Abstract
Pulmonary embolism and splenic infarction are rare in patients with polycythemia vera. We herein describe a man in his early 60s whose main symptoms were chest tightness, cough, and sputum expectoration. Antibiotics, bronchodilators, and mucoactive agents did not improve his symptoms. Pulmonary artery computed tomography angiography showed pulmonary embolism, and abdominal computed tomography showed multiple hypodense foci in the spleen. Bone marrow aspiration cytology, biopsy, and genetic testing confirmed polycythemia vera. The patient’s symptoms were relieved after treatment with hydroxyurea and rivaroxaban. This case emphasizes that although pulmonary embolism and splenic infarction are relatively rare in patients with polycythemia vera, the possibility of polycythemia vera should be considered in clinical practice.
Keywords: Polycythemia vera, pulmonary embolism, splenic infarction, case report, computed tomography, bone marrow examination, genetic testing
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm (MPN) characterized by clonal erythrocytosis. PV can be accompanied by leukocytosis, elevated platelets, splenomegaly, and thrombosis. Thrombosis is the most important cause of death in patients with PV, and cerebral artery embolism is the most common thrombotic manifestation. More rarely, splenic infarction, pulmonary artery embolism, and mesenteric artery embolism have been reported. 1 In an effort to reduce the rate of misdiagnosis, we herein report a rare case of pulmonary embolism (PE) and splenic infarction caused by PV.
Case presentation
Ethics approval was not required for publication of this case report. Additionally, because all patient details were deidentified, consent for publication from the patient was deemed unnecessary. The patient provided verbal informed consent for treatment. The reporting of this study conforms to the CARE guidelines. 2
Twenty days before presentation, the patient developed chest tightness and a productive cough after being cold. His symptoms were not substantially relieved by resting. He visited the Red Cross Hospital of Qinghai Province and was diagnosed with chronic bronchitis, emphysema, arrhythmia, and splenomegaly. Two weeks later, the symptoms worsened. The chest tightness and productive cough were not accompanied by chest pain, hemoptysis, abdominal distension, or abdominal pain. For further treatment, he visited Qinghai University Affiliated Hospital.
Physical examination showed a temperature of 38°C, pulse rate of 86 beats/minute, respiratory rate of 30 breaths/minute, and blood pressure of 140/90 mmHg. The patient had limited consciousness, a poor mental state, cyanotic lips, barrel chest, symmetrical voice tremor on palpation, and evidence of air blebs on auscultation of both lungs. His heart rhythm was regular, and there were no abnormal murmurs in any valve area. The jugular vein was distended, and the hepatojugular reflux sign was positive. Abdominal tension and rebound pain were negative and the liver was not palpable, but the spleen was palpable subcostally (line I measured 36.7 mm, line II measured 78 mm, and line III measured 21 mm). Moreover, both lower limbs were edematous, and this was more severe in the left limb.
The white blood cell count was 21.37 ×109/L, neutrophil count was 18.52 × 109/L, red blood cell count was 6.90 × 1012/L, hemoglobin level was 173 g/L, hematocrit was 51.30%, platelet count was 704 × 109/L, lactate dehydrogenase level was 695 U/L, uric acid level was 506 µmol/L, D-dimer level was 19.5 mg/L, fibrinogen content was 4.100 g/L, C-reactive protein level was 67.70 mg/L, procalcitonin level was 0.45 ng/mL, troponin I level was 0.240 ng/mL, and N-terminal pro-brain natriuretic peptide level was 10900 ng/L.
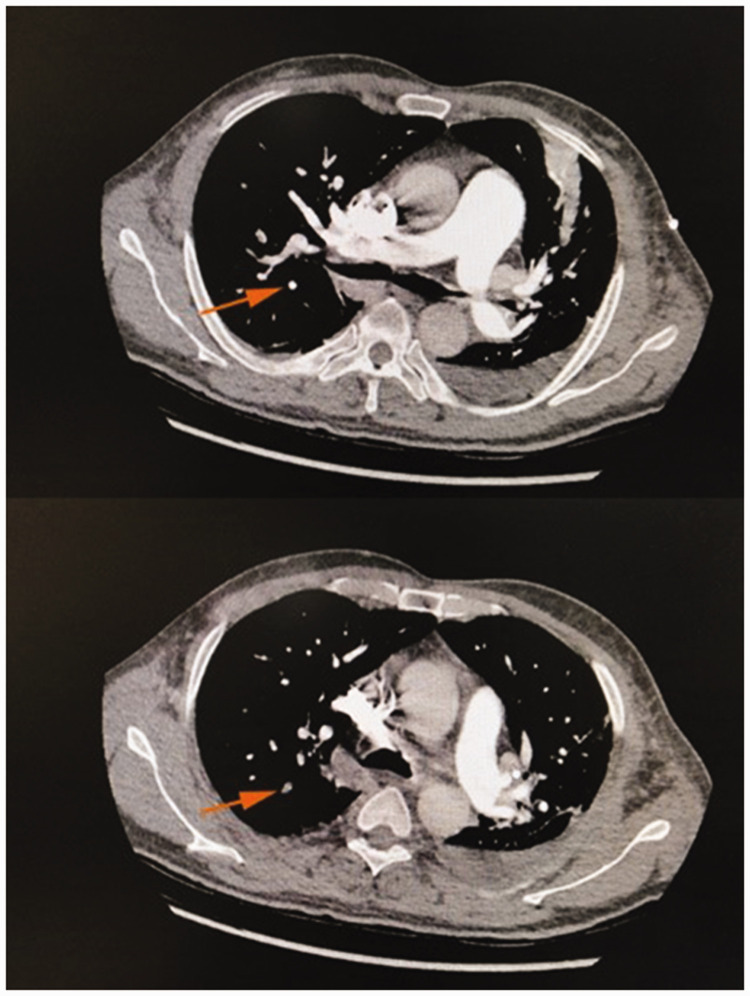
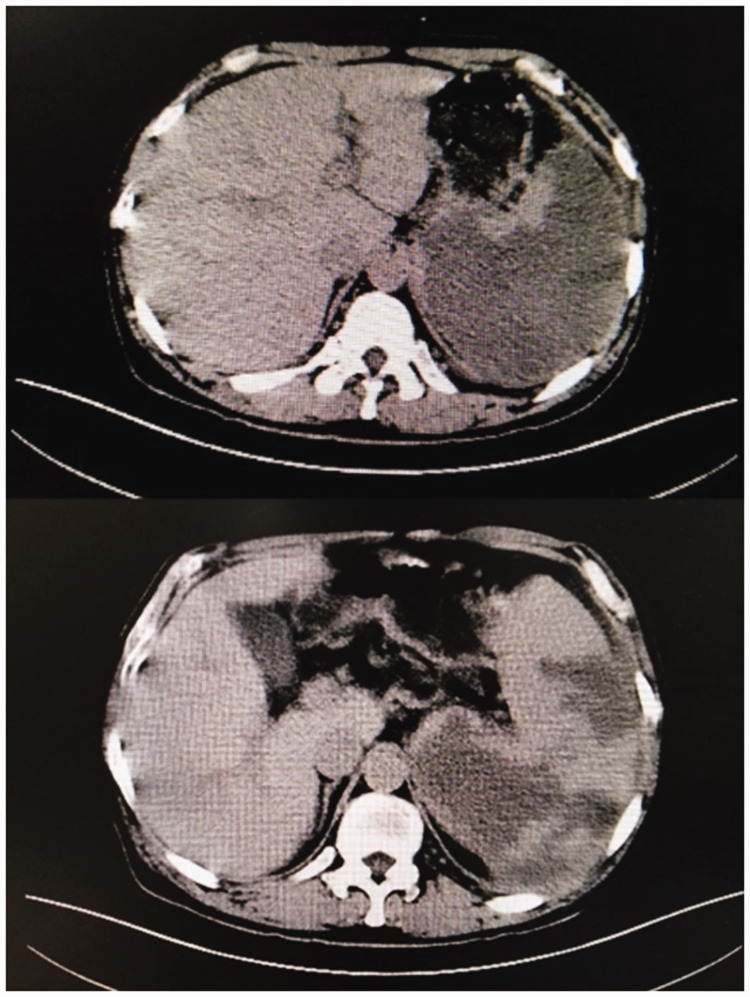
There were no obvious abnormalities in the deep veins of the lower extremities. Echocardiography showed that the right ventricular outflow tract was 32 mm, the left and right diameter of the right ventricle was 35 mm, the main pulmonary artery diameter was 24 mm, the left ventricular inner diameter was 34 to 49 mm, the ejection fraction was 54%, and the left ventricular fractional shortening was 30%. Pulmonary artery computed tomography angiography (CTA) showed segmental visualization of the remote pulmonary artery of the posterior right upper lobe. The diameter of the main pulmonary artery was about 38 mm (Figure 1). A plain CT scan of the abdomen showed fluid in the abdominal cavity, splenomegaly with multiple hypodense foci in the spleen, and portal hypertension (Figure 3). Abdominal ultrasonography showed that the thickness of the spleen was 52 mm and the length was 164 mm, the echo of the spleen section was uneven, and a low-density lesion of about 9.3 × 2.6 mm was present at the upper pole of the spleen. The patient refused to undergo further enhanced CT examination of the abdomen. Therefore, he was given low-molecular-weight heparin at 6000 AXaIU (once every 12 hours), cefoperazone sodium and sulbactam sodium at 3.0 g intravenously (once every 8 hours), a vasodilator, diuresis, and nasal cannula oxygen therapy. His symptoms improved thereafter.
Figure 1.
Pulmonary artery computed tomography angiography at the beginning of the disease course. Segmental visualization of the remote pulmonary artery of the posterior right upper lobe is seen in the lower image (orange arrow).
Figure 3.
Plain computed tomography scan of the abdomen. Images show obviously enlarged spleen and multiple hypodense foci in the spleen.
Bone marrow aspiration and biopsy were performed. The results of the biopsy showed the possibility of MPN. The patient was recommended to undergo relevant examinations to exclude MPN. The BCR-ABL fusion gene qualitative test was negative. The pathologic report from the bone marrow tissue examination showed that the hematopoietic tissue was obviously active; granulocytes, erythroid cells, and megakaryocytes were all proliferating; and macronuclei and multi-megakaryocytes were seen. A genetic test showed JAK2 V617F positivity. The patient was diagnosed with PV and treated with 100 mg of aspirin per day and 0.25 g of hydroxyurea per day. After hospital discharge, the heparin was replaced with the oral anticoagulant rivaroxaban (10 mg/day).
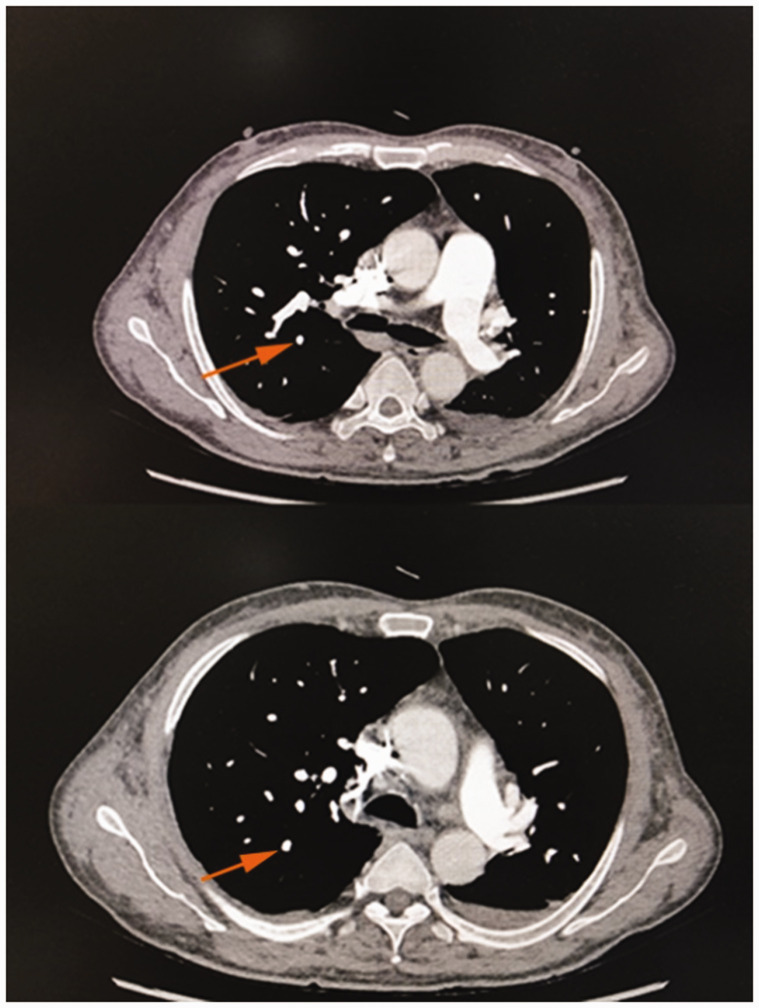
One month later, the patient’s symptoms had subsided further, and repeat pulmonary artery CTA showed disappearance of the segmental visualization of the remote pulmonary artery of the posterior right upper lobe. Additionally, the diameter of the main pulmonary artery was about 32.5 mm (Figure 2). The white blood cell count was 10.60 × 109/L, neutrophil count was 8.45 × 109/L, neutrophil percentage was 79.7%, red blood cell count was 5.24 × 1012/L, hemoglobin level was 157 g/L, hematocrit was 49.2%, and platelet count was 233 × 109/L. However, the patient still refused to undergo an enhanced abdominal CT examination. He was recommended to continue treatment with rivaroxaban, aspirin, and hydroxyurea outside the hospital.
Figure 2.
Pulmonary artery computed tomography angiography 1 month later. Images show disappearance of the segmental visualization of the remote pulmonary artery of the posterior right upper lobe compared with the lower image of Figure 1 (orange arrow).
Discussion and Conclusions
PV is a type of chronic MPN that is mainly characterized by an abnormal increase in red blood cells. It can also be accompanied by leukocytosis, elevated platelets, and splenomegaly. Patients with PV are prone to develop arteriovenous thrombosis or abnormal bleeding, especially patients with JAK2 V617F gene mutation.3,4 Many studies have shown that the occurrence of thrombosis in patients with PV is more common in those with stroke and myocardial infarction, whereas PE and splenic infarction are relatively rare.1,5 Patients with MPN present with symptoms mainly related to thrombosis and/or hemorrhage. 6 Benmalek et al. 7 described a patient with chest pain and headache due to coronary artery and cerebral venous thrombosis secondary to MPN.
The pathogenesis of the acquired thrombophilic state in patients with PV is complex and multifactorial. However, it can be summarized by the three main elements necessary for thrombosis: vascular endothelial damage, blood stasis, and hypercoagulability. Blood flow stasis and hypercoagulability are caused by an abnormal increase in red blood cells, platelets, and tissue factor secondary to abnormal clonal proliferation; vascular endothelial damage by inflammatory factors, such as interleukin-6; and the increased adhesion between red blood cells and endothelial cells. Together, these phenomena promote thrombosis. The mechanism may be related to activation of the JAK-STAT signaling pathway and the increased levels of Lu/BCAM expression and phosphorylation in red blood cells.8,9
Our patient had recurrent chest tightness, cough, and sputum production as the first symptoms, and the symptoms recurred after treatment at another hospital. The possibility of PE could not be excluded because of the patient’s elevated D-dimer level and symptoms; therefore, pulmonary artery CTA was performed, and he was diagnosed with PE (middle-risk group). In addition, it was necessary for us to consider the possibility of blood system diseases because of the multiple hypodense foci and splenomegaly combined with the increased blood cell counts; therefore, we also performed bone marrow cytology and genetic tests. The final diagnosis was PV. This case indicates that the possibility of blood system diseases should be considered in patients with coexisting arterial and venous thrombosis accompanied by abnormal blood cells.
According to the mechanism and clinical manifestations of PV, the purpose of treatment is to control the blood cell counts, reduce the risk of thrombosis, and prevent the transformation from PV to myelofibrosis and leukemia. Treatment measures include cytoreductive therapy, oral aspirin, and phlebotomy. 10 Because embolism is the main cause of death in patients with PV, anticoagulant treatment should be given based on the above treatment measures for patients with thrombosis as soon as possible. Additionally, studies have shown that direct oral anticoagulants are more effective than vitamin K antagonists in preventing the recurrence of blood clots and do not increase the risk of bleeding in patients with PV. 11 A systematic review showed that anticoagulation combined with cytoreductive surgery was the best measure to prevent the recurrence of thrombosis for patients with MPN. 12 Therefore, after our patient’s hospital discharge, we asked him to continue treatment with hydroxyurea and rivaroxaban. One month later, his routine blood parameters had improved, and there was no obvious filling defect on pulmonary artery CTA. Therefore, the patient was recommended to continue treatment with hydroxyurea and rivaroxaban.
In conclusion, clinicians should be aware of this rare anomaly of the coexistence PE and splenic infarction in patients with PV. Additionally, blood system diseases should be considered in patients with coexisting arterial and venous thrombosis accompanied by abnormal blood cells.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Ping Huang https://orcid.org/0000-0003-2315-9594
Yuhong Li https://orcid.org/0000-0002-0312-7168
References
- 1.Bai J, Shi H, Ai L, et al. [Survival and prognosis of patients with polycythemia vera: a study of 816 patients in a single Chinese center]. Zhonghua Yi Xue Za Zhi 2015; 95: 1364–1368. [PubMed] [Google Scholar]
- 2.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhou Y, Wang Y, et al. Thrombosis among 1537 patients with JAK2(V617F) -mutated myeloproliferative neoplasms: risk factors and development of a predictive model. Cancer Med 2020; 9: 2096–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dentali F, Ageno W, Rumi E, et al. Cerebral venous thrombosis and myeloproliferative neoplasms: results from two large databases. Thromb Res 2014; 134: 41–43. [DOI] [PubMed] [Google Scholar]
- 5.Enblom A, Lindskog E, Hasselbalch H, et al. High rate of abnormal blood values and vascular complications before diagnosis of myeloproliferative neoplasms. Eur J Intern Med 2015; 26: 344–347. [DOI] [PubMed] [Google Scholar]
- 6.Rungjirajittranon T, Owattanapanich W, Ungprasert P, et al. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 2019; 19: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmalek R, Mechal H, Zahidi H, et al. Combined venous and arterial thrombosis revealing underlying myeloproliferative disorder in a young patient: a case report. J Med Case Rep 2021; 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleppe M, Kwak M, Koppikar P, et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 2015; 5: 316–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wautier MP, El Nemer W, Gane P, et al. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood 2007; 110: 894–901. [DOI] [PubMed] [Google Scholar]
- 10.McMullin MF, Harrison CN, Ali S, et al. A guideline for the diagnosis and management of polycythaemia vera. A British Society for Haematology Guideline. Br J Haematol 2019; 184: 176–191. [DOI] [PubMed] [Google Scholar]
- 11.Huenerbein K, Sadjadian P, Becker T, et al. Direct oral anticoagulants (DOAC) for prevention of recurrent arterial or venous thromboembolic events (ATE/VTE) in myeloproliferative neoplasms. Ann Hematol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamulyák EN, Daams JG, Leebeek F, et al. A systematic review of antithrombotic treatment of venous thromboembolism in patients with myeloproliferative neoplasms. Blood Adv 2021; 5: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]