Abstract
Introduction:
Cesarean section is one of the most common obstetrical interventions that has been performed at an increasing rate globally, due to both medical and non-medical reasons. This study aims to develop a prediction tool for pregnant women potentially needing c-section, such that necessary preparations from the mothers, families, and health providers can be made.
Methods:
A total of 603 pregnant women were recruited in the first phase of c-section prediction tool development. The association between the maternal and fetal factors on the risk of c-section were analyzed, followed by a stepwise multivariate regression analysis. In the next phase, 61 pregnant women were enrolled for external validation. Discrimination was assessed using area under the curve. The calibration plot was then made and assessed using the Hosmer–Lemeshow test.
Results:
There were 251 (41.6%) cases of vaginal delivery and 352 (58.4%) of c-section assessed. Multivariate analysis showed that gestational age < 37 wg (OR: 1.66, 95% CI: 1.10–2.51), pre-pregnancy body mass index (underweight) (OR: 0.40, 95% CI: 0.22–0.76), no history of vaginal delivery (OR: 2.66, 95% CI: 1.76–4.02), history of uterine surgery (OR: 8.34, 95% CI: 4.54–15.30), obstetrical complications (OR: 5.61, 95% CI: 3.53–8.90), birthweight ⩾ 3500 g (OR: 4.28, 95% CI: 2.16–8.47), and non-cephalic presentation (OR: 2.74, 95% CI: 1.53–4.89) were independently associated with c-section delivery. Those parameters were included in a 7-item scoring tool, with consecutive predictive scores of 1,–1,2,3,3,2,2,1. The area under the curve result was 0.813 (95% CI: 0.779–0.847), indicating a good predictive ability. The external validation showed AUC: 0.806, 95% CI: 0.694–0.917, Hosmer–Lemeshow test p = 0.666 and calibration plot coefficient of r = 0.939.
Conclusion:
A total of 7 maternal-fetal factors were found to be strongly associated with c-section delivery, including gestational age < 37, maternal underweight body mass index, previous uterine surgery, obstetrical complications, birthweight ⩾ 3500, history of vaginal delivery, and non-cephalic presentation. Using these factors, a prediction tool was developed and validated with good quality.
Keywords: cesarean section, maternal-fetal characteristics, mode of delivery, prediction, scoring system
Introduction
Cesarean section, or more commonly known as c-section, has become the main alternative delivery method in pregnancy with life-threatening complications. 1 The decision to perform c-section should be made under conditions where vaginal delivery is impossible, or poses more risks, and is therefore taken only with certain maternal or fetal indications. 2
Based on the Statement on Cesarean Section Rates by the WHO, 3 a systematic review and ecological analysis have found that a population-based c-section rates above 10% does not correlate with reductions in maternal and neonatal mortality, thus is considered non-optimal, considering the adverse complications in future pregnancies.4,5 Nevertheless, in the last decade, WHO found that the rates of c-section has dramatically increased from 7% in 1990 to more than 1 in 5 childbirths (21%) in 2021, and is projected to reach 29% in 2030, globally. If the trend continues, Eastern Asia and Latin America are projected to reach the highest rates at 63% and 54% respectively. 6
Although c-section can be an imperative, lifesaving surgery in certain cases, one concerning reason behind the trend is the increasing c-section by maternal request, without any medical indications. 7 A systematic review by Begum et al. 8 in 2020 found that c-section by maternal request makes up 0.2%–42% of all childbirths, and 0.9%–60% of all c-sections, with 11-fold increase in c-section by maternal request in upper middle-income countries compared with either high or lower-middle income countries.
In Indonesia, the rate of c-section mimics the global trend, as it increased from 9.8% in 2013 to 17.6% in 2018, with the highest rate found in Jakarta (31.1%). 9 In recent years, the rate of c-section in Cipto Mangunkusumo National Referral Hospital alone reached almost 50%. Despite advanced surgical techniques, c-section poses short-term and long-term complications. Several risks are associated with c-section, including miscarriage and stillbirth, placenta previa, and placenta accreta in the following pregnancy, as well as development of childhood asthma. 10 In Cipto Mangunkusumo hospital, the cases of placenta accreta was found to be at 76 out of 2660 c-section deliveries (2.86%) in 2019. 11 A multi-country survey has also found that c-section performed without medical indications increases risks for severe maternal outcome. 12
Furthermore, in Indonesia, the high maternal morbidity and mortality rates were highly influenced by the poor infrastructure of the healthcare system in remote areas as well as poor awareness of the pregnant mothers, resulting in delayed referrals. 13 In developing countries like Indonesia, poor awareness of the early signs of obstetrical complications also contributes to late consultation to obstetricians. This could eventually delay the c-section, resulting in life-threatening conditions. Therefore, by educating pregnant women with regards to their risks of c-section, maternal and fetal outcomes could potentially be improved.
With varying trends in c-section and its medical and non-medical reasons, the medical risk factors behind today’s trend of c-section becomes unclear. Numbers of scoring system related maternal and fetal characteristics to predict the risk of c-sections have been developed for obstetricians in order to ensure the procedure was done only if indicated.14,15 However, the existing scoring systems were built for obstetrician, hence are too difficult for pregnant women in general population to comprehend, thus many pregnant women remain unaware of their obstetrical condition. Therefore, this study aims to assess the maternal and fetal risk factors of c-section and develop a prediction tool for mothers potentially needing c-section. Hence, necessary preparations from the mothers and families, especially in third trimester, and health providers can be made.
Methods
Study setting
The study was carried out at Cipto Mangunkusumo Hospital, Fatmawati Hospital, and Tangerang General hospital, located in Jakarta-Tangerang, Indonesia. All of the hospitals are tertiary referral hospital, which receive and treat referred cases from primary and secondary healthcare facilities. Moreover, Cipto Mangunkusumo Hospital is a national referral hospital in Indonesia, handling patients not only from Jakarta, Indonesia, but also other referred patients from other provinces in Indonesia, mainly the ones with adverse pregnancy complications. These hospitals are also teaching hospitals, where examination and procedures were performed by residents of the Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Indonesia, under close supervision by highly qualified Obstetrics and Gynecology subspecialists and consultants.
Study design and sample recruitment
This was a retrospective cohort study using data from the hospital’s medical records. The first phase of the study was development of a scoring system, which took place in Cipto Mangunkusumo Hospital and Fatmawati Hospital. The minimum sample size calculated was 273; on the basis incidence of c-section rate in Jakarta, 2018 (31.1%), 9 along with 95% confidence interval (CI), and 80% power. 16 The study was restricted to women delivered in those two hospitals from January to April 2019, with gestational age of 22–42 weeks. A total of 753 cases met the criteria. We excluded deliveries with babies weighing 500 g or less (n = 13), cases of intrauterine fetal death at less than 28 weeks (n = 28), and cases with incomplete antenatal data (n = 109). After exclusions, a total of 603 cases complied our eligibility criteria and were put to analysis.
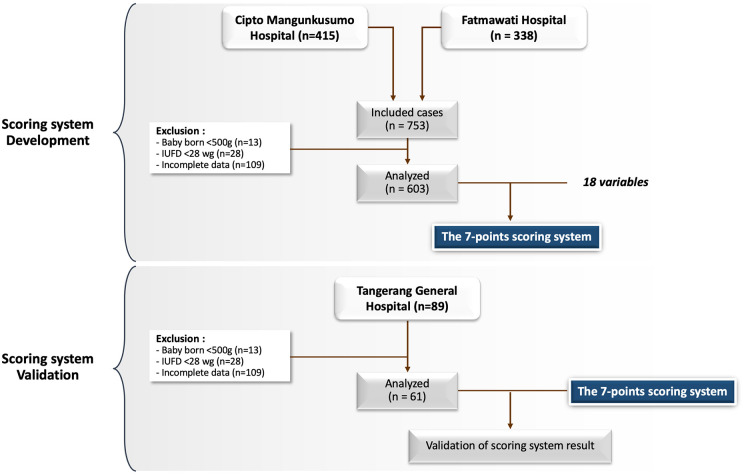
The next phase was a validation of the scoring system, which took place in Tangerang General Hospital. The minimum sample size was also calculated with 95% CI and 80% power. 16 With the effect size 0.26 (low-risk and high-risk difference from the first part of study), the minimum sample requirement for the external validation was 46. Applying similar inclusion and exclusion criteria, the study was restricted to women delivered in the hospital from July to August 2021, with gestational age of 22–42 weeks. Within the period of study, a total 89 cases met the criteria. We excluded deliveries with babies weighing 500 g or less (n = 5), cases of intrauterine fetal death at less than 28 weeks (n = 4), and cases with incomplete antenatal data (n = 19). We continued the external validation using 61 samples. Figure 1 shows the workflow diagram.
Figure 1.
The workflow diagram.
This study has been approved by The Ethical Committee for Research in Humans from The Faculty of Medicine, Universitas Indonesia (KET-1491/UN2.F1/ETIK/PPM.00.02/2020). Since this was a retrospective study, we extracted only clinically relevant information from medical records with ensuring patient’s privacy protection. This study also did not affect patients treatment and health, thus written informed consent from all participants was waived by the Ethical Committee.
Outcome measures
All data extracted from medical records were classified into demographic characteristics, pregnancy history, current pregnancy characteristics, and neonatal features. Demographic characteristics; maternal age, gestational age, body height, body weight before pregnancy and during last trimester, body mass index (BMI) before pregnancy and during last trimester which was categorized based on Asia-Pacific BMI criteria including underweight (< 18.5), normal (18.5–22.9), overweight (23–24.9), and obese (⩾ 25). Pregnancy history; gravidity, parity, previous uterine surgery (c-section or myomectomy), history of vaginal delivery. Current pregnancy characteristics; antenatal care (ANC) visit, types of pregnancy (single or twin), pregnancy program, presence of chronic diseases (diabetes, hypertension, heart disease, kidney disease, autoimmune diseases, infections including syphilis, human immunodeficiency virus (HIV), or hepatitis B, and cancer), obstetrical complications [hypertensive disorder in pregnancy, 17 gestational diabetes mellitus (severe hyperglycaemia in pregnancy), 18 intrauterine growth restriction (i.e. estimated fetal weight or abdominal circumference below the 10th percentile, abnormal doppler/amniotic fluid index/biophysical profile), 19 placenta previa, and placental abruption], and the presence of premature rupture of membrane (later than 6 or 12 h). Neonatal features: birth weight, and fetal presentation at the last trimester.
All women were classified into two groups: vaginal delivery and c-section. C-section included both emergency and elective c-section. Vaginal delivery included spontaneous delivery, with or without induction, vacuum extraction, or forceps delivery. The primary outcome of this study was to identify the possible independent factors associated with c-section, which are comprehensible for both non-health care workers and health-care providers.
Statistical analysis
Data analysis was performed using SPSS statistics, version 25 (SPSS Inc, Chicago, Illinois, USA). Mean and standard deviation were used to describe continuous variables with normal distribution, while median and interquartile range for non-normal distribution data. Comparison of proportions was performed by Pearson’s chi-square (χ 2 ) for categorial variables. Variables with p-value < 0.250 in bivariate analysis were put into logistic regression analysis. Each odds ratio (OR) with multivariable log-binomial regression models was estimated with 95% confidence intervals (CIs). A p-value of less than 5% was considered statistically significant.
A predictive scoring tool was developed through stepwise calculations: (1) dividing each prognostic factor’s coefficient B by its standard error (coefficient B/SE); (2) choosing the lowest B/SE value as a reference (3) dividing each B/SE value by the reference value; and (4) picking the rounded number nearest to the result from step 3. In order to evaluate the performance of our scoring system, we analyzed calibration score using the Hosmer–Lemeshow test and discrimination score using receiver operating characteristic (ROC) and area under receiver operating characteristic curve (AUC). These were followed by internal validation using repeated backward logistic regression model for each of predictors with 1000 bootstrap resampling. Finally, the result of external validation of the scoring system were evaluated using repeated discrimination and calibration test using the same method.
Results
Characteristics of study population
There were 603 cases assessed in this study, including 251 (41.6%) cases with vaginal delivery and 352 (58.4%) with c-section delivery. The characteristics of study population is shown in Table 1. Bivariate and multivariate analysis were performed to evaluate the significances of variables associated with mode of delivery. Among the 18 variables which were analyzed for their association with the risk of c-section, 11 variables found to be significant (Table 2). These 11 variables were then included in the logistic regression analysis, resulting in 7 variables found to be significantly associated with c-section delivery (p < 0.05). The odds ratio of the 11 variables are showed in Table 3.
Table 1.
Baseline characteristics of study population.
| Variables | Study population | |
|---|---|---|
| Median (IQR) | n (%) | |
| Maternal age (years) | 29 (25–35) | |
| Gestational age (weeks) | 36 (33–38) | |
| Gravidity | ||
| Primigravidity | 228 (37.8) | |
| Multigravidas | 375 (62.2) | |
| Parity | ||
| Nulliparous | 244 (40.5) | |
| Multiparous | 239 (59.5) | |
| Pregnancy interval | 2 (0–6) | |
| Height (cm) | 155 (152–159) | |
| Weight before pregnancy (g) | 55 (49–64) | |
| Weight at last trimester (g) | 65 (58–75) | |
| BMI before pregnancy | 23 (20.3–26.7) | |
| BMI at last trimester | 27.30 (24.42–31.22) | |
| Presence of chronic disease | ||
| Yes | 125 (20.7) | |
| No | 487 (79.3) | |
| History of vaginal delivery | ||
| Yes | 240 (39.8) | |
| No | 363 (60.2) | |
| History of uterine surgery | ||
| Yes | 146 (24.2) | |
| No | 457 (75.8) | |
| Systolic BP | 120 (110–140) | |
| Diastolic BP | 80 (70–90) | |
| Pregnancy program | ||
| Yes | 1 (0.2) | |
| No | 602 (99.8) | |
| Types of pregnancy | ||
| Single | 569 (94.4) | |
| Twin | 34 (5.6) | |
| Premature rupture of membranes | ||
| No | 428 (71.1) | |
| Yes | 174 (28.9) | |
| Obstetrical complications | ||
| No | 396 (65.7) | |
| Yes | 207 (34.3) | |
| Preeclampsia/eclampsia | 144 | |
| Gestational diabetes mellitus | 11 | |
| IUGR | 75 | |
| Placenta previa/Placental abruption | 40 | |
| Birth weight (gram) | 2550 (1900–3050) | |
| Cephalic presentation | ||
| No | 95 (15.7) | |
| Yes | 508 (84.3) | |
IQR: interquartile range; BMI: body mass index; BP: blood pressure.
Table 2.
Bivariate analysis of factors associated with c-section.
| Variables | SC | PV | OR (95% CI) | p value |
|---|---|---|---|---|
| n (%) | ||||
| Maternal age | ||||
| ⩾ 35 | 98 (27.8) | 53 (21.1) | 1.44 (0.98–2.11) | 0.074* |
| < 35 | 254 (72.2) | 198 (78.9) | ||
| Gestational age | ||||
| < 37 | 197 (56.0) | 116 (46.2) | 1.48 (1.07–2.05) | 0.023* |
| ⩾ 37 | 155 (44.0) | 135 (53.8) | ||
| Gravidity | ||||
| Primigravidity | 123 (34.9) | 105 (41.8) | 0.75 (0.53–1.04) | 0.102* |
| Multigravidas | 229 (61.1) | 146 (58.2) | ||
| Body height | ||||
| ⩽ 149 | 36 (10.2) | 21 (8.4) | 1.25 (0.71–2.19) | 0.53 |
| ⩾ 150 | 316 (89.8) | 230 (91.6) | ||
| Pre-pregnancy BMI | ||||
| Underweight | 28 (8.0) | 46 (18.3) | < 0.001* | |
| Normal | 129 (36.6) | 94 (37.5) | ||
| Overweight | 59 (16.8) | 41 (16.3) | ||
| Obese | 136 (38.6) | 70 (27.9) | ||
| Excessive weight gain | ||||
| Yes | 48 (13.6) | 23 (9.2) | 1.56 (0.92–2.69) | 0.121* |
| No | 304 (86.4) | 228 (90.8) | ||
| ANC > 4 times | ||||
| Yes | 330 (93.8) | 233 (92.8) | 1.16 (0.61–2.21) | 0.778 |
| No | 22 (6.3) | 18 (7.2) | ||
| Twin pregnancy | ||||
| Yes | 21 (6.0) | 13 (5.2) | 1.16 (0.57–2.37) | 0.815 |
| No | 331 (94.0) | 238 (94.8) | ||
| Presence chronic disease | ||||
| Yes | 83 (23.6) | 42 (16.7) | 1.53 (1.02–2.32) | 0.052* |
| No | 269 (76.4) | 209 (83.3) | ||
| History of vaginal delivery | ||||
| No | 237 (67.3) | 126 (50.2) | 2.04 (1.46–2.85) | < 0.001* |
| Yes | 115 (32.7) | 125 (49.8) | ||
| Previous uterine surgery | ||||
| Yes | 131 (37.2) | 15 (6.0) | 9.33 (5.30–16.41) | < 0.001* |
| No | 221 (62.8) | 236 (94.0) | ||
| Previous c-section < 19 months | ||||
| Yes | 5 (1.4) | 0 (0.0) | – | 0.079 |
| No | 347 (98.6) | 251 (100) | ||
| Pregnancy program | ||||
| Yes | 1 (0.3) | 0 (0) | – | 1 |
| No | 351 (99.7) | 251 (100) | ||
| Obstetrical complications a | ||||
| Yes | 167 (47.4) | 40 (15.9) | 4.76 (3.20–7.09) | < 0.001* |
| No | 185 (52.6) | 211 (84.1) | ||
| Birth weight (gram) | ||||
| ⩾ 3500 | 50 (14.2) | 18 (7.2) | 2.14 (1.22–3.77) | 0.01* |
| < 3500 | 302 (85.8) | 233 (92.8) | ||
| Cephalic presentation | ||||
| No | 70 (19.9) | 25 (10.0) | 2.24 (1.38–3.66) | 0.001* |
| Yes | 282 (80.1) | 226 (90.0) | ||
| PROM ⩾ 6 h | ||||
| Yes | 87 (24,7) | 66 (26.3) | 0.92 (0.63–1.33) | 0.731 |
| No | 265 (75,3) | 185 (73.7) | ||
| PROM ⩾ 12 h | ||||
| Yes | 65 (18.5) | 49 (19.5) | 0.93 (0.62–1.41) | 0.825 |
| No | 287 (81.5) | 202 (80.5) | ||
OR: odds ratio; CI: confidence interval; BMI: body mass index; ANC: antenatal care.
Hypertension in pregnancy, gestational diabetes mellitus, intrauterine growth restrictions, placenta previa, and placental abruption.
Variables with p < 0.25 were selected for the multivariate analysis.
Table 3.
Odds ratio for independent variables in multivariate logistic regression analysis.
| Variables | OR (95% CI) | p |
|---|---|---|
| Maternal age ⩾ 35 y.o. | 1.00 (0.59–1.70) | 0.994 |
| Gestational age < 37 wg | 1.67 (1.11–2.54) | 0.015 |
| Primigravidity | 1.31 (0.63–2.74) | 0.456 |
| Pre-pregnancy BMI (underweight) | 0.42 (0.22–0.78) | 0.007 |
| Pre-pregnancy BMI (overweight) | 0.87 (0.49–1.55) | 0.641 |
| Pre-pregnancy BMI (obese) | 0.93 (0.59–1.70) | 0.781 |
| Excessive weight gain | 1.09 (0.54–2.20) | 0.798 |
| Presence chronic disease | 1.18 (0.72–1.94) | 0.512 |
| No history of vaginal delivery | 2.14 (1.06–4.34) | 0.034 |
| Previous uterine surgery | 9.75 (4.64–20.51) | < 0.001 |
| Obstetrical complications | 5.67 (3.54–9.09) | < 0.001 |
| Birth weight ⩾ 3500 g | 4.25 (2.13–8.49) | < 0.001 |
| Non-cephalic presentation | 2.75 (1.53–4.95) | 0.001 |
OR: odds ratio; CI: confidence interval; wg: weeks of gestation; BMI: body mass index.
Development of c-section scoring system
There were seven variables identified in development of scoring system for the final model (Table 4), including gestational age < 37 weeks, underweight pre-pregnancy BMI, previous uterine surgery, obstetrical complications, birth weight ⩾ 3500 g, no history of vaginal delivery, and non-cephalic presentation. Following that, a 7-item scoring system was developed (gestational age < 37 weeks = 1, underweight pre-pregnancy BMI = –1, non-cephalic presentation = 1, no history of vaginal delivery = 2, birthweight ⩾ 3500 g = 2, previous uterine surgery = 3, and obstetrical complications = 3), with a total score 11. The full scoring system is shown in Table 5.
Table 4.
Derivation of 7-point scoring system to the risk of c-section from stepwise multivariate analysis.
| Variable | Coefficient B | SE | B/SE | OR (95% CI) | Score |
|---|---|---|---|---|---|
| Gestational age < 37 wg | 0.508 | 0.211 | 2.408 | 1.66 (1.10–2.51) | 1 |
| Pre-pregnancy BMI (underweight) | –0.9 | 0.322 | –2.795 | 0.40 (0.22–0.76) | –1 |
| No history of vaginal delivery | 0.978 | 0.211 | 4.635 | 2.66 (1.76–4.02) | 2 |
| History of uterine surgery | 2.121 | 0.310 | 6.842 | 8.34 (4.54–15.30) | 3 |
| Obstetrical complications | 1.724 | 0.236 | 7.305 | 5.61 (3.53–8.90) | 3 |
| Birthweight ⩾ 3500 g | 1.453 | 0.349 | 4.163 | 4.28 (2.16–8.47) | 2 |
| Non-cephalic presentation | 1.007 | 0.296 | 3.402 | 2.74 (1.53–4.89) | 1 |
SE: standard error; OR: odds ratio; CI: confidence interval; wg: weeks of gestation; BMI: body mass index.
Table 5.
Sensitivity, specificity, and probability analysis of the scoring system.
| Category | Total score | Sensitivity (%) | Specificity (%) | Probability (%) |
|---|---|---|---|---|
| Low risk | –1 | 100 | 0 | 8.91 |
| 0 | 100 | 3.6 | 15.29 | |
| 1 | 97.7 | 13.9 | 24.98 | |
| 2 | 94.0 | 37.8 | 38.06 | |
| High risk | 3 | 81.3 | 62.9 | 53.13 |
| 4 | 69.0 | 80.9 | 67.66 | |
| 5 | 51.7 | 90.8 | 79.43 | |
| 6 | 37.5 | 95.2 | 87.69 | |
| 7 | 22.4 | 98.4 | 92.93 | |
| 8 | 15.9 | 98.8 | 96.04 | |
| 9 | 9.4 | 99.2 | 97.82 |
Furthermore, the area of AUC was 0.813 (95% CI: 0.779–0.847). (Figure 2). This was considered good as sensitivity of 81% were shown when the score ⩾3 was categorized as high risk for c-section, with a probability score of 53.13% (Table 5). Calibration using Hosmer–Lemeshow showed a good calibration score with p = 0.555 (p > 0.05). An internal validation using 1000× boostraping also showed the same p value (p = 0.555). As the p values of before and after bootstrapping are the same, the scoring system can likely be expected to have similar results in a bigger population.
Figure 2.
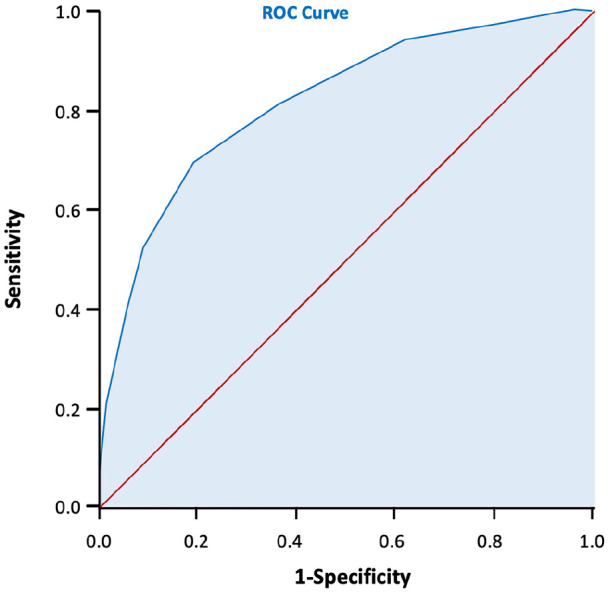
The ROC curve of the scoring system development the AUC = 0.813, 95% CI: 0.779–0.847.
External validation
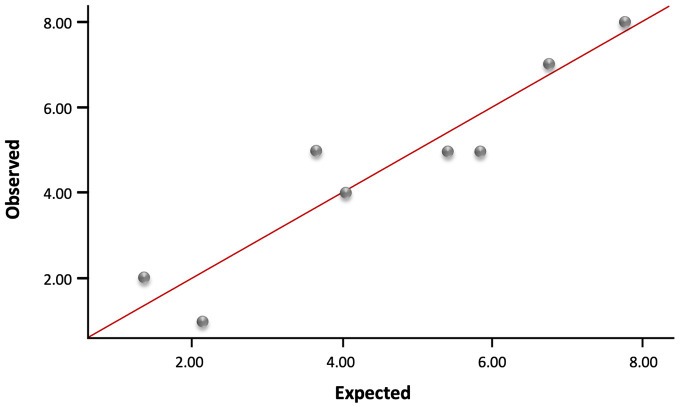
We then enrolled 61 subjects for external validation of the scoring tool. Among them, 24 subjects underwent vaginal delivery and 37 subjects underwent c-section delivery. The performance of c-section risk scoring tool was assessed for calibration and discrimination results. As the calibration plot showed a coefficient of r = 0.939 (Figure 3), and the Hosmer–Lemeshow test showed p = 0.624, the scoring system was considered to have a good calibration. In Figure 4, The AUC was 0.806, with 95% CI (0.694–0.917), showing an excellent discrimination with no big difference from the first part of the study.
Figure 3.
The plot calibration diagram of the scoring system in external validation (r = 0.939).
Figure 4.
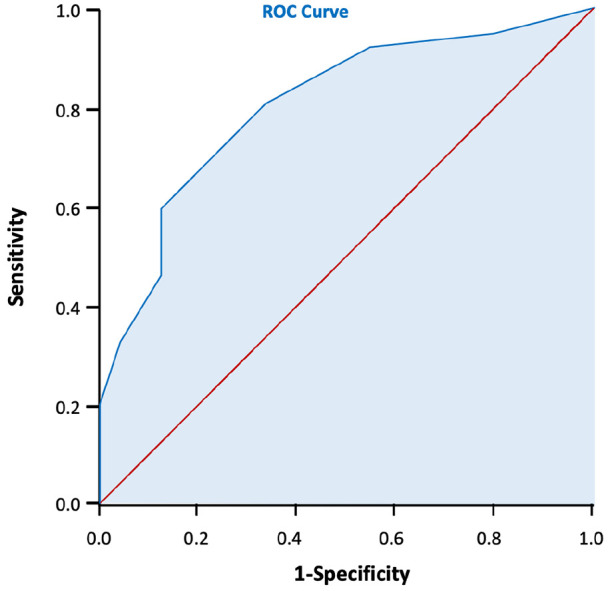
The ROC curve of the scoring system in external validation. The AUC = 0.806, 95% CI: 0.694–0.917.
Discussion
There were seven variables identified to be independently associated with c-section delivery, including gestational age < 37 weeks, maternal underweight pre-pregnancy, previous uterine surgery, obstetrical complications, no history of vaginal delivery, birth weight ⩾ 3500 g, and non-cephalic presentation. From these variables, a scoring system for the risk of c-section has been developed. Since this scoring system was intended for those without medical background, certain variables which were deemed too technical for general population to understand were not included in the analysis. Therefore, variables such as characteristics of amniotic fluid or umbilical cord were not included in the analysis despite their known associations with the risk of c-section based on previous studies. 14
Among the maternal demographical characteristics, maternal underweight BMI pre-pregnancy was the only variable to independently reduce the risk of c-section by OR: 0.40, 95% CI: 0.22–0.76. This finding was consistent with numbers of study from Asia to Europe, where pre-pregnancy underweight BMI were found to lower the risk of cesarean delivery by almost half, with OR: 0.45–0.66.20–22 In contrast, obese patients who were thought to have increased risks for c-section, showed no significant difference in our study. This was surprising as a previous study had suggested that nulliparous obese pregnant women might have increased risk of c-section. 23 A different cut off value for BMI category between the two criteria, Asian-Pacific and WHO criteria, might have influenced the differing results.
In terms of the obstetrical history, the history of uterine surgery and no history of vaginal delivery were found to be highly associated with c-section. Our study found that the history of uterine surgery was one of two variables with the highest scores in predicting c-section. This finding is in agreement with previous studies which found that a history of c-section in previous births increased the risk of c-section in the following pregnancy, with RR 4.30 (4.24–4.36) and OR: 3.5 (3.4–3.6).15,24 This value may also be increased in deliveries with a history of previous c-section with a gestational distance of less than 19 months.25,26 Although a history of previous c-section is not an absolute indication of c-section in subsequent pregnancies, vaginal birth after cesarean (VBAC) might cause numerous adverse effects including uterine rupture, fetal death, or fetal brain damage due to hypoxia. In addition, other previous uterine surgeries such as myomectomy or resection of adenomyosis were also known as a risk factor for c-section, and are considered indications for c-section in subsequent deliveries. 27 Moreover, history of vaginal delivery also affects the risk of c-section. Mothers in their first pregnancy have a greater risk of having a cesarean delivery compared to those who have already had a vaginal delivery before. This is because the pelvic of multiparous women with previous vaginal delivery was considered to be more flexible and easier to undergo vaginal delivery in the following pregnancies.15,23,28
Furthermore, obstetrical complications are also well-established major risk factors of c-section procedure. In this study, we included preeclampsia/eclampsia, gestational diabetes mellitus, IUGR, placenta previa, and placental abruption, since those are the most common pregnancy problems in Indonesia. 9 Previous studies have suggested that those complications of pregnancy had relative risk of around 1.45–1.75 for c-section.14,15,24 Our study found that preterm birth (gestational age < 37 wg) increased the risk of c-section. This was consistent with another previous study which showed that birth at < 37 wg increased the risk of c-section with an OR: 1.45, 95% CI: 1.16–1.72. 29 This findings imply that pregnant women who felt the signs of labor such as uterine contractions, bloody mucous discharge or water breaking before 37 weeks of gestation, were predicted to had increased risk of c-section.
The characteristics of the fetus during pregnancy may also affect the risk of c-section delivery, especially during the third trimester. Our study found that birthweight ⩾ 3500 g had OR of 4.28 (95% CI: 2.16–8.47), thus is given a score of “2” in our prediction tool. This is in agreement with a previous study which found that heavier fetal weight was associated with the increased risk of c-section.14,30 Another previous study also supports our finding, suggesting that a total of 60.7% of pregnancies with a fetal weight more than 3500 were delivered by c-section, compared to 39.3% for a fetus weighing < 3500 g. 28 Although our study used the clinical birthweight rather than the estimated fetal weight, previous studies have found that there was no significant difference between estimated fetal weight and actual birth weight in normal weight population. 31 Significant difference of fetal weight usually found in small for gestational age fetus, with differences up to 200 g. 32 Thus, a clinically-determined estimated fetal weight of ⩾ 3500 g, can still be a predictive factor for an increased risk of c-section. In addition, our study also found that non-cephalic presentation is associated with increased risk of c-section. This was not surprising, as numbers of studies have also found its association with increased risk of c-section. A previous study found that the incidence of c-section in non-cephalic presentation was 93.3% (p < 0.001) compared to head presentation, the incidence of which is 37.3%. 28 Nevertheless, studies have suggested that non-cephalic presentation was best diagnosed at 36 weeks of gestational age. 33 Therefore, pregnant women who uses our scoring tool with diagnosed fetal presentation before 36 weeks of gestation, is recommended to repeat the examination in the subsequent weeks of pregnancy. Moreover, interestingly, in our first part of study, we also found 37.18 per 1000 rate of early fetal death. This high number was due to the fact that our study was conducted in tertiary and national referral hospitals, thus the number of cases of adverse pregnancy complications was higher than the national data. 9
To the best of our knowledge, this is the first study to propose a scoring system in the risk of c-section for both non-medical and medical personnel, with variables which were considered simple and easy to evaluate. The internal and external validations have reflected satisfactory calibration and discrimination values of the scoring system. Nevertheless, there were certain limitations of the study. Pregnant women that could confidently use this scoring were the ones with late trimester, as most of variables could only be evaluated during the last trimester. Also, this was a retrospective study, thus the conclusions were limited by the results of this present study. Further studies should explore the application of this predictive scoring tool in a wider range of population with a prospective cohort design.
Conclusion
There were seven independent factors found to be highly associated with c-section delivery, including gestational age < 37, underweight pre-pregnancy BMI, previous uterine surgery, no history of vaginal delivery, obstetrical complications, birthweight ⩾ 3500, and non-cephalic presentation. A predictive scoring tool has been developed and validated with a good quality. Pregnant women were expected to use this scoring tool as a self-administered questionnaire so they are able to self-assess their risks of c-section. High risk of c-section results would encourage mothers, families, and healthcare professionals to arrange for early consultations with obstetricians and make better preparations for the mothers to deliver at the hospitals.
Acknowledgments
We thank Danone Specialized Nutrition Indonesia for supporting this research, as they will further create an online application for the tools.
Footnotes
Author contributions: R.I. and N.W. designed the study. R.H. and A.W.L. conducted the research, performed the analysis, interpretation of data, and wrote the manuscript. R.I. validated the data, revised the paper, and had primary responsibility for the final content. All authors agreed to the published version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rima Irwinda  https://orcid.org/0000-0002-6260-6273
https://orcid.org/0000-0002-6260-6273
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Teguete I, Maiga AW, Leppert PC. Maternal and neonatal outcomes of grand multiparas over two decades in Mali. Acta Obstet Gynecol Scand 2012; 91(5): 580–586. [DOI] [PubMed] [Google Scholar]
- 2. Gedefaw G, Demis A, Alemnew B, et al. Prevalence, indications, and outcomes of caesarean section deliveries in Ethiopia: a systematic review and meta-analysis. Patient Saf Surg 2020; 14: 11–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Department of Reproductive Health and Research World Health Organization. WHO statement on caesarean section rates. Geneva: World Health Organization, 2015. [Google Scholar]
- 4. Ye J, Zhang J, Mikolajczyk R, et al. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG 2016; 123(5): 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Betran AP, Torloni MR, Zhang J, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health 2015; 12(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Caesarean section rates continue to rise, amid growing inequalities in access. Geneva: World Health Organization, 2021. [Google Scholar]
- 7. Chien P. Global rising rates of caesarean sections. BJOG 2021; 128(5): 781–782. [DOI] [PubMed] [Google Scholar]
- 8. Begum T, Saif-Ur-Rahman KM, Yaqoot F, et al. Global incidence of caesarean deliveries on maternal request: a systematic review and meta-regression. BJOG 2021; 128(5): 798–806. [DOI] [PubMed] [Google Scholar]
- 9. Kementerian Kesehatan Republik Indonesia. Laporan Nasional Riset Kesehatan Dasar. Jakarta, Indonesia: Kementeri Kesehat RI, 2018, pp. 1–582. [Google Scholar]
- 10. Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med 2018; 15(1): e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Putri RA, Dilmy MAF, Purwosunu Y, et al. Surgical management outcome of placenta accreta spectrum at Cipto Mangunkusumo General Hospital (2017-2019). In: Annual Scientific Meeting of Indonesian Maternal Fetal Medicine Association. Surabaya, 13–8 march 2020. [Google Scholar]
- 12. Souza J, Gülmezoglu A, Lumbiganon P, et al. Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: the 2004-2008 WHO Global Survey on Maternal and Perinatal Health. BMC Med 2010; 8(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahmood MA, Hendarto H, Laksana MAC, et al. Health system and quality of care factors contributing to maternal deaths in East Java, Indonesia. PLoS ONE 2021; 16(2): e0247911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan P, Tang F, Sun G, et al. Prediction of emergency cesarean section by measurable maternal and fetal characteristics. J Investig Med 2020; 68(3): 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossi RM, Requarth E, Warshak CR, et al. Risk calculator to predict cesarean delivery among women undergoing induction of labor. Obstet Gynecol 2020; 135(3): 559–568. [DOI] [PubMed] [Google Scholar]
- 16. Sastroasmoro S, Ismael S. Dasar-dasar Metodologi Penelitian Klinis. Jakarta, Indonesia: Sagung Seto, 2011. [Google Scholar]
- 17. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122(5): 1122–1131. [DOI] [PubMed] [Google Scholar]
- 18. Riddle MC. Standards of medical care in diabetes: response to position statement of the American Diabetes Association. Diabetes Care 2020; 43(1): 476.31744812 [Google Scholar]
- 19. Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 2014; 36(2): 86–98. [DOI] [PubMed] [Google Scholar]
- 20. Teshome AA, Li Q, Garoma W, et al. Gestational diabetes mellitus, pre-pregnancy body mass index and gestational weight gain predicts fetal growth and neonatal outcomes. Clin Nutr ESPEN 2021; 42: 307–312. [DOI] [PubMed] [Google Scholar]
- 21. Zhao RF, Zhang WY, Zhou L. Relationship between the risk of emergency cesarean section for nullipara with the prepregnancy body mass index or gestational weight gain. Zhonghua Fu Chan Ke Za Zhi 2017; 52(11): 757–764. [DOI] [PubMed] [Google Scholar]
- 22. Vilar Sánchez Á, Fernández Alba JJ, González Macías MDC, et al. Maternal underweight and perinatal outcomes: a restrospective cohort study. Nutr Hosp 2017; 34(3): 647–653. [DOI] [PubMed] [Google Scholar]
- 23. Papoutsis D, Antonakou A, Gornall A, et al. The SaTH risk-assessment tool for the prediction of emergency cesarean section in women having induction of labor for all indications: a large-cohort based study. Arch Gynecol Obstet 2017; 295(1): 59–66. [DOI] [PubMed] [Google Scholar]
- 24. Janoudi G, Kelly S, Yasseen A, et al. Factors associated with increased rates of caesarean section in women of advanced maternal age. J Obstet Gynaecol Can 2015; 37(6): 517–526. [DOI] [PubMed] [Google Scholar]
- 25. Mohsin FF, Skaeer IH, Mohammed Ali HK. Inter-delivery interval and the success of vaginal birth after caesarean delivery in Babylon maternity and pediatric hospital. Int J Contemp Med Res 2019; 6(3): C16–C21. [Google Scholar]
- 26. Huang WH, Nakashima DK, Rumney PJ, et al. Interdelivery interval and the success of vaginal birth after cesarean delivery. Obstet Gynecol 2002; 99(1): 41–44. [DOI] [PubMed] [Google Scholar]
- 27. Mylonas I, Friese K. Indications for and risks of elective cesarean section. Dtsch Arztebl Int 2015; 112(29–30): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotha S, Kushtagi P, Radhakrishnan K. Prediction of mode of delivery in term pregnancies: development of scoring system. Int J Reprod Contracept Obstet Gynecol 2015; 4(5): 1283–1290. [Google Scholar]
- 29. Cheyney M, Burcher P, Vedam S. A crusade against home birth. Birth 2014; 41(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 30. Froehlich RJ, Sandoval G, Bailit JL, et al. Association of recorded estimated fetal weight and cesarean delivery in attempted vaginal delivery at term. Obstet Gynecol 2016; 128(3): 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eze CU, Abonyi LC, Njoku J, et al. Correlation of ultrasonographic estimated fetal weight with actual birth weight in a tertiary hospital in Lagos, Nigeria. Afr Health Sci 2015; 15(4): 1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stephens K, Al-Memar M, Beattie-Jones S, et al. Comparing the relation between ultrasound-estimated fetal weight and birthweight in cohort of small-for-gestational-age fetuses. Acta Obstet Gynecol Scand 2019; 98(11): 1435–1441. [DOI] [PubMed] [Google Scholar]
- 33. Nicholson JM. Non-cephalic presentation in late pregnancy. BMJ 2006; 333(7568): 562–563. [DOI] [PMC free article] [PubMed] [Google Scholar]