Abstract
Corynebacterium matruchotii has been the subject of numerous dental pathogenesis studies. The purpose of the present study was to resolve concerns about diversity within the reference strains of C. matruchotii through analysis of seven strains procured from the American Type Culture Collection (ATCC). Analysis of whole-cell fatty acid profiles with the library generation software of Microbial ID Inc. revealed that three types of organisms have been deposited in the ATCC as C. matruchotii. These three groups of organisms were also distinguishable by DNA-DNA dot blot hybridization, by sequences of two hypervariable regions of the 16S rRNA gene, and by the pyrrolidonyl arylamidase test. These studies indicate that two C. matruchotii reference strains, ATCC 33449 and ATCC 33822, are members of the recently proposed species, Corynebacterium durum. The colonial morphology and biochemical reactions of the C. durum strains are more diverse than originally reported. Strain ATCC 43833 is unique and represents a novel species. In addition to the type strain, ATCC 14266, true members of the species C. matruchotii include ATCC strains 14265, 33806, and 43832 plus two reference strains, L2 and Richardson 13, which comprise the vast majority of strains used in dental pathogenesis research with this species.
The seminal paper by Gilmour et al. (13) provided an accurate description of Bacterionema matruchotii and clearly distinguished it from members of the genus Leptotrichia and from other organisms with which it had been confused, including Cladothrix matruchotii, Leptothrix buccalis, Leptotrichia buccalis, and Leptotrichia dentium. Based on chemotaxonomic characteristics, Bacterionema matruchotii was assigned to the genus Corynebacterium by Collins et al. in 1982 (5).
When this laboratory evaluated the API Rapid CORYNE identification system (Biomérieux Vitek, Inc., Hazelwood, Mo.), it was discovered that reactions of Corynebacterium matruchotii had been omitted from its database (12). Since the database included most other recognized Corynebacterium species of human origin, we suspected that the developers had encountered some unresolvable discrepancies with representative strains of this species. To explore this possibility, we purchased all strains of C. matruchotii available at the American Type Culture Collection (ATCC). Because a large body of work has been published on the possible role of C. matruchotii in the pathogenesis of dental plaque, caries, and periodontitis, we also included two additional strains of C. matruchotii that were used in many of these studies, namely strain L2 and Richardson's strain 13 (22, 23).
The present study was designed to examine the taxonomic relatedness of commercially available and other reference strains of C. matruchotii. The question was addressed on the basis of whole-cell fatty acid analyses, DNA-DNA dot blot hybridizations, sequencing of two hypervariable regions of the 16S rRNA gene, and biochemical reactions. The results indicate that the seven ATCC strains deposited as C. matruchotii comprise three different taxa, including four true C. matruchotii strains, two strains of the recently proposed species Corynebacterium durum, and one representative of a novel taxon.
MATERIALS AND METHODS
Strains.
Strains analyzed in the present study included seven strains deposited in the ATCC as C. matruchotii: ATCC 14266 (type strain), ATCC 14265, ATCC 33449, ATCC 33806, ATCC 33822, ATCC 43832, and ATCC 43833. C. matruchotii strains L2 and Richardson 13 were generously provided by I. Takazoe, Tokyo, Japan. Three strains, LCDC 81-379, LCDC 86-376, and LCDC 91-086, from the laboratory of K. A. Bernard, were also studied because they are similar to the C. matruchotii reference strains ATCC 33449 and ATCC 33822.
Media and biochemical tests.
The broth culture medium was heart infusion broth (HIB; Difco Laboratories, Detroit, Mich.) supplemented with 0.2% Tween 80 (Difco Laboratories) (HIB-T). For strains that grew poorly in HIB-T broth, a half-strength recipe of HIB-T supplemented with 0.2% yeast extract (Difco Laboratories) and 0.2 μg of hemin/ml (Sigma Chemical Co., St. Louis, Mo.) (0.5 HIB-T/YH) was prepared as recommended by Marion Gilmour (personal communication). Heart infusion agar supplemented with 5% sheep blood (University Hospital Microbiology Media Room) was used for routine subcultures and for overnight cultures that were used to inoculate conventional biochemical tests. Trypticase soy agar supplemented with 5% sheep blood (TSBA; Prepared Media Labs, Tualatin, Oreg.) was used for all morphology studies.
Conventional biochemical tests were done as described by Krech and Hollis (18), including enteric fermentation media with Andrade indicator. Inocula for the Rapid CORYNE strips were harvested from overnight cultures on two to four heart infusion blood agar plates. As previously described by Gavin et al., all strains were tested with the CORYNE strips according to the manufacturer's instructions (12). For confirmation of 24-h results, the carbohydrate reactions in the strip also were read after incubation for 48 h. API profile numbers were referenced to the version 2 database.
GLC.
Whole-cell fatty acid analyses were performed as previously described (7). The organisms were grown on TSBA. To obtain sufficient biomass of early-stationary-phase cells, one to three plates were inoculated and incubated at 35°C in an aerobic atmosphere with 5% CO2 for up to 3 days, depending on each strain's growth rate. Whole-cell fatty acid composition was determined by gas-liquid chromatography (GLC) with the Microbial ID, Inc. (MIDI) system, and data were analyzed with the library generation software of MIDI, which is a program that provides two-dimensional cluster plots as well as dendrograms based on cluster analysis. The two-dimensional plots were based on principal component analysis. Principal Component 1 is the component responsible for the greatest degree of variability among the samples tested and is represented on the horizontal axis. Principal Component 2 is responsible for the second greatest degree of variability and is displayed on the vertical axis. The scale for both axes is the Euclidean distance.
DNA-DNA hybridization.
Chromosomal DNA was prepared from cells that were incubated by shaking at 37°C for 48 h in 200 mL of HIB-T or the 0.5 HIB-T/YH broth. The DNA extraction procedure was described previously (6). The DNA-DNA hybridization method, using the NEBlot Phototope kit (New England Biolabs, Inc., Beverly, Mass.) and the PolarPlex Chemiluminescent Blotting kit (New England Biolabs, Inc.), was performed as described by Springer et al. (20) by using established conditions that allowed 26 to 27% mismatch for DNA with G+C content of 55 to 58 mol%. So that target DNAs immobilized on the membrane could be reused for subsequent DNA-DNA hybridizations, hybridized probe DNA was removed according to the manufacturer's instructions.
16S rRNA gene sequencing.
In order to select hypervariable regions likely to be unique for each taxon, the 16S rRNA gene sequences in nine Corynebacterium species were compared using the Baylor College of Medicine search launcher ClustalW (version 1.6) alignment method. Two regions of over 40 bp in length were chosen and termed hypervariable region 1, corresponding to Escherichia coli 16S rRNA positions 177 to 218, and hypervariable region 2, corresponding to positions 988 to 1034.
Two primer sets were designed for the amplification of hypervariable region 1 and hypervariable region 2: Primer 1 (5′GGTGAGTAACACGTGGGTGA3′, corresponding to E. coli 16S rRNA positions 103 to 122); Primer 1R (5′ATTACCCCACCAACAAGCTG3′, corresponding to positions 255 to 236); Primer 2 (5′CCGCAAGGCTAAAACTCAAA3′, corresponding to positions 887 to 906); and Primer 2R (5′CCAACATCTCACGACACGAG3′, corresponding to positions 1078 to 1059).
The GeneAmp PCR Core Reagents kit (Perkin-Elmer Corporation, Foster City, Calif.) was used according to the guidelines provided by the manufacturer, allowing for a MgCl2 concentration of 1.5 mM. The Thermal Cycler 480 (Perkin-Elmer Cetus Instruments, Foster City, Calif.) was used for temperature cycling. The initial template melting step (95°C, 2 min) was followed by 35 cycles of melting (94°C, 1 min), annealing (55°C, 1 min for hypervariable region 1; and 50°C, 1 min for hypervariable region 2), and extending (72°C, 2 min). The final extension time was increased to 8 min, and the resulting products were stored at 4°C.
The sequencing of the entire 16S rRNA gene of strain ATCC 43833 was done as described by Kattar et al. (16).
After amplification, mineral oil was removed by the addition of chloroform (J.T. Baker, Phillipsburg, N.J.). Microcon-50 microconcentrators (Amicon, Inc., Beverly, Mass.) were used according to the manufacturer's instructions to remove excess primers and deoxynucleoside triphosphates from the PCR-generated amplicons.
The ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer Corporation) was used for sequencing reactions, according to the manufacturer's instructions. MicroSpin G-50 columns (Pharmacia Biotech, Piscataway, N.J.) were used to remove unincorporated dye-labeled dideoxynucleotide terminators.
Depending upon their availability for service, automated sequencing was carried out by the University of Washington Sequencing Facility of the Department of Biochemistry or the DNA Core Facility of the Department of Pharmacology using the ABI Prism 377 and the ABI Prism 373 DNA sequencers, respectively.
Nucleotide sequence accession number.
The full sequence of the 16S rRNA gene of strain ATCC 43833 has been deposited in GenBank with accession number AF262996.
RESULTS
Whole-cell fatty acid analyses.
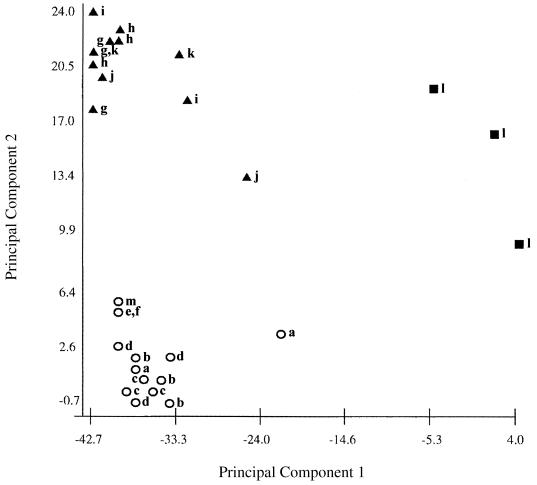
Fatty acid profiles of all strains were analyzed with the library generation software of MIDI, resulting in the two-dimensional plot shown in Fig. 1. The computerized cluster analysis demonstrated three defined clusters, which were assigned the designations of group A (ATCC 14266T, ATCC 14265, ATCC 33806, ATCC 43832, L2, and Richardson 13), group B (ATCC 33449, ATCC 33822, LCDC 81-379, LCDC 86-376, and LCDC 91-086), and group C (ATCC 43833). A dental clinical isolate of C. matruchotii is included in the figure. The major fatty acid peaks of the group A (true C. matruchotii) strains and the group B (true C. durum) strains were palmitic, oleic, and stearic acid (Table 1). The remaining fatty acids detected by the MIDI system (not listed) are believed to be the products of mycolic acid degradation, which occurs at the high temperature in the system's injection port (11).
FIG. 1.
Two-dimensional plot generated by principal component analysis of fatty acid profiles showing the distribution of strains that are listed in Table 1. Circles, group A C. matruchotii; triangles, group B C. durum; squares, group C strain ATCC 43833. Data from repeat analyses are included. Strains: a, ATCC 14266; b, ATCC 14265; c, ATCC 33806; d, ATCC 43832; e, L2; f, Richardson's 13; g, ATCC 33449; h, ATCC 33822; i, LCDC 81-379; j, LCDC 86-376; k, LCDC 91-086; l, ATCC 43833; m, a dental isolate of C. matruchotii. Numbers on the axes indicate the Euclidean distance.
TABLE 1.
Major cellular fatty acids
| GLC groupa | Speciesb | Fatty acid content [mean % (range)]
|
||
|---|---|---|---|---|
| 16:0, palmitic | 18:1ω9c, oleic | 18:0, stearic | ||
| Ac | C. matruchotii | 41.3 (36.0–46.0) | 33.2 (27.5–38.6) | 9.5 (7.4–12.5) |
| Bd | C. durum | 32.1 (27.7–37.4) | 49.2 (45.8–55.2) | 6.6 (4.4–9.0) |
Groups were based on cluster analysis by the MIDI system. See Fig. 1.
Final identification from this study.
Strains: ATCC 14266T, ATCC 14265, ATCC 33806, ATCC 43832, L2, and Richardson 13.
Strains: ATCC 33449, ATCC 33822, LCDC 81-379, LCDC 86-376, and LCDC 91-086.
In the unique strain, ATCC 43833, the major cellular fatty acid (39%) was one of two compounds that the MIDI system cannot distinguish, either 15:0 ISO 2OH/16:1ω7t or 16:1ω7t/15i2OH. Other major peaks were 16:0 (27%), 18:1ω9c (20%), and 16:1ω5c (6%).
DNA-DNA hybridization.
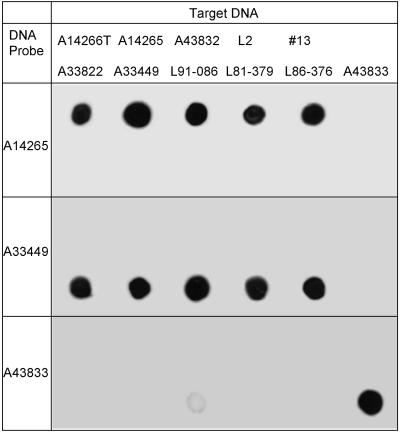
Representative lumigraphs of DNA dot blots for 11 of the 12 strains in this study are shown in Fig. 2. Because the DNA yield was low from strain ATCC 33806, it was excluded from the dot blot study. Whole-cell DNA probes for hybridization were selected on the basis of GLC groupings. DNA from strains in each GLC group hybridized only with the DNA probes from strains in their respective group. DNA from the single group C strain (ATCC 43833) hybridized only with autologous DNA. The DNA dot blot results were in complete agreement with the groupings indicated by the MIDI cluster analysis from cellular fatty acid profiles.
FIG. 2.
DNA-DNA homology studies with solid-phase dot blot hybridizations. Probe and target DNAs are indicated.
16S rRNA sequencing.
Sequencing of the two selected hypervariable regions confirmed the GLC and DNA group divisions (Fig. 3). The sequences from GLC group A strains correlated well with the published sequence of the C. matruchotii type strain. For four of the five strains in group B, the sequences of the two variable regions were almost identical to that published for C. durum (19). The sequence of variable region 1 in strain ATCC 33822 differed from that of C. durum by eight bases. The single strain in group C (ATCC 43833) has a unique sequence differing from any sequences accessible by BLAST searches of all nonredundant GenBank, EMBL (European Bioinformatics Institute), and DDBJ (DNA Data Bank of Japan) sequences.
FIG. 3.
Alignment of sequences of two variable regions in the 16S rRNA gene. GenBank sequences of C. matruchotii and C. durum were used as references and compared with those found in this study. Bold bases for C. durum indicate differences from the C. matruchotii sequence. The first and last nucleotides of regions 1 and 2 correspond to E. coli 16S rRNA positions 177 to 218 and 988 to 1034, respectively.
Morphology.
When grown in air or a CO2-enriched atmosphere for 48 h at 37°C on TSBA, all strains in this study produced colonies about 1 mm in diameter. Four of the six true C. matruchotii colonies were white, cream, or gray with a smooth matte surface and creamy texture when touched with a loop. Colonies of strains ATCC 33806 and Richardson's 13 had a crinkled surface and lifted as a single dry colony when touched by a wire loop. When grown in the presence of CO2, these two strains produced distinct pits in the agar.
The C. durum strains (ATCC 33449, ATCC 33822, LCDC 81-379, LCDC 86-376, and LCDC 91-086) produced tiny gray-white or cream-colored colonies with a dry or matte surface. When C. durum strains were cultured in an aerobic atmosphere, all of the colonies had a gummy texture and four of the five strains caused pitting of the agar medium. When grown in a CO2-enriched atmosphere, the C. durum colonies had a creamy texture on their surface while the remainder of each colony was gummy and adherent to the medium. Only strain LCDC 86-376 produced pits when grown in the presence of CO2. Colonies of strain ATCC 43833 were pinpoint to 0.1 mm in diameter, gray-white, and had a smooth, nonadherent texture.
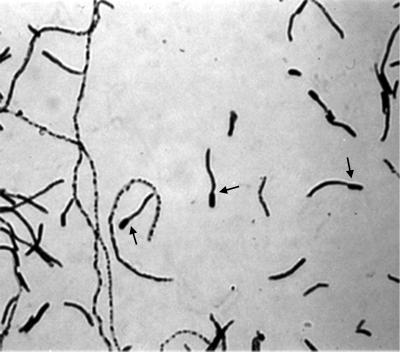
During the first 6 months of this study, the three taxa had distinct Gram stain morphologies that showed little variance with time and growth media. The six true C. matruchotii strains demonstrated the whip handle that is typical of this species (Fig. 4). The five C. durum strains produced pleomorphic coryneform rods with granules or beads but not filaments. In an aerobic atmosphere the novel strain, ATCC 43833, produced very short but relatively broad gram-positive rods in diphtheroid-like clusters. In the presence of CO2, this strain produced pleomorphic rods in a variety of sizes. Following multiple serial subcultures during a 2-year period, the C. matruchotii strains lost their characteristic whip handle cellular morphology, with the exception of rare filaments.
FIG. 4.
The Gram stain morphology of C. matruchotii after growth on blood agar in a CO2-enriched atmosphere at 37°C for 48 h. Arrows indicate whip handle morphology.
All the strains in this study grew better in 0.5 HIB-T/YH than in HIB or HIB-T. The greatest growth enhancement in 0.5 HIB-T/YH broth occurred with the true C. matruchotii strains.
Biochemical tests.
The results from conventional biochemical tests and the numerical profiles of the reactions from the Rapid CORYNE system are shown in Table 2. The production of pyrrolidonyl arylamidase was the only trait that consistently distinguished C. matruchotii strains from C. durum strains. The atypical strain, ATCC 43833, was unique in its failure to reduce nitrate and its production of alkaline phosphatase. The conventional esculin test was negative for all strains, whereas it was positive in the CORYNE strip for 9 of the 12 strains. In contrast, the CORYNE strip did not detect urease activity in two of the four C. durum strains that were urease positive in the conventional test. Two of the five C. durum isolates yielded both positive and negative reactions when tested three times in conventional mannitol broth. With the exception of one isolate, repeat testing of C. durum isolates for production of acid in galactose also yielded contradictory results.
TABLE 2.
| Final identification of strain | API reactionc
|
API profile | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nit | Urea | Esc | Glu | Malt | Suc | Manl | Xyl | Gal | ||
| C. matruchotii | ||||||||||
| ATCC 14266T | + | − | − | + | + | + | − | − | − | 7050325 |
| ATCC 14265 | + | − | − | + | + | + | − | − | − | 7050325 |
| ATCC 33806 | + | − | − | + | + | + | − | − | − | 7000325 |
| ATCC 43832 | + | − | − | + | + | + | − | − | − | 7050325 |
| L-2 | + | − | − | + | + | + | − | − | − | 7010325 |
| Richardson's 13 | + | − | − | + | + | + | − | − | − | 7000325 |
| C. durum | ||||||||||
| ATCC 33449 | + | + | − | + | + | + | − | − | vd | 3040325 |
| ATCC 33822 | + | − | − | + | + | + | v | − | v | 3040335 |
| LCDC 81-379 | + | + | − | + | + | + | + | − | v | 3441335 |
| LCDC 86-376 | + | + | − | + | + | + | v | − | + | 3440335 |
| LCDC 91-086 | + | + | − | + | + | + | + | − | v | 3441335 |
| Novel strain | ||||||||||
| ATCC 43833 | − | − | − | + | + | + | − | − | − | 2140325 |
API reactions were read after 48 h and conventional reactions after 7 days of incubation.
The LCDC isolates originally had been identified as closely resembling C. matruchotii.
The Rapid CORYNE strips contain reactive ingredients in ordered groups of three as follows: nitrate reduction (NIT), pyrazinamidase, and pyrrolidonyl arylamidase; alkaline phosphatase, β-glucuronidase, and β-galactosidase; α-glucosidase, N-acetyl-β-glucosaminidase, and esculin (ESC); urease, gelatin hydrolysis, and fermentation control; glucose, ribose, and xylose; mannitol, maltose, and lactose; and sucrose and glycogen (catalase was the 21st test).
v, repeat tests gave inconsistent results.
DISCUSSION
Evidence from biochemical reactions, fatty acid analysis, DNA hybridization, and 16S rRNA hypervariable region sequencing shows that the seven strains deposited in the ATCC as C. matruchotii actually are diverse organisms including four true C. matruchotii strains, two C. durum strains, and a single unique strain that probably represents a novel taxon. The group corresponding to the C. matruchotii type strain was clearly and correctly described by Marion Gilmour and colleagues (13).
Gilmour and Turner described C. matruchotii as a pleomorphic organism whose variability in colonial morphology is a consequence of mode of reproduction, culture age, and cultivation conditions (14). They described a rough-to-intermediate-to-smooth morphological variation. When our biochemical results are examined, it is apparent that the true C. matruchotii strains have the biochemical reactions described in Bergey's Manual of Systematic Bacteriology (15). The API system, which does not include C. matruchotii, provides no identification for many of the strains in this study. Strains ATCC 33806, ATCC 43833, and Richardson's 13 are assigned to the genus Corynebacterium, and strain ATCC 33822 has a “good identification” as Listeria grayi.
Biochemical tests do not readily distinguish C. durum from C. matruchotii. The only API CORYNE test in which they consistently differ is the pyrrolidonyl arylamidase reaction, which occurred with the six C. matruchotii strains but with none of the five C. durum strains in this study. This difference is consistent with two earlier studies that tested a total of 63 C. durum isolates, none of which had pyrrolidonyl arylamidase activity (19, 24). The C. durum species is biochemically diverse. Within isolates from 58 cultures of healthy throats, von Graevenitz et al. found 8 API CORYNE profile numbers, none of which is identical to the four profile numbers in this study (24).
Riegel et al. noted that all five of their C. durum isolates produced acid from mannitol and galactose, which helped to distinguish them from C. matruchotii (19). However, when we tested our C. durum isolates on Hugh Leifson's oxidation-fermentation medium as used by Reigel, no acid was detected in mannitol or galactose. Using the in-house peptone water fermentation broth with Andrade indicator that the Centers for Disease Control and Prevention recommends for testing coryneforms (18), we did repeat tests with mannitol and galactose. Two of the C. durum strains were consistently positive with mannitol, one was negative, and results with two strains were not reproducible. Four of the C. durum strains also had inconsistent results in repeat galactose testing (Table 2). An additional study of these strains' reactions on both mannitol and galactose in peptone water broth from two providers revealed differences in the frequency of positive results (data not shown). The differences in urease activity and esculin hydrolysis observed between the Rapid CORYNE system and conventional tests might be attributed to the different basal media in these two systems.
It was surprising to find that our strains of C. durum did not fit the colonial morphology described by Riegel et al., who selected the species name to reflect its strong adherence when grown in an aerobic atmosphere on TSBA (19). When Riegel's strains were cultured on TSBA in the presence of CO2, they were not adherent. Our C. durum strains on TSBA responded to the atmosphere in the reverse manner, with gummy adherence seen only in the presence of CO2. The differences between our strains and Riegel's strains of C. durum might be attributed to the fact that our strains originally were deposited in the ATCC as C. matruchotii or were reported to closely resemble C. matruchotii, which might represent a unique subset of C. durum strains. Furthermore, the original specimens for our strains and the Riegel strains were quite different. In contrast to the five C. durum isolates described by Riegel et al., only one of the C. durum strains in the present study (LCDC 81-379) had been recovered from sputum. ATCC strains 33449 and 33822 were from a gingival margin and subgingival plaque, respectively, and LCDC strains 91-086 and 86-376 were isolated from blood and a foot abscess, respectively.
Within the true C. matruchotii strains, the sequences of hypervariable regions 1 and 2 plus flanking regions differed at only one position, in which the guanine of the type strain was replaced by an adenine in the other five strains. All but one of the C. durum strains also exhibited highly conserved sequences that varied in only two contiguous bases that were either two cytosines or two thymidines. However, the sequence of hypervariable region 1 in C. durum ATCC 33822 differed from that of the type strain by eight bases. Because this strain was consistent with the species C. durum in DNA dot blots, fatty acid analyses, and biochemical reactions, it seems likely that this is the correct identification. Intraspecies variation in the sequences of 16S rRNA has been reviewed by Clayton et al., who concluded that some of the sequence heterogeneity observed within species may be due to interoperon differences (4). We have notified the ATCC of the corrected identifications of C. durum strains ATCC 33449 and ATCC 33822.
C. matruchotii has been used by a number of researchers as a microbiologic model for intracellular calcification (1, 3, 8, 9, 21, 25). The organism has provided valuable information regarding calcification of bioprostheses (17), dental plaque (10), and the principles of membrane-mediated proteolipid-dependent calcification in vertebrate systems (2). It is important to note that the diversity found in this study does not affect the conclusions of pathogenesis studies with C. matruchotii. The vast majority of earlier studies have relied on results from one or more of four strains, namely ATCC 14265, ATCC 14266, L2, and Richardson 13, all of which we have confirmed to be true C. matruchotii strains.
ACKNOWLEDGMENT
We thank Marion N. Gilmour for providing reprints and her invaluable advice for acquiring reference strains and optimizing the growth of C. matruchotii.
REFERENCES
- 1.Boyan B D, Boskey A L. Co-isolation of proteolipids and calcium-phospholipid-phospate complexes. Calcif Tissue Int. 1984;36:214–218. doi: 10.1007/BF02405320. [DOI] [PubMed] [Google Scholar]
- 2.Boyan B D, Landis W J, Knight J, Dereszewski G, Zeagler J. Microbial hydroxyapatite formation as a model of proteolipid-dependent membrane-mediated calcification. Scanning Electron Microsc. 1984;4:1793–1800. [PubMed] [Google Scholar]
- 3.Boyan-Salyers B D, Boskey A L. Relationship between proteolipids and calcium-phosphate complexes in Bacterionema matruchotii calcification. Calcif Tissue Int. 1980;30:167–174. doi: 10.1007/BF02408622. [DOI] [PubMed] [Google Scholar]
- 4.Clayton R A, Sutton G, Hinkle P S, Jr, Bult C, Fields C. Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa. Int J Syst Bacteriol. 1995;45:595–599. doi: 10.1099/00207713-45-3-595. [DOI] [PubMed] [Google Scholar]
- 5.Collins M D, Goodfellow M, Minnikin D E. A survey of the structures of mycolic acids of the genus Corynebacterium and possibly related taxa. J Gen Microbiol. 1982;128:129–149. doi: 10.1099/00221287-128-1-129. [DOI] [PubMed] [Google Scholar]
- 6.Coyle M B, Groman N B, Russell J Q, Harnisch J P, Rabin M, Holmes K K. The molecular epidemiology of three biotypes of Corynebacterium diphtheriae in the Seattle outbreak of 1972–1982. J Infect Dis. 1989;159:670–679. doi: 10.1093/infdis/159.4.670. [DOI] [PubMed] [Google Scholar]
- 7.Coyle M B, Leonard R B, Nowowiejski D J, Malekniazi A, Finn D J. Evidence of multiple taxa within commercially available reference strains of Corynebacterium xerosis. J Clin Microbiol. 1993;31:1788–1793. doi: 10.1128/jcm.31.7.1788-1793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damen J J M, Ten Cate J M. Silica-induced precipitation of calcium-phosphate in the presence of inhibitors of hydroxyapatite formation. J Dent Res. 1992;71:453–457. doi: 10.1177/00220345920710030601. [DOI] [PubMed] [Google Scholar]
- 9.Ennever J. Intracellular calcification by oral filamentous microorganisms. J Periodontol. 1960;31:304. [Google Scholar]
- 10.Ennever J, Vogel J, Rider L, Paoloski S. Proteolipid and calculus matrix calcification in vitro. J Dent Res. 1977;56:140–142. doi: 10.1177/00220345770560020701. [DOI] [PubMed] [Google Scholar]
- 11.Funke G, Lawson P A, Collins M D. Heterogeneity within human-derived Centers for Disease Control and Prevention (CDC) coryneform group ANF-1-like bacteria and description of Corynebacterium auris sp. nov. Int J Syst Bacteriol. 1995;45:735–739. doi: 10.1099/00207713-45-4-735. [DOI] [PubMed] [Google Scholar]
- 12.Gavin S E, Leonard R B, Briselden A M, Coyle M B. Evaluation of the Rapid CORYNE identification system for Corynebacterium species and other coryneforms. J Clin Microbiol. 1992;30:1692–1695. doi: 10.1128/jcm.30.7.1692-1695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmour M N, Howell A, Jr, Bibby B G. The classification of organisms termed Leptotrichia (Leptothrix) buccalis. Bacteriol Rev. 1961;25:131–141. doi: 10.1128/br.25.2.131-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmour M N, Turner P A. Culture purity assessments and morphological dissociation in the pleomorphic microorganisms Bacterionema matruchotii. Appl Microbiol. 1974;27:1134–1141. doi: 10.1128/am.27.6.1134-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones D, Collins M D. Irregular, nonsporing gram-positive rods. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins Co.; 1986. pp. 1261–1266. [Google Scholar]
- 16.Kattar M M, Chavez J F, Limaye A P, Rassoulian-Barrett S L, Yarfitz S L, Carlson L C, Houze Y, Swanzy S, Wood B L, Cookson B T. Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J Clin Microbiol. 2000;38:789–794. doi: 10.1128/jcm.38.2.789-794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight J P, Torell J A, Hunter R E. Bacterial associated porcine heterograft heart valve calcification. Am J Cardiol. 1984;53:370–372. doi: 10.1016/0002-9149(84)90473-9. [DOI] [PubMed] [Google Scholar]
- 18.Krech T, Hollis D G. Corynebacterium and related organisms. In: Balows A, Hausler W L Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 277–286. [Google Scholar]
- 19.Riegel P, Heller R, Prevost G, Jehl F, Monteil H. Corynebacterium durum sp. nov. from human clinical specimens. Int J Syst Bacteriol. 1997;47:1107–1111. doi: 10.1099/00207713-47-4-1107. [DOI] [PubMed] [Google Scholar]
- 20.Springer B, Wu W K, Bodmer T, Haase G, Pfyffer G E, Kroppenstedt R M, Schroder K-H, Emler S, Kilburn J O, Kirschner P, Telenti A, Coyle M B, Botter E C. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J Clin Microbiol. 1996;34:1100–1107. doi: 10.1128/jcm.34.5.1100-1107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain L D, Boyan B D. Ion-translocating properties of calcifiable proteolipids. J Dent Res. 1988;67:526–530. doi: 10.1177/00220345880670030101. [DOI] [PubMed] [Google Scholar]
- 22.Takazoe I, Itoyama T. Analytical electron microscopy of Bacterionema matruchotii calcification. J Dent Res. 1980;59:1090–1094. doi: 10.1177/00220345800590062101. [DOI] [PubMed] [Google Scholar]
- 23.Takazoe I, Nakamura T. The relation between metachromatic granules and intracellular calcification of Bacterionema matruchotii. Bull Tokyo Dent Coll. 1965;6:29–42. [PubMed] [Google Scholar]
- 24.von Graevenitz A, Punter-Streit V, Riegel P, Funke G. Coryneform bacteria in throat cultures of healthy individuals. J Clin Microbiol. 1998;36:2087–2088. doi: 10.1128/jcm.36.7.2087-2088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilhelmus K R, Robinson N M, Jones D B. Bacterionema matruchotii ocular infections. Am J Ophthalmol. 1979;87:143–147. doi: 10.1016/0002-9394(79)90132-6. [DOI] [PubMed] [Google Scholar]