Abstract
Foveal hypoplasia is the absence of a foveal depression and the presence of the ganglion cell layer in the foveola. A spectrum of clinical characteristics, including normal or variably decreased visual acuity, has been described in patients with blunted foveal contours. Multiple systemic and ophthalmologic conditions including albinism, aniridia, nanophthalmos, prematurity, and fovea plana have been associated with this anomaly. This article illustrates select clinical conditions characterized by a blunted foveal contour. Given the heterogeneity of findings, a thorough medical history and detailed physical and ocular examinations are usually sufficient for the clinician to make the correct diagnosis.
Keywords: blunted foveal contour, fovea, retina
Introduction
Foveal hypoplasia (FH) is the absence of a foveal depression and the presence of the ganglion cell layer (GCL) in the foveola.1,2 In 1913, blunted foveal contour was first reported in patients with albinism, who typically had poor visual acuity (VA) and nystagmus. However, spectral domain optical coherence tomography (SD-OCT) has expanded the definition of FH and has revealed more patients with blunted foveal contour than were previously recognized clinically.3,4
Currently, there is a spectrum of clinical characteristics, including normal or variably decreased VA, described among patients with a blunted foveal contour.5–9 Multiple systemic and ophthalmologic conditions including albinism, aniridia, nanophthalmos, prematurity, and fovea plana have also been associated with this anomaly.1–3,5,7,10–12
This article illustrates various clinical findings associated with a blunted foveal contour in the adult and pediatric population.
The development of the fovea
Foveal development starts between weeks 14 and 22 of gestation and may extend until 13 years of age.4,13–15 Foveal maturation is characterized by three chronological processes: (1) the inward displacement of inner retinal layers, (2) cone photoreceptor specialization, and (3) outward migration of cone photoreceptors.4,16
By weeks 20 to 22 of gestation, the fovea is identified by the presence of a single layer of cones in the outer nuclear layer (ONL). 4 Starting at week 25 of gestation, the outward displacement of the ganglion cell, inner plexiform, and inner nuclear layers (INLs) begin to form the foveal pit. 4 By weeks 28 and 29 of gestation, the foveal pit is more prominent, marked by a growth in length of the cone inner segments and the fibers of Henle, as well as a thinning of the INL. 14 At 5 to 8 days postpartum, the foveal pit has increased in depth due to the continued lateral displacement of the GCL and INL. 14 After birth, cone inward displacement occurs causing the pit to become wider and shallower and the foveal ONL to become thicker. 4 The pit appears complete by 13 to 15 months of age with a single layer of neurons in the center. 4 Cone density continues to increase between 15 months and 13 years of age. 4
Disruption in any of the stages of foveal development may result in FH. 16 However, the mechanisms leading to foveal differentiation are not completely understood. 17 Although subjects born prematurely may have an arrest in the centrifugal displacement of the inner retinal layers, subjects born at term may also display a blunted foveal contour.9,18 These findings are typically bilateral and symmetric, but unilateral cases have been recently reported with OCT.7,11,19–21 SD-OCT has been an important tool to improve understanding of retinal development.4,16
Clinical characteristics of patients with a blunted foveal contour
Recent findings
Grading of FH and SD-OCT findings
The current structural grading system for FH is based on foveal development and SD-OCT findings. 3 The characteristic feature of FH on SD-OCT is the persistence of the inner retinal layers at the foveal center. 3 FH can be divided into typical FH and atypical FH. 15
Atypical FH is characterized by a disruption of the ellipsoid zone, a sign of photoreceptor degeneration.3,15 This is shown on SD-OCT as a shallow pit with decreased retinal and ONL thickness and deeper foveal depth. 3
Typical FH is divided in four grades. Grade 1 FH is characterized developmentally by having partial centrifugal displacement of the inner retinal layers, as well as the presence of cone photoreceptor specialization and centripetal migration of cone photoreceptors. 3 This is depicted on SD-OCT as a shallow pit with the presence of outer segment (OS) lengthening and ONL widening, respectfully.3,15 Grade 2 FH has all the features associated with Grade 1, except that centrifugal displacement of the inner retinal layers fails to occur.3,15 SD-OCT shows no pit formation for Grade 2 FH. 3 Grade 3 FH has all the characteristics of Grade 2, except that cone photoreceptor specialization does not occur.3,15 SD-OCT depicts absence of OS lengthening for Grade 3 FH.3,15 Grade 4 FH has all the features of Grade 3, but the absence of centripetal migration of cone photoreceptors.3,15 On SD-OCT, there is no ONL widening on Grade 4 FH.3,15 Grading of FH gives an indication of the stage at which foveal development was arrested and may be used to predict VA.3,15,16
Grading of FH and VA
The persistence of inner retinal layers at the foveal center in patients with a blunted foveal depression does not preclude excellent VA. Central VA is impacted mostly by foveal photoreceptor differentiation independent of the foveal depression.6–9,18 On SD-OCT, the presence of photoreceptor elongation and outer nuclear segment widening has been reported as the most predictive factor for preserved visual function.6–9 Grade 1 FH has been associated with better VA, whereas grades 2, 3, and 4 have been correlated with progressively poorer VA.3,16 Furthermore, patients with atypical FH usually have worse visual prognosis as compared with those with typical FH due to photoreceptor degeneration. 3
FH and other imaging studies
Several other tomographic, angiographic, and autofluorescence patterns have been used to further describe patients with FH.22,23 FH is characterized on fundus autofluorescence (FAF) by variable degrees of reduced foveal attenuation of autofluorescence. 23 Fundus fluorescein angiography (FFA) may show absence of foveal avascular zone in patients with FH. 23 However, FAF and FFA patterns seem less sensitive to make the diagnosis. 23 OCT angiography (OCT-A) has been used to further characterize the organization of the retinal capillary plexuses in patients with FH. 22 FH is characterized on OCT-A by the absence of a foveal avascular zone, but preserved fusion of both the superficial and deep capillary plexuses around the foveal center. 22 Furthermore, Ramtohul and Freund 24 recently described a novel concentric macular ring (CMR) sign on ultra-widefield (UWF) retinal images to detect FH. They concluded that this CMR sign, present in all their patients with FH, may support the diagnosis of FH, especially in patients in whom OCT cannot be performed. 24
Disorders associated with a blunted foveal contour
Atypical FH has been associated with photoreceptor dysfunction syndromes such as achromatopsia and other cone–rod dystrophies.3,15 Albinism, PAX6 mutations, SLC38A8 mutations, retinopathy of prematurity (ROP), optic nerve hypoplasia, and isolated cases have been more commonly associated to typical FH.3,15 Furthermore, Ramtohul and Freund 24 recently discovered previously unreported diagnoses associated with FH including neurofibromatosis type 1 and alternating hemiplegia of childhood with ATP1A3 mutation. Ramtohul and Freund 24 also recently found a novel paracentral acute middle maculopathy lesion associated with Grade 3 FH. They hypothesized that the underdeveloped vascular anatomy at the fovea may be related to the atypical occurrence of ellipsoid zone/interdigitation zone disruption in this eye with central acute middle maculopathy. 24
All these new findings highlight the fact that FH is still a topic of ongoing research. This article describes select clinical entities that have been associated with a blunted foveal contour.
Albinism
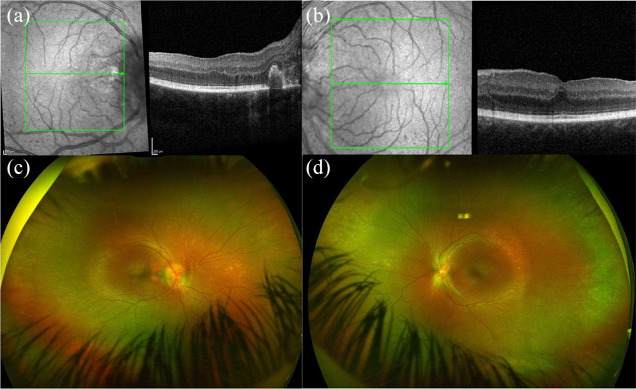
Albinism is a genetic disorder characterized by reduced or absent melanin synthesis that may affect the eyes, skin, and hair. 25 Patients could have fair skin and white-, yellow-, or red-colored hair. 26 Ocular albinism is usually caused by an X-linked mutation in G protein-coupled receptor 143 (GPR143), which affects the growth of melanosomes. 27 However, oculocutaneous albinism is typically caused by autosomal recessive mutations in tyrosinase (TYR), OCA2 melanosomal transmembrane protein (OCA2), tyrosinase related protein 1 (TYRP1), and solute carrier family 45 member 2 (SLC45A2), which affect the production of melanin. 26 Several visual pathway alterations, including an increased number of nerve fibers at the optic chiasm and changes in cortical structures, have been described among patients with albinism.28,29 Furthermore, ocular and oculocutaneous variants of albinism have been associated with decreased VA, usually worse than 20/200, iris transillumination, and nystagmus.8,25 Most patients with albinism have FH. 15 Most patients with albinism present with Grade 2 or worse of FH, and have poor BCVA.3,15 Fundoscopic findings include a variable degree of retinal pigment epithelial hypopigmentation, a blunted foveal contour, and prominent visualization of the choroidal vasculature.1,2 Clinical examples are shown in Figures 1 and 2.
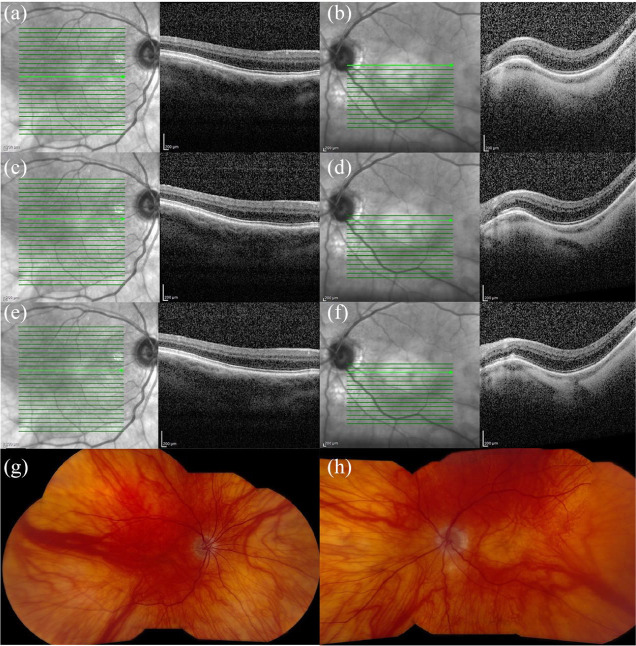
Figure 1.
Spectral-domain optical coherence tomography (SD-OCT) and fundus photography of a 75-year-old male with oculocutaneous albinism, nystagmus, bilateral pseudophakia, and a best corrected visual acuity of 20/100 in each eye. SD-OCT of the (a, c, and e) right and (b, d, and f) left eyes shows blunted foveal contours. (g) Fundus photography of the right eye shows a blunted foveal reflex and decreased pigment. (h) Fundus photography of the left eye shows similar findings plus a macular staphyloma.
Figure 2.
Spectral-domain optical coherence tomography (SD-OCT), fundus photography, and anterior segment photography of a 57-year-old female with ocular albinism, nystagmus, bilateral nuclear sclerosis, and a best corrected visual acuity of 20/40 in the right eye and 20/60 in the left eye. SD-OCT of the (a) right and (b) left eyes shows blunted foveal contours. Fundus photography of (c) right and (d) left eyes shows a blunted foveal reflex and decreased foveal pigment. Anterior segment photography of (e) right and (f) left eyes shows hypopigmentation of the iris.
Aniridia
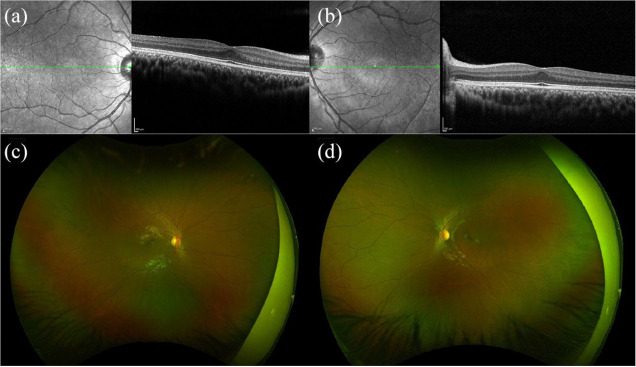
Aniridia is caused by an autosomal dominant mutation in paired box 6 (PAX6), which affects the embryonic development of many organs, including the iris. 30 Various phenotypes are reported, ranging from hypoplasia to complete aplasia of the iris.31,32 Aniridia has been associated with decreased VA, strabismus, nystagmus, and limbal stem cell deficiency.20,31 Most patients with aniridia have FH. 33 The foveal structure can be variable in patients with PAX6 mutations, with FH ranging from grades 1 to 4.3,15,33 Clinical examples are shown in Figures 3 and 4.
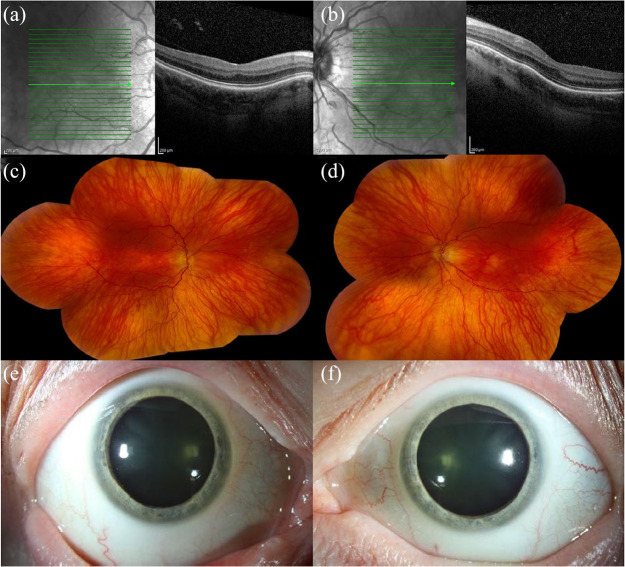
Figure 3.
Spectral-domain optical coherence tomography (SD-OCT) and anterior segment photography of a 29-year-old male with aniridia, nystagmus, bilateral limbal stem cell deficiency, and pseudophakia, and a best corrected visual acuity of 20/400 in the right eye and 20/200 in the left eye. SD-OCT of (a) right and (b) left eyes is technically suboptimal due to nystagmus and inability to fixate, but shows blunted foveal contours. Anterior segment photography of the (c) right and (d) left eyes shows aniridia, corneal neovascularization, and decentered in-the-bag posterior chamber intraocular lenses.
Figure 4.
Spectral-domain optical coherence tomography (SD-OCT) of a 69-year-old male with mild aniridia, and a best corrected visual acuity of 20/30 in the right eye and 20/40 in the left eye. SD-OCT of the (a) right and (b) left eyes shows blunted foveal contours.
Nanophthalmos
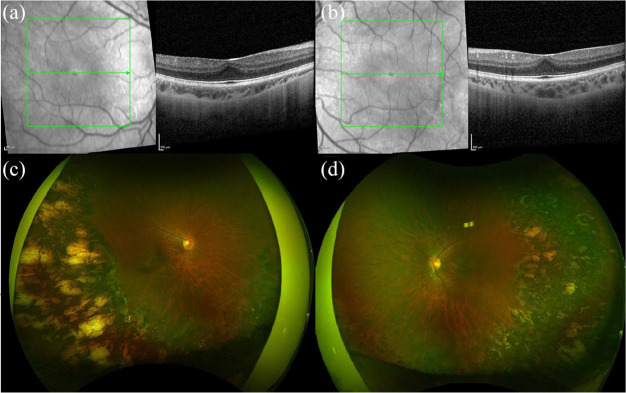
Nanophthalmos is a condition in which a halt in the development of the eye causes an anatomically small eye with preserved structural organization.34,35 Nanophthalmos is usually inherited in an autosomal dominant or recessive pattern, but sporadic cases have been described. 35 Nanophthalmos has been associated with mutations in transmembrane protein 98 (TMEM98), protease serine 56 (PRSS56), Crumbs homologue 1 (CRB1), membrane-type frizzled-related protein (MFRP), and bestrophin 1 (BEST1), which affect the growth of the eye and several ocular structures, including the retina and sclera. 35 Nanophthalmos is characterized by decreased axial length, hyperopia, increased lens/eye volume ratio, angle-closure glaucoma, and diminished VA, usually worse than 20/40.10,34,35 Some of the fundoscopic findings described include retinal cysts, absent or primitive foveal avascular zone, retinal detachment, macular hypoplasia, retinoschisis, optic disk drusen, increased choroidal thickness, uveal effusion, and pigmentary retinal dystrophy.10,34,35 A clinical example is shown in Figure 5.
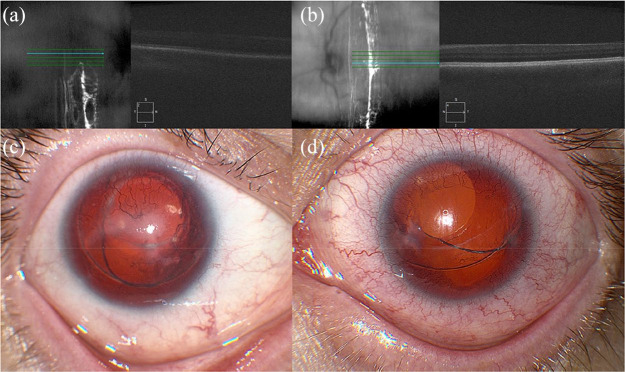
Figure 5.
Spectral-domain optical coherence tomography (SD-OCT) and fundus photography of an 8-year-old female with nanophthlamos, bilateral bullous retinoschisis, and a best corrected visual acuity of 20/50 in the right eye and 20/40 in the left eye. SD-OCT of the (a) right and (b) left eyes shows blunted foveal contours. (c) Fundus photography of the right eye shows a blunted foveal reflex, a subretinal neovascular membrane, and optic disc drusen. (d) Fundus photography of the left eye shows a blunted foveal reflex and optic disc drusen.
History of prematurity
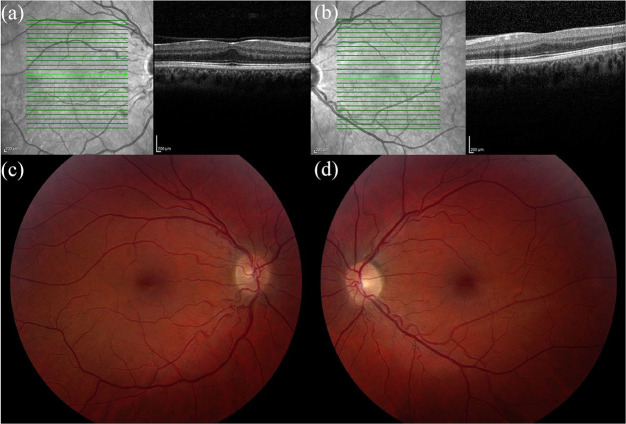
ROP is a disorder that affects the maturation of the retinal vasculature causing delayed and anomalous vascularization in premature infants.36–38 Higher incidences of strabismus, cataracts, glaucoma, retinal detachment, macular ectopia, and decreased VA have been described.9,36–38 Patients with a history of prematurity, with or without a diagnosis of ROP, and with or without macular ectopia, may show a characteristic blunting of the foveal contour. Patients born prematurely may have retention of the inner retinal layers at the foveal center with ONL widening and photoreceptor elongation, which is typically asymptomatic with normal or near-normal VA.9,18,19,39 Clinical examples are shown in Figures 6 and 7.
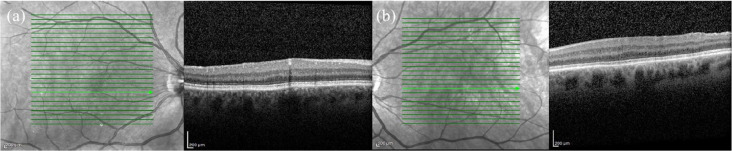
Figure 6.
Spectral-domain optical coherence tomography (SD-OCT) and fundus photography of a 34-year-old female with a history of prematurity and a best corrected visual acuity of 20/20 bilaterally. SD-OCT of the (a) right and (b) left eyes shows a blunted foveal contour. (c) Fundus photography of the right eye shows a blunted foveal reflex and macular ectopia. (d) Fundus photography of left eye shows similar findings plus temporal vessel straightening.
Figure 7.
Spectral-domain optical coherence tomography (SD-OCT) and fundus photography of a 14-year-old female with history of bilateral aggressive posterior retinopathy of prematurity treated with laser, cerebral infarction, strabismus, and a best corrected visual acuity of 20/30 in the right eye and 20/50 in the left eye. SD-OCT of (a) right and (b) left eyes shows blunted foveal contours. Fundus photography of (c) right and (d) left eyes shows blunted foveal reflex, temporal vessel straightening, and retinal laser ablation.
Fovea plana
The term ‘isolated foveal hypoplasia’ has been used to describe patients with the absence of other ocular diseases, low VA, nystagmus, and typical ophthalmic and angiographic findings such as abnormal maculofoveal reflexes, unclear definition of the maculofoveal area, and capillaries running abnormally close to the presumed macular area. 19 However, a blunted foveal contour has also been described among patients with excellent VA and otherwise normal ophthalmic and systemic examinations.7,19,20 In 2008, Marmor et al. 8 introduced the term ‘fovea plana’ to describe eyes showing a blunted foveal contour with normal visual function, and used the term ‘foveal hypoplasia’ for those patients with visual impairment. 22
SD-OCT of patients with fovea plana may also show retention of the inner retinal layers at the foveal center with ONL widening and photoreceptor elongation. Fovea plana remains a diagnosis of exclusion. A clinical example is shown in Figure 8.
Figure 8.
Spectral-domain optical coherence tomography (SD-OCT) and fundus photography of a 16-year-old male with autism and a best corrected visual acuity of 20/20 bilaterally. SD-OCT of (a) right and (b) left eyes shows blunted foveal countours. Fundus photography of (c) right and (d) left eyes shows blunted foveal reflexes.
Conclusion
A blunted foveal contour is associated with multiple etiologies including albinism, aniridia, nanophthalmos, prematurity, and fovea plana among others.6–8 VA may vary depending on the development of the foveal photoreceptors and structural grading of FH. Nystagmus may also be present but may not preclude excellent VA. Given the heterogeneity of findings in patients with a blunted foveal contour, a thorough medical history and detailed physical and ocular examinations are typically sufficient to establish the correct diagnosis.
Footnotes
Author contributions: Sofía M. Muns: Formal analysis; Writing – original draft.
Victor M. Villegas: Data curation; Formal anlysis; Writing - review and editing.
Stephen G. Schwartz: Conceptualization; Data curation; Formal analysis; Writing – review and editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH Center Core grant P30-EY014801 and an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York, NY, USA.
ORCID iDs: Sofía M. Muns  https://orcid.org/0000-0002-1713-8474
https://orcid.org/0000-0002-1713-8474
Stephen G. Schwartz  https://orcid.org/0000-0002-1441-9473
https://orcid.org/0000-0002-1441-9473
Contributor Information
Sofía M. Muns, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico
Victor M. Villegas, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico Department of Ophthalmology, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico; Department of Ophthalmology, Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami, Miami, FL, USA; Department of Surgery, Ponce Health Sciences University, Ponce, Puerto Rico.
Stephen G. Schwartz, Department of Ophthalmology, Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami, 3880 Tamiami Trail North, Naples, FL 34103, USA.
References
- 1. Naumann GO, Lerche W, Schroeder W. Foveolar aplasia in tyrosinase-positive oculocutaneous albinism. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1976; 200: 39–50. [DOI] [PubMed] [Google Scholar]
- 2. Elschnig A. Zur Anatomie des menschlichen Albinoauges. Graefes Archiv für Ophthalmologie 1913; 84: 401–419. [Google Scholar]
- 3. Thomas MG, Kumar A, Mohammad S, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity. Ophthalmology 2011; 118: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hendrickson A, Possin D, Vajzovic L, et al. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol 2012; 154: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg MF, Custis PH. Retinal and other manifestations of incontinentia pigmenti (Bloch-Sulzberger syndrome). Ophthalmology 1993; 100: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 6. Villegas VM, Schwartz SG, Berrocal AM, et al. Widefield optical coherence tomography of foveal dragging in retinopathy of prematurity. Int J Ophthalmol 2019; 12: 1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villegas VM, Schwartz SG, Hamet TD, et al. Variable clinical profile of fovea plana in normal children. Ophthalmic Surg Lasers Imaging Retina 2018; 49: 251–257. [DOI] [PubMed] [Google Scholar]
- 8. Marmor MF, Choi SS, Zawadzki RJ, et al. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol 2008; 126: 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villegas VM, Capó H, Cavuoto K, et al. Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol 2014; 158: 508–512. [DOI] [PubMed] [Google Scholar]
- 10. Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol 2007; 143: 1067–1068. [DOI] [PubMed] [Google Scholar]
- 11. Shields RA, Cavuoto KM, McKeown CA, et al. Unilateral foveal hypoplasia in a child with bilateral anterior segment dysgenesis. Clin Case Rep 2015; 3: 676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol 1997; 124: 587–626. [DOI] [PubMed] [Google Scholar]
- 13. Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol 2008; 126: 507–511. [DOI] [PubMed] [Google Scholar]
- 14. Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology 1984; 91: 603–612. [DOI] [PubMed] [Google Scholar]
- 15. Thomas MG, Papageorgiou E, Kuht HJ, et al. Normal and abnormal foveal development. Br J Ophthalmol. Epub ahead of print 4 November 2020. DOI: 10.1136/bjophthalmol-2020-316348. [DOI] [PubMed] [Google Scholar]
- 16. Ramtohul P, Comet A, Denis D. Multimodal imaging correlation of the concentric macular rings sign in foveal hypoplasia: a distinctive Henle fiber layer geometry. Ophthalmol Retina 2020; 4: 946–953. [DOI] [PubMed] [Google Scholar]
- 17. Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology 2011; 118: 2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu WC, Lin RI, Shih CP, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 2012; 119: 1907–1916. [DOI] [PubMed] [Google Scholar]
- 19. Oliver MD, Dotan SA, Chemke J, et al. Isolated foveal hypoplasia. Br J Ophthalmol 1987; 71: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curran RE, Robb RM. Isolated foveal hypoplasia. Arch Ophthalmol 1976; 94: 48–50. [DOI] [PubMed] [Google Scholar]
- 21. Bazvand F, Karkhaneh R, Roohipoor R, et al. Optical coherence tomography angiography in foveal hypoplasia. Ophthalmic Surg Lasers Imaging Retina 2016; 47: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 22. Dolz-Marco R, Phasukkijwatana N, Sarraf D, et al. Optical coherence tomography angiography in fovea plana. Ophthalmic Surg Lasers Imaging Retina 2016; 47: 670–673. [DOI] [PubMed] [Google Scholar]
- 23. Mota A, Fonseca S, Carneiro A, et al. Isolated foveal hypoplasia: tomographic, angiographic and autofluorescence patterns. Case Rep Ophthalmol Med 2012; 2012: 864958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramtohul P, Freund KB. Central acute middle maculopathy: a novel variant of paracentral acute middle maculopathy in foveal hypoplasia. Ophthalmol Retina 2020; 4: 344–347. [DOI] [PubMed] [Google Scholar]
- 25. Brücher VC, Heiduschka P, Grenzebach U, et al. Distribution of macular ganglion cell layer thickness in foveal hypoplasia: a new diagnostic criterion for ocular albinism. PLoS ONE 2019; 14: e0224410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institutes of Health. Oculocutaneous albinism, https://ghr.nlm.nih.gov/condition/oculocutaneous-albinism#definition (2015, accessed 26 May 2020).
- 27. National Institutes of Health. Ocular albinism, https://ghr.nlm.nih.gov/gene/GPR143 (2007, accessed 28 May 2020).
- 28. Welton T, Ather S, Proudlock FA, et al. Altered whole-brain connectivity in albinism. Hum Brain Mapp 2017; 38: 740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bridge H, von dem Hagen EA, Davies G, et al. Changes in brain morphology in albinism reflect reduced visual acuity. Cortex 2014; 56: 64–72. [DOI] [PubMed] [Google Scholar]
- 30. National Institutes of Health. Aniridia, https://ghr.nlm.nih.gov/condition/aniridia#inheritance (2009, accessed 28 May 2020).
- 31. Calvão-Pires P, Santos-Silva R, Falcão-Reis F, et al. Congenital aniridia: clinic, genetics, therapeutics, and prognosis. Int Sch Res Notices 2014; 2014: 305350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yokoi T, Nishina S, Fukami M, et al. Genotype-phenotype correlation of PAX6 gene mutations in aniridia. Hum Genome Var 2016; 3: 15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lima Cunha D, Arno G, Corton M, et al. The spectrum of PAX6 mutations and genotype-phenotype correlations in the eye. Genes (Basel) 2019; 10: 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Serrano JC1, Hodgkins PR, Taylor DS, et al. The nanophthalmic macula. Br J Ophthalmol 1998; 82: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carricondo PC, Andrade T, Prasov L, et al. Nanophthalmos: a review of the clinical spectrum and genetics. J Ophthalmol 2018; 2018: 2735465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carroll L, Owen LA. Current evidence and outcomes for retinopathy of prematurity prevention: insight into novel maternal and placental contributions. Explor Med 2020; 1: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azami M, Jaafari Z, Rahmati S, et al. Prevalence and risk factors of retinopathy of prematurity in Iran: a systematic review and meta-analysis. BMC Ophthalmol 2018; 18: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pétursdóttir D, Holmström G, Larsson E. Visual function is reduced in young adults formerly born prematurely: a population-based study. Br J Ophthalmol 2020; 104: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol 2012; 130: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]