Abstract
Objectives: Ankle brachial pressure index (ABPI) is limited for diabetic patients. This can have costly impacts upon patient's quality of life along with healthcare budgets, with diabetic care equating to approximately 10% of NHS expenditure.11 We aimed to determine whether ultrasound waveform parameters are an alternative for quantifying lower extremity peripheral arterial disease (PAD) where ABPI is unreliable. Design: This was a prospective, observational study. Waveform parameters, systolic rise time (SRT), maximal systolic acceleration (AccMax) and peak systolic velocity (PSV) were recorded at ankle and compared to the ABPI and an aorta-ankle duplex ultrasound scan (DUS) as gold standard. Setting: Measurements were obtained by a Clinical Vascular Scientist at the Royal Free Hospital. Participants: Participants (≥18yrs) with known PAD, but without previous vascular intervention were allocated to non-diabetic control (n = 24) and diabetic test groups (n = 22). Outcome measures: The primary outcome measure was the correlation of novel ultrasound derived indices to PAD severity. The secondary outcome was the efficacy of this correlation in the diabetic population. Results: AccMax was most powerful in detecting PAD in both groups when compared to ABPI in the controls (r = 0.805; p < 0.01) and to DUS in control and test groups (r = −0.633 to −0.643; p < 0.01). In the test group, PSV did not consistently quantify PAD. SRT measurements were inconclusive throughout. Conclusion: AccMax is a rapid alternative tool for diagnosing PAD in diabetic patients. With further research, this simple test may prove useful for monitoring PAD progression in patients unsuitable for ABPI, reducing the need for lengthy repeat duplex scans.
Keywords: lower extremity arterial disease, maximal systolic acceleration, diabetes mellitus, duplex ultrasound
Introduction
People living with Type I or Type II diabetes are twice as likely to develop cardiovascular disease, such as lower extremity peripheral arterial disease (hereafter, ‘PAD’), when compared to the non-diabetic population. 1 As a consequence, National Institute of Health and Care Excellence (NICE) guidelines recommend regular assessments for patients living with diabetes to foster early identification and treatment of PAD. 2 Diabetes coupled with PAD can lead to as much as a 30-fold increase in lower limb amputation when compared to the general, non-diabetic population 3 and despite investment into diabetic foot care, diabetes remains a leading contributor to lower limb amputations.4–6
Ankle Brachial Pressure Index (ABPI) is the current first line tool in the diagnosis and quantification of PAD. If abnormal, a duplex ultrasound (DUS) assessment can follow which will anatomically locate the lesion impacting the blood flow to aid endovascular or surgical intervention. However, ABPI reliability and accuracy can be limited for people living with Mönckeberg's sclerosis secondary to diabetes7,8 due to arterial calcification increasing the external pressure needed for a blood pressure cuff to compress the artery. This can lead to falsely elevated ABPI results, which may underrepresent the severity or even the presence of PAD for the diabetic patient. The Toe Brachial Index (TBI) is a possible alternative to ABPI, however the microvasculature is not immune from calcification7,9 and the TBI is subject to the same limitations as ABPI when ulcers, calluses, fragile skin and toe amputations are present. 10
The late diagnosis or undetected progression of PAD can shorten the available window for preventative management having costly impacts upon a patient's quality of life and life expectancy, along with healthcare budgets; current estimates for diabetic care equate to 10% of NHS expenditure. 11 With the limitations of ABPI/TBI in mind, an alternative to cuff compression techniques is therefore ideal and the use of DUS derived blood waveform analysis may be the answer.
The back bone of DUS is its assessment of real time haemodynamic information using spectral Doppler; a technique that is widely used by Clinical Vascular Scientists (CVS) in routine clinical practice. Performing DUS assessments to locate arterial disease is often detailed and lengthy, even when performed by CVS. Qualitative analysis of spectral Doppler waveforms at ankle level provides a quick alternative to obtain cursory information on the presence of PAD however, a quantitative approach providing information similar to an ABPI is not well studied. Studies investigating DUS derived, one-spot, quantitative techniques have primarily focused on detecting proximal disease in the aorto-iliac and renal arteries.12–18 Few studies have explored its use in diagnosing and grading disease of the full lower limb arterial tree from aorta to ankle.19,20
Bardelli et al. 18 published data on a novel parameter of routinely acquired Doppler waveforms called the maximum systolic acceleration (AccMax); the initial slope of systolic acceleration or slope of systolic acceleration until any significant change in acceleration occurs (Figure 1), and determined its conceivable use as a quantitative measure of proximal renal artery health. Subsequently, van Tongeren et al. 19 transferred this technique to ankle level with the hope of finding a diagnostic tool to grade PAD akin to ABPI/TBI. Excitingly, they concluded AccMax to be an accurate tool. Despite this promising data, their methodology was limited as AccMax was compared to the ABPI as gold standard, which is flawed in the diabetic population.
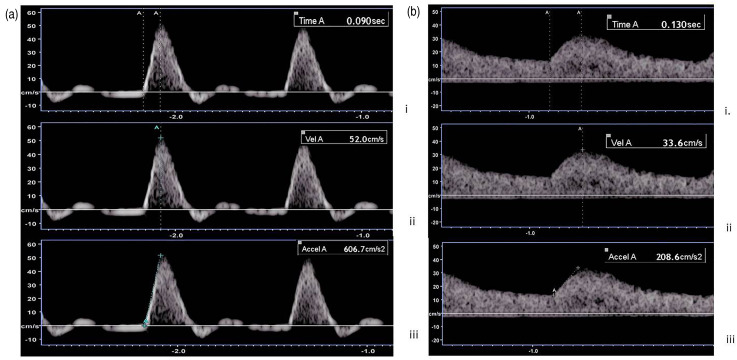
Figure 1.
Spectral Doppler waveform trace of the posterior tibial artery at ankle level. Calipers were placed on a normal (a.) and abnormal (b.) waveform trace to record the systolic rise time (i), peak systolic velocity (ii) and maximal systolic acceleration (iii).
This study aimed to determine the diagnostic capabilities of ultrasound derived Doppler waveform measurements at ankle in the diabetic population by comparing the waveform parameters to a full lower limb arterial DUS as gold standard. ABPI would usually be the gold standard for applying a quantifiable value to PAD however, as these measurements are unreliable in the diabetic population a Modified Rutherford score (MRS) based on DUS was used as an alternative, which was validated against ABPI in the non-diabetic population. Through this method we aimed to gain further information on whether ultrasound derived Doppler waveform analysis at the ankle can be used as an alternative tool to quantify PAD for patients where traditional ABPI measurements are unreliable.
Methodology
This research was conducted as a prospective, observational cohort study following ethical review from HRA East of England Essex Research Ethics Committee (Ref: 15/EE/0436).
Participants
1904 patients aged ≥18 years with known aorta to ankle arterial disease were screened from Diabetic Foot and Vascular Studies outpatient clinics. Patients were deemed unsuitable to participate if they were currently undergoing renal replacement therapy, had previously undergone endovascular or surgical treatment, were unable to lay supine and/or were unable to provide informed consent.
Following review, 120 patients were invited to the study via a public and peer reviewed participant information leaflet. Informed consent was obtained both verbally and in written format. All patients were approached and selected in a consecutive fashion from the Diabetic Foot and Vascular Studies outpatient lists and therefore it was assumed that the potential participants these lists served would provide a representative sample of the local diabetic population.
Environment
To minimise external influences on vasodilation or constriction, the research environment was kept at a constant temperature of 23 ± 0.5 degree Celsius, free from any potential draft sources or heaters. 15 min were allowed for each participant to acclimatise to room temperature and they were laid supine for 5 min prior to data collection.
Dependent Variable: Doppler waveform parameters
The following Doppler waveform parameters were recorded at ankle from the anterior tibial artery (ATA) and posterior tibial artery (PTA) by a single CVS using a Toshiba Aplio 500 ultrasound machine and a PLT-7.5 MHz linear array transducer: AccMax, peak systolic velocity (PSV) and systolic rise time (SRT). AccMax was defined according to Bardelli et al. 18 and SRT was defined as the time taken for the systolic curve to reach PSV. AccMax, SRT and PSV were measured visually without the aid of automated caliper placement software (Figure 1).
In a longitudinal section, colour and spectral Doppler were used to insonate the target vessel at an angle of 60 degrees or less in the direction of blood flow. Three freeze frames of the Doppler trace were acquired at the same location, approximately 10–20 s apart, to obtain three recordings for each parameter. An average value for each parameter was calculated. Time taken to obtain all parameters was recorded.
Independent Variable: Lower limb doppler ultrasound assessment (DUS)
The abdomen and lower limb(s) arteries were scanned by a single CVS using a Toshiba Aplio 500 ultrasound machine with a PLT-7.5 MHz linear array and a 3.5 MHz curvi-linear transducer where increased penetration for imaging was required. The following arteries were interrogated for data collection: abdominal aorta, common iliac, external iliac, common femoral, superficial femoral, popliteal, tibioperoneal trunk, anterior tibial, posterior tibial and peroneal.
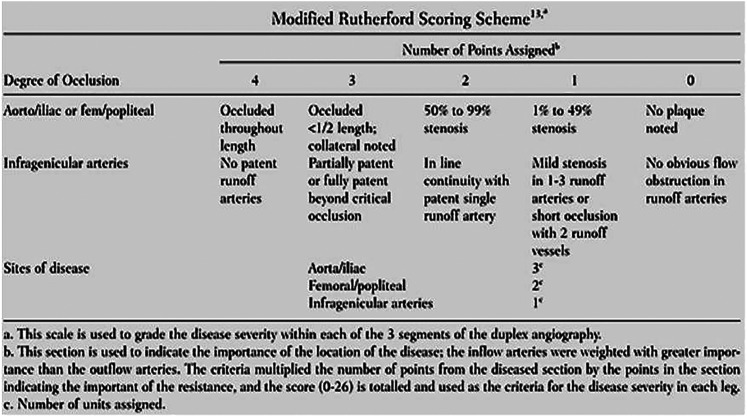
Arterial pathology was recorded on a blank anatomical template. Stenoses were graded, following departmental protocol, where a ≥50% stenosis was based on a x2 fold increase in PSV pre and post stenosis and a ≥75% stenosis was based on a x4 fold increase in PSV. Arterial pathology was quantified using the MRS as presented in Alnaeb et al.'s work (Figure 2). 21
Figure 2.
Modified rutherford scoring scheme as presented by Alnaeb et al. 21
Scores were calculated by grading the severity of each stenosis and then multiplying the points by the weighting factor assigned to the corresponding segment of the arterial tree. For example, if a 50–99% stenosis was graded in the common iliac artery, a 1–49% stenosis in the popliteal artery and a 50–99% stenosis in the ATA, the lower limb would be calculated as follows: MRS = (2 × 3) + (1 × 2) + (2 × 1) = 16.
The total score was then placed in a category of disease severity as defined by Alnaeb et al.: 21
Score Severity of Disease
0 Disease ‘free’
1–6 Mild disease
>7 Significant disease
There is limited literature available on the sensitivity and specificity of MRS and DUS is known to be highly operator dependent. MRS scores were evaluated for correlation with the ABPI measurements obtained in the non-diabetic patients to provide a local validation of this technique.
Independent Variable: ABPI
A resting ABPI was acquired by a single CVS and performed according to NICE guidelines and the severity of disease was graded according to their following classifications: 22
Resting ABPI Severity of Disease
>1.3 Calcification may be present
<0.9 PAD present
<0.5 Chronic limb-threatening ischaemia
Statistical analysis
Data analysis was performed using IBM SPSS, version 22. A series of probability-probability plots and histograms were produced and showed a normal distribution of data suitable for parametric testing. Significance for all tests was set at P < 0.05.
Simple linear regression was performed and a scatter plot produced to observe the magnitude of any relationships between AccMax, SRT or PSV (dependent variables) and the independent variables, resting ABPI and MRS. This statistical analysis was also performed to elucidate the relationship between ABPI and MRS in the non-diabetic control group to locally validate MRS for use as the gold standard in the diabetic test group where ABPI is known to be flawed. Pearson's correlation coefficient was then applied to determine the statistical significance of the above relationships in both control and test groups.
A paired t test was performed to identify any differences in AccMax, SRT, PSV and ABPI between participants with and without diabetic neuropathy.
Intra-rater reliability was calculated using Cronbach's alpha.
Results
Participants
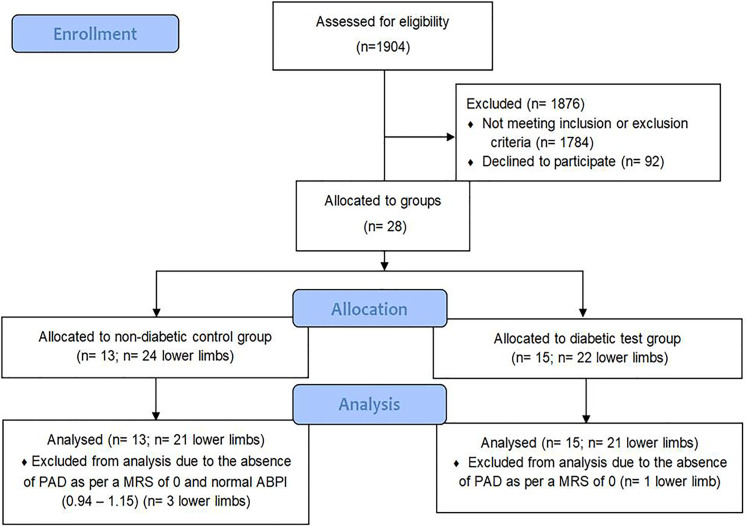
28 consenting participants took part in the study; 23% of 120 invited (Table 1; participant characteristics) and were allocated to a non-diabetic control group (n = 13; lower limb = 24; age = 62–81yrs) and a diabetic test group (n = 15; lower limb = 22; age = 49–83yrs). Of the participants, 3 lower limbs from the control group and 1 lower limb from the test group were excluded from statistical analysis due to the absence of PAD as per a normal ABPI (0.94–1.15) and/or MRS of 0 (see Figure 3).
Table 1.
Participant characteristics.
| Characteristics | N | Percentage (%) |
|---|---|---|
| Sex | ||
| Female | 7 | 25 |
| Male | 21 | 75 |
| Age Range | ||
| 49–60 | 2 | 7 |
| 61–72 | 18 | 64 |
| 73–83 | 8 | 29 |
| Past Medical History | ||
| Diabetes Mellitus | 15 | 54 |
| Hypertension | 16 | 57 |
| Irregular Cardiac Output | 4 | 14 |
Figure 3.
Participant flow diagram.
Collection of Doppler waveform parameters
The average time taken to collect three measurements each for AccMax, SRT and PSV was 4minutes 10seconds, ranging from 1 min 3 s to 4 min 50 s.
Statistical analysis
MRS was validated against ABPI in the control group and showed a moderate to strong, negative relationship (p < 0.01; r = −0.716).
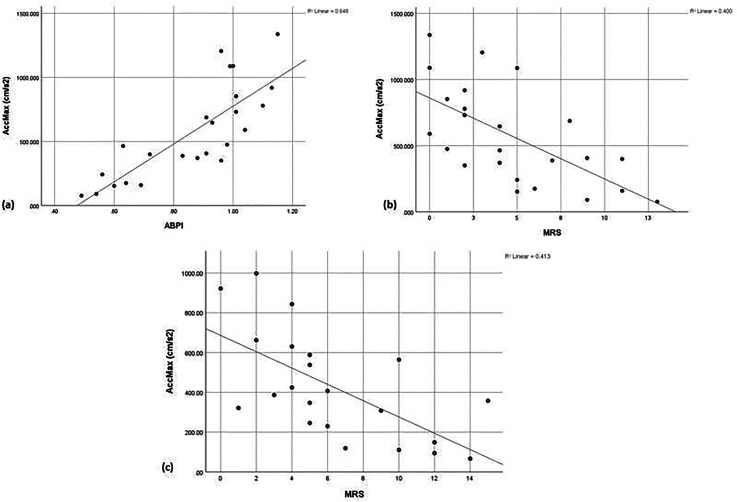
AccMax was found to be a powerful parameter in detecting PAD diagnosed by both ABPI and MRS in non-diabetic patients (p < 0.01; r = 0.805 and r = −0.633, respectively) showcasing its efficacy. In the diabetic test group, AccMax compared to MRS also exhibited a moderate relationship (p < 0.01; r = −0.643, respectively) with remarkably similar correlation to the control group highlighting its preserved efficacy in the test group. In corroboration, regression analysis showed that as PAD worsened AccMax similarly worsened by showing a trend of decreasing acceleration (Figure 4).
Figure 4.
Simple scattergraphs showing the relationship between worsening PAD and the Doppler derived AccMax. (a) AccMax versus ABPI in the non-diabetic control group; (b) AccMax versus duplex ultrasound MRS in the non-diabetic control group; (c) AccMax versus duplex ultrasound MRS in the diabetic test group.
Intra-rater analysis showed excellent levels of reliability (α = 0.936–0.968) for AccMax based on three repeated measurements taken per artery per limb.
In the non-diabetic control group, PSV was shown to compare favourably to both ABPI (p < 0.01; r = 0.861) and the MRS (p < 0.01; r = −0.732). However, the converse was found in the diabetic test group where weak and insignificant relationships were found when compared to MRS (p > 0.05; r = −0.343). SRT measurements did not show a consistent relationship to ABPI and MRS in the controls (p > 0.05; r = −0.319, r = 0.112) or to MRS in the test group (p < 0.05; r = −0.482).
Discussion
The results from this study highlight the superiority of AccMax in diagnosing PAD in the diabetic population, adding to the growing evidence of using AccMax as an alternative to ABPI.19,20,23
By expanding upon previous methodology, 19 our study compared Doppler waveform parameters to a locally validated DUS score as gold standard and demonstrates the use of AccMax as an alternative to ABPI for the diabetic population. The ability to acquire AccMax quickly, at an average of 4 min 10 s compared to approximately 13.7 min for ABPI, 24 and with excellent intra-rater reliability (α = 0.936–0.968), further supports the promise of AccMax.
In accordance with Bernoulli's principle of fluid dynamics (Appendix 1), as blood passes through a stenosis flow velocity increases. This results in an increase of the Reynolds number (Appendix 2), which predicts the transition of blood flow from laminar to turbulent. As flow exits the stenosis and the vessel widens, there is a further increase in Reynolds number and turbulent flow develops resulting in a loss of energy from the system. Laminar flow will usually be restored a short distance from the stenosis however, the energy lost due to turbulence at the exit of the stenosis results in a drop in pressure across this region of the circulation. Acceleration can be derived from the instantaneous maximal derivative of arterial pressure 20 and therefore it can be reasonably assumed that the more severe the proximal disease, thus the greater the pressure drop, the slower AccMax will be and this is corroborated by our results. The body is far more complex than this reductionist view and there will be multiple factors within the systemic circulation impacting the waveform shape. However, our results have provided encouraging data that AccMax can work as a simplified parameter to diagnose and grade proximal PAD.
Our results for PSV and SRT reinforce the superiority of AccMax. PSV was found to correlate well to disease severity in the control group, but it failed to perform in the diabetic test group. It was initially hypothesised that PSV may be affected by loss of compliance in the diabetic population however, Sung et al. 25 have shown no influence of compliance upon PSV. It is reasonable to therefore suggest that diabetic neuropathy may be at play here. In ‘healthy’ controls the distal vascular bed will vasodilate in response to a pressure drop across a significant stenosis to recruit more blood down the affected blood vessel to preserve vascular functions. It is possible that in the diabetic group many may have been experiencing an impaired vasodilatory response due to the presence of neuropathy. A subset analysis of patients with diabetic neuropathy was performed in this study, as we hypothesised that neuropathic vasoconstriction may affect the waveform parameters measured however, due to a small sample size our study was limited and unable to provide data on the impact of neuropathy upon the parameters.
SRT showed no consistent or reliable relationships to the severity of PAD. The inadequacy of SRT has also been reported by Bardelli et al. 18 where variations in the morphology of the systolic curve, for example double peaks, led to false positives of renal artery stenosis. In an in vitro setting, Sung et al. 25 additionally found that the time to reach the systolic peak also showed no correlation with the severity of proximal stenosis. As mentioned, the autoregulatory system of the human body is complex and it is therefore likely that various systemic factors such as, cardiac output, resistance, and vasodilation/constriction, will have additional impacts on SRT over each haemodynamic cycle. As AccMax just represents the instantaneous systolic acceleration onset, not the time taken for blood to reach the PSV, it is plausible that AccMax is not similarly affected due to its more focused scope of measurement.
To eliminate the inadequacies of ABPI for the diabetic test group, we sought to use DUS as the gold standard and locally validated the MRS to score disease present on DUS. Despite its advantage over ABPI, it is important to note that the MRS could be improved. The literature did not clearly state how multiple stenoses within one segment were graded for the MRS. 21 Thus, our interpretation led us to score only the most severe stenosis in each segment, which may have underestimated the quantification of disease present. The scoring system also did not consider the significance of the length of stenosis upon the resulting pressure drop distal to a lesion, however helpfully this has recently been addressed by Brouwers et al. 23 who found no statistically significant difference in AccMax between different stenosis lengths in an in vitro setting. Brouwers et al. 23 have provided important in vitro data to support the use of AccMax by comparing to direct intraluminal mean pressure gradients as gold standard. This method is ideal and along with larger sample sizes future studies would benefit from comparing AccMax to intraluminal mean pressure gradients in vivo as gold standard. This would help account for any flaws encountered by indirect measurements such as ABPI, TBI or the grading systems of lower limb arterial disease by DUS or angiographic assessments. Future studies may also benefit from following patients prospectively over time to understand whether AccMax similarly worsens as disease worsens.
A possible user dependent limitation of measuring AccMax may be found in accurate caliper placement. Although inter-rater reliability was not investigated in this study, intra-rater reliability was found to be excellent (α = 0.936–0.968). We expect with sufficient training, this excellent inter-rater reliability can be similarly achieved. To achieve this, it is important to ensure correct caliper placement. During initial study design we found it a limitation to measure SRT and AccMax simultaneously in the presence of abnormal waveform shapes and we therefore advise that both parameters are measured independently from each other and that the difference between SRT and AccMax is well understood prior to data collection.
Conclusion
Our in-vivo study has demonstrated the diagnostic importance of AccMax in the clinical setting, providing new evidence to validate its efficacy as an alternative diagnostic tool for PAD for patients unsuitable for ABPI/TBI measurements.
Recording of AccMax at ankle is a novel interpretation of routinely acquired Doppler ultrasound waveforms proving that its use can be readily and easily implemented into the already established routine without the need for extra equipment or software, therefore eliminating any cost burden to healthcare providers who already possess ultrasound machines. With AccMax also taking ≤ 5 min to perform, it is a desirable alternative to a costly and time consuming full lower limb DUS assessment for patients who cannot receive an ABPI/TBI assessment.
This quick and simple improvement in cardiovascular diagnostics may have big potential. By aiding the early diagnosis and surveillance of PAD it could reduce patient morbidity and therefore improve quality of life for patients already harshly affected by diabetes and indeed other patients where ABPI is unsuitable, such as those with gross lower limb swelling, extensive lower limb ulcers, or Mönckeberg's sclerosis secondary to chronic renal disease.
The data that support the findings of this study are available from the corresponding author upon reasonable request
Acknowledgements
We would like to acknowledge and thank the Royal Free London Vascular Studies Unit for accommodating the study, and the Royal Free London Podiatry Department for their support during participant recruitment.
Appendices
Appendix 1.
Bernoulli Equation:
ET = total fluid energy; P = pressure; ρ = density, g = gravity,; h = height; v = velocity.
Appendix 2.
Reynold's Number (Re):.
.
Appendix 3.
Raw Data Sets.:
| NON-DIABETIC PARTICIPANTS (CONTROL GROUP) | |||||
|---|---|---|---|---|---|
| AccMax (cm/s2) | SRT ( s ) | PSV (cm/s) | ABPI | MRS | |
| 1 | 464.36 | 0.076 | x | 0.63 | 4 |
| 2 | 646.4 | 0.07 | 50.6 | 0.93 | 4 |
| 3 | 1337.03 | 0.072 | 103.6 | 1.15 | 0 |
| 4 | 590 | 0.1 | 64.6 | 1.04 | 0 |
| 5 | 388.07 | 0.074 | 29.93 | 0.83 | 7 |
| 6 | 242.27 | 0.078 | 19.13 | 0.56 | 5 |
| 7 | 159 | 0.076 | 15.03 | 0.69 | 11 |
| 8 | 76.47 | 0.113 | 15.03 | 0.49 | 13 |
| 9 | 399.4 | 0.067 | 26.6 | 0.72 | 11 |
| 10 | 406.43 | 0.065 | 35.53 | 0.91 | 9 |
| 11 | 370.6 | 0.098 | 62.03 | 0.88 | 4 |
| 12 | 1088.73 | 0.073 | x | 1 | 0 |
| 13 | 152.96 | 0.078 | x | 0.6 | 5 |
| 14 | 779.26 | 0.085 | 66.5 | 1.1 | 2 |
| 15 | 918.73 | 0.076 | 80.06 | 1.13 | 2 |
| 16 | 350.7 | 0.07 | 24.46 | 0.96 | 2 |
| 17 | 731.17 | 0.055 | 46.37 | 1.01 | 2 |
| 18 | 1205.2 | 0.052 | 65.4 | 0.96 | 3 |
| 19 | 174.97 | 0.074 | 13.47 | 0.64 | 6 |
| 20 | 90.43 | 0.102 | 13.33 | 0.54 | 9 |
| 21 | 1087.03 | 0.05 | 56.06 | 0.99 | 5 |
| 22 | 687.433 | 0.065 | 44.43 | 0.91 | 8 |
| 23 | 475.2 | 0.096 | 46.5 | 0.98 | 1 |
| 24 | 852.4 | 0.085 | 72.87 | 1.01 | 1 |
| DIABETIC PARTICIPANTS (TEST GROUP) | |||||
|---|---|---|---|---|---|
| AccMax (cm/s2) | SRT ( s ) | PSV (cm/s) | ABPI | MRS | |
| 1 | 843.43 | 0.081 | x | 0.97 | 4 |
| 2 | 148.9 | 0.104 | x | 0.58 | 12 |
| 3 | 245.9 | 0.098 | x | 0.55 | 5 |
| 4 | 537.9 | 0.116 | 77.97 | x | 5 |
| 5 | 66.7 | 0.124 | 11.9 | 0.44 | 14 |
| 6 | 119.01 | 0.031 | 17.27 | 0.43 | 7 |
| 7 | 424.27 | 0.076 | 38.33 | 0.65 | 4 |
| 8 | 588.17 | 0.074 | 45.67 | 0.77 | 5 |
| 9 | 357.47 | 0.135 | 72.4 | 0.73 | 15 |
| 10 | 630.26 | 0.031 | x | 0.95 | 4 |
| 11 | 997.8 | 0.057 | x | 1.03 | 2 |
| 12 | 921.83 | 0.059 | x | 0.94 | 0 |
| 13 | 308.06 | 0.122 | 37.33 | 0.65 | 9 |
| 14 | 230.13 | 0.131 | 38.47 | x | 6 |
| 15 | 94.47 | 0.121 | 9.7 | 0.47 | 12 |
| 16 | 564.1 | 0.087 | 48.9 | 0.76 | 10 |
| 17 | 662.27 | 0.107 | 93.13 | x | 2 |
| 18 | 347.47 | 0.096 | 37.4 | 0.7 | 5 |
| 19 | 407.07 | 0.081 | 65 | x | 6 |
| 20 | 110.5 | 0.154 | 24.2 | x | 10 |
| 21 | 321.4 | 0.122 | 44.8 | 1.37 | 1 |
| 22 | 386.53 | 0.096 | 37.07 | 1.47 | 3 |
Footnotes
Ethical approval: This research was conducted following ethical review from HRA East of England Essex Research Ethics Committee (Ref: 15/EE/0436)
Guarantor: Hannah Michelle Williamson - Corresponding Author
Contributorship: Hannah Michelle Williamson: Concept and design of study and data analysis technique. Data acquisition. Article draft, revision and approval.
Matthew Bartlett: Concept and design of study and data analysis technique. Article revision and approval.
Mital Desai: Design of study. Article revision and approval.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) would like to thank the Royal Free Charity for the financial support to enable open-access publication.
ORCID iD: Hannah Michelle Williamson https://orcid.org/0000-0001-6582-288X
References
- 1.Emerging Risk Factors Collaboration (ERFC). Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Health and Care Excellence (NICE). Clinical Knowledge Summary Diabetes – type 2, https://cks.nice.org.uk/topics/diabetes-type-2/ (2020, accessed September 2020).
- 3.Khanolkar M, Bain S, Stephens J. The diabetic foot. QJM 2008; 101: 685–695. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Fact sheets: diabetes. World Health Organization, https://www.who.int/news-room/fact-sheets/detail/diabetes (2020, accessed September 2020). [Google Scholar]
- 5.Low Wang C, Blomster J, Heizer G, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol 2018; 25;72: 3274–3284. [DOI] [PubMed] [Google Scholar]
- 6.Moxey P, Gogalniceanu P, Hinchliffe R, et al. Lower extremity amputations — a review of global variability in incidence. Diabet Med 2011; 28: 1144–1153. [DOI] [PubMed] [Google Scholar]
- 7.Potier L, Abi Khalil C, Mohammedi K, et al. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc 2011; 41: 110–116. [DOI] [PubMed] [Google Scholar]
- 8.Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med 2016; 21: 382–389. [DOI] [PubMed] [Google Scholar]
- 9.An S, Son Y, Kim S, et al. Vascular calcification score on plain radiographs of the feet as a predictor of peripheral arterial disease in patients with chronic kidney disease. Int Urol Nephrol 2010; 42: 773–780. [DOI] [PubMed] [Google Scholar]
- 10.Toursarkissian B, Alejandro M, Smilanich R, et al. Noninvasive localisation of infrainguinal arterial occlusive disesase in diabetics. Ann Vasc Surg 2001; 15: 73–78. [DOI] [PubMed] [Google Scholar]
- 11.National Institute of Health and Care Excellence (NICE). Nice guidance: Type 2 diabetes in adults, https://www.nice.org.uk/guidance/ng28/chapter/Introduction (2019, accessed September 2020).
- 12.Coffi S, Ubbink D, Zwiers I, et al. The value of peak systolic velocity ratio in the assessment of haemodynamically significance of subcritical iliac artery stenoses. Eur J Vasc Endovasc 2001; 22: 424–428. [DOI] [PubMed] [Google Scholar]
- 13.Coffi S, Ubbink D, Zwiers I, et al. Improved assessment of the hemodynamic significance of borderline iliac stenoses with use of hyperemic duplex scanning. J Vasc Surg 2002; 36: 575–580. [DOI] [PubMed] [Google Scholar]
- 14.Coffi S, Ubbink D, Legemate D. Nonivasive techniques to detect subcritical iliac artery stenoses. Eur J Vasc Endovasc 2005; 29: 305–307. [DOI] [PubMed] [Google Scholar]
- 15.Elsman B, Legemate D, de Vos H, et al. Hyperaemic colour Duplex scanning for the detection of aortoiliac stenoses. A comparative study with infra-arterial pressure measurement. Eur J Vasc Endovasc 1997; 14: 462–467. [DOI] [PubMed] [Google Scholar]
- 16.Shaalan W, French-Sherry E, Castilla M, et al. Reliability of common femoral artery hemodynamics in assessing the severity of aortoiliac inflow disease. J Vasc Surg 2003; 37: 960–969. [DOI] [PubMed] [Google Scholar]
- 17.Van Asten W N, Beijneveld W J, Pieters B R, et al. Assessment of aortoiliac obstructive disease by Doppler spectrum analysis of blood flow velocities in the common femoral artery at rest and during reactive hyperaemia. Surgery 1991; 109: 633–639. [PubMed] [Google Scholar]
- 18.Bardelli M, Veglio F, Arosio E, et al. New intrarenal echo-Doppler velocimetric indices for the diagnosis of renal artery stenosis. Kidney Int 2006; 69: 580–587. [DOI] [PubMed] [Google Scholar]
- 19.Van Tongeren R, Bastiaansen A, van Wissen R, et al. A comparison of the doppler-derived maximal systolic acceleration versus the ankle-brachial pressure index of detecting and quantifying peripheral arterial occlusive disease in diabetic patients. J Cardiovasc Surg 2010; 51: 391–398. [PubMed] [Google Scholar]
- 20.Buschmann E, Li L, Brix M, et al. A novel computer-aided diagnostic approach for detecting peripheral arterial disease in patients with diabetes. PloS one 2018; 13: e0199374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alnaeb M, Boutin A, Crabtree V, et al. Assessment of lower extremity peripheral arterial disease using a novel automated optical device. Vasc Endovasc Surg 2007; 41: 522–527. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Health and Care Excellence (NICE). CKS: Peripheral arterial disease: How should I assess a person with suspected peripheral arterial disease? https://cks.nice.org.uk/topics/peripheral-arterial-disease/diagnosis/assessment/ (2019, access September 2020).
- 23.Brouwers JJ, van Doorn LP, van Wissen RC, et al. Using maximal systolic acceleration to diagnose and assess the severity of peripheral artery disease in a flow model study. J Vasc Surg 2020; 71: 242–249. [DOI] [PubMed] [Google Scholar]
- 24.Doubeni C, Yood R, Emani S, et al. Identifying unrecognized peripheral arterial. Disease among asymptomatic patients in the primary care setting. Angiol 2006; 57: 171–180. [DOI] [PubMed] [Google Scholar]
- 25.Sung CK, Lee KH, Kim SH. Evaluation of factors influencing arterial Doppler waveforms in an in vitro flow phantom. Ultrasonography 2017; 36: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]