Abstract
The novel severe acute respiratory syndrome viral disease outbreak due to SARS-CoV-2 is a rapidly evolving disease and represents one of the greatest medical challenges in recent times. It is believed that SARS-CoV-2 has migrated from bats to an intermediate host and then to humans. This article aims at the mechanism and management of prothrombotic state in COVID-19 positive patients. We tried to present how the SARS-CoV-2 virus can induce thromboembolic events and the incidence of these thromboembolic events. We also tried to depict anticoagulation management in these patients as well as postdischarge plan and follow-up. Invasion of type 2 pneumocytes by the SARS-CoV-2 virus is critical in the course of illness because it results in activation of immune cells leading to elevation of cytokines. The subsequent activation of T cells and macrophages infiltrates the infected myocardial cells causing direct myocardiocyte toxicity and development of arrhythmia. Hypoxia or hypotension during the clinical course causes a mismatch between myocyte oxygen supply and workload demand resulting in cardiac distress. SARS-CoV-2 affects endothelial cells and pericytes that lead to severe micro and macrovascular dysfunction, and together with oxygen supply-demand mismatch, immune hyperresponsivity can potentially cause destabilization and plaque rupture causing acute coronary syndromes. Other mechanisms of injury include myocarditis, pericarditis, stress cardiomyopathy, vasculitis, and DIC (Disseminated intravascular coagulation)/microthrombi. SARS-CoV-2 enters the cells by the Spike protein S whose surface unit, S1, binds to the ACE2 receptor on the host cell. The type II transmembrane serine proteases TMPRSS2 and histone acetyltransferases (HAT) are host cell proteases that are recruited by the virus to cleave ACE2 surface protein S which facilitates the viral entry. Therefore, TMPRSS2 and HAT could be targeted for potential drugs against SARS-CoV-2. SARS-CoV-2 uses an RNA-dependent RNA polymerase for proliferation, which is targeted by remdesivir that is currently approved for emergency use by Food and Drug Administration (FDA). We need to adopt a multifaceted approach when combating SARS-CoV-2 because it presents several challenges including medical, psychological, socioeconomic, and ethical. COVID-19 is the biggest calamity during the 21st century, we need to have a keen understanding of its pathophysiology and clinical implications for the development of preventive measures and therapeutic modalities.
Keywords: anticoagulation of choice, cardiovascular complications, COVID-19, prothrombotic state
Introduction
Coronavirus disease-2019 is a spectrum of disease ranging from severe pneumonia complicated with acute respiratory distress syndrome (ARDS) to mild flu like symptoms, anemia or even no symptoms. Corona means “crown” or “garland” in Latin; it was used to describe the virus when it was first discovered due to its spike-like capsid. It is an enveloped, single-stranded (positive-sense) RNA virus, associated with a nucleoprotein within a capsid composed of matrix protein. Most human coronaviruses are one of two stereotypes: OC43-like and 229E-like. Although initially we have seen cases with pneumonia, ARDS and respiratory failure are the complications of SARS-COV-2, but later in the course we have seen many cases with multiorgan failure.
SARS-CoV-2 binds to angiotensin converting enzyme type-2 (ACE-2) receptors of human cells, and after entering into the cells, it leads to the down regulation of ACE2 receptors. 1 As ACE2 receptors are broadly distributed in the heart and vascular systems, which makes these systems prospective targets for SARS-CoV-2 infection. So, ACE2-related signaling pathways could possibly play the key role in mediating myocardial injury. Mechanisms that also contribute in myocardial and vascular injuries include endothelial cell dysfunction, with an elevated level of von Willebrand factor as well as systemic inflammation, by Toll-like receptor activation; and a pro-coagulatory state, induced by tissue factor pathway activation. Thromboembolic manifestations include pulmonary embolism, acute coronary syndrome, deep vein thrombosis (DVT), and CVA (cerebrovascular accident). A study showed an overall 24% [95% confidence interval (CI), 17–32%] incidence of acute pulmonary embolism in patients with SARS-CoV-2 infection, where 50% cases are in intensive care unit (ICU) and 18% cases in other patients.
The incidence of venous thromboembolism (VTE) on the wards (10%) is lower than in the ICU (48%) at day 14. Another study of 143 patients hospitalized with SARS-CoV-2 infection with age range of 63 ± 14 years, 74 (51.7%) men, 66 patients had lower extremity DVT [among which 23 (34.8%) with proximal DVT and 43 (65.2%) with distal DVT]. In China, there are also some reports showing neurologic symptoms in almost 36% of patients hospitalized with SARS-CoV-2 infection. 2
Pathophysiology
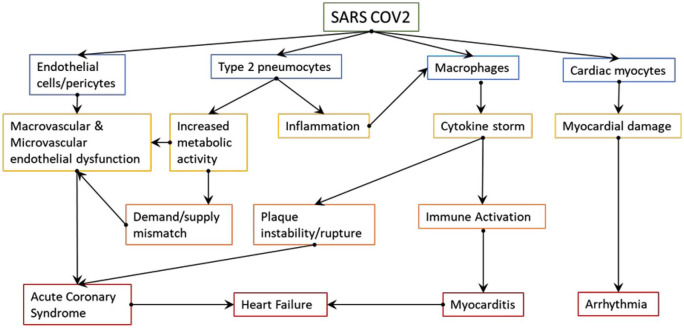
There is growing evidence of information on thrombosis related to SARS-Cov-2 infection. Patients often are admitted with coagulopathy and venous, or much less frequently, arterial thrombosis and resultant ischemia, during their recovery phase or much earlier in systemic inflammation phase. 3 While there are occasional case reports about patients displaying antiphospholipid antibody syndrome or other inherited thrombophilia, but the majority are due to various mechanisms ranging from vasculitis/capillaries and thrombotic microangiopathy to sepsis-induced coagulopathy and life-threatening diffuse intravascular coagulation. 4 The mechanisms of activation of the coagulation system in SARS-CoV-2 infection are not so different from those seen in infections with other viruses such as HIV, Ebola, or dengue, but unlike the latter two, SARS-Cov-2 does not appear to cause hemorrhagic manifestations or significant bleeding. Coronavirus-induced VTE occurs through various pathophysiological mechanisms set into motion by “cytokine storm” including severe systemic inflammation caused by activation of Toll-like receptors, endothelial dysfunction, characterized by an increase of intracellular Von Willebrand factor and finally by activation of intrinsic coagulation cascade through tissue factor release facilitated by a procoagulant state. Vascular response to hypoxia regulated mainly by hypoxia-inducible transcription factors, seen in models of thrombosis in animal and human as well and indirect mechanisms involving immune-mediated thrombosis through antiphospholipid antibodies, are also speculated to be involved (Figure 1).
Figure 1.
Pathophysiology of SARS-CoV-2 mediated cardiac injury-key manifestations. 5
Inflammatory hyperresponsiveness in SARS-CoV-2 infection can be evident with increased interleukin 6 (IL-6), C-reactive protein, and fibrinogen levels. 5 This inflammation causes elevated D-dimer levels because of the prothrombotic state, worsened by endothelial dysfunction and damage. Early studies from Wuhan noted that these pro-inflammatory cytokines are much elevated in ICU than in non-ICU patients. The higher the systemic inflammation is at presentation, primarily characterized by SIRS (Systemic Inflammatory Response Syndrome) markers such as Ferritin, LDH (Lactate Dehydrogenase), CRP (C-Reactive Protein), the higher are the D-dimer levels. Moreover, elevated d-dimer levels at admission or worsening D-dimer levels during the illness are associated with increased mortality. The degree of elevation of IL-6 directly correlates with the rise of D-dimers, establishing a link between inflammation associated with SARS-CoV-2 infection and procoagulant state. Pathogen-associated thrombosis happens through activation of various host defense systems leading to further activation of coagulation cascade and thrombosis. Polyphosphates derived from organisms, complement activation, and neutrophil extracellular traps in thrombi capturing cell-free DNA and histones lead to activation of platelets, mast cells, and factor XII further igniting the intrinsic coagulation cascade and thrombosis. Endothelial cell invasion by SARS-CoV-2 leads to inflammatory cell invasion, activation of transcription factors influencing apoptotic cell death, and release of procoagulant contents. Circulating serine protease inhibitors such as Protein C, antithrombin, and C1 esterase inhibitor decrease in severe inflammation. Critically ill patients who received specific serine protease inhibitors such as antithrombin or thrombomodulin may have improved survival in post hoc analyses. As coagulation continues, the resultant decrease in coagulation factors associated with increased fibrinolysis may be similar to the fibrinolytic phase in DIC, which might occur in the advanced period of illness by rising levels of D-dimers and clinically concomitant worsening sepsis with multiorgan failure (Table 1).
Table 1.
High sensitivity cardiac troponin concentrations (ng/L) as quantitative variable. 6
| High sensitivity cardiac Troponin I (ng/L) | COVID-19 severity | Associated condition |
|---|---|---|
| 0 | COVID-19 mild | Healthy (no preexisting cardiac condition) |
| ⩽20 | COVID-19 mild | |
| >20–100 | COVID-19: severe | Small T1MI, PE, HF, ARDS |
| >100 & above | T1MI, Myocarditis, Takotsubo, Shock |
ARDS, acute respiratory distress syndrome; HF, heart failure; PE, pulmonary embolism; T1MI, type 1 myocardial infarction.
Mild elevations (up to 100 ng/L) can be explained by prior cardiac disease and COVID-19-induced cardiac injury in case of mild to severe COVID-19. But >100 ng/L should raise the concern of T1MI, Myocarditis, Takotsubo, Shock T1MI (type 1 MI), pulmonary embolism (PE), heart failure (HF), and acute respiratory distress syndrome (ARDS).
Coronavirus infection as in other systemic infections like influenza that cause severe inflammation, hypoxia, hypovolemia, and thrombosis has profound effects on the cardiac muscle as elucidated above. Cardiac troponins which is a marker of cardiac myocyte injury or death is elevated most often than not in patients, especially who have had a prior cardiac illness, that suffered moderate to severe infection causing ICU admission or death. In these patients, the troponins have increased in level in conjunction with the progression of disease to ARDS. Moreover, marked elevations of high sensitivity troponins (>5 times ULN – upper limit of normal) were seen in patients with acute respiratory failure needing mechanical ventilation, T1 myocardial infarction, severe dilated cardiomyopathy, Takotsubo, or myocarditis. Concentrations of troponin T/I remained near normal or normal in majority of patients who had no or mild infection.
B-type natriuretic peptide/N-terminal pro B-type natriuretic peptide (BNP/NT-ProBNP) also are shown to be elevated as is expected when there is increased hemodynamic strain, heart failure, or cardiomyopathy causing ventricular dilation.In addition, worsening pulmonary status causing right ventricular pressure or volume overload, existing heart failure or cardiovascular disease in patients who contracted COVID seemed to exhibit higher levels of these peptides. The exact mechanisms involved in elevation of cardiac markers is still unknown, but a fair assessment of these markers will provide early opportunities to detect and manage acute cardiac events that may otherwise be masked by overwhelming inflammation and end-organ damage often associated with severe coronavirus infection.
The vascular endothelial dysfunction causing microcirculatory changes in SARS-CoV-2 infection may have significant effects on cardiac myocardium. In addition to sepsis-induced coagulopathy and cytokine storm, the direct viral tropism to and replication in endothelial cells, demonstrated by viral inclusions ultimately leading to endothelial apoptosis and mononuclear and polymorphonuclear infiltration as described on post mortem studies. 7 Thrombotic microangiopathy due to procoagulant state and activation of tissue factor pathway, mismatch of myocardial oxygen supply and demand due to hypotension or hypoxia, thrombotic microangiopathy involving coronary microcirculation or acute thrombosis or rupture of atherosclerotic plaque affecting coronary macro circulation or more subtle changes such as eccentric left ventricular hypertrophy and dilated cardiomyopathy lead to decreased cardiac output, cardiogenic shock, end-organ hypoperfusion, and injury. 8 The same mechanisms may also be involved in causing acute cerebrovascular accidents in these patients. It is essential to understand the pathophysiology of thrombosis and resultant cardiomyocyte injury to diagnose, design, and implement specific treatment modalities in patients afflicted with coronavirus infection. 9
Receptor signaling
Studying receptor signaling provides a wealth of information about the entry of SARS-CoV-2 virus in human host cells, its pathogenesis and infectivity, potential targets for immune surveillance, and intervention strategies. Coronaviruses are a positive-polarity, single-stranded RNA viruses whose genome encodes for four structural proteins, nuclear capsid (N), matrix (M), envelope (E), and surface glycoprotein (S, comprising two subunits S1 and S2). The trimeric spike surface protein facilitates viral entry by interacting with host cell surface receptors. Although ACE2 is widely known as a primary host cell receptor for viral entry, CD147 (BSG) is emerging as a possible SARS-CoV-2 receptor, distributed in all cell types suggesting the presence of other routes of viral entry.
As the virus prepares to invade the host cell, it uses the host cell proteases to cleave its surface protein into two domains (also called priming); the S1 ectodomain recognizes and attaches to the ACE2 receptor while the S2 C-terminal domain facilitates cell binding and subsequent entry of the virus. 10 This mechanism is not uncommon in other infections such as MERS (Middle East Respiratory Syndrome), paramyxoviruses, influenza, or the SARS-CoV-2, which also use lysosomal proteases (such as cathepsins B, L) for their entry. Thus, the level of these proteases in specific tissues determines the infectivity of that tissue, hence the primary involvement of the lung and gastrointestinal tract in SARS-CoV-2 infection, which exhibits high protease activity. There are various proteases involved at various stages of the SARS-CoV-2 virus-cell cycle, such as type II transmembrane serine proteases (TTSP) family (serine TMPRSS2 and TMPRSS4 transmembrane proteases, HAT), neutrophil elastase. It is important to note that Camostat is a serine protease inhibitor that may be protective.
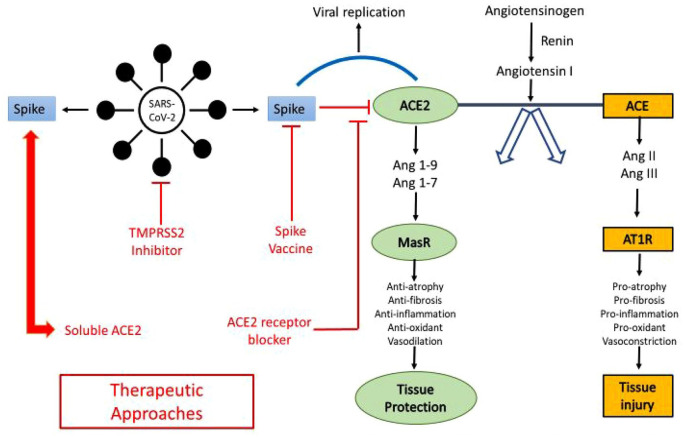
It is unknown if ACE2 levels compatible with proneness to SARS-CoV-2 infection. ACE2 plays a significant role in attenuating inflammation and fibrosis, and also assists in cardiorenal homeostasis of blood pressure by controlling the renin-angiotensin-aldosterone axis through the conversion of angiotensin II to angiotensin (1–7). angiotensin (1–7) inhibits inflammation by impeding the resistin/TLR4 (Toll Like Receptor 4)/MAPK (Mitogen-activated Protein kinase)/NF-kB (Nuclear Factor Kappa B) pathway (Figure 2).
Figure 2.
SARS-CoV-2 invasion into host cells. 1
Zhu et al. 11 ACE2 is expressed higher in heart than in the kidneys and more so when there is preexisting coronary artery disease or heart failure. In a rat model of ischemic heart disease, administering an ACE2 activator such as diminazene aceturate (DIZE), an anti-trypanosomal drug subcutaneously, increased myocardial ACE2 mRNA expression and protease activity, decreased ACE mRNA expression and ameliorated cardiac remodeling. Once SARS-CoV-2 binds to the ACE2 receptor, it removes the anti-inflammatory properties of ACE2 by limiting its catalytic activity. The resultant lack of angiotensin (1–7) production may exacerbate lung insult and cardiovascular morbidity. In a murine model, excess lung inflammation worsened the abdominal aortic aneurysms induced by angiotensin II. Also, mice lacking in the TLR3/TLR4 adaptor TRIF (TIR-domain-containing adapter-inducing interferon b had a higher infection rate and inflammation due to SARS-CoV. 12 Acute myocardial injury by SARS-CoV-2 might be the outcome of inhibition of ACE2 activity. Understandably, ACE inhibitors and ARBs (Angiotensin Receptor Blockers) may be protective by curbing the proinflammatory effect of angiotensin II. However, patients with severe COVID infection and morbidity have concurrent diabetes with nephropathy, hypertension, or preexisting coronary artery disease and were already on these medications leading to speculation that they might be affecting adversely in COVID infection. Nevertheless, many international societies of cardiology debunked this hypothesis.
Upon entry into host cells, coronaviruses, unlike other viral, bacterial, or parasitic organisms, activate aryl hydrocarbon receptors (AhRs) directly independent of IDO1-kynurenine-AhR signaling pathway. These AhRs potentiate their activity via the IDO1-kynurenine-AhR positive feedback loop. The direct activation and potentiation of AhRs through indoleamine 2,3-dioxygenase-1 (IDO1) independent mechanism causes subsequent rapid activation of various downstream effectors, devolving into systemic AhR activation syndrome, leading to systemic hyper inflammation, thrombosis, and fibrosis and culminating in multiorgan failure and death. Activation of AhRs depends on various host factors, including age, gender, comorbidities, physical activity, diet, and other environmental activities, each of which can stimulate AhRs separately. There are no specific therapies currently available against the AhR gene or IDO1 currently, but it is interesting to note that dexamethasone works against AhR and IDO1 genes, vitamin D3 can downregulate the AhR gene, and Vitamin E may suppress IDO1 gene. Lack of physical activity also stimulates the IDO1-kynurenine-AhR signaling pathway and increases the risk of SARS-CoV-2, infection or its complications, and therefore, physical exercise during the COVID pandemic outbreaks should be encouraged. 13
SARS-CoV-2 invades enterocytes and other intestinal cells and affects the activation of mediators associated with type I and III interferons. The sentinel event in innate immune response marked by activation of interferon-I (IFN-I)-associated mediators is suppressed in SARS-CoV-2 infection, thereby facilitating viral replication. This relative postponement of IFN-I signaling pathways results in abnormal immune response, extensive vascular leak, impaired T-cell activation to virus-specific components, and consequent damage to vital organs. Increasing extracellular ATP is shown to facilitate IFN-I secretion through P38/JNK/ATF-2 signaling pathway, and cells deprived of ATP during hypoxia or hypotension are more vulnerable in SARS-CoV and possibly in COVID-19 infection. In addition, pretreatment with IFN-I affects the replication of SARS-CoV-2. Prevention of extracellular ATP from hydrolysis and enhancing the building of adenosine to maintain the ATP levels, thereby ensuring IFN-I activation, the earliest phase of the innate immune response, can be potentially be achieved using antibodies to CD39 and CD73 ectonucleotidases.14,15 Alternatively, the administration of Anti-CD73 monoclonal antibodies, together with A2AR antagonists, which remove the compensatory inhibition of A2AR that increases CD73 levels, has demonstrated a synergistic effect in the treatment of metastatic tumors and can also be beneficial in the treatment of COVID-19 infection.
The control of SARS-CoV-2 infection needs a tailored and highly synchronized host immune response, determined by the activation of receptor named pattern recognition receptors (PRR) induced by pathogen-associated molecular patterns (PAMPs). 11 Viral RNA is a PAMP that activates toll-like receptors, which are one type of PRR. Although TLR3 senses double-stranded RNA, TLR7, and TLR8 sense single-stranded RNA, RIG-I senses short dsRNA and specific patterns on ssRNA and MDA-5 senses long dsRNA, it remains mostly unknown which sensors are involved in identifying SARS-CoV-2 genomic material. However, in vitro studies showed, the Matrix protein of SARS-CoV-2 induces interferon-beta through toll-like-receptor-related TRAF3 (Tumor Necrosis Factor Receptor–Associated Factor) independent mechanism. In an in vitro study conducted by Haga et al., inhibiting TACE prevents host cell entry of SARS-CoV. In an in vivo study done in pairs, France, 16 poly IC, a TLR3 ligand, strengthens the signaling potential of the receptors through the expression of TMPRSS2 and resultantly inducing the expression of various proinflammatory mediators such as CCL2 (Chemokine Ligand 2), KC, and IL-1. TLR3 and TLR4 agonists may work synergistically to give protection against COVID infection and simultaneous induction of TLR pathways through TRIF and MyD88, both of which are critical to host immune response.
Disease severity determines the expression of genes involved in up trending of inflammatory chemokine genes in SPP1+ monocyte-derived macrophages populations (CCL2, CCL3, CCL4, CCL7, and CCL8), and expression of genes like HMOX1 and HIF1A are responsible for hypoxia as well as oxidative stress, and reduction of major histocompatibility complex (MHC) class II (HLA-A and HLA-DQA1) and some type I IFN genes (IFIT1 and OAS1). Dust cells also showed a severity-associated increase in the level of the chemokines CCL18 and CCL4 L2 and the cathepsins CTSL and CTSB. Interleukins such as IL-6 and IL-8 are involved in exaggerated host immune response seen in severe COVID-19 infection. These dissimilarities between the mild and severe COVID-19, patients account for a specific inflammatory response and can serve as a target for potential immunotherapy treatment of severe infection.
Presentation
Most of the patients with the SARS-CoV-2 have usually mild to moderate clinical presentation. Severe presentations have mainly pulmonary involvement. Cardiac complications from COVID-19 infection have given an understanding into other major predictors of mortality. Cardiac involvement manifests as myocardial injury and myocarditis, pericarditis, acute myocardial infarction (AMI), HF and cardiomyopathy, shock, arrhythmias and cardiac arrest, and venous thromboembolic event. 17
SARS-CoV-2 mediated severe systemic inflammation may cause AMI by disruption of atherosclerotic plaque. A study conducted back in 2018 showed that influenza and some other viruses were associated with an increased risk of AMI within the first 7 days of a viral illness. The incidence ratio was 6.1 and 2.8 for influenza and other viruses, respectively. A different study on patients hospitalized with community-acquired pneumonia showed an elevated risk of active cerebrovascular diseases that remained present for many years after hospital admission. SARS-CoV-2 induces immense inflammation and hypercoagulability, which leads to AMI. 10
Patients with COVID-19 are also at an elevated risk of developing VTE, most commonly pulmonary emboli. If patients with COVID-19 infection present with sudden onset of hypoxia, respiratory distress, hypotension, it may suggest pulmonary embolism. 17 An elevation in D-dimer values supports clinical findings. Although D-dimer is a nonspecific acute-phase reactant, elevated D-dimer level has been used to identify those severe COVID-19 patients at increased VTE risk. A study conducted on 25 patients with COVID-19 pneumonia showed an elevated D-dimer level in all patients (a median of 6.06 micrograms/ml), with 10 of them having a pulmonary embolism (PE) diagnosed by computed tomography pulmonary angiography (CTPA). Patients with confirmed PE on CTPA were found to have an average D-dimer level of 11.07 micrograms/ml. An elevated risk of death is observed in COVID-19 patients with a D-Dimer level greater than 1 μg/ml during hospitalization (odds ratio 18.4)
Another study conducted in the Netherlands included 184 critically ill patients; among them, 27% of patients had imaging-confirmed venous thromboembolic events, 81% of whom (25 patients) had a pulmonary embolism (8) whereas, DVT was detected in one patient.18,19
A study conducted on a group of 5,119 patients with COVID-19 disease revealed 44 patients (0.86%) had ischemic stroke, and 18 (41%) received this diagnosis during the 14-day risk interval. The incidence ratio of ischemic stroke as compared with the control period was 12.9 (95% CI, 7.1–23.5) in the risk period. Seventeen patients (0.33%) had AMI, and four (23.5%) received this diagnosis during the 14-day risk interval. The incidence ratio of AMI as compared with control period was 5.9 (95% CI, 1.9–18.2) in this risk period. 18
COVID-19-related extremity/digital ischemia is another thromboembolic manifestation we have encountered. In Wuhan, China, seven cases of limb ischemia were observed in severely ill patients with COVID pneumonia. None of them met the criteria for shock and were not any vasopressors. They had different degrees of acral ischemia, seen in the form of plantar plaques and acrophytic bruises. 20 Although digital/extremity ischemia is seen in ICU /critically ill patients, we also have seen an exceptional case of digital ischemia of the right thumb in our hospital, who presented with only digital ischemia without having any respiratory symptoms. Laboratory evidence was supported by an elevation in the D-Dimer, serum ferritin, and CRP levels.
Management
The cytokine storms induced by proinflammatory cytokines were observed in critically ill patients with COVID-19. Cytokine storms lead to cardiovascular collapse, multiple organ dysfunction, and death. A study in China suggests COVID-19-induced cytokine storms are mainly regulated by IL-6.
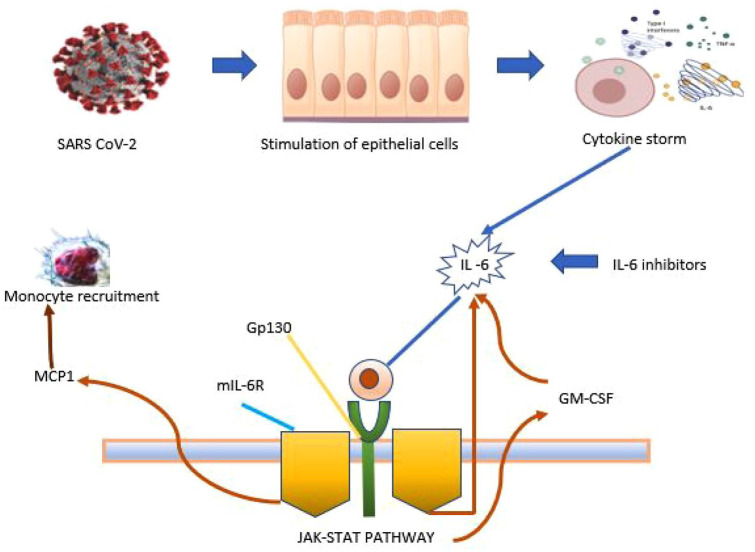
Tocilizumab, an IL-6 receptor blocker that recognizes the IL-6 binding site of the human IL-6 receptor and inhibits the IL-6 signaling pathway through the IL-6 binding site’s competitive blockade, hence found to be beneficial for patients with severe COVID-19 infection (Figure 3).
Figure 3.
Mechanism of action of tocilizumab. 19
GM-CSF, granulocyte-macrophage-colony stimulating factor; Gp130, glycoprotein 130; IL-6, interleukin 6; MCP1, monocyte chemoattractant protein-1; mIL-6R, membrane interleukin-6 receptor.
In a clinical trial on 21 patients in China, all 21 patients included met the criteria for severe COVID-19, including one of the following: shortness of breath; respiratory rate > 30 bpm; oxygen saturation < 93% while resting; or PaO2/FiO2 ratio ⩽ 300 mmHg. Critical criteria included one of the following: Respiratory failure needing mechanical ventilation/patients with Shock/requiring admission to ICU with organ failure. After few days of treatment with tocilizumab, the body temperature of patients with fever (all 21 patients initially had a fever) returned to normal with a noticeable improvement in all other symptoms. Of 21 patients, 15 (75%) had reduced oxygen requirement, and 1 patient did not require oxygen. Computed tomography (CT) showed that 90.5% (19/21) of the patients had resolved pulmonary insults. Laboratory examination showed lymphocytes, and CRP returned to normal. This study is inadequate as the IL-6 level before the treatment of tocilizumab is reported, whereas the level of IL-6 after treatment was not.
Another study suggested patients treated with tocilizumab had a significantly greater survival rate than control patients after adjusting for other baseline clinical criteria. Two out of 62 patients died in the tocilizumab group, whereas 11 out of 23 died in the control group. The recovery rate in patients discharged on tocilizumab was 92% compared with 42% in the control group. Respiratory function improvement was noted in 64.8% of the hospitalized patients on tocilizumab, whereas 100% of controls deteriorated and required mechanical ventilation.
Remdesivir, a nucleotide analogue that inhibits viral RNA polymerases has shown in vitro activity against SARS-CoV-2. In a cohort of hospitalized patients with severe COVID-19 infection, treated with remdesivir, 36 patients out of 53(68%) showed improvement. COVID-19 coagulopathy management is a multifaced approach. Diagnostic approaches start with clinical examinations and are confirmed by imaging studies (venous echo-Doppler ultrasound, echocardiography, and CTPA). All patients with COVID-19 should undergo coagulopathy screening on admission, especially D-dimer, PT, and platelet count. Trending levels of these coagulopathy markers are suggested as there is a high risk of coagulopathy in these patients over the course. On admission, patient should be started on prophylactic dose of LMWH (Low Molecular Weight Heparin), unless any contraindication such as AKI (Acute Kidney Injury) where unfractionated heparin is the treatment of choice.
A study showed the usual 75–80 units/kg loading dose and 18 units/kg/h maintenance dose for treatment of VTE for patients with severe renal dysfunction is associated with supra-therapeutic levels. A loading dose of 60 units/kg and 12 units/kg/h maintenance dose is used for patients with severe kidney dysfunction. This dosing regimen is recommended by acute coronary syndrome (ACS) treatment guidelines where a lower target APTT (Activated Partial Thromboplastin Time) range of 1.5–2 is desired. This dose minimization is still expected to achieve minimum therapeutic anticoagulation [APTT > 1.5] for patients treated for VTE, even though at the lower end of the range, despite the 33% dose reduction. Eventually, doses should be adjusted to maintain a therapeutic level (APTT 1.5–2.5). 20
An observational study suggested that higher anticoagulation targets for thromboprophylaxis of severe COVID-19 patients could decrease the rate of thrombosis. In this study, prophylactic group patients were treated with standard or reinforced prophylactic dosage of heparin (low molecular weight heparin LMWH-enoxaparin – up to 6000 IU/12 h SC(SUBCUTANEOUS), and therapeutic group patients were treated with LMWH at dose 100 IU/kg/12 h SC(SUBCUTANEOUS) based on weight, without exceeding 10,000 IU/12 h. 21
Khan et al. reported the use of 1.5 mg/kg LMWH (enoxaparin) daily in patients with D-dimer levels > 1000 μg/L in the ICU. LMWH (enoxaparin) is frequently used anticoagulation in VTE prophylaxis. Different doses of LMWH were used in patients with COVID-19 admitted to the hospital due to the lack of sufficient data on the dose of LMWH. In a recent study, the association between the prophylactic (0.5 mg/kg twice daily) and therapeutic dose (1 mg/kg twice daily) of LMWH, and mortality in COVID-19 patients was searched retrospectively, and it was verified that the use of the therapeutic dose of enoxaparin reduced mortality in patients with severe COVID-19.22,23
Another study conducted on hospitalized patient with COVID-19 showed both prophylactic- and treatment-dose anticoagulation were associated with lower in-hospital mortality compared with no anticoagulation. 24
The beneficial effect of prophylactic heparin is well described in a study by Dr. Ning Tang, where 449 patients with severe COVID-19 were included; 99 patients received LMWH at prophylactic doses. No difference was observed in the 28-day mortality in patients who received heparin compared with those who did not; when a SIC (sepsis-induced coagulopathy) score ⩾ of 4 was to be applied to the patients, anticoagulant therapy with LMWH appears to be associated with a better prognosis to mortality (40.0% versus 64.2%, p = 0.029). Patients with COVID-19 infection with a D-dimer > six-fold of the upper limit of normal (32.8% versus 52.4%, p = 0.017) were shown improvement with LMWH use.
LMWH has several nonanticoagulant properties, such as it decreases the release and biological activity of IL-6. Low-molecular-weight heparin binds to SARS-CoV-2 and blocks viral replication. Patients infected with COVID-19 who are treated with LMWH have lower IL-6 levels and higher lymphocyte counts than those who did not receive LMWH.
In addition, a significant reduction in the D-dimer level and fibrinogen degradation products is observed with LMWH use, indicating an improvement in these patients’ hypercoagulable states. LMWH is beneficial as it inhibits the release of IL-6 along with the increase in lymphocyte count that hinders the deadly cytokine storm.
In the critical care unit, unfractionated heparin is the treatment of choice for its short duration of action and no proven interactions with the experimental drugs used in COVID-19 treatment. Trending aPTT is a good way to monitor, but it may be misleading by increased acute-phase protein FVIII. The aXa test is the alternate option to aPTT (Tables 2 and 3).
Table 2.
Pharmacotherapies used in COVID-19 infection (ClinicalTrials.gov).
| Pharmacotherapy | Mechanism of action | Trial number | Trial | Primary outcome |
|---|---|---|---|---|
| Hydroxychloroquine and chloroquine | It increases endosomal pH and inhibits viral fusion to the cell membrane thus blocks viral entry | NCT04358081 | Hydroxychloroquine Therapy Alone and in Combination With Azithromycin in Moderate to Severe COVID-19 Infection. | To show in patients receiving standard of care that the percentage who achieve clinical improvement with hydroxychloroquine or hydroxychloroquine and azithromycin is better than placebo at day 15. |
| Hinders glycosylation of ACE2 and decreases affinity of ACE2 receptor for SARS-Cov-2 virus | ||||
| Favipiravir | Inhibit RNA polymerase thus inhibits viral replication | NCT04358549 | Study on the Use of Favipiravir in Subjects With COVID-19 Who Required Hospitalization | To decide on the effect of favipiravir + SOC versus SOC on clearance of COVID-19 virus; measured by nasopharyngeal and oropharyngeal sampling. |
| Lopinavir | Protease inhibitor, inhibits viral replication and viral release from host cells | NCT04328012 | COVID MED Trial – Comparison of Different Therapeutics for Hospitalized Patients With SARS-CoV-2 Infection. | The difference in NCOSS scores between the various treatment groups. |
| Remdesivir | Inhibit RNA-dependent RNA polymerase | NCT04292899 | Study to Determine the Safety and Antiviral Activity of Remdesivir in Subjects With Severe COVID-19 Infection | The odds magnitude relation depicts the chances of improvement within the ordinal scale between the treatment teams. The ordinal scale is an associate assessment of the clinical standing on a given day. Each day, the worst score from the previous day are recorded and documented. The dimensions are as follows: (1) death; (2) hospitalized, on invasive mechanical ventilation or extracorporeal membrane action (ECMO); (3) hospitalized, on BiPAP/CPAP or high flow oxygen; (4) hospitalized, requiring low flow supplemental atomic number; (5) hospitalized, not on supplemental oxygen but requiring current treatment (coronavirus (COVID-19) connected or otherwise); (6) hospitalized, not on supplemental oxygen, and not needed current treatment (other than per-protocol remdesivir administration; (7) no hospitalization |
| IL-6 inhibitors | Block IL-6 receptor thus prevents inflammatory cascades | NCT04356937 | To Determine the Effectiveness of Tocilizumab on Patients With COVID-19 Infection | The primary outcome is the time from administering the investigational therapeutics (or placebo) to requiring intubation or death of subjects who die before intubation. |
| Eculizumab | Eculizumab binds to the terminal complement component C5, thus hinders prothrombotic and proinflammatory sequences mediated by C5. | NCT04346797 | CORIMUNO19-ECU: Trial on Efficaciousness and Safety of Eculizumab (Soliris) in Patients With COVID-19 Infection, Nested Within the CORIMUNO-19 Cohort | Survival and not requiring of intubation, events considered are intubation or death. |
| Convalescent plasma | Exhibit neutralization activity against virus | NCT04343755 | Treatment With Convalescent Plasma for Hospitalized Subjects With COVID-19 Infection | Patients hospitalized with COVID-19 however not intubated [time frame: 7 days], Mechanical ventilation rate at day 7 from beginning treatment in hospitalized patients |
| The primary objective for patients with COVID-19 already intubated [time frame: 30 days], Mortality rate at 30 days from starting treatment for patients with COVID-19. |
ACE2, angiotensin converting enzyme type-2; ECMO, extracorporeal membrane oxygenation; IL-6, interleukin 6; NCOSS, NIAID COVID-19 Ordinal Severity Scale; RNA, ribonucleic acid; SOC, Standard of Care.
Table 3.
Ongoing trials on use of nebulized UFH (Unfractionated Heparin) in COVID-19 infection (ClinicalTrials.gov Identifier: NCT04397510).
| Nebulized unfractionated heparin inhibits fibrin deposition and sequential microvascular thrombosis. There are trials in patients with acute lung insult, and related conditions showed that inhaled unfractionated heparin reduced pulmonary dead space and coagulation activation, thereby preventing microvascular thrombosis and clinical deterioration, resulting in increased time free of mechanical ventilation. | ||
| NCT04530578 | Percentage of patients requiring mechanical ventilation [Time Frame: 15 days] PaO2 / FiO2 < 200 on blood gas (or the inability to maintain a SpO2 of 92% with a venturi mask). Acute respiratory failure (pH lower than 7.35 with PaCO2 more than 45 mmHg) | Nebulized Heparin in COVID-19-related acute severe respiratory syndrome (NEBUHEPA) |
| NCT04545541 | Alive and Mechanical Ventilator Free Score [time frame: day
28] Validated hierarchical composite outcome, depending on mortality and ventilator free days, which is less likely to favor a treatment with discordant effects on survival and days free of mechanical ventilation. |
Nebulized heparin in patients with severe COVID-19 |
| NCT04397510 | Mean daily PaO2 to FiO2 ratio [time frame: 10 days] | Treatment of COVID-19induced lung insult by nebulized heparin |
Oral anticoagulants, such as warfarin, dabigatran (direct thrombin inhibitor), apixaban (factor Xa inhibitors), rivaroxaban, edoxaban, and betrixaban are not used in the treatment of COVID-19-related thrombosis as there is a possible interaction with antiviral therapeutics. Antiplatelet therapy (e.g. aspirin/DAPT) shows no beneficial effect as VTE prophylaxis in COVID-19 patients who needed hospitalization.
Patients present with signs of massive or high-risk PE as like hypotension or hemodynamic instability; a bedside echocardiogram followed by thrombolytic therapy is the right choice of action. If there is refractory circulatory collapse or cardiac arrest, extracorporeal membrane oxygenation (ECMO) is preferred, along with surgical intervention (embolectomy or catheter-directed procedure).
Swiss Medical Weekly states,
In patients in intensive care with a large increase in D-dimers, severe inflammation, or signs of hepatic or renal dysfunction or imminent respiratory failure, intermediate or therapeutic dosing of LMWH or unfractionated heparin should be considered, according to the bleeding risk.
As per National Institutes of Health (NIH) guideline, for nonhospitalized patients with COVID-19, there are no recent data to support the measurement of coagulation markers (e.g. D-dimers, prothrombin time, platelet count, fibrinogen). For patients with COVID-19 who do not require hospitalization, anticoagulants and antiplatelet therapy should not be started for the prevention of venous or arterial thromboembolism unless the patient has other indications for anticoagulation or is participating in a clinical trial.
Prognosis
A New York–based study showed the effect of therapeutic dose anticoagulation in patients with COVID-19. Out of 2773 hospitalized patients, 786 (23%) were on therapeutic AC. This study’s outcome is different on patients who were on mechanical ventilation than those who were not. In patients on mechanical ventilation, in-hospital mortality was lower for those receiving anticoagulation than those not receiving anticoagulation. The general population of patients who received anticoagulation showed in-hospital mortality of 22.5% with a median survival of 21 days, compared with 22.8% and 14 days in those not receiving anticoagulation.
Another small case series of three patients on mechanical ventilation in the intensive care unit reported the use of systemic tPA (25 mg over 2 hours followed by another 25 mg over the subsequent 22 hours. There is an improvement in ventilatory parameters, but the long-term outcome of using tPA is yet unknown.
A second case series on the effect of aerosolized freeze-dried plasminogen in COVID-19 patients of different stages of illness (moderate/severe/critical). There is a significant improvement in oxygenation and ventilatory parameters. 21
Postdischarge plan
No study showed a beneficial effect of routine postdischarge use of prophylactic anticoagulation is not recommended in COVID-19 patients. Food and Drug Administration approved postdischarge prophylaxis for some high-risk patients; in these cases, rivaroxaban 10 mg daily for 31 to 39 days, and betrixaban 160 mg on Day 1, followed by betrixaban 80 mg once a day for 35 to 42 days are preferred.
Modified IMPROVE-VTE score, age, D-dimer level are included as selection criteria for this study. Postdischarge use of VTE prophylaxis depends on the individual patient’s risk factors, including reduced mobility, bleeding risks, and feasibility.
Conclusion
The incidence of major thrombotic events in patients with COVID-19 is raising concern in treating this patient population. The level of D-dimer and fibrinogen degradation product in COVID-19 patients is proportionate to the degree of illness and an indicator of mortality. COVID-19-related coagulopathy may reflect unregulated hemostasis and is different from sepsis-induced disseminated intravascular coagulopathy. Multiple factors are related to COVID-19-associated coagulopathy; hence, knowing the pathophysiology will help in proper management.
Acknowledgments
I hereby acknowledge the contributions from all authors mentioned above in writing, editing, reviewing the manuscript.
Footnotes
Author contributions: Trishna Acherjee, Aparna Behara, Muhammad Saad and Timothy J. Vittorio
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Trishna Acherjee  https://orcid.org/0000-0002-7571-2075
https://orcid.org/0000-0002-7571-2075
Contributor Information
Trishna Acherjee, Internal Medicine, BronxCare Health System, Bronx, NY-10457, USA.
Aparna Behara, Internal Medicine, BronxCare Health System, Bronx, NY, USA.
Muhammad Saad, Internal Medicine, BronxCare Health System, Bronx, NY, USA.
Timothy J. Vittorio, Department of Cardiology, BronxCare Health System, Bronx, NY, USA
References
- 1. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 2020; 117: 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med 2020; 25: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020; 20: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Michieli L, Ola O, Knott J, et al. High-sensitivity cardiac troponin T for the detection of myocardial injury and risk stratification in COVID-19. Clin Chem 2021; 67: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bandyopadhyay D, Akhtar T, Hajra A, et al. COVID-19 pandemic: cardiovascular complications and future implications. Am J Cardiovasc Drugs 2020; 20: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation 2020; 141: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 10. Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect 2020; 53: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu H, Rhee JW, Cheng P, et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep 2020; 22: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 2015; 6: e00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turski WA, Wnorowski A, Turski GN, et al. AhR and IDO1 in pathogenesis of Covid-19 and the ‘systemic AhR activation syndrome:’ a translational review and therapeutic perspectives. Restor Neurol Neurosci 2020; 38: 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abouelkhair MA. Targeting adenosinergic pathway and adenosine A2A receptor signaling for the treatment of COVID-19: a hypothesis. Med Hypotheses 2020; 144: 110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bost P, Giladi A, Liu Y, et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell 2020; 181: 1475–1488.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sallenave JM, Guillot L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in Covid-19: key therapeutic targets. Front Immunol 2020; 11: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020; 116: 1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020; 142: 2080–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19 – immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol 2020; 11: 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes S, Szeki I, Nash MJ, et al. Anticoagulation in chronic kidney disease patients – the practical aspects. Clin Kidney J 2014; 7: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helms J, Severac F, Merdji H, et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-center cohort study. Ann Intensive Care 2021; 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan IH, Savarimuthu S, Leung MST, et al. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg 2020; 72: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canoglu K, Saylan B. Therapeutic dosing of low-molecular-weight heparin may decrease mortality in patients with severe COVID-19 infection. Ann Saudi Med 2020; 40: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattioli M, Benfaremo D, Mancini M, et al. Safety of intermediate dose of low molecular weight heparin in COVID-19 patients. J Thromb Thrombolysis 2020; 51: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]