Abstract
Introduction
The extracellular matrix (ECM) plays an integral role in wound healing. It provides both structure and growth factors that allow for the organised cell proliferation. Large or complex tissue defects may compromise host ECM, creating an environment that is unfavourable for the recovery of anatomical function and appearance. Acellular dermal matrices (ADMs) have been developed from a variety of sources, including human (HADM), porcine (PADM) and bovine (BADM), with multiple different processing protocols. The objective of this report is to provide an overview of current literature assessing the clinical utility of ADMs across a broad spectrum of applications.
Methods
PubMed, MEDLINE, EMBASE, Scopus, Cochrane and Web of Science were searched using keywords ‘acellular dermal matrix’, ‘acellular dermal matrices’ and brand names for commercially available ADMs. Our search was limited to English language articles published from 1999 to 2020 and focused on clinical data.
Results
A total of 2443 records underwent screening. After removing non-clinical studies and correspondence, 222 were assessed for eligibility. Of these, 170 were included in our synthesis of the literature. While the earliest ADMs were used in severe burn injuries, usage has expanded to a number of surgical subspecialties and procedures, including orthopaedic surgery (e.g. tendon and ligament reconstructions), otolaryngology, oral surgery (e.g. treating gingival recession), abdominal wall surgery (e.g. hernia repair), plastic surgery (e.g. breast reconstruction and penile augmentation), and chronic wounds (e.g. diabetic ulcers).
Conclusion
Our understanding of ADM’s clinical utility continues to evolve. More research is needed to determine which ADM has the best outcomes for each clinical scenario.
Lay Summary
Large or complex wounds present unique reconstructive and healing challenges. In normal healing, the extracellular matrix (ECM) provides both structural and growth factors that allow tissue to regenerate in an organised fashion to close the wound. In difficult or large soft-tissue defects, however, the ECM is often compromised. Acellular dermal matrix (ADM) products have been developed to mimic the benefits of host ECM, allowing for improved outcomes in a variety of clinical scenarios. This review summarises the current clinical evidence regarding commercially available ADMs in a wide variety of clinical contexts.
Keywords: Acellular dermal matrix, complex wound, soft-tissue defects, wound closure, wound healing, breast reconstruction, hernia repair, skin substitute
Introduction
Historically, large and/or complex soft-tissue defects have been treated with techniques including full and split-thickness skin grafts (FTSG and STSG), local flap coverage and free tissue transfer. Each of these has disadvantages such as donor site morbidity, risk of flap/graft complications or even failure. 1 In some cases, such as excessive wound depth or specialised function of tissue needing repair, patient and/or wound characteristics may preclude the use of traditional techniques for soft-tissue coverage. 1
Successful wound healing depends largely on the interactions of proliferating cells with the extracellular matrix (ECM) in a process known as dynamic reciprocity. 2 The ECM—composed of proteoglycans, hyaluronic acid, collagen and elastin—directs tissue regeneration and differentiation via mechanical cues and signalling molecules. 2 In traumatic or chronic wounds, the ECM is often damaged to the extent that it no longer adequately supports healing. Acellular dermal matrices (ADMs) were developed in an attempt to capitalise on the properties of native ECM and promote organised regeneration of host tissue in a wide variety of clinical contexts. 2
When ADMs are placed, host cells are incorporated into the matrix and directed by preserved growth factors and mechanical cues in the matrix structure.2,3 A variety of cells invade the ADM, including fibroblasts, myofibroblasts, lymphocytes, macrophages, granulocytes, mast cells and others.3,4 After inflammatory cell infiltration, the matrix undergoes remodelling, collagen and elastin levels increase, and revascularisation is initiated.3,5–7 Lymphangiogenesis is possible, but is slower. 3 Essentially, the ADM acts as a scaffold to promote host tissue growth. 1
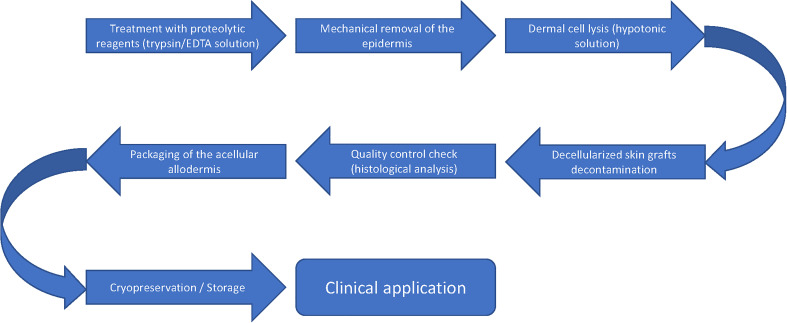
ADMs were initially used to treat burn wounds in the 1990s and have since become a valuable addition to reconstructive algorithms as they are available off the shelf and have superior biocompatibility compared to synthetic soft-tissue grafts.8,9 All ADMs are decellularised and antigenic components have been removed to prevent immune rejection 4 (Figure 1).
Figure 1.
Schematic representation of ADM preparation. ADM, acellular dermal matrix.
Given the success of early ADM applications, interest has evolved to include a variety of procedures spanning multiple surgical subspecialties. 10 Over the past two decades, a number of commercially available ADMs have been developed that vary both in origin of tissue and level of processing (Table 1, Figure 2). 11 Human cadaver (HADM), bovine (BADM) and porcine (PADM) tissues have been used in a variety of different clinical contexts, with results differing by product and application. 11 Products are further distinguished by tissue type (Figure 2), additives (e.g. antibiotics or surfactants) and preparation regulations. 12 In this article, we review the current literature assessing the clinical utility of ADM across a broad spectrum of applications.
Table 1.
Common commercially available ADMs.
| Product | Description |
|---|---|
| AlloDerm® (LifeCell Corp., Bridgewater, NJ, USA) | Human, non-cross-linked |
| AlloMax® (CR Bard/Davol Inc., Cranston, RI, USA) | Human, non-cross-linked |
| DermACELL® (LifeNet Health Inc., Virginia Beach, VA, USA) | Human, non-cross-linked |
| FlexHD® (Ethicon, Inc., Somerville, NJ, USA) | Human, non-cross-linked |
| Cortiva® (RTI Surgical, Alachua, FL, USA) | Human, non-cross-linked |
| Integra™ (Integra Life Sciences, Princeton, NJ, USA) | Bovine, cross-linked |
| MatriDerm® (Dr Suwelack AG, Billerbeck, Germany) | Bovine, non-cross-linked |
| SurgiMend® (Integra Life Sciences, Princeton, NJ, USA) | Bovine, non-cross-linked |
| Strattice® (Allergan, Madison, NJ, USA) | Porcine, non-cross-linked |
| Permacol™ (Medtronic, Minneapolis, MN, USA) | Porcine, cross-linked |
| CollaMend™ (CR Bard/Davol Inc., Cranston, RI, USA) | Porcine, cross-linked |
ADM, acellular dermal matrix.
Figure 2.
Diagrams showing common sources of ADM tissue. Yellow highlighted portions represent the area harvested for processing. ADM, acellular dermal matrix.
Methods
The authors performed a review of the PubMed, MEDLINE, EMBASE, Scopus, Cochrane and Web of Science databases using keywords ‘acellular dermal matrix’, ‘acellular dermal matrices’ and brand names for commercially available ADMs shown in Table 1. Articles were screened by title and abstract, then by full text for inclusion. Our search was limited to English language articles (or those with available English translations) published from January 1999 to September 2020. This review is focused on recent clinical data with special attention to studies comparing different ADMS.
Summary findings of included studies are presented in tables divided by clinical context (Tables 2–9). Within each table, articles are grouped by level of evidence (e.g. case report/series, retrospective study, prospective study, meta-analysis).
Table 2.
Clinical evidence for ADMs in burn wounds.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Retrospective studies | |||
| Guo et al. (2016) | Unspecified PADM | Deep dermal burn wound treatment 60 adult burned patients Excluded: older than 65 years, those with naturally occurring musculoskeletal or visceral injuries, and those already admitted into hospital 3 + days after injury |
|
| Prospective studies | |||
| Heimbach et al. (2003) | Integra™, BADM | 216 burn injury patients who were treated at 13 burn care facilities in the USA. The mean TBSA burned was 36.5% (range = 1%–95%). Integra™ was applied to fresh, clean, surgically excised burn wounds |
|
| Nguyen et al. (2010) | Integra™, BADM | 6 adult patients who had been successfully treated with Integra™ ± STSG |
|
| Moiemen et al. (2010) | Integra™, BADM | 8 patients (9 reconstruction sites) had unmeshed Integra™ with TNP therapy between the 1st and the 2nd stages. Patients underwent serial biopsies on days 7, 14, 21 and 28 after application |
|
| Angspatt et al. (2017) | PoreSkin®, HADM | Burn scar treatment 8 patients, 11 hypertrophic burn scars |
|
| Demircan et al. (2015) | Matriderm®, BADM | 15 paediatric patients with full-thickness facial burns. In all patients, TBSA burnt was >50% |
|
| Bloemen et al. (2010) | Matriderm®, BADM | 46 patients, 69 pairs of BADM and conventionally treated (no BADM) sites |
|
| Okuno et al. (2018) | PELNAC®, HADM |
Studying yeast culture properties isolated from burn wounds in vitro 36 of 273 patients fulfilled inclusion criteria to isolate burn wounds tissue for analysis |
|
ADM, acellular dermal matrix; BADM, bovine acellular dermal matrix; CI, confidence interval; HADM, human acellular dermal matrix; mVSS, modified Vancouver Scar Scale; PADM, porcine acellular dermal matrix; STSG, split-thickness skin graft; TBSA, total body surface area; TNP, topical negative pressure; VSS, Vancouver Scar Scale.
Table 9.
Clinical evidence for ADMs in urology.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Case series and case reports | |||
| Bonitz et al. (2016) | AlloDerm®, HADM AlloMax®, HADM |
CBE 6 male patients, born with CBE, and who had abdominal wall defects. 2 children, aged 6 and 8 years, with unrepaired bladder exstrophy plates and large abdominal wall defects (8 and 12 cm wide). Both had their bladders reconstructed, placed within the pelvis, and HADM was used to replace the absent abdominal wall (bridged repair) without the use of pelvic osteotomy. In 3 other patients, HADM reinforced the native fascial repair (bolster repair). HADM also served as a filler for the abdominal depression that was present after initial staged repair. Where HAD was used for bridged or bolster repair, the edges of the allograft were extended 2–3 cm beyond the perimeter of the defect |
|
ADM, acellular dermal matrix; CBE, classic bladder exstrophy; HADM, human acellular dermal matrix.
Results
After duplicates were removed, there were 2443 records identified that underwent screening. After screening, 170 articles were included in our synthesis of the literature. A total of 19 articles were included in Burn, 18 in Wound Care, 30 in Breast Reconstruction, 9 in Andrology, 11 in Gynecology and Gynecological Oncology, 26 in Orthopaedic Surgery, 18 in Oral and Maxillofacial Surgery, 9 in Craniofacial Surgery, 16 in Abdominal Wall / Hernia and 7 in Otolaryngology/Ear, Nose, and Throat (ENT).
Plastic and reconstructive surgery—burn
ADMs have been used as an adjunct for tissue modification and enhancement following severe burns (Table 2).13–16 ADM application to self-assembled skin substitute (SASS) has been shown to increase cell proliferation, preserve intrinsic properties and reduce likelihood of rejection. 13
Researchers have manipulated the biological signalling pathway via either direct application of signalling cells to an ADM or by combining a deep-degree burned dermal matrix (DDBDM) harvested from the host with an ADM.14,17 ADMs impregnated with signalling cells or DDBDMs had higher probability of maintaining integrity, histocompatibility and stability. 14
Integra™ (BADM) is the most commonly used ADM for treating severe burns.18,19 In a large, multicentre study, Integra™ showed improvements in hypertrophic scarring compared to controls. 20 While subsequent studies have confirmed its efficacy in improving appearance, elasticity and functional outcomes, 21 infection rates remain a concern.22,23 The use of antimicrobial dressings and/or negative pressure wound therapy (NPWT) in conjunction with Integra™ has led to improved infection rates.19,24 Recently, MatriDerm (BADM) has been used in pediatric 25 and adult populations26–28 to treat burns via a single staged procedure. 19 Compared to Integra™, MatriDerm has demonstrated increased neovascularisation and higher degradation rates. 19 The concurrent use of NPWT with MatriDerm has improved clinical outcomes. 18 While early clinical data on MatriDerm are promising, the literature lacks direct clinical comparisons of MatriDerm and Integra™. 18
One case study described HADM application to infant calvarial burns involving the brain. 29 Recommended treatments typically involve high speed drilling for massive calvarial exposure or coverage with adjacent vascularised scalp tissue,30,31 but these techniques prove challenging with immature cranial development. However, in this case, HADM (AlloDerm) was used to reconstruct a large dural defect and calvarial burn, which successfully prevented cerebrospinal fluid leakage, facilitating dural reconstruction and efficient revascularisation of tissues. 29 Candida parapsilosis, a common exogenous yeast that resides in burn wounds, has been observed proliferating on HADM (Pelnac®) after seven days of incubation as well as penetrating and crossing the ADM within three days. 16
While existing literature is limited, PADM has been used in treating burn wounds due to its ability to support proliferation and enhanced epidermal cell attachment given the partial conservation of basement membra. Dermabrasion used in conjunction with PADM resulted in wound healing duration of 22.5 days, whereas those treated conservatively without PADM required 30.3 days. 15 Limited use of PADM in this context may be attributed to high cost and/or concern for transmitting infection from source tissue. 19
Wound care
Lower limb skin and tissue are extremely thin, especially from the foot and ankle, which poses a challenge for obtaining wound closure (Table 3). Reverse sural adipofascial flaps (RSAF) are commonly used to achieve coverage, but when used in conjunction with STSG, healing is prolonged.32,33 One report detailed a method of RSAF application in which ADM was successfully (25% faster healing) used in concert with NPWT. 34 ADMs may be a useful adjunct to RSAF as they increase tissue vascularisation and support early fibroblast and endothelial cell growth.22,35 A retrospective study of eight patients with foot and ankle wounds reported healing in an average of 104.5 days when treated with ADM and NPWT before STSG and RSAF compared to 141.2 days with STSG and RSAF alone. 34 Other reports have shown that patients treated conservatively (nano-silver dressing alone) or with dermabrasion + nano-silver dressing had a longer average hospital stay than those treated with dermabrasion + PADM. 15
Table 3.
Clinical evidence for ADMs in wound care and ulcers.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Case series and case reports | |||
| Pontell et al. (2018) | Integra™, BADM |
Lower-extremity wound reconstruction 8 patients: 4 with all components and 4 with only RSAF and STSG |
|
| Retrospective studies | |||
| Paredes et al. (2017) | PriMatrix®, FBADM | Chronic, large venous leg ulcers 33 patients, 40 total wounds Excluded: Those with non- CEAP class 6 wounds |
|
| Prospective studies | |||
| Cazzell et al. (2019) | DermACELL®, HADM |
VLUs 28 patients: 18 utilising D-ADM and 10 without |
|
| Kavros et al. (2014) | PriMatrix®, FBADM | DFUs 46 patients completed (out of 55) Mean age: 61 ± 14 years Mean BMI: 28.9 ± 4.3 BMI of 38 or less Those with comorbidities were excluded |
|
ADM, acellular dermal matrix; BADM, bovine acellular dermal matrix; CEAP, clinical aetiology anatomy pathophysiology; DFU, diabetic foot ulcer; FBADM, fetal bovine acellular dermal matrix; HADM, human acellular dermal matrix; NPWT, negative pressure wound therapy; RSAF, reverse sural adipofascial flap; STSG, split-thickness skin graft; TBSA, total body surface area; VLU, venous leg ulcer.
ADM application for upper-limb wounds has resulted in improved elasticity and range of motion (ROM) compared to wounds treated with skin graft alone. 36 Axillary and cubital joint dermis wounds are associated with high rates of contracture and severe scarring. 37 However, with ADM application, one retrospective study of 89 patients reported patient satisfaction with pain relief, ROM and aesthetic outcome in 82%, and 75% had good-excellent physician-reported functionality and ROM. 38
ADMs are particularly useful when treating exposed tendons and bones that may be unsuitable for skin graft coverage.37,39 In radial forearm flap donor site closure, ADM application has led to minimal scar contracture and complications, as well as normal ROM, grip and pinch.37,40,41 In tumour resection surgery, skin contracture is a common complication; however, application of ADM rather than skin grafts alone has improved final ROM. 42
Skin grafting can be difficult in lower-limb wounds as limited available tissue may lead to dermal tension. However, one study of 30 lower-limb injuries treated with combination ADM and STSG reported successful grafting in 29 wounds and an average of 56.4 days to complete healing. 43 Success in these patients may be attributed to ADM’s ability to maintain elasticity and tensile strength while promoting vascularisation and preventing infection.
ADMs have shown efficacy as an adjunct in lower limb ulcers treatment.2,44–46 One randomised controlled trial showed that HADM resulted in greater reduction in wound size at 24 weeks (59.6% HADM vs. 8.1% control). 44 In the same study, 100% of HADM-treated wounds remained closed at four weeks postoperatively and 75% remained closed at 12 weeks compared to 66.7% at four weeks and 33.3% at 12 weeks in the control group. 44 Fetal BADM has been used in treatments for diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs).45,46 BADM has improved DFU healing outcomes by 40% and led to a wound closure of 76% in 53.1 ± 21.9 days in one cohort. 45 In VLUs, BADM application resulted in a median reduction of 23.5% in the wound area at four weeks postoperatively. 46 When compared to advanced moist wound therapy (AMWT), such as foams and gels, HADM application in DFUs resulted in complete closure in 69.6% compared to 46.2% with AMWT. 2
In addition to wound closure, ADMs have been shown to improve the aesthetic properties of skin. In burn scars, HADM has been used to achieve significant improvement of burn scar quality as measured by the Vancouver Scar Scale. 47
ADMs have been associated with complications, including the following: hypopigmentation; lack of vascularisation and lymphatics; absence of hair follicles, sweat and sebaceous glands; and incomplete innervation. 17 Increased duration of treatments raises costs associated with ADMs.2,45,48,49 When incorporating BADM into DFU treatment, 42.9% of patients needed multiple applications with an average wait of 23 days between appplications. 45 ADM + STSG treatment is a staged process in which STSG is performed 3–4 weeks after ADM application. 34 When incorporating ADM in wound treatment algorithms, the effects of treatment duration should be considered.
Breast reconstruction
ADMs have become a popular adjunct to enhance wound healing, organised tissue regeneration and cosmesis in breast reconstruction and augmentation (Table 4).2,50,51 While individual reports vary, aggregate data indicate that complications are rare.52–57 A systematic review of 1039 breast reconstructions with either PADM or HADM showed low overall rates of skin and nipple necrosis (11% and 5%, respectively), infection (12%), hematoma (1%) and seroma (5%), with only 9% of patients requiring reoperation. 52 This success may be attributed to ADMs’ ability to fully integrate into host tissue with neovascularisation, cell repopulation and lack of inflammatory cells observed at both short- and long-term follow-up.56,58
Table 4.
Clinical evidence for ADMs in breast reconstruction.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Case series and case reports | |||
| Knabben et al. (2016) | Permacol®, PADM |
Breast reconstruction post skin-sparing mastectomy in those with breast cancer 10 patients Mean age: 50.9 years (range = 37–64 years) Mean BMI: 21.1 kg/m2 (17.6–26.5 kg/m2) 2 had a history of smoking, 1 had history of diabetes mellitus |
|
| Kornstein A (2013) | Strattice®, PADM |
Case 1: 40-year-old white female, postpartum soft-tissue laxity and grade II ptosis Case 2: 30-year-old white woman, congenital soft-tissue laxity and grade 1 ptosis Case 3: 49-year-old white woman, postpartum and post-weight-loss induced laxity and grade III ptosis |
|
| Retrospective studies | |||
| Butterfield (2013) | SurgiMend®, FBADM AlloDerm®, HADM |
Breast reconstruction 440 reconstructions SurgiMend® (79%) AlloDerm® (21%) |
|
| Ricci et al. (2016) | SurgiMend®, FBADM AlloDerm®, HADM |
Breast reconstruction 952 reconstructions SurgiMend® (39%) AlloDerm® (61%) |
|
| Mazari et al. (2018) | SurgiMend®, FBADM Strattice®, PADM, non-cross-linked |
Breast reconstruction 97 reconstructions SurgiMend® (56%) AlloDerm® (44%) |
|
| Gabriel et al. (2018) | Alloderm RTU® (ready to use) Sterile version of AlloDerm®, HADM |
Breast reconstruction 68 patients, 116 biopsy specimens Mean age: 53 years Mean BMI: 26 kg/m2 43% of patients having had chemotherapy and 17% radiotherapy |
|
| Ranganathan et al. (2016) | FlexHD®, HADM AlloDerm®, HADM |
Breast reconstruction 309 patients FlexHD (60.2%) AlloDerm (39.8%) |
|
| Keifer et al. (2016) | AlloDerm®, HADM (58.4%) Cortiva®, HADM (41.6%) |
Prosthetic-based breast reconstruction 166 patients, 298 total breast reconstructions Cortiva patients, on average, weighed 1.7 kg more and were 1.6 years older |
|
| Qureshi et al. (2016) | AlloDerm®, HADM | Breast reconstruction 367 patients (265 ADM and 102 non-ADM) Mean age: 50 years for both groups BMI: 28.2 kg/m2 |
|
| Rose et al. (2016) | AlloDerm®, HADM |
Expander-based breast reconstruction 55 patients, 77 ADM-based tissue expander reconstruction Mean age: 48.1 years Mean BMI: 25.9 kg/m2 |
|
| Salzberg et al. (2016) | AlloDerm®, HADM (93%) Strattice®, non-cross-linked PADM (6.9%) FlexHD®, HADM (0.1%) |
Breast reconstruction 863 patients, 1584 total reconstructions Mean age: 47.0 years 14% current/former smokers, 10% other co-morbidities, 25% using chemotherapy, 10% using radiotherapy |
|
| Prospective studies | |||
| Bullocks et al. (2014) | DermACELL®, HADM | Two-stage breast reconstruction 10 female patients, 18 total breasts Age range: 33–59 years 2 smokers |
|
| Vu et al. (2015) | FlexHD Pliable®, HADM | Breast reconstruction 41 patients, 72 breasts Age: at least 18 years No patients who experienced complications from previous surgeries or previously underwent reconstruction with tissue expander No patients with BMI >40 kg/m2 or who had previous radiation treatment |
|
| Systematic reviews and meta-analyses | |||
| Adetayo et al. (2016) |
Alloderm®, HADM | Breast reconstruction and abdominal wall |
|
ADM, acellular dermal matrix; BADM, bovine acellular dermal matrix; BMI, body mass index; FBADM, fetal bovine acellular dermal matrix; HADM, human acellular dermal matrix; JP, Jackson Pratt; OR, odds ratio; PADM, porcine acellular dermal matrix.
ADMs have been applied to revision augmentations, as they adequately reinforce the soft tissue and implant pocket, thereby decreasing rates of capsular contracture.53–55 A retrospective review of 850 breast reconstructions reported that out of 450 breast reconstructions using PADM, there was a total complication rate of 33.2%: 12.2% developed seromas; 5.2% major infections; and 6.5% minor infectons. 59 One study of 3189 breast reconstructions noted that if antibiotics were administered for <24 h after operation, the infection rate was 2.48%, whereas regimens that lasted >24 h had an infection rate of 13.21%. 60 In a prospective study of 27 patients, ultrasound detected lymphoceles in only three patients; one patient experienced infection, and all three cases of seroma resolved by 12 months. 61
A 13-year cumulative study of 1584 breast reconstructions with ADM reported capsular contracture of 0.8% in the entire cohort and 1.9% in irradiated breasts. 53 A separate study of 455 breasts revealed minimal contracture 21 months after breast reconstruction surgery. 54 By facilitating an optimal breast pocket, ADMs help to obtain symmetrical coverage, enhance the aesthetic outcome, decrease pain from pectoralis muscle mobilisation and reduce scarring.57,62 Limited capsule contracture and scarring may also be attributed to the decreased inflammatory response associated with ADMs.63,64 Additionally, it is more difficult for scar tissue or capsules to develop on the ADM surface. Once revascularised, the ADM-treated tissue will exhibit improvements, such as enhanced elasticity, that minimise contracture. 65
One case study described the use of ADM to rescue a non-ADM reconstructed breast from complications. 66 A patient undergoing radiotherapy after breast reconstruction developed a radiation ulcer, and by utilising PADM with Becker’s 50 expander as reinforcement, the breast and ulcer were resolved. 66
While ADM appears more costly in the short term ($6686 ADM vs. $5615 non-ADM), ADM has been associated with lower total cost at two years postoperatively ($11,862 vs. $12,319). 67 ADM integration with fenestrations and perforations led to decreased risk of infection, duration of tissue draining and length of hospital stay. 68
Thick ADM implants (≥1.2 mm), compared to thin ADM implants, have been linked to increased rates of necrosis (+3.5%), seroma (+3.5%), infection (+8.8%) and need for drainage two weeks postoperatively (+15%).60,69 This may be the result of reduced neovascularisation in thicker ADMs. 69 One study showed that implants <400 mL were 10.3 times more likely to experience capsular contracture. 53
Implants from different manufacturers (with different processing protocols) may produce different outcomes. In two comparative studies of HADMs, FlexHD resulted in more complications than AlloDerm and Cortiva.70,71 In studies directly comparing ADMs of different origins, results indicate that BADM may be more suitable for breast reconstruction compared to HADM and PADM.12,71–73
Patient lifestyle factors are known to affect outcomes in ADM procedures.53,58,69,74,75 Smoking status, chemotherapy or radiation, and diabetes mellitus have been associated with increased risk for seroma, cellulitis, wound infection and implant failure.53,58,69,74,75 Body mass index (BMI) has been identified as a predictor of tissue drainage time. 69
Surgeon expertise appears to influence outcomes of ADM procedures.70,76,77 Multiple factors must be carefully considered when performing ADM breast procedures, including the following: pectoralis muscle anatomy; flap conditions; skin excess; sentinel-node status; flap vascularity; BMI; and the type of tumour, if present. 78
Andrology
Following application in breast reconstructions, ADM usage evolved to include penile augmentations, erectile dysfunction (ED) treatments and phalloplasties (Table 5).79,80 Advantages of ADM include lower risk of necrosis, shorter operation time and more subtle incisions.79–81
Table 5.
Clinical evidence for ADM in andrology.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Retrospective studies | |||
| Xu et al. (2019) | Unspecified PADM | Penile girth enhancement 78 patients Mean age: 31.14 years (age range = 21–66 years) Exclusion: patients aged 70+ years, history of mental illness, coagulopathy or type I/II diabetes |
|
| Prospective studies | |||
| Alei et al. (2012) | Unspecified PADM | Penile girth augmentation 69 patients |
|
| Tealab et al. (2013) | Pelvicol™, PADM |
Penile augmentation 18 patients Mean age: 24 years (age range = 19–38 years) |
|
| Zhang J et al. (2004) | Unspecified PADM | Penile augmentation 12 patients |
|
| Zhang X et al. (2018) | Unspecified PADM | Penile augmentation for improvements in premature ejaculation 39 patients Mean age at the time of operation: 29 years (age range = 24–37) Excluded: those with penis deformities, bleeding disorders, keloid formation; those who were on medication for ejaculatory function |
|
| Zhang Z et al. (2015) | Unspecified PADM | Wound healing of Fournier gangrene 36 total patients, 17 experimental (those with XADM) and 19 controls |
|
ADM, acellular dermal matrix; IELT, intravaginal ejaculation latency time; PADM, porcine acellular dermal matrix; XADM, xenographic acellular dermal matrix.
One study of 69 patients described a technique in which PADM was placed circumferentially from the groove between cavernous and spongious bodies on one side to the other and secured to Bucks fascia. 79 One year postoperatively, penile circumference increased 3.1 cm while flaccid and 2.4 cm while erect. 79 A retrospective study of 78 patients who received ADM administrations as filler material also showed increased measurements (mean + 1.1 cm). 82 A pilot study assessing an acellular collagen matrix reported results varying by ADM insertion method, with a bilayer inserted through V-Y suprapubic incision producing greater circumference increases and patient satisfaction. 83
ADMs may contribute to improved erectile function and reduced premature ejaculation in penile augmentation patients.81,84 One study followed 39 patients seeking treatment for ED, and after six months, flaccid girth increased >1 cm, erect girth by >2 cm and intravaginal ejaculatory latency time increased by 200 s on average. 84
PADM has been used as an adjunct in Fournier gangrene treatment. 85 In one study, average wound preparation time was 13.6 days when using PADM whereas non-PADM treatment required 22.4 days. 85 Overall hospitalisation decreased by 14 days on average. PADM was shown to promote granulation tissue growth, with maximum retention of penile and perineum function, morphology and protective features. 85
While aphallia is a condition typically addressed by either the De Castro technique or a scrotal flap phalloplasty,86,87 one case report has detailed usage of ADM in this procedure for an infant, with the goal of supplying additional support and girth to the phallus as well as increased vascularisation. 80 After harvesting scrotal skin flap for neophallus construction, the ADM was sutured to the pubic symphysis, then covered with a layer of tunica vaginalis. Twelve months postoperatively, the patient had no complications and good cosmetic outcome. 80 Aphallia in children, however, is a rare disease with limited published data, and further research is needed to assess efficacy of ADM in this context.
One study reported that 60.3% of patients experienced erectile discomfort and 12.8% had no obvious augmentation effects when treated with ADM. 82 Reported complications include severe penile oedema, ischemic shaft ulcers, hematomas and wound infections.82,83 Upon suturing the ADM to Buck’s fascia, micro-branches of the dorsal nerve of the penis may become covered and lead to less receptor threshold. 84 Additionally, a thick ADM may affect proprioception receptors in the deep tissue and on the skin surface, leading to abnormal temperature and pressure differences. 84
Gynaecology and gynaecological oncology
Historically, vaginoplasties were performed using peritoneal tissue, STSGs or allogenic epidermal sheets.88,89 However, ADM has recently been utilised to reduce postoperative pain, procedural complexity and preserve the vaginal mucosa histology. 90 In one study, 16 patients diagnosed with uterine cervix carcinoma underwent vaginal repair using ADM after radical hysterectomy and radiotherapy. 90 Two weeks postoperatively, normal epithelial tissue and vaginal mucosa histology were observed whereas histology previously revealed granulation and inflammatory cells.
Published reports suggest that vaginal length <7 cm is correlated to less sexual satisfaction with lower sexual function. 91 After vaginoplasty with ADM secondary to carcinoma resection of the cervix, vaginal length was improved to an average final length of 9.25 cm, with 75% of patients reporting improved sexual satisfaction. 90 By utilising the ADM in the cervical repair, the superior end of the vagina is preserved, thus retaining cells for future cervical screening tests. 90 Unlike biological tissue, which is at risk of defects and infections when exposed to radiation, a synthetic ADM mesh may be better suited for harsh environments and minimise inflammation and malformations in surrounding tissue.92–94 Of note, successful integration of ADM relies on implantation within highly vascularised tissue. 94
Recurrent gynaecological cancer is traditionally treated with pelvic exenteration using synthetic mesh, myocutaneous flaps from the abdomen or thigh, or pedicled greater omental flaps (PGOF) to secure the pelvic floor. 95 However, a recent case report described the use of PGOF, HADM, and autologous adipose-derived cells to improve pelvic cavity support and volume. 96 Success in this case may be attributed to accelerated angiogenesis and the favourable environment for adipose-derived stem cell incorporation provided by HADM. 97 Another patient with osteoradionecrosis and recurrent vulvar squamous cell carcinoma underwent exenteration, and pelvic floor reconstruction incorporated HADM with bilateral, thigh-based tissue flaps. The carcinogenic and bacterial-infected wounds resolved without complication. 98
Orthopaedic surgery
Foot and ankle
ADMs have been used to promote bony regrowth and periosteum replacement, ultimately leading to cell proliferation, neovascularisation and resolution of bone defects (Table 6).99,100 ADMs have gained popularity in foot and ankle procedures as they lack the disadvantages inherent in many human auto- or allografts, xenografts or synthetic grafts. 101
Table 6.
Clinical evidence for ADMs in orthopaedic surgery.
| Authors | Product name(s), Material |
Usage, Population |
Summary findings |
|---|---|---|---|
| (A) Achilles tendon repair | |||
| Case series and case reports | |||
| Bertasi et al. (2017) | DermACELL®, M-ADM |
Achilles tendon repair 35-year-old fell and re-ruptured native tendon 2 months after repair with M-ADM. Histology specimens were obtained 1 month after re-rupture |
|
| Retrospective studies | |||
| Cole et al. (2018) | ArthroFlex®, M-ADM |
Achilles tendon repair 9 patients Mean age: 58.3 years Mean follow-up: 14.4 months |
|
| (B) Foot/Ankle arthroplasty | |||
| Case series and case reports | |||
| Carpenter et al. (2017) | Arthroflex® | Interpositional ankle arthroplasty 4 patients (age range = 32–42 years) |
|
| Retrospective studies | |||
| Berlet et al. (2008) | ?? | Interpositional arthroplasty of first MTP joint 9 patients, 5 female Mean age: 53.3 years |
|
| (C) Foot/Ankle | |||
| Prospective studies | |||
| Pontell et al. (2018) | ADM, Integra™; Ethicon Inc | RSAFs 8 patients, 4 with an immediate STSG and 4 with a delayed STSG |
|
| (D) Rotator cuff repair | |||
| Case studies and case series | |||
| Neumann et al. (2017) | Porcine dermal matrix xenograft | 60 patients (61 shoulders) were observed for an average of 50.3 months |
|
| Mirzayan et al. (2019) | 25 shoulders (during 2006–2016) with massive rotator cuff tears underwent a procedure with an ADM Mean patient age: 61 years |
|
|
| Retrospective studies | |||
| Hohn et al. (2018) | ?? | From 2008 to 2014, 23 patients who received a revision RC repair augmented with ADM with >2 years of follow-up Mean age: 60.1 years |
|
| (E) Glenoid resurfacing | |||
| Case study and case series | |||
| Namdari et al. (2013) | Graftjacket® | 2 patients who had hemi-arthroplasty and biologic glenoid resurfacing |
|
| (F) Forearm/Hand/Wrist | |||
| Laboratory study | |||
| Ehsan et al. (2012) | Arthroflex, LifeNet Health | Scaphoid and lunate with the scapholunate ligament were taken from 15 cadaveric specimens 5 specimens were kept intact, 5 were reconstructed with a 1.0-mm-thick dermal matrix, and 5 were reconstructed with a 1.5-mm-thick dermal matrix |
|
| Case study and case series | |||
| Gould et al. (2019) | 2 female patients treated to prevent recurrence of distal radioulnar heterotopic ossification |
|
|
| Peterson and Adham (2006) | ADM (AlloDerm) | 5 patients with postoperative and 5 patients with post-traumatic neuropathic pain at the wrist had a neuroma excision and/or neurolysis with interposition of an ADM between skin and nerve |
|
| Retrospective studies | |||
| Terry et al. (2014) | Alloderm; LifeCell, Bridgewater, NJ, USA | 43 patients who had open fasciotomies between 2005 and 2012. 23 treated with ADM Median age: 66.5 years |
|
| Prospective studies | |||
| Hoang et al. (2019) | ADM (FlexHD) | 132 patients having an open fasciotomy for Dupuytren’s disease. 28 patients were treated with the ADM Median age: 67 years |
|
| Kokkalis et al. (2009) | GraftJacket (ADM) | 100 thumbs with trapeziometacarpal osteoarthritis had surgery with ADM instead of the flexor carpi radialis tendon autograft |
|
| (G) Hip | |||
| Prospective studies | |||
| Rao et al. (2016) | Graft Jacket; Wright Medical Technology, Arlington, TN, USA | 12 patients who had a transosseous repair of the gluteus medius and minimus insertions augmented with ADM |
|
ADM, acellular dermal matrix; AOFAS, Association of Orthopaedic Foot and Ankle Society; ASES, American Shoulder and Elbow Surgeons; FFI-R, Foot Function Index-Revised; M-ADM, human dermis processed with Matracell®; MTP, metatarsophalangeal; PADM, porcine acellular dermal matrix; PAS, Periodic acid–Schiff; ROM, range of motion; RSAF, reverse sural adipofascial flap; STSG, split-thickness skin graft; VAS, Visual Analogue Scale.
Multiple case reports describe ADM augmentation of Achilles tendon repairs with no instances of tendon rerupture or complications.101,102 ADMs have been used in interpositional ankle arthroplasty, either as a way to resurface the talus 103 or as a spacer in the first metatarsophalangeal joint. 104 Both procedures were successful, with increased ROM, decreased pain, and no complications.103,104 ADMs have also been successfully utilised to facilitate ankle wound healing. 105 When combined with a reverse sural adipofascial flap (RSAF), ADMs led to 25% faster healing compared to RSAF alone. 34
Shoulder and upper extremity
In both primary and revision rotator cuff repairs, incorporation of ADMs has led to improvements in pain, ROM and muscle strength.106,107 These repairs remained intact at long-term follow-ups. 107 In irreparable rotator cuff tears, ADMs have been used to cover the exposed bone, leading to decreased pain and better functional scores. 108 ADM augmentation of distal biceps repair in a tendon-deficient model led to a stronger tendon than without ADM. 109
ADMs have been shown to improve interface strength and decrease re-tear rates when applied at the suture-tendon interface of rotator cuff repairs. 110 Though the procedure is technically challenging, surgeons have also utilised ADMs in superior capsular reconstructions, resulting in improved pain and shoulder function. 111
ADM use has been described in glenoid resurfacing with improved outcomes in most cases. 112 However, foreign body reactions have been reported and should be considered if there is significant postoperative pain. 112 ADMs have been interposed between the radius and ulna to prevent heterotopic ossification after a forearm injury, leading to improvements in ROM and no recurrence. 113 ADMs are also thought to be more resistant to infection than silicone and collagen-based alternatives. 113
Hand and wrist
ADMs have been used to reconstruct ligaments in arthritic hands. 114 Historical methods utilised donor tendon, but were associated with scarring, pain, tendon rupture, tendonitis and neuroma formation. 114 Xenografts addressed some of these disadvantages, but caused immunologic reactions in some patients. 114 In a cadaveric scapholunate reconstruction model, HADM provided tensile strength comparable to traditional techniques and may potentially decrease donor site morbidity in these repairs. 115 A study of 100 ligament reconstructions in patients with thumb carpometacarpal arthritis found that, when using ADM, there were no adverse effects, foreign body reactions or infections. 114
When used for Dupuytren’s disease, ADMs have been shown to decrease the rate of recurrence, presumably via ADM-mediated inhibition of myofibroblasts that might otherwise create contractures.116,117 ADMs have been used in proximal row carpectomies to prevent degradation of the radiocapitate space and have been effective in treating radiocarpal arthritis. 118 ADMs have demonstrated efficacy as an adjunct in treating neuropathic wrist pain. 119 In these cases, ADM was used to cushion the nerve as an alternative to the traditional flap coverage. 119
Hip and pelvis
ADMs have been used effectively to augment gluteus medius and minimus repairs. 120 This technique is thought to decrease re-tear rates by providing structural strength to the repair, better tendon-bone healing and increased tensile strength due to revascularisation of the graft. 120
Capsular defects have been filled using ADMs to create hip stability in hip reconstructions. 121 ADMs have shown some utility in addressing shortcomings of common hip abductor repair methods. 122 Traditional techniques are associated with unpredictable results with extended periods of rehabilitation. 122 However, patients receiving ADM treatments had significant improvement in Visual Analogue Scale pain and Harris Hip scores across groups. 122
Significant complications are common in pelvic reconstructions due to the complex anatomy, multi-level organ involvement and microbial environment associated with these procedures. 96 Historical attempts to improve outcomes such as synthetic meshes led to adhesions and infections, and myocutaneous flaps from the thigh were too invasive in many cases. 96 One case report described a less-invasive technique using HADM combined with a pedicled omental flap and autologous adipose derived cells that led to fewer adhesions. 96 Other cases have described the successful use of HADM in pelvic floor reconstruction after total exenteration or cylindrical abdominoperineal resection.96,98,123
Oral and maxillofacial surgery
The current gold standard for the treatment of gingival recession is the bilaminar technique using subepithelial connective tissue graft (SCTG); however, this technique has limitations, including the following: lack of available grafts; need for a second surgical site; pain after the surgery; proximity to the palatine neurovascular bundle; and suboptimal aesthetic outcomes. 124 ADMs have been shown to reduce the need for donor tissue and surgical time, and increase patient acceptance (Table 7). 125
Table 7.
Clinical evidence for ADM in oral and maxillofacial surgery.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| (A) Gingival recessions | |||
| Case series and case reports | |||
| Fickl et al. (2013) | Unspecified PADM | 6 patients with 28 gingival recessions had a procedure with a modified tunnelling technique and ADM |
|
| Prospective studies | |||
| Godavsarthi et al. (2016) | AlloDerm®, HADM |
14 patients with Miller Class I or II gingival recessions 3 women Mean age: 41.4 years Randomly assigned to PPG with CAF or ADM with CAF |
|
| Abou-Arraj et al. (2017) | AlloDerm®, HADM Puros Dermis® Solvent-dehydrated HADM |
17 patients with Miller Class I gingival recessions Randomly assigned to AlloDerm® or Puros Dermis® groups |
|
| Cosgarea et al. (2016) | Mucoderm®, PADM |
12 patients with at least two Miller Class I, II or III gingival recessions treated with a modified coronally advanced tunnel technique and then with an ADM 9 women, mean age: 34 years |
|
| Chaparro et al. (2015) | Unspecified PADM | 24 patients with 93 gingival recessions were treated with the tunnel procedure and ADM |
|
| Costa et al. (2016) | AlloDerm®, HADM |
19 smokers with bilateral Miller Class I or II gingival recessions were randomly assigned to received ADM and EMD or ADM alone |
|
| Mahn et al. (2015) | AlloDerm®, HADM |
50 patients with Class I and II gingival recessions were treated with an ADM with a CAF |
|
| Ozenci et al. (2015) | AlloDerm®, HADM |
20 patients with 58 Miller Class I gingival recessions were divided into receiving either ADM with tunnel technique or ADM with CAF |
|
| Wang et al. (2015) | AlloDerm®, HADM Puros Dermis®, Solvent-dehydrated HADM |
20 patients with Miller Class I and II gingival recessions were treated with either FDADM or SDADM |
|
| De Resende et al. (2019) | AlloDerm®, HADM |
25 patients with 50 recession sites were treated with either a FGG or ADM |
|
| (B) Gingival fenestration | |||
| Case series and case reports | |||
| Breault et al. (2016) | AlloDerm®, HADM |
ADM used to treat one gingival fenestration |
|
| (C) Parotid fistula | |||
| Case studies | |||
| Blythe et al. (2016) | AlloDerm®, HADM |
One patient with a parotid fistula |
|
| (D) Alveolar bone grafts | |||
| Retrospective studies | |||
| Clavijo-Alvarez et al. (2010) | AlloDerm®, HADM |
35 patients included from a retrospective review from 2005 to 2007 15 patients (4 girls) received ADM augmentation Mean age at surgery was 10 years |
|
| (E) Regenerate bone/soft tissue in dental implants | |||
| Case studies and case series | |||
| Momen-Heravi et al. (2018) | PerioDerm®, HADM |
A patient with a successful soft-tissue and bone regeneration of dehiscence in the maxillary incisor region using ADM |
|
| Prospective studies | |||
| Fischer et al. (2019) | Derma®, PADM |
20 patients undergoing implant surgery with soft-tissue augmentation (24 total cases) with ADM Mean age: 50.2 ± 11.9 years |
|
| Papi and Pompa (2018) | Mucoderm®, PADM |
12 patients received a dental implant in the upper premolar area and the ADM was inserted 8 weeks later |
|
| Fernandes et al. (2016) | AlloDerm®, HADM |
19 patients undergoing extraction of maxillary teeth were randomly assigned to ADM and mineralised AB or ADM only |
|
| (F) Repair ora-antral fistulas | |||
| Prospective studies | |||
| Li et al. (2018) | Heal-all®, HADM |
9 patients with oro-antral fistulas had the defects repaired with ADM and acellular bone matrix |
|
AB, bone allograft; ADM, acellular dermal matrix; CAF, coronally advanced flap; EMD, enamel matrix derivative; FDADM, freeze-dried ADM; FGG, free gingival graft; HADM, human acellular dermal matrix; KMW, keratinised mucosa width; PADM, porcine acellular dermal matrix; PPG, periosteal pedicle graft; SDADM, solvent-dehydrated ADM.
ADMs have shown increased efficacy in treating Miller Class I, II and III gingival recessions in non-smokers124–127 and Miller Class I and II in smokers 128 compared to non-ADM controls. ADMs were also an effective adjunct when used in conjunction with a coronally advanced flap in Miller Class I and II recessions.129,130
Multiple formulations of ADMs have been used in gingival recession treatment, with both freeze-dried and solvent-dehydrated ADMs successfully achieving root coverage. 131 HADMs have produced superior aesthetic outcomes when compared to autogenous free gingival graft but were associated with delayed healing. 132 Of note, one study reported complete root coverage in only 42.86% of patients using PADM. 133
There is a growing body of research evaluating ADMs in other conditions, including gingival fenestrations, 134 persistent parotid fistulas, 135 ora-antral fistulas, 136 alveolar bone loss/grafting137,138 and dental implants.139–141 Generally, ADMs were used to limit donor tissue harvest, 134 promote soft tissue growth137,139 and improve aesthetic outcomes. 141
Craniofacial surgery
ADMs have been used in craniofacial surgery to treat soft-tissue defects secondary to congenital conditions, disease and surgical wounds.142–150 In aplasia cutis congenita (ACC), ADMs have shown utility both as an adjunct to grafting and as a conservative treatment for scalp coverage.142,143
BADM has been used as a spacer graft for upper eyelid retraction procedures secondary to thyroid eye disease. 145 In a study of 32 eyelids in 26 patients, average upper margin reflex distance was lowered from 7.7 mm to 3.3 mm with 69% of patients achieving perfect results. 145 ADMs have also been used to manage nasal lining deficiency in Le Fort 1 osteotomy, prevent Frey syndrome after parotid neoplasm surgery and as an implant for dorsal augmentation in rhinoplasty.146–148 In patients with cranial defects, ADM has been used to improve bone regeneration and closure of chronic wounds after skull defect reconstruction.149,150
Abdominal wall/hernia
Ventral hernias, though common, continue to present surgical challenges, and there is no consensus regarding optimal treatment (Table 8).151,152 Biological mesh composed of ADM has recently been used in efforts to address the shortcomings of synthetic materials in abdominal wall reconstructions.74,153,154 In 2010, the Ventral Hernia Working Group published a grading system with recommendations for use of either synthetic (Grade 1) or biological mesh (Grades 2–4), with grades designated by risk of postoperative complications.152,155
Table 8.
Clinical evidence for ADMs in AWR.
| Authors | Product name(s), Material | Usage, Population | Summary findings |
|---|---|---|---|
| Case reports | |||
| King et al. (2013) | Strattice™, non-cross-linked PADM |
45-year-old obese white man |
|
| Retrospective studies | |||
| Begum et al. (2016) | Strattice™, non-cross-linked PADM |
Paediatric AWR and chest wall reconstruction 13 patients over a 3-year period. 11 had AWR and two underwent chest wall reconstruction. 7 procedures were contaminated at the time of surgery |
|
| Caso Maestro et al. (2014) | Strattice™, non-cross-linked PADM |
Paediatric delayed closure after liver transplant 6 paediatric patients underwent delayed abdominal wall closure with a biological mesh after liver transplant |
|
| Clemens et al. (2013) | Strattice™, non-cross-linked PADM SurgiMend®, FBADM |
234 consecutive cancer patients who underwent AWR for ventral hernia or musculofascial resection defects with underlay bioprosthetic mesh (porcine or bovine acellular dermal matrix) and complete midline musculofascial closure. 120 patients underwent a non-bridged, inlay AWR with PADM (n = 59/120) or BADM (n = 51/120). |
|
| Garvey et al. (2017) | Alloderm®, HADM |
191 patients |
|
| Giordano et al. (2017) | Strattice™, non-cross-linked PADM |
511 consecutive patients who underwent complex AWR using ADM. Propensity scoring done for multivariable analysis and one-to-one matching |
|
| Giordano et al. (2018) | Strattice™, non-cross-linked PADM |
452 patients (mean age: 59 years) underwent AWR with ADM |
|
| Gowda et al. (2016) | Strattice™, non-cross-linked PADM Alloderm®, HADM |
87 patients who underwent hernia repair after pancreas and/or renal transplant 27 underwent ventral hernia repair with PADM, 34 patients with HADM and 26 with synthetic mesh |
|
| Guerra et al. (2014) | Strattice™, non-cross-linked PADM |
13 adults (mean age: 60 years; 8 women) underwent single-stage ventral herniorrhaphy involving removal of infected synthetic mesh and repair with PADM 54% (7/13) were obese and 46% (6/13) had chronic obstructive pulmonary disease/emphysema. 6 patients had undergone ≥2 previous repairs |
|
| Guerra et al. (2014) | Strattice™, non-cross-linked PADM |
44 patients (mean age: 57.5 years) with complex ventral abdominal wall hernias were repaired using PADM. 45 single-stage repairs (3 primary; 42 incisional) 17 had previously placed synthetic mesh removed. In 40 cases, primary fascial closure was achieved; in 5 cases, PADM was used as a bridge. Vacuum-assisted closure was used for 38/45 cases: 19 closed incisions, 16 cases using the ‘French fry’ technique, and 3 cases with open incisions |
|
| Systematic reviews and meta-analyses | |||
| Adetayo et al. (2016) | Alloderm®, HADM |
AWR and breast reconstruction 53 studies for meta-analysis. Majority (68.6%) were retrospective |
|
ADM, acellular dermal matrix; AWR, abdominal wall reconstruction; BADM, bovine acellular dermal matrix; FBADM, fetal bovine acellular dermal matrix; HADM, human acellular dermal matrix; PADM, porcine acellular dermal matrix.
Compared to synthetic mesh, ADM has been associated with decreased rates of infection, extrusion, erosion and adhesion formation. 154 In studies comparing different types of ADM, PADM and BADM appear to outperform HADM.153,154,156 While HADM may be equally effective in reducing infection, recurrence rates are higher than in PADM or BADM.74,153,154,156,157 The high elastin content of HADM is believed to contribute to relaxation over time and may be the cause of increased reports of laxity and/or bulging.154,156–158
PADM has been used effectively in giant and recurrent hernias, as well as in both elderly and paediatric patients and recurrent hernias.155,159,160 One study comparing PADM and BADM showed similar outcomes between the two formulations. 161
The literature assessing PADM and BADM is lacking in some surgical procedures such as bladder exstrophy repair, urethral reconstruction and treatments for premature ejaculation; however, HADM has shown utility in these procedures (Table 9).4,162,163
Of note, one case report described a delayed type IV hypersensitivity reaction to PADM. 164 When infection is suspected after ADM placement, hypersensitivity should be considered as part of the diagnostic algorithm. 164
Otolaryngology/ear, nose and throat (ENT)
HADM and xenogeneic ADMs have been used in laryngotracheal and pharyngeal reconstruction as they are relatively thin and flexible compared to myocutaneous flaps.165–169 ADM grafts carry lower risk of fistula and stricture formation and avoid donor site complications associated with flap harvest. 165 Thin ADMs are more often used for partial superficial defects in the trachea, larynx or hypopharynx. Thicker sheet ADMs were typically used for complex pharyngeal fistula closure and partial pharyngoplasty for stage III–IV carcinomas. 165 Other successful uses of ADM in otorhinolaryngology include closure of hard palatal fistula and tympanoplasty.170,171
Discussion
The current literature indicates that there is not a single ADM that has proven superiority in every clinical context.70,153,154,156 In burn wounds, BADMs have produced the most favourable outcomes (compared to PADM and HADM). While Integra™ is the most popular option for coverage, MatriDerm® has seen increased utilisation as it can be applied in a single-stage procedure and provides improved neovascularisation and degradation. 19 Further studies are needed to compare PADM and BADM.
In breast reconstruction, multiple studies have been performed to directly compare ADMs of different tissue origins.12,73 There is no clear aggregate trend indicating that one tissue source consistently produces favourable outcomes.12,72,73 However, of the available HADMs, AlloDerm® may outperform other HADMs in breast reconstruction.71,75 In these procedures, increased implant size and/or thickness (≥1.2 mm) appears to negatively impact outcomes.53,60,69
In procedures such as abdominal wall reconstruction, structural components of ADM play a role in the stability of the repair. Aside from providing mechanical cues, properties that confer rigidity, such as lower elastin content, may influence the success of a repair.154,156–158
As previously mentioned, ADMs are frequently used to treat gingival recessions and provide improved cosmesis compared to traditional autograft techniques. HADM outcomes are generally superior to PADM, though the literature is lacking in studies with direct comparisons.132,133 Of note, standardised ADM graft size may skew outcomes as individual patients have highly variable gingival defects. 132
In orthopaedic procedures, ADMs have primarily been used in orthoplastic reconstructions (e.g. reverse sural flap) and in tendon repairs where limited vascularisation and/or adhesion is a concern. 120 Future applications of ADM in orthopaedic applications may incorporate more injectable formulations.
Many ADMs have been treated with different products, cells and signalling molecules.6,7 For example, ADMs pretreated with bFGF had better recruitment of mesenchymal stem cells, 71 proliferation and differentiation compared with a matrix pretreated with BMP-2 (though both were better than controls).6,172 Further studies are needed to assess the clinical utility of various treated ADMs.
ADMs have been produced from a variety of sources including human, porcine and bovine tissue and can be further classified by tissue source (dermis, intestinal submucosa, urinary bladder, pericardium, etc.).173,174 The variable efficacy between different allogenic or xenogenic ADMs may be attributed to advantages and/or disadvantage of each in providing barrier function, vascular ingrowth, innervation potential, growth factors and mechanical cues to induce site-appropriate healing. 175 In addition to the origins of each ADM, products vary by type and level of processing and/or sterilisation. 176 Some studies have suggested that aseptic processing is more beneficial than sterilisation, but others have found them to be equivalent, and there is currently no consensus on optimal processing.177,178
In addition to efficacy considerations, practical and ethical constraints must be considered in discussions of ADM products. Xenogenic ADMs are more readily available (compared to HADMs). However, patients belonging to certain ethnic and/or religious groups may hold beliefs that preclude the use of products with certain tissue origins. 179 Patients’ belief systems often require a nuanced understanding of religious and cultural norms. 179 For example, while Jewish and Islamic dietary restrictions may not translate to tissue implantation, Buddhists and Seventh-day Adventists often practice veganism, which could lead them to refuse xenogenic tissue products. 180 Furthermore, some Hindus and Sikhs are opposed to all allogenic and xenogenic products, but other Hindus allow the use of donated allogenic tissues.179,180
Given that ADMs represent a relatively new addition to the reconstructive ladder, datum is limited regarding the efficacy of different commercially available ADMs in specific clinical contexts. This review does not include quantitative meta-analyses and is limited by the quality and/or amount of clinical data available for some injury patterns. Additional studies are needed for direct comparison of various ADMs. While formulations and clinical uses of ADM continue to evolve, this review provides a broad overview to better define our current understanding of its clinical utility.
Conclusion
ADMs have been used in a variety of clinical contexts, utilising the properties of the ECM to aid in organised native tissue regeneration. There is clinical evidence to support ADM usage in various subspecialties and procedures, including orthopaedic surgery, breast reconstruction, burn, wound care, andrology, oral and maxillofacial surgery, craniofacial surgery, abdominal wall/hernia repair and otolaryngology. Early reports on ADMs are promising, and further research is needed to determine their place in current reconstructive algorithms.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SBH1038313. Though they are not directly related to this case report, the authors would like to disclose the following support for Brendan MacKay: Paid teaching for TriMed. Paid teaching and consulting, as well as research support from AxoGen. Paid consulting for Baxter/Synovis and GLG. The remaining authors have nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Cameron T Cox https://orcid.org/0000-0003-0026-9272
Supplemental material: Supplemental material for this article is available online.
How to cite this article
Petrie K, Cox CT, Becker BC and MacKay BJ. Clinical applications of acellular dermal matrices: A review. Scars, Burns & Healing, Volume 7, 2021. DOI: 10.1177/ 20595131211038313.
References
- 1.Janis JE, Nahabedian MY. Acellular dermal matrices in surgery. Plast Reconstr Surg 2012; 130(Suppl 2): 7s–8s. [DOI] [PubMed] [Google Scholar]
- 2.Kirsner RS, Bohn G, Driver VR, et al. Human acellular dermal wound matrix: evidence and experience. Int Wound J 2015; 12: 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohac M, Danisovic L, Koller Jet al. et al. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur J Histochem 2018; 62: 2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin ZC, Yang BC, Li M, et al. [Appllication of human acellular dermal matrix in surgical treatment of genitourinary disease]. Beijing Da Xue Xue Bao Yi Xue Ban 2019; 51: 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Kim HG, Lee WJ. Characterization and tissue incorporation of cross-linked human acellular dermal matrix. Biomaterials 2015; 44: 195–205. [DOI] [PubMed] [Google Scholar]
- 6.Mirzaei-Parsa MJ, Ghanbari H, Alipoor Bet al. et al. Nanofiber-acellular dermal matrix as a bilayer scaffold containing mesenchymal stem cell for healing of full-thickness skin wounds. Cell Tissue Res 2019; 375: 709–721. [DOI] [PubMed] [Google Scholar]
- 7.Ye K, Traianedes K, Choong PFet al. et al. Chondrogenesis of human infrapatellar fat pad stem cells on acellular dermal matrix. Front Surg 2016; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janis JE, O’Neill AC, Ahmad Jet al. et al. Acellular dermal matrices in abdominal wall reconstruction: a systematic review of the current evidence. Plast Reconstr Surg 2012; 130(Suppl 2): 183s–193s.. [DOI] [PubMed] [Google Scholar]
- 9.DeLong MR, Tandon VJ, Farajzadeh M, et al. Systematic review of the impact of acellular dermal matrix on aesthetics and patient satisfaction in tissue expander-to-implant breast reconstructions. Plast Reconstr Surg 2019; 144: 967e–974e. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher SI, Matthews DC. Acellular dermal matrix and subepithelial connective tissue grafts for root coverage: a systematic review. J Indian Soc Periodontol 2017; 21: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dussoyer M, Michopoulou A, Rousselle P. Decellularized scaffolds for skin repair and regeneration. Appl Sci 2020; 10: 3435. [Google Scholar]
- 12.Ricci JA, Treiser MD, Tao R, et al. Predictors of complications and comparison of outcomes using SurgiMend Fetal Bovine and AlloDerm human cadaveric acellular dermal matrices in implant-based breast reconstruction. Plast Reconstr Surg 2016; 138: 583e–591e. [DOI] [PubMed] [Google Scholar]
- 13.Cloutier C B, Guignard R, Bernard G, et al. Production of a bilayered self-assembled skin substitute using a tissue-engineered acellular dermal matrix. Tissue Eng Part C Methods 2015; 21: 1297–1305. [DOI] [PubMed] [Google Scholar]
- 14.Yu G, Ye L, Tan Wet al. et al. A novel dermal matrix generated from burned skin as a promising substitute for deep-degree burns therapy. Mol Med Rep 2016; 13: 2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo ZQ, Qiu L, Gao Y, et al. Use of porcine acellular dermal matrix following early dermabrasion reduces length of stay in extensive deep dermal burns. Burns 2016; 42: 598–604. [DOI] [PubMed] [Google Scholar]
- 16.Okuno E, Jarros IC, Bonfim-Mendonca PSet al. et al. Candida parapsilosis isolates from burn wounds can penetrate an acellular dermal matrix. Microb Pathog 2018; 118: 330–335. [DOI] [PubMed] [Google Scholar]
- 17.Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 2018; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Zuijlen P, Gardien K, Jaspers M, et al. Tissue engineering in burn scar reconstruction. Burns Trauma 2015; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua AWC, Khoo YC, Tan BKet al. et al. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 2016; 4: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg 1988; 208: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen DQ, Potokar TS, Price P. An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns 2010; 36: 23–28. [DOI] [PubMed] [Google Scholar]
- 22.Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 2003; 24: 42–48. [DOI] [PubMed] [Google Scholar]
- 23.Shirley R, Teare L, Dziewulski Pet al. et al. A fatal case of toxic shock syndrome associated with skin substitute. Burns 2010; 36: e96–e98. [DOI] [PubMed] [Google Scholar]
- 24.Moiemen NS, Yarrow J, Kamel Det al. et al. Topical negative pressure therapy: does it accelerate neovascularisation within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns 2010; 36: 764–768. [DOI] [PubMed] [Google Scholar]
- 25.Demircan M, Cicek T, Yetis MI. Preliminary results in single-step wound closure procedure of full-thickness facial burns in children by using the collagen-elastin matrix and review of pediatric facial burns. Burns 2015; 41: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 26.Haslik W, Kamolz LP, Manna Fet al. et al. Management of full-thickness skin defects in the hand and wrist region: first long-term experiences with the dermal matrix Matriderm. J Plast Reconstr Aesthet Surg 2010; 63: 360–364. [DOI] [PubMed] [Google Scholar]
- 27.van Zuijlen PP, van Trier AJ, Vloemans JFet al. et al. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast Reconstr Surg 2000; 106: 615–623. [DOI] [PubMed] [Google Scholar]
- 28.Bloemen MCT, van Leeuwen MCE, van Vucht NEet al. et al. Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. Plast Reconstr Surg 2010; 125: 1450–1459. [DOI] [PubMed] [Google Scholar]
- 29.Barret JP, Dziewulski P, McCauley RLet al. et al. Dural reconstruction of a class IV calvarial burn with decellularized human dermis. Burns 1999; 25: 459–462. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan RL, Choucair RJ, Donelan MB. Management of massive calvarial exposure in young children. J Burn Care Rehabil 1998; 19: 29–32. [DOI] [PubMed] [Google Scholar]
- 31.Bizhko IP, Slesarenko SV. Operative treatment of deep burns of the scalp and skull. Burns 1992; 18: 220–223. [DOI] [PubMed] [Google Scholar]
- 32.Parodi PC, De Biasio F, Rampino Cordaro Eet al. et al. Distally-based superficial sural flap: advantages of the adipofascial over the fasciocutaneous flap. Scand J Plast Reconstr Surg Hand Surg 2010; 44: 37–43. [DOI] [PubMed] [Google Scholar]
- 33.Bocchi A, Merelli S, Morellini Aet al. et al. Reverse fasciosubcutaneous flap versus distally pedicled sural island flap: two elective methods for distal-third leg reconstruction. Ann Plast Surg 2000; 45: 284–291. [DOI] [PubMed] [Google Scholar]
- 34.Pontell ME, Saad N, Winters BSet al. et al. Reverse sural adipofascial flaps with acellular dermal matrix and negative-pressure wound therapy. Adv Skin Wound Care 2018; 31: 612–617. [DOI] [PubMed] [Google Scholar]
- 35.Molnar JA, DeFranzo AJ, Hadaegh Aet al. et al. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg 2004; 113: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 36.Ryssel H, Germann G, Kloeters Oet al. et al. Dermal substitution with Matriderm(®) in burns on the dorsum of the hand. Burns 2010; 36: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 37.Rehim SA, Singhal M, Chung KC. Dermal skin substitutes for upper limb reconstruction: current status, indications, and contraindications. Hand Clin 2014; 30: 239–52, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frame JD, Still J, Lakhel-LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg 2004; 113: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 39.Helgeson MD, Potter BK, Evans KNet al. et al. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat-related soft tissue wounds. J Orthop Trauma 2007; 21: 394–399. [DOI] [PubMed] [Google Scholar]
- 40.Murray RC, Gordin EA, Saigal Ket al. et al. Reconstruction of the radial forearm free flap donor site using integra artificial dermis. Microsurgery 2011; 31: 104–108. [DOI] [PubMed] [Google Scholar]
- 41.Gravvanis AI, Tsoutsos DA, Iconomou Tet al. et al. The use of integra artificial dermis to minimize donor-site morbidity after suprafascial dissection of the radial forearm flap. Microsurgery 2007; 27: 583–587. [DOI] [PubMed] [Google Scholar]
- 42.Chalmers RL, Smock E, Geh JL. Experience of Integra(®) in cancer reconstructive surgery. J Plast Reconstr Aesthet Surg 2010; 63: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 43.Kim YH, Hwang KT, Kim KHet al. et al. Application of acellular human dermis and skin grafts for lower extremity reconstruction. J Wound Care 2019; 28: S12–S17. [DOI] [PubMed] [Google Scholar]
- 44.Cazzell S. A randomized controlled trial comparing a human acellular dermal matrix versus conventional care for the treatment of venous leg ulcers. Wounds 2019; 31: 68–74. [PubMed] [Google Scholar]
- 45.Kavros SJ, Dutra T, Gonzalez-Cruz R, et al. The use of PriMatrix, a fetal bovine acellular dermal matrix, in healing chronic diabetic foot ulcers: a prospective multicenter study. Adv Skin Wound Care 2014; 27: 356–362. [DOI] [PubMed] [Google Scholar]
- 46.Paredes JA, Bhagwandin S, Polanco Tet al. et al. Managing real world venous leg ulcers with fetal bovine acellular dermal matrix: a single centre retrospective study. J Wound Care 2017; 26: S12–s19. [DOI] [PubMed] [Google Scholar]
- 47.Angspatt A, Termwattanaphakdee T, Muangma Pet al. et al. Pilot clinical evaluation of PoreSkin: a human acellular dermal matrix in burn scars. J Med Assoc Thai 2017; 100: 441–446. [PubMed] [Google Scholar]
- 48.Böttcher-Haberzeth S, Biedermann T, Reichmann E. Tissue engineering of skin. Burns 2010; 36: 450–460. [DOI] [PubMed] [Google Scholar]
- 49.Yu P, Qi Z. Prospective randomized comparison of scar appearances between cograft of acellular dermal matrix with autologous split-thickness skin and autologous split-thickness skin graft alone for full-thickness skin defects of the extremities. Plast Reconstr Surg 2016; 137: 906e. [DOI] [PubMed] [Google Scholar]
- 50.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006; 57: 1–5. [DOI] [PubMed] [Google Scholar]
- 51.Uroskie TW, Colen LB. History of breast reconstruction. Semin Plast Surg 2004; 18: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heidemann LN, Gunnarsson GL, Salzberg CAet al. et al. Complications following nipple-sparing mastectomy and immediate acellular dermal matrix implant-based breast reconstruction-a systematic review and meta-analysis. Plast Reconstr Surg Glob Open 2018; 6: e1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzberg CA, Ashikari AY, Berry Cet al. et al. Acellular dermal matrix-assisted direct-to-implant breast reconstruction and capsular contracture: a 13-year experience. Plast Reconstr Surg 2016; 138: 329–337. [DOI] [PubMed] [Google Scholar]
- 54.Basu CB, Jeffers L. The role of acellular dermal matrices in capsular contracture: a review of the evidence. Plast Reconstr Surg 2012; 130: 118s–124s. [DOI] [PubMed] [Google Scholar]
- 55.Maxwell GP, Gabriel A. Acellular dermal matrix for reoperative breast augmentation. Plast Reconstr Surg 2014; 134: 932–938. [DOI] [PubMed] [Google Scholar]
- 56.Gabriel A, Maxwell GP. Alloderm RTU integration and clinical outcomes when used for reconstructive breast surgery. Plast Reconstr Surg Glob Open 2018; 6: e1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knabben L, Kanagalingam G, Imboden Set al. et al. Acellular dermal matrix (permacol((R))) for heterologous immediate breast reconstruction after skin-sparing mastectomy in patients with breast cancer: a single-institution experience and a review of the literature. Front Med (Lausanne) 2016; 3: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bullocks JM. DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg 2014; 37: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loo YL, Haider S. The use of porcine acellular dermal matrix in single-stage, implant-based immediate breast reconstruction: a 2-center retrospective outcome study. Plast Reconstr Surg Glob Open 2018; 6: e1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips B, Bishawi M, Dagum Aet al. et al. A systematic review of infection rates and associated antibiotic duration in acellular dermal matrix breast reconstruction. Eplasty 2014; 14: e42. [PMC free article] [PubMed] [Google Scholar]
- 61.Laura B, Alice C, Silvia G, et al. Postsurgical ultrasound evaluation of patients with prosthesis in acellular dermal matrix: results from monocentric experience. Int J Surg Oncol 2019; 2019: 7437324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srinivasa DR, Holland M, Sbitany H. Optimizing perioperative strategies to maximize success with prepectoral breast reconstruction. Gland Surg 2019; 8: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orenstein S, Qiao Y, Kaur Met al. et al. In vitro activation of human peripheral blood mononuclear cells induced by human biologic Meshes1. J Surg Res 2010; 158: 10–14. [DOI] [PubMed] [Google Scholar]
- 64.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010; 126: 1842–1847. [DOI] [PubMed] [Google Scholar]
- 65.Nahabedian MY. Discussion: treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg 2013; 132: 530–531. [DOI] [PubMed] [Google Scholar]
- 66.Naseem S, Patel AD, Devalia H. Pioneering technique using acellular dermal matrix in the rescue of a radiation ulcer. G Chir 2016; 37: 46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi AA, Broderick K, Funk Set al. et al. Direct hospital cost of outcome pathways in implant-based reconstruction with acellular dermal matrices. Plast Reconstr Surg Glob Open 2016; 4: e831–e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JYS, Mlodinow AS. What's new in acellular dermal matrix and soft-tissue support for prosthetic breast reconstruction. Plast Reconstr Surg 2017; 140: 30s–43s. [DOI] [PubMed] [Google Scholar]
- 69.Rose JF, Zafar SN, Ellsworth WA, IV. Does acellular dermal matrix thickness affect complication rate in tissue expander based breast reconstruction? Plast Surg Int 2016; 2016: 2867097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffman L, Small K, Talmor M. Use of acellular dermal matrix in postmastectomy breast reconstruction: are all acellular dermal matrices created equal? Plast Reconstr Surg 2016; 138: 148e–149e. [DOI] [PubMed] [Google Scholar]
- 71.Ranganathan K, Santosa KB, Lyons DA, et al. Use of acellular dermal matrix in postmastectomy breast reconstruction: are All acellular dermal matrices created equal? Plast Reconstr Surg 2015; 136: 647–653. [DOI] [PubMed] [Google Scholar]
- 72.Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg 2013; 131: 940–951. [DOI] [PubMed] [Google Scholar]
- 73.Mazari FAK, Wattoo GM, Kazzazi NH, et al. The comparison of strattice and SurgiMend in acellular dermal matrix-assisted, implant-based immediate breast reconstruction. Plast Reconstr Surg 2018; 141: 283–293. [DOI] [PubMed] [Google Scholar]
- 74.Adetayo OA, Salcedo SE, Bahjri Ket al. et al. A meta-analysis of outcomes using acellular dermal matrix in breast and abdominal wall reconstructions: event rates and risk factors predictive of complications. Ann Plast Surg 2016; 77: e31–e38. [DOI] [PubMed] [Google Scholar]
- 75.Keifer OP J, Page EK, Hart Aet al. et al. A complication analysis of 2 acellular dermal matrices in prosthetic-based breast reconstruction. Plast Reconstr Surg Glob Open 2016; 4: e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019; 13: 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vu MM, Kim JY. Current opinions on indications and algorithms for acellular dermal matrix use in primary prosthetic breast reconstruction. Gland Surg 2015; 4: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zenn M, Venturi M, Pittman T, et al. Optimizing outcomes of postmastectomy breast reconstruction with acellular dermal matrix: a review of recent clinical data. Eplasty 2017; 17: e18. [PMC free article] [PubMed] [Google Scholar]
- 79.Alei G, Letizia P, Ricottilli F, et al. Original technique for penile girth augmentation through porcine dermal acellular grafts: results in a 69-patient series. J Sex Med 2012; 9: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhry R, Theisen KM, Dangle PPet al. et al. Congenital aphallia: novel use of acellular dermal matrix during scrotal flap phalloplasty. Urology 2017; 105: 167–170. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J-m, Cui Y-y, Pan S-jet al. et al. [Penile augmentation using acellular dermal matrix]. Zhonghua Zheng Xing Wai Ke Za Zhi 2004; 20: 418–420. [PubMed] [Google Scholar]
- 82.Xu TM, Zhang XW, Zhang GXet al. et al. [Complications and management for penile augmentation with acellular dermal matrix]. Beijing Da Xue Xue Bao Yi Xue Ban 2019; 51: 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tealab AA, Maarouf AM, Habous Met al. et al. The use of an acellular collagen matrix in penile augmentation: a pilot study in Saudi Arabia. Arab J Urol 2013; 11: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, Wu Y, Zhang M, et al. Acellular dermal matrix in premature ejaculation: a preliminary study. Medicine (Baltimore) 2018; 97: e13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, Lv L, Mamat M, et al. Xenogenic (porcine) acellular dermal matrix promotes growth of granulation tissues in the wound healing of Fournier gangrene. Am Surg 2015; 81: 92–95. [PubMed] [Google Scholar]
- 86.De Castro R, Rondon A, Barroso Uet al. et al. Phalloplasty and urethroplasty in a boy with penile agenesis. J Pediatr Urol 2013; 9: 108.e1–108.e2. [DOI] [PubMed] [Google Scholar]
- 87.Bajpai M. Scrotal phalloplasty: a novel surgical technique for aphallia during infancy and childhood by pre-anal anterior coronal approach. J Indian Assoc Pediatr Surg 2012; 17: 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carranza-Lira S, Sauer-Ramírez R, Bolívar-Flores YJ. Neovagina with cultured allogenic epidermal sheets. Eur J Obstet Gynecol Reprod Biol 1999; 82: 77–79. [DOI] [PubMed] [Google Scholar]
- 89.Seccia A, Salgarello M, Sturla Met al. et al. Neovaginal reconstruction with the modified McIndoe technique: a review of 32 cases. Ann Plast Surg 2002; 49: 379–384. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z, Huang J, Zeng Aet al. et al. Vaginoplasty with acellular dermal matrix after radical resection for carcinoma of the uterine cervix. J Invest Surg 2019; 32: 180–185. [DOI] [PubMed] [Google Scholar]
- 91.Liu X, Liu M, Hua Ket al. et al. Sexuality after laparoscopic peritoneal vaginoplasty in women with Mayer-Rokitansky-Kuster-Hauser Syndrome. J Minim Invasive Gynecol 2009; 16: 720–729. [DOI] [PubMed] [Google Scholar]
- 92.Komorowska-Timek E, Oberg KC, Timek TAet al. et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg 2009; 123: 807–816. [DOI] [PubMed] [Google Scholar]
- 93.Seth A, Hirsch E, Fine Net al. et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg 2012; 130: 750–758. [DOI] [PubMed] [Google Scholar]
- 94.Tork S, Jefferson RC, Janis JE. Acellular dermal matrices: applications in plastic surgery. Semin Plast Surg 2019; 33: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolehmainen M, Suominen S, Pelvic TE. Perineal and genital reconstructions. Scand J Surg 2013; 102: 25–31. [DOI] [PubMed] [Google Scholar]
- 96.Perrone AM, Livi A, Fini M, et al. A surgical multi-layer technique for pelvic reconstruction after total exenteration using a combination of pedicled omental flap, human acellular dermal matrix and autologous adipose derived cells. Gynecol Oncol Rep 2016; 18: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Komatsu I, Yang J, Zhang Y, et al. Interstitial engraftment of adipose-derived stem cells into an acellular dermal matrix results in improved inward angiogenesis and tissue incorporation. J Biomed Mater Res Part A 2013; 101: 2939–2947. [DOI] [PubMed] [Google Scholar]
- 98.Said HK, Bevers M, Butler CE. Reconstruction of the pelvic floor and perineum with human acellular dermal matrix and thigh flaps following pelvic exenteration. Gynecol Oncol 2007; 107: 578–582. [DOI] [PubMed] [Google Scholar]
- 99.Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics 2003; 26(5 Suppl): s591–s596. [DOI] [PubMed] [Google Scholar]
- 100.Shamian B, Hinds RM, Capo JT. Novel use of synthetic acellular dermal matrix for coverage of a tibial defect following resection of an osteochondroma: a case report. Int J Low Extrem Wounds 2016; 15: 82–85. [DOI] [PubMed] [Google Scholar]
- 101.Bertasi G, Cole W, Samsell Bet al. et al. Biological incorporation of human acellular dermal matrix used in Achilles tendon repair. Cell Tissue Bank 2017; 18: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cole W, Samsell B, Moore MA. Achilles tendon augmented repair using human acellular dermal matrix: a case series. J Foot Ankle Surg 2018; 57: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 103.Carpenter B, Duncan K, Ernst Jet al. et al. Interposition ankle arthroplasty using acellular dermal matrix: a small series. J Foot Ankle Surg 2017; 56: 894–897. [DOI] [PubMed] [Google Scholar]
- 104.Berlet GC, Hyer CF, Lee THet al. et al. Interpositional arthroplasty of the first MTP joint using a regenerative tissue matrix for the treatment of advanced hallux rigidus. Foot Ankle Int 2008; 29: 10–21. [DOI] [PubMed] [Google Scholar]
- 105.Hutchison RL, Craw JR. Use of acellular dermal regeneration template combined with NPWT to treat complicated extremity wounds in children. J Wound Care 2013; 22: 708–712. [DOI] [PubMed] [Google Scholar]
- 106.Hohn EA, Gillette BP, Burns JP. Outcomes of arthroscopic revision rotator cuff repair with acellular human dermal matrix allograft augmentation. J Shoulder Elbow Surg 2018; 27: 816–823. [DOI] [PubMed] [Google Scholar]
- 107.Neumann JA, Zgonis MH, Rickert KD, et al. Interposition dermal matrix xenografts: a successful alternative to traditional treatment of massive rotator cuff tears. Am J Sports Med 2017; 45: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 108.Mirzayan R, Stone MA, Batech Met al. et al. Failed dermal allograft procedures for irreparable rotator cuff tears can still improve pain and function: the “biologic tuberoplasty effect”. Orthop J Sports Med 2019; 7: 2325967119863432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conroy C, Sethi P, Macken C, et al. Augmentation of distal biceps repair with an acellular dermal graft restores native biomechanical properties in a tendon-deficient model. Am J Sports Med 2017; 45: 2028–2033. [DOI] [PubMed] [Google Scholar]
- 110.Cooper J, Mirzayan R. Acellular dermal matrix in rotator cuff surgery. Am J Orthop (Belle Mead NJ) 2016; 45: 301–305. [PubMed] [Google Scholar]
- 111.Laskovski JR, Boyd JA, Peterson EEet al. et al. Simplified technique for superior capsular reconstruction using an acellular dermal allograft. Arthrosc Tech 2018; 7: e1089–e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Namdari S, Melnic C, Huffman GR. Foreign body reaction to acellular dermal matrix allograft in biologic glenoid resurfacing. Clin Orthop Relat Res 2013; 471: 2455–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gould DJ, Rahgozar P, Nagengast ESet al. et al. Use of acellular dermal matrix to prevent recurrence of radioulnar heterotopic ossification. Plast Reconstr Surg Glob Open 2019; 7: e2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kokkalis ZT, Zanaros G, Sotereanos DG. Ligament reconstruction with tendon interposition using an acellular dermal allograft for thumb carpometacarpal arthritis. Tech Hand Up Extrem Surg 2009; 13: 41–46. [DOI] [PubMed] [Google Scholar]
- 115.Ehsan A, Lee DG, Bakker AJet al. et al. Scapholunate ligament reconstruction using an acellular dermal matrix: a mechanical study. J Hand Surg Am 2012; 37: 1538–1542. [DOI] [PubMed] [Google Scholar]
- 116.Hoang D, Steven P, Chen VWet al. et al. Use of acellular dermal matrix following fasciectomy for the treatment of Dupuytren's disease. Plast Reconstr Surg Glob Open 2019; 7: e2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Terry MJ, Sue GR, Goldberg Cet al. et al. Hueston revisited: use of acellular dermal matrix following fasciectomy for the treatment of Dupuytren's disease. Ann Plast Surg 2014; 73: S178–S180. [DOI] [PubMed] [Google Scholar]
- 118.Rabinovich RV, Lee SJ. Proximal row carpectomy using decellularized dermal allograft. J Hand Surg Am 2018; 43: 392.e1–392.e9. [DOI] [PubMed] [Google Scholar]
- 119.Peterson SL, Adham MN. Acellular dermal matrix as an adjunct in treatment of neuropathic pain at the wrist. J Trauma 2006; 61: 392–395. [DOI] [PubMed] [Google Scholar]
- 120.Laskovski J, Urchek R. Endoscopic gluteus medius and minimus repair with allograft augmentation using acellular human dermis. Arthrosc Tech 2018; 7: e225–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perets I, Hartigan DE, Walsh JPet al. et al. Arthroscopic capsular reconstruction of the hip with acellular dermal extracellular matrix: surgical technique. Arthrosc Tech 2016; 5: e1001–e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rao BM, Kamal TT, Vafaye Jet al. et al. Surgical repair of hip abductors. A new technique using Graft Jacket allograft acellular human dermal matrix. Int Orthop 2012; 36: 2049–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han JG, Wang ZJ, Gao ZGet al. et al. Human acellular dermal matrix for pelvic floor reconstruction after cylindrical abdominoperineal resection. Hepatogastroenterology 2011; 58: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 124.Godavarthi L, Murthy KR, Pavankumar S. A comparison of acellular dermal matrix allograft and periosteal pedicle graft covered by coronally advanced flap in the treatment of gingival recession: 1-year follow-up study. Int J Periodontics Restorative Dent 2016; 36: e67–e75. [DOI] [PubMed] [Google Scholar]
- 125.Abou-Arraj RV, Kaur M, Vassilopoulos PJet al. et al. Creation of a zone of immobile connective tissue with acellular dermal matrix allografts. Int J Periodontics Restorative Dent 2017; 37: 571–579. [DOI] [PubMed] [Google Scholar]
- 126.Cosgarea R, Juncar R, Arweiler Net al. et al. Clinical evaluation of a porcine acellular dermal matrix for the treatment of multiple adjacent class I, II, and III gingival recessions using the modified coronally advanced tunnel technique. Quintessence Int 2016; 47: 739–747. [DOI] [PubMed] [Google Scholar]
- 127.Chaparro A, De la Fuente M, Albers D, et al. Root coverage of multiple miller class I and II recession defects using acellular dermal matrix and tunneling technique in maxilla and mandible: a 1-year report. Int J Periodontics Restorative Dent 2015; 35: 639–645.– [DOI] [PubMed] [Google Scholar]
- 128.Costa PP, Alves LB, Souza SL, et al. Root coverage in smokers with acellular dermal matrix graft and enamel matrix derivative: a 12-month randomized clinical trial. Int J Periodontics Restorative Dent 2016; 36: 525–531. [DOI] [PubMed] [Google Scholar]
- 129.Mahn DH. A double-layer technique using an acellular dermal matrix for the treatment of Miller Class I and II gingival recession defects: 1-year results of 50 consecutive cases. Int J Periodontics Restorative Dent 2015; 35: 257–262. [DOI] [PubMed] [Google Scholar]
- 130.Ozenci I, Ipci SD, Cakar Get al. et al. Tunnel technique versus coronally advanced flap with acellular dermal matrix graft in the treatment of multiple gingival recessions. J Clin Periodontol 2015; 42: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 131.Wang HL, Suarez-Lopez Del Amo F, Layher Met al. et al. Comparison of freeze-dried and solvent-dehydrated acellular dermal matrix for root coverage: a randomized controlled trial. Int J Periodontics Restorative Dent 2015; 35: 811–817. [DOI] [PubMed] [Google Scholar]
- 132.de Resende DRB, Greghi SLA, Siqueira AFet al. et al. Acellular dermal matrix allograft versus free gingival graft: a histological evaluation and split-mouth randomized clinical trial. Clin Oral Investig 2019; 23: 539–550. [DOI] [PubMed] [Google Scholar]
- 133.Fickl S, Jockel-Schneider Y, Lincke Tet al. et al. Porcine dermal matrix for covering of recession type defects: a case series. Quintessence Int 2013; 44: 243–246. [DOI] [PubMed] [Google Scholar]
- 134.Breault LG, Brentson RC, Fowler EBet al. et al. Repair of a gingival fenestration using an acellular dermal matrix allograft. US Army Med Dep J 2016: 81–84. [PubMed] [Google Scholar]
- 135.Blythe JN, Koraitim M, Arcuri Fet al. et al. Novel approach in the treatment of a persistent iatrogenic parotid fistula using AlloDerm(R)–an allogenic acellular dermal matrix. Br J Oral Maxillofac Surg 2016; 54: 109–110. [DOI] [PubMed] [Google Scholar]
- 136.Li XY, Wu J, Cao Jet al. et al. [Clinical analysis of acellular dermal matrix and acellular bone matrix in oro-antral fistula repair]. Hua Xi Kou Qiang Yi Xue Za Zhi 2018; 36: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clavijo-Alvarez JA, Vecchione L, DeCesare G, et al. Autologous bone grafting with adjunctive use of acellular dermal matrix for alveolar cleft defects: early outcomes. Cleft Palate Craniofac J 2010; 47: 116–121. [DOI] [PubMed] [Google Scholar]
- 138.Fernandes PG, Muglia VA, Reino DM, et al. Socket preservation therapy with acellular dermal matrix and mineralized bone allograft after tooth extraction in humans: a clinical and histomorphometric study. Int J Periodontics Restorative Dent 2016; 36: e16–e25. [DOI] [PubMed] [Google Scholar]
- 139.Fischer KR, Testori T, Wachtel Het al. et al. Soft tissue augmentation applying a collagenated porcine dermal matrix during second stage surgery: a prospective multicenter case series. Clin Implant Dent Relat Res 2019; 21: 923–930. [DOI] [PubMed] [Google Scholar]
- 140.Momen-Heravi F, Peters SM, Garfinkle Let al. et al. Acellular dermal matrix as a barrier for guided bone regeneration of dehiscence defects around dental implants: a clinical and histological report. Implant Dent 2018; 27: 521–524. [DOI] [PubMed] [Google Scholar]
- 141.Papi P, Pompa G. The use of a novel porcine derived acellular dermal matrix (Mucoderm) in peri-implant soft tissue augmentation: preliminary results of a prospective pilot cohort study. Biomed Res Int 2018; 2018: 6406051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mericli AF, Chen K, Murariu Det al. et al. Calvarial regeneration with use of acellular dermal matrix in aplasia cutis congenita. J Craniofac Surg 2015; 26: 1960–1962. [DOI] [PubMed] [Google Scholar]
- 143.Park ES, Park JH, Shin HSet al. et al. Clinical application of acellular dermal matrix in the treatment of aplasia cutis congenita on scalp. J Craniofac Surg 2017; 28: e788–e789. [DOI] [PubMed] [Google Scholar]
- 144.Ozkan A, Topkara A, Ozcan RHet al. et al. The use of the PlasmaBlade and acellular dermal matrix in rhinophyma surgery: a case report. J Cutan Med Surg 2016; 20: 155–158. [DOI] [PubMed] [Google Scholar]
- 145.Sun J, Liu X, Zhang Y, et al. Bovine acellular dermal matrix for levator lengthening in thyroid-related upper-eyelid retraction. Med Sci Monit 2018; 24: 2728–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Susarla SM, MacIsaac ZM, Swanson Eet al. et al. Acellular dermal matrix as an adjunct material in Cleft Le Fort I Osteotomies. J Craniofac Surg 2017; 28: 225–226. [DOI] [PubMed] [Google Scholar]
- 147.Wang W, Fan J-c, Sun C-J, et al. Systematic evaluation on the use of acellular dermis matrix graft in prevention frey syndrome after parotid neoplasm surgery. J Craniofac Surg 2013; 24: 1526–1529. [DOI] [PubMed] [Google Scholar]
- 148.Yang CE, Kim SJ, Kim JHet al. et al. Usefulness of cross-linked human acellular dermal matrix as an implant for dorsal augmentation in rhinoplasty. Aesthetic Plast Surg 2018; 42: 288–294. [DOI] [PubMed] [Google Scholar]
- 149.Jeong WH, Roh TS, Kim YS, et al. Acceleration of osteogenesis by platelet-rich plasma with acellular dermal matrix in a calvarial defect model. Childs Nerv Syst 2016; 32: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 150.Luo X, Lin C, Wang X, et al. Acellular dermal matrix combined with autologous skin grafts for closure of chronic wounds after reconstruction of skull defects with titanium mesh. J Neurol Surg A Cent Eur Neurosurg 2016; 77: 297–299. [DOI] [PubMed] [Google Scholar]
- 151.Renard Y, de Mestier L, Henriques J, et al. Absorbable polyglactin vs. non-cross-linked porcine biological mesh for the surgical treatment of infected incisional hernia. J Gastrointest Surg 2020; 24: 435–443. [DOI] [PubMed] [Google Scholar]
- 152.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 2010; 148: 544–558. [DOI] [PubMed] [Google Scholar]
- 153.Gowda AU, McNichols CH, Asokan I, et al. Porcine acellular dermal matrix for hernia repair in transplant patients. Ann Plast Surg 2016; 77: 674–677. [DOI] [PubMed] [Google Scholar]
- 154.Garvey PB, Giordano SA, Baumann DPet al. et al. Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg 2017; 224: 341–350. [DOI] [PubMed] [Google Scholar]
- 155.Guerra O, Maclin MM. Non-crosslinked porcine-derived acellular dermal matrix for the management of complex ventral abdominal wall hernias: a report of 45 cases. Hernia 2014; 18: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bochicchio GV, De Castro GP, Bochicchio KMet al. et al. Comparison study of acellular dermal matrices in complicated hernia surgery. J Am Coll Surg 2013; 217: 606–613. [DOI] [PubMed] [Google Scholar]
- 157.Kissane NA, Itani KM. A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: what have we learned? Plast Reconstr Surg 2012; 130: 194s–202s. [DOI] [PubMed] [Google Scholar]
- 158.Campbell KT, Burns NK, Ensor Jet al. et al. Metrics of cellular and vascular infiltration of human acellular dermal matrix in ventral hernia repairs. Plast Reconstr Surg 2012; 129: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Giordano S, Schaverien M, Garvey PBet al. et al. Advanced age does not affect abdominal wall reconstruction outcomes using acellular dermal matrix: a comparative study using propensity score analysis. Am J Surg 2017; 213: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 160.Guerra O. Noncrosslinked porcine-derived acellular dermal matrix for single-stage complex abdominal wall herniorrhaphy after removal of infected synthetic mesh: a retrospective review. Am Surg 2014; 80: 489–495. [PubMed] [Google Scholar]
- 161.Clemens MW, Selber JC, Liu J, et al. Bovine versus porcine acellular dermal matrix for complex abdominal wall reconstruction. Plast Reconstr Surg 2013; 131: 71–79. [DOI] [PubMed] [Google Scholar]
- 162.Bonitz RP, Hanna MK. Use of human acellular dermal matrix during classic bladder exstrophy repair. J Pediatr Urol 2016; 12: 114.e1–114.e5. [DOI] [PubMed] [Google Scholar]
- 163.Alharthi M, Liberman D, Richard C. Using acellular dermal matrix during transanal repair of rectourethral fistula: surgical technique. J Visc Surg 2018; 155: 223–227. [DOI] [PubMed] [Google Scholar]
- 164.Vedak P, St John J, Watson A, et al. Delayed type IV hypersensitivity reaction to porcine acellular dermal matrix masquerading as infection resulting in multiple debridements. Hernia 2017; 21: 489–492. [DOI] [PubMed] [Google Scholar]
- 165.Hui A, Hong P, Bezuhly M. Use of acellular dermal matrices in laryngotracheal and pharyngeal reconstruction: systematic review. J Laryngol Otol 2017; 131: 585–592. [DOI] [PubMed] [Google Scholar]
- 166.He J, Tian Y, Wu P, et al. Heterogeneous bovine acellular dermal matrix for mucosal repair in reconstructive surgery for laryngeal and hypopharyngeal carcinoma. Oncol Res Treat 2015; 38: 282–284. [DOI] [PubMed] [Google Scholar]
- 167.Li P, Li S, Tang Q, et al. Reconstruction of human oncological tracheal defects with xenogenic acellular dermal matrix. Auris Nasus Larynx 2017; 44: 237–240. [DOI] [PubMed] [Google Scholar]
- 168.Yin D, Tang Q, Wang S, et al. Xenogeneic acellular dermal matrix in combination with pectoralis major myocutaneous flap reconstructs hypopharynx and cervical esophagus. Eur Arch Otorhinolaryngol 2015; 272: 3457–3461. [DOI] [PubMed] [Google Scholar]
- 169.Yang L, Yang F, Li Wet al. et al. [Experience of applying acellular dermal matrix in the head and neck tumor surgery]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2015; 50: 579–582. [PubMed] [Google Scholar]
- 170.Zhang B, Li J, Sarma Det al. et al. The use of heterogeneous acellular dermal matrix in the closure of hard palatal fistula. Int J Pediatr Otorhinolaryngol 2014; 78: 75–78. [DOI] [PubMed] [Google Scholar]
- 171.Lee JM, Seo YJ, Shim DBet al. et al. Surgical outcomes of tympanoplasty using a sterile acellular dermal allograft: a prospective randomised controlled study. Acta Otorhinolaryngol Ital 2018; 38: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Du M, Zhu T, Duan X, et al. Acellular dermal matrix loading with bFGF achieves similar acceleration of bone regeneration to BMP-2 via differential effects on recruitment, proliferation and sustained osteodifferentiation of mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl 2017; 70: 62–70. [DOI] [PubMed] [Google Scholar]
- 173.Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 2009; 26: 507–523. [DOI] [PubMed] [Google Scholar]
- 174.Deeken CR, Eliason BJ, Pichert MDet al. et al. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann Surg 2012; 255: 595–604. [DOI] [PubMed] [Google Scholar]
- 175.Urciuolo F, Casale C, Imparato Get al. et al. Bioengineered skin substitutes: the role of extracellular matrix and vascularization in the healing of deep wounds. J Clin Med 2019; 8: 2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adelman DM, Selber JC, Butler CE. Bovine versus porcine acellular dermal matrix: a comparison of mechanical properties. Plast Reconstr Surg Glob Open 2014; 2: e155–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nilsen TJ, Dasgupta A, Huang YCet al. et al. Do processing methods make a difference in acellular dermal matrix properties? Aesthet Surg J 2016; 36(Suppl. 2): S7–S22. [DOI] [PubMed] [Google Scholar]
- 178.Klein GM, Nasser AE, Phillips BT, et al. Is sterile better than aseptic? Comparing the microbiology of acellular dermal matrices. Plast Reconstr Surg Glob Open 2016; 4: e761.– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eriksson A, Burcharth J, Rosenberg J. Animal derived products may conflict with religious patients’ beliefs. BMC Med Ethics 2013; 14: 48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jenkins ED, Yip M, Melman Let al. et al. Informed consent: cultural and religious issues associated with the use of allogeneic and xenogeneic mesh products. J Am Coll Surg 2010; 210: 402–410. [DOI] [PubMed] [Google Scholar]