Abstract
It is well known that exercise increases the activity of thyroid glands and raises the blood level of melatonin. The increase of melatonin during exercise may be linked to a rise in thyroid-stimulating hormone (TSH). No previous study has investigated the combined effects of melatonin ingestion and acute submaximal exercise on thyroid hormones’ responses. The purpose of this pilot study was to explore the effects of daytime ingestion of melatonin on thyroid hormones’ responses to acute submaximal exercise. After 50 min of either melatonin (6 mg) or placebo ingestion, eight physical education students (mean ± standard deviation of age: 22 ± 1 years) were asked to run for 45 min at 60% of their maximum aerobic speed. Free thyroxine (fT4) and TSH were measured in plasma samples before and immediately after exercise. After submaximal exercise, TSH increased by 54% in both placebo and melatonin conditions. There was no significant (Condition × Exercise) interaction, and no significant condition effect for TSH. The fT4 remained unchanged before/after submaximal exercise in both placebo [15.2 (1.9) and 15.0 (1.6) pmol/L, respectively, p > .05], and melatonin [16.7 (2.7) and 16.3 (2.7) pmol/L, respectively, p > .05] conditions. There was no significant (Condition × Exercise) interaction, no significant exercise effect, and no significant condition effect for fT4. To conclude, acute melatonin ingestion did not affect thyroid hormones’ responses to submaximal exercise.
Keywords: biological rhythm, inflammation, metabolism, oxidative stress, thyrotropic axis
Introduction
It is well known that glands activity and hormones production (i.e., cortisol and thyroid hormones) are affected by physical activity (Deligiannis et al., 1993; Dergaa, Ben Saad, et al., 2021; Souissi et al., 2021). The thyroid is one of the most affected glands by the submaximal exercise (Deligiannis et al., 1993; Gagnon et al., 2014; Hackney & Saeidi, 2019). Triiodothyronine (T3) and thyroxine (T4) are both produced by the thyroid gland and can be detected in the free forms (i.e., fT3, fT4—free thyroxine). Thyroid hormones are widely known for their role in thermoregulation and fatty acid oxidation (Gullu et al., 2004). Thyrotropin-releasing hormone is produced by the hypothalamus, which stimulates the pituitary gland to generate thyroid-stimulating hormone (TSH; Gullu et al., 2004). The adenohypophysis produces TSH, which stimulates the production of thyroid hormones (i.e., T3 and T4). Therefore, TSH is a thyroid function marker (Kosack et al., 2012). TSH influences fat synthesis, mobilization, and breakdown, as well as the amount of catecholamine receptors on cell surfaces, which influences heart rate (Mullur et al., 2014; Souissi et al., 2021). In trained athletes, increased activity of the thyroid hormones, as well as the adrenal cortex, plays a major role in adaptations to physical exercise (Deligiannis et al., 1993). Submaximal exercise results in elevated blood TSH levels (Ciloglu et al., 2005). It has been reported that TSH secretion possibly increases both during and hours after prolonged submaximal exercise to increase metabolism (Canali & Kruel, 2001; Ciloglu et al., 2005; Pardini, 2001).
Interestingly, the increase of TSH after exercise may be associated with an increase in melatonin secretion (Escames et al., 2012; Van Reeth et al., 1994). However, the relation between melatonin, exercise, and TSH in the morning is not yet well established. Melatonin (N-acetyl-5-methoxytryptamine), which is the pineal gland’s primary product, regulates circadian rhythm, sleep, and sexual behavior (Dergaa, Varma, et al., 2021; Pandi-Perumal et al., 2008; Souissi et al., 2019). Melatonin is known to have antioxidant and anti-inflammatory effects (Escames et al., 2012; Kruk et al., 2021; Souissi et al., 2018, 2019; Watson et al., 2016). Several researchers have looked into the effects of melatonin on endurance exercise (Alonso et al., 2006; Maldonado et al., 2012; Souissi et al., 2018, 2019; Veneroso et al., 2009). A single dose of exogenous melatonin, given before submaximal exercise, was reported to reduce oxidative stress, inflammation, and muscle damage (Alonso et al., 2006; Kruk et al., 2021; Maldonado et al., 2012; Veneroso et al., 2009).
To resume, because physical exercise increases the activity of thyroid glands (Deligiannis et al., 1993; Dergaa, Ben Saad, et al., 2021; Souissi et al., 2021) and rises the blood level of melatonin (Escames et al., 2012), the increase of melatonin during exercise may be linked to a rise in TSH. However, to the best of the authors’ knowledge, no previous study has investigated the acute combined effects of daytime melatonin ingestion and exercise on humans’ thyroid hormones’ responses. The present pilot study examined the possible effects of daytime acute ingestion of melatonin on fT4 and TSH responses to submaximal exercise. We hypothesized that melatonin ingestion may favor the rise of TSH during exercise.
Population and Methods
Participants
Only healthy physical education male students participated in this study. Participants were physically active and had normal corpulence status (body mass index: 18.5–24.9 kg/m2). The choice of student is argued by our tendency to focus on population who trained moderately. Participants were asked to refrain from exercise, alcohol, and caffeine-containing drinks for at least 24 hr before the measurements started. A cardiologist checked participants’ medical history and reported that all participants were healthy and nonsmokers. The absence of any participant during the second/third sessions of the protocol was applied as an exclusion criterion. Participants were classified as neither morning nor evening type according to the Horne and Ostberg (1976) questionnaire. Participants have signed an informed consent, after receiving a complete verbal description of the protocol. The study protocol was in accordance with the Helsinki Declaration for conducting human experimentation and was approved by the Farhat HACHED ethical committee, Sousse, Tunisia (FH/1609021).
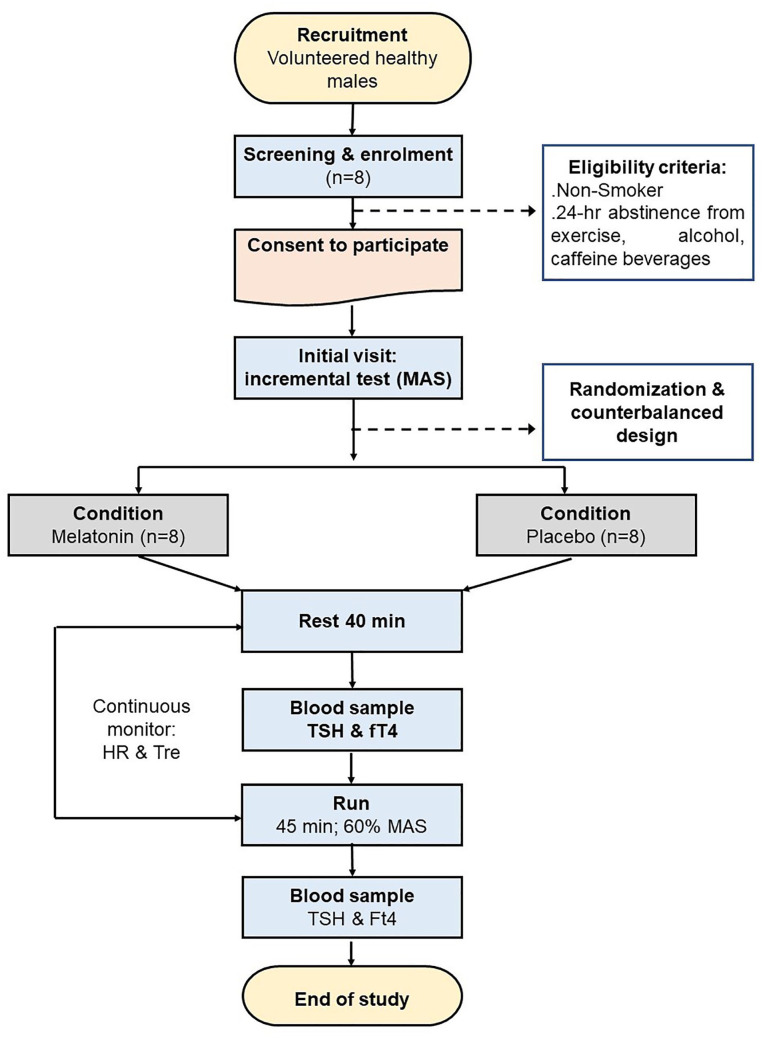
At 09:50 of the preliminary visit, an incremental test to exhaustion was used to determine maximum aerobic speed (MAS; Hagin et al., 2015). The experimental conditions (placebo or melatonin) were randomized and counterbalanced. The intervals between conditions were at least equal to 24 hr. Exercise is performed at 23°C ± 0.1°C (60% ± 3% humidity). All conditions were performed indoors at the same time of the day (i.e., between 8:00 and 10:35) to minimize the effects of diurnal variations in the aerobic and anaerobic contributions to physical performance (Dergaa, Varma, et al., 2021; Souissi et al., 2020). Figure 1 illustrates the study design.
Figure 1.
Flowchart of the Study’s Methodology
Note. MAS = maximum aerobic speed; HR = heart rate; Tre = rectal temperature; TSH = thyroid-stimulating hormone; fT4 = free thyroxine.
Sample Size
The sample size was calculated according to the following formula (Kang et al., 2008): N = Zα2 s2/d2, where “s” is the standard deviation (SD = 0.415 µIU/mL) and “d” is the accuracy of estimate or how close it is to the true mean (M = 0.29 µIU/mL). Given the pioneer character of our study, the “s” was collected from the study of Deligiannis et al. (1993), including 15 athletes who swam front crawl for 30 min at a moderate speed. At 20°C, their TSH increased by 90.4%, from 1.15 ± 0.42 µIU/mL (pre-exercise) to 2.19 ± 0.41 µIU/mL (post-exercise). “Zα” is the normal deviate for a one-tailed alternative hypothesis at a level of significance (Zα is equal to 1.64 at an error rate of 0.05%). The appraised sample size gives a sample of six participants. The assumption of 30% of nonattendance during the conditions gives a revised sample of eight participants [= 5.5/(1 – 0.30)].
Experimental Protocol
From 08:20 to 09:00, participants rested in a seated position. At 09:00, the participant ingested whether the 6 mg of quick-release vegetable melatonin (Jamieson Laboratories, Canada) or placebo capsule with 500 mL of water before resting for 50 min (Figure 1). A heart rate monitor (Polar RS800, Polar Electro, FIN-90440 KEMPELE, Finland) and a rectal probe (Universal YSI400 Adults, China) were used to continually record the heart rate and rectal temperature, respectively. Heart rate was expressed as a percentage of the predicted maximal heart rate (= 220 – age (year)) (Robergs & Landwehr, 2002). Blood samples were collected at 09:40 (before exercise). The treadmill (Finnlo by HAMMER, Germany) workout began at 09:50. Participants ran at 60% of their MAS for 45 min. Then, blood samples were recollected (after exercise) for measuring TSH and fT4 levels using immune-electrochemiluminescence on an automated Cobas e411 machine (Roche Diagnostics, USA).
Statistical Analyses
All the statistical analyses were performed using Statistical Software, Version 10.0, for Windows (StatSoft, Maisons-Alfort, France). The results were presented as the mean ± standard deviation throughout the text. The data were compared using repeated-measures analysis of variance. When necessary, the Bonferroni post hoc was applied to identify significant differences. Effect sizes were calculated as partial eta-squared η . The level of significance was predetermined to be p < .05 for all statistical analyses.
Results
Eight healthy physical education students were included. Table 1 illustrates their anthropometric parameters. All the participants undergoing submaximal exercise successfully completed the exercise. Rectal temperature exceeds 38°C in both conditions. Heart rate increased progressively in both conditions without reaching the predicted maximal heart rate. In placebo condition, the heart rate increased from 74% of predicted maximal heart rate (at 5 min of exercise) to 85% of predicted maximal heart rate (at the end of the exercise). In melatonin condition, the heart rate increased from 73% of predicted maximal heart rate (at 5 min of exercise) to 83% of predicted maximal heart rate (at the end of the exercise). Participants seem to be under the same physiological stress in both conditions during the exercise.
Table 1.
Characteristics of Participants (N = 8).
| Data | Mean ± SD |
|---|---|
| Age (years) | 22 ± 1 |
| Height (cm) | 178 ± 2 |
| Weight (kg) | 67.1 ± 2.8 |
| Body fat (%) | 20.7 ± 2.1 |
| Muscle mass (%) | 47.1 ± 1.5 |
| Bone mass (kg) | 9.3 ± 0.3 |
Note. Data were mean ± standard deviation.
fT4 was stable before/after submaximal exercise in both placebo [15.2 (1.9) and 15.0 (1.6) pmol/L, respectively, p > .05] and melatonin [16.7 (2.7) and 16.3 (2.7) pmol/L, respectively, p > .05] conditions (Figure 2). There was no significant (Condition × Exercise) interaction, F(1, 7) = 0.26, p = .62, = 0.03; no significant exercise effect, F(1, 7) = 0.79, p = .40, = 0.10; and no significant condition effect, F(1, 7) = 1.81, p = .21, = 0.20.
Figure 2.
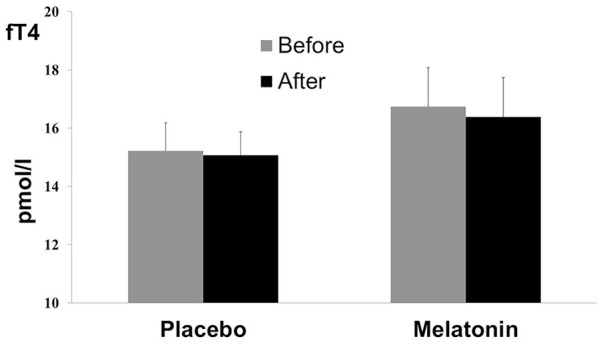
Free Thyroxine (fT4) Measures (Mean ± Standard Deviation) Before and After Acute Submaximal Exercise of 45 Min in Placebo and Melatonin Conditions
TSH was increased after submaximal exercise in both placebo [by 54%: from 1.18 (0.99) to 1.82 (1.90) µIU/mL, p < .001] and melatonin [by 54%: from 1.08 (0.99) to 1.67 (1.83) µIU/mL, p < .001] conditions (Figure 3). There was no significant (Condition × Exercise) interaction, F(1, 7) = 0.25, p = .63, = 0.03, and no significant condition effect, F(1, 7) = 1.27, p = .29, = 0.15.
Figure 3.
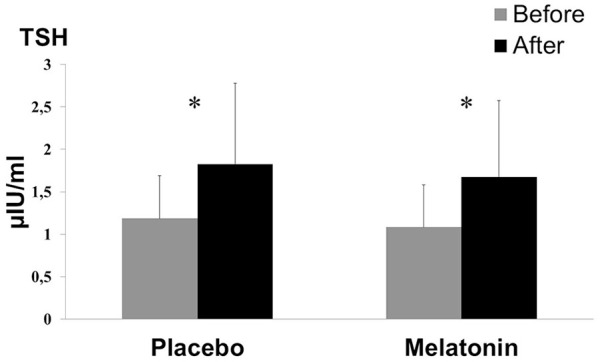
Thyroid-Stimulating Hormone (TSH) Measures (Mean ± Standard Deviation) Before and After Acute Submaximal Exercise of 45 Min in Placebo and Melatonin Conditions
*Significant difference compared with rest (p < .001).
Discussion
To the finest of the authors’ knowledge, this is the first investigation that evaluated the effects of daytime ingestion of melatonin on thyroid hormones’ responses to acute submaximal exercise in healthy active males. The main result of the present study was that daytime melatonin ingestion did not affect thyroid hormones’ responses to acute submaximal exercise.
In conditions with two levels (i.e., placebo and melatonin), TSH increased by 54% after submaximal exercise (p < .001). This finding is in line with the one of Deligiannis et al. (1993), who reported that swimming for 30 min at 20°C boosted TSH levels considerably. Furthermore, Deligiannis et al. (1993) identified that TSH increased by 90.4% after swimming at 20°C, remained unchanged at 26°C, and then declined at 32°C (Deligiannis et al., 1993). In fact, it has been reported that when exposed to cold, metabolic rates increase, whereas when exposed to heat and/or during exercise-induced hyperthermia (Febbraio et al., 1996; Souissi et al., 2019, 2021), metabolic rates drop (Dempsey & Astwood, 1943; Febbraio, 2001; Febbraio et al., 1996). Thyroid hormones stimulate sympathetic nerves that innervate brown adipose tissue to produce metabolic heat and act on skeletal muscle to produce heat (Cannon & Nedergaard, 2010). Thyroid hormones can act as a barrier to effective heat dissipation in hyperthermia conditions (Bowen et al., 1984; McMurray & Hackney, 2000). Therefore, the decrease in fT4 during submaximal exercise-induced hyperthermia by muscle working (Souissi et al., 2021) could be interpreted as an adaptive mechanism to hyperthermia. However, our finding indicated that fT4 did not show a significant decrease after submaximal exercise. Indeed, it is still unknown whether a longer duration of exercise or a hot condition than the ones used in our investigation would have decreased significantly plasma fT4. We highlight that thyroid function depends according to the exercise intensity, thermoregulatory control, and perhaps to other factors such as specific characteristics of the participants (Ciloglu et al., 2005; Deligiannis et al., 1993).
Given the pioneer character of our study, which aims to explore the impacts of acute daytime ingestion of melatonin on TSH and fT4 responses to submaximal exercise, a part of the discussion will concern the chronic effects of melatonin ingestion. The literature reported evidence for a net beneficial effect of chronic melatonin administration (El-Gendy et al., 2018; Potes et al., 2019). The latter is reported to decrease pituitary levels and increase plasma levels of TSH in animals (Gordon et al., 1980; Panda & Turner, 1968). Animal studies have also demonstrated that melatonin interacts with other hormones to alleviate heat stress possibly with T4 and successfully modifies the adrenal function to relieve thermal stress (Sejian & Srivastava, 2010). Contrary to chronic melatonin ingestion, the results of the present study indicated that acute melatonin ingestion has no effect on plasma TSH levels. Therefore, it would be interesting to investigate in the future the effects of chronic melatonin ingestion on plasma TSH response to submaximal exercise.
Study Limitations
Although our study has direct practical implications, because it is the first one coupling the effects of melatonin and submaximal exercise on thyroid hormones’ secretion, its major limitation was that end-exercise values of melatonin, TSH, and fT4 were not corrected for plasma volume changes (Alis et al., 2015). In addition, it was better to analyze thyroid hormones, before and after (e.g., 30 or 60 min after the end of the exercise) the physical exercise, not only at the end of the exercise, as done in our study.
To conclude, the daytime ingestion of melatonin before 50 min of acute submaximal exercise did not favor the increase in TSH in response to exercise.
Acknowledgments
The authors would like to thank all the students for their participation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Amine Souissi  https://orcid.org/0000-0003-2072-2425
https://orcid.org/0000-0003-2072-2425
Helmi Ben Saad  https://orcid.org/0000-0002-7477-2965
https://orcid.org/0000-0002-7477-2965
References
- Alis R., Sanchis-Gomar F., Primo-Carrau C., Lozano-Calve S., Dipalo M., Aloe R., Blesa J. R., Romagnoli M., Lippi G. (2015). Hemoconcentration induced by exercise: Revisiting the Dill and Costill equation. Scandinavian Journal of Medicine & Science in Sports, 25(6), e630–e637. 10.1111/sms.12393 [DOI] [PubMed] [Google Scholar]
- Alonso M., Collado P. S., Gonzalez-Gallego J. (2006). Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. Journal of Pineal Research, 41(1), 8–14. 10.1111/j.1600-079X.2006.00323.x [DOI] [PubMed] [Google Scholar]
- Bowen S. J., Washburn K. W., Huston T. M. (1984). Involvement of the thyroid gland in the response of young chickens to heat stress. Poultry Science, 63(1), 66–69. 10.3382/ps.0630066 [DOI] [PubMed] [Google Scholar]
- Canali E., Kruel L. (2001). Hormonal responses to exercise. Revista Paulista de Educação Física, 15(2), 141–153. [Google Scholar]
- Cannon B., Nedergaard J. (2010). Thyroid hormones: Igniting brown fat via the brain. Nature Medicine, 16(9), 965–967. 10.1038/nm0910-965 [DOI] [PubMed] [Google Scholar]
- Ciloglu F., Peker I., Pehlivan A., Karacabey K., Ilhan N., Saygin O., Ozmerdivenli R. (2005). Exercise intensity and its effects on thyroid hormones. Neuroendocrinology Letters, 26(6), 830–834. https://www.ncbi.nlm.nih.gov/pubmed/16380698 [PubMed] [Google Scholar]
- Deligiannis A., Karamouzis M., Kouidi E., Mougios V., Kallaras C. (1993). Plasma TSH, T3, T4 and cortisol responses to swimming at varying water temperatures. British Journal of Sports Medicine, 27(4), 247–250. 10.1136/bjsm.27.4.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey E. W., Astwood E. B. (1943). A determination of the rate of thyroid hormone secretion at various environmental temperatures. Endocrinology, 32(6), 509–518. 10.1210/endo-32-6-509 [DOI] [Google Scholar]
- Dergaa I., Ben Saad H., Romdhani M., Souissi A., Fessi M. S., Yousfi N., Masmoudi T., Souissi N., Ammar A., Hammouda O. (2021). Biological responses to short-term maximal exercise in male police officers. American Journal of Men’s Health, 15(4), 15579883211040920. 10.1177/15579883211040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergaa I., Varma A., Musa S., Chaabane M., Ben Salem A., Fessi M. (2021). Diurnal variation: Does it affect short-term maximal performance and biological parameters in police officers? International Journal of Sport Studies for Health, 3(2). https://www.scilit.net/article/bb3b36da053802886913e61f90943c2c [Google Scholar]
- El-Gendy F. M., El-Hawy M. A., Hassan M. G. (2018). Beneficial effect of melatonin in the treatment of neonatal sepsis. The Journal of Maternal-Fetal & Neonatal Medicine, 31(17), 2299–2303. 10.1080/14767058.2017.1342794 [DOI] [PubMed] [Google Scholar]
- Escames G., Ozturk G., Bano-Otalora B., Pozo M. J., Madrid J. A., Reiter R. J., Serrano E., Concepcion M., Acuna-Castroviejo D. (2012). Exercise and melatonin in humans: Reciprocal benefits. Journal of Pineal Research, 52(1), 1–11. 10.1111/j.1600-079X.2011.00924.x [DOI] [PubMed] [Google Scholar]
- Febbraio M. A. (2001). Alterations in energy metabolism during exercise and heat stress. Sports Medicine, 31(1), 47–59. 10.2165/00007256-200131010-00004 [DOI] [PubMed] [Google Scholar]
- Febbraio M. A., Snow R. J., Stathis C. G., Hargreaves M., Carey M. F. (1996). Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Experimental Physiology, 81(4), 685–693. 10.1113/expphysiol.1996.sp003969 [DOI] [PubMed] [Google Scholar]
- Gagnon D. D., Gagnon S. S., Rintamaki H., Tormakangas T., Puukka K., Herzig K. H., Kyrolainen H. (2014). The effects of cold exposure on leukocytes, hormones and cytokines during acute exercise in humans. PLOS ONE, 9(10), Article e110774. 10.1371/journal.pone.0110774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Morley J. E., Hershman J. M. (1980). Melatonin and the thyroid. Hormone and Metabolic Research, 12(2), 71–73. 10.1055/s-2007-996203 [DOI] [PubMed] [Google Scholar]
- Gullu S., Altuntas F., Dincer I., Erol C., Kamel N. (2004). Effects of TSH-suppressive therapy on cardiac morphology and function: Beneficial effects of the addition of beta-blockade on diastolic dysfunction. European Journal of Endocrinology, 150(5), 655–661. 10.1530/eje.0.1500655 [DOI] [PubMed] [Google Scholar]
- Hackney A. C., Saeidi A. (2019). The thyroid axis, prolactin, and exercise in humans. Current Opinion in Endocrine and Metabolic Research, 9, 45–50. 10.1016/j.coemr.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagin V., Gonzales B. R., Groslambert A. (2015). Effects of cognitive stimulation with a self-modeling video on time to exhaustion while running at maximal aerobic velocity: A pilot study. Perceptual and Motor Skills, 120(2), 491–501. 10.2466/26.25.PMS.120v18x5 [DOI] [PubMed] [Google Scholar]
- Horne J. A., Ostberg O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. https://www.ncbi.nlm.nih.gov/pubmed/1027738 [PubMed] [Google Scholar]
- Kang M., Ragan B. G., Park J. H. (2008). Issues in outcomes research: An overview of randomization techniques for clinical trials. Journal of Athletic Training, 43(2), 215–221. 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosack C. S., Page A. L., Van Hulsteijn L. T., Lentjes E. G. (2012). TSH-CHECK-1 test: Diagnostic accuracy and potential application to initiating treatment for hypothyroidism in patients on anti-tuberculosis drugs. PLOS ONE, 7(3), Article e33704. 10.1371/journal.pone.0033704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk J., Aboul-Enein B. H., Duchnik E. (2021). Exercise-induced oxidative stress and melatonin supplementation: Current evidence. The Journal of Physiological Sciences, 71(1), Article 27. 10.1186/s12576-021-00812-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado M. D., Manfredi M., Ribas-Serna J., Garcia-Moreno H., Calvo J. R. (2012). Melatonin administrated immediately before an intense exercise reverses oxidative stress, improves immunological defenses and lipid metabolism in football players. Physiology & Behavior, 105(5), 1099–1103. 10.1016/j.physbeh.2011.12.015 [DOI] [PubMed] [Google Scholar]
- McMurray R., Hackney A. (2000). Endocrine responses to exercise and training. In Garrett W. E., Kirkendall D. T. (Eds.), Exercise and sport science (pp. 135–161). Lippincott Williams & Wilkins. [Google Scholar]
- Mullur R., Liu Y. Y., Brent G. A. (2014). Thyroid hormone regulation of metabolism. Physiological Reviews, 94(2), 355–382. 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda J. N., Turner C. W. (1968). The role of melatonin in the regulation of thyrotrophin secretion. European Journal of Endocrinology, 57(3), 363–373. 10.1530/acta.0.0570363 [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal S. R., Trakht I., Spence D. W., Srinivasan V., Dagan Y., Cardinali D. P. (2008). The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nature Clinical Practice Neurology, 4(8), 436–447. 10.1038/ncpneuro0847 [DOI] [PubMed] [Google Scholar]
- Pardini D. (2001). Hormonal changes in athlete women. Arquivos Brasileiros de Endocrinologia & Metabologia, 45(4), 343–351. 10.1590/S0004-27302001000400006 [DOI] [Google Scholar]
- Potes Y., de Luxán-Delgado B., Rubio-González A., Reiter R. J., Coto Montes A. (2019). Dose-dependent beneficial effect of melatonin on obesity; interaction of melatonin and leptin. Melatonin Research, 2(1), 1–8. 10.32794/mr11250008 [DOI] [Google Scholar]
- Robergs R. A., Landwehr R. (2002). The surprising history of the “HRmax=220-age” equation [Review]. Journal of Exercise Physiology Online, 5(2), 1–10. https://www.scopus.com/inward/record.uri?eid=2-s2.0-3242723231&partnerID=40&md5=263020382ffbceae9225b5ee6edee13e [Google Scholar]
- Sejian V., Srivastava R. S. (2010). Effects of melatonin on adrenal cortical functions of Indian goats under thermal stress. Veterinary Medicine International, 2010, 348919. 10.4061/2010/348919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souissi A., Haddad M., Dergaa I., Ben Saad H., Chamari K. (2021). A new perspective on cardiovascular drift during prolonged exercise. Life Sciences, 287, 120109. 10.1016/j.lfs.2021.120109 [DOI] [PubMed] [Google Scholar]
- Souissi A., Souissi N., Dabboubi R., Souissi N. (2018). Effect of melatonin on inflammatory response to prolonged exercise. Biological Rhythm Research, 51(4), 560–565. 10.1080/09291016.2018.1543638 [DOI] [Google Scholar]
- Souissi A., Yousfi N., Dabboubi R., Aloui G., Haddad M., Souissi N. (2019). Effect of acute melatonin administration on physiological response to prolonged exercise. Biological Rhythm Research, 51(6), 980–987. 10.1080/09291016.2019.1573462 [DOI] [Google Scholar]
- Souissi A., Yousfi N., Souissi N., Haddad M., Driss T. (2020). The effect of diurnal variation on the performance of exhaustive continuous and alternated-intensity cycling exercises. PLOS ONE, 15(12), Article e0244191. 10.1371/journal.pone.0244191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth O., Sturis J., Byrne M. M., Blackman J. D., L’Hermite-Baleriaux M., Leproult R., Oliner C., Refetoff S., Turek F. W., Van Cauter E. (1994). Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. American Journal of Physiology, 266(6 Pt. 1), E964–E974. 10.1152/ajpendo.1994.266.6.E964 [DOI] [PubMed] [Google Scholar]
- Veneroso C., Tunon M. J., Gonzalez-Gallego J., Collado P. S. (2009). Melatonin reduces cardiac inflammatory injury induced by acute exercise. Journal of Pineal Research, 47(2), 184–191. 10.1111/j.1600-079X.2009.00699.x [DOI] [PubMed] [Google Scholar]
- Watson N., Diamandis T., Gonzales-Portillo C., Reyes S., Borlongan C. V. (2016). Melatonin as an antioxidant for stroke neuroprotection. Cell Transplantation, 25(5), 883–891. 10.3727/096368915X689749 [DOI] [PubMed] [Google Scholar]