Abstract
Background:
This systematic review and meta-analysis assesses the efficacy of regular, moderate to vigorous physical activity (MVPA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents in randomized controlled trials (RCTs).
Methods:
RCTs including children and adolescents with clinically diagnosed ADHD, implementing regular MVPA, and assessing ADHD core-symptoms on a valid rating scale post-intervention (primary outcome) were included. Outcomes were pooled through random-effects meta-analysis. Prospero registration: CRD42019142166.
Results:
MVPA had a small effect on total ADHD core symptoms (n = 11; g = −0.33; 95% CI [−0.63; −0.02]; p = .037).
Conclusions:
MVPA could serve as an alternative treatment for ADHD. New RCTs are necessary to increase the understanding of the effect regarding frequency, intensity, type of MVPA interventions, and differential effects on age groups.
Keywords: ADHD, exercise, physical activity, attention-deficit/hyperactivity disorder, attention deficit disorder
Introduction
Attention deficit hyperactivity disorder (ADHD) has a prevalence of 5.5% worldwide (Erskine et al., 2017). ADHD is characterized by at least six symptoms of inattention or hyperactivity/impulsivity, which persist for at least 6 months to a degree inconsistent with the developmental level, having a negative impact on social and academic activities (American Psychiatric Association, & DSM Task Force, 2017). Several symptoms need to be present prior to the age of 12 years, in two or more settings, and interfere with social or academic functioning (American Psychiatric Association, & DSM Task Force, 2017). ADHD is associated with a higher risk of comorbid mental and substance use disorders as well as social, academic and occupational impairment (Erskine et al., 2016).
Physical activity guidelines recommend 60 minutes of moderate to vigorous physical activity (MVPA) per day for children and adolescents (American College of Sports Medicine, 2017). Physical activity is defined as bodily movement of any kind, produced by skeletal muscles. This movement must result in energy expenditure (Caspersen et al., 1985). Energy expenditure can be measured in terms of metabolic equivalent of task (MET) with moderate to vigorous physical activity being more than 2.99 MET (Ainsworth et al., 2011; Anastasopoulou et al., 2012; Anastasopoulou et al., 2014). Moderate to vigorous activity can also be operationalized as 64-95% of maximal heart rate or 46% to 90% of maximum rate of oxygen consumption (VO2max; Garber et al., 2011). Only 25% of children and adolescents in Europe and North America meet this recommendation (World Health Organization, 2016). Compared to healthy controls, the probability of meeting these guidelines is reduced by 21% for ADHD patients (Mercurio et al., 2019).
Regular MVPA could be a viable treatment option for ADHD, since previous research supports beneficial effects on assumed underlying symptom dynamics of ADHD: executive functions and a dysfunctional motivational style (Davis et al., 2011; Kim et al., 2011; Ko et al., 2013; Sonuga-Barke, 2002, 2003; Xue et al., 2019). Two recent meta-analyses investigate the effect of physical activity on ADHD symptoms (Cerrillo-Urbina et al., 2015; Zang, 2019). Cerrillo-Urbina et al. (2015) have reported large effects of physical activity compared to control groups on inattentive symptoms, and medium-sized effects on hyperactive symptoms. Zang (2019) did not report a significant difference of ADHD symptoms between physical activity and a control group at post treatment. These results should be regarded with caution since these meta-analyses contain several methodological limitations and both studies rely on a different sample of included trials. Both meta-analyses included non-randomized trials (e.g., McKune et al., 2003; Verret et al., 2012); Verret et al., 2010; Zang, 2019). However, in healthcare trials, randomization is strongly recommended to prevent systematic baseline differences between groups and distortion of effect sizes (Kunz et al., 2007). Effects for subjective outcomes are more likely exaggerated in non-randomized trials compared to randomized controlled trials (RCTs) (Wood et al., 2008). Further, both meta-analyses pooled performance-based measures of executive functions (e.g., Chang et al., 2012 Chou & Huang, 2017; Memarmoghaddam et al., 2016; Pan et al., 2014; Silva et al., 2019; Zang, 2019) with outcomes on a clinical rating scale. To combine outcomes in a meta-analysis, the same underlying construct should be measured (Higgins et al., 2019). Studies show that executive functions are not adequate measurements of clinical symptoms (Schwartz & Verhaeghen, 2008). In addition, Zang (2019) included a study, where participants did not fulfil the full criteria of an ADHD diagnosis (Hoza et al., 2015). Interventions further include acute as well as regular physical activity (e.g., Cerrillo-Urbina et al., 2015: Chang, Liu, et al., 2012; Pontifex, 2013; Tantillo et al., 2002); (e.g., Chang, Liu, et al., 2012; Fritz & O’Connor, 2016; Zang, 2019). Acute physical activity might have immediate benefits on ADHD symptoms such as the induction of exercise-related changes in brain activity (Neudecker et al., 2015) and the fascilitation of attention (Chang et al., 2012) or self-esteem (Dale et al., 2019). Accumulating these acute effects, regular exercise might lead to long-lasting improvements in ADHD symptoms through (psycho-) physiological or psychosocial adaptations such as increased fitness, executive functioning (Muntaner-Mas et al., 2021; Rivera-Brown & Frontera, 2012; Scudder et al., 2014), better peer relationships or social skills (Balish et al., 2014; Lee et al., 2014; Storebø et al., 2019). While the effects of acute and regular exercise are both important in the treatment of ADHD patients, they are difficult to combine in meta-analytic approaches. To provide an alternative treatment for ADHD, physical activity should be provided regularly to achieve lasting effects that match the long-term nature of ADHD.
Due to the methodological problems described above, one cannot draw firm conclusions about the efficacy of physical activity as a treatment for clinical ADHD symptoms from these previous meta-analyses (Schünemann et al., 2013). Therefore, the use of these results in evidence-based guidelines (Erickson et al., 2019; Feldman et al., 2018) is questionable. Moreover, several recent RCTs were not included in both meta-analyses (Bahram et al., 2014; Benzing & Schmidt, 2019; Davis et al., 2017; Garcia-Gomez et al., 2016; Gelade et al., 2016; Pan et al., 2016; Soori et al., 2020). To overcome these limitations, we performed a systematic review and meta-analysis of RCTs that included regular MVPA interventions for children and adolescents with ADHD and also measured post-differences in symptoms between intervention and control groups on a clinically valid ADHD rating scale.
Method
This review was conducted in accordance to the PRISMA statement (Moher et al., 2009), see Online Resource 1, and registered with PROSPERO (CRD42019142166).
Eligibility Criteria
We included studies that met the following eligibility criteria: (1) Participants between 5 and 21 years old, diagnosed with ADHD by a qualified health professional according to DSM-III, DSM-IV, DSM-IV-TR, DSM-5, ICD-9, or ICD-10 criteria. (2) Interventions implementing regular MVPA (≥2× per week over a course of ≥4 weeks). Moderate to vigorous intensity was defined as exceeding energy expenditure of 2.99 metabolic equivalent of tasks (MET) (Ainsworth et al., 2011; Anastasopoulou et al., 2014). If MET values were not reported in a paper, heart rate or VO2max values were transformed into equivalent MET values (Garber et al., 2011). If no measure of energy expenditure was reported, the average intensity of the intervention was derived from a compendium of physical activities (Butte et al., 2018; Garber et al., 2011). (3) Comparison of MVPA interventions to any standard treatment (e.g., psychotherapy, medication) or passive control group (e.g., waiting list, supportive therapy). Trials implementing MVPA as add-on treatment were included if the main intervention was equal across groups. (4) Outcomes reporting a total score of ADHD core symptoms on an observer-rated, psychometrically valid rating scale at the end of the intervention. Alternatively, reported subscale-scores (inattention, hyperactivity/impulsivity) had to allow the calculation of a valid total score. (5) RCT design. (6) Studies of all languages were included.
Literature Search
The primary literature search was conducted in June 2019, with the last update being in March 2020 in the following electronic databases: Pubmed, Cochrane, Embase, Web of Science, Academic Search Premier, PsychInfo, Eric, and CINAHL. In addition, language specific databases (Fachportal Pädagogik and LILACS) were searched. An unpublished literature search was conducted on national and international trial registers (ClinicalTrials.gov, www.controlled-trial.com, DRKS). Grey literature was searched on OpenGrey. The review also included results of dissertations searched on ProQuest, Open Access Dissertation and Theses, WorldCat, DissOnline, and EBSCO Open Dissertations. We did not set date restrictions. An exemplary search strategy for PubMed is provided in Online Resource 2. Reference lists of prior relevant reviews and identified studies were scanned for additional published trials.
Study Selection
Two researchers (B.S., S.W.) independently screened titles and abstracts of the search results. For selected trials, both researchers independently applied inclusion and exclusion criteria. If information on any of the inclusion criteria was unclear or omitted in the paper, we contacted study authors at least three times. Disagreement was resolved through discussion or consultation with a third reviewer (M.H., R.U.).
Data Extraction and Outcome Measures
Two researchers (B.S., S.W.) independently performed data extraction. All authors used data collection forms for intervention reviews for RCTs provided by the Cochrane group. Information was summarized into a spread sheet (Higgins et al., 2019). Disagreement was resolved through discussion. If data was incomplete or unclear, we made attempts to contact the authors for clarification (at least three times).
The primary outcome of this meta-analysis is the difference in a total score of ADHD core symptoms between the MVPA intervention and the control group, at post-intervention. Post-intervention was defined at the time-point closest to the last session of the intervention. Secondary outcomes include the difference between the intervention and control group in functional impairment in the social context (post-intervention) and dropout. We originally planned to include ADHD core symptoms at follow-up, functional impairment in the academic context and adverse events as secondary outcomes. We could not perform these analyses, as only one included study reported ADHD symptoms at follow-up (Gelade et al., 2018). No included study reported functional impairment in the academic context and adverse events (see Table 1).
Table 1.
Summary of characteristics of included studies.
| Authors | Design | Participants | Intervention | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Design (points of measurement) | Control condition | n | Age (M, SD), gender | Diagnosis | Content | Length (minutes) | Frequency, duration, intensity a | Co-intervention | Scales (1. ADHD 2. Social impairment) | Rater (included in analysis, reported) | |
| Bahram et al. (2014) | RCT (pre, post) | No inter-vention | 30 | M = 9.47 | ADHD, all subtypes | Warm-up, cool-down | 5–7 | 3×/week 12 weeks Moderate b |
None | 1. CSI-4
c
2. n.a. |
Teacher, parent |
| SD = 1.98 | Running (group, investigator) | 20–35 | |||||||||
| 0% male | |||||||||||
| Benzing and Schmidt (2019) | RCT (pre, post) | Waiting-list | 51 | M = 10.43 | ADHD, all subtypes | Exergaming (individual, parent) | 30 | 3×/week 8 weeks Moderate d |
Standard treatment (75% medicated) | 1. Conners 3 2. n.a. |
Parent |
| SD = 1.37 | |||||||||||
| 82% male | |||||||||||
| Choi et al. (2015) | RCT (pre, post) | Supportive therapy | 35 | M = 15.9 | ADHD, all subtypes | Stretching, feedback and cooling down | 20 | 3×/week 6 weeks Moderatee,f |
Methyl-phenidate (100% medicated) | 1. K-ARS 2. n.a. |
Parent g |
| SD = 1.2 | Aerobic exercise (running, jumping rope, basketball) (group, professional trainers) | 60 | |||||||||
| 100% male | |||||||||||
| Davis et al. (2017) | RCT (pre, post) | Waiting-list | 8 | M = 10.27 | ADHD-H; ADHD-C | Aerobic activities (e.g., walking, jogging, various games and dance forms) (group, investigator) | 40 | 2×/week 4 weeks Moderate h |
Unclear | 1. DBD | Teacher g , parent |
| SD = 1.26 | 2. SDQ | ||||||||||
| 88% male | |||||||||||
| Felmet (1998) | RCT (post) | Waiting-list | 40 | M = 9.7 | ADHD, all subtypes | Karate lessons (group, professional trainer) | 45 | 2–3×/week 8 weeks Moderate to vigorous i |
Standard treatment (80% medicated) | 1. ADDES 2. n.a. |
Parent |
| SD = 1.36 | |||||||||||
| 100% male | |||||||||||
| Garcia-Gomez et al. (2016) | RCT (pre, post) | Waiting-list | 18 | M = 10.49 | ADHD, all subtypes | Horseback riding + unmounted activities (group-based, professional trainer) | 45 | 2×/week 12 weeks Moderate to vigorous j |
Standard treatment (unclear amount medicated) | 1. BASC 2. n.a. |
Teacher |
| SD = 1.78 | |||||||||||
| 67% male | |||||||||||
| Gelade et al. (2016); Gelade et al. (2018) | RCT (pre, post, follow-up) | 1. Methyl-phenidate | 112 | M = 6.63 | ADHD, all subtypes | Warm up, cool down | 10 | 3×/week 10–12 weeks Moderate to maximal k |
None | 1. SWAN
c
2. n.a. |
Teacher, parent |
| 2. Neuro-feedback | SD = 1.76 | High intensity exercise at 70–80% of Hrmax | 10 | ||||||||
| 75% male | High intensity exercise at 80–100% of Hrmax (individual, investigator) | 10 | |||||||||
| Kang et al. (2011) | RCT (pre, post) | Supportive therapy | 32 | M = 8.49 | ADHD, all subtypes | Goal directed activities (e.g., darts) | 20 | 2×/week 6 weeks Mainly moderate to vigorous f |
Methyl-phenidate (100% medicated) | 1. K-ARS 2. CBCL |
Parent g |
| SD = 1.03 | Shuttle runs | 15 | |||||||||
| 100% male | Jump roping (group-based, professional trainer) | 20 | |||||||||
| Oh et al. (2018) | RCT (pre, post) | Methyl-phenidate or Atomo-xetine | 34 | M = 8.15 | ADHD, all subtypes | Horseback Riding + unmounted activities (group-based, professional trainer) | 60 | 2×/week 12 weeks Moderate to vigorous j |
None | 1. K-ARS 2. CBCL |
Psycho-logist |
| SD = 1.57 | |||||||||||
| 91% male | |||||||||||
| Pan et al. (2016) | RCT (2 × 2 crossover) | Waiting-list | 32 | M = 8.9 | ADHD, all subtypes | Warm up, cool down | 10 | 2×/week 12 weeks Moderate l |
Standard treatment (56% medicated) | 1./2. CBCL c | Parent |
| SD = 1.5 | Motor skill practice | 20 | |||||||||
| 100% male | Executive function focused table tennis exercise (individual, professional trainer) | 40 | |||||||||
| Soori et al. (2020) | RCT (pre, post) | No inter-vention | 56 | M = 12.53 | ADHD, all subtypes | Warm-up, cool-down | 10 | 3×/week 6 weeks Vigorous m |
None | 1. CPRS-R
c
2. n.a. |
Parent |
| SD = 0.30 | High intensity interval training: shuttle runs (group-based, investigator) | Approx. 10 | |||||||||
| 47% male | |||||||||||
Note. ADHD: attention deficit hyperactivity disorder core symptoms; ADDES: attention deficits disorders evaluation scale, second edition, home version; BASC: behavior assessment system for children; bpm: beats per minute; CBCL: the Chinese version of the child behavior checklist; CSI-4: child symptom inventory-4; Conners3-P: Conner’s 3rd edition; CPRS-R: Conner’s parent rating scale (revised version); DBD: disruptive behavior disorder rating scale; FI: functional impairment; HRmax: maximal heart rate; K-ARS: Dupaul attention deficit hyperactivity disorder rating scale–Korean version; M: mean; MET: metabolic equivalent of tasks; SD: standard deviation; SDQ: strengths and difficulties questionnaire; SWAN: behavior assessment system for children.
Intensity was categorized according to Garber et al. (2011).
Target heart rate 150–160 bmp (approx. 71%–76% HRmax Fox et al., 1971).
Only total score of ADHD core symptoms available.
3.1–4.8 MET Butte et al. (2018).
Target heart rate: 60% of Hrmax.
Jump rope: 6.9 MET; running: 5.5–7.3 MET (Butte et al., 2018).
Outcome assessor was blinded.
Heart rate >150 bpm (approx. 71% HRmax Fox et al., 1971).
5.3–10.3 MET (Ainsworth et al., 2011).
3.8–7.3 MET (Ainsworth et al., 2011).
70%–80% HRmax to 80%–100% Hrmax.
4.2 MET (Butte et al., 2018).
Target heart rate: 85% of Hrmax.
Study Quality Assessment
Quality of included studies was assessed using the Cochrane “Risk of Bias” assessment tool (RoB2) (Sterne et al., 2019). Two researchers (B.S., S.W.) independently judged the risk of bias of the effect of assignment to intervention for the primary outcome in each domain (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome data, selection of the reported result) for each trial. We contacted authors if any information was unclear (minimum of three attempts).
Data Synthesis and Meta-analyses
Statistical analysis was performed using the meta package (Schwarzer, 2007) in the Statistical Analysis Software R (version 3.6.0) and RStudio (version 1.2.1335). We calculated effect measures of the difference in outcome score between the experimental and control groups at post-intervention according to Hedges’ g (Hedges, 2016) and reported a 95% confidence interval (CI) for ADHD core symptoms and functional impairment. Differences in dropout at post-intervention were calculated as odds ratio (OR) and reported with a 95% CI. Data were synthesized using random-effects meta-analyses (DerSimonian & Laird, 1986). The conventional p-value of <.05 was considered as a threshold for statistical significance. When only separate measures of inattention, impulsivity and hyperactivity were available for the primary outcome, they were combined into a total score of ADHD core symptoms as a mean score of subscales according to Cochrane standards (Higgins et al., 2019). If both teacher and parent ratings were available, we used teacher ratings for interventions delivered in home contexts and parent ratings for interventions delivered in the school context, in order to ensure the maximum possible blinding of outcome assessments. If multiple relevant control groups were reported in a single trial, we combined the outcomes of the control groups as proposed in the Cochrane guidelines (Higgins et al., 2019). We multiplied scores by −1 for scales in which increase indicated improved outcomes, as to maintain consistency in direction of the scales. Statistical heterogeneity was analysed by calculation of Cochran’s Q, with a significance level of α = 0.1. Heterogeneity was quantified through I2 statistics. Values <40% were considered as unlikely to represent important heterogeneity (Higgins et al., 2019). The quality of evidence for primary and secondary outcomes were rated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann et al., 2013).
Planned Subgroup Analyses
We performed the following planned subgroup analyses for the primary outcomes, when at least two studies provided data for the analysis: type of treatment (standalone, add-on), duration of MVPA (<45 minutes, ≥45 minutes), frequency of MVPA (=2×/week, >2×/week), and intensity of MVPA (moderate, moderate to vigorous). We originally planned to analyse the efficacy of MVPA depending on the type of control group (active, passive, standard treatment) separately for both MVPA as standalone and MVPA as adjunctive treatment. However, since less than five comparisons to estimate an effect would likely yield imprecise results (Borenstein et al., 2011), we decided to perform a subgroup analysis to compare the effects of all types of treatment (standalone and add-on) differentiated by the type of control group (active, passive). Control groups were defined as active if a standard treatment (National Guideline Centre (UK), 2018; Wolraich et al., 2019) was implemented. Control groups were defined as passive if they implemented no intervention, a waitlist control, or any semi-active treatment, defined as any treatment not recommended as first-in-line treatment by current guidelines (National Guideline Centre (UK), 2018; Wolraich et al., 2019). We conducted five subgroup analyses, including a total of 10 subgroups. Therefore, a Bonferroni adjusted alpha level of <.005 was chosen. Differences in effects-sizes for subgroup comparisons were considered significant if 95% CIs did not overlap.
Sensitivity Analyses
We performed separate sensitivity analyses for the efficacy of MVPA intervention on inattentive and hyperactive symptoms to investigate whether the result was robust for the single symptom dimensions. We did not perform the planned sensitivity analysis including only studies that were judged as low risk of bias, since only one study fulfilled the pre-specified criteria. Instead, we performed a subgroup analysis to estimate the effect of MVPA on the primary outcome depending on the blinding of outcome-assessors. Blinding was defined as outcome assessors being unaware of group allocation and study hypotheses, and was confirmed by personal communication with the trialists in all cases. Due to substantial heterogeneity in the study samples and the interventions, we conducted further subgroup analyses including providers (professional trainers vs. parents or investigators) and delivery (individual vs. group-based) of the intervention. We conducted three additional subgroup analyses, including a total of six subgroups. Therefore, a Bonferroni adjusted alpha level of p < .008 was chosen. Differences in effects-sizes for subgroup comparisons were considered significant if 95% CIs did not overlap. In addition, we performed univariate meta-regressions. The mean age of the sample, gender (% of male participants in the study), and medication status (% of medicated participants in the study) were included as predictors.
Publication Bias
Publication bias was assessed using Egger’s test for asymmetry of funnel plots for the primary outcome (Egger et al., 1997). In case of a significant small-study effect, the asymmetrical funnel plot was corrected by using the trim and fill algorithm. The procedure was applied on the right and left side of the plot to add or remove studies that contribute to imbalance, so that an unbiased estimate of effect could be provided (Duval & Tweedie, 2000).
Results
Selection and Characteristics of Studies
Figure 1 represents the PRISMA flow diagram for the study selection. Eleven RCTs including nine trials published in peer-reviewed journals (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Garcia-Gomez et al., 2016; Gelade et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020), one conference abstract (Davis et al., 2017), and one dissertation (Felmet, 1998) were included in the analysis. Two studies were conducted in Iran (Bahram et al., 2014; Soori et al., 2020), one each in the Netherlands (Gelade et al., 2016), Spain (Garcia-Gomez et al., 2016), Switzerland (Benzing & Schmidt, 2019), and Taiwan (Pan et al., 2016), three in the Republic of Korea (Choi et al., 2015; Kang et al., 2011; Oh et al., 2018), and two in the United States of America (Davis et al., 2017; Felmet, 1998). One study was translated from Persian by a Persian to English interpreter (Bahram et al., 2014). Trials included a total of 448 patients randomized (416 patients analysed). The sample size ranged from 8 to 112 participants (Msample = 40.73; SDsample = 27.11). The age range of included participants was 6 to 18 years (Mage = 9.47 years; SDage = 2.94), and 24% were female. All included studies used an outpatient sample. Length of implemented MVPA ranged from 10 to 60 minutes (Mlength = 35.68 min, SDlength = 16.21 min). Five studies employed the intervention twice per week (Davis et al., 2017; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016), one study two to three times per week (Felmet, 1998), and five studies three times per week (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Gelade et al., 2016; Soori et al., 2020). Intensity of the intervention was mainly moderate in five studies (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Davis et al., 2017; Pan et al., 2016), moderate to vigorous in five studies (Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Soori et al., 2020), and moderate to maximal in one study (Gelade et al., 2016). Duration of treatment ranged from 4 to 12 weeks (Mduration = 8.81 weeks, SDduration = 3.06 weeks). Five studies delivered the intervention as standalone treatment (Bahram et al., 2014; Davis et al., 2017; Gelade et al., 2016; Oh et al., 2018; Soori et al., 2020), six delivered the intervention as adjunctive treatment to standard care (Benzing & Schmidt, 2019; Choi et al., 2015; Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Pan et al., 2016). One study included an active control group (standard care; Oh et al., 2018), one an active (methylphenidate) as well as a semi-active (neurofeedback) control group (Gelade et al., 2016), and nine a passive control group: supportive therapy (Choi et al., 2015; Kang et al., 2011), waiting list (Benzing & Schmidt, 2019; Felmet (1998); Davis et al., 2017; Garcia-Gomez et al., 2016; Pan et al., 2016), no intervention (Bahram et al., 2014; Soori et al., 2020). Outcome assessors were blinded in three studies (Choi et al., 2015; Davis et al., 2017; Kang et al., 2011). Interventions were delivered group-based in nine studies (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Soori et al., 2020), and individually in two studies (Gelade et al., 2016; Gelade et al., 2018; Pan et al., 2016). Interventions were delivered by the investigators or related study personnel in four studies (Bahram et al., 2014; Davis et al., 2017; Gelade et al., 2016, 2018; Soori et al., 2020), by the parents in one study (Benzing & Schmidt, 2019), and by a professional trainer in six studies (Choi et al., 2015; Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016). A summary of the characteristics of all included studies is represented in Table 1.
Figure 1.
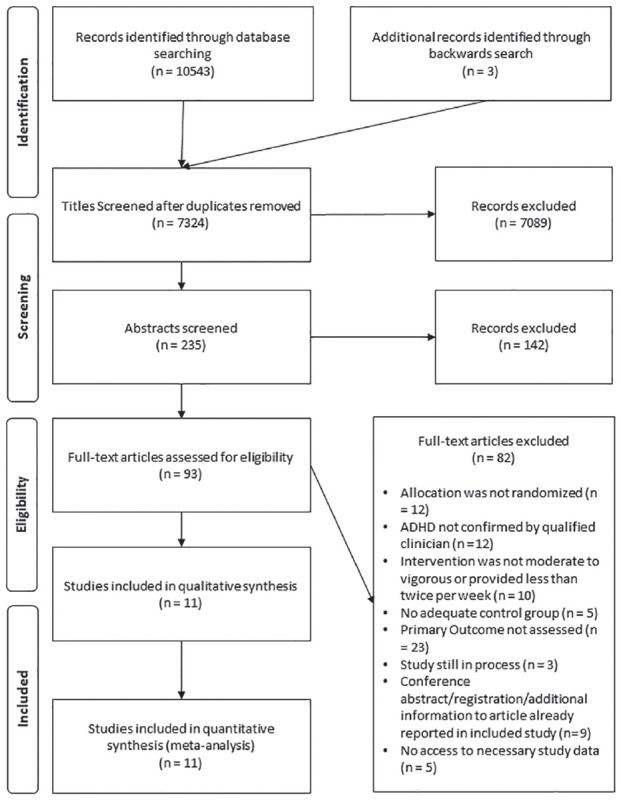
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of included studies. Exclusion of full-text studies was performed sequentially, according to the following sequence: randomized allocation, ADHD diagnosis, intervention, control group, primary outcome, completed study, duplication or additional information, no access to necessary study data. Studies that were included in previous meta-analyses (Cerrillo-Urbina et al., 2015; Zang, 2019), but excluded in the screening process of this meta-analysis are reported in Online Resource 4.
Risk of Bias Assessment
Risk of bias assessment is presented in Figure 2. Two RCTs were judged as raising some concerns (Davis et al., 2017; Gelade et al., 2016). All remaining RCTs (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020) were rated at an overall high risk of bias. All RCTs (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Gelade et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020) were judged as being at low risk of bias arising from the allocation process, since the allocation sequence was random and concealed until participants were enrolled and assigned to the intervention. All RCTs (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Gelade et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020) raised concerns because participants and providers were aware of the allocation to MVPA intervention or control group. However, one RCT was judged as being at low risk of bias (Gelade et al., 2016) since the trial provided three active or semi-active interventions and was therfore likely to mask treatment expectations (Higgins et al., 2019). Four RCTs (Choi et al., 2015; Felmet, 1998; Garcia-Gomez et al., 2016; Soori et al., 2020) were judged as being at high risk of bias for missing outcome data, since missing data was unequally distributed across groups and participants with missing outome data were not included in the analysis. Only three RCTs reported blinding of outcome assessors (Choi et al., 2015; Davis et al., 2017; Kang et al., 2011). One RCT raised some concerns for the assessment of the outcome being influenced by knowledge of the intervention, since outcome assessors were aware of the allocation. However, the RCT provided three active or semi-active interventions. Treatment expectations might therefore have been concealed (Gelade et al., 2016). All other RCTs were judged as being at high risk for knowledge of intervention influencing the measurement of the outcome, since outcome assessors were parents, teachers or psychologists with knowledge about the hypotheses and allocation of the participants (Bahram et al., 2014; Benzing & Schmidt, 2019; Felmet, 1998; Garcia-Gomez et al., 2016; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020). Finally, all (Bahram et al., 2014; Choi et al., 2015; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020) but two (Benzing & Schmidt, 2019; Gelade et al., 2016) RCTs raised concerns for selection of outcome data by not providing an a priori trial registration.
Figure 2.
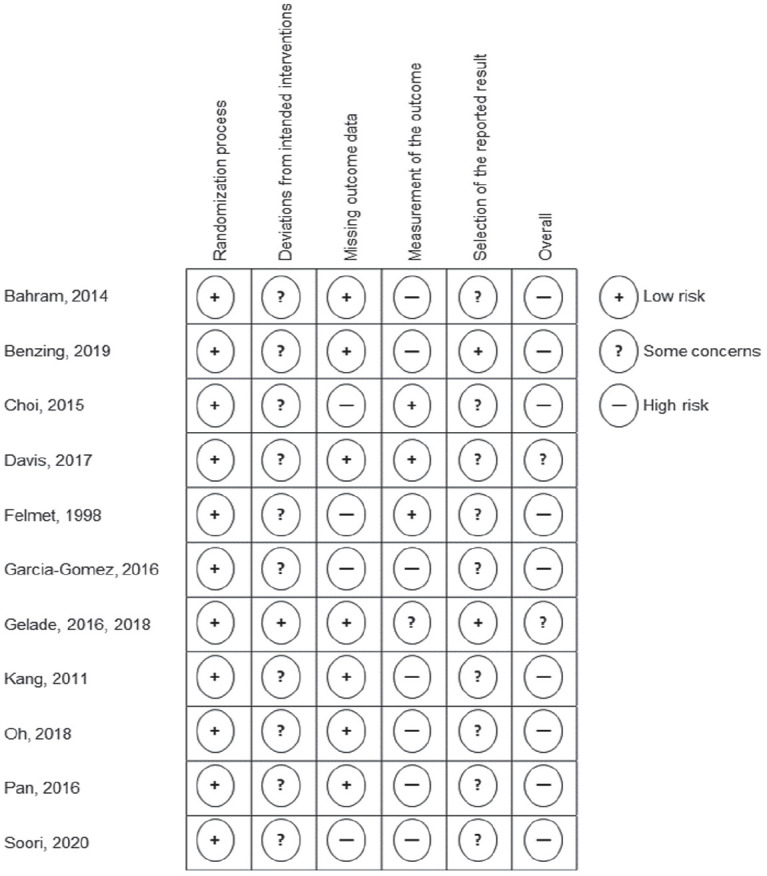
Risk of bias rating for the included randomized controlled trials for all subdomains as well as overall risk of bias.
Primary Outcome
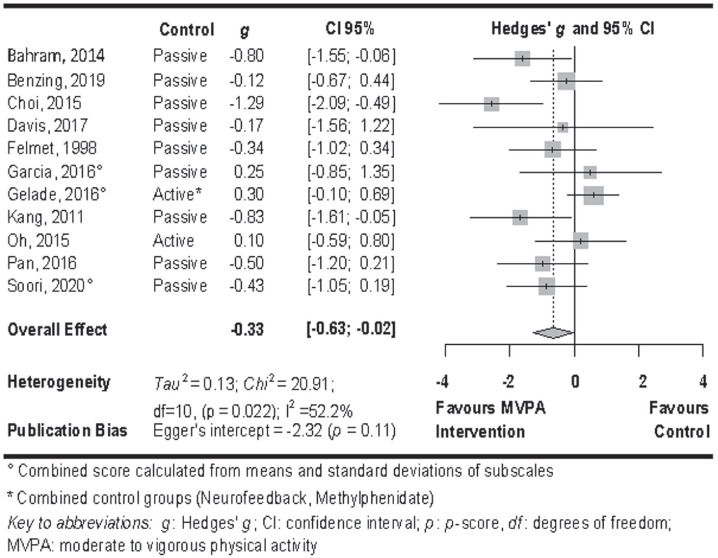
Random-effects meta-analysis of total ADHD core symptoms at post-treatment showed evidence of a significant, small effect in favour of MVPA interventions in comparison to any control condition (g = 0.33; 95% CI [−0.63; −0.02]; p = 0.037, see figure 3). Heterogeneity was substantial (I2 = 52.2%; χ2 = 20.91; df = 10, p = .022; τ2 = 0.13). Eggers’ tests did not indicate publication bias (Intercept = −2.32; p = .115). Therefore, a trim and fill analysis was not computed. Quality of evidence was rated as low since most trials were judged to be at high risk of bias and the CI around the effect estimate included both a meaningful effect and no effect.
Figure 3.
Forest plot of the meta-analysis of ADHD total core symptoms.
Secondary Outcomes
Functional impairment
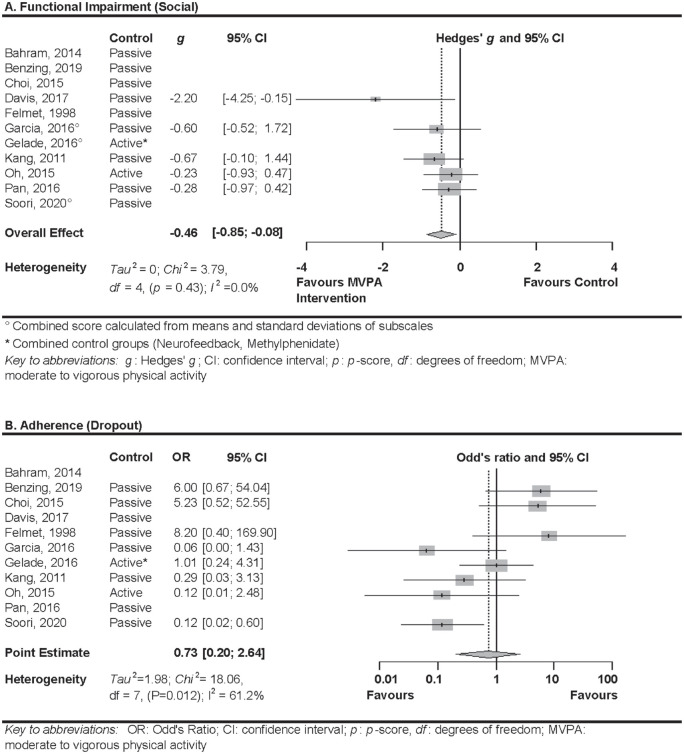
Six RCTs assessed functional impairment in the social context (Davis et al., 2017; Garcia-Gomez et al., 2016; Gelade et al., 2018; Kang et al., 2011; Oh et al., 2018; Pan et al., 2016). Authors of one RCT (Gelade et al., 2018) were not able to provide the data of the necessary subscale of the rating scale. Therefore, only five studies were included in the analysis. Random effects meta-analysis showed a significant medium effect of MVPA in comparison to any control group (g = −0.46; 95% CI [−0.85; −0.08]; p = .017; see Figure 4a). I2 was 0% (χ2 = 3.79; df = 4, p = .435; τ2 = 0), suggesting heterogeneity was negligible. Quality of evidence was rated as low, due to the high risk of bias of all included trials and the CI around the effect estimate including both a large effect and no effect. Egger’s test indicated possibility for publication bias (Intercept = −2.436; p = .040). Two studies were added when applying the trim and fill algorithm on either side of the mean, resulting in a small effect of MVPA in comparison to any control group (g = −0.32; 95% CI [−0.74; −0.11]; p = .14). I2 was 29.7% (χ2 = 8.54; df = 6, p = .2014; τ2 = 0.09), suggesting heterogeneity was still negligible.
Figure 4.
Forest plots of the meta-analyses of social impairment (a) and dropout (b).
Dropout
Eight RCTs reported dropout over the course of the study (Benzing & Schmidt, 2019; Choi et al., 2015; Felmet, 1998; Garcia-Gomez et al., 2016; Gelade et al., 2016; Kang et al., 2011; Oh et al., 2018; Soori et al., 2020). Random-effects meta-analysis did not show a significant difference in the odds of dropout in MVPA in comparison to any control group (OR = 0.73; 95% CI [0.20; 2.64]; p = .637; see Figure 4b). Heterogeneity was substantial (I2 = 61.2%; χ2 = 18.06; df = 7, p = .012; τ2 = 1.98). Egger’s test did not indicate publication bias (Intercept = 0.32; p = .890). Quality of evidence was rated as very low, due to high risk of bias of the majority of trials, a large amount of heterogeneity that could not be explained by trial characteristics, and the CI around the effect estimate including both meaningful effects estimates in favour and opposed to the intervention.
Subgroup Analyses
Five subgroup analyses were conducted. Full statistics are presented in Table 2. Subgroup analyses resulted in a significant, moderate-sized effect in favour of the MVPA intervention for trials that tested against a passive control group (g = −0.40; 95% CI [−0.67]; −0.13; p = .004). The effect of MVPA interventions in comparison to an active control group did not reach significance (g = 0.41; 95% CI [−0.03; 0.86]; p = .071) and can be considered significantly different to the effect against a passive control group, since confidence intervals did not overlap. Two subgroup analyses trended towards significance. Namely, MVPA implemented as adjunctive treatment resulted in a moderate effect (g = −0.48; 95% CI [−0.87; −0.09]; p = .015). In comparison, interventions implemented as stand-alone treatment resulted in a non-significant small effect (g = −0.15; 95% CI [−0.59; 0.29]; p = .509). The effect for RCTs implementing moderate physical activity interventions trended towards significance (g = −0.57; 95% CI [−1.01; −0.14]; p = .010). The effect of moderate to vigorous/maximal physical activity was small and nonsignificant (g = −0.14; 95% CI [−0.51; 0.24]; p = .475). No other subgroup analysis yielded significant results.
Table 2.
Meta-analytic findings in subgroup analyses.
| Subgroup | n | g | 95% CI | p | τ2 | χ2 | df | p | I2 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control group a | Active b | 2 | 0.41 | [−0.03; 0.86] | 0.071 | 0.02 | 1.24 | 1 | 0.265 | 19.6 |
| Passive b | 10 | −0.40 | [−0.67; −0.13] | 0.004* | 0.06 | 13.04 | 9 | 0.161 | 31.0 | |
| Type of treatment | Add–On | 6 | −0.48 | [−0.87; −0.09] | 0.015 | 0.09 | 8.21 | 5 | 0.145 | 39.1 |
| Standalone | 5 | −0.15 | [−0.59; 0.29] | 0.509 | 0.13 | 8.52 | 4 | 0.074 | 53.1 | |
| Length | <45 minutes | 7 | −0.31 | [−0.67; 0.05] | 0.093 | 0.12 | 12.43 | 6 | 0.053 | 51.7 |
| ≥45 minutes | 4 | −0.34 | [−1.00; 0.31] | 0.301 | 0.27 | 8.01 | 3 | 0.046 | 62.6 | |
| Frequency | >2×/week | 6 | −0.38 | [−0.83; 0.07] | 0.098 | 0.21 | 16.43 | 5 | 0.006 | 69.6 |
| =2×/week | 5 | −0.29 | [−0.68; 0.11] | 0.158 | 0.02 | 4.37 | 4 | 0.358 | 8.5 | |
| Intensity | moderate | 5 | −0.57 | [−1.01; −0.14] | 0.010 | 0.09 | 6.37 | 4 | 0.173 | 37.2 |
| vigorous | 6 | −0.14 | [−0.51; 0.24] | 0.475 | 0.10 | 9.46 | 5 | 0.092 | 47.2 |
Note. n: number of studies; g: Hedges’ g; CI: confidence interval; df: degrees of freedom.
For Gelade et al. (2016), the comparison against methylphenidate was included in the analysis of active control groups, the comparison against neurofeedback was included in the analysis of passive control groups.
Significantly different to comparison group: confidence intervals do not overlap.
p < 0.005.
Sensitivity Analyses
The separate sensitivity analyses for the effect of MVPA interventions on inattentive and hyperactive ADHD symptoms included nine RCTs that reported separate scores of inattention and hyperactivity (Bahram et al., 2014; Benzing & Schmidt, 2019; Choi et al., 2015; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Gelade et al., 2016; Gelade et al., 2018; Kang et al., 2011; Oh et al., 2018). Random-effects meta-analysis resulted in a medium effect in favour of the MVPA intervention, trending towards significance for inattentive ADHD symptoms (g = −0.60; 95% CI [−1.26; 0.06]; p = .07). Heterogeneity was significant and substantial (τ2 = 0.82; χ2 = 56.4; df = 8, p = .000; I2 = 85.8%). Full statistics are presented in Online Resource 3.A. Random-effects meta-analysis for hyperactive ADHD symptoms resulted in a non-significant, small effect in favour of the MVPA intervention (g = −0.25; 95% CI [−0.54; 0.05]; p = .10). Heterogeneity was negligible (τ2 = 0.07; χ2 = 12.58; df = 8, p = .127; I2 = 36.4%).
Further subgroup analyses resulted in trends towards significance for studies that reported blinding of outcome assessors. An analysis including these subgroups resulted in a large effect (n = 3; g = −0.93; 95% CI [−1.45; −0.41]; p = .000) for improvement of total ADHD core symptoms in the MVPA group in comparison to any other control group. The analysis including eight trials with non-blinded outcome assessors resulted in a non-significant small effect (n = 8; g = −0.16; 95% CI [−0.44; 0.12]; p = .259). The difference between the two subgroups was considered as trending towards significance due to the small overlap of confidence intervals. The subgroup analysis investigating the delivery of the intervention resulted in a medium, significant effect for the subgroup of group-based delivery (n = 9; g = −0.42; 95% CI [−0.72; −0.12]; p = .007). Group-based delivery was not considered significantly different to the subgroup delivering the intervention individually (n = 2; g = −0.04; 95% CI [−0.81; 0.73]; p = .914), due to the large overlap of confidence intervals (see Online Resource 3.B). Only the univariate meta-regression including age as predictor for the difference of ADHD total symptoms between groups at post-treatment resulted in a significant result (n = 11; QM(1) = 8.77; p = .003; R2 = 88.67%). Age was negatively associated with the effect size (β = −0.13, 95% CI [−0.23; −0.05], p = .016). Since a smaller value of effect size indicates higher symptom improvement, this means that the intervention seems to be more effective for older age groups. We visually inspected the plot of the meta-regressions for outliers. One study (Choi et al., 2015) was identified as a potential data point with high leverage through visual inspection (see Online Resource 3.C) and Grubbs test for outliers (G = 2.37; U = 0.38; p = .023). To ensure that high leverage exerted by this data point did not simply drive the association between mean baseline age and ADHD core symptoms at post-treatment, we reran the meta-regression model excluding this study with an adolescent sample (Choi et al., 2015). After removal of that study, the meta-regression no longer reached significance (n = 10; QM(1) = 2.29; p = .130; R2 = 55.07%) (for full analysis refer to Online Resource 3.C).
Discussion
This meta-analysis revealed a significant, small effect of MVPA on total ADHD core symptoms. The analysis of the secondary outcomes resulted in a significant, moderate effect in favour of MVPA for functional impairment in the social context and no difference in odds of dropout between MVPA and control groups. We had insufficient data to assess the effect of MVPA on ADHD core symptoms at follow up, functional impairment in the academic context, and adverse events. Subgroup analyses indicated a significant, moderate effect of MVPA on total ADHD core symptoms, when testing against passive control groups. The comparison of MVPA interventions versus active control groups (pharmacotherapy) resulted in a medium-sized, non-significant effect in favour of pharmacotherapy. The subgroup analyses resulted in moderate effects trending towards significance for trials implementing mainly physical activity of moderate intensity, and for trials implementing MVPA as an add-on treatment, compared to all control groups. The effects for interventions of mainly vigorous or maximal intensity and standalone interventions were small and non-significant. However, both trials that compared physical activity interventions to an active control group implemented mainly vigorous or maximal physical activity as a standalone treatment. It is unclear if the inferior results are explained by the intensity of the intervention, delivery as a standalone or add-on treatment, or the comparison to active control groups. No other subgroup analysis yielded significant results. The sensitivity analyses that considered inattentive and hyperactive impulsive symptoms separately led to a reduction in heterogeneity compared to the main analysis and resulted in a non-significant moderate effect for inattentive symptoms and a non-significant small effect for hyperactive symptoms. However, a smaller sample was included in these analyses than in the main analysis, since two RCTs only provided a total score of ADHD symptoms (Pan et al., 2016; Soori et al., 2020). The further subgroup analysis comparing studies with blinded outcome assessors (resulting in a large, significant effect in favour of MVPA interventions) versus studies with unblinded assessment (resulting in a small non-significant effect in favour of MVPA interventions) indicated a marginally significant difference between these subgroups. In addition, the subgroup analysis for group-based interventions resulted in a moderate, significant effect in favour of MVPA interventions and reduction in heterogeneity. However, only two studies were included in the analysis of individually delivered interventions (resulting in a negligible, non-significant effect in favour of MVPA interventions) and the difference between these two groups could not be considered significantly different, due to the overlap of confidence intervals.
Univariate meta-regressions including age, gender, and medication status identified only age as a relevant predictor of the primary outcome’s effect size. Higher age was associated with a larger effect size in favour of MVPA interventions. However, the one study’s effect size, including an adolescent sample, was identified as a data point with high leverage. After removal of this data point, age did no longer remain a significant predictor of effect size. This additional analysis highlights the need for further studies investigating the effect of MVPA on ADHD in adolescent samples.
Due to inclusion criteria based on high methodological standards and the integration of RCTs that were more recently published (Bahram et al., 2014; Benzing & Schmidt, 2019; Davis et al., 2017; Felmet, 1998; Garcia-Gomez et al., 2016; Gelade et al., 2016; Oh et al., 2018; Pan et al., 2016; Soori et al., 2020), our sample differs to those of previously published meta-analyses. Compared to Cerrillo-Urbina et al. (2015), our analysis resulted in smaller effect estimates for ADHD core symptoms. Results of the meta-analysis of to Cerrillo-Urbina et al. (2015) might differ due to inclusion of non-RCTs (McKune et al., 2003; Verret et al., 2012), which tend to overestimate effects (Wood et al., 2008), and performance-based measures of attention as primary outcomes (Chang, Liu, et al., 2012). The null-effect reported by Zang (2019) might be explained by the inclusion of trials with participants not meeting all criteria of an ADHD diagnosis (Hoza et al., 2015) and including effect-measures of a trial implementing physical activity of light intensity (Ainsworth et al., 2011; Jensen & Kenny, 2004). Evidence suggests physical activity should be at least of moderate intensity to show positive effects on underlying mechanisms of ADHD (de Greeff et al., 2018) and on other mental disorders (Carter et al., 2016), while evidence for the efficacy of light intensity is scarce.
Current guidelines recommend pharmacotherapy and psychosocial interventions as treatment for ADHD, either alone or in combination (National Guideline Centre (UK), 2018; Wolraich et al., 2019). Regarding psychosocial treatments, meta-analyses report small effects on ADHD symptoms and medium effects on functional impairment in the social context (peer-problems) if outcomes of various psychosocial interventions (i.e., cognitive behavioural interventions, behavioural interventions, social skills training, parent coaching, self-control training, organizational skills training, cognitive training, neurofeedback) are pooled (Daley et al., 2014; Sonuga-Barke et al., 2013). A meta-analysis investigating (cognitive) behavioural therapy for externalizing disorders reports medium effects on ADHD symptoms in children with an ADHD diagnosis (Battagliese et al., 2015). For parent-administered interventions, meta-analytic results also suggest medium effects on ADHD symptoms (Coates et al., 2015). No meta-analysis included trials implementing pharmacotherapy as a control group. With a small effect of MVPA compared to all control groups (including pharmacotherapy) and a medium effect of MVPA compared to passive control groups, MVPA seems to result in comparable effects on ADHD core symptoms to psychosocial interventions. In addition, the medium effect on functional impairment in the social context seems to fall into the same range as those of psychosocial treatment (Daley et al., 2014). However, this comparison is merely observational. Effects of psychosocial interventions seem to disappear when using assessments that were most probably blinded (Daley et al., 2014; Sonuga-Barke et al., 2013). In our meta-analysis, effects of MVPA became even larger when only trials blinding outcome assessors were included. Nevertheless, due to the inclusion of much more studies in meta-analyses regarding psychosocial treatments, these effects can be judged as more robust compared to the effects of MVPA for ADHD.
In direct comparison to pharmacotherapy, our results indicated no significant difference in effect. Since only two trials were included in the analysis, this was likely due to a lack of statistical power rather than non-inferiority. The findings of previous meta-analyses indicate medium to large effects of pharmacotherapy on ADHD core symptoms (Cortese et al., 2018; Storebo et al., 2015). The observational comparison to the small effect in our meta-analysis indicates inferior effects of MVPA compared to pharmacotherapy.
Due to our pre-defined inclusion criteria, one RCT of high methodological quality was not included in the meta-analysis, that we consider worth mentioning. Hoza et al. (2015) conducted a RCT, implementing moderate to vigorous physical activity (30 minutes, 5 days a week for twelve weeks) in comparison to a sedentary occupational intervention. Outcome assessors were blinded to expectations of effectiveness of the interventions. Results indicated moderate effects for improvement of ADHD symptoms and peer-behaviour in the MVPA group, while between-group differences at post-intervention were neglectable. However, the study was excluded, because the sample was classified as “at risk” for ADHD but did not fulfil the entire criteria for an ADHD diagnosis.
While this is the first meta-analysis investigating the efficacy of MVPA interventions for ADHD including only RCTs, there are still important methodological limitations of the included studies. First, only three studies reported blinding of outcome assessors (Choi et al., 2015; Davis et al., 2017; Kang et al., 2011). The results of the remaining trials might therefore be distorted by treatment expectations. Most studies implemented a waitlist or no-intervention control group. It is nevertheless unlikely that the reported results are solely placebo effects, since the two studies that implemented a psychoeducation control group (Choi et al., 2015; Kang et al., 2011) as well as our sensitivity analysis including only studies, in which outcome assessors were blinded (Choi et al., 2015; Davis et al., 2017; Kang et al., 2011) reported large treatment effects in favour of MVPA. Only two studies performed intention-to-treat analyses (Benzing & Schmidt, 2019; Gelade et al., 2016). These studies resulted in smaller effects compared to those studies performing per-protocol analysis. It remains possible that participants dropped out of the intervention because they experienced inferior efficacy, which would have distorted the results. Dropout rates were highly heterogeneous across studies. However, with 9% overall dropout rate in the MVPA intervention (Mdropout = 8%; SDdropout = 0.08), the discontinuation rate can be considered small compared to an average dropout rate of 20% for pharmacotherapy reported in a recent meta-analysis (Riera et al., 2017) and 3% to 34% for psychosocial treatments reported by RCTs (Bor, Sanders, & Markie-Dadds, 2002; Pfiffner et al., 2007), and in comparison to a recent meta-analysis investigating dropout-rates in physical activity interventions for ADHD (17.5% dropout; Vancampfort et al., 2016). Lastly, since children and adolescents with ADHD show higher rates of disqualification, aggression, and emotional reactivity in sports, the implementation of a MVPA intervention might present unique challenges (Johnson & Rosen, 2016). Other than treatment discontinuation, none of the studies analysed the success of implementing the intervention through process evaluation. It remains unclear if all participants received the intended dose of the intervention.
Limitations of our systematic review and meta-analysis include a large heterogeneity of the implemented interventions. The American College of Sports Medicine classifies exercise prescription according to five characteristics: Frequency, Intensity, Time, and Type (American College of Sports Medicine, 2017). The interventions implemented in the included studies were similar in frequency (2–3 times per week) and intensity (moderate to vigorous). While time spent performing MVPA differed in the studies (10–60 minutes), the main difference was the type of physical activity performed. The interventions varied from horseback riding (de Greeff et al., 2018; Garcia-Gomez et al., 2016) to racket sports (Pan et al., 2016), high intensity interval training (Gelade et al., 2016; Soori et al., 2020) to endurance training (Choi et al., 2015; Davis et al., 2017; Kang et al., 2011), and from exergaming (Benzing & Schmidt, 2019) to martial arts (Felmet, 1998). This diversity in interventions is likely to have increased heterogeneity and causes one to question if the characteristics of the intervention influenced the efficacy.
Remarkably, the planned and additional subgroup analyses investigating the interventions’ characteristics lead to a considerable reduction in heterogeneity. It seems like interventions of moderate to vigorous intensity that are delivered in groups and by professional trainers lead to the best results. Current evidence describes physical activity interventions of moderate to vigorous intensity as most efficacious also for other disorders, such as depression and anxiety as well as on executive function in healthy individuals (Ahn & Fedewa, 2011; de Greeff et al., 2018). Group-based interventions could be especially efficient since they increase motivation in participation and therefore improve the delivery of the adequate dose, frequency and intensity of the intervention (Balish et al., 2014), as well as social skills through the social contacts in the group (Lee et al., 2014). On the other hand, supportive and personal coaching strategies, which might be more pronounced in trained professionals, seem to be critical factors for a positive experience and adequate delivery of the intervention in the intended dose (Lee et al., 2014). Repeated experiences of these positive psycho-social effects might increase social skills and have a positive effect on ADHD symptoms (Storebø et al., 2019).
As an additional limitation, we were not able to retrieve full-text information on three studies that were included after the abstract screening (Becker, 1998; De Castro et al., 2015; Hendry-Adams, 2010) and outcome data on the primary outcome of two studies included after the full text screening (Garcia, 2016; Jalali et al., 2015), even though we contacted every study author at least three times. Secondary outcome data of one included trial could not be retrieved (Gelade et al., 2016). Only one study (Choi et al., 2015) included an adolescent sample. The applicability of the results on adolescent ADHD is therefore questionable. We were unable to perform meta-analyses for long-term outcomes and functional impairment in the academic context of MVPA for ADHD. However, ADHD symptoms are associated with higher odds of failure to complete high school, grade retention and suspension (Erskine et al., 2016) and seem to at least partially persist into adulthood (Faraone et al., 2006). Evidence from other mental disorders suggests that only those who continue with MVPA after exercise interventions show long-lasting effects in catamnestic evaluations (Blumenthal et al., 2007). Consequently, assessing long-lasting effects of MVPA would be of great interest.
This systematic review and meta-analysis presents preliminary evidence for small to moderate effects of MVPA interventions for ADHD core symptoms and functional impairment. With additional beneficial effects on associated impairment, MVPA could serve as a holistic treatment approach. However, heterogeneity of effects over all studies is high and the included studies present methodological limitations. Subgroup analyses indicate positive effects and reduced heterogeneity especially in group-based interventions of moderate intensity, delivered by professional trainers. However, to confirm this preliminary evidence, further RCTs investigating the effects of delivery, trainer and intensity of MVPA are needed. To create more meaningful data, future research should rely on high methodological standards, such as blinding of outcome assessors, intention-to-treat analyses, and comparison against standard treatment or a placebo control group. In addition, follow-up effects of MVPA interventions, and the efficacy of MVPA for adolescent ADHD are of interest.
Supplemental Material
Supplemental material, sj-pdf-1-jad-10.1177_10870547211017982 for The Efficacy of Physical Activity for Children with Attention Deficit Hyperactivity Disorder: A Meta-Analysis of Randomized Controlled Trials by Britta Seiffer, Martin Hautzinger, Rolf Ulrich and Sebastian Wolf in Journal of Attention Disorders
Author Biographies
Britta Seiffer is a research associate and PhD student at the Eberhard Karls-University of Tuebingen. Her research interests focus on the integration of physical actitity in the care of patients with various mental illnesses and the development of targeted physical activity interventions for attention deficit hyperactivity disorders.
Martin Hautzinger is a senior university professor of clicical psychology and psychotherapy at the Eberhard Karls-University of Tuebingen. Besides, he is member of the expert group of the Institute for Medical, Pharmaceutical and Psychotherapeutical Examination Questions, member of the expert council for psychology at the German Research Foundation, member of the executive board of the private Tuebingen Academy for behaviour therapy, instructor and supervisor at various training schools for psychotherapy. His research focuses on intervention research (Psychotherapy, Prevention), affective disorders, anxiety disorders, alcohol addiction, psycho-physiological disorders, physical and psychological disorders at infancy and adolescence among other issues.
Rolf Ulrich is a full university professor for cognitive psychology at the Eberhard Karls-University of Tuebingen. Besides, he is a fellow of the American Association of Psychological Science, member of the German Academy of Sciences Leopoldina, and president of the Wilhelm-Wundt Society. His research focuses on Cognition, Mathematical Modeling, Statistical Methods, and Psychophysics.
Sebastian Wolf is the leader of the junior research group “Exercise and Mental Health” at the Eberhard Karls-University of Tuebingen. Besides, he is a licenced psychotherapist as well as instructor and supervisor at the private Tuebingen Academy for behaviour therapy. His research interest focuses on the integration of physical actitity in the care of patients with various mental illnesses.
Footnotes
Availability of Data and Material: The datasets generated during and/or analyzed during the current study are available under the following DOI: 10.23668/psycharchives.3007.
Authors’ Contributions: Britta Seiffer and Sebastian Wolf developed the idea for the systematic review and meta-analysis. All authors were involved in the conceptualization and design. The literature search and data analysis were performed by Britta Seiffer. The screening and data extraction were performed by Britta Seiffer and Sebastian Wolf. The first draft of the manuscript was written by Britta Seiffer and all authors critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ms. Seiffer, Prof. Hautzinger, Prof. Ulrich, and Dr. Wolf report no financial interests or potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Britta Seiffer  https://orcid.org/0000-0001-6088-8265
https://orcid.org/0000-0001-6088-8265
Sebastian Wolf  https://orcid.org/0000-0001-8238-5861
https://orcid.org/0000-0001-8238-5861
Supplemental material: Supplemental material for this article is available online.
References
- Ahn S., Fedewa A. L. (2011). A meta-analysis of the relationship between children's physical activity and mental health. J Pediatr Psychol, 36(4), 385-397. doi: 10.1093/jpepsy/jsq107 [DOI] [PubMed] [Google Scholar]
- Ainsworth B. E., Haskell W. L., Herrmann S. D., Meckes N., Bassett D. R., Jr., Tudor-Locke C., Leon A. S. (2011). 2011 compendium of physical activities: A second update of codes and MET values. Medicine & Science in Sports & Exercise, 43(8), 1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2017). ACSM’s guidelines for exercise testing and prescription. Alphen aan den Rijn: Wolters Kluwer. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, & DSM Task Force. (2017). Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association. [Google Scholar]
- Anastasopoulou P., Tansella M., Stumpp J., Shammas L., Hey S. (2012). Classification of human physical activity and energy expenditure estimation by accelerometry and barometry. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012, 6451–6454. 10.1109/EMBC.2012.6347471 [DOI] [PubMed] [Google Scholar]
- Anastasopoulou P., Tubic M., Schmidt S., Neumann R., Woll A., Hartel S. (2014). Validation and comparison of two methods to assess human energy expenditure during free-living activities. PLoS One, 9(2), e90606. 10.1371/journal.pone.0090606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram M. E., Assarian F., Atoof F., Taghadosi M., Akkasheh N., Akkasheh G. (2014). Effect of a 12-week interval running program on female primary school students with ADHD. Feyz Journal of Kashan University of Medical Sciences, 18(2), 151–158. [Google Scholar]
- Balish S. M., McLaren C., Rainham D., Blanchard C. (2014). Correlates of youth sport attrition: A review and future directions. Psychology of Sport and Exercise, 15(4), 429–439. 10.1016/j.psychsport.2014.04.003 [DOI] [Google Scholar]
- Battagliese G., Caccetta M., Luppino O. I., Baglioni C., Cardi V., Mancini F., Buonanno C. (2015). Cognitive-behavioral therapy for externalizing disorders: A meta-analysis of treatment effectiveness. Behaviour Research and Therapy, 75, 60–71. 10.1016/j.brat.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Becker L. M. (1998). The effects of exercise versus methylphenidate on attention and behavior in children with attention deficit hyperactivity disorder, predominantly inattentive type. (Vol. 58). ProQuest Information & Learning. [Google Scholar]
- Benzing V., Schmidt M. (2019). The effect of exergaming on executive functions in children with ADHD: A randomized clinical trial. The Scandinavian Journal of Medicine & Science in Sports, 29(8), 1243–1253. 10.1111/sms.13446 [DOI] [PubMed] [Google Scholar]
- Blumenthal J. A., Babyak M. A., Doraiswamy P. M., Watkins L., Hoffman B. M., Barbour K. A., Sherwood A. (2007). Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic Medicine, 69(7), 587–596. 10.1097/PSY.0b013e318148c19a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor W., Sanders M. R., Markie-Dadds C. (2002). The effects of the Triple P-positive parenting program on preschool children with co-occurring disruptive behavior and attentional/hyperactive difficulties. Journal of Abnormal Child Psychology, 30(6), 571–587. 10.1023/a:1020807613155 [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. (2011). Introduction to meta-analysis. Wiley. [Google Scholar]
- Butte N. F., Watson K. B., Ridley K., Zakeri I. F., McMurray R. G., Pfeiffer K. A., Fulton J. E. (2018). A youth compendium of physical activities: Activity codes and metabolic intensities. Medicine & Science in Sports & Exercise, 50(2), 246–256. 10.1249/MSS.0000000000001430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T., Morres I. D., Meade O., Callaghan P. (2016). The effect of exercise on depressive symptoms in adolescents: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 580–590. 10.1016/j.jaac.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Caspersen C. J., Powell K. E., Christenson G. M. (1985). Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports, 100(2), 126–131. [PMC free article] [PubMed] [Google Scholar]
- Cerrillo-Urbina A. J., Garcia-Hermoso A., Sanchez-Lopez M., Pardo-Guijarro M. J., Santos Gomez J. L., Martinez-Vizcaino V. (2015). The effects of physical exercise in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis of randomized control trials. Child: Care, Health and Development, 41(6), 779–788. 10.1111/cch.12255 [DOI] [PubMed] [Google Scholar]
- Chang Y. K., Labban J. D., Gapin J. I., Etnier J. L. (2012). The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research, 1453, 87–101. 10.1016/j.brainres.2012.02.068 [DOI] [PubMed] [Google Scholar]
- Chang Y. K., Liu S. Y., Yu H. H., Lee Y. H. (2012). Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Archives of Clinical Neuropsychology, 27(2), 225–237. 10.1093/arclin/acr094 [DOI] [PubMed] [Google Scholar]
- Choi J. W., Han D. H., Kang K. D., Jung H. Y., Renshaw P. F. (2015). Aerobic exercise and attention deficit hyperactivity disorder: Brain research. Medicine & Science in Sports & Exercise, 47(1), 33–39. 10.1249/MSS.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. C., Huang C. J. (2017). Effects of an 8-week yoga program on sustained attention and discrimination function in children with attention deficit hyperactivity disorder. PeerJ, 5, e2883. 10.7717/peerj.2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J., Taylor J. A., Sayal K. (2015). Parenting interventions for ADHD: A systematic literature review and meta-analysis. Journal of Attention Disorders, 19(10), 831–843. 10.1177/1087054714535952 [DOI] [PubMed] [Google Scholar]
- Cortese S., Adamo N., Del Giovane C., Mohr-Jensen C., Hayes A. J., Carucci S., Cipriani A. (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry, 5(9), 727–738. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L. P., Vanderloo L., Moore S., Faulkner G. (2019). Physical activity and depression, anxiety, and self-esteem in children and youth: An umbrella systematic review. Mental Health and Physical Activity, 16, 66–79. 10.1016/j.mhpa.2018.12.001 [DOI] [Google Scholar]
- Daley D., van der Oord S., Ferrin M., Danckaerts M., Doepfner M., Cortese S., European A. G. G. (2014). Behavioral interventions in attention-deficit/hyperactivity disorder: A meta-analysis of randomized controlled trials across multiple outcome domains. Journal of the American Academy of Child and Adolescent Psychiatry, 53(8), 835–847. 10.1016/j.jaac.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Davis C. L., Premji S., Ahn Y. J., Williams C., Looney J., Drescher C. F., Bustamante E. E. (2017). Effects of aerobic exercise on cognition and mental health symptoms in children with attention-deficit hyperactivity disorder. Annals of Behavioral Medicine, 51, S1005. [Google Scholar]
- Davis C. L., Tomporowski P. D., McDowell J. E., Austin B. P., Miller P. H., Yanasak N. E., Naglieri J. A. (2011). Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychology, 30(1), 91–98. 10.1037/a0021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro F. R. A., Vargas M. A. S. D., Rodriguez E. V. M. D., Tria G. E. D., Agnes M. C. A. D., Lopez A. V. D., Madrunio M. R. D. (2015). The effects of psychoeducation-cognitive exercises program on the executive functioning of children with signs and symptoms of attention deficit hyperactivity disorder. [Google Scholar]
- de Greeff J. W., Bosker R. J., Oosterlaan J., Visscher C., Hartman E. (2018). Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. The Journal of Science and Medicine in Sport, 21(5), 501–507. 10.1016/j.jsams.2017.09.595 [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Duval S., Tweedie R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Hillman C., Stillman C. M., Ballard R. M., Bloodgood B., Conroy D. E., & For Physical Activity Guidelines Advisory. (2019). Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Medicine & Science in Sports & Exercise, 51(6), 1242–1251. 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine H. E., Baxter A. J., Patton G., Moffitt T. E., Patel V., Whiteford H. A., Scott J. G. (2017). The global coverage of prevalence data for mental disorders in children and adolescents. Epidemiology and Psychiatric Sciences, 26(4), 395–402. 10.1017/S2045796015001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine H. E., Norman R. E., Ferrari A. J., Chan G. C., Copeland W. E., Whiteford H. A., Scott J. G. (2016). Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 55(10), 841–850. 10.1016/j.jaac.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Faraone S. V., Biederman J., Mick E. (2006). The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine, 36(2), 159–165. 10.1017/S003329170500471X [DOI] [PubMed] [Google Scholar]
- Feldman M. E., Charach A., Belanger S. A. (2018). ADHD in children and youth: Part 2-treatment. Paediatrics & Child Health, 23(7), 462–472. 10.1093/pch/pxy113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmet M. B. (1998). The effects of karate training on the levels of attention and impulsivity of children with attention deficit/hyperactivity disorder. University of Toledo. [Google Scholar]
- Fox S. M., Naughton J. P., Haskell W. L. (1971). Physical activity and the prevention of coronary heart disease. Annals of Clinical Research, 3(6), 404–432. [PubMed] [Google Scholar]
- Fritz K. M., O’Connor P. J. (2016). Acute exercise improves mood and motivation in young men with ADHD symptoms. Medicine & Science in Sports & Exercise, 48(6), 1153–1160. 10.1249/MSS.0000000000000864 [DOI] [PubMed] [Google Scholar]
- Garber C. E., Blissmer B., Deschenes M. R., Franklin B. A., Lamonte M. J., Lee I. M., & American College of Sports. (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine & Science in Sports & Exercise, 43(7), 1334–1359. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Garcia C. R. (2016). Fencing after school as a didactic proposal for attention deficit/hyperactivity disorder (ADHD). Cultura Ciencia Y Deporte, 11(33), 217–224. [Google Scholar]
- Garcia-Gomez A., Rodriguez-Jimenez M., Guerrero-Barona E., Rubio-Jimenez J. C., Garcia-Pena I., Moreno-Manso J. M. (2016). Benefits of an experimental program of equestrian therapy for children with ADHD. Research in Developmental Disabilities, 59, 176–185. 10.1016/j.ridd.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Gelade K., Janssen T. W. P., Bink M., Twisk J. W. R., van Mourik R., Maras A., Oosterlaan J. (2018). A 6-month follow-up of an RCT on behavioral and neurocognitive effects of neurofeedback in children with ADHD. European Child & Adolescent Psychiatry, 27(5), 581–593. 10.1007/s00787-017-1072-1 [DOI] [PubMed] [Google Scholar]
- Gelade K., Janssen T. W., Bink M., van Mourik R., Maras A., Oosterlaan J. (2016). Behavioral effects of neurofeedback compared to stimulants and physical activity in attention-deficit/hyperactivity disorder: A randomized controlled trial. The Journal of Clinical Psychiatry, 77(10), e1270–e1277. 10.4088/JCP.15m10149 [DOI] [PubMed] [Google Scholar]
- Hedges L. V. (2016). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics, 6(2), 107–128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- Hendry-Adams N. (2010). An experimental study on the effects of exercise on the attention and behaviour of primary age children with EBD and ADHD. [Google Scholar]
- Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M. (Eds.). (2019). Cochrane handbook for systematic reviews of interventions [version 6.0, updated July 2019]. www.training.cochrane.org/handbook
- Hoza B., Smith A. L., Shoulberg E. K., Linnea K. S., Dorsch T. E., Blazo J. A., McCabe G. P. (2015). A randomized trial examining the effects of aerobic physical activity on attention-deficit/hyperactivity disorder symptoms in young children. Journal of Abnormal Child Psychology, 43(4), 655–667. 10.1007/s10802-014-9929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali N., Shahrbabaki M. E., Sahebozamani M. (2015). The effect of exercise program in reducing symptoms of attention deficit/hyperactivity disorder in children. Iranian Journal of Psychiatry and Clinical Psychology, 20(4), 309–316. [Google Scholar]
- Jensen P. S., Kenny D. T. (2004). The effects of yoga on the attention and behavior of boys with attention-deficit/hyperactivity disorder (ADHD). Journal of Attention Disorders, 7(4), 205–216. 10.1177/108705470400700403 [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Rosen L. A. (2016). Sports behavior of ADHD children. Journal of Attention Disorders, 4(3), 150–160. 10.1177/108705470000400302 [DOI] [Google Scholar]
- Kang K. D., Choi J. W., Kang S. G., Han D. H. (2011). Sports therapy for attention, cognitions and sociality. International Journal of Sports Medicine, 32(12), 953–959. 10.1055/s-0031-1283175 [DOI] [PubMed] [Google Scholar]
- Kim H., Heo H. I., Kim D. H., Ko I. G., Lee S. S., Kim S. E., Kim C. J. (2011). Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neuroscience Letters, 504(1), 35–39. 10.1016/j.neulet.2011.08.052 [DOI] [PubMed] [Google Scholar]
- Ko I. G., Kim S. E., Kim T. W., Ji E. S., Shin M. S., Kim C. J., Bahn G. H. (2013). Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Molecular Medicine Reports, 8(2), 393–400. 10.3892/mmr.2013.1531 [DOI] [PubMed] [Google Scholar]
- Kunz R., Vist G., Oxman A. D. (2007). Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews, 2: MR000012. 10.1002/14651858.MR000012.pub2 [DOI] [PubMed] [Google Scholar]
- Lee H., Causgrove Dunn J., Holt N. L. (2014). Youth sport experiences of individuals with attention deficit/hyperactivity disorder. Adapted Physical Activity Quarterly, 31(4), 343–361. 10.1123/apaq.2014-0142 [DOI] [PubMed] [Google Scholar]
- McKune A. J., Pautz J., Lomjbard J. (2003). Behavioural response to exercise in children with attention-deficit/hyperactivity disorder. South African Journal of Sports Medicine, 15(3), 17–21. 10.17159/2078-516X/2003/v15i3a223 [DOI] [Google Scholar]
- Memarmoghaddam M., Torbati H. T., Sohrabi M., Mashhadi A., Kashi A. (2016). Effects of a selected exercise programon executive function of children with attention deficit hyperactivity disorder. The Journal of Medicine and Life, 9(4), 373–379. [PMC free article] [PubMed] [Google Scholar]
- Mercurio L. Y., Amanullah S., Gill N., Gjelsvik A. (2019). Children with ADHD engage in less physical activity. Journal of Attention Disorders, 25(8): 1187–1195. 10.1177/1087054719887789 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntaner-Mas A., Ortega F. B., Femia P., Kiive E., Eensoo D., Mäestu J., Harro J. (2021). Low cardiorespiratory fitness and obesity for ADHD in childhood and adolescence: A 6-year cohort study. Scandinavian Journal of Medicine & Science in Sports, 31(4), 903–913. 10.1111/sms.13905 [DOI] [PubMed] [Google Scholar]
- National Guideline Centre (UK). (2018). NICE guideline No. 87: Attention deficit hyperactivity disorder: Diagnosis and management (Vol. 12). National Institute for Health and Care Excellence (UK). [PubMed] [Google Scholar]
- Neudecker C., Mewes N., Reimers A. K., Woll A. (2015). Exercise interventions in children and adolescents with ADHD: A systematic review. Journal of Attention Disorders, 23(4), 307–324. 10.1177/1087054715584053 [DOI] [PubMed] [Google Scholar]
- Oh Y., Joung Y. S., Jang B., Yoo J. H., Song J., Kim J., Jeong B. (2018). Efficacy of hippotherapy versus pharmacotherapy in attention-deficit/hyperactivity disorder: A randomized clinical trial. Journal of Alternative and Complementary Medicine, 24(5), 463–471. 10.1089/acm.2017.0358 [DOI] [PubMed] [Google Scholar]
- Pan C. Y., Chang Y. K., Tsai C. L., Chu C. H. (2014). Effects of exercise intervention in youths with attention-deficit hyperactivity disorder. Research Quarterly for Exercise and Sport, 85, 83–84. [Google Scholar]
- Pan C. Y., Chu C. H., Tsai C. L., Lo S. Y., Cheng Y. W., Liu Y. J. (2016). A racket-sport intervention improves behavioral and cognitive performance in children with attention-deficit/hyperactivity disorder. Research in Developmental Disabilities, 57, 1–10. 10.1016/j.ridd.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Pfiffner L. J., Yee Mikami A., Huang-Pollock C., Easterlin B., Zalecki C., McBurnett K. (2007). A randomized, controlled trial of integrated home-school behavioral treatment for ADHD, predominantly inattentive type. Journal of the American Academy of Child and Adolescent Psychiatry, 46(8), 1041–1050. 10.1097/chi.0b013e318064675f [DOI] [PubMed] [Google Scholar]
- Pontifex M. B. (2013). Transient modulations of inhibitory control in children with ADHD: The effect of a single bout of physical activity (Vol. 73). ProQuest Information & Learning. [Google Scholar]
- Riera M., Castells X., Tobias A., Cunill R., Blanco L., Capella D. (2017). Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: Meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology, 234(17), 2657–2671. 10.1007/s00213-017-4662-1 [DOI] [PubMed] [Google Scholar]
- Rivera-Brown A. M., Frontera W. R. (2012). Principles of exercise physiology: Responses to acute exercise and long-term adaptations to training. PM&R, 4(11), 797–804. 10.1016/j.pmrj.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Schünemann H., Brożek J., Guyatt G., Oxman A. (2013). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. guidelinedevelopment.org/handbook
- Schwartz K., Verhaeghen P. (2008). ADHD and Stroop interference from age 9 to age 41 years: A meta-analysis of developmental effects. Psychological Medicine, 38(11), 1607–1616. 10.1017/S003329170700267X [DOI] [PubMed] [Google Scholar]
- Schwarzer G. (2007). Meta: An R package for meta-analysis. R News, 7(3), 40–45. [Google Scholar]
- Scudder M. R., Lambourne K., Drollette E. S., Herrmann S. D., Washburn R. A., Donnelly J. E., Hillman C. H. (2014). Aerobic capacity and cognitive control in elementary school-age children. Medicine & Science in Sports & Exercise, 46(5), 1025–1035. 10.1249/mss.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L. A. D., Doyenart R., Henrique Salvan P., Rodrigues W., Felipe Lopes J., Gomes K., Silveira P. C. (2019). Swimming training improves mental health parameters, cognition and motor coordination in children with attention deficit hyperactivity disorder. International Journal of Environmental Health Research, 30(5), 1–9. 10.1080/09603123.2019.1612041 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J. (2002). Psychological heterogeneity in AD/HD–A dual pathway model of behaviour and cognition. Behavioural Brain Research, 130(1–2), 29–36. 10.1016/s0166-4328(01)00432-6 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J. (2003). The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neuroscience & Biobehavioral Reviews, 27(7), 593–604. 10.1016/j.neubiorev.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J., Brandeis D., Cortese S., Daley D., Ferrin M., Holtmann M., European A. G. G. (2013). Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. The American Journal of Psychiatry, 170(3), 275–289. 10.1176/appi.ajp.2012.12070991 [DOI] [PubMed] [Google Scholar]
- Soori R., Goodarzvand F., Akbarnejad A., Effatpanah M., Ramezankhani A., Teixeira A. L., Ghram A. (2020). Effect of high-intensity interval training on clinical and laboratory parameters of adolescents with attention deficit hyperactivity disorder. Science & Sports, 35, 4. 10.1016/j.scispo.2019.08.002 [DOI] [Google Scholar]
- Sterne J. A. C., Savovic J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., Higgins J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ, 366, 14898. 10.1136/bmj.14898 [DOI] [PubMed] [Google Scholar]
- Storebø O. J., Elmose Andersen M., Skoog M., Joost Hansen S., Simonsen E., Pedersen N., Gluud C. (2019). Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. The Cochrane Database of Systematic Reviews, 6(6), Cd008223. 10.1002/14651858.CD008223.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebo O. J., Ramstad E., Krogh H. B., Nilausen T. D., Skoog M., Holmskov M., Gluud C. (2015). Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). The Cochrane Database of Systematic Reviews, 11, CD009885. 10.1002/14651858.CD009885.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantillo M., Kesick C. M., Hynd G. W., Dishman R. K. (2002). The effects of exercise on children with attention-deficit hyperactivity disorder. Medicine & Science in Sports & Exercise, 34(2), 203-212. 10.1097/00005768-200202000-00004 [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Firth J., Schuch F. B., Rosenbaum S., Probst M., Ward P. B., Stubbs B. (2016). Dropout from physical activity interventions in children and adolescents with attention deficit hyperactivity disorder: A systematic review and meta-analysis. Mental Health and Physical Activity, 11, 46–52. 10.1016/j.mhpa.2016.09.002 [DOI] [Google Scholar]
- Verret C., Gardiner P., Beliveau L. (2010). Fitness level and gross motor performance of children with attention-deficit hyperactivity disorder. Adapted Physical Activity Quarterly, 27(4), 337–351. 10.1123/apaq.27.4.337 [DOI] [PubMed] [Google Scholar]
- Verret C., Guay M. C., Berthiaume C., Gardiner P., Beliveau L. (2012). A physical activity program improves behavior and cognitive functions in children with ADHD: An exploratory study. Journal of Attention Disorders, 16(1), 71–80. 10.1177/1087054710379735 [DOI] [PubMed] [Google Scholar]
- Wolraich M. L., Hagan J. F., Jr., Allan C., Chan E., Davison D., Earls M. Adolescents with Attention-Deficit/Hyperactive. (2019). Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics, 144(4), e20192528. 10.1542/peds.2019-2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L., Egger M., Gluud L. L., Schulz K. F., Juni P., Altman D. G., Sterne J. A. (2008). Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ, 336(7644), 601–605. 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Physical activity in adolescents: Key facts and figures. http://www.euro.who.int/__data/assets/pdf_file/0018/303480/HBSC-No.7_factsheet_Physical.pdf?ua=1
- Xue Y., Yang Y., Huang T. (2019). Effects of chronic exercise interventions on executive function among children and adolescents: A systematic review with meta-analysis. British Journal of Sports Medicine, 53(22), 1397–1404. 10.1136/bjsports-2018-099825 [DOI] [PubMed] [Google Scholar]
- Zang Y. (2019). Impact of physical exercise on children with attention deficit hyperactivity disorders: Evidence through a meta-analysis. Medicine (Baltimore), 98(46), e17980. 10.1097/MD.0000000000017980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jad-10.1177_10870547211017982 for The Efficacy of Physical Activity for Children with Attention Deficit Hyperactivity Disorder: A Meta-Analysis of Randomized Controlled Trials by Britta Seiffer, Martin Hautzinger, Rolf Ulrich and Sebastian Wolf in Journal of Attention Disorders