Abstract
Pancreatic islet cells develop mature physiological responses to glucose and other fuels postnatally. In this study, we used fluorescence imaging techniques to measure changes in intracellular calcium ([Ca2+]i) to compare islets isolated from mice on postnatal days 0, 4, and 12 with islets from adult CD-1 mice. In addition, we used publicly available RNA-sequencing data to compare expression levels of key genes in β-cell physiology with [Ca2+]i data across these ages. We show that islets isolated from mice on postnatal day 0 displayed elevated [Ca2+]i in basal glucose (≤4mM) but lower [Ca2+]i responses to stimulation by 12-20mM glucose compared to adult. Neonatal islets displayed more adult-like [Ca2+]i in basal glucose by day 4 but continued to show lower [Ca2+]i responses to 16 and 20mM glucose stimulation up to at least day 12. A right shift in glucose sensing (EC50) correlated with lower fragment-per-kilobase-of-transcript-per-million-reads-mapped (FPKM) of Slc2a2 (glut2) and Actn3 and increased FPKM for Galk1 and Nupr1. Differences in [Ca2+]i responses to additional stimuli were also observed. Calcium levels in the endoplasmic reticulum were elevated on day 0 but became adult-like by day 4, which corresponded with reduced expression in Atp2a2 (SERCA2) and novel K+-channel Ktd17, increased expression of Pml, Wfs1, Thada, and Herpud1, and basal [Ca2+]i maturing to adult levels. Ion-channel activity also matured rapidly, but RNA sequencing data mining did not yield strong leads. In conclusion, the maturation of islet [Ca2+]i signaling is complex and multifaceted; several possible gene targets were identified that may participate in this process.
Keywords: neonatal, calcium, postnatal, development, calcium signaling, β cell, maturation, islet, RNA sequencing, FPKM, endoplasmic reticulum, ion channels, glucose, diabetes
Graphical Abstract:
1. Introduction
Pancreatic β-cells are contained within micro-organs of the pancreas called islets of Langerhans [1]. The main function of the β-cells is to synthesize and secrete insulin in order to regulate blood glucose and help to maintain energy balance. There are many steps in the mechanistic process of insulin secretion [2]. The first step is the uptake of glucose by the β-cell followed by glycolysis driven by glucokinase phosphorylation. This leads to the production of two ATP plus pyruvate as end products for every glucose molecule to fuel additional ATP production by the mitochondria in the cell. This increase in the ATP:ADP ratio causes ATP-gated potassium (KATP) channels in the plasma membrane to close, causing the plasma membrane to depolarize. Subsequently, voltage-gated L-type calcium channels in the plasma membrane open to allow calcium to flow into the β-cell. This influx of calcium then causes insulin release from the β-cell. The amount of calcium taken up is associated with the amount of insulin secreted by the islet [3,4]. Note that additional insulin secretion through metabolic amplifying pathways can also occur independently of changes in intracellular calcium ([Ca2+]i) [5,6]. On balance, [Ca2+]i can be considered an indirect indicator of insulin secretion and can provide a reasonable characterization of the physiology of a β-cell [7].
When measuring [Ca2+]i in a β-cell, several aspects of the stimulus-secretion coupling pathway can be observed by exposing islets to varying glucose concentrations and other stimuli. At glucose concentrations under ~5mM in normally functioning adult mouse islets, low [Ca2+]i levels are observed with no obvious stimulatory activity. Increasing glucose to a higher concentration results in a sharp peak in calcium influx, which is also known as the 1st phase response. This sharp peak eventually plateaus, and steady oscillations are observed in what is known as 2nd phase response. This biphasic response to glucose is observed In vitro among individual dispersed β-cells [8,9], intact islets [10,11] and perifused pancreas [12]. Biphasic release of insulin can even be measured in the blood following an intravenous glucose tolerance test [13], suggesting the importance of this feature to insulin secretion. Finally, exposure to still higher glucose concentrations results in a hyper-stimulatory environment that saturates the response to a point of maximal continuous calcium influx.
Involvement of metabolic and transcriptional remodeling contributes to the complexity of β-cell maturation [14,15]. Several metabolic elements have been found to be necessary for proper functional development of β-cells. These include the presence of estrogen related receptor γ (ERRγ) [15], induction of the insulin transactivator NeuroD [16], and expression of urocortin 3 (UCN3), which is a key marker in mature β-cells [17]. The overexpression of MAFA in neonatal rat β-cells also has shown to result in increased glucose responsiveness indicating that this increase in MAFA expression plays a substantial role in the β-cell maturation process [18]. Furthermore, the process of weaning is associated with improved glucose-stimulated oxidative phosphorylation and insulin secretion [19]. Lastly, it has been reported that β-cell signaling switches from the nutrient sensor target of rapamycin (mTOR1) in neonatal mice to the cellular energy sensor 5′-adenosine monophosphate–activated protein kinase (AMPK) [20]. This change is critical for the appropriate maturation of β-cells.
Although these developmental regulators are important in β-cell maturation, it is less clear how these developmental changes lead to the islet’s physiological response to nutrient stimulation, especially in terms of [Ca2+]i. The present study is focused on [Ca2+]i and genes involved in [Ca2+]i signaling. A great deal is known about the physiological aspects of calcium handling in adult islets as reviewed in [7]. However, the early stages of postnatal islet maturation have not been extensively studied, especially in terms of the calcium signaling that is critical to stimulus-secretion coupling in the β-cell. Prior work suggests that ion channels involved with [Ca2+]i mature quickly [21], however, the coupling of [Ca2+]i to glucose-stimulated insulin secretion matures more gradually [22]. Studying the effects on [Ca2+]i when islets are treated with compounds that modify the steps in the insulin secretion process can provide data on when specific aspects of this process mature. Elucidating the functional maturation of beta cells is important to the improvement of stem-cell-derived beta-cell therapies for type 1 diabetes [23] and to better understanding the beta-cell de-differentiation process in type 2 diabetes [24].
In this study, we made a systematic comparison of islet function at postnatal (PN) days 0, 4, and 12 of the maturation process in direct comparison with adult controls. Specifically, we examined changes in [Ca2+]i in response to glucose stimulation, depolarization by potassium chloride, and cyclopiazonic-acid-induced calcium release from the endoplasmic reticulum (ER). These different experiments were utilized to determine at what age these postnatal responses become adult-like. We also include targeted analysis of a previously deposited RNA-sequencing study to provide a list of genes related to these key functions in the β-cell as a potential starting point to explore novel signaling pathways related to early islet maturation.
2. Methods
2.1. Mice
CD-1 mice (Envigo, Indianapolis, IN) were used throughout and were also utilized in the study from which RNA sequencing data were obtained [22]. All procedures involving mice were approved by the Ohio University Animal Care and Use Committee. Breeder pairs consisting of one female and one male mouse were housed together and used solely for breeding purposes. The adult islets were taken from adult CD-1 male mice ranging from 12-20 (mean ~15) weeks of age. Each trial utilized mice of a single age. Trial-to-trial differences in [Ca2+]i values were observed by one-way ANOVA due to inherent biological variability from mouse to mouse [25]. However, no correlation was observed between age and [Ca2+]i response to glucose stimulation among adult islets (first phase EC50, R2=.28, P=.15; second phase EC50, R2=.12, P=.35). Adult mice were housed in a separate room with 1-2 mice per cage. The neonates were kept with the parents until needed for experimentation. They were used at ages varying from day of birth (PN day 0) to PN day 12. Male adult mice were used in these studies to avoid variations in endocrine function from estrous cycle changes. Since neonates are prepubertal, this was not a concern. Thus, both female and male neonatal mice were used for these experiments. The adults were euthanized using a carbon dioxide chamber. The neonates, due to underdevelopment of lungs, were euthanized by cervical dislocation instead of exposure to carbon dioxide.
2.2. Islet isolation
Pancreatic islets from adult mice were isolated by collagenase-P digestion (Roche Diagnostics, Indianapolis, IN) followed by centrifugation with Histopaque 1100 (Sigma-Aldrich, St. Louis, MO) as previously described [26]. Islets were incubated overnight in RPMI 1640 medium containing 11 mM glucose (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin to allow recovery from collagenase digestion before further treatment. Media with 11mM glucose is standard for optimal viability of islets in culture [27]. This recovery time is necessary for islets to regain the ability to functionally respond to glucose stimulation. Neonatal islets were isolated using the protocol described by Huang and Gu [28] except that collagenase Type XI was used instead of Type IV to digest the connective tissue of the pancreas. Following isolation, neonatal islets were treated the same as adult islets.
2.3. Stimuli and other reagents
Stimuli used to test islet function in this study include potassium chloride (KCl, Fisher, Hampton), nifedipine (MilliporeSigma, Burlington), and cyclopiazonic acid (MilliporeSigma, Burlington). KCl was made into a 1M stock in deionized water. The other stimuli were prepared in stock solutions in DMSO and diluted by at least 1:1000 for the final concentration used in this study.
2.4. [Ca2+]i Imaging
Fura-2 AM fluorescence imaging was utilized to measure [Ca2+]i levels as previously described [29,30]. Briefly, islets were imaged using a Hamamatsu ORCA-Flash4.0 digital camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan, Model C11440-22CU) mounted on a BX51WIF fluorescence microscope with a 10x objective (Olympus, Tokyo, Japan). Excitation light was provided by a xenon burner supplied to the image field through a light pipe and filter wheel (Sutter Instrument Co., Novato CA, Model LB-LS/30) with a Lambda 10-3 Optical Controller (Sutter Instrument Co., Novato, CA, Model LB10-3-1572). Images were taken sequentially from 340 nm and 380 nm excitation to produce each [Ca2+]i ratio from emitted light at 510 nm. Data were analyzed using CellSens Dimension 1.13 imaging software (Olympus, Tokyo, Japan). All islets were loaded in a modified KRB solution with 1 μM fura-2 AM and allowed to incubate in the dye for 30 minutes before experimentation. Note that the modified KRB solutions used for fura-2 loading always contained the glucose concentration used at the start of each [Ca2+]i experiment [9]. Cell Tracker Red at 200nM was also included during fura-2 loading for one of the two treatment groups in order to pair together and simultaneously image islets from postnatal vs. adult groups as published previously [31]. By recording control and test groups simultaneously, this approach controls for temperature, perifusion rate, and other variables, which allows us to identify subtle changes in islet function including signs of ER stress by examining the latency, amplitude, and slope of islet [Ca2+]i responses to glucose stimulation [31–33]. Each experiment was conducted on multiple islets for three independent trials performed on separate dates with islets isolated from different litters of mice.
2.5. Data mining of RNA sequencing data and analysis
RNA sequencing data for analysis originally came from MipeGFP mouse islets (controls) from P0 to P60 that were hand-picked and further dissociated with trypsin into single cells, followed by FACS to isolate eGFP+ β-cells using an Aria III Platform (BD) [22]. Total RNA was isolated with TRIzol reagent (Life Technologies) followed by a DNA free RNA™ kit (Zymo Research). RNA quality was quantified using Agilent Bioanalyzer 2100. RNAs with RINs above 8 were utilized for expression analysis. Library construction with 3 biological replicates for sequencing data was performed using standard Illumina protocols and HiSeq-2000. Raw reads (37-85 million per sample) were aligned (on average 81.3% of total reads) to the mouse genome (mm9) and transcriptome using RNA-Seq unified mapper (RUM, [34]). Expression levels were quantified as the number of mapped fragments overlapping the corresponding gene per million reads sequenced, per kb of gene length (FPKM).
We data mined this RNA sequencing data set utilizing a database of mouse genes from Jackson Laboratories (http://www.informatics.jax.org/). In order to determine which genes may be associated with changes in specific aspects of islet function, the database was searched using defined vocabulary terms related to metabolism: ‘glycolytic process’ (GO:0006096) resulting in 92 genes and 139 annotations, ‘tricarboxylic acid cycle’ (GO:0006099) resulting in 32 genes and 44 annotations, ‘mitochondrial DNA metabolic process’ (GO:0032042) resulting in 16 genes and 20 annotations, ‘electron transport chain’ (GO:0022900) resulting in 89 genes and 122 annotations, ‘oxidative phosphorylation’ (GO:0006119) resulting in 109 genes and 137 annotations. We also searched ‘endoplasmic reticulum calcium ion homeostasis’ (GO:0032469) resulting in 28 genes and 39 annotations and ‘ion channel’ (GO:0005216) resulting in 425 genes and 1761 annotations. These lists of genes based on vocabulary terms within the database were then exported to excel and cross-referenced with the RNA sequencing dataset to generate tables of genes that 1) were found at frequencies >1 FPKM for at least one neonatal age and 2) produced significant changes in FPKM between PN day 0 and day 12. For the “glycolytic process’ gene table, we also included glucose transporter genes from the Slc2a family as part of this process (Slc2a2, Slc2a6, and Slc2a13). Due to a large number of genes on the ‘ion channel’ list, an additional restriction was made to focus on genes displaying a two-fold increase or decrease in gene expression between PN days 0 and 12.
Data Availability:
The RNA-seq datasets (raw, processed and results files) together with the experiment annotation are available at the Array Express public repository (http://www.ebi.ac.uk/arrayexpress/) with accession number E-MTAB-2266. Note that postnatal days 1, 5, and 13 in that dataset correspond to days 0, 4, and 12, respectively, in the present study.
2.6. Statistical Analysis
One-way ANOVA with Tukey HSD post hoc test were used for all comparisons of islets from PN days 0, 4, 12, and adult mice, unless otherwise stated. The EC50s were calculated for each of three trials for each postnatal age and the total of nine corresponding adult controls using a 2-point moving average to account for possible differences between each set of glucose steps. Curve fitting analysis to calculate the half-maximal [Ca2+]i response to glucose stimulation was provided by “Quest Graph™ EC50 Calculator.” AAT Bioquest, Inc, https://www.aatbio.com/tools/ec50-calculator.
3. Results and Discussion
3.1. [Ca2+]i responses to glucose stimulation In neonatal Islets
As a starting point in systematically characterizing neonatal islet physiology, we examined [Ca2+]i responses to a range of glucose concentrations from 0mM (no stimulation) to 20mM, which produces a maximal [Ca2+]i response. As shown in Figure 1, islets from neonatal and adult islets were observed in 0 mM glucose and then stimulated to 8 and then 16 mM glucose. At PN day 0, no differences were found in [Ca2+]i in 0 mM glucose, but neonatal islets had significantly increased [Ca2+]i compared to adult islets when stimulated with 8mM glucose (Figure 1A). When exposed to higher glucose stimulation (16mM), PN day 0 islets did not show any additional increase in [Ca2+]i as seen in the adults, which indicates an overall reduced response to glucose. Neonatal islets at PN day 4 had a similar pattern to those at PN day 0 for this glucose dose response curve (Figure 1B). By PN day 12, the neonatal islets began to produce a more attenuated response to 8mM glucose similar to the adults, although the neonatal 2nd phase response was still elevated. Also, islets at PN day 12 displayed a much larger response to 16mM glucose (Figure 1C) compared to PN day 0 and PN day 4.
Figure 1. Neonatal [Ca2+]i response varies from adult response to stepwise glucose stimulation.
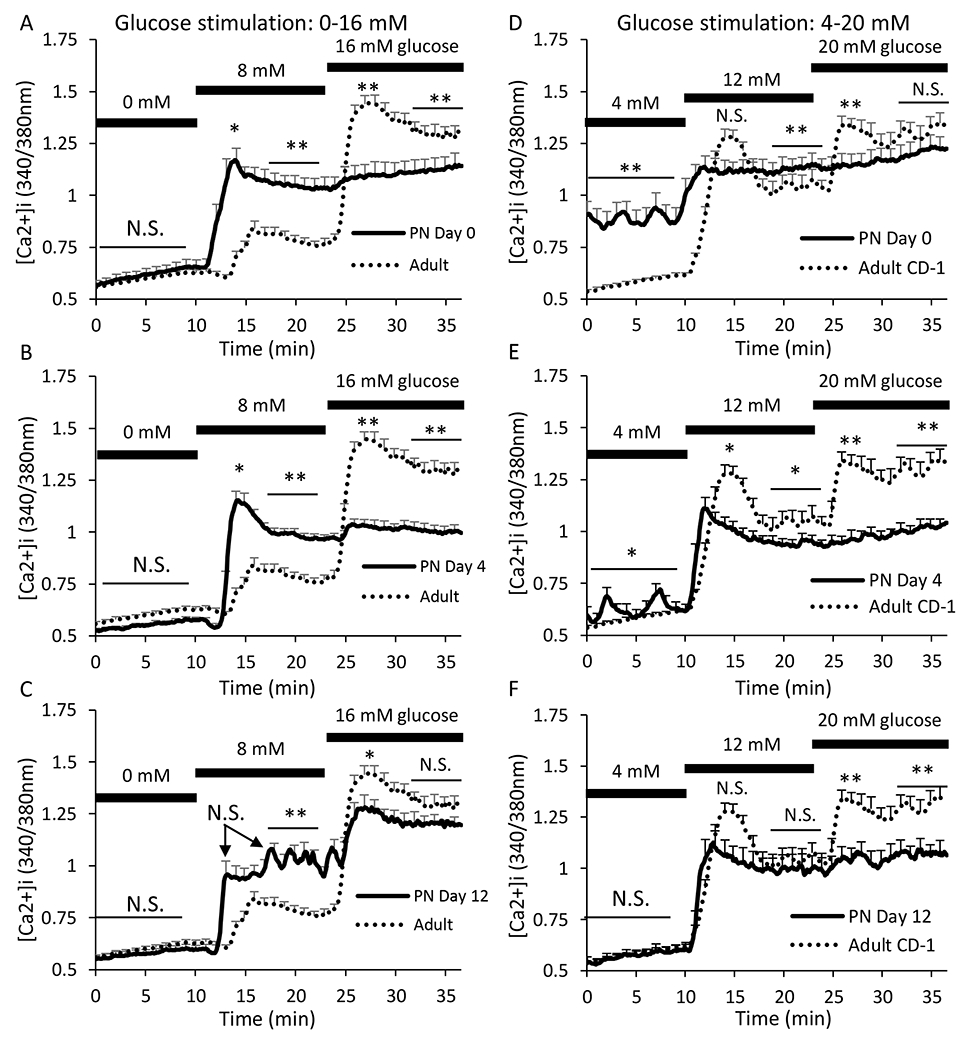
A-C. Traces showing the [Ca2+]i response to glucose stimulation from 0 to 8 to 16 mM for islets from neonatal mice at postnatal day 0 (A, N=22 islets), day 4 (B, N=26 islets), and day 12 (C, N=23 islets) based on N=3 trials for each neonatal age. Adult islets were paired with each neonatal recording for a total of N=80 adult islets derived from 9 different trials. D-F. Traces showing [Ca2+]i response to glucose stimulation from 4 to 12 to 20 mM for islets from neonatal mice at postnatal day 0 (A, N=29 islets), day 4 (B, N=24 islets), and day 12 (C, N=29 islets). Adult islets were paired with each neonatal recording for a total of N=85 adult islets derived from 9 different trials. Adult traces (dotted black lines) are presented in all panels for reference. Asterisks (*P<0.05, **P<0.01, N.S. not significant) indicate differences between adult and neonatal [Ca2+]i traces for each neonatal day shown left to right as basal in 0mM glucose, 1st phase peak and 2nd phase plateau in 8mM glucose, and 1st phase peak and 2nd phase plateau in 16mM glucose. First phase is calculated as the maximum [Ca2+]i level during stimulation and plateau is the mean [Ca2+]i level indicated during a 5-min span indicated by bar.
Neonatal responses to glucose stimulation were observed again, this time using glucose concentrations of 4mM, 12mM, and 20mM. The starting glucose concentration of 4mM is considered sub-stimulatory but close to the threshold of activation for adult mouse islets [35,36]. In 4mM glucose, the basal [Ca2+]i response was substantially higher for islets at PN day 0 compared to the adult islets (Figure 1D). Oscillations were visible at this age, which is typically not observed until exposure to a higher concentration of glucose in adult islets. At PN day 4, basal [Ca2+]i was no longer elevated, but oscillations persisted (Figure 1E). By PN day 12, this activity completely disappeared, and the basal response was the same as what is seen in adult islets (Figure 1F). Therefore, by PN day 12, the maturation process for the basal [Ca2+]i response in neonatal islets could be considered complete.
The 1st phase response, characterized by the initial peak when exposed to 12mM glucose, was almost indistinguishable from the 2nd phase at PN day 0 (Figure 1D). As the islets mature to PN day 4, the classic biphasic response became more recognizable, but the 1st phase response did not appear as robust as in adult islets (Figure 1E). By PN day 12, both 1st and 2nd phase are considered adult-like by statistical analysis, although the 1st phase still appeared slightly attenuated in Figure 1F. When exposed to 20mM glucose (Figure 1D–F), neonatal islets did not produce any response at any postnatal stage tested.
These results suggest that neonatal islets are overly active in low glucose concentrations but unable to respond to high levels of glucose, which is consistent with the view that glucose metabolism is a key mechanism in controlling β-cell maturation/function [37,38]. This gradually resolves into sharper glucose responses more closely resembling that of healthy adult islets. Of interest, the only known study of human neonatal islets found similar findings of high insulin secretion in low glucose but minimal response to glucose stimulation [39], which is consistent with our PN day 0 data.
3.2. Glucose sensing gradually right shifts with age in neonatal islets
To further quantify these [Ca2+]i responses to glucose stimulation, the maximum peak point (1st phase, Figure 2A) and average plateau value (2nd phase, Figure 2B) were plotted at each glucose concentration for each age. When comparing the 1st phase response of the neonatal islets to the adult islets, PN days 0 and 4 showed higher maximum peaks in lower glucose but lower maximum peaks in high glucose (relative to adults). At PN day 12, there was a right shift in the maximum peaks that brings the low glucose response closer to the adult response. In high glucose, islets at PN day 12 continued to have a lower maximum peak compared to adults.
Figure 2. Combined glucose curves show left-shifted pattern.
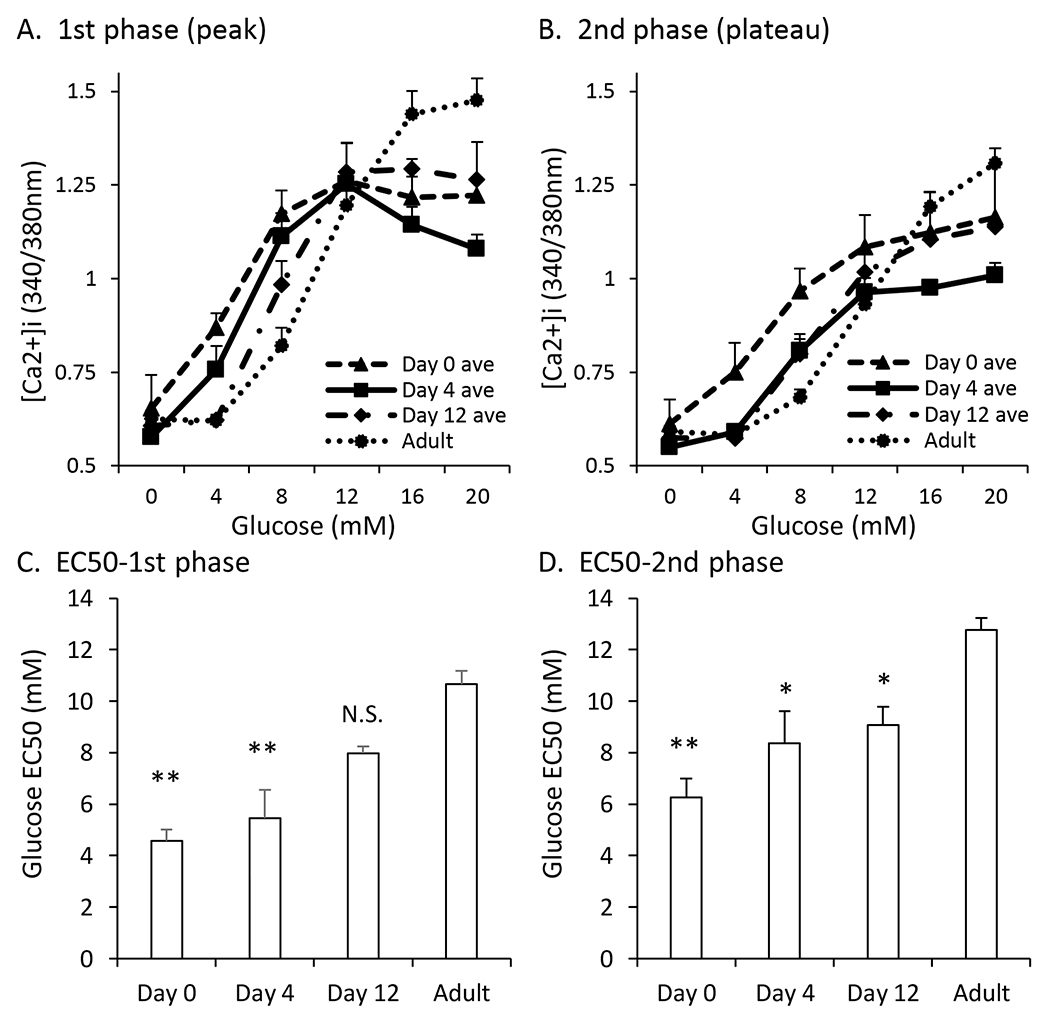
A-B. Traces showing 1st phase peak (A) and 2nd phase plateau (B) [Ca2+]i responses to glucose stimulation from 0 to 4 to 8 to 12 to 16 to 20mM for islets aged postnatal day 0 , day 4, day 12, and adult (dotted black). C-D. EC50 values of the maximum peak (C) and plateau (D) of islets at postnatal day 0, day 4, day 12, and adult. Asterisks (*) indicate differences between adult and neonatal [Ca2+]i traces based on N=3 sets of islets for each neonatal day and N=9 for control. *P<0.05, **P<0.01, N.S. Not significant.
When looking at the average plateau value (2nd phase response) in each glucose concentration, postnatal islets showed a similar pattern of higher [Ca2+]i in lower glucose and lower [Ca2+]i in higher glucose relative to adult islets (Figure 2B). One exception was that [Ca2+]i levels in 4mM glucose appeared adult-like as early as PN day 4. This results in a much sharper right shift in the sigmoid curve from PN day 0 to day 4. One other interesting observation is that the [Ca2+]i levels in high glucose (16 and 20mM) observed in the 2nd phase response approached adult levels as early as PN day 0, then much lower at PN day 4, and finally bounced back at PN day 12. This was a consistent observation across three trials.
We performed curve-fitting analysis to estimate the half-maximal [Ca2+]i response to glucose stimulation (EC50) during islet maturation. As shown in Figure 2C, the 1st phase response to glucose gradually shifted to higher glucose concentrations from PN day 0 to adulthood, indicating a right shift as islets mature. By PN day 12, the EC50 for glucose was not statistically different from the adult response. The EC50 for the 2nd phase response to glucose was significantly lower for PN days 0, 4, and 12 compared to adult (Figure 2D).
3.3. Gene expression related to glucose metabolism in maturing islets
We performed targeted analysis of a publicly available RNA sequencing database to identify possible genes of interest with regard to calcium signaling related to glucose stimulation [22]. Specifically, we used the keywords ‘glycolysis’, ‘TCA cycle’, ‘electron transport chain (ETC)’, and ‘oxidative phosphorylation’ in the Jackson Laboratories database to pull genes related to glycolysis in mice. As described in the Methods, we identified several genes with differential expression during early neonatal development as detected by fragment abundance in FPKM. A total of 46 genes were identified with significant expression differences between postnatal day 0 and 12. Genes with expression changes greater than ±25% are shown in Table 1.
Table 1. RNA sequencing expression of glycolytic genes.
Gene list for RNA transcript abundance in fragments per kilobase million (FPKM). From a list of 92 genes related to ‘glycolytic process’ (GO:0006096), the 29 genes in this table showed statistically significant differences between postnatal day 0 and day 12. P-values vs. day 0: *P<0.05, **P<0.01, ***P<0.001.
| Gene Symbol | PN Day 0 | PN Day 4 | PN Day 12 | P12/P0 |
|---|---|---|---|---|
|
| ||||
| Actn3 | 3.8 +/− 0.1 | ***13 +/− 0.1 | ***0.6 +/− 0.1 | 15.9 |
| Cox7a1 | 13.3 +/− 3.3 | 7.2 +/− 1.4 | *3.2 +/− 0.8 | 24.1 |
| Cox6a2 | 8.5 +/− 2.7 | *16.1 +/− 0.7 | 2.2 +/− 2.6 | 25.3 |
| Gpd1 | 21.3 +/− 1.8 | 17.6 +/− 0.9 | **13.0 +/− 1.1 | 61.2 |
| Slc2a2 | 133.1 +/− 6.5 | **99.9 +/− 5.6 | **82.9 +/− 5.2 | 62.3 |
| Mpi | 9.4 +/− 0.3 | 8.5 +/− 0.4 | **5.9 +/− 0.5 | 63.0 |
| Ogdhl | 55.8 +/− 1.4 | **37.5 +/− 2.8 | **37.1 +/− 2.7 | 66.5 |
| Entpd5 | 6.3 +/− 0.4 | 6.1 +/− 0.2 | **4.3 +/− 0.2 | 67.9 |
| Sesn2 | 16.2 +/− 0.3 | 15.0 +/− 0.7 | **11.2 +/− 0.8 | 69.3 |
| Pgam1 | 112.9 +/− 6.8 | *94.2 +/− 1.6 | **81.3 +/− 4.2 | 72.0 |
| Flcn | 18.8 +/− 0.5 | **14.7 +/− 0.9 | **13.6 +/− 1.0 | 72.5 |
| Pfkfb2 | 44.7 +/− 1.5 | 43.6 +/− 3.7 | **32.6 +/− 2.5 | 72.9 |
| Prkag2 | 15.6 +/− 0.5 | 14.6 +/− 0.9 | *11.4 +/− 1.1 | 73.3 |
| Chchd4 | 14.1 +/− 0.1 | 13.7 +/− 0.5 | **10.4 +/− 0.7 | 73.8 |
| Stox1 | 3.4 +/− 0.2 | 3.7 +/− 0.1 | *2.5 +/− 0.2 | 74.4 |
|
| ||||
| Gale | 12.5 +/− 0.7 | **18.6 +/− 1.2 | *16.1 +/− 0.8 | 128.5 |
| Eno2 | 2.8 +/− 0.1 | **4.1 +/− 0.1 | *3.9 +/− 0.4 | 137.2 |
| Pfkp | 1.9 +/− 0.1 | 2.2 +/− 0.0 | *3.2 +/− 0.3 | 165.8 |
| Myc | 9.0 +/− 0.2 | *6.3 +/− 0.8 | ***17.0 +/− 1.1 | 189.4 |
| Slc2a13 | 1.3 +/− 0.3 | 1.4 +/− 0.1 | **3.2 +/− 0.3 | 246.2 |
| Pde2a | 0.4 +/− 0.5 | 0.6 +/− 0.8 | *1.8 +/− 0.1 | 474.9 |
| Galk1 | 0.5 +/− 0.1 | ***27 +/− 0.3 | *4.4 +/− 1.0 | 947.3 |
| Nupr1 | 0.9 +/− 0.5 | ***19.9 +/− 2.1 | ***30.0 +/− 3.8 | 3220.1 |
Genetic expression driving neonatal islet maturation may reside in well-known glycolytic modulators such as Slc2a2 and glucokinase (Gck). Slc2a2 is the gene responsible for coding the glucose transporter glut2, which facilitates glucose uptake by the β-cell [40]. Expression of this gene showed a significant decline in fragments per kilobase million, which was 37.7% from day 0 to day 12 (see Table 1). Gck, the rate-limiting step in glycolysis [41,42], also declined mildly with age but the reduction in expression was not quite significant (P<0.10). Higher expression of these genes at PN day 0 suggests that islets are taking up more glucose and may have an increased rate of glycolysis at birth. This may explain, in part, the hyperactivity observed in basal glucose in neonatal islets.
Several novel metabolic genes were also identified by RNA-sequencing analysis that could play a role in the maturation of glucose-stimulated insulin secretion (Table 1). Alpha-actinin-3 (Actn3) codes for a microfilament protein that is associated with performance advantages among elite athletes [43]. RNA sequencing data suggest that Actn3 is rapidly downregulated by 64% by day 4 and by 84% by day 12 compared to FPKM on day 0. Actin remodeling is a well-established mechanism of modulating insulin exocytosis [44], however, nothing is known about Actn3 in the pancreas. Cytochrome C oxidase genes Cox7a1 and Cox6a2 were also substantially downregulated. Reduced cytochrome C is an essential regulator of sustained insulin secretion, however, this appears to be independent of [Ca2+]i changes [45,46]. Nuclear protein transcriptional regulator 1 (Nupr1), a substantially upregulated gene during maturation, has been shown to improve insulin secretion in the face of inflammatory stress [47], but deletion of Nupr1 has also been shown to improve insulin sensitivity and increase β-cell mass [48,49]. Overall, none of these genes are clearly linked with the observed [Ca2+]i changes. However, any changes in glycolysis or mitochondrial activity that result in a change in ATP/ADP would likely impact the KATP-channel-dependent pathway of [Ca2+]i-dependent insulin secretion.
3.4. Depolarization by KCl shows ion channel role in maturation
To examine the [Ca2+]i response to depolarization of the plasma membrane in the β-cell, we used potassium chloride (KCl) to bypass the increase in ATP/ADP required for glucose stimulated β-cell depolarization and voltage-gated calcium influx and force the voltage-gated calcium channels to open in low concentrations of glucose. After recording islets in low glucose conditions (3 mM) for two minutes, the islets were exposed to an additional 30mM KCl for 3 minutes and then washed back to 3mM glucose solution. Neonatal islets at PN day 0 had elevated [Ca2+]i prior to stimulation and a diminished response to KCl compared to the adult islets (Figure 3A). At PN day 4, the response to KCl continued to be slightly lower than adults (Figure 3B) though not significantly. At PN day 12, the average KCl response was slightly elevated compared to adult islets although not signifcantly (Figure 3C). The amplitude of the KCl response is shown for each postnatal stage in Figure 3D. Of interest, the same pattern of depolarization-induced [Ca2+]i was observed by Huang et al., including the slightly enhanced response on PN day 12 relative to adult [22]. Our findings thus confirm previous observations [21,22].
Figure 3. Response to KCl stimulation is decreased at early neonate stages.
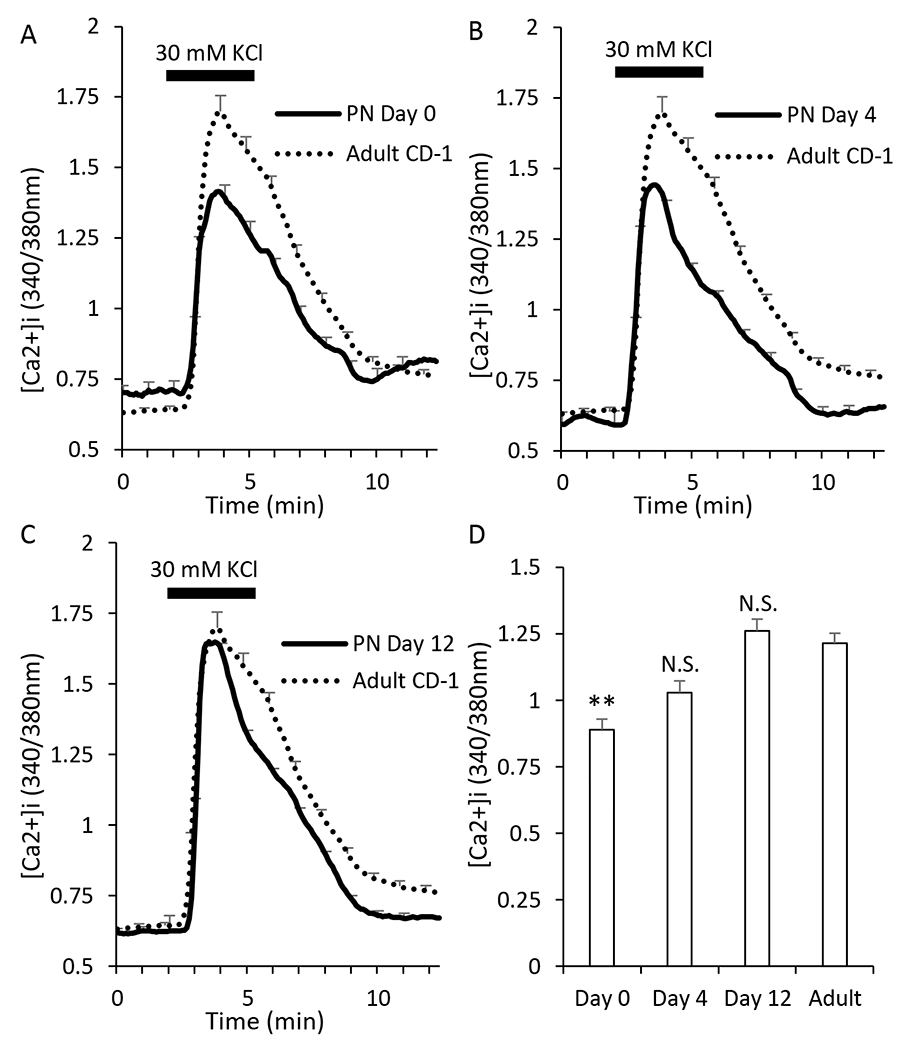
A-C. Traces showing the response to electrical stimulation (KCl) after exposure to 3mM glucose for neonatal islets at postnatal day 0 (A, N=23), day 4 (B, N=24), and day 12 (C, N=24). Adult traces (dotted black lines, N=86) are used for comparison to the neonatal progression. D. Bar graph of the amplitude of response to stimulation. Asterisks (*) indicate differences between adult and neonatal [Ca2+]i traces based on N=3 sets of islets for each neonatal day and N=9 for adult. **P<0.01, N.S. not significant.
3.5. Nifedipine exposure suggests rapidly maturing L-type calcium channels in neonatal islets
L-type calcium-channels play a key role in the insulin secretion process. To note, there are several other types of voltage-gated calcium channels such as N-type, R-type, and P/Q-type [50]. However, L-type calcium channels are the predominant type expressed in mouse β-cells. L-type calcium channels also dominantly contribute to the [Ca2+]i current of β-cells. Due to this dominance, L-type calcium channel activity was assessed by monitoring [Ca2+]i patterns in islets for 15 min in 11mM glucose and then treating islets with nifedipine to inhibit voltage-gated calcium-channel activity. Because of oscillatory activity in 11mM glucose, a long prestimulation phase was needed to get an accurate mean [Ca2+]i level. As shown in Figure 4, [Ca2+]i levels dropped rapidly in response to nifedipine exposure, as expected. Islets from PN day 0 mice had the lowest [Ca2+]i levels in 11mM glucose and the weakest response to nifedipine (Figure 4A). Islets from PN day 4 and day 12 had a much stronger response to nifedipine (Figure 4B–C), similar to that of adults. The amplitude of the response to nifedipine shown in Figure 4D was based on subtracting the mean [Ca2+]i levels during nifedipine exposure from the mean [Ca2+]i levels during the 15-min control period. This longer control period provided better signal averaging against the inherent variabiltity created by endogenous oscillatory activity in 11mM glucose. In summary, only islets from PN day 0 had responses to nifedipine that differed from adults, indicating that calcium-channel activity appears to mature very rapidly in islets.
Figure 4. Nifedipine does not completely repress neonatal [Ca2+]i.
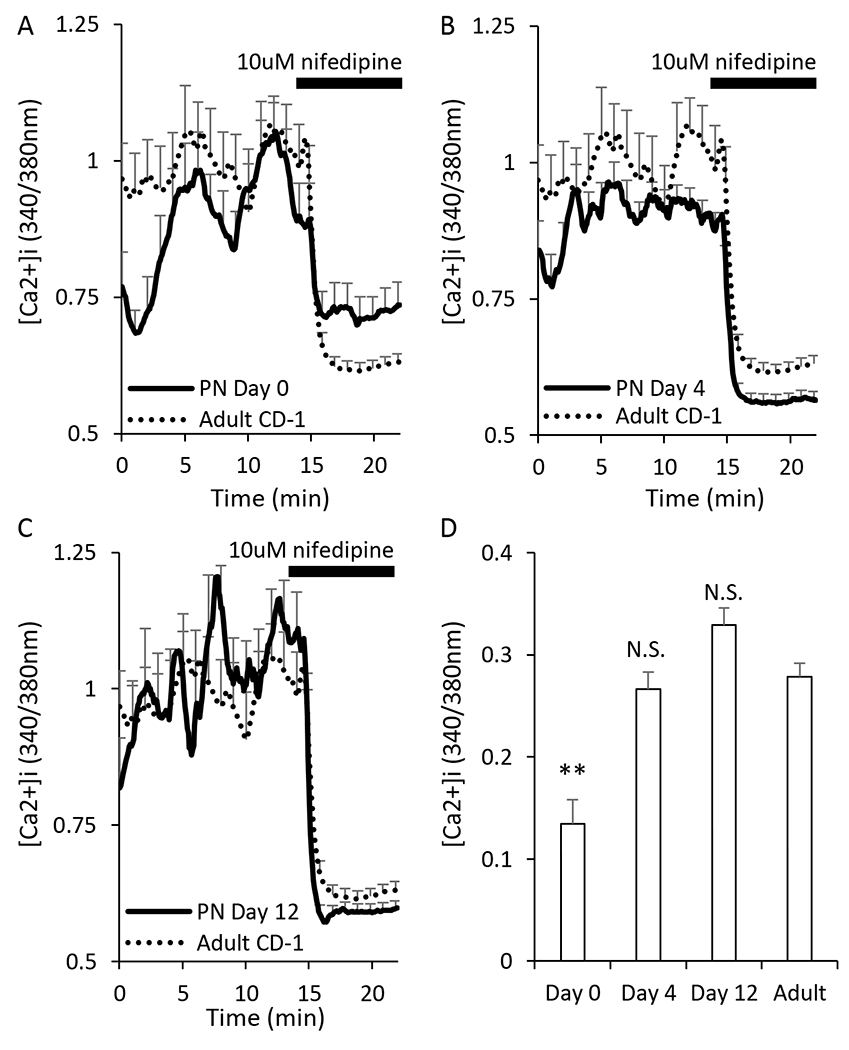
A-C. Traces showing the responses of islets to the calcium channel blocker nifedipine after exposure to 15mM glucose for neonatal islets at postnatal day 0 (A, N=28), day 4 (B, N=32), and day 12 (C, N=28). Adult traces (dotted black lines, N=86) are used for comparison to the neonatal progression. D. Bar graph of the amplitude of response to stimulation. Asterisks (*) indicate differences between adult and neonatal [Ca2+]i traces based on N=3 trials for each neonatal day and N=9 for adult. **P<0.01, N.S. not significant.
3.6. Gene expression related to ion channels in maturing islets
We performed targeted anaylsis of the public RNA sequencing dataset as previously described for glycolytic genes, but instead used the term ‘ion channel’ in the Jackson Laboratory database. Among 425 genes involved with ion channel activity that were identified, 25 genes were significantly decreased by greater than 50% (15 genes) or increased by greater than 100% in expression (i.e. doubled, 10 genes) between PN day 0 and day 12, as shown in Table 2. Many of these genes were fairly low expressing (<5 FPKM) throughout early islet maturation.
Table 2. RNA sequencing expression of genes encoding ion channels.
Gene list for RNA transcript abundance in FPKM. P-values vs. day 0: *P<0.05, **P<0.01, ***P<0.001. From a list of 425 genes related to ‘ion channel’ (GO:0005216), the 25 genes in this table showed statistically significant changes in FPKM between postnatal day 0 and day 12 of >200% or <50% of values on day 0. P-values vs. day 0: *P<0.05, **P<0.01, ***P<0.001.
| Gene Symbol | PN Day 0 | PN Day 4 | PN Day 12 | P12/P0 |
|---|---|---|---|---|
|
| ||||
| Chrna4 | 4.6 +/− 0.7 | 3.6 +/− 0.1 | **0.5 +/− 0.1 | 11.9 |
| Kcnk9 | 54.3 +/− 18.8 | 29.0 +/− 1.7 | *6.8 +/− 0.7 | 12.6 |
| Kcnc1 | 1.5 +/− 0.1 | ***0.52 +/− 0.1 | ***0.4 +/− 0.0 | 24.6 |
| Kcnn2 | 1.6 +/− 0.2 | *0.8 +/− 0.2 | **0.4 +/− 0.1 | 29.0 |
| Kcnmb4 | 4.0 +/− 0.3 | *2.9 +/− 0.1 | ***1.4 +/− 0.1 | 34.5 |
| Scn3a | 1.7 +/− 0.1 | 1.9 +/− 0.0 | **0.6 +/− 0.1 | 34.8 |
| Lrrc38 | 2.3 +/− 0.2 | *3.1 +/− 0.2 | **0.8 +/− 0.1 | 35.9 |
| Mcoln3 | 4.8 +/− 0.4 | **3.1 +/− 0.2 | ***17 +/− 0.1 | 36.2 |
| Kcnh8 | 1.9 +/− 0.3 | 1.5 +/− 0.1 | **0.7 +/− 0.1 | 39.0 |
| Panx1 | 5.5 +/− 0.1 | **4.2 +/− 0.3 | ***2.6 +/− 0.1 | 46.4 |
| Lrrc55 | 5.1 +/− 1.6 | *1.2 +/− 0.1 | 2.4 +/− 0.5 | 46.8 |
| Ttyh3 | 20.6 +/− 0.6 | **15.7 +/− 0.8 | ***10.1 +/− 0.3 | 49.1 |
| Kcnq4 | 6.7 +/− 0.2 | ***4.5 +/− 0.2 | ***3.3 +/− 0.2 | 49.1 |
| Kcnk3 | 3.7 +/− 0.4 | 3.5 +/− 0.0 | **1.8 +/− 0.1 | 49.4 |
| Kcnb2 | 3.0 +/− 0.2 | 2.5 +/− 0.2 | **1.5 +/− 0.1 | 49.8 |
|
| ||||
| Aqp1 | 1.7 +/− 0.5 | 2.7 +/− 0.7 | **3.8 +/− 0.2 | 220.5 |
| Rasa3 | 1.4 +/− 0.3 | 2.2 +/− 0.1 | **3.4 +/− 0.1 | 238.1 |
| Tmc8 | 0.4 +/− 0.3 | 0.6 +/− 0.1 | *1.1 +/− 0.1 | 275.3 |
| Kcnj5 | 3.5 +/− 0.1 | ***6.4 +/− 0.1 | **13.3 +/− 1.4 | 379.2 |
| Kcnn4 | 0.4 +/− 0.3 | *1.2 +/− 0.2 | **2.0 +/− 0.2 | 494.4 |
| Lrrc26 | 0.2 +/− 0.0 | *0.7 +/− 0.2 | **1.1 +/− 0.2 | 611.0 |
| Ano1 | 0.4 +/− 0.1 | ***15 +/− 0.1 | *2.6 +/− 0.7 | 653.7 |
| Gabra4 | 0.2 +/− 0.0 | **0.5 +/− 0.0 | *1.7 +/− 0.5 | 718.5 |
| Kcne3 | 0.2 +/− 0.1 | 0.2 +/− 0.3 | ***13 +/− 0.1 | 755.7 |
| P2rx1 | 0.2 +/− 0.1 | **2.4 +/− 0.4 | **4.9 +/− 1.2 | 2441.9 |
The most substantially downregulated genes were Chrna4 (down 89%) and Kcnk9 (down 88%). Chrna4 codes for the cholinergic receptor nicotinic alpha 4 subunit, which is expressed in islets at the protein level and associated with Mafa signaling [51]. How reduced expression of Chrna4 would impact islet calcium signaling is unclear, although of interest, cholinergic stimulation can synchronize calcium oscillations between islets [52]. Kcnk9 codes for the TASK-3 K+-channel that normally acts as a leak channel to hyperpolarize cells [53,54]. It is plausible that increased TASK-3-channel activity on PN day 0 could blunt, in part, the [Ca2+]i response to glucose, and there is evidence of such a role for TASK-1 in beta cells [55]. P2rx1 was the most strongly upregulated gene (up 2341%), which codes for a family of purinergic receptors that is known to regulate insulin secretion, proliferation, and islet survival [56]. Kcnj5 was the only other gene with increased expression >4 FPKM during neonatal maturation. Kcnj5 (up 279%) is a G-protein-activated inward rectifying K+-channel (GIRK channel). Little is known about the function of Kcnj5 in β-cells, although there is some evidence of GIRK-channels in β-cells [57]. Of note, Kcnj11, which codes for the Kir6.2 subunit of the KATP channel, showed mildly reduced FPKM between day 0 (41.3 ± 3.2) and day 12 (31.6 ± 1.9, P<.05). No other changes were found in genes coding for the two most dominant ion channels in the beta cell, the SUR1 subunit of the KATP channel (abcc8) or the subunits of the L-type calcium channel (Cacna1c and Cacna1d).
3.7. Estimated calcium levels in the ER
Lastly, cyclopiazonic acid (CPA) was used to estimate the free calcium being stored in the ER of islet cells as previously described [58]. Islets perifused in 11mM glucose KRB solution were first exposed to 10μM nifedipine to minimize calcium entry through L-type voltage-gated calcium channels, the dominant source of extracellular calcium entry in the β-cell. CPA was then added to block SERCA pumps within β-cells, causing calcium stored in the ER to be released, thus gauging the amount of calcium that is stored at the various ages. Fluorescent microscopy experiments showed that the CPA-induced calcium release from the ER was highest at PN day 0 (Figure 5A) but rapidly decreased as the pups aged. By PN day 4, these CPA responses were similar to adult responses (Figure 5B). CPA responses on PN day 12 were significantly lower than adult responses (Figure 5C). These effects are summarized in Figure 5D.
Figure 5. ER calcium storage is increased in early ages.
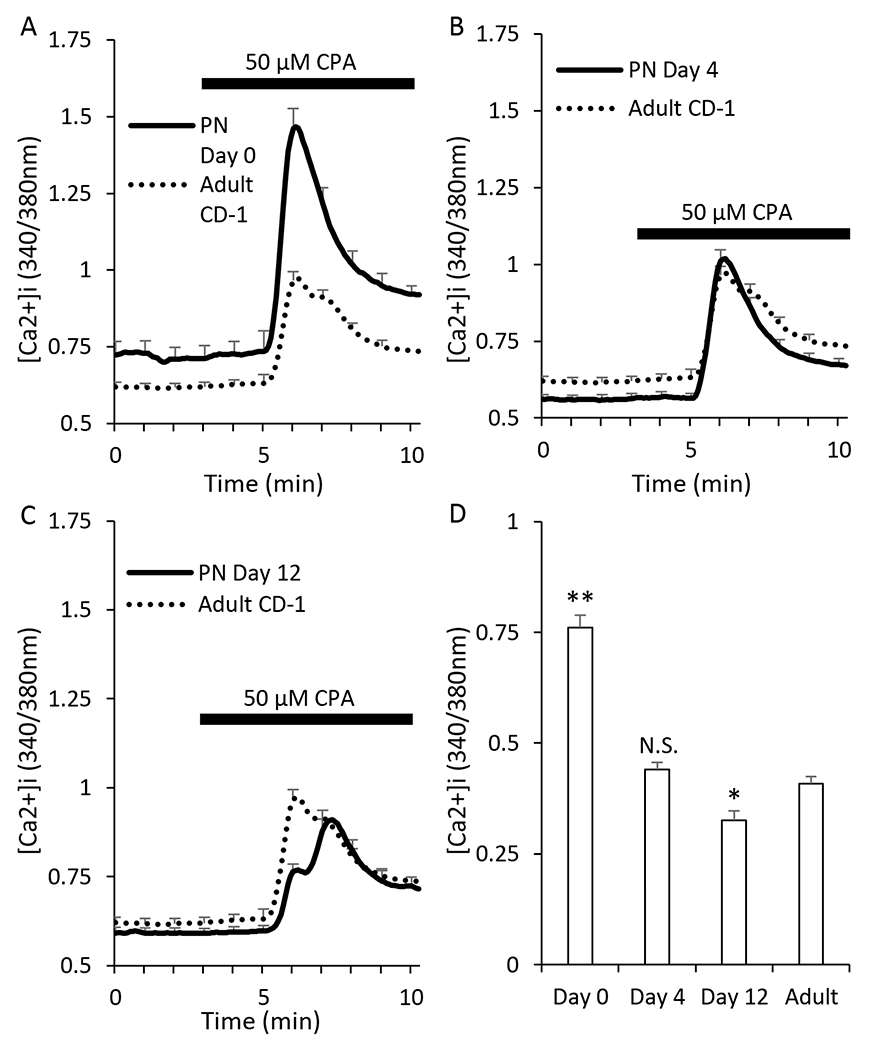
A-C. Traces showing islet response to cyclopiazonic acid (CPA) at postnatal day 0 (A, N=28), day 4 (B, N=32), and day 12 (C, N=28) in the presence of 11 mM glucose and 10uM nifedipine to prevent additional calcium influx through voltage-gated calcium channels during CPA exposure. Adult (dotted black lines, N=86) are used as a reference in panels A-C. D. Bar graph showing the peak CPA response at postnatal day 0, day 4, day 12, and adult (dotted black line). D. Bar graph of the amplitude of response to stimulation. Asterisks (*) indicate differences between adult and neonatal [Ca2+]i traces based on N=3 trials for each neonatal day and N=9 for adult. *P<0.05, **P<0.01, N.S. not significant.
ER calcium plays a critical role in regulating the unfolded protein response and protein folding itself [59]. Consequently, neonatal islets may need to synthesize more proteins early after birth and need more calcium present in the ER. Previous studies observing glucose-inducible protein biosynthesis in neonatal rat β-cells age PN day 2-3 support this hypothesis. Compared to 10-week old β-cells, the neonates synthesized up to seven times more protein in basal concentrations of glucose [38]. This hyperactivity in basal concentrations was also shown to be a stable property of these neonatal β-cells, as it was conserved even after 24-hours of exposure to basal glucose in vitro [38]. In our studies, we observed nearly double the ER calcium on day 0 than at any other age. ER calcium returned to adult levels by day 4 and was even slightly lower than adult on day 12.
3.8. Gene expression related to ER calcium in maturing islets
Again, the publicly available RNA sequencing Jackson Laboratories database was utilized as described previously using the search term ‘endoplasmic reticulum calcium ion homeostasis’. In examining potential genetic underpinnings of this enhanced ER calcium at birth, we identified several genes of interest. 14 genes total were found to significantly change in expression from PN day 0 to PN day 12. These genes are shown in Table 3. The genes ATP2a2 and ATP2a3, which code for sarcoendoplasmic reticulum Ca2+-ATPases (SERCA)2 and SERCA3, respectively, could clearly play a role. Both SERCAs are intracellular calcium pumps located in the ER that move calcium from the cytosol into the ER [60,61]. Both genes are downregulated with age, which is consistent with our observed decrease in ER calcium with age. However, detailed studies from Ravier et al. suggest that this activity alone may not be sufficient to explain the observed changes during maturation [62]. Another potential player is K+-channel tetramerization domain containing 17 (Kctd17). Using fura-2AM to measure thapsigargin-induced calcium release, Mencacci et al. showed that a mutation in Kctd17 can reduce ER calcium storage in fibroblasts [63]. Although Kctd17 has not been reported in islets to date, this study suggests that reduced Kctd17 expression could result in reduced ER calcium storage, which is consistent with our observations.
Table 3. RNA sequencing expression of ER calcium homeostasis genes.
Gene list for RNA transcript abundance in FPKM. From a list of 14 genes related to ‘endoplasmic reticulum calcium ion homeostasis’ (GO:0032469), the 16 genes in this table showed statistically significant differences between postnatal day 0 and day 12. P-values vs. day 0: *P<0.05, **P<0.01, ***P<0.001.
| Gene Symbol | PN day 0 | PN day 4 | PN day 12 | P12/P0 |
|---|---|---|---|---|
|
| ||||
| Kctd17 | 5.2 +/− 0.6 | 3.9 +/− 0.3 | *3.1 +/− 0.3 | 60.2 |
| Atp2a2 | 166.2 +/− 10.5 | *132.4 +/− 4.0 | **115.9 +/− 7.4 | 69.7 |
| Bak1 | 8.7 +/− 0.2 | 8.6 +/− 0.3 | **6.3 +/− 0.4 | 72.0 |
| Appbp2 | 16.8 +/− 0.8 | 16.4 +/− 0.5 | *12.5 +/− 1.1 | 74.5 |
| Atp2a3 | 133.9 +/− 3.8 | *118.7 +/− 5.0 | **105.2 +/− 5.0 | 78.6 |
| Pacs2 | 11.2 +/− 0.4 | *9.8 +/− 0.4 | *8.9 +/− 0.8 | 79.4 |
| Fis1 | 54.5 +/− 3.2 | 50.3 +/− 4.3 | *44.45 +/− 2.0 | 81.6 |
| Psen2 | 19.6 +/− 0.8 | 18.2 +/− 1.6 | *16.1 +/− 0.9 | 82.1 |
| Bax | 34.1 +/− 1.1 | *29.7 +/− 1.2 | **28.4 +/− 0.7 | 83.3 |
|
| ||||
| Rap1gds1 | 6.2 +/− 0.2 | *7.3 +/− 0.2 | 6.4 +/− 0.4 | 102.9 |
| Thada | 5.2 +/− 0.2 | **7.4 +/− 0.3 | *6.8 +/− 0.5 | 129.9 |
| Wfs1 | 27.0 +/− 1.6 | *37.6 +/− 2.6 | *35.3 +/− 2.2 | 130.6 |
| Herpud1 | 36.1 +/− 3.4 | *54.5 +/− 4.0 | *48.4 +/− 3.3 | 134.1 |
| Pml | 4.3 +/− 0.4 | **6.0 +/− 0.1 | *6.1 +/− 0.5 | 141.4 |
The most upregulated ER-calcium-related transcripts as mice aged were Pml (up 30%), Wfs1 (up 31%) Thada (up 34%) and Herpud1 (up 41%). Promylcytic leukemia (Pml) tumor suppressor has been shown to play a crucial role in IP3R phosphorylation and IP3R-mediated calcium release from the ER [64]. Thus, upregulation of Pml could, in part, contribute to reduced ER calcium levels as observed. Pml has only been observed in islets in association with FoxO-mediated protection of β-cells [65], so a potential role for Pml in β-cell ER function has not been explored. Wolfram syndrome 1 (Wfs1) is a well-established ER-stress responsive gene that negatively regulates SERCA expression [66], which is consistent with our findings. Thada codes for a protein that is involved in thermogenesis and is associated with SERCA [67] but little else is known. Finally, Herpud1 is involved with the unfolded protein response to ER stress [68]. Herpud1 has also been implicated in a novel mechanism regulating insulin secretion [69]. Although none of these transcript changes were very large, the combined effects could impact ER calcium homeostasis during islet maturation.
It should be noted that ER calcium storage levels are also highly sensitive to changes in glucose metabolism. Glucose concentration is a major determinant of ER calcium filling [62,70]. Thus, the observed left shift in glucose sensitivity in neonatal β-cells could drive increased ER calcium on its own. This possibility cannot be discounted and could explain, at least in part, our observed differences. Future studies will attempt to parse this out.
3.9. Potential significance of islet maturation to understanding and treating diabetes.
Several advances in recent years have led to stem cell therapies becoming a strong research focus for the potential treatment of type 1 diabetes [71–73]. The autoimmune targeting of β-cells by the body results in a decrease in β-cell mass, and therefore, a decrease in insulin production that leads to hyperglycemia [74]. Genetically manipulating stem cells to differentiate into β-cells is viewed as a potential solution to insulin insufficiency. Progress has been made by closely mimicking the pathway that pluripotent stem cells take during pancreatic β-cell formation [23]. It has even been shown that a major determinant of β-cell function is its communication with other cell types within the islet [75]. These points exemplify the sensitivity of a β-cell to a specific developmental process and environmental cues to properly function. Thus, to create a more realistic and functional β-cell, it is necessary to induce β-cells to go through the same maturation process that they normally undergo during maturation after birth. Thoroughly characterizing this maturation process is key to engineering β-cells that are as similar to those produced during development as possible. Providing a detailed map of [Ca2+]i changes as islets mature can contribute to enhancing this knowledge.
Improved knowledge of the maturation process of β-cells may also be useful to the field of type 2 diabetes. Recent work has provided strong evidence that dysfunction in β-cells in type 2 diabetes may be due to a dedifferentiation-like pattern that appears to be observed in the islets [24,76,77]. In both diabetic islets from db/db mice and neonatal islets, we have shown that the [Ca2+]i response is increased in lower concentrations of glucose but islets are unable to fully respond to higher glucose concentrations [78,79]. Similar results have been reported following chronic exposure to high glucose [35] or free fatty acids [80] in β-cells. Although speculative, it is possible that type 2 diabetic islets revert to a neonatal form but either do not or cannot go through a rematuration process to reach correct adult-like physiology.
3.10. Limitations of this study
Several limitations to this study should be noted. First, [Ca2+]i changes do not correlate one-to-one with insulin secretion due to additional amplifying pathways [2,6] and other dissociations between insulin and [Ca2+]i [81]. However, [Ca2+]i can provide valuable data on the dynamics of stimulation at the level of individual islets or even individual β-cells, and appropriate changes in [Ca2+]i are still integral to properly functional β-cells. This level of detail is not possible using standard static or perifusion methods of measuring insulin secretion. Importantly, our overall findings agree with prior studies that demonstrated elevated basal insulin secretion and immature glucose sensing in neonatal islets [22,82]. Second, our analysis of RNA sequencing data was focused on specific cellular functions (glycolysis, ER, and ion channels) to compare these data with our [Ca2+]i studies; genes important to other aspects of β-cell identity, viability and function may not have been identified in this analysis. Third, estimated mRNA from RNA-sequencing does not necessarily translate into functional proteins to be used by the β-cell and does not account for post-translational modifications. Consequently, these data are not completely representative of what is functional and actively being used in neonatal β-cells. Western blotting or other immunoassays would be necessary to identify proteins that match the mRNA expression levels. Along with this, there were technical differences, such as culturing conditions, duration of isolation and recovery, different mouse strains as islet sources (CD1 vs. MIP-cre utilizing human growth hormone mini-gene and doxycycline), and intact islets used in calcium studies vs dispersed β-cells used for RNA sequencing. Thus, direct gene-inactivation in β-cells would be needed to elucidate the physiological functions of at least some of the candidate genes, which we envision to present enormous challenges due to the need of deriving new mouse lines and the potential redundancy/compensation issues amongst gene family members. Ideally, qPCR would be used to confirm gene expression. However, due to the extremely small amount of extractable mRNA available in PN day 0 neonatal mice, this is beyond our capabilities. Although these are substantial limitations, our purpose was to provide a first attempt at identifying possible genes that support the observed [Ca2+]i findings for future study. This study has just scratched the surface of determining genes underling how β-cells respond to stimuli throughout the early stages of life.
4. Conclusions
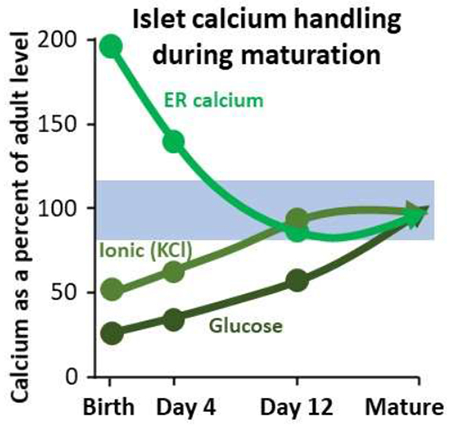
There are many underlying factors that help a β-cell correctly carry out its function. Therefore, as neonates are maturing, there is undoubtedly a great deal of machinery changing and maturing as well, which this study has begun to explore. Figure 6 summarizes physiological responses to various stimuli between birth and PN day 12 by displaying the islet response to (A) glucose, (B) KCl-induced depolarization (C) and other stimuli at each postnatal stage as the percent of the normal adult response. We found that different physiological mechanisms that are important in the functioning of β-cells matured at various points throughout the first few days of life in neonatal mice. Among the mechanisms studied, the ion-channel activity and ER calcium storage were the first to be fully matured at approximately PN day 4. Neonatal [Ca2+]i responses to glucose stimulation are not fully adult-like by PN day 12 and require additional time to mature. Many of these changes most likely result from changes in gene expression throughout this maturation period. Therefore, it is important to identify genes that are playing a key role in the maturation process using techniques such as RNA sequencing. Elucidating the natural islet maturation process may aid in our understanding of the pathophysiology of and potential therapies for type 1 and type 2 diabetes.
Figure 6. Summary of characteristics of maturing islets.
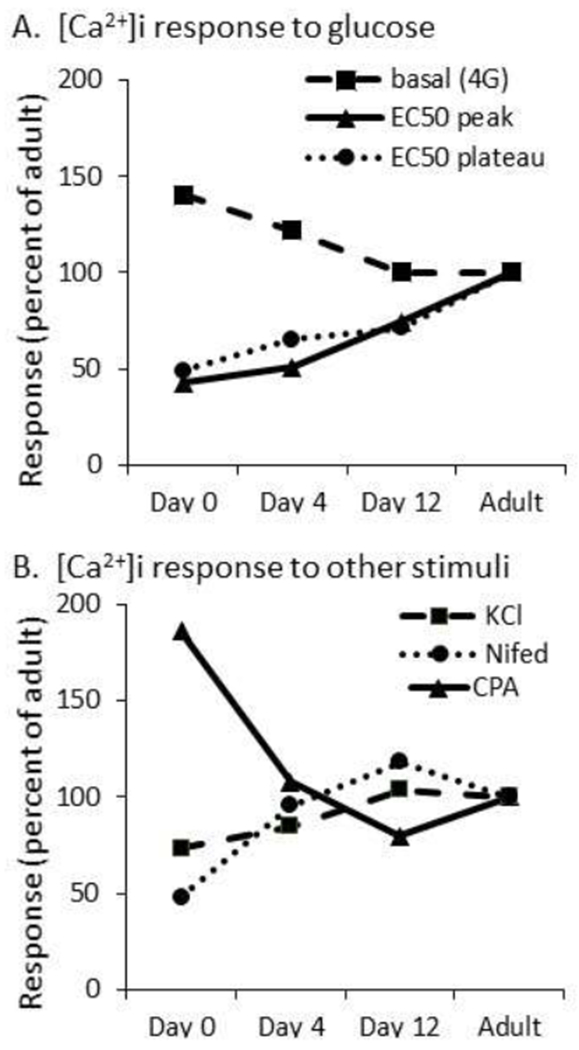
A-B. Data summary of postnatal responses normalized as percent of mature adult response for glucose (A: basal, peak, and plateau) and for other responses (B: KCl, nifedipine, and CPA).
Highlights:
Distinct adult-like features of [Ca2+]i signaling develop at different times in neonatal mouse islets
[Ca2+]i responses to glucose remained left-shifted and attenuated beyond postnatal day 12
Calcium handling in the ER and ion channels matured rapidly to adult-like patterns
RNA sequencing data mining identifies possible candidate genes involved with maturing [Ca2+]i signaling
Acknowledgments
Funding for this work was provided by the NIDDK Mouse Metabolic Phenotyping Centers (National MMPC, RRID:SCR_008997, www.mmpc.org) under the MICROMouse Program (19AU3974), the Osteopathic Heritage Foundation (OHF), and Ohio University Heritage College of Osteopathic Medicine (OU-HCOM). Additional funding to H.L.W. from the Student Enhancement Award and the John J. Kopchick Award for undergraduate students at Ohio University. Thanks to the Research and Scholarly Advancement Fellowship program for providing Lauren Donovan the opportunity to participate in this project. Special thanks to David Jacobson and Maureen Gannon at Vanderbilt University for their input and guidance for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
Conflict of Interest Statement.
On behalf of all co-authors, I declare there are no competing interests or conflicts of interest with any author for the submitted manuscript “Postnatal maturation of calcium signaling in islets of Langerhans from neonatal mice” by Hannah L. West at al.
References
- [1].Slack JM, Developmental biology of the pancreas, Development. 121 (1995) 1569–1580. [DOI] [PubMed] [Google Scholar]
- [2].Henquin JC, Regulation of insulin secretion: a matter of phase control and amplitude modulation, Diabetologia. 52 (2009) 739–751. [DOI] [PubMed] [Google Scholar]
- [3].Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, Wollheim CB, Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells, J. Clin. Invest 98 (1996) 2524–2538. 10.1172/JCI119071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P, Hierarchy of the beta-cell signals controlling insulin secretion, Eur J Clin Invest. 33 (2003) 742–750. [DOI] [PubMed] [Google Scholar]
- [5].Straub SG, James RF, Dunne MJ, Sharp GW, Glucose activates both K(ATP) channel-dependent and K(ATP) channel-independent signaling pathways in human islets, Diabetes. 47 (1998) 758–763. [DOI] [PubMed] [Google Scholar]
- [6].Kalwat MA, Cobb MH, Mechanisms of the amplifying pathway of insulin secretion in the beta cell., Pharmacol Ther. 179 (2017) 17–30. 10.1016/j.pharmthera.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Idevall-Hagren O, Tengholm A, Metabolic regulation of calcium signaling in beta cells, Semin. Cell Dev. Biol 103 (2020) 20–30. 10.1016/j.semcdb.2020.01.008. [DOI] [PubMed] [Google Scholar]
- [8].Mears D, Jr NFS, Atwater I, Rojas E, Bertram R, Sherman A, Evidence that calcium release-activated current mediates the biphasic electrical activity of mouse pancreatic beta-cells, J Membr Biol. 155 (1997) 47–59. [DOI] [PubMed] [Google Scholar]
- [9].Scarl RT, Corbin KL, Vann NW, Smith HM, Satin LS, Sherman A, Nunemaker CS, Intact pancreatic islets and dispersed beta-cells both generate intracellular calcium oscillations but differ in their responsiveness to glucose, Cell Calcium. 83 (2019) 102081. 10.1016/j.ceca.2019.102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lacy PE, Walker MM, Fink CJ, Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin, Diabetes. 21 (1972) 987–998. 10.2337/diab.21.10.987. [DOI] [PubMed] [Google Scholar]
- [11].Henquin J-C, Dufrane D, Kerr-Conte J, Nenquin M, Dynamics of glucose-induced insulin secretion in normal human islets, Am. J. Physiol. Endocrinol. Metab 309 (2015) E640–650. 10.1152/ajpendo.00251.2015. [DOI] [PubMed] [Google Scholar]
- [12].Curry DL, Bennett LL, Grodsky GM, Dynamics of insulin secretion by the perfused rat pancreas, Endocrinology. 83 (1968) 572–584. 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- [13].Getty L, Hamilton-Wessler M, Ader M, Dea MK, Bergman RN, Biphasic insulin secretion during intravenous glucose tolerance test promotes optimal interstitial insulin profile, Diabetes. 47 (1998) 1941–1947. 10.2337/diabetes.47.12.1941. [DOI] [PubMed] [Google Scholar]
- [14].Dhawan S, Tschen S-I, Zeng C, Guo T, Hebrok M, Matveyenko A, Bhushan A, DNA methylation directs functional maturation of pancreatic β cells, J. Clin. Invest 125 (2015) 2851–2860. 10.1172/JCI79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, Atkins AR, Downes M, Evans RM, ERRγ Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells, Cell Metab. 23 (2016) 622–634. 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gu C, Stein GH, Pan N, Goebbels S, Hürnberg H, Nave K-A, Herrera P, White P, Kaestner KH, Sussel L, Lee JE, Pancreatic beta cells require NeuroD to achieve and maintain functional maturity, Cell Metab. 11 (2010) 298–310. 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA, Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3, Nat. Biotechnol 30 (2012) 261–264. 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aguayo-Mazzucato C, Koh A, El Khattabi I, Li W-C, Toschi E, Jermendy A, Juhl K, Mao K, Weir GC, Sharma A, Bonner-Weir S, Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells, Diabetologia. 54 (2011) 583–593. 10.1007/s00125-010-2026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stolovich-Rain M, Enk J, Vikesa J, Nielsen FC, Saada A, Glaser B, Dor Y, Weaning triggers a maturation step of pancreatic β cells, Dev. Cell 32 (2015) 535–545. 10.1016/j.devcel.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [20].Jaafar R, Tran S, Shah AN, Sun G, Valdearcos M, Marchetti P, Masini M, Swisa A, Giacometti S, Bernal-Mizrachi E, Matveyenko A, Hebrok M, Dor Y, Rutter GA, Koliwad SK, Bhushan A, mTORC1 to AMPK switching underlies β-cell metabolic plasticity during maturation and diabetes, J. Clin. Invest 129 (2019) 4124–4137. 10.1172/JCI127021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang S-B, Rupnik M, Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas, Ann. N. Y. Acad. Sci 1152 (2009) 53–62. 10.1111/j.1749-6632.2008.04003.x. [DOI] [PubMed] [Google Scholar]
- [22].Huang C, Walker EM, Dadi PK, Hu R, Xu Y, Zhang W, Sanavia T, Mun J, Liu J, Nair GG, Tan HYA, Wang S, Magnuson MA, Stoeckert CJJ, Hebrok M, Gannon M, Han W, Stein R, Jacobson DA, Gu G, Synaptotagmin 4 Regulates Pancreatic beta Cell Maturation by Modulating the Ca(2+) Sensitivity of Insulin Secretion Vesicles., Dev Cell. 45 (2018) 347–361. e5. 10.1016/j.devcel.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M, Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges, Cell Stem Cell. 22 (2018) 810–823. 10.1016/j.stem.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Talchai C, Xuan S, Lin HV, Sussel L, Accili D, Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure., Cell. 150 (2012) 1223–1234. 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, Sherman A, Satin LS, Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo?, Diabetes. 54 (2005) 3517–3522. [DOI] [PubMed] [Google Scholar]
- [26].Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS, A Practical Guide to Rodent Islet Isolation and Assessment, Biol Proced Online. 11 (2009) 3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO, Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration, J Biol Chem. 273 (1998) 33501–33507. [DOI] [PubMed] [Google Scholar]
- [28].Huang C, Gu G, Effective Isolation of Functional Islets from Neonatal Mouse Pancreas, J Vis Exp. (2017). 10.3791/55160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Whitticar NB, Strahler EW, Rajan P, Kaya S, Nunemaker CS, An Automated Perifusion System for Modifying Cell Culture Conditions over Time., Biol Proced Online. 18 (2016) 19. 10.1186/s12575-016-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gelin L, Li J, Corbin KL, Jahan I, Nunemaker CS, Metformin Inhibits Mouse Islet Insulin Secretion and Alters Intracellular Calcium in a Concentration-Dependent and Duration-Dependent Manner near the Circulating Range., J Diabetes Res. 2018 (2018) 9163052. 10.1155/2018/9163052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Corbin KL, Hall TE, Haile R, Nunemaker CS, A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro, Islets. 3 (2011) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crim WS, Wu R, Carter JD, Cole BK, Trace AP, Mirmira RG, Kunsch C, Nadler JL, Nunemaker CS, AGI-1067, a novel antioxidant and anti-inflammatory agent, enhances insulin release and protects mouse islets, Molecular and Cellular Endocrinology. 323 (2010) 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Law NC, Marinelli I, Bertram R, Corbin KL, Schildmeyer C, Nunemaker CS, Chronic stimulation induces adaptive potassium channel activity that restores calcium oscillations in pancreatic islets in vitro, Am. J. Physiol. Endocrinol. Metab 318(2020) E554–E563. 10.1152/ajpendo.00482.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, Stoeckert CJ, Hogenesch JB, Pierce EA, Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM), Bioinformatics. 27 (2011) 2518–2528. 10.1093/bioinformatics/btr427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Glynn E, Thompson B, Vadrevu S, Lu S, Kennedy RT, Ha J, Sherman A, Satin LS, Chronic glucose exposure systematically shifts the oscillatory threshold of mouse islets: Experimental evidence for an early intrinsic mechanism of compensation for hyperglycemia., Endocrinology. (2015) en20151563. 10.1210/en.2015-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Henquin J-C, Dufrane D, Nenquin M, Nutrient control of insulin secretion in isolated normal human islets, Diabetes. 55 (2006) 3470–3477. 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- [37].Jermendy A, Toschi E, Aye T, Koh A, Aguayo-Mazzucato C, Sharma A, Weir GC, Sgroi D, Bonner-Weir S, Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells., Diabetologia. 54 (2011) 594–604. 10.1007/s00125-010-2036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martens GA, Motté E, Kramer G, Stangé G, Gaarn LW, Hellemans K, Nielsen JH, Aerts JM, Ling Z, Pipeleers D, Functional characteristics of neonatal rat β cells with distinct markers, J. Mol. Endocrinol 52 (2014) 11–28. 10.1530/JME-13-0106. [DOI] [PubMed] [Google Scholar]
- [39].Henquin J-C, Nenquin M, Immaturity of insulin secretion by pancreatic islets isolated from one human neonate, J Diabetes Investig. 9 (2018) 270–273. 10.1111/jdi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thorens B, GLUT2, glucose sensing and glucose homeostasis, Diabetologia. 58 (2015) 221–232. 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- [41].Liang Y, Najafi H, Matschinsky FM, Glucose regulates glucokinase activity in cultured islets from rat pancreas, J. Biol. Chem 265 (1990) 16863–16866. [PubMed] [Google Scholar]
- [42].Matschinsky FM, Assessing the potential of glucokinase activators in diabetes therapy., Nat Rev Drug Discov. 8 (2009) 399–416. 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- [43].Eynon N, Hanson ED, Lucia A, Houweling PJ, Garton F, North KN, Bishop DJ, Genes for elite power and sprint performance: ACTN3 leads the way, Sports Med. 43 (2013) 803–817. 10.1007/s40279-013-0059-4. [DOI] [PubMed] [Google Scholar]
- [44].Howell SL, Tyhurst M, Microtubules, microfilaments and insulin-secretion, Diabetologia. 22 (1982) 301–308. 10.1007/BF00253571. [DOI] [PubMed] [Google Scholar]
- [45].Jung S-R, Kuok ITD, Couron D, Rizzo N, Margineantu DH, Hockenbery DM, Kim F, Sweet IR, Reduced cytochrome C is an essential regulator of sustained insulin secretion by pancreatic islets, J Biol Chem. 286 (2011) 17422–17434. 10.1074/jbc.M110.202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rountree AM, Neal AS, Lisowski M, Rizzo N, Radtke J, White S, Luciani DS, Kim F, Hampe CS, Sweet IR, Control of insulin secretion by cytochrome C and calcium signaling in islets with impaired metabolism, J Biol Chem. 289 (2014) 19110–19119. 10.1074/jbc.M114.556050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Päth G, Mehana AE, Pilz IH, Alt M, Baumann J, Sommerer I, Hoffmeister A, Seufert J, NUPR1 preserves insulin secretion of pancreatic β-cells during inflammatory stress by multiple low-dose streptozotocin and high-fat diet, Am. J. Physiol. Endocrinol. Metab 319 (2020) E338–E344. 10.1152/ajpendo.00088.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barbosa-Sampaio HC, Liu B, Drynda R, Rodriguez de Ledesma AM, King AJ, Bowe JE, Malicet C, lovanna JL, Jones PM, Persaud SJ, Muller DS, Nupr1 deletion protects against glucose intolerance by increasing beta cell mass, Diabetologia. 56 (2013) 2477–2486. 10.1007/s00125-013-3006-x. [DOI] [PubMed] [Google Scholar]
- [49].Barbosa-Sampaio HC, Drynda R, Liu B, Rodriguez De Ledesma AM, Malicet C, lovanna JL, Jones PM, Muller DS, Persaud SJ, Reduced nuclear protein 1 expression improves insulin sensitivity and protects against diet-induced glucose intolerance through up-regulation of heat shock protein 70, Biochim. Biophys. Acta 1852 (2015) 962–969. 10.1016/j.bbadis.2015.01.013. [DOI] [PubMed] [Google Scholar]
- [50].Rorsman P, Ashcroft FM, Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men, Physiol Rev. 98 (2018) 117–214. 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ganic E, Singh T, Luan C, Fadista J, Johansson JK, Cyphert HA, Bennet H, Storm P, Prost G, Ahlenius H, Renström E, Stein R, Groop L, Fex M, Artner I, MafA-Controlled Nicotinic Receptor Expression Is Essential for Insulin Secretion and Is Impaired in Patients with Type 2 Diabetes, Cell Rep. 14 (2016) 1991–2002. 10.1016/j.celrep.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang M, Fendler B, Peercy B, Goel P, Bertram R, Sherman A, Satin L, Long lasting synchronization of calcium oscillations by cholinergic stimulation in isolated pancreatic islets, Biophys. J 95 (2008) 4676–4688. 10.1529/biophysj.107.125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kovács I, Pocsai K, Czifra G, Sarkadi L, Szucs G, Nemes Z, Rusznák Z, TASK-3 immunoreactivity shows differential distribution in the human gastrointestinal tract, Virchows Arch. 446 (2005) 402–410. 10.1007/s00428-005-1205-7. [DOI] [PubMed] [Google Scholar]
- [54].Yoshida K, Shi S, Ukai-Tadenuma M, Fujishima H, Ohno R-I, Ueda HR, Leak potassium channels regulate sleep duration, Proc. Natl. Acad. Sci. U.S.A 115 (2018) E9459–E9468. 10.1073/pnas.1806486115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dadi PK, Vierra NC, Jacobson DA, Pancreatic β-cell-specific ablation of TASK-1 channels augments glucose-stimulated calcium entry and insulin secretion, improving glucose tolerance, Endocrinology. 155 (2014) 3757–3768. 10.1210/en.2013-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ohtani M, Ohura K, Oka T, Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic β-cells, Cell. Physiol. Biochem 28 (2011) 355–366. 10.1159/000331752. [DOI] [PubMed] [Google Scholar]
- [57].Iwanir S, Reuveny E, Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels, Pflugers Arch. 456 (2008) 1097–1108. 10.1007/s00424-008-0479-4. [DOI] [PubMed] [Google Scholar]
- [58].O’Neill CM, Lu C, Corbin KL, Sharma PR, Dula SB, Carter JD, Ramadan JW, Xin W, Lee JK, Nunemaker CS, Circulating Levels of IL-1B+IL-6 Cause ER Stress and Dysfunction in Islets From Prediabetic Male Mice, Endocrinology. 154 (2013) 3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang IX, Raghavan M, Satin LS, The Endoplasmic Reticulum and Calcium Homeostasis in Pancreatic Beta Cells, Endocrinology. 161 (2020). 10.1210/endocr/bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Varadi A, Molnar E, Ashcroft SJ, A unique combination of plasma membrane Ca2+-ATPase isoforms is expressed in islets of Langerhans and pancreatic beta-cell lines, Biochem J. 314 ( Pt 2) (1996) 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Beauvois MC, Merezak C, Jonas J-C, Ravier MA, Henquin J-C, Gilon P, Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum, Am. J. Physiol., Cell Physiol 290 (2006) C1503–1511. 10.1152/ajpcell.00400.2005. [DOI] [PubMed] [Google Scholar]
- [62].Ravier MA, Daro D, Roma LP, Jonas J-C, Cheng-Xue R, Schuit FC, Gilon P, Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic β-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3, Diabetes. 60 (2011) 2533–2545. 10.2337/db10-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mencacci NE, Rubio-Agusti I, Zdebik A, Asmus F, Ludtmann MHR, Ryten M, Plagnol V, Hauser A-K, Bandres-Ciga S, Bettencourt C, Forabosco P, Hughes D, Soutar MMP, Peall K, Morris HR, Trabzuni D, Tekman M, Stanescu HC, Kleta R, Carecchio M, Zorzi G, Nardocci N, Garavaglia B, Lohmann E, Weissbach A, Klein C, Hardy J, Pittman AM, Foltynie T, Abramov AY, Gasser T, Bhatia KP, Wood NW, A missense mutation in KCTD17 causes autosomal dominant myoclonus-dystonia, Am. J. Hum. Genet 96 (2015) 938–947. 10.1016/j.ajhg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Giorgi C, Ito K, Lin H-K, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, Rizzuto R, Tacchetti C, Pinton P, Pandolfi PP, PML regulates apoptosis at endoplasmic reticulum by modulating calcium release, Science. 330 (2010) 1247–1251. 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kitamura YI, Kitamura T, Kruse J-P, Raum JC, Stein R, Gu W, Accili D, FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction, Cell Metab. 2 (2005) 153–163. 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [66].Zatyka M, Da Silva Xavier G, Bellomo EA, Leadbeater W, Astuti D, Smith J, Michelangeli F, Rutter GA, Barrett TG, Sarco(endo)plasmic reticulum ATPase is a molecular partner of Wolfram syndrome 1 protein, which negatively regulates its expression, Hum. Mol. Genet 24 (2015) 814–827. 10.1093/hmg/ddu499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Moraru A, Cakan-Akdogan G, Strassburger K, Males M, Mueller S, Jabs M, Muelleder M, Frejno M, Braeckman BP, Raiser M, Teleman AA, THADA Regulates the Organismal Balance between Energy Storage and Heat Production, Dev. Cell 41 (2017) 72–81. e6. 10.1016/j.devcel.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu X, Guo Y, Wang J, Zhu L, Gao L, Dysregulation in the Unfolded Protein Response in the FGR Rat Pancreas, Int J Endocrinol. 2020. (2020) 5759182. 10.1155/2020/5759182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wong N, Morahan G, Stathopoulos M, Proietto J, Andrikopoulos S, A novel mechanism regulating insulin secretion involving Herpud1 in mice, Diabetologia. 56 (2013) 1569–1576. 10.1007/s00125-013-2908-y. [DOI] [PubMed] [Google Scholar]
- [70].Tengholm A, Heilman B, Gylfe E, The endoplasmic reticulum is a glucose-modulated high-affinity sink for Ca2+ in mouse pancreatic beta-cells, J Physiol. 530 (2001) 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Weir GC, Cavelti-Weder C, Bonner-Weir S, Stem Cell Approaches for Diabetes: Towards Beta Cell Replacement, Genome Medicine. (2011). 10.1186/gm277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Melton DA, Using stem cells to study and possibly treat type 1 diabetes, Philos. Trans. R. Soc. Lond., B, Biol. Sci 366 (2011) 2307–2311. 10.1098/rstb.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang Y-P, Thorel F, Boyer DF, Herrera PL, Wright CVE, Context-specific α- to-β-cell reprogramming by forced Pdx1 expression, Genes Dev. 25 (2011) 1680–1685. 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Medina A, Parween S, Ullsten S, Vishnu N, Siu YT, Quach M, Bennet H, Balhuizen A, Åkesson L, Wierup N, Carlsson PO, Ahlgren U, Lernmark Å, Fex M, Early deficits in insulin secretion, beta cell mass and islet blood perfusion precede onset of autoimmune type 1 diabetes in BioBreeding rats, Diabetologia. 61 (2018) 896–905. 10.1007/s00125-017-4512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hayden MR, Patel K, Habibi J, Gupta D, Tekwani SS, Whaley-Connell A, Sowers JR, Attenuation of endocrine-exocrine pancreatic communication in type 2 diabetes: pancreatic extracellular matrix ultrastructural abnormalities, J Cardiometab Syndr. 3 (2008) 234–243. 10.1111/j.1559-4572.2008.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R, Inactivation of specific β cell transcription factors in type 2 diabetes, J. Clin. Invest 123 (2013) 3305–3316. 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang Z, York NW, Nichols CG, Remedi MS, Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy, Cell Metab. 19 (2014) 872–882. 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jahan I, Corbin KL, Bogart AM, Whitticar NB, Waters CD, Schildmeyer C, Vann NW, West HL, Law NC, Wiseman JS, Nunemaker CS, Reducing Glucokinase Activity Restores Endogenous Pulsatility and Enhances Insulin Secretion in Islets From db/db Mice., Endocrinology. 159 (2018) 3747–3760. 10.1210/en.2018-00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Whitticar NB, Nunemaker CS, Reducing Glucokinase Activity to Enhance Insulin Secretion: A Counterintuitive Theory to Preserve Cellular Function and Glucose Homeostasis, Frontiers in Endocrinology. (2020). 10.3389/fendo.2020.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT, Chronic Exposure to Excess Nutrients Left-shifts the Concentration Dependence of Glucose-stimulated Insulin Secretion in Pancreatic beta-Cells., J Biol Chem. 290 (2015) 16191–16201. 10.1074/jbc.M114.620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Qureshi FM, Dejene EA, Corbin KL, Nunemaker CS, Stress-induced dissociations between intracellular calcium signaling and insulin secretion in pancreatic islets, Cell Calcium. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Helman A, Cangelosi AL, Davis JC, Pham Q, Rothman A, Faust AL, Straubhaar JR, Sabatini DM, Melton DA, A Nutrient-Sensing Transition at Birth Triggers Glucose-Responsive Insulin Secretion, Cell Metab. 31 (2020) 1004–1016. e5. 10.1016/j.cmet.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The RNA-seq datasets (raw, processed and results files) together with the experiment annotation are available at the Array Express public repository (http://www.ebi.ac.uk/arrayexpress/) with accession number E-MTAB-2266. Note that postnatal days 1, 5, and 13 in that dataset correspond to days 0, 4, and 12, respectively, in the present study.


