Abstract
The effect of spinal cord stimulation (SCS) using differential target multiplexed programming (DTMP) on proteins involved in the regulation of ion transport in spinal cord (SC) tissue of an animal model of neuropathic pain was evaluated in comparison to low rate (LR) SCS. Rats subjected to the spared nerve injury model (SNI) and implanted with a SCS lead were assigned to DTMP or LR and stimulated for 48 h. A No-SCS group received no stimulation, and a Sham group received no SNI or stimulation. Proteins in the dorsal ipsilateral quadrant of the stimulated SC were identified and quantified using mass spectrometry. Proteins significantly modulated by DTMP or LR relative to No-SCS were identified. Bioinformatic tools were used to identify proteins related to ion transport regulation. DTMP modulated a larger number of proteins than LR. More than 40 proteins significantly involved in the regulation of chloride (Cl−), potassium (K+), sodium (Na+), or calcium (Ca2+) ions were identified. SNI affected proteins that promote the increase of intracellular Ca2+, Na+, and K+ and decrease of intracellular Cl−. DTMP modulated proteins involved in glial response to neural injury that affect Ca2+ signaling. DTMP decreased levels of proteins related to Ca2+ transport that may result in the reduction of intracellular Ca2+. Presynaptic proteins involved in GABA vesicle formation and release were upregulated by DTMP. DTMP also upregulated postsynaptic proteins involved with elevated intracellular Cl−, while modulating proteins, expressed by astrocytes, that regulate postsynaptic Cl− inhibition. DTMP downregulated K+ regulatory proteins affected by SNI that affect neuronal depolarization, and upregulated proteins that are associated with a decrease of intracellular neuronal K+ and astrocyte uptake of extracellular K+. DTMP treatment modulated the expression of proteins with the potential to facilitate a reversal of dysregulation of ion transport and signaling associated with a model of neuropathic pain.
Keywords: spinal cord stimulation, differential target multiplexed programming, proteomics, ion transport regulation, neuropathic pain model
Introduction
Chronic pain greatly impacts the quality of life and socioeconomic welfare of hundreds of millions of patients worldwide. 1 The financial burden associated with the treatment of chronic pain dictates the need for improving the effectiveness of available treatments. Conventional management uses pharmacological agents, physical therapy, or minimally invasive procedures. When these treatments fail, spinal cord stimulation (SCS) becomes a safe and highly effective option.
The optimization of this treatment requires the understanding of chronic pain and how SCS parameters affects it. Chronic pain involves the activation of neuroinflammatory processes at the spinal cord level, driven by glial cells.2,3 Following peripheral nerve injury, ectopic neuronal action potentials lead to neurotransmitter release at synapses, generating action potentials and activating surrounding glial cells. These processes lead to phenotypic changes involving many genes relevant to pain-related processes.4,5 It has been shown that glial activation is fundamental in the development and maintenance of chronic neuropathic pain via the disruption of biological processes that are key in maintaining balanced neuron-glial interactions.6,7 Among these processes, ion regulation across cell membranes plays a critical role in cell communication at the neuron-glial interaction and in intracellular cascades triggered by activation of metabotropic and ionotropic receptors. It is, therefore, worth evaluating how electrical signals affect those processes to increase our understanding of the mechanism of action of SCS. For example, changes in intracellular calcium ion (Ca2+) concentrations are associated with inflammatory states leading to peripheral and central sensitization. 8 Intracellular Ca2+ levels are especially crucial in astrocyte’s signaling and regulation of both excitatory and inhibitory synaptic transmission on multiple synaptic connections. Many cation-permeable ionotropic receptors for neurotransmitters like glutamate (GLU), acetylcholine, and adenosine triphosphate (ATP), present at presynaptic, postsynaptic, extrasynaptic, and glial cell membranes, are permeable to Ca2+ and can contribute to synaptic function at different levels. 9 Furthermore, astrocytes communicate with each other via propagating Ca2+ waves released from the endoplasmic reticulum mediated by inositol triphosphate through gap junctions allowing for intercellular signaling. Perisynaptic astrocytic processes are endowed with neurotransmitter transporters that maintain neurotransmitter homeostasis. These processes are rich in ionotropic cation channels and sodium-dependent pumps (Na+/K+ pump) that may complement Ca2+ signaling providing a basis for the complex bidirectional astrocyte-neuron communication in the tripartite synapse. 10
Ion channels and transporters have been shown to be involved in a variety of both microglial11,12 and astroglial 13 functions. Glial cells help to maintain an ionic homeostatic state and as such respond to changes in the electrochemical environment. Regulation of Ca2+ is important for a number of intracellular cascades within both glial cell types. 14 Potassium ion (K+) regulation within microglia has been shown to be involved in modulating inflammatory cytokine expression as well as helping to regulate extracellular potassium levels. 15 Astrocytes play a key role in gamma-aminobutyric acid (GABA) signaling both by regulating chloride (Cl−) transport as well as GABA clearance and recycling back to the neuron for repackaging into presynaptic vesicles. 16 GABA receptors are expressed in neurons and glia, exerting their inhibitory function by regulating intracellular Cl− transport resulting in neuronal hyperpolarization. It has been shown that SCS modulates the electrophysiological GABAergic response and attenuates pain in an animal model. 17 Also relevant are sodium ion (Na+) and K+ channels, essential for the initiation and propagation of action potentials that critically influence the ability of the central nervous system to respond to diverse stimuli. Abnormal Na+ and K+ channel function has been linked to neuropathic pain.18,19
Unlike neurons, which generate action potentials when exposed to pulsed electrical signals of enough intensity and duration, glial cells do not generate action potentials upon membrane depolarization. Still, glial depolarization depends on electrical signal parameters such as frequency, pulse width, intensity, and charge balancing.20–22 Recently, we showed that a SCS programming approach using multiple signals (differential target multiplexed programming, DTMP) provided significant relief of pain-like behavior in the spared nerve injury (SNI) animal model of neuropathic pain while modulating gene expression of biological processes such as immune system, synaptic transmission, and ion transport toward levels of naive animals. 23 Furthermore, we showed that DTMP highly correlates with naïve animals upon the reversal of transcriptomic markers differentially expressed by neurons, microglia, astrocytes, and oligodendrocytes.23–25 The effect of SCS using DTMP on gene expression and pain-like behavior was more pronounced than that obtained with low rate SCS (LR).
Considering the crucial importance of ion dysregulation underlying chronic pain development, a burning question remains of how different modalities of spinal cord stimulation may affect proteins involved in ion transport regulation in the stimulated spinal cord tissue. This work presents the proteome of spinal cord tissue exposed to DTMP or LR in an animal model of neuropathic pain to better understand the effects of these two therapeutic modalities on ion transport regulation and cell communication pathways at the neuron-glial interface.
Materials and Methods
Animals, Surgical Manipulations and SCS
A description of the experimental design is provided elsewhere. 23 Briefly, the study was approved by the Institutional Animal Care and Use Committee at Illinois Wesleyan University. Animals were male Sprague-Dawley rats (Envigo RMS, Indianapolis, US) weighing in the 275–315 g range (10–12 weeks old). After acclimation to the environment, and following baseline assessments, animals were randomly assigned to a SCS group (DTMP or LR), a No-SCS group (implanted but untreated), or a Sham group (implanted and uninjured). Animals in the DTMP (n = 10), LR (n = 10) and No-SCS (n = 10) groups were subjected to the spared nerve Injury (SNI) model of neuropathic pain and implanted with a miniaturized cylindrical quadrupolar SCS lead as previously described in detail by our group. 23 Five days after surgical intervention, animals in the SCS groups (DTMP and LR) that successfully developed the pain model were continuously stimulated for 48 h, while those in the No-SCS group were sham-stimulated for the same period. Animals in the Sham group (n = 10) were subjected to a sham surgery for the SNI model and implanted with the SCS lead, and although not stimulated, they were assessed in parallel with animals in the other groups. Animals were housed individually in a temperature and humidity control room and subjected to a 12 h light/dark cycle. Food and water were supplied ad libitum. DTMP utilizes multiplexed charge-balanced pulsed signals with components at 50 Hz (150 µs pulse width, PW) and 1200 Hz (50 µs PW), distributed over the contacts of the lead. LR utilizes a single charge-balanced signal at 50 Hz (150 µs PW). Signal intensities in the 0.03–0.10 mA range and corresponded to about 70% of the motor threshold. Programs were not duty cycled and initial intensities were kept throughout stimulation.
Protein Isolation and Quantification
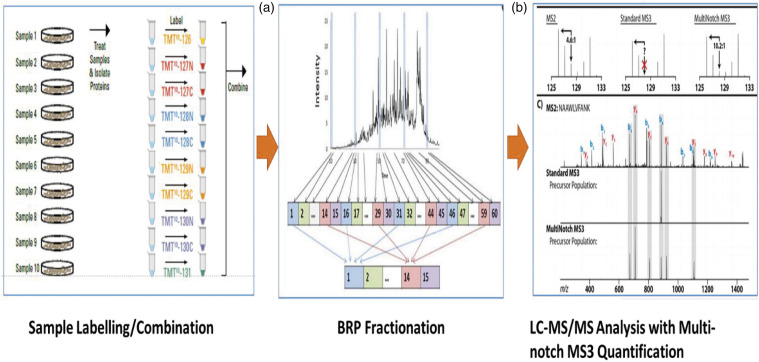
After SCS, animals were euthanized and the ipsilateral dorsal quadrant of the L1-L2 segment of the spinal cord underneath the SCS lead was harvested, washed with cold saline, and snap frozen before storage at −80°C. Representative samples (n = 4) from subjects that responded to the pain model and treatment, based on behavioral results previously reported, 23 were used in the proteomic analysis. Proteins were extracted from homogenized tissue and trypsinized using standard methods.26,27 Peptides from all experimental groups were tagged with isotopic labels unique to each group and analyzed using liquid chromatography-tandem mass spectrometry using standard methods (Cell Signaling Technology, Danvers, MA). Figure 1 illustrates the sample flow. Tissue was suspended in a 9M urea buffer free of detergents and enriched with protease inhibitors, followed by sonication and centrifugation to separate the proteins out. Total protein concentration was measured, and proteins digested with trypsin following alkylation of cysteine residues under appropriate buffering. Tryptic peptides were isotopically labeled using a tandem mass tag (TMT) system, in which each tagged sample consisted of a pool of biological specimens (n = 3–4) of a given experimental group. The TMT system allows for simultaneous identification, quantification, and comparison of multiple experimental groups. 28 Labeled peptides were combined and loaded onto a 50 cm x 100 μm PicoFrit capillary column packed with C18 reversed-phase resin and fractionated via reverse column liquid chromatography (LC) into 96 fractions. The column was developed with a 150-min linear gradient of acetonitrile in 0.125% formic acid delivered at 280 nL/min. Fractions were combined non-sequentially to 12 fractions, and their mass spectra obtained in a LC-tandem mass spectrometry (LC-MS/MS/MS) instrument, allowing the highest number of identifications possible and most accurate quantification via multi-notch MS3 methodology 26 with parameters optimized under protocols developed at Cell Signaling Technology. Prior to running samples, instrument performance was evaluated by running a control trypsin digested MKN45 whole cell lysate standard. Instrument performance was assessed by the number of unique peptide identifications from the control versus a quality cutoff. A four-protein mix peptide standard (Waters 186002866; 50 fmol/injection) was also added to each autosampler insert prior to LC-MS/MS/MS analyses to assess instrument performance and changes in instrument performance during all runs. Tandem mass spectra were evaluated using SEQUEST and the Core platform from Harvard University. 29 Protein searches were performed against the most recent update of the Uniprot rat database 30 with mass accuracy of ±50 ppm for precursor ions and 0.02 Da for product ions. Results were filtered with mass accuracy of ±5 ppm on precursor ions. Results were further filtered to a 1% protein level false discovery rate (FDR). Fold changes were obtained from comparison of the normalized spectral intensities (log2 scale) of the tagged peptides uniquely assigned to each protein. Significance of the normalized fold changes was calculated using a two tailed t-test for each protein identified and quantified. Proteins significantly modulated (p < 0.05) between Sham and No-SCS were isolated, and the effect of SCS treatment relative to No-SCS was followed. The combination of mass tagging of the samples, the multi-notch MS3 method, and a strong algorithm for accurate protein identification from reporter unique peptide ions provides reliable quantitative results. The StringDB bioinformatics tool 31 was used to build protein-protein interaction networks for significantly modulated proteins. Gene ontology enrichment analysis (GOEA) with the Panther database 32 was used to obtain biological processes that were modulated by the pain model and DTMP treatment.
Figure 1.
A sample scheme of the proteomics flow for isolation, purification, and quantification (courtesy of Cell Signaling Technology). Left: Trypsinized peptides from each experimental group are isotopically labeled and combined. Center: Labeled peptides are fractioned using high pressure liquid chromatography (HPLC). Right: Each fraction is analyzed in a tandem mass spectrometer (LC/MS/MS) to identify and quantify labeled peptides, which will be differentially assigned to a sample via isotopic shifting in the mass/charge (m/z) according to their respective label.
Results
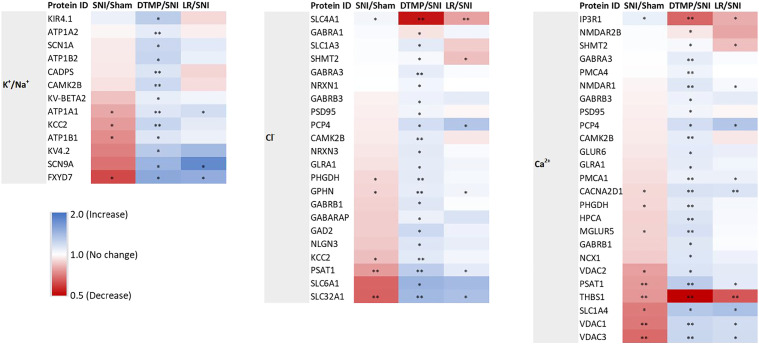
About 7200 proteins were uniquely identified and quantified in this study. Of these, 1222 and 705 were significantly modulated by DTMP and LR SCS, respectively. Table 1 and Figure 2 compile proteins enriched, via the GOEA, in biological processes that involve regulation of Na+/K+, Cl−, and Ca2+, that were significantly modulated by DTMP in comparison to the effect of treatment with LR. Table 1 lists statistically different fold change values, while Figure 2 contains heat maps illustrating the general trend of expression changes after either DTMP in comparison with LR treatment and in relation to the effect of the pain model. Given that the number of proteins significantly modulated by DTMP are almost twice the number of those modulated by LR, it is not surprising to find that a fraction of proteins involved in regulation of Na+/K+, Cl−, and Ca2+ significantly modulated by DTMP treatment were significantly modulated also by LR treatment.
Table 1.
Significantly modulated proteins (p value <0.05) and their respective expression fold changes (FC) due to injury model (SNI:SHAM) or following DTMP (DTMP:SNI) or LR (LR:SNI) relative to injury.
| Ion | Protein name | Protein ID | FC SNI:Sham | FC DTMP:SNI | FC LR:SNI | Cell type |
|---|---|---|---|---|---|---|
| K+/Na+ | Calcium/calmodulin-dependent protein kinase type II subunit beta isoform 2 | CAMK2B | 1.14* | N | ||
| Potassium voltage-gated channel subfamily D member 2 precursor | KV4.2 | — | 1.19 | — | N | |
| FXYD domain-containing ion transport regulator 7 | FXYD7 | −1.31 | 1.31 | 1.29 | N | |
| Sodium/potassium-transporting ATPase subunit alpha-1 precursor | ATP1A1 | −1.13 | 1.09* | 1.12 | N | |
| Sodium/potassium-transporting ATPase subunit beta-1 | ATP1B1 | −1.17 | 1.09 | — | N | |
| Voltage-gated potassium channel subunit beta-2 | KV-BETA2 | — | 1.06 | — | N | |
| Solute carrier family 12 member 5 | KCC2 | −1.15 | 1.15* | — | A, N | |
| Calcium-dependent secretion activator 1 | CADPS | — | 1.10* | — | N | |
| ATP-sensitive inward rectifier potassium channel 10 | KIR4.1 | — | 1.18 | — | A | |
| Sodium/potassium-transporting ATPase subunit alpha-2 precursor | ATP1A2 | — | 1.07* | — | A | |
| Sodium/potassium-transporting ATPase subunit beta-2 | ATP1B2 | — | 1.15 | — | A | |
| Sodium channel protein type 1 subunit alpha | SCN1A | — | 1.14 | — | M, N | |
| Sodium channel protein type 1 subunit alpha | SCN9A | — | 1.27 | 1.42 | N | |
| Cl− | Calmodulin regulator protein PCP4 isoform PEP19 | PCP4 | — | 1.24 | 1.41 | N |
| Gamma-aminobutyric acid receptor-associated protein | GABARAP | — | 1.11 | — | N | |
| Disks large homolog 4 | PSD95 | — | 1.08 | — | N | |
| Neuroligin-3 precursor | NLGN3 | — | 1.18 | — | A, N | |
| Neurexin-1 precursor | NRXN1 | — | 1.07 | — | A, N | |
| Neurexin-3 precursor | NRXN3 | — | 1.15 | — | N | |
| Gephyrin | GPHN | −1.09 | 1.13* | 1.08 | N | |
| Gamma-aminobutyric acid receptor subunit beta-1 precursor | GABRB1 | — | 1.18 | — | N | |
| Gamma-aminobutyric acid receptor subunit beta-3 precursor | GABRB3 | — | 1.10 | — | N | |
| Gamma-aminobutyric acid receptor subunit alpha-1 precursor | GABRA1 | — | −1.06 | — | A, N | |
| Gamma-aminobutyric acid receptor subunit alpha-3 precursor | GABRA3 | — | 1.13* | — | N | |
| Glycine receptor subunit alpha-1 precursor | GLRA1 | — | 1.14 | — | N | |
| Vesicular inhibitory amino acid transporter | SLC32A1 | −1.45* | 1.50* | 1.45 | A, N | |
| Glutamate decarboxylase 2 | GAD2 | — | 1.27 | — | N | |
| Excitatory amino acid transporter 1 isoform 3 | SLC1A3 | — | 1.15 | — | A | |
| Sodium- and chloride-dependent GABA transporter 1 | SLC6A1 | — | 1.54 | — | A, N | |
| Sodium-driven chloride bicarbonate exchanger isoform X3 | SLC4A1 | 1.07 | −1.85* | −1.22* | A, N | |
| Ca2+ | Plasma membrane calcium-transporting ATPase 1 | PMCA1 | — | 1.10* | 1.11 | A, N |
| Plasma membrane calcium-transporting ATPase 4 | PMCA4 | — | 1.09* | — | A, N | |
| Voltage-dependent anion-selective channel protein 1 | VDAC1 | −1.36* | 1.29* | 1.24 | N | |
| Voltage-dependent anion-selective channel protein 2 | VDAC2 | −1.23 | 1.20 | — | N | |
| Voltage-dependent anion-selective channel protein 3 isoform X1 | VDAC3 | −1.41* | 1.29* | 1.25 | M, N | |
| Sodium/calcium exchanger 1 isoform 9 precursor | NCX1 | — | 1.18 | — | A, M, N | |
| Neuron-specific calcium-binding protein hippocalcin | HPCA | — | 1.19* | — | N | |
| Glutamate receptor ionotropic, NMDA 1 isoform 3a precursor | NMDAR1 | — | 1.17* | 1.05 | A, M, N | |
| Glutamate receptor ionotropic, NMDA 2B precursor | NMDAR2B | — | −1.05 | — | A, M, N | |
| Glutamate receptor ionotropic, kainate 2 precursor | GLUR6 | — | 1.15 | — | A, M, N | |
| Metabotropic glutamate receptor 5 precursor | MGLUR5 | −1.10 | 1.22* | — | A, M, N | |
| Voltage-dependent calcium channel subunit alpha-2/delta-1 isoform 1 precursor | CACNA2D1 | −1.08 | 1.21* | 1.19* | M, N | |
| D-3-phosphoglycerate dehydrogenase | PHGDH | −1.08 | 1.15* | — | A | |
| Phosphoserine aminotransferase | PSAT1 | −1.24* | 1.36* | 1.15 | A, M | |
| Inositol 1,4,5-trisphosphate receptor type 1 isoform 3 | IP3R1 | 1.11 | −1.43* | −1.19 | A, N | |
| Serine hydroxymethyltransferase, mitochondrial | SHMT2 | — | 1.05 | −1.14 | A, N | |
| Thrombospondin-1 precursor | THBS1 | −1.26* | −2.40* | −1.49* | A | |
| Neutral amino acid transporter A | SLC1A4 | −1.34 | 1.39 | 1.45 | A |
Cellular expression from either astrocytes (A), microglia (M), or neurons (N) is also listed. Negative values indicate a decrease in expression level.
*Denotes p < 0.001.
Figure 2.
Heat maps illustrating fold changes in expression levels of proteins involved in regulation of K+/Na+ (left), Cl− (center) and Ca2+ (right) by DTMP and LR SCS relative to the pain model (DTMP/SNI and LR/SNI, respectively). The effect of the pain model relative to no injury is also included (SNI/Sham) to compare the effect of SCS treatments. For instance, pain tend to decrease gene expression, while DTMP tends to increase it, thus reversing the effect of pain. * denotes p < 0.05, ** denotes p < 0.001.
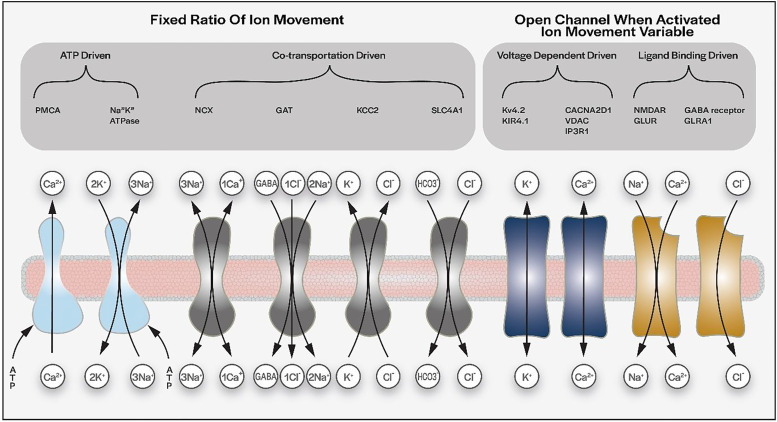
SCS Regulation of Potassium and Sodium Ion Transport
Of the proteins enriched via the GOEA, 10 were identified as being involved in regulation of K+ transport across cell membranes (Table 1; Figure 3). Multiple isoforms of Na+/K+ co-transporters were significantly modulated by DTMP, including some unaffected by the pain model. Of those regulated by DTMP, only ATPA1, FXYD7, and SCN9A were significantly modulated by LR. Na+/K+ ATPase subunits ATP1A1 and ATP1B1, primarily expressed by neurons, were downregulated by the pain model, and reversed toward Sham levels following DTMP stimulation. FXYD7, a support protein that helps to localize the ATP1B1 subunit to the plasma membrane of neurons, was also downregulated by the pain model and upregulated by DTMP. The expression of the subunits ATP1A2 and ATP1B2, primarily found in astrocytes, were unaffected by the pain model, but significantly increased following DTMP. The K+/Cl− cotransporter protein KCC2, only expressed in neuron cells, was also significantly downregulated by SNI and upregulated by DTMP. DTMP increased the expression of CADPS, which facilitates release of BDNF. 33 Following nerve injury, BDNF was shown to increase KCC2 activity, helping to restore Cl− homeostasis. 34
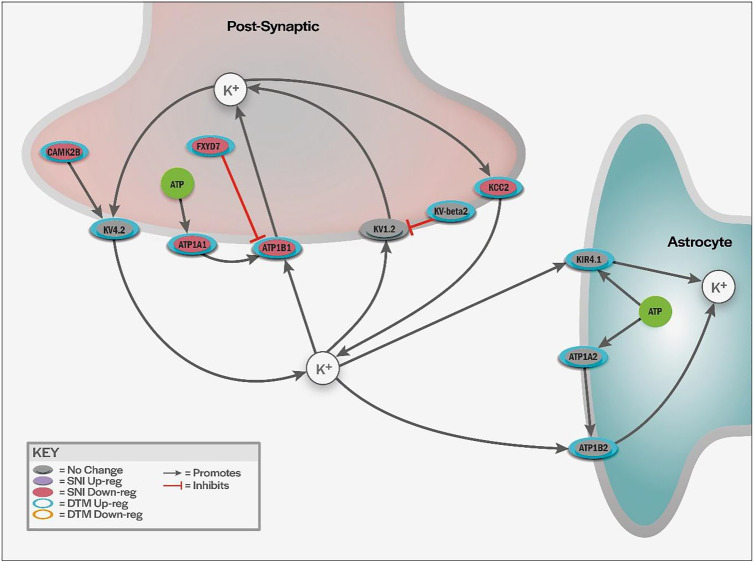
Figure 3.
Illustration of proposed modulation of K+ transport. Proteins modulated by DTMP that are involved in K+ regulation in postsynaptic neurons and surrounding astrocyte cells. K+ leak channels and pumps are important in resetting membrane potential and limiting action potential characteristics. Reduction in K+ efflux, induced by the pain model, promotes depolarization and increased hypersensitivity. DTMP reversed changes in K+ regulation induced by the pain model. Some proteins that further promote K+ regulation, though unaffected by SNI, were also increased following DTMP.
Ion channels that facilitate rapid K+ transport across cell membranes, which were significantly upregulated by DTMP, include KV4.2, expressed by neurons, and KIR4.1, expressed by astrocytes. KV4.2 allows the efflux of K+ out of the neuron thus reducing excitability. Though not an actual K+ transporter, KV-BETA2 was found to be upregulated by DTMP. This is neuronally expressed and known to inhibit the K+ transporter KV1.2. The inhibition of KV1.2 mediated by KV-BETA2 reduces neuronal excitability by reducing intracellular cation presence. Among the known 9 α-subunit voltage-gated sodium channel (VGSC) subtypes (Nav1.1 to Nav1.9), Nav1.1 (SCN1A) and Nav1.7 (SCN9A) were significantly modulated by DTMP when compared to SNI.
SCS Regulation of Chloride Transport
There were 15 proteins identified by the GOEA that are involved in regulation of Cl− transport (Figure 4; Table 1). Proteins significantly reduced by SNI and subsequently modulated by DTMP include GPHN, CAMK2B, KCC2, and SLC32A1. LR also significantly modulated GPHN and SLC32A1. GPHN and CAMK2B promote GABA receptor signaling. GPHN is a structural protein shown to localize GABA receptors to cell membranes in neurons. 35 CAMK2B promotes activation of the GABA receptor. SLC32A1 is found presynaptically and loads GABA into vesicles ready for release into the synapse. Another presynaptic protein upregulated by DTMP is GAD2, which converts GLU into GABA and is involved in GABA vesicle-loading. KCC2 clears excess Cl− from the neuron and allows resetting of the Cl− electrochemical equilibrium.
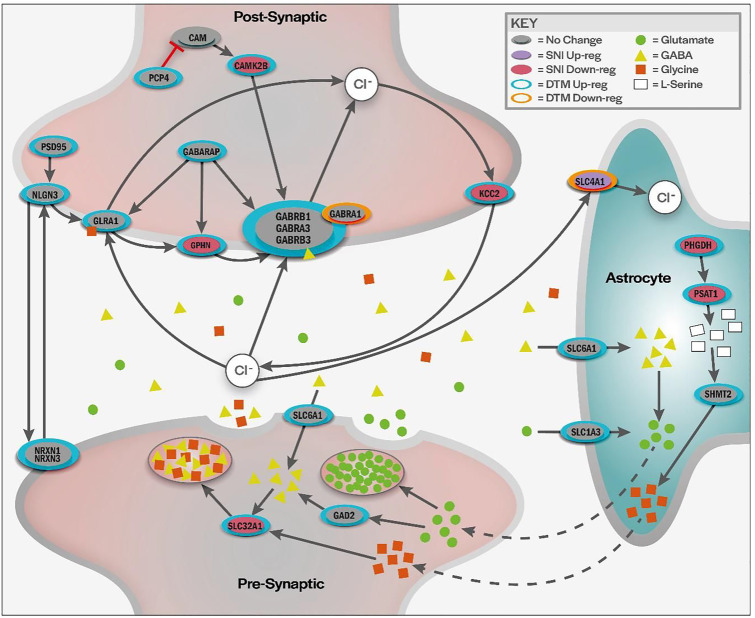
Figure 4.
Illustration of proposed modulation of Cl− transport. Proteins involved in Cl− regulation that are modulated by DTMP treatment. GABA receptors and the glycine receptors are key proteins allowing Cl− into the cell for inhibitory control and subsequent pain relief. The presynaptic release and recycling of GABA, with the aid of neighboring astrocytes, facilitates continued inhibition and pain control as evidenced by a shift in protein expression that would facilitate increased intracellular Cl−. Regarding proteins involved in GABA signaling and Cl− hyperpolarization, DTMP was able to reverse expression of proteomic changes induced by the pain model, as well as to increase expression of proteins unaffected by this but important to GABA and Cl− signaling pathway.
PSD95, NLGN3, NRXN, and GABARAP, involved in anchoring GABA receptors to the cell membrane, were upregulated by DTMP. GABA receptor subunits, GABRB3, GABRA3, and GABRB1 were upregulated by DTMP, whereas GABRA1 was downregulated. When activated, GABA receptors allow the influx of Cl− into the neuron resulting in neuron hyperpolarization and pain signaling inhibition. The glycine receptor, GLRA1, which facilitates Cl− influx, was found to be upregulated by DTMP. Transporters, such as SLC6A1, SLC1A3 (mainly expressed by astrocytes36,37), and SLC4A1, were upregulated by DTMP treatment. Only SLC4A1 was significantly upregulated by LR. These transporters play an indirect role in Cl− signaling or regulation.
SCS Regulation of Calcium Ion Transport
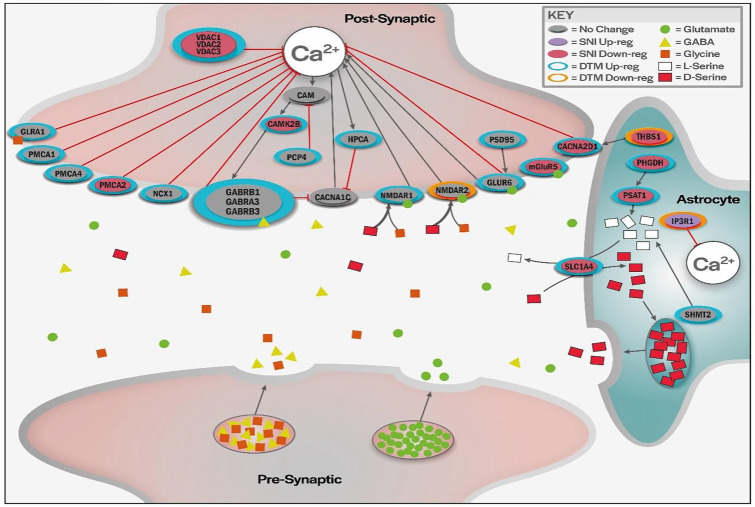
Proteins related to Ca2+ regulation and their interactions are depicted in Figure 5 and Table 1. The increase of cytoplasmic Ca2+ triggers events that lead to various cascades involved with neuronal sensitization. Three main types of proteins regulate Ca2+ cytoplasmic concentrations and consist of: (1) channels that when opened allow the free passage of Ca2+, (2) transporters that require energy and/or a secondary component to transport Ca2+ across a membrane, and (3) secondary proteins that do not directly facilitate Ca2+ movement but are intrinsically linked to the previous types of proteins.
Figure 5.
Illustration of proposed modulation of Ca2+ transport. Proteins involved in synaptic Ca2+ concentrations that were modulated by DTMP.
Expression levels of channel-related proteins VDAC1/2/3, CACNA2D1, and IP3R1 were significantly changed by the pain model and reversed by DTMP treatment. LR treatment also reversed levels of these proteins significantly, except for VDAC2. Interestingly, NMDAR1/2 and GLUR6 were not affected by pain, but significantly modulated by DTMP. It was observed that NMDAR1 was upregulated whereas NMDAR2 was downregulated in response to DTMP. The transporters PMCA1/2/44 and NCX1 were also modulated. Of the 3 PMCA isoforms, only PMCA2 was found to be significantly downregulated by the pain model, although all three were significantly upregulated by DTMP, while LR only upregulated PCMA1 significantly.
Levels of secondary proteins involved in regulation of Ca2+ transport, such as mGLUR5 and CAMK2B, were found to be significantly decreased by the pain model and only significantly reversed by DTMP. Other secondary proteins significantly upregulated by DTMP include the G-coupled GABA receptors GABRB1 and GABRB3 that generate cascade effects to modulate cytoplasmic Ca2+ and is affected by other DTMP modulated proteins like HPCA, CADPS PCP4, and PSD95.
PHGDH, PSAT1, and SHMT2, which are part of L-serine synthesis and are found within astrocytes, were upregulated by DTMP, reversing significant downregulation of PHGDH and PSAT1 induced by the pain model. Interestingly, LR also reversed PSAT1, but further downregulated SNI induced expression of SHMT2. Another signaling protein linked to astrocytes is THBS1, which was the only protein found related to calcium regulation that had decreased expression from the pain model and was reversed by DTMP and LR treatment.
Discussion
Our previous work 23 showed that DTMP modulates the expression of genes in the rat spinal cord segments (L1-L2) in which the sciatic nerve originates. The effect is distinctive from conventional SCS programs that use a single signal either at low rate (50 Hz) or high rate (1.2 kHz). That work also showed that, although SCS in general modulates ion transport regulation, the effect of DTMP is more significant at balancing neuron-glia interactions toward a pre-injury state. The results of the present study suggest that the mechanism of action for DTMP-based SCS may involve modulation of ion transport regulation. Although LR SCS likewise modulates proteins involved in regulation of K+, Na+, Cl−, and Ca2+ transport, the effect of DTMP was significantly larger (see Table 1 for complete list). These ions are responsible for establishing membrane potentials in neurons. Figure 6 summarizes the ionic channel types modulated by the pain model and/or DTMP. In general, increased intracellular concentrations of Na+ and Ca2+ result in neuron depolarization, contributing to enhanced pain signaling. Conversely, increased intracellular concentrations of K+ are fundamental in restoring the resting membrane potential, while Cl− plays an inhibitory role in neuronal firing. Although the present study did not directly measure or assess membrane permeability to specific ions, it is noteworthy that DTMP significantly upregulated proteins involved in the generation of K+ rectifiers and inward Cl− currents and the efflux of cytoplasmic Ca2+ and Na+, which may result in decreased excitability. Further studies to elucidate the changes in neuronal and glial transmembrane ion trafficking and electrophysiological changes associated with DTMP or other SCS waveforms are certainly warranted to advance our understanding of the mechanisms of actions of the various SCS modalities.
Figure 6.
A scheme depicting key membrane proteins that are involved with ion flux and that have been modulated by treatment with DTMP.
Studies have shown that neuropathic pain can, in part, be characterized by suppression or reduced expression of K+ channels. 38 Neural activity induces elevated extracellular K+ concentrations, and the reuptake of K+ is crucial to reestablishing the membrane potential. 39 KIR4.1, upregulated by DTMP, in addition to facilitating release of BDNF from astrocytes also allows entry of K+ facilitating K+ synaptic clearance. Consistent with those studies, our analyses show that the pain model decreased the expression of proteins involved in regulation of K+ membrane gradients such as ATP1B1, ATP1A1, FXYD7, CAMK2B, and KCC2. These proteins facilitate reestablishing the membrane potential either directly, as with the Na+/K+ ATPase ATP1B1, or via secondary components like FXYD7, which interacts with ATP1B1 reducing its binding affinity to K+, thereby allowing faster release of K+ and increasing the rate of K+ influx into the neuron. 40 Na+/K+ ATPase proteins transport 3 Na+ out of the cell and bring 2 K+ into the cells to reestablish electrochemical equilibrium and decrease neuronal excitability and reestablish membrane potential. Additionally, KCC2 removes K+ and Cl− from the cell, thus reestablishing the electrochemical equilibrium and allowing continued repolarization of neurons, which is primarily governed by the entry of Cl−. Furthermore, the influx of K+ into astrocytes, mediated by Na+/K+ ATPase, has been shown to inhibit BDNF release, 41 facilitating the increased activity of neuronal KCC2 and inhibiting hyperalgesia. 42 DTMP increased, thus reversed, the expression levels of all K+-related proteins (Table 1). These expression changes, taken together, have the potential to reestablish the electrophysiological properties of the neural tissues that were altered by the pain model.
At rest, neuronal intracellular Na+ levels are low relative to the extracellular space. Na+ efflux plays a crucial role in maintaining the membrane potential, and its influx determines membrane depolarization during action potential generation. This process is tightly regulated by Na+/K+ ATPases and Na+/Ca2+exchangers. As such, a large percentage of the neurons energy, up to 70%, is used to drive Na+/K+ ATPases, in the form of ATP, 43 to reestablish the Na+ and K+ gradient that resets the membrane potential after depolarization. Ion gradients can also drive the flux of other ions across the membrane. For example, we found increased expression of the GABA transporter protein SLC6A1 following DTMP stimulation, which is found in both neurons and glial cells. SLC6A1 uses the Na+ gradient to provide reuptake of GABA from the extracellular space and is important in resetting inhibitory signaling. Interestingly, cytosolic Na+ concentration in astrocytes is twice as high as that in neurons, setting a reversal potential for many Na+-dependent transporter/exchangers. Furthermore, mechanical or chemical stimulation of astrocytes induces complex changes in Na+ waves spreading through the astrocyte syncytium. 44 Beyond Na+ transporters and exchangers, relevant VGSCs, like Nav1.1 (SCN1A) and Nav1.7 (SCN9A), which play a critical role in neuropathic and nociceptive pain, were significantly modulated by DTMP or LR (only for SCN9A). VGSCs are integral membrane proteins consisting of a central α-subunit associated with one or more auxiliary β-subunits that support electrogenesis in neurons and in other cell types including astrocytes, and microglia, where they regulate phagocytosis, motility, Na+/K+-ATPase activity, and secretion of cytokines. 45 Human genetic evidence supports the crucial involvement of SCN9A to pain sensation. Loss of SCN9A function leads to congenital insensitivity to pain, whereas gain-of-function mutations in the encoding gene cause painful neuropathies, such as inherited erythromelalgia. 46 Furthermore, recent evidence showed that activation of SCN1A in modality-specific nociceptive fibers elicits robust mechanical pain but not thermal hypersensitivity without neurogenic inflammation. 47 Interestingly, while the α-subunit forms the ion conducting pore and the channel gate for activation and inactivation, the beta subunit dictates the insertion of the ion channel into the membrane. Evaluation of these highly regulated processes with phosphoproteomic techniques may provide valuable data to further understand the biological processes modulated by DTMP.
It is known that high levels of cytosolic Ca2+ result in neuron depolarization and activate pathways that result in neural sensitization. 48 Many proteins, such as HPCA, CADPS PCP4, and PSD95, which were modulated by DTMP, bind to Ca2+ to elicit their effects. PCP4, for example, interacts with the calcium-binding protein calmodulin to modulate Ca2+ binding affinity. CAMK2B is a kinase downstream of Ca2+-activated calmodulin and has been shown to interact with GABAB receptors promoting their activity. PSD95 is an anchor for excitatory- or cation-related channels and transporters such as NMDAR, AMPAR, GluR, K+ channels, and PMCA. Its role is similar to that of GPHN, which anchors inhibitory related proteins to the synaptic membrane and is also involved in Cl− regulation. Additionally, high levels of cytosolic Ca2+ in glial cells induce inflammatory pathways leading to increased neuroinflammatory expression and neuronal sensitization. 49 As Ca2+ is key in generating neuronal sensitization, it is not surprising that most proteins identified with regulation of ion transport were related to Ca2+. The exchange of Ca2+ is crucial for typical neural functions, and several proteins involved in Ca2+ influx were affected by the pain model. Many proteins involved in modulating cytosolic Ca2+ levels identified by the GOEA are involved in regulating intracellular Ca2+ presence and predominantly within neural cells (Figure 5). For example, expression levels of three VDAC isoforms were found to be significantly decreased by the pain model. DTMP reversed the effect of the pain model (Table 1) in these 3 isoforms, and LR in two of them. VDAC proteins are expressed in the mitochondrial membrane and clear excess Ca2+. Conversely, the level of IP3R1, a channel protein in the endoplasmic reticulum that functions to release Ca2+ into the cytoplasm, was increased by the pain model. DTMP and LR significantly reversed the expression of this protein relative to the pain model, possibly mitigating the effect on intracellular calcium concentration exerted by the pain model. Proteins that inhibit neuronal Ca2+ influx were upregulated following DTMP with some, such as the Ca2+ exporter PMCA2, also shown to be downregulated in response to the injury model. NMDAR receptors were differentially regulated by DTMP though they can promote different pathways. 50 The expression of IP3R1, a channel protein in the endoplasmic reticulum that functions to release Ca2+ into the cytoplasm, was increased due to the pain model and significantly reversed by DTMP. D-Serine is a potent agonist of NMDARs and increased expression of a protein, such as SLC1A4, provides its clearance from the synapse further demonstrating the capability of DTMP to reduce Ca2+ influx.
Serine biosynthesis has been previously linked to neuropathic pain. Enzymes engaged in L-serine biosynthesis by astrocytes, 51 such as PHGDH and PSAT1, underwent reversed expression with DTMP relative to the pain model. This reversal points towards a critical impact on the modulation of synaptic plasticity by glial cells. Neurons cannot synthesize L-serine in the synapse because they do not express PHGDH. Others have reported the reduction of PHGDH in the dorsal root ganglion (DRG) of neuropathic pain models.52,53 In a paclitaxel-induced peripheral neuropathy model, the decrease of PHGDH in DRG glial cells led to a decrease in L-serine, which modulated pain-like behavior when administered intraperitoneally. L-serine is the main source of the neurotransmitters glycine and D-serine, and is essential for the synthesis of phospholipids. 52 D-serine and glycine are co-agonists in the binding of GLU to NMDAR. D-serine is synthesized from L-serine by serine racemase, 54 while glycine is synthesized from L-serine by SHMT, 55 which was upregulated by DTMP and downregulated by LR. The L-serine biosynthetic pathway in the metabolic and catabolic homeostasis by astrocytes plays an important role in the activity and plasticity of the synapse. It is noteworthy to emphasize that DTMP modulated critical enzymes in this process with the potential to reestablish homeostatic levels of L-serine. The potential role of DTMP in modulating astrocytes to balance biological processes is further emphasized by the regulation of ionotropic and metabotropic GLU receptors by DTMP that modulate intracellular second messenger pathways, including Ca2+. 56
In terms of direct inhibitory signaling, the increase of intracellular Cl− leads to hyperpolarization, and reduces and/or prevents action potential generation. Many analgesics target chloride-permeable receptors, specifically GABA receptors, to treat neuropathic pain conditions. We identified that the pain model induced a decrease in the expression of channel proteins involved in reestablishing Cl− homeostasis, such as KCC2, and secondary players such as GPHN. GPHN is an anchoring protein that helps translocating GABA receptors to the plasma membrane in neurons. Many proteins identified as being involved in regulation of Cl− transport was significantly increased following treatment with DTMP (Figure 4). This includes Cl− channels and transporters like KCC2, GLRA1, and GABA receptors (GABRB3, GABRA3, and GABRB1). GPHN, also upregulated by DTMP, has been shown to localize GLRA1 to the membrane. 35 Other components involved in regulating Cl− transport found to be significantly upregulated by DTMP treatment are related to presynaptic GABA vesicle production and release (GAD2, SLC32A1) and astrocyte reuptake of excess synaptic GABA (SLC6A1). SLC6A1 allows astrocytes to take up excess GABA, which can be converted to GLU, which in turn may be transported back to the neuron for either GLU-induced excitability or converted back to GABA for induced inhibition. Similarly, SLC1A3 takes up excess GLU from the synapse, which can be recycled back to the neuron. Expression of SLC4A1, a Cl− transporter found in astrocytes, was upregulated by the pain model, and significantly reversed by DTMP and LR treatment, although at a larger extent by DTMP. Given that SLC4A1 facilitates Cl− clearance and enhances neuronal sensitization, DTMP may limit Cl− clearance, and presumably enhance inhibitory signaling.
Changes in the membrane potential is the functional aspect of neurons that allow the propagation of signals through the body and cause a response or outcome, as in the case of pain perception. As such, regulation of ion transport at synapses is typically found to be slightly modified in response to nerve injury, but not drastically. Indeed, drastic ion concentration changes in the synaptic or extracellular spaces typically lead to cell death or diseased states. Though some studies have shown changes in the activity of ion channels following induction of a neuropathic pain model, 57 this does not necessarily translate into a measurable change in the concentration of those proteins within neural cells, as the regulation by phosphorylation or other mediators is more common. Many proteins maintain a basal level of expression, even following nerve injury, and get incorporated within or removed from a membrane upon interacting with a secondary protein or messenger, leading to activation or inhibition. Indeed, we observed no significant change in expression levels in many of the proteins due to the pain model, although DTMP treatment modulated them. This is plausible because channel activity is constantly required for normal signaling, whereas inhibitory pathways are not constantly upregulated without some chronic stimulus, whether it be pharmacological or electrical.
The intrinsic nature of protein function requires the study of post-translational modifications, such as phosphorylation and acetylation, which are out of this study’s scope. Besides this inherent limitation of the general proteomics method, there are other limitations pertinent to this study, such as the inclusion of only male subjects. It is plausible that results with female subjects would render different protein expression profiles based on differences previously found in gender-based responses to pain-like behavior and gene expression.58,59 The extensive data acquired and presented here corresponds to an early snapshot in the evolution of chronic neuropathic pain in the SNI animal model. It is conceivable that changes in protein expression due to the pain model, and the effects of treatment would differ at later stages. Current efforts aimed at discovering the effect of time on the neuropathic animal model and effect of DTMP are ongoing in our laboratory. Also, 48 h of continuous SCS treatment does not reflect stimulation periods used clinically, which are much longer. Longer durations of DTMP treatment may have resulted in different modulation patterns. This work also limits the analysis to the dorsal ipsilateral quadrant of the stimulated cord (L1-L2), which is the origin of the sciatic nerve in the rat. 60 Proteomic analysis in the cord at the level of the L4-L6 sciatic nerve roots and DRGs could provide additional insight into the non-local effects of DTMP.
Despite its limitations, this study supports the idea that the mechanism of action of DTMP stimulation involves, at least in part, modulation of ion regulation at the tripartite synapse. The proposed net effect of DTMP, and to a lesser extent LR, on reestablishing homeostatic ionic balance via the modulation of expression levels of proteins involved in the regulation of ion transport is proposed in Figure 7. This study also suggests that the modulatory effect of DTMP is stronger than that provided by LR SCS, in agreement with previous transcriptomics-based analysis.23–25 Inflammatory cascades are activated by depolarizing events, such as prolonged increases in cytosolic Ca2+, which result in increased expression of inflammatory mediators. The overall trend observed in this study is the modulation of the expression of proteins that facilitate an increase in K+ permeability and cytosolic Cl− and a decrease in cytosolic Ca2+, which potentially may lead to inhibition at the neuron-glial interaction, promoting decreased excitability, and thereby providing analgesia. The reversal by DTMP treatment of changes in expression levels of multiple ion-regulated channels expressed on neuronal and glial membranes induced by the pain model highlights the relevance of preclinical research to understand the effect of electrical signals on pain processes. Our future work will be focused on characterizing the proteomic and phosphoproteomic changes observed in the neural tissue during neuroinflammation.
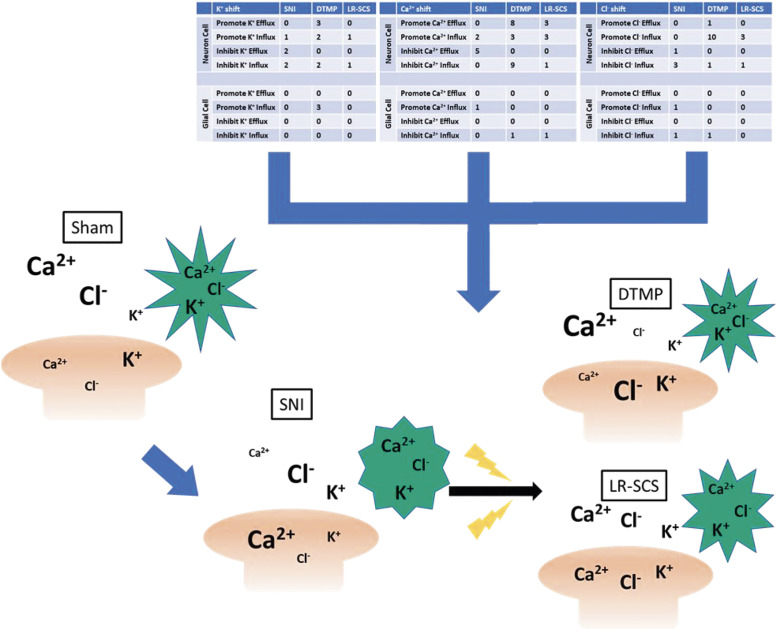
Figure 7.
Illustration of proposed regulation of ion transport across neuron, glial, and extracellular space based on proteomic changes due to SNI model, DTMP, or LR. In the uninjured Sham state, extracellular concentrations of Ca2+ and Cl−are elevated with K+ concentration being higher intracellularly in the neuron. The pain model caused a shift in inward Ca2+ currents and inhibiting Cl−influx. This shift in ion regulatory proteins was reversed toward Sham levels in DTMP treated animals with a similar, but less robust, shift due to LR.
Acknowledgments
Thanks to Stimgenics and Millennium Pain Center for funding this research.
Footnotes
Author Contributions: D.L.C. and R.V. designed the study. All authors analyzed the data and contributed to the drafting and editing of the final draft.
Conflict of Interests: D.L.C. and R.V. are paid consultants and advisory board members of Medtronic Inc. They are co-inventors in patents related to differential target multiplexed spinal cord stimulation. Other authors declare no conflicts.
Funding: Stimgenics LLC and Millennium Pain Center.
ORCID iD
David L. Cedeño https://orcid.org/0000-0001-5421-802X
References
- 1.Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the global burden of disease study 2017. Ann Transl Med 2020; 8: 299. DOI: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertozzi MM, Rossaneis AC, Fattori V, et al. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem Biol Interact 2017; 273: 180–189. DOI: 10.1016/j.cbi.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003; 306: 624–630. DOI: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 4.Grace PM, Hutchinson MR, Maier SF, et al. Pathological pain and the neuroimmune interface. Nat Reviews Immunol 2014; 14: 217–231. DOI: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012; 73: 638–652. DOI: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens KE, Chen Z, Sivanesan E, et al. RNA-seq of spinal cord from nerve-injured rats after spinal cord stimulation. Mol Pain 2018; 14: 174480691881742. DOI: 10.1177/1744806918817429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallejo R, Tilley DM, Cedeño DL, et al. Genomics of the effect of spinal cord stimulation on an animal model of neuropathic pain. Neuromodulation 2016; 19: 576–586. DOI: 10.1111/ner.12465. [DOI] [PubMed] [Google Scholar]
- 8.Patel R, Montagut-Bordas C, Dickenson AH. Calcium channel modulation as a target in chronic pain control. Br J Pharmacol 2018; 175: 2173–2184. DOI: 10.1111/bph.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pankratov Y, Lalo U. Calcium permeability of ligand-gated Ca2+ channels. Eur J Pharmacol 2014; 739: 60–73. DOI: 10.1016/j.ejphar.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Verkhratsky A, Noda M, Parpura V, et al. Sodium fluxes and astroglial function. Adv Exp Med Biol 2013; 961: 295–305. DOI: 10.1007/978-1-4614-4756-6_25. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo P, Attwell D, Madry C. Ion channels and receptors as determinants of microglial function. Trends Neurosci 2019; 42: 278–292. DOI: 10.1016/j.tins.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Ono C, Aiba S, et al. Therapeutic concentration of lithium stimulates complement C3 production in dendritic cells and microglia via GSK-3 inhibition. Glia 2015; 63: 257–270. DOI: 10.1002/glia.22749. [DOI] [PubMed] [Google Scholar]
- 13.Hatton GI, Parpura V. Ion Channels in Astrocytes. Boston, MA: Springer, 2004. [Google Scholar]
- 14.Mizoguchi Y, Kato TA, Seki Y, et al. Brain-derived neurotrophic factor (BDNF) induces sustained intracellular Ca2+ elevation through the up-regulation of surface transient receptor potential 3 (TRPC3) channels in rodent microglia. J Biol Chem 2014; 289: 18549–18555. DOI: 10.1074/jbc.M114.555334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Lu J, Wu CY, et al. Expression of Kv1.2 in microglia and its putative roles in modulating production of proinflammatory cytokines and reactive oxygen species. J Neurochem 2008; 106: 2093–2105. DOI: 10.1111/j.1471-4159.2008.05559.x. [DOI] [PubMed] [Google Scholar]
- 16.Elorza-Vidal X, Gaitán-Peñas H, Estévez R. Chloride channels in astrocytes: structure, roles in brain homeostasis and implications in disease. Int J Mol Sci 2019; 20: 1034. DOI: 10.3390/ijms20051034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuwissen KPV, Vries LE, Gu JW, et al. Burst and tonic spinal cord stimulation both activate spinal GABAergic mechanisms to attenuate pain in a rat model of chronic neuropathic pain. Pain Pract 2020; 20: 75–87. DOI: 10.1111/papr.12831.2019/08/20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 2014; 37: 146–158. DOI: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace MS. Calcium and sodium channel antagonists for the treatment of pain. Clin J Pain 2000; 16: S80–S85. DOI: 10.1097/00002508-200006001-00014. [DOI] [PubMed] [Google Scholar]
- 20.Agnesi F, Blaha CD, Lin J, et al. Local glutamate release in the rat ventral lateral thalamus evoked by high-frequency stimulation. J Neural Eng 2010; 7: 026009. DOI: 10.1088/1741-2560/7/2/026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roitbak AI, Fanardjian VV. Depolarization of cortical glial cells in response to electrical stimulation of the cortical surface. Neuroscience 1981; 6: 2529–2537. DOI: 10.1016/0306-4522(81)90098-1. [DOI] [PubMed] [Google Scholar]
- 22.Tawfik VL, Chang SY, Hitti FL, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery 2010; 67: 367–375. DOI: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallejo R, Kelley CA, Gupta A, et al. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain 2020; 16: 174480692091805. DOI: 10.1177/1744806920918057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cedeño DL, Smith WJ, Kelley CA, et al. Spinal cord stimulation using differential target multiplexed programming modulates neural cell-specific transcriptomes in an animal model of neuropathic pain. Mol Pain 2020. Jan-Dec; 16: 1744806920964360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith WJ, Cedeño DL, Thomas SM, et al. Modulation of microglial activation states by spinal cord stimulation in an animal model of neuropathic pain: Comparing high rate, low rate, and differential target multiplexed programming. Mol Pain 2021; 17: 1744806921999013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAlister GC, Nusinow DP, Jedrychowski MP, et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem 2014; 86: 7150–7158. DOI: 10.1021/ac502040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting L, Rad R, Gygi SP, et al. MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 2011; 8: 937–940. DOI: 10.1038/nmeth.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Elias JE. Relative protein quantification using tandem mass tag mass spectrometry. Methods Mol Biol 2017; 1550: 185–198. DOI: 10.1007/978-1-4939-6747-6_14. [DOI] [PubMed] [Google Scholar]
- 29.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 1994; 5: 976–989. DOI: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 30.The UniProt Consortium . UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 2019; 47: D506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019; 47: D607–D613. DOI: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 2003; 13: 2129–2141. DOI: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinoda Y, Sadakata T, Nakao K, et al. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci 2011; 108: 373–378. DOI: 10.1073/pnas.1012220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beverungen H, Klaszky SC, Klaszky M, et al. Rehabilitation decreases spasticity by restoring chloride homeostasis through the brain-derived neurotrophic factor-KCC2 pathway after spinal cord injury. J Neurotrauma 2020; 37: 846–859. DOI: 10.1089/neu.2019.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choii G, Ko J. Gephyrin: a central GABAergic synapse organizer. Exp Mol Med 2015; 47: e158. DOI: 10.1038/emm.2015.5. [DOI] [PubMed] [Google Scholar]
- 36.Pirttimaki T, Parri HR, Crunelli V. Astrocytic GABA transporter GAT-1 dysfunction in experimental absence seizures. J Physiol 2013; 591: 823–833. DOI: 10.1113/jphysiol.2012.242016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13: 713–725. DOI: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 38.Du X, Gamper N. Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmaco 2013; 11: 621–640. DOI: 10.2174/1570159X113119990042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beguin P, Crambert G, Monnet-Tschudi F, et al. FXYD7 is a brain-specific regulator of Na,K-ATPase alpha1-beta isozymes. EMBO J 2002; 21: 3264–3273. DOI: 10.1093/emboj/cdf330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crambert G, Geering K. FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci STKE 2003; 2003: RE1–re1. DOI: 10.1126/stke.2003.166.re1. [DOI] [PubMed] [Google Scholar]
- 41.Kinboshi M, Mukai T, Nagao Y, et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front Mol Neurosci 2017; 10: 408. DOI: 10.3389/fnmol.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitayama T.. The Role of K+-Cl−-cotransporter-2 in neuropathic pain. Neurochem Res 2018; 43: 110–115. DOI: 10.1007/s11064-017-2344-3. [DOI] [PubMed] [Google Scholar]
- 43.Ronquist G, Waldenstrom A. Imbalance of plasma membrane ion leak and pump relationship as a new aetiological basis of certain disease states. J Intern Med 2003; 254: 517–526. DOI: 10.1111/j.1365-2796.2003.01235.x. [DOI] [PubMed] [Google Scholar]
- 44.Parpura V, Verkhratsky A. Homeostatic function of astrocytes: Ca2+ and Na+ signalling. Transl Neurosci 2012; 3: 334–344. DOI: 10.2478/s13380-012-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black JA, Waxman SG. Noncanonical roles of voltage-gated sodium channels. Neuron 2013; 80: 280–291. DOI: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Shields SD, Deng L, Reese RM, et al. Insensitivity to pain upon adult-onset deletion of Nav1.7 or its blockade with selective inhibitors. J Neurosci 2018; 38: 10180–10201. DOI: 10.1523/JNEUROSCI.1049-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osteen JD, Herzig V, Gilchrist J, et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016; 534: 494–499. DOI: 10.1038/nature17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleichmann M, Mattson MP. Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal 2011; 14: 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sompol P, Norris CM. Ca2+, astrocyte activation and calcineurin/NFAT signaling in age-related neurodegenerative diseases. Front Aging Neurosci 2018; 10: 199. DOI: 10.3389/fnagi.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss SW, Albers DS, Iadarola MJ, et al. NMDAR1 glutamate receptor subunit isoforms in neostriatal, neocortical, and hippocampal nitric oxide synthase neurons. J Neurosci 1998; 18: 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Koning TJ, Snell K, Duran M, et al. L-serine in disease and development. Biochem J 2003; 371: 653–661. DOI: 10.1042/BJ20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiya T, Kawamata T, Namiki A, et al. Role of satellite cell-derived L-serine in the dorsal root ganglion in paclitaxel-induced painful peripheral neuropathy. Neuroscience 2011; 174: 190–199. DOI: 10.1016/j.neuroscience.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 53.Komori N, Takemori N, Kim HK, et al. Proteomics study of neuropathic and nonneuropathic dorsal root ganglia: altered protein regulation following segmental spinal nerve ligation injury. Physiol Genomics 2007; 29: 215–230. DOI: 10.1152/physiolgenomics.00255.2006. [DOI] [PubMed] [Google Scholar]
- 54.Tabata-Imai A, Inoue R, Mori H. Increased sensitivity to inflammatory pain induced by subcutaneous formalin injection in serine racemase knock-out mice. PLoS One 2014; 9: e105282. DOI: 10.1371/journal.pone.0105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neame S, Safory H, Radzishevsky I, et al. The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc Natl Acad Sci 2019; 116: 20736–20742. DOI: 10.1073/pnas.1909458116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliet SHR, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience 2009; 158: 275–283. DOI: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 57.Vicario N, Turnaturi R, Spitale FM, et al. Intercellular communication and ion channels in neuroptahic pain chronicization. Inflamm Res 2020; 69: 841–850. [DOI] [PubMed] [Google Scholar]
- 58.Ji RR, Nackley A, Huh Y, et al. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. DOI: 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorge RE, Mapplebeck JCS, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neuroscience 2015; 18: 1081–1083. DOI: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelderd JB, Chopin SF. The vertebral level of origin of spinal nerves in the rat. Anat Rec 1977; 188: 45–47. DOI: 10.1002/ar.1091880106. [DOI] [PubMed] [Google Scholar]