Abstract
Most of the reviews on histoplasmosis documented in literature have been in the adult population. Very few studies highlight the peculiarities associated with histoplasmosis in Africa especially in the pediatric population. This review addresses the above concerns with clinical summaries and diagnosis of some case reports of histoplasmosis in African children. We highlighted 44 case reports of histoplasmosis in African children (1950–2021) distributed across Western Africa (38.6%, n = 17), Eastern Africa (9.1%, n = 4), Southern Africa (9.1%, n = 4), and Central Africa (43.2%, n = 19). No case report was found from Northern Africa. The age range was 1–17 years, with a mean of 9.2. Of the 44 case reports, 8 cases (18.2%, 8/44) were caused by Histoplasma capsulatum var capsulatum, 33 cases (75%, 33/44) were caused by Histoplasma capsulatum var duboisii, and specie identification was not found in 3 cases. Only three (6.8%) cases were HIV positive; 56.8% (25/44) were disseminated histoplasmosis, pulmonary histoplasmosis accounted for just one case (2.3%, 1/44). Extrapulmonary presentation included skin lesions (ulcers, fistulas, nodules, patches, pigmentations, papules, and abscesses), bone lesions, osteoarthritis, and fractures. The commonest sites affected were skin (n = 29, 65.9%), bones (n = 20, 45.5%), and lymph nodes (n = 15, 34.1%). Histopathology was the commonest diagnostic method (n = 33, 75%). Amphotericin B was first-line therapy in 45.5% of the cases (n = 20) followed by ketoconazole (20.5%, n = 9); 27 cases (61.4%) had favorable outcomes, 8 cases (18.2%) had fatal outcomes, while in 9 cases, the outcome was not revealed. This review revealed several cases of histoplasmosis misdiagnosed as other conditions including tuberculosis (n = 3, 6.8%), pneumonia (n = 1, 2.3%), cancers (n = 4, 9.1%), nephritic syndrome (n = 1, 2.3%), leishmaniasis (n = 1, 2.3%), and hyperreactive malarial splenomegaly syndrome (n = 1, 2.3%). In addition, histoplasmosis was not considered in some case reports even when symptoms were suggestive. Diagnosis of histoplasmosis was made at autopsy with postmortem findings suggestive of histoplasmosis (n = 3, 6.8%). This report highlights the need for a paradigm shift on the part of pediatricians in Africa. They need to look beyond clinical conditions considered common in our environment for this age group and evaluate for other diseases including histoplasmosis.
Keywords: Africa, children, disseminated histoplasmosis, Histoplasma duboisii, histoplasmosis, tuberculosis
Introduction
The HIV pandemic and the increasing use of immunosuppressive therapies, such as calcineurin and tumor necrosis factor inhibitors, in patients with autoimmune conditions have also resulted in more cases of histoplasmosis.1–3 Infections are usually self-limiting in immunocompetent children and rarely require treatment. 4 Infections may occur by inhalation of microconidia following human activities that disrupt the soil which tend to aerosolize the hyphae and conidia of the organism or orally, causing infection in the intestines.1,5 Perinatal and congenital infections have also been reported in children living with HIV.6,7 Histoplasmosis in African children is not uncommon. Oladele et al. 8 in a review article on histoplasmosis in Africa reported 37 pediatric cases out of 470 cases documented between 1952 and 2016. In addition, a case series study by Pakasa et al. 9 revealed a high incidence (44.4%, 16/36) of histoplasmosis in young children, within the age range of 3–7 years. In a more recent review by Amona et al., 10 of the 54 cases reported in Congo, 17 (31.5%) were from the pediatric age group. However, there still exist occurrences of histoplasmosis being misdiagnosed as other clinical conditions like tuberculosis (TB), pneumonia, cancers, nephrotic syndrome and hyperreactive malarial splenomegaly syndrome.11–13 In yet some cases, there is a delay in diagnosis of histoplasmosis with other common conditions like TB and cancers topping the list of differential diagnosis, resulting in prolonged hospital stay, economic losses, time wastage, inappropriate use of antibiotics and mortalities.14–16 In addition, children have a high rate of asymptomatic infection (about 95%) even when exposed to outbreaks. 17 The reviews aim to document the number of case reports of histoplasmosis in the pediatric population in Africa and identify the drivers, clinical manifestation and treatment outcomes of the cases. This will hopefully drive awareness with improved finding of cases and good clinical outcomes.
The organism
Histoplasma species pathogenic to humans are Histoplasma capsulatum var capsulatum (Hcc) and Histoplasma capsulatum var duboisii (Hcd). 18 H. duboisii is commonly found in Africa; however, few cases have been reported in Europe, in African patients/immigrants’ seeking care in Europe. 19 H. capsulatum is the causative agent of classical histoplasmosis; a dimorphic fungal pathogen with two distinct morphological forms: the mold form which grows at ambient temperatures and the yeast form which grows at body temperature.18,20 The fungus is primarily found in soil enriched with bird or bat guano, where it exists in the mold form. The mold form is composed of hyaline septate hyphae that are 1 to 2.5 μm diameter.18,20 Hyphae produce two different hyaline asexual reproduction structures: macroconidia and microconidia. The macroconidia or tuberculate conidia measures 8 to 15 μm in diameter and are characterized by a thick wall with distinctive projections on the surface, while the microconidia are tiny, smooth structures that are 2 to 4 μm in diameter. Microconidia are small enough to be lodged in the alveoli when inhaled and are the infectious forms, while the macroconidia aid in the identification.18,20,21 The yeast is the pathogenic form of the organism and measures 2 to 4 μm in diameter; found in the tissues of infected individuals or when the organism is grown at ⩾37°C in vitro.18,20,21 Histoplasma duboissi is a variant predominantly found in Africa and produces larger yeast cells (8–15 μm in length).18,20,21
Materials and methods
A systematic literature search was conducted using PubMed, Google Scholar, AJOL, Cochrane Library, Africa-Wide: NiPAD, CINAHL (accessed via EBSCO Host) databases and gray literature to identify all published papers regarding the topic ‘between 1 January 1950 and 1 January 2021’. The search terms such as ‘histoplasmosis in children’ OR ‘case reports of histoplasmosis in children’ were used. References in all relevant papers were also reviewed for additional publications (‘snow balling’) on histoplasmosis in children that may not have been published in the searched databases. Publications without patients’ country of origin were excluded. Publications reporting cases of histoplasmosis in individuals greater than 18 years of age were excluded from the review, as well as studies involving histoplasmosis in nonhumans; cases in which the age was not specified were also excluded. Publications without abstracts were excluded. Diagnosis of histoplasmosis was defined as identification of Histoplasma spp. via histopathological examination of tissue samples or culture or molecular methods and/or positive Histoplasma antigen and/or positive Histoplasma antibody detection. Case reports without specified diagnostic modality were excluded. Data extracted from each case included the following: age, gender, disease type (single organ versus disseminated disease), sites of infection, comorbidities, clinical features, diagnostic test results, treatment and patient outcome. Case reports with undefined HIV status were assumed to be negative, while case reports with defined HIV status (that is positive or negative) were documented as such.
Results
Patient characteristics
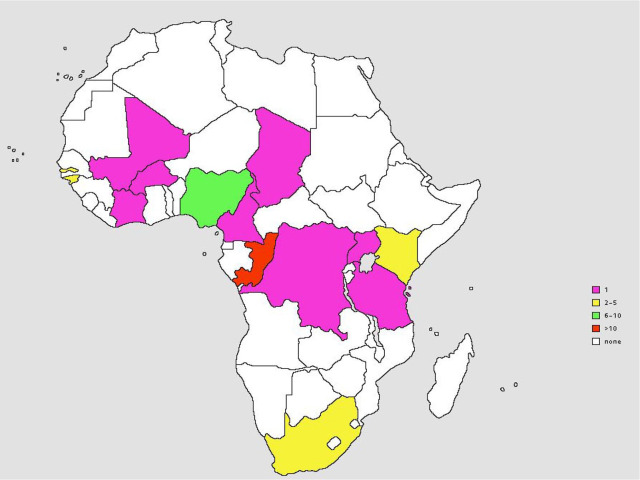
Our extensive literature search yielded a total of 64 publications on childhood histoplasmosis in Africa of which 20 publications were excluded and 44 included in this review (Table 1). The 44 cases were distributed across all the subregions of Africa except northern Africa, with highest number of cases from central Africa (n = 19), followed by Western Africa (n = 17), Southern Africa (n = 4) and Eastern Africa (n = 4) (Table 2, Figure 1). Of the 44, 26 were males (26/44, 59.1%), with the sex ratio (M/F) at 1.4. The mean age (±standard deviation) at presentation was 9.2 ± 4.6 years, with a median age of 9 years; range: 1–17 years. Of the 44 patients, 3 (6.8%) were infants (⩽2 years); 9 (20.5%) cases were from urban areas, 6 (13.6%) were from rural areas and 29 were not specified. Three of the case reports were immigrants in Europe as at the time of diagnosis; a 12-year-old female from Cameroon, a 7-year-old female from Guinea Bissau, and 7-year-old male from South Africa; 6.8% (3/44) of these patients were HIV positive. No statistically significant relationship was observed when comparing the relationship between histoplasmosis and HIV status of patients (p > 0.05, Fisher’s exact test). Underlying conditions were only identified in 4.5% (2/44) of the patients who had mesenteric chylous cyst and malnutrition. The only case with occupational history was a 15-year-old male involved in crushing stones for 2 years (Table 3).
Table 1.
Inclusion and exclusion criteria that were used in the literature search.
| <18 years | Exclusively ⩾ 18 years |
| Diagnosis of histoplasmosis | No diagnosis of histoplasmosis |
| Published 1 January 1950–1 January 2021 | Published before 1 January 1950 |
| Publications with patients’ country of origin | Publications without patients’ country of origin |
| Patient is of African origin | Patient is of non-African origin |
| Case reports with abstracts | Case reports without abstracts |
| Original case reports of histoplasmosis | No original case reports of histoplasmosis |
Table 2.
Distribution of selected case reports of pediatric histoplasmosis in Africa.
| Sub region | Number of cases per country | n | % |
|---|---|---|---|
| Western Africa | Cote d′voire (1), Gambia (3), Guinea-Bissau (2), Mali (1), Nigeria (9), Burkina Faso (1) | 17 | 38.6 |
| Central Africa | Cameroon (1), Chad (1), Zaire (1), Congo (16) | 19 | 43.2 |
| Southern Africa | South Africa (4) | 4 | 9.1 |
| Eastern Africa | Kenya (2), Uganda (1), Tanzania (1) | 4 | 9.1 |
| Northern Africa | – | – | – |
| Total | 44 | 44 | 100 |
n, number of cases; %, percentage of cases.
Figure 1.
Distribution of selected case reports of histoplasmosis in African children.
Table 3.
Description of 44 cases of histoplasmosis in African children (1950–2021).
| Sex/age (years) | Hcc/Hcd | Setting | HIV | Site of infection | DH | Treatment | Ocm | References |
|---|---|---|---|---|---|---|---|---|
| Zaire | ||||||||
| a F/4 | NR | NS | N | Liver, spleen, lymph nodes | YES | Ketoconazole | F | Lamey and Parisien 22 |
| Uganda | ||||||||
| b M/4 | Hcc | NS | N | Mucosa | NO | Surgery/Itraconazole | F | Kweyamba et al. 23 |
| Tanzania | ||||||||
| c M/15 | Hcc | NS | N | Lungs | NO | None | D | Kabangila et al. 24 |
| South Africa | ||||||||
| F/16 | Hcc | NS | N | Bones | NO | NR | NR | Suleman and Scheepers 25 |
| d M/7 | Hcc | Rural | N | Lymph nodes, spleen | YES | AmB, Ketoconazole, Itraconazole | F | Daubenton and Beatty 26 |
| F/8 | Hcc | NS | P | Skin, lymph nodes, tongue | YES | None | D | Pillay et al. 27 |
| F/11 | Hcc | NS | P | Skin, lung, lymph nodes, liver, spleen | YES | AmB | NR | Mosam et al. 13 |
| Nigeria | ||||||||
| M/6 | Hcd | NS | N | Skin, muscles, lymph nodes, bones | NO | AmB/Ketoconazole | F | Akpuaka et al. 28 |
| b M/11 | NR | NS | N | Skin, muscles | NO | AmB/ketoconazole | F | Akpuaka et al. 28 |
| M/10 | Hcd | NS | N | Skin, lymph nodes, bone | NO | Fluconazole | F | Onwuasoigwe 29 |
| M/12 | Hcd | NS | N | Bone | NO | AmB | F | Mace 15 |
| M/9 | Hcd | NS | N | Skin, bone | NO | NR | NR | Khalil et al. 12 |
| M/14 | Hcd | NS | N | Lymph node | NO | NR | NR | Khalil et al. 12 |
| M/9 | Hcd | NS | N | Skin, muscles | NO | Surgery/Itraconazole | F | Katchy et al. 30 |
| M/13 | Hcd | NS | N | Skull | NO | AmB | F | Shoroye and Oyedeji 16 |
| F/13 | Hcd | NS | N | Skin, lymph nodes | YES | None | D | Ubesie et al. 14 |
| Mali | ||||||||
| M/6 | Hcd | Rural | N | Skin, mucosa, lymph nodes, bones | YES | Ketoconazole | F | Minta et al. 31 |
| Kenya | ||||||||
| F/6 | Hcc | NS | P | Liver, spleen | YES | NR | NR | Pamnani et al. 11 |
| F/1.9 | Hcc | NS | N | Liver, spleen | YES | NR | NR | Pamnani et al. 11 |
| Guinea Bissau | ||||||||
| M/12 | Hcd | NS | N | Skin, muscles, fistulas | NO | Posaconazole | F | Gonçalves et al. 32 |
| e F/7 | NR | NS | N | Skin, muscle, bone | NO | NR | NR | Guimarães et al. 20 |
| Gambia | ||||||||
| F/4 | Hcd | NS | N | Skin, bone | YES | AmB/Ketoconazole | F | Mabey and Hay 33 |
| F/5 | Hcd | NS | N | Lymph node | YES | AmB/Ketoconazole | F | Mabey and Hay 33 |
| M/10 | Hcd | NS | N | Skin | YES | Surgery/AmB/Ketoconazole | F | Mabey and Hay 33 |
| Cote d′voire | ||||||||
| M/6 | Hcd | NS | N | Skin, bone | NO | Terbinafine | F | Bankolé et al. 34 |
| Congo | ||||||||
| M/17 | Hcd | Urban | N | Skin, bone lesions | YES | AmB | F | N’Golet et al. 35 |
| F/15 | Hcd | Rural | N | Skin | NR | NR | NR | Destombes et al. 36 |
| F/6 | Hcd | Rural | N | Skin | NR | AmB | F | Carme et al. 37 |
| F/13 | Hcd | Rural | N | Mucosa, bone | NO | AmB | F | Carme et al. 37 |
| M/11 | Hcd | Urban | N | Skin, bone | YES | AmB | F | Griffet et al. 38 |
| F/2 | Hcd | NS | N | Skin, bone, eye | YES | AmB | F | Carme et al. 37 |
| M/13 | Hcd | NS | N | Skin, bone | YES | AmB | F | Carme et al. 37 |
| M/17 | Hcd | NS | N | Skin | NO | AmB | F | Carme et al. 37 |
| M/17 | Hcd | NS | N | Skin, mucosa | NO | AmB | F | Carme et al. 37 |
| M/13 | Hcd | Rural | N | Bone | YES | AmB | F | Moyikoua et al. 39 |
| F/7 | Hcd | Urban | N | Skin, bone | YES | Surgery/Itraconazole | F | Paugam et al. 40 |
| M/15 | Hcd | Urban | N | Skin, lung | YES | None | D | Okoko et al. 41 |
| M/4 | Hcd | Urban | N | Skin, lymph nodes | YES | Itraconazole | D | Chandenier et al. 42 |
| F/9 | Hcd | Urban | N | Lymph node, liver, spleen | YES | Ketoconazole | D | Mabiala et al. 43 |
| M/3 | Hcd | Urban | N | Skin, lymph node, bone | YES | Itraconazole | D | Mabiala et al. 43 |
| M/4 | Hcd | Urban | N | Bone lesions | YES | Itraconazole | D | Mabiala et al. 43 |
| Chad | ||||||||
| F/10 | Hcd | NS | N | Skin | YES | AmB | F | Garcia-Guiñon et al. 44 |
| Cameroon | ||||||||
| f F/12 | Hcd | NS | N | Skin, lymph nodes, bone lesions | YES | NR | NR | André et al. 45 |
| Burkina Faso | ||||||||
| M/1 | Hcd | Urban | N | Skin, liver, spleen, lymph nodes, bones | YES | Fluconazole/AmB | F | Barro/Traoré et al. 46 |
AmB, amphotericin B; D, death; DH, disseminated histoplasmosis; EPH, extrapulmonary histoplasmosis; F, favorable clinical outcome; F, female; Hcc, Histoplasma capsulatum; Hcd, Histoplasma duboisii; M, male; N, negative; NR, not revealed; NS, not specified; Ocm, outcome; P, positive; PH, pulmonary histoplasmosis.
Patient recovered but died of measles.
Case reports with underlying conditions (mesenteric chylous cyst, malnutrition).
A case report of pulmonary histoplasmosis in a 15-year-old male involved in crushing stones for 2 years.
Paediatric histoplasmosis reported in London in an indigene of South Africa.
Paediatric histoplasmosis reported in Portugal in an indigene of Guinea Bissau.
Paediatric histoplasmosis reported in France in an indigene of Cameroon.
Disease characteristics
Of the 44 cases, disseminated histoplasmosis (DH) constituted 56.8% (25/44): a single case (2.3%, 1/44) of pulmonary histoplasmosis (PH), the other cases were extrapulmonary forms presenting with skin lesions (ulcers, nodules, sinuses and fistulas), abscesses, bone lesions, fractures, osteoarthritis and generalized/diffused lymphadenopathy; 8 cases (18.2%, 8/44) were caused by Hcc, 33 cases (75%, 33/44) were caused by Hcd, and specie identification was not found in 3 cases. The affected sites were skin (n = 29, 65.9%), bones (n = 20, 45.5%), lymph nodes (n = 15, 34.1%), spleen (n = 7, 15.9%), muscles (n = 5, 11.4%), liver (n = 6, 13.6%), mucosa (n = 4, 9.1%), lungs (n = 3, 6.8%), eyes (n = 1, 2.3%) and tongue (n = 1, 2.3%) as shown in Table 3.
Pulmonary histoplasmosis misdiagnosed as TB
The clinical and radiological features seen in histoplasmosis have similarities with findings in TB and may account for misdiagnosis of cases of histoplasmosis as TB (Table 4).11–13 Even though histoplasmosis and TB are AIDS-defining illnesses, a diagnosis of histoplasmosis is rarely considered when patients with pediatric AIDS present with respiratory symptoms.11,13 The high burden of TB in Africa is also a major factor that may have relegated other clinical conditions like histoplasmosis to the background. 47 In some cases, instead of considering the possibility of other diseases, anti-TB therapy is still commenced despite having negative acid-fast bacilli (AFB) results.11,13 In a case report by Mosam et al., 13 anti-TB therapy and antibiotics were commenced in a child with HIV who was negative for Mantoux test and sputum AFB test based on the presence of constitutional symptoms (fever, weight loss, cough, and generalized lymphadenopathy). Histoplasmosis was diagnosed later on biopsy of skin lesions which revealed intracytoplasmic fungal yeast cells, 2–4 μm in diameter morphologically similar to H. capsulatum. Child was commenced on IV amphotericin B for disseminated histoplasmosis but died a week later. 13 Pulmonary histoplasmosis may also be misdiagnosed as pneumonia or bronchiectasis which may warrant initiation of empirical antibiotics, thereby increasing length of hospital stay for the patient, worsening of patient’s clinical condition, increased risk of mortality and death eventually, if appropriate diagnosis is not made on time.13,27
Table 4.
Case reports of histoplasmosis in African children misdiagnosed as tuberculosis.
| Sex/Age/Country | Clinical findings | HIV | Initial diagnosis | Diagnostic method/final diagnosis | Treatment/outcome | References |
|---|---|---|---|---|---|---|
| F/6/ Kenya | Fever, cough, abdominal pain. Examination findings: ill-looking, pale, wasted, febrile with bilateral pitting edema of the legs, hepatosplenomegaly, bilateral basal crepitations. Laboratory findings: Hb: 7.8 g/dL, ESR: 47 mm/1st hr. Sputum was negative for acid-fast bacilli (AFB), chest x-ray showed patchy pneumonic infiltrate. | P | Tuberculosis/leishmaniasis | Bone marrow aspirate cytology /DH | Not revealed | Pamnani et al. 11 |
| M/14/ Nigeria |
A painless lymph node swelling in the anterior neck region. | N | Tuberculosis lymphadenitis with a cold abscess/Lipoma | Biopsy of the lymph node swelling/African histoplasmosis | Not revealed | Khalil et al. 12 |
| F/11/ South Africa |
Weight loss, cough, and skin lesions. Examination findings: Pale, febrile, generalized lymphadenopathy and hepatosplenomegaly. Emaciated with hyperpigmented cutaneous plaques and nodules on her face, arms, and thighs. Hb = 91 g/L, WBC = 48 × 109/L, Platelet count = 94 × 109/L. Mantoux test: negative, sputum AFB: negative for acid-fast bacilli | P | Tuberculosis | Skin biopsy/DH (Hcc) | No improvement with anti-TB therapy IV AmB was commenced. She died a week later | Mosam et al. 13 |
AFB, acid-fast bacilli; AmB, amphotericin B; DH, disseminated histoplasmosis; N, negative; P, positive; TB, tuberculosis.
TB and histoplasmosis co-occurrence
Of the 44 cases, 3 (6.8%) were coinfected with histoplasmosis and TB. Co-occurrence of TB and histoplasmosis have been reported across all age groups but however rare in children (Table 5).27,32 While an immunocompromised status may encourage the co-occurrence of these two opportunistic infections in an individual, case reports have also been documented in nonimmunocompromised patients. 32 Persistence of respiratory symptoms despite the commencement or completion of anti-TB drugs is suggestive of histoplasmosis. It may present as treatment failure, relapse, or multidrug-resistant (MDR) TB in a patient with confirmed diagnosis of TB who may have taken complete anti-TB therapy but with no clinical response.32,48 A high index of suspicion and extensive range of investigations including sputum AFB, Gene Xpert, lymph node biopsy, sputum culture, bone marrow aspirate examination, and PCR are needed for prompt diagnosis and timely intervention, otherwise may result in a misdiagnosis and inappropriate therapy.13,32,48
Table 5.
Co-occurrence of pediatric histoplasmosis with TB/pneumonia.
| Sex/Age/Country | Clinical findings | HIV | Initial diagnosis | Diagnostic method/Final diagnosis | Treatment/outcome | References |
|---|---|---|---|---|---|---|
| M/12/ Guinea-Bissau |
Multiple cervical nodules, axillary, and inguinal cutaneous fistulas. Painful supra pubic abdominal mass. CT scan: micro-nodules and hilar calcifications on the left lower lobe. Sputum culture for TB was positive. | N | Lymphoma/Pulmonary TB | Cervical nodular biopsy: Histoplasma duboisii, swab cultures of the exudates: Histoplasma duboisii | 6 months course of anti-TB therapy but did not improve. Patient recovered with Posaconazole | Gonçalves et al. 32 |
| M/12/ Tanzania |
Productive cough and intermittent low-grade evening fevers for 1 year and shortness of breath of 1 week duration | N | TB/Recurrent TB | AFB; initially positive, then became negative after completion of anti-TB therapy. Post-mortem findings showed yeast cells suggestive of Histoplasma capsulatum | No improvement after commencement of anti TB therapy and repeated anti-TB therapy. Patient died | Kabangila et al. 24 |
| F/8/ South Africa |
Fever, malaise, and respiratory distress. Examination findings: pallor with generalized lymphadenopathy. Punched out painless ulcer on her left lower leg, ulcerative lesions on the tip of her tongue and the angle of her mouth; tender hepatomegaly. Chest radiograph: right upper lobe consolidation with cavitation. Direct immunofluorescence and culture of the oral lesions yielded herpes simplex type 1. | P | Herpesvirus infection, severe community-acquired pneumonia, Pneumocystis carinii infection | Bone marrow aspirate and trephine biopsy (revealed yeast forms of H. capsulatum /DH | Patient died before antifungal therapy could be commenced. Postmortem specimens from lung, liver, lower limb skin lesion and lymph node demonstrated histoplasmosis. | Pillay et al. 27 |
AFB, acid-fast bacilli; CT, computed tomography; DH, disseminated histoplasmosis; N, negative; P, positive; TB, tuberculosis.
DH
DH is one of the commonest clinical manifestations of histoplasmosis in children with AIDS and is often fatal if untreated.13,26,27 It can also occur in nonimmunocompromised children and has been documented even before the advent of HIV/AIDS. 26 DH results from the hematogeneous spread of a primary infection or a reactivation of infections from the lungs due to compromised cell-mediated immunity. Other risk factors include environmental exposures, anti-tumor necrosis factor therapy, CD4 lymphopenia, solid organ transplants, hematological malignancy, defects in IL-12, and interferon gamma pathways.4,5,26,48 Clinical manifestations include the following: malaise, prolonged fever, cough, dyspnea, interstitial pneumonitis, weight loss, diarrhea, abdominal pain, peripheral edema, generalized lymphadenopathy, angina, headache, seizures, abnormal gait, altered consciousness, cytopenia(s), coagulopathy, oropharyngeal/gastrointestinal ulcerations, erythematous nodular/ulcerative cutaneous lesions, and failure to thrive.22,35,43–45 Symptoms are nonspecific and can mimic many other clinical conditions including malaria, sepsis, hepatic failure, hematologic disorders, and malignancies.22,26,30,35,43–45 Commonly affected sites include liver, spleen, bone marrow, gastrointestinal tract, heart, adrenal glands, central nervous system, eyes, and skin.11,13,22,26,30,35,43–45 Chest radiograph may be normal, or it may reveal interstitial, reticulonodular, or miliary infiltrates.11,13,27
Histoplasmosis mimicking malignancies (lymphoma)
Findings of generalized lymphadenopathy, weakness, wasting, painful facial and body swellings, abdominal distention, hepatomegaly, splenomegaly, deranged liver function tests, and abnormal hematological findings in a patient with DH are highly suggestive of a malignancy and may be misdiagnosed as such.11,12,15,16 Table 6 shows some of the diagnosis considered in cases highlighted including osteosarcoma, lymphomas, neuroblastoma with orbital secondaries, facial tumor, and septicemia subsequently followed by a diagnosis of DH after a bone marrow examination or tissue biopsy. DH was not considered as a provisional or differential diagnosis in all the cases highlighted. In a case report by Mace, 15 IV cyclophosphamide was already commenced as therapy for Burkitt’s lymphoma and later discontinued after histology result showed findings (giant cells with yeasts spread throughout the cytoplasm) suggestive of African histoplasmosis. Patient, however, recovered after receiving IV amphotericin B therapy. 11 One wonders how many cases of DH that are being misdiagnosed as malignancies especially where investigations like bone marrow aspirate cytology and tissue biopsy cannot be done or are not affordable like in low-resource settings.
Table 6.
Histoplasmosis mimicking malignancies/nephritic syndrome/malarial splenomegaly syndrome.
| Sex/age/country | Clinical findings | HIV | Initial diagnosis | Diagnostic method/final diagnosis | Treatment/outcome | References |
|---|---|---|---|---|---|---|
| F/21 months/Kenya | Anorexia, abdominal swelling, and passing of black colored stools. Examination findings: pale, with hepatosplenomegaly | N | Malarial splenomegaly syndrome/visceral leishmaniasis | Bone marrow aspirate cytology/DH | Not revealed | Pamnani et al. 11 |
| M/9/Nigeria | Large, painful swelling on the middle half of the right clavicle. Radiograph: Bone destruction | N | Osteomyelitis/osteosarcoma | Biopsy of the swelling/African histoplasmosis | Not revealed | Khalil et al. 12 |
| F/13/Nigeria | Neck masses, generalized body swelling, reduction in urinary output. Examination findings: Patient was in respiratory distress, pale, generalized body swelling and generalized lymphadenopathy. Bp, 160/100 mm Hg, oxygen saturation <88%. Ascitic fluid cytology was negative for malignant cells. Mantoux test were negative. Abd. USS; bilateral nephritis | N | Nephritic syndrome secondary to a lymphoma/(HIV)-associated nephropathy/ disseminated tuberculosis. | Lymph node biopsy: H. capsulatum. | Blood pressure and control of edema which became refractory. She died on the 16th day of admission | Ubesie et al. 14 |
| M/12/Nigeria | Soft, tender, and no fluctuant swelling on the left side of the face adjacent to the mental region of the mandible. Radiographs showed diffuse osteolysis of the alveolus in the lower left premolar region. | N | Burkitt’s lymphoma. | Histology of biopsied specimen had the appearance of African histoplasmosis | Recovered with AmB therapy after Cyclo was discontinued | Mace 15 |
| M/13/Nigeria | Swelling involving the left supraorbital and mid-frontal region of the skull. Examination findings: firm and tender oval swelling, which measured about 6.5 cm by 2.5 cm and occupied the left supraorbital and midfrontal regions of the skull | N | Burkitt’s lymphoma/neuroblastoma with orbital secondaries/septicemia with abscess formation | Microscopic examination of biopsy material revealed numerous yeast typical of Histoplasma duboisii. | AmB therapy; Patient recovered | Shoroye and Oyedeji 16 |
AmB, amphotericin B; Cyclo, cyclophosphamide; DH, disseminated histoplasmosis; N, negative; P, positive.
Histoplasmosis and nephrotic syndrome
DH involving the kidneys can present as nephrotic syndrome. Unfortunately, DH is rarely considered as a diagnosis. 14 TB and malignancies are usually top on the list as differentials. 14 Ubesie et al. 14 reported a 13-year-old female with complaints of neck masses, generalized body swelling, and a reduction in urinary output. Clinical diagnosis considered until patient’s demise was nephritic syndrome secondary to lymphoma, human immunodeficiency virus (HIV)-associated nephropathy, and disseminated TB. Findings at autopsy revealed H. capsulatum var capsulatum infection (Table 6). 14 The poor awareness on the side of pediatricians about the occurrence and clinical presentation of DH in children is really a challenge to health care and has caused preventable mortalities. The timing at which diagnosis is made is very critical to the outcome of patient’s management. DH should be ruled out whenever diagnosis of malignancies, TB, or other chronic illnesses are made.
Histoplasmosis mimicking hyperreactive malarial splenomegaly syndrome
Hyperreactive malarial splenomegaly syndrome, previously called tropical splenomegaly syndrome, is an exaggerated immune response to recurrent or persistent malarial infection. 49 It is commonly characterized by massive splenomegaly and hepatomegaly, features which also characterize DH.11,49 In the African pediatric population, chronic hepatosplenomegaly is commonly associated with malarial infections and schistosomiasis. 50 Pamnani et al. 11 documented a case of DH misdiagnosed as splenomegaly syndrome even when malaria parasite was negative. Unfortunately, the management and outcome were not discussed. A high index of suspicion is therefore needed when reviewing such cases to investigate for or rule out histoplasmosis, we may never know how many cases that have been missed and mortalities suffered (Table 6).
Histoplasmosis presenting as localized (cutaneous) lesions
Unlike classical histoplasmosis which involves the lungs, African histoplasmosis commonly involves the skin and may progress to cause bone damage.12,19,25,31 Localized lesions are commonly seen in immunocompetent population and may present as boils, swellings, ulcers, papules, subcutaneous abscesses, cutaneous fistulas, limb deformity, tumors, or enlarged lymph nodes.37,38,40,43,45 Lesions may be painful or painless.37,38,40,43,45 Common radiological findings include osteolytic lesions, periosteal reaction, cortical erosion, and cauterization.15,16,25,26,29 The presence of lymphadenopathy on clinical examination in the pediatric age group is most often associated with TB, malignancies, and sepsis.12,13,32,44 Garcia-Guiñon et al. 44 report a case of DH misdiagnosed as TB in a 10-year-old girl who presented with painless suppurative lesions on the head, face, the upper body, and the armpit. A diagnosis of Histoplasma duboisii infection was subsequently made from Giemsa smear of aspirates obtained from the lesions. Patient recovered after receiving IV amphotericin. 44
Diagnosis
Diagnostic modalities for histoplasmosis used in the cases reviewed were mostly histopathology (n = 33, 75%). Others included microscopy (n = 3,6.8%), bone marrow aspirate and cytology (n = 3,6.8%), culture (n = 1,2.3%), and serology (n = 1,2.3%)11–16,22–35,37,41–44,46 Molecular diagnosis was not used in any of the cases. The diagnosis of histoplasmosis in children can be challenging. In African children, the unavailability of both basic diagnostic tools such as fungal culture or serology as well as more advanced molecular techniques make the situation further complex. Pediatricians and clinical mycologists have to depend on a high index of suspicion undergirded by a knowledge of predisposing factors such as severe acute malnutrition, HIV/AIDS and relevant exposures, and available diagnostic tools to establish a diagnosis.11,13,26–28 A proven diagnosis of histoplasmosis is established by the recovery of the organism by culture from an affected site or blood while a probable diagnosis is established by the presence of a host factor that confers increased risk, clinical picture consistent with histoplasmosis infection, and mycological evidence such as a positive antigen test result from urine, blood or, CSF.1,23,24,26,51 A higher likelihood of serologic evidence of infection including higher rates of antigenemia and antigenuria were found in children who are immunocompromised. This is because immunocompromised children are also more likely to present with disseminated disease while pulmonary disease is commoner in immunocompetent children.26,44,51,52 It has also been suggested that using multiple diagnostic methods would improve the diagnostic yield of the tests.51,52
Radiologic investigations such as a chest x-ray or a chest computed tomography (CT) scan may be used as an initial diagnostic investigation in children with suspected pulmonary disease. While chest radiographic findings in histoplasmosis are often nonspecific and overlap with other chronic pulmonary conditions like sarcoidosis or TB, an abnormal chest radiograph is common and may be the first clue to childhood histoplasmosis.27,32,51,52 Common radiographic abnormalities in childhood histoplasmosis include lymphadenopathy (hilar, perihilar, mediastinal, subcarinal, and paratracheal), pulmonary nodules, consolidation, infiltrates, ground glass opacification, pleural effusion, and cavitatory lesions. Bronchial or vascular compression, calcifications, and rarely, pericarditis may be seen.27,32,52,53 In a case series from Colombia, 83% of all patients had pulmonary infiltrates on chest radiographs and Quellete et al. reported that 71% and 84% of their patients had an abnormal chest radiograph and chest CT, respectively.53,54 A patient with relevant host factors and an abnormal chest radiograph or CT does not, however, qualify as a probable case under current diagnostic criteria. This is probably due to significant overlap of radiographic abnormalities with other similar conditions.4,52
Fungal culture is the microbiological gold standard for the diagnosis of childhood histoplasmosis.20,26,51,52 Fungal cultures may be done from various specimens including sputum, bronchoalveolar lavage fluid, blood, or bone marrow aspirates. Cultures are more likely to be positive in patients with disseminated disease or chronic pulmonary illness and the yield of cultures in these cases can be as high as 85%, especially when blood or bone marrow samples are used.54,55 Cultures of sputum are positive in two thirds of patients with cavitatory disease and in less than one-third of those with noncavitatory disease. Most pediatric patients fall into the latter category and thus sputum and other respiratory samples may not be very reliable in children.52,54,55 Cultures are done in brain heart infusion to enhance the conversion of Histoplasma capsulatum to the yeast form which is more easily identified and may take at least 2 weeks.52,54,55 Cultures were found to be positive in 80% of patients with histoplasmosis in a case series from Colombia. Almost two thirds of all the patients in the series had progressive disseminated disease. 53 Cytologic examination with direct visualization of Histoplasma yeast cells may lead to a high proportion of false negatives as the small yeast cells may be missed. This is especially true in children where induced samples (bronchoalveolar lavage, induced sputum) may be required. When direct examination is done, Histoplasma capsulatum appears as small 2–4 μm oval yeast cells with fungal staining. Grocott-Gomori methenamine silver nitrate and periodic acid Schiff (PAS) are the most useful stains.1,52,54
Serologic tests are typically positive in up to 90–95% of all children with symptomatic histoplasmosis.1,4,51 Agar gel immunodiffusion (ID) and complement fixation tests (CFTs) are the most commonly used and have a comparable sensitivity of about 80%. Doing both tests on the same patients increases the likelihood of making a diagnosis.4,51 The complement fixation test has a slightly better sensitivity than the immunodiffusion test especially early in the disease but the immunodiffusion test is more specific. False negatives are common in patients with HIV infection or other immunodeficiency and TB may cause a false-positive reaction as can coccidioidomycosis and paracoccidioidomycosis.1,4,51,52,54 Antigen assays are most useful in acute pulmonary disease and disseminated disease. They are rarely positive in chronic pulmonary infection but are positive in as many as 80% of all patients with acute pulmonary disease.51–55 Urine or serum may be used for antigen detection but urine testing is more sensitive especially in children with progressive disseminated disease. Antigenemia and/or antigenuria is found to be more common in HIV-infected children with histoplasmosis. Blastomycosis, coccidioidomycosis, and paracoccidioidomycosis may all result in false-positive antigen tests.51–55
The role of molecular methods in the diagnosis of childhood histoplasmosis is uncertain, probably due to the lack of consensus on gene targets.52,53 An assay developed at the Mayo clinic was reported to be positive in only one third of culture-positive bronchoalveolar lavage specimens. 56 Another study reported a less than 10% PCR positivity rate in antigen-positive urine specimens. 57
Direct microscopy using Giemsa stain was interestingly used in the diagnosis of histoplasmosis. This is very important especially in low-resource settings where affordability and accessibility to diagnostic tools has remained a major challenge. Garcia-Guiñon et al. 44 reported a case of disseminated histoplasmosis in a 10-year-old diagnosed using Giemsa stain of purulent secretions. Yeast-like ovoid cells measuring 10–14 μm in diameter morphologically similar to Hcd as well as short mycelium were seen. 44 However, sensitivity is low and may result in false-positive results due to the difficulty in differentiating Histoplasma microscopically from other yeasts such as Candida species, Cryptococcus species, P. brasiliensis, Pneumocystis jirovecii, Leishmania donovani, and Toxoplasma gondii. 58
Treatment
Amphotericin B was the first-line therapy in 45.5% of the cases followed by ketoconazole (20.5%, n = 9). Other antifungal drugs used were itraconazole, posaconazole, fluconazole, and terbinafine. Despite using a combination of amphotericin B and azoles, treatment failure was observed in some cases. Gonçalves et al. 32 reported a case of disseminated African histoplasmosis in a 12-year-old boy from Guinea Bissau on amphotericin B and itraconazole maintenance therapy with progressive clinical deterioration. Improvement was observed with posaconazole therapy. He completed 12 months of therapy with no relapse during and 3 months after treatment. 32 In yet another case report by Daubenton and Beatty, 26 amphotericin B and ketoconazole maintenance therapy in a 7-year-old boy with disseminated histoplasmosis was not effective. Improvement was observed with oral itraconazole. 26 Although antifungal resistance in Histoplasma is not currently a concern, our review has revealed a likelihood of an emerging resistance. Patients on amphotericin B therapy for childhood histoplasmosis should therefore be closely monitored for possible change of medications if there is no improvement.
Not all forms of childhood histoplasmosis warrant antifungal therapy as the disease is self-limiting and resolves spontaneously in most healthy individuals. Immunocompromised children and children with acute pulmonary or disseminated histoplasmosis, however, should always be treated.1,4,59 Standard guidelines for the treatment of histoplasmosis were published in 2007 and have been adapted for pediatric use.4,59 The antifungals that are preferred for use in childhood histoplasmosis are amphotericin B deoxycholate, liposomal amphotericin B, amphotericin B lipid complex, and itraconazole. Definite indications for antifungal therapy include acute diffuse symptomatic pulmonary infections, chronic cavitatory pulmonary infections, central nervous system infections, and progressive disseminated histoplasmosis. Asymptomatic or mildly symptomatic acute pulmonary infections, mediastinal lymphadenitis, or granuloma may benefit from antifungal therapy but this is not definitively recommended. Antifungal treatment is not recommended for mediastinal fibrosis, pulmonary nodules, broncholithiasis, and presumed ocular histoplasmosis syndrome.4,59 A 4- to 6-week course of amphotericin B deoxycholate at a daily dose of 1 mg/kg/day is usually curative in children with acute pulmonary or disseminated disease; 2–4 weeks of amphotericin followed by 5–10 mg/kg/day of itraconazole for another 8–10 weeks is a suitable alternative.4,58 Amphotericin B deoxycholate is better tolerated in children than in adults where the lipid-based forms are more in use. 4 A longer duration of antifungal therapy may be required in children with HIV/AIDs or other immunodeficient states and in those with more severe disease. 4 Lifelong itraconazole suppressive therapy may be required in chronically immunosuppressed patients. 4 Prolonged itraconazole therapy for 12–24 months is the regimen of choice for chronic pulmonary histoplasmosis. 59 Antigen levels are used to monitor treatment and monitoring should continue for 12 months after therapy to monitor for relapse.4,59
Conclusion
Diagnosis of histoplasmosis in children requires a high index of suspicion and much awareness on the side of the clinicians. The low number of pediatric histoplasmosis documented in literature especially in Africa compared with the number of cases reported in adults and in other regions of the world may be due to the challenge of misdiagnosis as seen in some of the cases presented above. There is a gross knowledge gap accounting for this which has to be addressed urgently.
Footnotes
Author contributions: Bassey Ewa Ekeng: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Validation, and Writing – original draft, review, and editing.
Kevin Edem: Data curation, Resources, and Writing – review and editing.
Patricia Akintan: Resources and Writing – review and editing.
Rita O. Oladele: Conceptualization, Methodology, Resources, and Writing – review and editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical statement: Our study did not require an ethical board approval because of its design of literature review of published data. Our study did not require consent because no individual patient data were included.
ORCID iD: Bassey Ewa Ekeng  https://orcid.org/0000-0002-0509-2480
https://orcid.org/0000-0002-0509-2480
Contributor Information
Bassey Ewa Ekeng, Department of Medical Microbiology and Parasitology, University of Calabar Teaching Hospital, Calabar 540271, Nigeria; Medical Mycology Society of Nigeria, Lagos, Nigeria.
Kevin Edem, Department of Paediatrics, University of Uyo Teaching Hospital, Uyo, Nigeria.
Patricia Akintan, Department of Paediatrics, College of Medicine, University of Lagos, Lagos, Nigeria.
Rita O. Oladele, Department of Medical Microbiology and Parasitology, Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, Lagos, NigeriaMedical Mycology Society of Nigeria, Nigeria
References
- 1. Wheat LJ, Azar MM, Bahr NC, et al. Histoplasmosis. Infect Dis Clin North Am 2016; 30: 207–227. [DOI] [PubMed] [Google Scholar]
- 2. Muñoz-Oca JE, Morales MLV, Nieves-Rodriguez A, et al. Concomitant disseminated histoplasmosis and disseminated tuberculosis after tumor necrosis factor inhibitor treatment: a case report. BMC Infect Dis 2017; 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sifuentes-Osornio J, Corzo-León DE, Ponce-de-León LA. Epidemiology of invasive fungal infections in Latin America. Curr Fungal Infect Rep 2012; 6: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fischer GB, Mocelin H, Severo CB, et al. Histoplasmosis in children. Paediatr Respir Rev 2009; 10: 72–177. [DOI] [PubMed] [Google Scholar]
- 5. Zanotti P, Chirico C, Gulletta M, et al. Disseminated histoplasmosis as AIDS-presentation. Case report and comprehensive review of current literature. Mediterr J Hematol Infect Dis 2018; 10: e2018040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schutze GE, Tucker NC, Jacobs RF. Histoplasmosis and perinatal human immunodeficiency virus. Pediatr Infect Dis J 1992; 11: 501–502. [DOI] [PubMed] [Google Scholar]
- 7. Alverson B, Alexander N, LeGolvan MP, et al. A human immunodeficiency virus-positive infant with probable congenital histoplasmosis in a nonendemic area. Pediatr Infect Dis J 2010; 29: 1055–1057. [DOI] [PubMed] [Google Scholar]
- 8. Oladele RO, Ayanlowo OO, Richardson MD, et al. Histoplasmosis in Africa: an emerging or a neglected disease? PLoS Negl Trop Dis 2018; 12: e0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pakasa N, Biber A, Nsiangana S, et al. African histoplasmosis in HIV-negative patients, Kimpese, Democratic Republic of the Congo. Emerg Infect Dis 2018; 24: 2068–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amona FM, Denning D, Moukassa D, et al. Histoplasmosis in the Republic of Congo dominated by African histoplasmosis, Histoplasma capsulatum var. duboisii. PLoS Negl Trop Dis 2021; 15: e0009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pamnani R, Rajab J, Githang’a J, et al. Disseminated histoplasmosis diagnosed on bone marrow aspirate cytology: report of four cases. East Afr Med J 2010; 86: 102–105. [DOI] [PubMed] [Google Scholar]
- 12. Khalil MA, Hassan AW, Gugnani HC. African histoplasmosis: report of four cases from north- eastern Nigeria. Mycoses 1997; 41: 293–295. [DOI] [PubMed] [Google Scholar]
- 13. Mosam A, Moodley V, Ramdial PK. Persistent pyrexia and plaques: a perplexing puzzle. Lancet 2006; 368: 551. [DOI] [PubMed] [Google Scholar]
- 14. Ubesie AC, Okafo OC, Ibeziako NS, et al. Disseminated histoplasmosis in a 13-year-old girl: a case report. Afr Health Sci 2013; 13: 518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mace MC. Oral African histoplasmosis resembling Burkitt’s lymphoma. Oral Surg 1978; 46: 407–412. [DOI] [PubMed] [Google Scholar]
- 16. Shoroye A, Oyedeji GA. African histoplasmosis presenting as a facial tumour in a child. Ann Trop Paediatr 1982; 2: 147–149. [DOI] [PubMed] [Google Scholar]
- 17. Adderson E. Histoplasmosis. Pediatr Infect Dis J 2006; 25: 73–74. [DOI] [PubMed] [Google Scholar]
- 18. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esteves C, Costa FR, Macedo C, et al. Histoplasmose Africana. Acta Radiol Port 2016; 28: 51–55l4. [Google Scholar]
- 20. Guimarães AJ, de Cerqueira MD, Nosanchuk JD. Surface architecture of Histoplasma capsulatum. Front Microbiol 2011; 2: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mihu MR, Nosanchuk JD. Histoplasma virulence and host responses. Int J Microbiol 2012; 2012: 268123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamey B, Parisien G. Disseminated form of histoplasmosis caused by Histoplasma capsulatum in a Zairian child. Med Trop 1982; 42: 557–559. [PubMed] [Google Scholar]
- 23. Kweyamba V, Apiyo M, Olika B, et al. A case of a 4-year-old boy with a mesenteric chylous cyst infected with Histoplasma capsulatum. Case Rep Surg 2016; 2016: 4296059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabangila R, Semvua K, Rambau P, et al. Pulmonary histoplasmosis presenting as chronic productive cough, fever, and massive unilateral consolidation in a 15-year-old immune-competent boy: a case report. J Med Case Rep 2011; 5: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suleman FE, Scheepers PA. Multiple skeletal lesions and pleural effusion owing to Histoplasma capsulatum infection in an immunocompetent patient from a nonendemic region. S Afr J Radiol 2011; 15: 82–84. [Google Scholar]
- 26. Daubenton JD, Beatty DW. Disseminated histoplasmosis in an immunocompetent child. S Afr Med J 1998; 88: 270–271. [PubMed] [Google Scholar]
- 27. Pillay T, Pillay DG, Bramdev A. Disseminated histoplasmosis in a human immunodeficiency virus infected African child. Pediatr Infect Dis J 1997; 16: 417–418. [DOI] [PubMed] [Google Scholar]
- 28. Akpuaka FC, Gugnani HC, Iregbulam LM. African histoplasmosis: report of two patients treated with amphotericin B and ketoconazole. Mycoses 1998; 41: 363–364. [DOI] [PubMed] [Google Scholar]
- 29. Onwuasoigwe O. Fluconazole in the therapy of multiple osteomyelitis in African histoplasmosis. Int Orthop 1999; 23: 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katchy AU, Eyesan SU, Awotunde TO, et al. Histoplasma duboisii of the femoral bone. J Res Med Sci 2019; 24: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minta DK, Sylla M, Traore AM, et al. Première observation malienne d’histoplasmose africaine disséminée à predominance osseuse chez un enfant VIH négatif. Revue de la littérature. J Mycol Méd 2014; 24: 152–157. [DOI] [PubMed] [Google Scholar]
- 32. Gonçalves D, Ferraz C, Vaz L. Posaconazole as rescue therapy in African histoplasmosis. Braz J Infect Dis 2013; 17: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mabey DCW, Hay RJ. Further studies on the treatment of African histoplasmosis with ketoconazole. Trans R Soc Trop Med Hyg 1989; 83: 560–562. [DOI] [PubMed] [Google Scholar]
- 34. Bankolé SR, Denoulet C, Coulibaly B, et al. A propos d’un cas ivoirien d’histoplasmose osseuse et cutanée à Histoplasma capsulatum var. duboisii [Apropos of 1 Ivoirian case of osseus and cutaneous histoplasmosis by Histoplasma capsulatum var. duboisii]. Bull Soc Pathol Exot 1998; 91: 151–153. [PubMed] [Google Scholar]
- 35. N’Golet A, N’Gouoni BG, Moukassa D, et al. Maxillary African histoplasmosis: unusual diagnostic problems of an unusual presentation. Pathol Res Pract 2005; 200: 841–844. [DOI] [PubMed] [Google Scholar]
- 36. Destombes P, Ravisse P, Nazimoff O. Assessment of deep mycoses established in twenty years of histopathology at the Pasteur Institute of Brazzaville. Bull Soc Pathol Exot 1970; 63: 315–323. [PubMed] [Google Scholar]
- 37. Carme B, Hayette M, Ngaporo AI, et al. African histoplasmosis due to Histoplasma duboisii (Histoplasma capsulatum var. duboisii): fourteen Congolese cases observed in 10 years (1981–1990). J Mycol Med 1993; 3: 67–73. [Google Scholar]
- 38. Griffet P, Poaty-Mapakou C, Bouyou-Mananga E, et al. African histoplasmosis due to Histoplasma duboisii: report of an exemplary case with cutaneous and bone localization. Med Armee 1984; 12: 679. [Google Scholar]
- 39. Moyikoua A, Carme B, Ngolet A, et al. African histoplasmosis: report of a case of osteoarthritis of the shoulder. Med Afr Noire 1991; 3S: 372–376. [Google Scholar]
- 40. Paugam A, de Pécoulas PE, Bourée P. African young girl with sores of the elbow, cold abcesses and Molluscum-like cutaneous lesions. Lett Infect 2014; 7: 126–129. [Google Scholar]
- 41. Okoko A, Bowassa GE, Oko A. Generalized histoplasmosis in a child immunocompetent to HIV. Med Afr Noire 2010; 57: 590–592. [Google Scholar]
- 42. Chandenier J, Goma D, Moyen G, et al. African histoplasmosis due to Histoplasmosis capsulatum var. duboisii: relationship with AIDS in recent Congolese cases. Sante 1995; 5: 227–234. [PubMed] [Google Scholar]
- 43. Mabiala JR, Mandavo CM, Evrard RN, et al. Trois cas pédiatriques d’histoplasmose africaine à Brazzaville. J Mycol Méd 2017; 27: 133–138. [DOI] [PubMed] [Google Scholar]
- 44. Garcia-Guiñon A, Torres-Rodríguez JM, Ndidongarte DT, et al. Disseminated histoplasmosis by Histoplasma capsulatum var. duboisii in a paediatric patient from the Chad Republic, Africa. Eur J Clin Microbiol Infect Dis 2009; 28: 697–699. [DOI] [PubMed] [Google Scholar]
- 45. André C, Badoual J, Kalifa G, et al. African histoplasmosis. A case. Arch Fr Pediatr 1984; 41: 429–431. [PubMed] [Google Scholar]
- 46. Barro/Traoré F, Sanwidi M, Dao F, et al. Histoplasmose africaine disséminée chez un enfant immunocompétent au Burkina Faso: un cas [Disseminated African Histoplasmosis in an immunocompetent child in Burkina Faso: one case]. Our Dermatol Online 2013; 4: 361–368. [Google Scholar]
- 47. Corbett EL, Marston B, Churchyard GJ, et al. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006; 367: 926–937. [DOI] [PubMed] [Google Scholar]
- 48. Mandengue CE, Ekeng BE, Oladele RO. Disseminated histoplasmosis; a threat in advanced HIV disease population in sub-Saharan Africa? J Adv Med Med Res 2021; 33: 115–144. [Google Scholar]
- 49. McGregor A, Doherty T, Lowe P, et al. Hyperreactive malarial splenomegaly syndrome – can the diagnostic criteria be improved? Am J Trop Med Hyg 2015; 93: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson S, Vennervald BJ, Dunne DW. Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria. PLoS Negl Trop Dis 2011; 5: e1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donnelly JP, Chen SC, Kauffmsn CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71: 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quellete CP, Stanek JR, Leber A, et al. Paediatric histoplasmosis in an area of endemicity: a contemporary analysis. J Pediatric Infect Dis Soc 2019; 8: 400–407. [DOI] [PubMed] [Google Scholar]
- 53. Lopez LF, Valencia Y, Tobon AM, et al. Childhood histoplasmosis in Colombia: clinical and laboratory observations of 45 patients. Med Mycol 2016; 54: 677–683. [DOI] [PubMed] [Google Scholar]
- 54. Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 2017; 55: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scheel CM, Gomez BL. Diagnostic methods for histoplasmosis: focus on endemic countries with variable infrastructure levels. Curr Trop Med Rep 2014; 1: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Babady NE, Buckwalter SP, Hall L, et al. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011; 49: 3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang YW, Li H, Durkin MM, et al. Urine polymerase chain reaction is not as sensitive as urine antigen for the diagnosis of disseminated histoplasmosis. Diagn Microbiol Infect Dis 2006; 54: 283–287. [DOI] [PubMed] [Google Scholar]
- 58. Dantas KC, de Freitas RS, da Silva MV, et al. Comparison of diagnostic methods to detect Histoplasma capsulatum in serum and blood samples from AIDS patients. PLoS ONE 2018; 13: e0190408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45: 807–825. [DOI] [PubMed] [Google Scholar]