Abstracts
Introduction
Some patients with coronavirus disease 2019 (COVID-19) have acute abdomen and need surgery. However, surgery in the acute phase of COVID-19 is associated with worse postoperative outcomes and an increased risk of mortality. We report a case of a patient with COVID-19 who developed intestinal perforation that was treated acutely with antibiotics and delayed surgical intervention.
Presentation of case
A 79-year-old man with COVID-19 was treated with remdesivir and dexamethasone, and his respiratory symptoms and hypoxia improved. However, abdominal symptoms developed, and intestinal perforation occurred. As the nasopharyngeal swab PCR test was positive for SARS-CoV-2, conservative treatment with tazobactam/piperacillin was started to avoid surgery in the acute phase of COVID-19. An intraperitoneal abscess was confirmed on follow-up computed tomography. Emergent laparoscopic lavage and drainage, and transverse colon stoma construction were performed with medical staff using full personal protective equipment. Bacterial culture from the ascites detected Escherichia coli and Bacteroides. The SARS-CoV-2 PCR test of the ascites sample was negative. No infection was observed in the medical staff.
Discussion
COVID-19 has been associated with a higher perioperative risk and postoperative mortality. There has also been a report of ascitic fluid testing positive for SARS-CoV-2 on PCR, suggesting the possibility of intraoperative aerosolization. Avoiding surgical treatment in the acute phase of COVID-19 may reduce deaths from perioperative complications.
Conclusion
Our case suggests that in acute COVID-19 lung infection, careful observation and delayed surgical treatment could prevent worsening of the COVID-19 and reduce the risk of infection to the medical staff.
Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TAZ/PIPC, tazobactam/piperacillin
Keywords: SARS-CoV-2, COVID-19, Perforation, Ascites, Diverticula
Highlights
-
•
Surgery in the acute phase of COVID-19 results in worse postoperative outcome.
-
•
Careful observation and delayed surgery prevented worsening of COVID-19.
-
•
This may help reduce the risk of infection to medical staff.
1. Introduction
Coronavirus disease 2019 (COVID-19) causes lower respiratory symptoms, such as fever, cough, and dyspnea, and can lead to death due to respiratory failure [1]. Non-respiratory complications, such as musculoskeletal, gastrointestinal, hepatic, cardiovascular, and neurological symptoms, have occasionally been observed. Gastrointestinal symptoms have been reported in 18.6% of patients [2].
Some patients with coronavirus disease 2019 (COVID-19) have acute abdomen and need surgery. However, surgery in the acute phase of COVID-19 is associated with worse postoperative outcomes and an increased risk of mortality [3].
Herein, we report a case of a patient with COVID-19 who developed intestinal perforation that was acutely treated with antibiotics and successfully treated with delayed laparoscopic lavage and drainage and transverse colostomy construction. This report is in line with the SCARE criteria [4].
2. Presentation of case
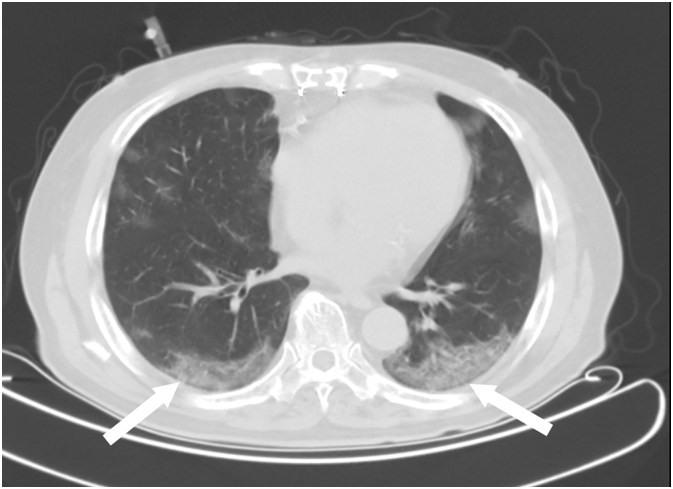
A 79-year-old Japanese man presented with dyspnea on exertion. His severe acute respiratory syndrome coronaviraus-2 (SARS-CoV-2) polymerase chain reaction (PCR) test result was positive. Chest computed tomography (CT) showed peripheral predominant ground-glass opacities in the bilateral lung (Fig. 1), and pneumonia and hypoxia due to COVID-19 were diagnosed. His medical history included diabetes mellitus, stage 4 chronic kidney disease, and coronary artery disease treated with coronary artery bypass graft surgery.
Fig. 1.
Chest computed tomography showing peripheral predominant ground-glass opacities in the bilateral lung (white arrows).
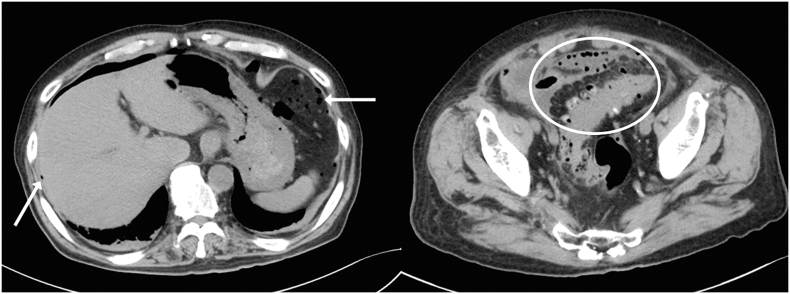
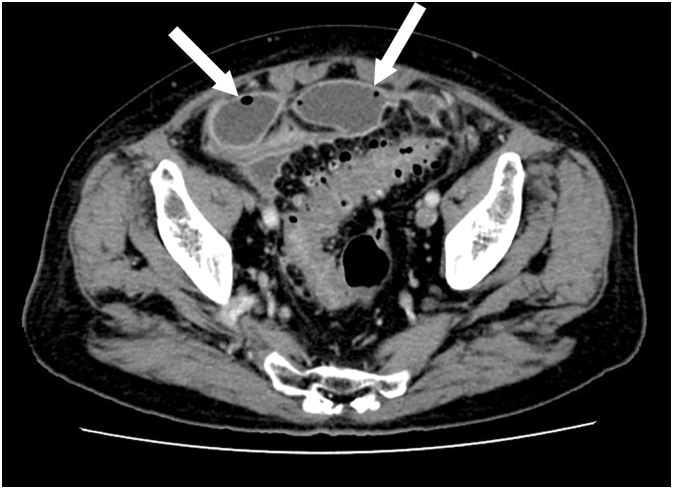
Remdesivir 200 mg/day on the first day and 100 mg/day on the second to fifth day, and dexamethasone 6 mg/day were started after admission. Vonoprazan was used for the prevention of intestinal ulcers. Respiratory symptoms and hypoxia improved. Remdesivir and dexamethasone treatment was stopped on the fifth day. However, anorexia and lower abdominal pain with rebound tenderness appeared on the sixth day. The physical examination revealed that the heel-drop sign was positive. As the abdominal radiograph showed a fecal mass and the symptoms improved with defecation, the patient was placed under observation. Although without abdominal symptoms, elevated white blood cell (WBC) counts and C-reactive protein (CRP) levels were observed on the eighth day. Abdominal CT showed multiple colonic diverticula and free air (Fig. 2), and intestinal perforation was diagnosed. As the SARS-CoV-2 PCR test was positive on the same day, 4.5 g intravenous tazobactam/piperacillin (TAZ/PIPC) every 8 h was started to avoid surgery in the acute phase of COVID-19. A follow-up CT revealed intraperitoneal abscess formation on the 13th day (Fig. 3), and peritonitis due to perforation of the sigmoid colon diverticulum was diagnosed. Emergent laparoscopic lavage and drainage and transverse colon stoma construction were performed by medical staff wearing full personal protective equipment. First, the pneumoperitoneum gas was evacuated with a laparoscopic aspirator. The perforation site could not be identified because of the large number of abscesses and extensive intestinal adhesions were observed. We did not choose another treatment method, such as resection and anastomosis, because we wanted to shorten the operation time. Bacterial cultures from the ascites detected E. coli and Bacteroides. SARS-CoV-2 PCR test of the ascites sample was negative, while SARS-CoV-2 PCR test of a nasopharyngeal swab was positive. No infection was observed among the medical staff.
Fig. 2.
Abdominal computed tomography showing multiple colonic diverticula (white circle) and free air (white arrows).
Fig. 3.
Abdominal computed tomography showing intraperitoneal abscess (white arrows).
The patient's abdominal symptoms, WBC counts, and CRP improved. The SARS-CoV-2 PCR tests from nasopharyngeal swabs taken on the 17th and 20th days returned negative. The patient was transferred to the general ward. TAZ/PIPC was changed to levofloxacin 500 mg/day on the 21st day, but the laboratory inflammatory response flared up and levofloxacin was switched to amoxicillin/clavulanate on the 25th day. The inflammatory response improved, and he was discharged on the 32nd day.
Six months after discharge from our hospital, the patient underwent laparoscopic total colorectal resection for multiple colonic diverticuli and stoma reversal. The postoperative course was good.
3. Discussion
Laparoscopic lavage and drainage for intestinal perforation in a patient with COVID-19 was successfully performed after antibiotic therapy to avoid surgery in the acute phase of COVID-19. Since medical staff are also at risk of contracting COVID-19 from patients through aerosols during surgery, it is important to delay the timing of surgical procedures until the acute phase of COVID-19 has subsided.
Up to 50% of patients with SARS-CoV-2 infection develop gastrointestinal symptoms before respiratory symptoms [5]. The most frequent gastrointestinal symptoms of SARS-CoV-2 infection are anorexia (26.1%), followed by diarrhea (13.5%), nausea (7.5%), vomiting (6%), and abdominal pain (5.7%) [2]. Patients with gastrointestinal symptoms have a worse prognosis than those without gastrointestinal symptoms [6]. Serious gastrointestinal complications include gastrointestinal bleeding and perforation. In a previous study, out of 905 patients infected with SARS-CoV-2, 10 developed severe gastrointestinal complications, with a reported mortality rate of 50% [7].
Several hypotheses have been proposed to explain the mechanism by which SARS-CoV-2 causes gastrointestinal damage. SARS-CoV-2 is a virus that enters the host via the angiotensin-converting enzyme-2 (ACE2) receptor and causes inflammation of glandular cells in the stomach, duodenum, and rectal epithelium, which express abundant ACE-2 receptors [8]. SARS-CoV-2 infection has also been shown to cause coagulopathy, leading to intestinal ischemia and perforation due to mesenteric thrombosis [6]. The effects of therapeutic agents against SARS-CoV-2, such as steroids and tocilizumab, are also known to cause intestinal perforation. Corticosteroid use has been shown to significantly increase the mortality rate associated with intestinal perforation [9]. Intestinal perforation has been reported in approximately 2/1000 patients who have received at least one dose of tocilizumab [10]. In our case, there was no intraoperative intestinal ischemia. Therefore, the bowel perforation may have been caused by dexamethasone against the background of a fragile bowel wall due to colonic diverticulum.
Patients with COVID-19 who present with gastrointestinal symptoms tend to be diagnosed late and have a poor disease course [5], [6]. A case of gastrointestinal perforation was reported in which a CT could not be performed because the patient had severe SARS-CoV-2 [11]. In our patient, anorexia and lower abdominal pain with rebound tenderness appeared on the sixth day. These were considered signs of peritoneal irritation. As the symptoms were relieved by defecation and infection protection was necessary, bedside abdominal radiography was preferred. Patients with COVID-19 may also benefit from an early abdominal CT if they show signs of peritoneal irritation, given the high incidence of gastrointestinal perforation.
Acute diverticulitis with perforation is a life-threatening condition that requires urgent surgery. Moreover, 73.8% and 3.8% of critically ill patients with COVID-19 develop gastrointestinal complications and gastrointestinal ischemia, respectively [12]. Patients who are SARS-CoV-2-positive and undergo surgery have worse postoperative outcomes and increased mortality risk [3]. When patients with a SARS-CoV-2 infection undergo surgery, the release of pro-inflammatory cytokines also increases the risk of pulmonary complications and artificial respiration. An international cohort study concluded that half of the patients with SARS-CoV-2 develop pulmonary complications postoperatively, and consequently, mortality increases rapidly [13]. For our patient, conservative treatment was first selected because of the high perioperative risk of SARS-CoV-2 pneumonia and the risk of infection exposure among medical staff.
The usefulness of performing a SARS-CoV-2 PCR test on the ascites fluid is unclear. Although most have reported negative SARS-CoV-2 PCR test results from ascites [14], some have reported positive SARS-CoV-2 PCR test results, suggesting the possibility of intraoperative aerosolization [15]. The ascites sample SARS-CoV-2 PCR test was negative in our patient. However, medical staff should be aware of the possibility of aerosol infections.
In conclusion, our case suggests that in acute COVID-19 lung infection, careful observation and delayed surgical treatment could prevent worsening of COVID-19 and reduce the risk of infection to the medical staff.
Ethics approval and consent to participate
The case report had been prepared in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Kishiwada City Hospital. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
YM: Writing and medical treatment. YY: Figure generation and writing. AY, KT, SR: Supervision and medical treatment. YM: Supervision and surgical treatment. All authors have read and approved the manuscript.
Declaration of competing interest
The authors have no conflicts of interest directly relevant to the content of this article.
Acknowledgements
The authors thank all doctors, nurses, pharmacist, technicians, radiological technologist, and clerks fighting against COVID-19 in Kishiwada City hospital. We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Chams N., Chams S., Badran R., Shams A., Araji A., Raad M., et al. COVID-19: a multidisciplinary review. Front. Public Health. 2020;8:383. doi: 10.3389/fpubh.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suryana K.D., Simadibrata M., Renaldi K. Impact of COVID-19 on the gut: a review of the manifestations, pathology, management, and challenges. Acta Med Indones. 2021;53(1):96–104. [PubMed] [Google Scholar]
- 3.Aziz H., Filkins A., Kwon Y.K. Review of COVID-19 outcomes in surgical patients. Am. Surg. 2020;86(7):741–745. doi: 10.1177/0003134820934395. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Group S. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional,multicenter study. Am. J. Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estevez-Cerda S.C., Saldaña-Rodríguez J.A., Alam-Gidi A.G., Riojas-Garza A., Rodarte-Shade M., Velazco-de la Garza J., et al. Severe bowel complications in SARS-CoV-2 patients receiving protocolized care. Rev. Gastroenterol. Mex. 2021;86(4):378–386. doi: 10.1016/j.rgmxen.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotfis K., Skonieczna-Żydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52(2):171–172. doi: 10.5114/ait.2020.93867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broersen L.H.A., Horváth-Puhó E., Pereira A.M., Erichsen R., Dekkers O.M., Sørensen H.T. Corticosteroid use and mortality risk in patients with perforated colonic diverticular disease: a population-based cohort study. BMJ Open Gastroenterol. 2017;4(1) doi: 10.1136/bmjgast-2017-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiff M.H., Kremer J.M., Jahreis A., Vernon E., Isaacs J.D., van Vollenhoven R.F. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141. doi: 10.1186/ar3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kangas-Dick A., Prien C., Rojas K., Pu Q., Hamshow M., Wan E., et al. Gastrointestinal perforation in a critically ill patient with COVID-19 pneumonia. SAGE Open Med. Case Rep. 2020;8 doi: 10.1177/2050313X20940570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Moheb M., Naar L., Christensen M.A., Kapoen C., Maurer L.R., Farhat M., et al. Gastrointestinal complications in critically ill patients with and without COVID-19. JAMA. 2020;324(18):1899–1901. doi: 10.1001/jama.2020.19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative C.S. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safari S., Keyvani H., Malekpour Alamdari N., Dehghanian A., Razavi Hashemi M., Nemati Honar B., et al. Abdominal surgery in patients with COVID-19: detection of SARS-CoV-2 in abdominal and adipose tissues. Ann. Surg. 2020;272(3):e253–e256. doi: 10.1097/SLA.0000000000004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coccolini F., Tartaglia D., Puglisi A., Giordano C., Pistello M., Lodato M., et al. SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann. Surg. 2020;272(3):e240–e242. doi: 10.1097/SLA.0000000000004030. [DOI] [PMC free article] [PubMed] [Google Scholar]