandemic preparedness research in a world after COVID-19.
Keywords: Bioterrorism, COVID-19, Drug repositioning, Host-pathogen interaction, Pan-pathogen antimicrobial, Pandemic
1. Introduction
At the close of the 19th century, the work of Louis Pasteur and Robert Koch led to the ‘germ theory’ of disease, which stated that pathogens, too small to see without magnification, can cause disease [1]. This was reciprocated by Paul Ehrlich’s ‘magic bullet’, which described the need for chemical drugs that target the pathogen without harming the host [2]. The magic bullet hypothesis was successfully realised in the 20th century as antibiotics, antifungals, antiparasitics, and antivirals: therapeutics which treat infectious disease by targeting the disease-causing pathogen [3].
Nevertheless, over the ensuing decades, several limitations of the germ theory for disease have arisen, chief amongst which is the need to consider the host in determining disease outcome, encapsulated by the growing success of immunomodulatory therapies in treating infectious diseases [4]. Even today, an increased understanding of the immune system has facilitated the discovery and development of novel drug targets and approaches for immunomodulatory interventions [5]. This has led to more advanced types of immune therapies, such as monoclonal antibodies and cytokines, entering clinical use [6]. During COVID-19, a paucity of antivirals led to the most effective treatments emerging from anti-inflammatory drugs like dexamethasone [7], [8]. A further limitation of germ theory is the lack of consideration of mutable pathogen properties, such as antigenic determinants, replicative rates, and tropism, which stimulate immune responses to pathogens and in turn affect pathogenicity. A more inclusive approach to investigating pathogenesis must consider both the pathogen and host as complex systems that dynamically affect each other [9], [10].
Today, these limitations have been consolidated by the ‘host-pathogen interactome’ model, which recognises the contributions of both the host and pathogen in disease outcome [11]. This review highlights the development of host-modulating antimicrobials and the recent discovery of general anti-infective signalling pathways such as STING and MAPK, and contends that deliberation of the host-pathogen interactome model requires that antimicrobials should be defined not merely by their ability to inhibit a pathogen, but by their propensity to treat disease. This conception, argued henceforth, gives rise to a new class of antimicrobial, with significant implications for bioterrorist and pandemic preparedness research in the 21st century.
2. The host-pathogen interactome model
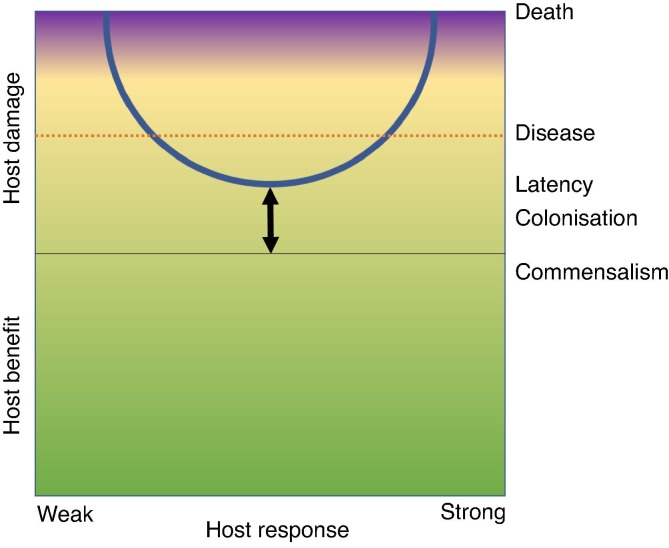
The use of immunomodulatory therapies to treat infectious disease, such as the recent success of dexamethasone to treat COVID-19, is indicative of the need for therapeutic development to consider not only the disease-causing pathogen but contributions of the host too. Casadevall and Pirofski’s seminal damage-response framework was propounded as an alternative to host-based and pathogen-based systems; it stated that microbial pathogenesis, whether bacterial, fungal, parasitic or viral is the outcome of interactions between host and microorganism [12]. Host damage is identified as a common principle with which to define and measure this interaction (Fig. 1 ) [13], [14], [15].
Fig. 1.
Casadevall and Pirofski’s damage-response framework of microbial pathogenesis. The y-axis denotes host damage as a function of the host response. Damage can occur throughout the host response, which is represented by a continuum from ‘weak’ to ‘strong’. Therapeutic intervention can shift the curve towards benefiting the host, as denoted by the arrow.
The need to consider both the host and pathogen in pathogenesis has implications for disease characterisation and antimicrobial development, the former of which has been addressed by Casadevall and Pirofski. Currently, classifications of microorganisms are based on phylogenetic groups (bacteria, fungi, parasites, viruses) [16], [17]. Casadevall and Pirofski argue this system is limited as most members of any group are not pathogenic in a host; of 150,000 fungal species, for example, only around 150 are pathogenic for humans [17]. However, classifications based on the perceived capacity of a microorganism to cause disease are equally inadequate as changes in host immune function, ecology, and/or behaviour can render them obsolete [18]. Similarly, as discussed later, classifying pathogens based on phylogenetic groups has been mirrored by the antimicrobial lexicon, which currently classifies antimicrobials according to their inhibitory activity against microbial phylogenetic groups (antibiotics, antifungals, antiparasitics, antivirals), encouraging a bias of therapeutic development towards pathogen-killing as opposed to host-pathogen interactome targeting and modulation [19].
The use of host damage as the common denominator with which to categorise pathogens allows pathogens that cause similar types of diseases to be grouped together despite differences in phylogeny and growth characteristics. According to Casadevall and Pirofski, pathogens grouped in a single ‘Class’ can share similarities with regard to the shape of the damage-response curve as a function of the host immune response [20], [21]. Ultimately, the host-pathogen interactome model crystallises the contemporary view of disease outcome as being determined both by the contributions of the host as well as the pathogen, a marked departure from the classical pathogen-centred view propounded in the early 20th century, with ramifications for microbial, immunological, and antimicrobial studies.
3. Host-modulating antimicrobials
The success of magic bullets and immunomodulatory therapies in the 20th century and the recent induction of the host-pathogen interactome model have propelled convergent research into antimicrobials with host-modulating properties over the last few decades [22]. Such ‘host-modulating antimicrobials’ have become a desideratum for all disciplines of modern antimicrobial development due to lower probabilities of drug interactions associated with higher patient compliance (compared to the use of immunomodulatory therapies in conjunction with antimicrobials), increased therapeutic range, and reduced contributions to antimicrobial resistance [23].
The last few years have seen a number of reviews describing various ways the host response can be modulated to maximise bacterial killing whilst minimising inflammatory tissue damage, reflecting a need for an orthogonal view of treating bacterial infection [24], [25], [26], [27]. Host-directed therapies for bacterial infections have also long been argued as a strategy to overcome antimicrobial resistance, even emerging as a promising approach to treat tuberculosis [28], [29].
In tandem with antibiotics, canonical antiviral drug development has been challenged, even before COVID-19. Traditional antivirals target viral proteins, incur higher development costs relative to antibiotics, offer limited therapeutic range, and are liable to escape mutant selection [30]. RNA viruses like SARS-CoV-2 are particularly limited in informational size, and have adapted to subvert multitasking host proteins [31]. Such solutions to the viral information economy paradox are conserved, offering the chance to leverage dependency on host proteins with host-directed antiviral therapies that are more effective, broad-acting, and inexpensive [32]. Furthermore, host-directed therapies can synergise with increased availability of bioactive compounds (as with the development of nitazoxanide), and recent advances in precision medicine, such as genome editing, targeted delivery methods, and RNAi [33]. These advances have been driven by a growing appreciation of host-virus interactions, the cornerstone of the emerging field of neo-virology [34]. Particularly in light of the recent pandemic, a successful antiviral development paradigm must complement rather than replace vaccine development for emerging viruses [35]; host-directed antivirals can reduce replication and tissue tropism whilst maintaining viral antigenicity for vaccines [36], [37].
As viruses are obligate parasites, similarities exist between antiviral and antiparasitic development [38]. For example, antimicrobials that directly target Leishmania parasites have been limited by the evolution of drug-resistant phenotypes, a property linked to its genome plasticity [39]. New strategies that are more refractory to the emergence of drug resistance target Leishmania viability indirectly via mechanisms targeting host-parasite interactions, including parasite-released ectokinases and host epigenetic regulation, which modulate host cell signalling and transcriptional regulation respectively [40]. Interestingly, several purported antivirals, including ivermectin, niclosamide, and nitazoxanide, were discovered as host-modulating antiparasitic agents.
The past 15 years have seen an acceleration in antifungal drug development, culminating in an armamentarium of systemic antifungal agents including including amphotericin B (AmB), the azoles, and the echinocandins [41]. Although their in vitro inhibitory and direct fungicidal effects are well characterised, antifungals also have indirect, immune system-mediated effects on fungi, which are only now coming to light [42]. Considering the substantial role of the host’s immune response in regulating fungal infection, a better understanding of these immunopharmacological properties have been argued to be potentially instrumental in designing rational drug therapies for invasive fungal infection (IFI) [43, 44].
Overall, Casadevall and Pirofski envisioned that a consequence of the host-pathogen interactome model would be the unification of a lexicon which emphasised differences between microbes and specific microbial attributes instead of highlighting commonalities. Without this unification, the disciplines of bacteriology, mycology, parasitology, and virology become increasingly insular, despite asking similar questions about the nature of infection. Yet, despite a concerted movement towards host-modulation in each of these disciplines, unification has been impeded by the antimicrobial lexicon, which has cemented the disciplines of antibiotic, antifungal, antiparasitic, and antiviral development by classifying antimicrobials according to the associated inhibited pathogen. Promisingly, however, the discovery of conserved targetable moieties within the host-pathogen interactome across pathogen classes may reignite the campaign for unification.
4. Host anti-infective responses
Recent biotechnological advancements have made possible the characterisation of signalling pathways that are conserved across infection types [45], [46]. For example, profiling global gene expression and aligning sequences to reference genomes have enabled isolation of differentially expressed genes pre- and post-infection [47], [48]. Selected genes are assessed against repositories and online databases to probe enrichment of functional biological pathways, and subnetworks are constructed by comparing and connecting identified genes to curated protein–protein interaction databases [49]. Traditional monolayer cell cultures have also been supplanted by human in vitro 3D models which probe functional multicellular interactions of epithelial and immune cells (dendritic cells, neutrophils) [50]. A consequence of such highly detailed mapping techniques is the discovery of general anti-infective signalling pathways that may be therapeutically targeted, particularly STING and MAPK.
To protect against infectious agents, the first line of defence by the host is activation of innate immune signalling pathways. Such pathways are multifactorial, primarily resolving to recognise pathogen-associated molecular patterns (PAMPs) [51], [52]. For example, detection of viral RNA particles, such as those associated with COVID-19, is achieved by RIG-I-like receptors (RLRs) [53]. Host defence countermeasures, including production of type I interferons (IFNs), are similarly triggered by microbial DNA from bacteria, viruses, and perhaps parasites, and are regulated by the cytosolic sensor, stimulator of interferon genes (STING) [54], [55]. The discovery of STING signalling has provided considerable insight into microbial pathogenesis, mechanisms of host defence, and causes of inflammatory disease and even cancer [56]. Regulation of the STING pathway has therefore been suggested as a pan-pathogen antimicrobial strategy [57]. Given the importance of STING as a modulator of both antiviral and pro-inflammatory responses to viral infection, it is interesting to consider last year it was shown to have a crucial role in RV-A and RV-C rhinoviral replication [58]. STING also exhibits tissue-specific localisation of expression in the lung, thus potentially contributing to protection against respiratory tract infection [59]. Considering the ability of azithromycin, a pan-pathogen antimicrobial, to upregulate virus-induced type I interferon responses, its use as a therapeutic for pulmonary bacterial infections, and the fact that it has been described as a ‘holy grail’ to prevent exacerbations in chronic respiratory disease, a molecular mechanism of azithromycin and similar macrolides via STING is conceivable, with exciting implications for developing future pan-pathogen antimicrobials with well-characterised host targets [60], [61].
The MAP kinases (MAPKs), which include ERK, JNK, and p38 families, comprise an integral part of the host intracellular signalling network, essential for signal transduction from receptors and stimuli to biological reaction [62], [63], [64], [65]. Appropriate functioning of MAPK signalling is critical to mount effective immune responses, and presents a broad-spectrum therapeutic target across pathogen classes, which drugs like macrolides may exploit [66], [67]. Macrolides, including azithromycin, are a class of diverse compounds which span antibiotics, antifungals, prokinetics, and immunosuppressants. The non-antimicrobial properties of macrolides have been suspected as far back as the 1960s and their successful treating of hyperinflammatory diseases such as diffuse panbronchiolitis (DPB) has served to extend their use to a number of chronic inflammatory diseases [68]. Macrolides have been shown to modulate intracellular MAPK, especially ERK1/2, and the NF-κB pathway downstream of ERK [69]. As these pathways exert plethoric cellular functions, including inflammatory cytokine production, cell proliferation, and mucin secretion, modulation of ERK1/2 and NF-κB can explain the majority of the reported immunomodulatory effects of macrolides [70], [71]. Intriguingly, however, specific proteins and receptors targeted by macrolides that affect MAPK/NF-κB signalling have not yet been identified, offering an avenue for experimental verification. Indeed, putative binding molecules may have multiple mechanisms of action. Overall, macrolide treatment of DPB, asthma, bronchiectasis, rhinosinusitis, and CF is made possible by polymodal modulation exerted at different levels of cellular signalling; yet among these, modulation of ERK1/2 and transcription factors is prominent, consistent, and clearly unrelated to antimicrobial properties [72].
Due to its broad-spectrum anti-infective effect against bacteria, parasites, and viruses, several studies have sought to delineate the underlying molecular mechanism of nitazoxanide, a thiazolide drug [73]. Tizoxanide, the main active metabolite of nitazoxanide, exerts anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines and suppressing activation of the NF-κB and the MAPK signalling pathways in LPS-treated macrophage cells [74]. Similarly, niclosamide, a potential pan-pathogen antimicrobial, was found to inhibit MAPK/ERK in human glioblastoma studies, indicative of crosstalk between anti-infectives and anti-cancer therapeutics [75]. Moreover, ivermectin, a potential treatment for COVID-19, reverses drug resistance in cancer cells via the EGFR/ERK/Akt/NF-κB pathway [76]. During viral pathogenesis, signalling pathways governing apoptosis, mitogenesis, cell proliferation, metabolism, and cytoskeletal reorganisation, which are regulated by ERK/MAPK signalling, are co-opted for biologic needs of the virus [77]. Development of new antiviral therapeutics based on clinical trials of ERK/MAPK inhibitors has thus been suggested for both DNA and RNA viruses, including for SARS-CoV-2 recently [78], [79].
As a corollary, it is of no surprise that host-directed anti-cancer therapies exhibit promising anti-infective properties with pan-pathogen potential; these include heat shock protein 90 (Hsp90) inhibitors, tamoxifen, and tyrosine kinase inhibitors such as diarylureas [80], [81], [82].
Autophagy signalling has also emerged as a host pharmacological target with broad-spectrum anti-infective potential. Recently, the Centers of Excellence for Translational Research (CETR) Program was founded to develop host-directed broad-spectrum anti-infective agents against pathogens with pandemic potential. According to their grant proposal, later funded by the National Institute of Allergy and Infectious Diseases (NIAID), ‘broad-spectrum host-directed therapeutics, once approved for clinical use, can be deployed for emerging pathogens, new outbreaks, and pathogens engineered with ill-intent’ [83]. The goal of this proposal is to generate autophagy pathway-directed compounds that are active against a range of taxonomically-unrelated pathogens. To accomplish this, a multitude of strategies are being employed including targeting of Beclin-1 complexes, genes and pathways for autophagy-dependent inhibition of bacterial infection, and Atg gene-dependent immunity [84], [85].
Virulence factors secreted by pathogens have co-evolved to manipulate host signalling pathways via a range of mechanisms, including constitutive pathway activation and subversion of critical signalling molecules. A major challenge is to discern the orchestra of factors within the host-pathogen interactome involved in successful infection, as well as their spatio-temporal regulation. Ultimately, the discovery of conserved anti-infective pathways is a landmark discovery, not only for the unification of the microbiological disciplines, but to mechanistically confirm the therapeutic success of existing antimicrobials which treat diseases pertaining to multiple pathogen classes.
5. Challenging the antimicrobial lexicon
The current taxonomy for antimicrobial drugs does not consider modern repositioning studies probing orthogonal uses of existing therapeutics. The term antibiotic – literally ‘opposing life’, derives from the Greek αντι anti, ‘against’ and βίος bios, ‘life’. This terminology has been extended to antifungal, antiparasitic, and antiviral drugs, reflecting a lexicon based on Ehrlich’s magic bullet. Though the lexicon does not accurately reflect the array of interactions of modern antimicrobials with the host-pathogen interactome, this has not been problematic. Macrolide antibiotics, for example, have been used to treat bacterial infections with the knowledge that their host-modulating properties play a crucial role in pathogen clearance and disease management. The lexicon is challenged, however, when 1) antimicrobials of one class exhibit inhibitory or host-modulating properties characteristic of another class or 2) antimicrobials are used clinically to treat diseases pertaining to another pathogen class. The ‘antibiotic’ azithromycin and the ‘antiparasitic’ agent nitazoxanide are examples of antimicrobials that have done both [86], [87], [88], [89]; azithromycin is clinically deployed against malarial parasites and nitazoxanide treats bacterial infections like H. pylori [90], [91].
Both azithromycin and nitazoxanide are immunomodulatory agents. Nitazoxanide treatment increases IFNγ- and IL-2-secreting CD4+ cells, TLR8-expressing monocytes, IFNα- and IFNβ-mRNA expression, mRNA specific for type I IFN inducible genes, and mRNA specific for genes involved in MHC class I presentation [92], [93]. The antiviral effects of nitazoxanide and its metabolite derivative tizoxanide result from stimulation of a strong antiviral immune response mediated by both native and acquired mechanisms. In its over 10 years’ clinical use there has been no reported drug resistance by nitazoxanide treatment, and attempts to produce resistance under laboratory conditions have failed [94]. The immunomodulatory effects of azithromycin are more well-established [95], [96]: treatment with azithromycin leads to reduced pro-inflammatory cytokine production and inflammation [97]. Consequently, azithromycin has been repositioned against a plethora of chronic pulmonary disorders including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), non-CF bronchiectasis, bronchiolitis obliterans syndrome, diffuse panbronchiolitis, and asthma [98]. It is conceivable that the immunomodulatory properties of azithromycin and nitazoxanide underpin their treatment of a range of infection types.
With such efficacy against a range of infectious diseases, to define azithromycin as an antibiotic or nitazoxanide as an antiparasitic agent oversimplifies their antimicrobial efficacy, precluding discovery of general infection mechanisms, rapid consideration for pandemics, and constructive unification of antimicrobial studies. Indeed, in the present pandemic, several studies addressed this by compiling pan-pathogen repositioning histories of therapeutic candidates [99]. In order to more accurately describe a candidate’s properties as well as hasten their consideration for pandemics, we previously propounded a system used to define antimicrobials based on both their ability to inhibit a pathogen in vitro and treat the corresponding disease in the clinical setting [100]. This system is based on Oprea and Overington’s Drug Repositioning Evidence Level (DREL) classification scheme, which assigns a numerical value to the quality of pharmacological evidence (Table 1 ) [101]. From this system we determined four antimicrobial types (antibiotics, antifungals, antiparasitics, and antivirals) can correspond to four DREL numbers for a given antimicrobial. An antimicrobial that is used clinically as an antimalarial and an antiviral but has no evidence of efficacy against bacteria or fungi is a 0:0:4:4 antimicrobial. The order of the DREL numbers here are: antibiotic = 0, antifungal = 0, antiparasitic = 4, antiviral = 4. If no investigations have been conducted for an antimicrobial class of a given therapeutic, an ‘X’ may be used to denote this.
Table 1.
Oprea and Overington’s DREL assessment of repositioning studies. A pan-pathogen antimicrobial is DREL 4 for two or more antimicrobial classes.
| Drug repositioning evidence level | Quality of scientific evidence |
|---|---|
| 0 | No evidence; includes in silico predictions without confirmation |
| 1 | In vitro studies with limited value for predicting in vivo/human situation |
| 2 | Animal studies with hypothetical relevance in humans |
| 3 | Incomplete studies in humans at the appropriate dose e.g. proof of concept; few cases from medical records; some clinical effects observed |
| 4 | Well-documented clinical end points observed for repositioned drug at doses within safety limits |
With an increasing number of repositioning studies being conducted worldwide, particularly in the midst of the current pandemic, a concomitant taxonomic structure can not only classify potential general antimicrobials, but also direct future repositioning studies, facilitate comparative therapeutic investigations, and inform treatment application in global health emergencies [102]. From our classification system based on DREL we determined azithromycin is a 4:0:4:3 antimicrobial (Table 2 ) [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117]. Pan-pathogen antimicrobials can therefore simply be defined as antimicrobials that are DREL 4 for two antimicrobial classes. Previously we propounded the term ‘broad-spectrum therapeutic’ to denote this; ‘pan-pathogen antimicrobial’ and ‘broad-spectrum anti-infective’ are preferred alternatives [118]. However, the term ‘broad-spectrum therapeutic’ may be used to describe a drug with pan-pathogen pharmacological properties that have not been fully tested in a clinical setting.
Table 2.
BFPV classification of potential pan-pathogen antimicrobials. Each number represents a DREL score for a particular antimicrobial class for a given therapeutic. ‘X’ denotes no investigations conducted. As repositioning studies ensue, DREL numbers for any given therapeutic are subject to change. DREL numbers highlight areas where more repositioning studies are needed. Cumulative repositioning of an antimicrobial against orthogonal infection types is an indicator of ‘pan-pathogen’ properties. All listed antimicrobials are broad-spectrum therapeutics (i.e. exhibit pan-pathogen pharmacological properties), but azithromycin is the first formally-recognised pan-pathogen antimicrobial. DREL values listed here are current as of July 2021.
Our system, hereby termed ‘BFPV classification’ (for antiBiotic, antiFungal, antiParasitic, antiViral; alternatively: Bacterial infection, Fungal infection, Parasitic infection, Viral infection) scores the effectiveness of an antimicrobial for a particular pathogen type against three major parameters: in vitro activity, in vivo activity, and clinical effectiveness. This represents a departure from the magic bullet-oriented lexicon by defining an antimicrobial not solely by its ability to inhibit a pathogen but by its ability to shift the damage-response curve towards mitigating damage within the physiological context. As the Casadevall-Pirofski model for disease considers contributions from both the pathogen and host, so the BFPV classification considers the ability of a given antimicrobial to treat a disease, not merely its capacity to inhibit a pathogen. This classification would also consider the effectiveness of non-antimicrobial therapeutics in treating infections, such as dexamethasone for COVID-19. As pan-pathogen antimicrobial development matures as a discipline in its own right, the DREL system can be replaced by a more accurate framework that classifies drugs according to the degree to which they reduce damage resulting from the host-pathogen interaction as a function of the host immune response, perhaps based on Casadevall and Pirofski’s ‘Class’ scheme for host-pathogen interactomes [20]. The inauguration of the term ‘pan-pathogen antimicrobial’ results from considering the contemporary view of disease and not only sequesters a novel set of drugs, but marries them with a particular application: to prepare for pandemics.
6. Bioterrorism and pandemics
The key advantage of pan-pathogen antimicrobials over single-target antimicrobials is the ability to account for diseases that have not yet emerged either by natural or engineered means. By being preparatory to pandemics and bioterrorism, the health and economic value of such drugs are of significance both for governments and enterprise.
Bioterrorism is a unique subject in the literature, appearing at the confluence of research publications and government mitigation strategy reports. The term ‘bioterrorism’ is distinct from ‘biowarfare’ with regard to the origin of the threat being from terrorist groups rather than nation states. In contrast to conventional warfare, where the mode of warfare is known, terrorism is more difficult to predict. While bioterrorism is often taken to mean acts that involve the use of biological materials such as bacteria, bacterial spores, and viruses, this is a limited definition. Indeed, terrorists can deploy a range of agents including classical chemical agents. However, for the scope of this review and in consideration of the ongoing COVID-19 pandemic, the definition is herein limited to biologically viable particles i.e. bacteria, fungi, parasites, and viruses.
In 2004, the British Association for Lung Research organised a symposium entitled ‘Bioterrorism: The Lung Under Attack’ in which the lung was identified as a physiological target for gaseous and aerosol-based compounds [119]. Understanding the effects of these substances on the lung was identified as a key consideration in the mitigation of bioterrorist threats [120]. In 2020, COVID-19 emerged as a respiratory viral pandemic, leading to the use of steroid treatments to curb acute respiratory distress and lung hyperinflammation in affected patients. Even prior to the pandemic the use of potential pan-pathogen antimicrobials to treat inflammation of the lung was increasing. For example, in vivo studies showed that ivermectin is an effective suppressor of inflammation, rationalising its use as a treatment for non-infectious respiratory inflammatory diseases such as allergic asthma, similar to azithromycin [121]. By inhibiting mucus and cytokine release, as well as inducing bronchorelaxation, niclosamide, another potential pan-pathogen antimicrobial, has also emerged as a therapeutic candidate for inflammatory airway diseases including cystic fibrosis, COPD, and asthma [122].
A novel mechanism of bronchodilating airways has emerged through the discovery of antagonists of the Ca2+-activated Cl- channel, TMEM16A, offering a new mechanism to block multiple contractiles operating in severe disease [123]. A recent screen of 580,000 compounds identified niclosamide and nitazoxanide as powerful TMEM16A antagonists preventing depolarisation and contraction of airway smooth muscle [124]. While isoproterenol, a canonical β-agonist, only showed partial bronchodilation of airways, niclosamide and nitazoxanide showed full effects, representing an important treatment for patients with severe asthma and COPD, in addition to rationalising current clinical trials against COVID-19. That current potential pan-pathogen antimicrobials are repositioned for a multitude of respiratory diseases is a further reason to consider their pharmacology for future outbreaks and emphasises the need for further research to unearth underlying mechanisms in relation to physiological context.
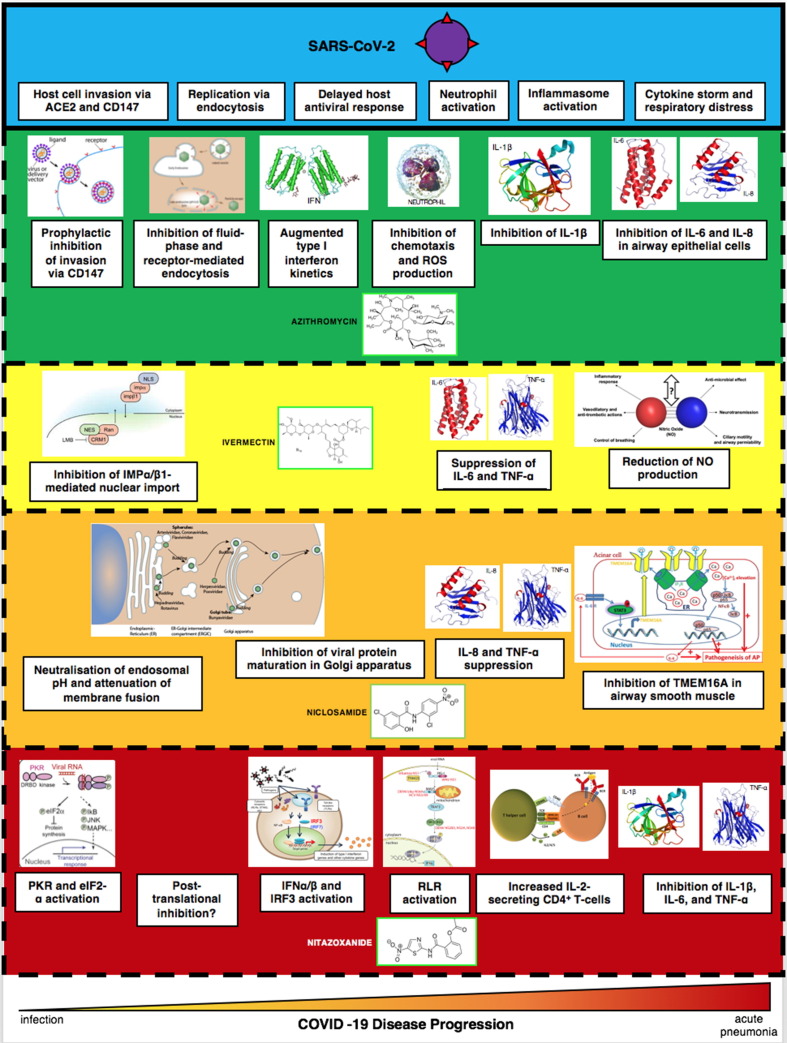
The concept of ‘general’ drugs for pandemics is not new. In 2007, the Strategic Plan for Biodefense Research by the U.S. Department of Health and Human Services (HHS) and NIAID stated that ‘anti-infectives with broad-spectrum activity directed at common, invariable, and essential components of different classes of microbes could potentially be effective against both traditional and non-traditional threats’ [125]. Similarly, the Transformational Medical Technologies (TMT) initiative, established by the U.S. Department of Defense in 2006, was conceived as a five-year, USD $1.5-billion project that would accelerate the development of countermeasures such as ‘broad-spectrum’ therapies that would work against multiple bacterial and viral pathogens, particularly haemorrhagic fever viruses such as Ebola and Marburg [126]. However, developing broad-spectrum drugs has been superseded by the industry-wide status quo of producing therapeutics that target a specific infectious disease i.e. ‘one drug-one bug’. There are no pan-pathogen inhibitors that target conserved properties across pathogen classes (the magic bullet paradigm). However, by targeting the host-pathogen interactome, host-modulating antimicrobials overcome this limitation, being able to treat diseases across infection types. This property is what makes host-modulating antimicrobials the first to display ‘pan-pathogen’ properties. Pan-pathogen antimicrobials, owing to their clinical safety profile for a myriad of diseases and anti-infective efficacy against a range of pathogens, satisfy the requirements for a new ‘general’ class of antimicrobials for pandemics (‘one drug-many bugs’), as stipulated by the current director of NIAID and chief medical advisor to the current U.S. President, Anthony Fauci (Fig. 2 ) [127].
Fig. 2.
Pharmacological profile of azithromycin, ivermectin, niclosamide, and nitazoxanideduring COVID-19 pneumonia pathogenesis. Establishment of pan-pathogen antimicrobials facilitates discovery of conserved pharmacological properties against multiple pathogen types. For example, lysosomotropicity has emerged as a desideratum for both antimalarial and antiviral therapeutics. Drug-disease interactions of each pan-pathogen antimicrobial can further provide mechanistic insights into the general nature of infection. In so doing, pan-pathogen antimicrobials can drive the unification of microbiological and immunological disciplines, first envisioned by Casadevall and Pirofski. Immunomodulation by cytokine suppression is a prospective hallmark of pan-pathogen antimicrobial pharmacology.
7. Discussion
For over a century, drug development has tailored to known diseases and pathogens. To prepare for a novel pathogen, a generalised drug development strategy is required, au courant with a range infection types. In theory, both magic bullet and ‘magic blanket’ paradigms can yield pan-pathogen antimicrobials. In reality, only one has; host-directed therapies that interfere with host cell mechanisms, enhance immune responses, reduce exacerbated inflammation, and balance host reactions at the site of pathology hold promise for the selective and symptomatic treatment of emerging infectious diseases. The success of host-modulating therapies alone represents a century-spanning, Kuhnian paradigm shift from the magic bullet. This review is not a rejection of Paul Ehrlich’s paradigm. Instead, as Einstein explicates: ‘the larger view encompasses rather than rejects the more restricted view’; azithromycin is still a magic bullet antibiotic, yet its host-modulating properties offer undetermined repositioning potential.
In viral infections such as COVID-19, targeting host cell factors and pathways that are required for productive replication and proliferation offers an opportunity for broad-acting treatments against both viral annexation of host cellular processes and ensuing pathophysiology. Indeed, all four highlighted antimicrobials exhibit host-directed, broad-spectrum antiviral properties, the desideratum of post-COVID-19 antiviral development [9]. In the future, knowledge of host cell factors and pathways commonly used by different pathogens can be greatly enhanced by probing host targets of the potential pan-pathogen antimicrobials identified in this review. Similarly, identification of conserved chemical moieties can lead to synthesis of novel, more potent pan-pathogen antimicrobials with reduced contributions to antimicrobial resistance. As antibiotics and antivirals of the 20th century became more specific for the bacterium and virus, so antimicrobials of the 21st will be increasingly specific for the host (Table 3 ).
Table 3.
Comparison of two antimicrobial paradigms. Classical antimicrobials contending with a single target exploit phenotypic differences between host and pathogen. Host-modulating antimicrobials target pathogen properties as developed through co-evolution with the host. Consequently, while broad-spectrum antivirals like remdesivir are magic bullets exclusively targeting viruses, host-directed properties of azithromycin, ivermectin, niclosamide, and nitazoxanide have rationalised their use against bacterial, parasitic, and viral infections. Pan-pathogen antimicrobials, therefore, have emerged from magic blanket, not magic bullet, development.
| Magic bullet | Magic blanket | |
|---|---|---|
| Drug class | Antimicrobial | Host-modulating antimicrobial |
| Target | Pathogen | Host-pathogen interactome |
| Pan-pathogen antimicrobials | No | Yes |
Development of drugs which target the host-pathogen interactome has more opportunity for growth relative to magic bullet development due to the number of target factors yet to be discovered and the need to curb antimicrobial resistance. In contrast to non-infectious disease types, great therapeutic potential also derives from the fact that pharmacological modulation of infectious diseases is considered within an acute, not chronic, pathological context, allowing for clinical application of more powerful modulators. A caveat, however, is the dynamic nature of the host-pathogen interactome across disease pathogenesis. Indeed, a crucial difference between targeting the host-pathogen interactome and targeting the pathogen is temporality, and great emphasis has been placed on the need to develop biomarkers that accurately reflect the host immunological signature in order to effectively inform clinical application of host modulators. Biomarkers indicate the stage of infection, allow the monitoring of treatment success or failure, provide information on organ involvement and type of inflammation, and permit patient stratification for selected immunomodulatory therapies. As biomarkers become increasingly accurate at reflecting immune status, so the effects of host-modulating antimicrobials can be better predicted. That being said, most immunomodulatory strategies have been developed without understanding the full complexity of their interactions with the host and hence the fact that we do not yet fully understand the complexity of the host-drug interaction of host-modulating antimicrobials need not preclude development and application of host-modulating therapies; rather identification of successful magic blankets can inspire further investigations into the nature and context of their pharmacological targets. As was the case for magic bullets a century ago, current understanding of host-modulating antimicrobials is still in its infancy, and offers an exciting avenue for further research.
8. Conclusion and future directions
This review represents the first time ‘pan-pathogen’ has been recognised as a pharmacological property. Azithromycin, ivermectin, niclosamide, and nitazoxanide assert an advantage over traditional antibiotics and antivirals in their ability to treat a wider range of infectious diseases by regulating the host-pathogen interactome, evidenced by their extensive repositioning history and recent spotlighting during the pandemic. While this review cannot endorse the use of these drugs either for the current pandemic or future biological events, such broad-acting drugs are nonetheless a terminus a quo for the research and development of ‘emergency drugs’ for future global health crises. The growing practice of repositioning existing drugs for infectious diseases can alternatively be considered the sole method of pan-pathogen antimicrobial discovery, such that modern antimicrobial repositioning programmes have profound contributions to 21st century antiviral development. Indeed, pan-pathogen antimicrobials are a novel antiviral class, one that is associated with pandemic preparedness research. Formal recognition of pan-pathogen antimicrobials can catalyse the long-campaigned unification of the disparate fields of bacteriology, fungology, parasitology, and virology, all while heralding a paradigm of antimicrobial development conceptually distinct from the antibiotic era of the 20th century. Finally, pan-pathogen antimicrobials are the most powerful antimicrobials ever conceived.
Acknowledgments
Acknowledgements
Many thanks to our colleagues at the Department of Biochemistry and Trinity College, University of Oxford. This review is dedicated to Friedemann T. Freund and to the memory of Steven P. Jobs.
Availability of data and material
Not applicable.
Authors’ contributions
PP conceived, wrote, and edited the manuscript.
Code availability
Not applicable.
Conflict of interest
The author declares no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Funding statement
This research received no funding.
References
- 1.Baxter A.G. Louis Pasteur's beer of revenge. Nat Rev Immunol. 2001;1(3):229–232. doi: 10.1038/35105083. PMID: 11905832. [DOI] [PubMed] [Google Scholar]
- 2.Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–480. doi: 10.1038/nrc2394. Epub 2008 May 12 PMID: 18469827. [DOI] [PubMed] [Google Scholar]
- 3.Neu H.C., Gootz T.D. In: Medical microbiology. 4th ed. Baron S., editor. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Antimicrobial Chemotherapy. Chapter 11. [Google Scholar]
- 4.McNaught W. Limitations of the germ theory. Lancet. 1968;2(7561):220. doi: 10.1016/s0140-6736(68)92655-x. PMID: 4173435. [DOI] [PubMed] [Google Scholar]
- 5.Pirofski L.-A., Casadevall A. Immunomodulators as an antimicrobial tool. Curr Opin Microbiol. 2006;9(5):489–495. doi: 10.1016/j.mib.2006.08.004. Epub 2006 Aug 22. PMID: 16931122; PMCID: PMC7108246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramamurthy D, Nundalall T, Cingo S, Mungra N, Karaan M, Naran K, Barth S. Recent advances in immunotherapies against infectious diseases. Immunotherapy Adv. 2020:ltaa007. doi: 10.1093/immadv/ltaa007. PMCID: PMC7717302. [DOI] [PMC free article] [PubMed]
- 7.Chitalia V.C., Munawar A.H. A painful lesson from the COVID-19 pandemic: the need for broad-spectrum, host-directed antivirals. J Transl Med. 2020;18(1):390. doi: 10.1186/s12967-020-02476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelleni M.T. Tocilizumab, remdesivir, favipiravir, and dexamethasone repurposed for COVID-19: a comprehensive clinical and pharmacovigilant reassessment. SN Compr Clin Med. 2021;3(4):1–5. doi: 10.1007/s42399-021-00824-4. Epub ahead of print. PMID: 33644693; PMCID: PMC7894610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathogenesis: of host and pathogen. Nat Immunol. 2006 Mar;7(3):217. doi: 10.1038/ni0306-217. PMID: 16482163. [DOI] [PubMed]
- 10.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10(7):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crua Asensio N., Muñoz Giner E., de Groot N.S., Torrent B.M. Centrality in the host-pathogen interactome is associated with pathogen fitness during infection. Nat Commun. 2017;16(8):14092. doi: 10.1038/ncomms14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoy D.A., Casadevall A., Pirofski L.A. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000;68(12):6511–6518. doi: 10.1128/IAI.68.12.6511-6518.2000. PMID: 11083759; PMCID: PMC97744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischetti V.A., Casadevall A., Pirofski L.A. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67(8):3703–3713. doi: 10.1128/IAI.67.8.3703-3713.1999. PMID: 10417127; PMCID: PMC96643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash A.A., Dalziel R.G., Fitzgerald J.R. Mechanisms of cell and tissue damage. Mims' Pathogenesis Infect Dis. 2015;171–231 doi: 10.1016/B978-0-12-397188-3.00008-1. Epub 2015 Feb 6. PMCID: PMC7158287. [DOI] [Google Scholar]
- 15.Sen R., Nayak L., De R.K. A review on host-pathogen interactions: classification and prediction. Eur J Clin Microbiol Infect Dis. 2016;35(10):1581–1599. doi: 10.1007/s10096-016-2716-7. Epub 2016 Jul 29 PMID: 27470504. [DOI] [PubMed] [Google Scholar]
- 16.Pitt T.L., Barer M.R. Classification, identification and typing of micro-organisms. Med Microbiol. 2012;24–38 doi: 10.1016/B978-0-7020-4089-4.00018-4. Epub 2012 May 24. PMCID: PMC7171901. [DOI] [Google Scholar]
- 17.Köhler JR, Casadevall A, Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med. 2014;5(1):a019273. doi: 10.1101/cshperspect.a019273. PMID: 25367975; PMCID: PMC4292074. [DOI] [PMC free article] [PubMed]
- 18.Pérez JC, Kumamoto CA, Johnson AD. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol. 2013;11(3):e1001510. doi: 10.1371/journal.pbio.1001510. Epub 2013 Mar 19. PMID: 23526879; PMCID: PMC3601966. [DOI] [PMC free article] [PubMed]
- 19.Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. Int J Antimicrob Agents. 2016;48(4):349–352. doi: 10.1016/j.ijantimicag.2016.07.004. Epub 2016 Aug 5. PMID: 27546219; PMCID: PMC7134768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadevall A., Pirofski L.A. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1(1):17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirofski L.-A., Casadevall A., Morrison T.E., Garsin D.A. Pathogenesis of COVID-19 from the perspective of the damage-response framework. mBio. 2020;11(4) doi: 10.1128/mBio.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauber S.C., Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1(1):68–79. PMID: 20021425. [PubMed] [Google Scholar]
- 23.Pasquale T.R., Tan J.S. Nonantimicrobial effects of antibacterial agents. Clin Infect Dis. 2005;40(1):127–135. doi: 10.1086/426545. Epub 2004 Dec 1 PMID: 15614702. [DOI] [PubMed] [Google Scholar]
- 24.Hawn T.R., Matheson A.I., Maley S.N., Vandal O. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol Rev. 2013;77(4):608–627. doi: 10.1128/MMBR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumla A.I., Gillespie S.H., Hoelscher M., Philips P.P.J., Cole S.T., Abubakar I., et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis. 2014;14(4):327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann S.H.E., Lange C., Rao M., Balaji K.N., Lotze M., Schito M., et al. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med. 2014;2(4):301–320. doi: 10.1016/S2213-2600(14)70033-5. Epub 2014 Mar 24. PMID: 24717627. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson R.J. Host-directed therapies against tuberculosis. Lancet Respir Med. 2014;2(2):85–87. doi: 10.1016/S2213-2600(13)70295-9. Epub 2014 Jan 9 PMID: 24503259. [DOI] [PubMed] [Google Scholar]
- 28.Chiang C.-Y., Uzoma I., Moore R.T., Gilbert M., Duplantier A.J., Panchal R.G., et al. Mitigating the impact of antibacterial drug resistance through host-directed therapies: current progress, outlook, and challenges. mBio. 2018;9(1) doi: 10.1128/mBio.01932-17. PMID: 29382729; PMCID: PMC5790911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin D.M. Host-directed therapies for tuberculosis. Cold Spring Harb Perspect Med. 2015;5(10) doi: 10.1101/cshperspect.a021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heaton S.M. Harnessing host-virus evolution in antiviral therapy and immunotherapy. Clin Transl Immunol. 2019;8(7):e1067. doi: 10.1002/cti2.1067. PMID: 31312450; PMCID: PMC6613463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16(8):e3000003. doi: 10.1371/journal.pbio.3000003. PMID: 30102691; PMCID: PMC6107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prussia A, Thepchatri P, Snyder JP, Plemper RK. Systematic approaches towards the development of host-directed antiviral therapeutics. Int J Mol Sci. 2011;12(6):4027-52. doi: 10.3390/ijms12064027. Epub 2011 Jun 15. PMID: 21747723; PMCID: PMC3131607. [DOI] [PMC free article] [PubMed]
- 33.Estrin M.A., Hussein I.T.M., Puryear W.B., Kuan A.C., Artim S.C., Runstadler J.A., et al. Host-directed combinatorial RNAi improves inhibition of diverse strains of influenza A virus in human respiratory epithelial cells. PLoS ONE. 2018;13(5):e0197246. doi: 10.1371/journal.pone.0197246. PMID: 29775471; PMCID: PMC5959063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe T, Kawaoka Y. [Neo-Virology: the raison d'etre of viruses]. Uirusu. 2016;66(2):155-162. Japanese. doi: 10.2222/jsv.66.155. PMID: 29081467. [DOI] [PubMed]
- 35.Wang Q.C., Nie Q.H., Feng Z.H. RNA interference: antiviral weapon and beyond. World J Gastroenterol. 2003;9(8):1657–1661. doi: 10.3748/wjg.v9.i8.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X., Carroll J.W., Macdonald M.R., Goff S.P., Gao G. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J Virol. 2004;78(23):12781–12787. doi: 10.1128/JVI.78.23.12781-12787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihara T., Nishimura Y., Shimizu Y., Nishiyama H., Yoshikawa G., Uehara H., et al. Linking virus genomes with host taxonomy. Viruses. 2016;8(3):66. doi: 10.3390/v8030066. PMID: 26938550; PMCID: PMC4810256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godkin A., Smith K.A. Chronic infections with viruses or parasites: breaking bad to make good. Immunology. 2017;150(4):389–396. doi: 10.1111/imm.12703. Epub 2017 Jan 19. PMID: 28009488; PMCID: PMC5343343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponte-Sucre A., Gamarro F., Dujardin J.C., Barrett M.P., López-Vélez R., García-Hernández R., et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. doi: 10.1371/journal.pntd.0006052. PMID: 29240765; PMCID: PMC5730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamotte S., Späth G.F., Rachidi N., Prina E., Pollastri M.P. The enemy within: targeting host-parasite interaction for antileishmanial drug discovery. PLoS Negl Trop Dis. 2017;11(6):e0005480. doi: 10.1371/journal.pntd.0005480. PMID: 28594938; PMCID: PMC5464532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You L., Yao C., Yang F., Yang Q., Lan J., Song X., et al. Echinocandins versus amphotericin B against Candida tropicalis fungemia in adult hematological patients with neutropenia: a multicenter retrospective cohort study. Infect Drug Resist. 2020;10(13):2229–2235. doi: 10.2147/IDR.S258744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sam Q.H., Yew W.S., Seneviratne C.J., Chang M.W., Chai L.Y.A. Immunomodulation as therapy for fungal infection: Are we closer? Front Microbiol. 2018;25(9):1612. doi: 10.3389/fmicb.2018.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ademe M. Immunomodulation for the treatment of fungal infections: opportunities and challenges. Front Cell Infect Microbiol. 2020;15(10):469. doi: 10.3389/fcimb.2020.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simitsopoulou M., Roilides E., Walsh T.J. Immunomodulatory properties of antifungal agents on phagocytic cells. Immunol Invest. 2011;40(7–8):809–824. doi: 10.3109/08820139.2011.615877. PMID: 21985307. [DOI] [PubMed] [Google Scholar]
- 45.Alto NM, Orth K. Subversion of cell signaling by pathogens. Cold Spring Harb Perspect Biol. 2012;4(9):a006114. doi: 10.1101/cshperspect.a006114. PMID: 22952390; PMCID: PMC3428769. [DOI] [PMC free article] [PubMed]
- 46.Pieters V.M., Co I.L., Wu N.C., McGuigan A.P. Applications of omics technologies for three-dimensional in vitro disease models. Tissue Eng Part C Methods. 2021;27(3):183–199. doi: 10.1089/ten.TEC.2020.0300. Epub 2021 Feb 22 PMID: 33406987. [DOI] [PubMed] [Google Scholar]
- 47.Ling K.M., Garratt L.W., Gill E.E., Lee A.H.Y., Agudelo-Romero P., Sutanto E.N., et al. Rhinovirus infection drives complex host airway molecular responses in children with cystic fibrosis. Front Immunol. 2020;11:1327. doi: 10.3389/fimmu.2020.01327. 10.3389/fimmu.2020.01327. PMID: 32765492; PMCID: PMC737839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattin K.A., Moore J.H. Role for protein-protein interaction databases in human genetics. Expert Rev Proteomics. 2009;6(6):647–659. doi: 10.1586/epr.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen C., Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;6(7):33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. 10.1128/CMR.00046-08. PMID: 19366914; PMCID: PMC2668232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gürtler C., Bowie A.G. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21(8):413–420. doi: 10.1016/j.tim.2013.04.004. Epub 2013 May 29. PMID: 23726320; PMCID: PMC3735846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehwinkel J., Gack M.U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3. Epub 2020 Mar 13. PMID: 32203325; PMCID: PMC7094958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Cai S., Sun Y., Li L., Ding S., Wang X. When STING meets viruses: sensing, trafficking and response. Front Immunol. 2020;11:2064. doi: 10.3389/fimmu.2020.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marinho F.V., Benmerzoug S., Oliveira S.C., Ryffel B., Quesniaux V.F.J. The emerging roles of STING in bacterial infections. Trends Microbiol. 2017;25(11):906–918. doi: 10.1016/j.tim.2017.05.008. Epub 2017 Jun 15. PMID: 28625530; PMCID: PMC5650497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su T., Zhang Y., Valerie K., Wang X.Y., Lin S., Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759–7771. doi: 10.7150/thno.37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahn J., Barber G.N. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51(12):1–10. doi: 10.1038/s12276-019-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKnight K.L., Swanson K.V., Austgen K., Richards C., Mitchell J.K., McGivern D.R., et al. Stimulator of interferon genes (STING) is an essential proviral host factor for human rhinovirus species A and C. Proc Natl Acad Sci USA. 2020;117(44):27598–27607. doi: 10.1073/pnas.2014940117. Epub 2020 Oct 15. PMID: 33060297; PMCID: PMC7959528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savigny F., Schricke C., Lacerda-Queiroz N., Meda M., Nascimento M., Huot-Marchand S., et al. Protective role of the nucleic acid sensor STING in pulmonary fibrosis. Front Immunol. 2021;11:588799. doi: 10.3389/fimmu.2020.588799. PMID: 33488589; PMCID: PMC7820752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welte T. Azithromycin: The holy grail to prevent exacerbations in chronic respiratory disease? Am J Respir Crit Care Med. 2019;200(3):269–270. doi: 10.1164/rccm.201903-0706ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiNicolantonio J.J., Barroso-Aranda J., McCarty M.F. Azithromycin and glucosamine may amplify the type 1 interferon response to RNA viruses in a complementary fashion. Immunol Lett. 2020;228:83–85. doi: 10.1016/j.imlet.2020.09.008. Epub 2020 Sep 28. PMID: 33002511; PMCID: PMC7521214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braicu C., Buse M., Busuioc C., Drula R., Gulei D., Raduly L., et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (Basel) 2019;11(10):1618. doi: 10.3390/cancers11101618. PMID: 31652660; PMCID: PMC6827047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuriakose S., Onyilagha C., Singh R., Olayinka-Adefemi F., Jia P., Uzonna J.E. TLR-2 and MyD88-dependent activation of MAPK and STAT proteins regulates proinflammatory cytokine response and immunity to experimental Trypanosoma congolense Infection. Front Immunol. 2019;10:2673. doi: 10.3389/fimmu.2019.02673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharp L.L., Schwarz D.A., Bott C.M., Marshall C.J., Hedrick S.M. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7(5):609–618. doi: 10.1016/s1074-7613(00)80382-9. PMID: 9390685. [DOI] [PubMed] [Google Scholar]
- 65.Rincón M., Flavell R.A., Davis R.J. Signal transduction by MAP kinases in T lymphocytes. Oncogene. 2001;20(19):2490–2497. doi: 10.1038/sj.onc.1204382. PMID: 11402343. [DOI] [PubMed] [Google Scholar]
- 66.Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011;195(7):1083-92. doi: 10.1083/jcb.201107132. Epub 2011 Nov 28. PMID: 22123833; PMCID: PMC3246894. [DOI] [PMC free article] [PubMed]
- 67.Kumar R., Khandelwal N., Thachamvally R., Tripathi B.N., Barua S., Kashyap S.K., et al. Role of MAPK/MNK1 signaling in virus replication. Virus Res. 2018;253:48–61. doi: 10.1016/j.virusres.2018.05.028. Epub 2018 Jun 1. PMID: 29864503; PMCID: PMC7114592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhanel G.G., Dueck M., Hoban D.J., Vercaigne L.M., Embil J.M., Gin A.S., et al. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs. 2001;61(4):443–498. doi: 10.2165/00003495-200161040-00003. [DOI] [PubMed] [Google Scholar]
- 69.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosnar M., Čužić S., Bošnjak B., Nujić K., Ergović G., Marjanović N., et al. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int Immunopharmacol. 2011;11(4):424–434. doi: 10.1016/j.intimp.2010.12.010. Epub 2010 Dec 30. PMID: 21195124. [DOI] [PubMed] [Google Scholar]
- 71.Yang J. Mechanism of azithromycin in airway diseases. J Int Med Res. 2020;48(6) doi: 10.1177/0300060520932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohanta TK, Arina P, Sharma N, Defilippi P. Role of azithromycin in antiviral treatment: enhancement of interferon-dependent antiviral pathways and mitigation of inflammation may rely on inhibition of the MAPK cascade? Am J Transl Res. 2020;12(12):7702-7708. PMID: 33437355; PMCID: PMC7791480. [PMC free article] [PubMed]
- 73.Singh N., Narayan S. Nitazoxanide: a broad spectrum antimicrobial. Med J Armed Forces India. 2011;67(1):67–68. doi: 10.1016/S0377-1237(11)80020-1. Epub 2011 Jul 21. PMID: 27365765; PMCID: PMC4920633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shou J., Kong X., Wang X., Tang Y., Wang C., Wang M.i., et al. Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-κB and MAPK activation. Inflammation. 2019;42(4):1336–1349. doi: 10.1007/s10753-019-00994-3. PMID: 30937840. [DOI] [PubMed] [Google Scholar]
- 75.Cheng B., Morales L.D., Zhang Y., Mito S., Tsin A., Pizzo S.V. Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line. PLoS ONE. 2017;12(9):e0184324. doi: 10.1371/journal.pone.0184324. 10.1371/journal.pone.0184324. PMID: 28877265; PMCID: PMC5587337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang L., Wang P., Sun Y.J., Wu Y.J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J Exp Clin Cancer Res. 2019;38(1):265. doi: 10.1186/s13046-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for middle east respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. Epub 2014 Dec 8. PMID: 25487801; PMCID: PMC4335870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DuShane J.K., Maginnis M.S. Human DNA virus exploitation of the MAPK-ERK cascade. Int J Mol Sci. 2019;20(14):3427. doi: 10.3390/ijms20143427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghasemnejad-Berenji M., Pashapour S. SARS-CoV-2 and the possible role of Raf/MEK/ERK pathway in viral survival: is this a potential therapeutic strategy for COVID-19? Pharmacology. 2021;106(1-2):119–122. doi: 10.1159/000511280. Epub 2020 Oct 2. PMID: 33011728; PMCID: PMC7573895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rochani A.K., Singh M., Tatu U. Heat shock protein 90 inhibitors as broad spectrum anti-infectives. Curr Pharm Des. 2013;19(3):377–386. doi: 10.2174/138161213804143608. PMID: 22920905. [DOI] [PubMed] [Google Scholar]
- 81.Montoya M.C., Krysan D.J., Garsin D.A. Repurposing estrogen receptor antagonists for the treatment of infectious disease. mBio. 2018;9(6) doi: 10.1128/mBio.02272-18. PMID: 30563895; PMCID: PMC6299222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catalano A., Iacopetta D., Pellegrino M., Aquaro S., Franchini C., Sinicropi M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics (Basel) 2021;10(1):92. doi: 10.3390/antibiotics10010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levine BC. Autophagy Modulators as Novel Broad-Spectrum Anti-Infective Agents. https://grantome.com/grant/NIH/U19-AI142784-01.
- 84.Münz C. Beclin-1 targeting for viral immune escape. Viruses. 2011 Jul;3(7):1166-78. doi: 10.3390/v3071166. Epub 2011 Jul 12. PMID: 21994775; PMCID: PMC3185790. [DOI] [PMC free article] [PubMed]
- 85.Sorouri M., Chang T., Jesudhasan P., Pinkham C., Elde N.C., Hancks D.C., et al. Signatures of host-pathogen evolutionary conflict reveal MISTR-A conserved mitochondrial STress response network. PLoS Biol. 2020;18(12):e3001045. doi: 10.1371/journal.pbio.3001045. PMID: 33370271; PMCID: PMC7793259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson D.W., Goodman C.D., Sleebs B.E., Weiss G.E., de Jong N.WM., Angrisano F., et al. Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 2015;13:52. doi: 10.1186/s12915-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kadappu K.K., Nagaraja M.V., Rao P.V., Shastry B.A. Azithromycin as treatment for cryptosporidiosis in human immunodeficiency virus disease. J Postgrad Med. 2002 PMID: 12432190. [PubMed] [Google Scholar]
- 88.Burns A.L., Sleebs B.E., Siddiqui G., De Paoli A.E., Anderson D., Liffner B., et al. Retargeting azithromycin analogues to have dual-modality antimalarial activity. BMC Biol. 2020;18(1):133. doi: 10.1186/s12915-020-00859-4. 10.1186/s12915-020-00859-4. PMID: 32993629; PMCID: PMC7526119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoffman P.S., Sisson G., Croxen M.A., Welch K., Harman W.D., Cremades N., et al. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother. 2007;51(3):868–876. doi: 10.1128/AAC.01159-06. Epub 2006 Dec 11. PMID: 17158936; PMCID: PMC1803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shakya A., Bhat H.R., Ghosh S.K. Update on nitazoxanide: a multifunctional chemotherapeutic agent. Curr Drug Discov Technol. 2018;15(3):201–213. doi: 10.2174/1570163814666170727130003. PMID: 28748751. [DOI] [PubMed] [Google Scholar]
- 91.Hickson S.E., Margineantu D., Hockenbery D.M., Simon J.A., Geballe A.P. Inhibition of vaccinia virus replication by nitazoxanide. Virology. 2018;518:398–405. doi: 10.1016/j.virol.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen T., Lee Y.-J., Osinusi A., Amorosa V.K., Wang C., Kang M., et al. Interferon stimulated gene expression in HIV/HCV coinfected patients treated with nitazoxanide/peginterferon-Alfa-2a and ribavirin. AIDS Res Hum Retroviruses. 2016;32(7):660–667. doi: 10.1089/aid.2015.0236. Epub 2016 Mar 14. PMID: 26974581; PMCID: PMC4931749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jasenosky L.D., Cadena C., Mire C.E., Borisevich V., Haridas V., Ranjbar S., et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–1290. doi: 10.1016/j.isci.2019.07.003. Epub 2019 Aug 8. PMID: 31402258; PMCID: PMC6831822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Müller J., Wastling J., Sanderson S., Müller N., Hemphill A. A Novel giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob Agents Chemother. 2007;51(6):1979–1986. doi: 10.1128/AAC.01548-06. Epub 2007 Apr 16. PMID: 17438059; PMCID: PMC1891416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amsden G.W. Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21. doi: 10.1093/jac/dkh519. Epub 2004 Dec 8 PMID: 15590715. [DOI] [PubMed] [Google Scholar]
- 96.Banjanac M., Munić Kos V., Nujić K., Vrančić M., Belamarić D., Crnković S., et al. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol Res. 2012;66(4):357–362. doi: 10.1016/j.phrs.2012.06.011. Epub 2012 Jul 3. PMID: 22766077. [DOI] [PubMed] [Google Scholar]
- 97.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li H., Zhou Y., Fan F., Zhang Y., Li X., Yu H., et al. Effect of azithromycin on patients with diffuse panbronchiolitis: retrospective study of 51 cases. Intern Med. 2011;50(16):1663–1669. doi: 10.2169/internalmedicine.50.4727. Epub 2011 Aug 15. PMID: 21841323. [DOI] [PubMed] [Google Scholar]
- 99.Oliver M.E., Hinks T.S.C. Azithromycin in viral infections. Rev Med Virol. 2021;31(2) doi: 10.1002/rmv.2163. Epub 2020 Sep 23. PMID: 32969125; PMCID: PMC7536932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Firth A., Prathapan P. Broad-spectrum therapeutics: a new antimicrobial class. Curr Res Pharmacol Drug Discov. 2021;2:100011. doi: 10.1016/j.crphar.2020.100011. Epub 2020 Dec 11. PMCID: PMC8035643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oprea T.I., Overington J.P. Computational and practical aspects of drug repositioning. Assay Drug Dev Technol. 2015;13(6):299–306. doi: 10.1089/adt.2015.29011.tiodrrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gatti M., De Ponti F. Drug repurposing in the COVID-19 era: insights from case studies showing pharmaceutical peculiarities. Pharmaceutics. 2021;13(3):302. doi: 10.3390/pharmaceutics13030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peters D.H., Friedel H.A., Azithromycin M.D. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1992;44(5):750–799. doi: 10.2165/00003495-199244050-00007. PMID: 1280567. [DOI] [PubMed] [Google Scholar]
- 104.Kshirsagar N.A., Gogtay N.J., Moran D., Utz G., Sethia A., Sarkar S., et al. Treatment of adults with acute uncomplicated malaria with azithromycin and chloroquine in India, Colombia, and Suriname. Res Rep Trop Med. 2017;13(8):85–104. doi: 10.2147/RRTM.S129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doan T, Hinterwirth A, Arzika AM, et al. Reduction of coronavirus burden with mass azithromycin distribution. Clin Infect Dis. 2020;ciaa606. doi:10.1093/cid/ciaa606. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 106.Kawamura K., Ichikado K., Takaki M., Eguchi Y., Anan K., Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int J Antimicrob Agents. 2018;51(6):918–924. doi: 10.1016/j.ijantimicag.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Lee N., Wong C.K., Chan M.C.W., Yeung E.S.L., Tam W.W.S., Tsang O.T.Y., et al. Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48–56. doi: 10.1016/j.antiviral.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Ashraf S., Chaudhry U., Raza A., et al. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob Resist Infect Control. 2018;7:27. doi: 10.1186/s13756-018-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.-J. Use of ivermectin is associated with lower mortality in hospitalized patients with Coronavirus disease 2019. Chest. 2021;159(1):85–92. doi: 10.1016/j.chest.2020.10.009. Epub 2020 Oct 13. PMID: 33065103; PMCID: PMC7550891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lv C., Liu W., Wang B., Dang R., Qiu L., Ren J., et al. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55–62. doi: 10.1016/j.antiviral.2018.09.010. Epub 2018 Sep 26. PMID: 30266338. [DOI] [PubMed] [Google Scholar]
- 111.Rajamuthiah R., Fuchs B.B., Conery A.L., Kim W., Jayamani E., Kwon B., et al. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS ONE. 2015;10(4):e0124595. doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Imperi F., Massai F., Ramachandran Pillai C., Longo F., Zennaro E., Rampioni G., et al. New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa Quorum Sensing. Antimicrob Agents Chemother. 2013;57(2):996–1005. doi: 10.1128/AAC.01952-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tam J., Hamza T., Ma B., Chen K., Beilhartz G.L., Ravel J., et al. Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nat Commun. 2018;9(1):5233. doi: 10.1038/s41467-018-07705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kao J.C., HuangFu W.C., Tsai T.T., Ho M.R., Jhan M.K., Shen T.J., et al. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Negl Trop Dis. 2018;12(8):e0006715. doi: 10.1371/journal.pntd.0006715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musher D.M., Logan N., Hamill R.J., DuPont H.L., Lentnek A., Gupta A., et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43(4):421–427. doi: 10.1086/506351. Epub 2006 Jul 11. PMID: 16838229. [DOI] [PubMed] [Google Scholar]
- 116.Musher D.M., Logan N., Bressler A.M., Johnson D.P., Rossignol J.F. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009;48(4):e41–e46. doi: 10.1086/596552. PMID: 19133801. [DOI] [PubMed] [Google Scholar]
- 117.Haffizulla J., Hartman A., Hoppers M., Resnick H., Samudrala S., Ginocchio C., et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14(7):609–618. doi: 10.1016/S1473-3099(14)70717-0. Epub 2014 May 19. PMID: 24852376; PMCID: PMC7164783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Firth A., Prathapan P. Azithromycin: the first broad-spectrum therapeutic. Eur J Med Chem. 2020;207:112739. doi: 10.1016/j.ejmech.2020.112739. Epub 2020 Aug 19. PMID: 32871342; PMCID: PMC7434625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maynard R.M., Tetley T.D. Bioterrorism: the lung under attack. Thorax. 2004;59:188–189. doi: 10.1136/thx.2003.016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waterer G.W., Robertson H. Bioterrorism for the respiratory physician. Respirology. 2009;14(1):5–11. doi: 10.1111/j.1440-1843.2008.01446.x. PMID: 19144044. [DOI] [PubMed] [Google Scholar]
- 121.Yan S., Ci X., Chen N., Chen C., Li X., Chu X., et al. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011;60(6):589–596. doi: 10.1007/s00011-011-0307-8. Epub 2011 Jan 29. PMID: 21279416. [DOI] [PubMed] [Google Scholar]
- 122.Cabrita I., Benedetto R., Schreiber R., Kunzelmann K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight. 2019;4(15) doi: 10.1172/jci.insight.128414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Danielsson J., Perez-Zoghbi J., Bernstein K., Barajas M.B., Zhang Y.i., Kumar S., et al. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology. 2015;123(3):569–581. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miner K., Labitzke K., Liu B., Wang P., Henckels K., Gaida K., et al. Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front Pharmacol. 2019;10:51. doi: 10.3389/fphar.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayden EC. Biodefence since 9/11: The price of protection. Nature. 2011;477(7363):150-2. 10.1038/477150a. PMID: 21900990. [DOI] [PubMed]
- 126.Hayden E.C. Pentagon rethinks bioterror effort. Nature. 2011;477(7365):380–381. doi: 10.1038/477380a. PMID: 21938042. [DOI] [PubMed] [Google Scholar]
- 127.Fauci A.S. Bioterrorism: defining a research agenda. Food Drug Law J. 2002;57(3):413–421. PMID: 12703508. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.