Abstract
The persistence of SARS-CoV-2 or its RNA on surfaces, points, or wastewaters may increase the risk of transmission of this virus. Therefore, we conducted this review to discuss the places and surfaces with the highest potential for infection and spread of the SARS-CoV-2 virus. Several common and public areas, hospitals, elevators, public transport, local markets, and surfaces such as public toilets, door handles, untreated and treated wastewaters, wastewater plants, and public washrooms are also considered major points for spreading of SARS-CoV-2. Highly contaminated surfaces or places often have materials or contain items made of materials on which the SARS-CoV-2 virus can persist (e.g., metal, wood, and plastic). For example, SARS-CoV-2 can exist up to 4 days on doorknobs made by those materials. For public places such as public transports, elevators, and local markets, crowding and enclosed spaces are major source for transmission. Several measures such as using copper alloy surfaces instead of metal surfaces, disinfectants, and suitable personal protective equipment have been suggested. Our research could be the basis to help develop studies on the existence and transmissibility of SARS-CoV-2 as well as its RNA to take measures to prevent and limit the harmful effects of COVID-19 pandemic.
Keywords: SARS-CoV-2, Covid-19, Contaminated surfaces and points, High-density viruses, Door handles, Public toilets, Hospital, Industrial zone, Public transports, Public elevators, Traditional or local markets, Public washrooms, Wastewater
1. Introduction
The World Health Organization (WHO) has declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a pandemic, and the disease has been named as COVID-19. COVID-19 has been considered a worldwide public health crisis since the early days of 2020 at a local wet market in Wuhan City, Hubei, China [1]. All the cases were reported with similar symptoms: fever, dyspnea, dry cough, and bilateral lung infiltration on radiographs [2]. This virus then quickly spread throughout mainland China and the rest of the world, and it infected millions and caused thousands of deaths globally. Until now, SARS-CoV-2 continues to spread, and more variants have been confirmed.
Due to the spike-like structure of the virus, it is easy for SARS-CoV-2 to mutate [3]. As of today, the WHO has classified the SARS-CoV-2 variants into variants of concern (VOCs) and variants of interest (VOIs) [4]. The VOIs consists of Eta (B.1.525), Iota (B.1.526), Kappa (B.1.617.1) and Lambda (C.37). In contrast, the VOCs consist of Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and most recently, Omicron (B.1.1.529) [5]. Both VOCs and VOIs have been proven to increase transmissibility, mortality and reduce the effectiveness of vaccines, as well as therapies [6].
The SARS-CoV-2 is believed to majorly spread from person to person with direct contact [7]. As reported by the United States Center for Disease and Control (CDC) [8], there are three primary ways of how SARS-CoV-2 spreads among people, which are (1) a person can be infected with SARS-CoV-2 when they breathe the nearby air of the infected person who is exhaling the small particles and droplets that have the viruses. (2) Infections can also occur from tiny droplets of the virus on the mouth, eyes, noses, primarily through splashes and spray like a sneeze or a cough, and (3) a person can also be infected when touching their eyes, noses, mouths with hands that contain the virus [2]. Furthermore, the COVID-19 can also spread from points, surfaces, and wastewater, which is a suitable environment for the half-life of the virus to survive and develop.
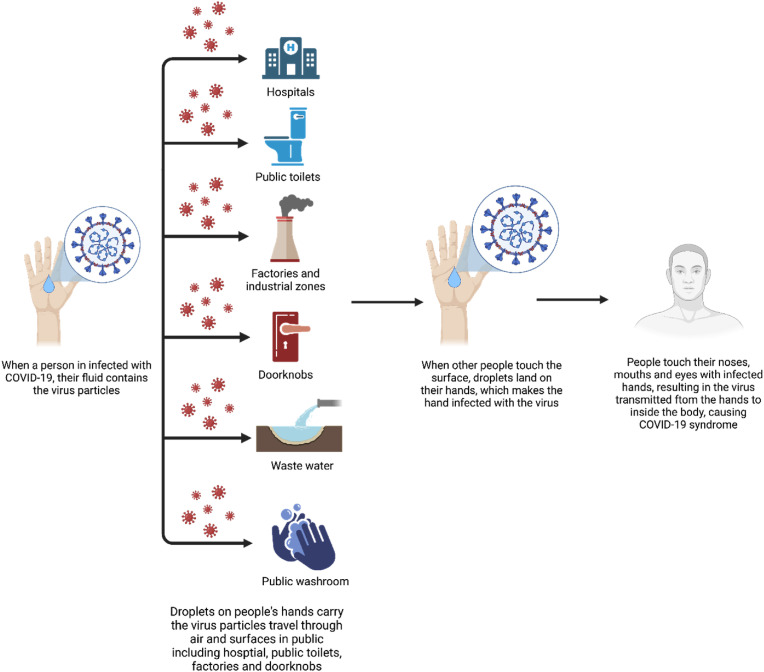
Despite the known spread, COVID-19 still poses threats as their particles tend to highly accumulate in public places [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. As we all know, a point is a spot where the virus can reside and spread, while a surface is a whole area where the virus stays. These places include door handles in public areas, public bathrooms, hospitals, medical centers, factories and industrial zones, public transports, public elevators, and local markets. Furthermore, the waste and wastewater services are potential ramifications of the COVID-19, thus monitoring viral RNA in wastewater may be useful to evaluate and control the prevalence of this disease [21]. Understanding how this virus spreads in these areas is important in managing and preventing the pandemic. In this review, we highlight recent studies that discuss the highest contaminated places and surfaces of the SARS-CoV-2 infections and spreading that should be taken care for preventing COVID-19. By this research, we hope health officials pay more attention to these routes of infections and diminish the exposure rate to lower COVID-19 cases in near future.
2. High-risk-contaminated points and surfaces for SARS-CoV-2
2.1. Door handles
It is believed that the virus only passed on directly by droplets through coughing or sneezing from person to person, but evidence has shown the virus may pass by inanimate objects due to its long half-life even after leaving the patient's body. Evidence showed that they could be a chain passed on by inanimate objects as the viruses can survive for a long period of time after leaving the patients' bodies. These viruses are delivered either by direct exposure to patients' droplets or by their indirect touch, carrying body fluids with billions of viruses to the surface. One of the most frequently touched objects universally used by everyone in households and public locations is door handles (or doorknobs). They can be highly potential fomites. Research showed that the virus could survive up to 5 days on metal, up to 4 days on wood, and up to 9 days on plastic [22]. Metal, wood, and plastic are common of virus spreading. These materials are commonly used to make a door handle or doorknob. In buildings, drawers and doorknobs have the most viruses [9]. These are contaminated surfaces that can spread viruses easily. In hospitals, surfaces, including door handles, have also been reported due to the presence of SARS-CoV-2 [10]. In addition, another research that scanned patients' rooms for the existence of SARS-CoV-2 on personal belongings, as well as living spaces, have found positive results for SARS-CoV-2 on personal belongings as well as living spaces such as door handles of toilets [23,24], door handles of patients' rooms can indicate a possibility of viruses on door handles that have been touched or used by COVID-19 patients. More specifically, another study suggested that the percentage of SARS-CoV-2 positive samples were taken from doorknobs is at the rate of 8.3% - the fourth-highest rate of contamination within a general COVID-19 ward in a hospital in Wuhan, China [25]. These studies might take samples where door handles were used at high frequency. Still, in public places where disinfection is not as frequently done, only one chance that viruses on a door handle can lead to the further uncontrollable spread of the disease. Research has shown that protecting surfaces such as door handles is a way to help seniors reduce their exposure to SARS-CoV-2 [26]. Various advice for disinfecting and cleaning surfaces have also been issued to reduce the risk of spreading viruses in hospitals [[27], [28], [29]].
2.2. Public toilets
Public restrooms represent a significant hazard in the direct human transmission of coronavirus. The route of transmission of SARS-CoV-2 is via three ways: inhalation of feces and/or aerosol urine from an individual who contains SARS-CoV-2; airborne respiratory transmission face-to-face our shortly after use among users; or from the transmission of fomite through sites of repeated contact such as doorknobs, washbasin faucets, ball balls or toilet roll dispensers [13]. A current systematic review attributed the coronavirus prevalence in hospitals and recorded 24% of air samples collected from toilets were tested positive, with mean viral RNA concentrations per m3. Air is higher than in any other areas that were sampled [30]. Destitute adherence to hand cleanliness encourages the survival and determination of hand infections for transmission to self or other planes [31]. Indeed, on the off chance that hands are wet washed, they may not essentially be appropriately dried, and damp hands can choose microscopic organisms from another surface they touch [32]. SARS-CoV-2 can survive on indoor natural textures, for example, plastic, glass, stainless steel, ceramics, elastic gloves, wood, and surgical veils [14,15]. Infection endures many hours later in feces and 3–4 days in pee [14]. Such studies suggest that the surface life of virus is sufficient for continued transmission. Dangers from toilet surfaces would contact surface sprinkles on can bowls, latrine bowls, or other adjacent surfaces.
While door handles are only objects that anybody within the population can touch. Still, a toilet is a closed space within which anyone can enter and where most of the excretion related to virus shedding happens. There are only a limited number of activities that one can do in a toilet. This means that toilets are exposed to many people. Toilet papers, toilet covers, toilet seats, washing basins, and of course, door handles of toilets are highly considerable [33]. The traces of viruses detected in were a study of patients’ ooms, indicating SARS-CoV-2 positive swabs from toilets’ door handles, toilets’ surfaces and sink [34]. The statistical significance of another study that sampled surfaces in hospitals suggested that toilet-related covers have an even higher rate of contamination, and samples were taken from the toilet ceiling-exhaust grille. Drainage stacks indicated a certain amount of condensed viral loads from aerosols that might be from toilet flushing. The contaminated surfaces suggested that these viruses may originate from the feces of patients as stool samples of patients (with or without diarrheas) are SARS-CoV-2 positive. In addition, materials in toilets are often made to be smooth for easy cleaning, but this increases the rate of surface-touch transferring [35]. Outside the bathrooms of hospitals, a case of transmission between 9 people in a public restroom was reported [36]. Compared to open spaces or rooms where the name does not define their activities, toilets are places where people have higher chances virus infections.
2.3. Hospitals or medical centers
At the end of February 2020, over 3,000 health workers were infected [30]. Hospitals have highest infected-patient density and where most samples were taken. Therefore, the dynamics of virus transmission should be closely examined to control the spread of COVID-19, with the unpredictable behaviors of viruses (spreading even at the asymptomatic phase). These people were negative to SARS-CoV-2 that might risk being infected by other silent spreaders through the direct contact or exposure to fomites [37]. Besides isolation rooms [23], other places could also have the possibility of being favorable to the coronavirus. It is easily transferred through treatment operations, especially endotracheal intubation and bronchoscopy [30,38]. Consequently, aerosol-generating procedures for general anesthesia have been reduced to avoid virus propagation [39]. In addition, virus can survive on contaminated surfaces in hospitals that are transferred to health care workers and spread around [40]. Not only is transmission within the same hospital, but middle east respiratory syndrome coronavirus (MERS, similar to SARS-CoV-2) can also be transmitted from hospital to hospital through the movement of cases [41]. This may be the route of transmission of SARS-CoV-2.
A study detected traces of SARS-CoV-2 on the floor in the area where no patients presented and on staffs’ shoes [25]. The study also showed traces of SARS-CoV-2 in doctor's office areas, suggestive warnings for more routine disinfection. For nurses' safety, the presence of viruses was also detected on gloves indicating risk factors for cross-contamination, so even with the aids of these tools, hand washing after contact with patients is still necessary. Doctors or nurses' communication with patients for regular checks or communication with staff members (security guards, receptionists, or janitors) with a silent carrier and other patients is essential for controlling the disease. During the pandemic, the management plan for pregnant women and cancer patients is an important task of the hospital [42,43]. Pregnant women have a high risk of viral infections. Infections during pregnancy are found in China [43]. In some cases, pregnant women with viral infections, fetal failure and premature birth have occurred [44]. The immune system of cancer patients is weakened. Therefore, hospital treatment puts patients at twice the risk of viral infections. Cancer patients infected with SARS-CoV-2 have a worse prognosis [45]. Face masks are recommended to limit the action of droplets, however, scarce evidence is available for the effectiveness of this method. The studies of swabbing samples from hospitals are good evidence of the contagiousness of disease. Still, it can go wilder in other public places as regular disinfection routines are not performed.
2.4. Industries and industrial zones
Industries and industrial zones are among the locations with a high risk of SARS-CoV-2 infection in the community. The increased transmission risk is due to a work culture where workers are in close contact [46]. The risk of spread depends on several factors: distance between workers, type of contact, duration of contact, other unique elements, and many. First, workers often have close physical contact during work, break time, changing rooms, etc. Second, the working time is about 8–12 hours per shift. Third, workers can be exposed to infectious diseases from many different sources: respiratory droplets in the air (coughing, sneezing) or through tools and work items in common spaces. Finally, other factors such as worker accommodation, public transport, etc., also increase the risk of SARS-CoV-2 infection to the community. One of the clearest examples of the spread of outbreaks in industries and industrial zones is in food processing and agricultural companies in the US; 8,978 employees have been confirmed with COVID-19, 55 workers were reported dead [8]. There is also the case of meat processing workers in Nebraska, USA, with 5,002 cases out of 26,000 suspected cases (19% attack rate) [47]. Faced with the situation and the risk of contagion, OSHA has advised factories, industrial parks, and workers to take preventive measures while not being vaccinated. Specifically, granting paid time off to employees going for vaccination; instructing infected, unvaccinated workers to contact the source of the disease and have symptoms to stay at home; implementing social distancing in the working area, providing masks and protective equipment for workers; educating and train workers on COVID 19 policies; maintain ventilation system; periodic disinfection and cleaning of working areas; implement retaliation protections and establish an anonymous process for workers to voice their concerns about hazards related to COVID-19.
2.5. Public transports
Public transports are exceedingly confined spaces; they make exceptionally perfect conditions for spreading COVID-19. The taxi, bus, train, subway metro, airplane and many do not have to discuss ventilation as proficient as in toilets or houses, and they are indeed more motile, moving from area to area, cities to cities and seats between travelers are not distant sufficient to preserve security remove. Passengers on public transports have a higher chance of coordinating contact with each other and a longer-term introduction to fomites than any other open place. The motility can advance obscure spreading carrying a noiseless spreader from one end to another, making it difficult to control the infection transmission. Traveling by public transport elevates the risk of COVID-19 disease [16]. Coronavirus infections are associated with flights and speed trains [48]. The relationship between the rate of infusion and the presence of a high-speed train station or an airport has been shown [48]. The foremost self-evident case of this implies transmission is widespread behavior of COVID-19, beginning from Wuhan, China, and presently in over 200 nations.
Studies on the history presentation of patients have recorded cases and cases of imported COVID-19 in nations (Coronavirus disease 2019 situation reports), and individuals who work as taxi drivers have to be detailed and also to be contaminated [49]. A study in China has shown that traveling by public transport (trains, buses) contributed to the spread of COVID-19 [50]. This depends on the frequency of travel and the distance between cities and the epidemic area. In India - a destination for many international tourists, there has been an increase in international cases [51]. Public trains are densely packed and use public rooms, food, and shared facilities, making it easy to spread the virus [52]. According to recent studies, the Diamond Princess has proven to have the ability to dominate transmission type between passengers and even consider asymptomatic infected groups. In addition, many cases in Tokyo in Japan were related to the party on the Yakatabune [53]. Another study had been used to compare the transmission patterns of SARS-CoV-2 and its similar previously pathogenic SARS-CoV-1. It can be spreading early at even an asymptomatic phase. The two viruses' survival and transmission behave the same way. All kinds of evidence suggested that public transport users should be decelerated to control the disease.
2.6. Public elevators
The elevator is one of the confined places with high risks of COVID-19 spreading. Ventilation plays an important role in virus accumulation. The elevator is a common space with a simple ventilation system that leads to unsafe ventilation (especially in hospitals) [17]. People have a high risk of getting viruses in the elevator because of close physical contact. On stainless-steel and plastic surfaces, SARS-CoV-2 was more stable than SARS-CoV-1 [15]. According to a recent study, SARS-CoV-2 can stay on metal surfaces for up to 5 days or 4 on plastic [22]. Both of which are often used on the lift floor panels. Even though there is no distinct correlation between the positive cases with contacting the buttons [54]. However, a high chance of getting the virus infections by touching these kinds of surfaces because of heavy traffic of these places, making us vulnerable to being in contact with virus. In addition, high rates of virus RNA samples were found on the elevator buttons in hospitals [55]. Besides, standing inside such contract space whose zones are around one and a half square meters with another individual isn't sufficient as beads can spread up to more than 2 m when hacking or sniffling. Hand washing is at that point suggested after leaving any lifts. It would be best to maintain a strategic distance from utilizing them.
2.7. Traditional and local markets
The outbreak of COVID-19 is considered to be originated from the Wuhan seafood market in China [56]. Therefore, it was of animal origin. There is a clear distinction between supermarket and street market in how it works. Supermarkets put everything on shacks, where people can easily find the items themselves, and the cashier is the only person customers truly need to communicate with. Otherwise, in the street market, many contiguous stalls are selling: fresh fruits, vegetables, meats, etc., and it's such the hustle and bustle area because of the noise from buyers and sellers. The spread of virus directly from human to human by droplets has been identified [57]. Exposure increases the risk of COVID-19 infection [58]. In addition, markets may circulate animals of unknown origin so that the consumer may be a source of viral infection, especially mammals [59,60]. Moreover, wet, polluted markets are good conditions for virus development [60]. At the beginning of epidemic, research in China showed a much higher incidence of disease originating from the market than human-to-human transmission [19]. A pandemic transmission model was developed to explain the unexpected appearance of the new viral strain in the Huanan (Southern China) Seafood Wholesale Market, where the first four cases of COVID-19 originated because most view emerging viruses gain its antigen for human receptors while circulating in zoonotic reservoirs.
In addition, following its previous example of SARS-CoV-1, bats – one of the local foods of the market were assumed to be the root of disease. A study in the origin of SARS-CoV-2 source posted on Nature [61] proves that the gene sequence of SARS-CoV-2 was different. Still, little information on pneumonia in animals is available, so the animal of origin is left undetected. Another hypothesis suggested an adaptation of the virus from passages through cell lines from previous studies of SARS-CoV-2 and escaped as well as circulated in the population while further adapting. Anyhow, its emergence is not on purpose, and the zoonotic reservoir of the virus is still not detected. In recent times, the return of COVID-19 in Beijing with many cases related to the Xinfadi market is evidence that the market is a favorable environment for virus spreading. Other wet markets than Huanan, China can minimize the transmission of COVID-19 by keeping distances between buyers with buyers, buyers with sellers, and sellers with sellers. At the time of disease's peak, closure can be recommended for the safety of all people.
2.8. Wastewater treatment plants
Sewage plants are also believed to be an important source for transmitting SARS-CoV-2. The formation of particles and air bubbles in wastewater can be the main virus transmission [62]. Aerosol viruses can be transmitted through the wind during wastewater treatment or sewage ponds for transmission in buildings [62]. During the SARS-CoV-1 outbreak, it was found that plumbing problems and inadequate wastewater treatment to remove the virus could cause the virus to spread from sewage plants [62]. During the outbreak of COVID-19, similar evidence was found when conventional wastewater plants could not wholly remove the viral load before discharge. In addition, reuse of wastewater for different purposes can increase the risk of transmission (e.g., water for crop culture) because up to 67% of fecal samples in wastewater are found to be positive with SARS-CoV-2 [62]. Fecal-oral transmission is common in low-income countries due to fecal waste or untreated sewage [62]. Some factors affecting the viability and transmission of SARS-CoV-2 through wastewater include temperature, organic content, solution pH. In lower temperatures (e.g., cold regions or temperate climates), SARS-CoV-2 can persist longer. As the organic content increases, the survival time decreases, for example, 10 days in a lake and 2 days in raw wastewater because antagonistic bacteria can inactivate the virus by activating extracellular enzymes [62]. Some organics in wastewater treatment can non-specifically adsorb to the virion envelope of SARS-CoV-2 to protect them from viral destruction processes [62]. No significant reduction was observed at pH 3–6 after 60 minutes. In addition, the high population is also a factor leading to the increased possibility of virus transmission through wastewater plants [62]. Because of the risk of virus transmission through wastewater plants, we need a risk assessment and management framework tailored to the transmission, including new tools for environmental monitoring and adequate disinfection [62]. Methods are currently used to detect and monitor wastewater: qualitative, quantitative, and in vitro counts by plaque-forming units (PFU). Plants can remove virions through physical, biological, and chemical processes. After primary treatment, the plant conducts secondary treatment to remove viruses by settling. The treated wastewater is then disinfected [62]. For wastewater reuse, compliance with wastewater reuse standards is essential because the connection of fecal–waterborne–foodborne transmission is widespread. Many developed countries have used tertiary treatment (sand filtration, managed aquifer recharge, UV radiation, advanced oxidation processes (AOP), and/or membrane technologies) or microfiltration and ultrafiltration to remove viruses [62]. Along with the virus removal process, early detection is also an important measure. Biosensor devices are applied, and early detection of viruses by quantification of nucleic acids [63]. The biosensors should have the following characteristics: cost-effectiveness, fast analysis time, high sensitivity, specificity, and low reagent volume [63]. In which the field-effect transistor (FET) biosensor is better than the SERS biosensor. Paper sensor is considered an economical and efficient method [64]. The transmission of virus is also dependent on infectivity. Therefore, qPCR (quantitative Polymerase Chain Reaction) molecular biology techniques have been used [65], in which Capsid-integrity PCR was used to quantify intact virions [65]. In addition, there are several other methods of determining the possibility of infection, such as plaque assay [65]. Studies on SARS-CoV-2 transmission through wastewater treatment plants have been of interest since the SARS-CoV-1 outbreak [62,64]. Based on that, studies on the transmission of SARS-CoV-2 were conducted. The existence of SARS-CoV-2 in human secretions has been detected [62]. Various methods for the elimination, early detection, and infectivity of SARS-CoV-2 have been investigated [64]. In the future, studies on this transmission route need to be focused on providing new findings and more effective measures in preventing transmission.
2.9. Public washrooms
A public washroom is also believed to be a common public area directly related to COVID-19 transmission. There are three potential transmission sources in the washroom, which are (1) the facial-oral route when the contaminated hands touch the food or face, (2) the respiratory route, when an individual is exposed to droplets or particles containing the virus, and (3) transmission through direct contact with infected surfaces [66]. There is a lot of evidence of the existence of SARS-CoV-2 on the surfaces in isolation ward washrooms in Singapore, Italy, and China, including sink, bow and lid, drain, tap, and toilet door handle [23,[67], [68], [69]]. In Guangzhou, China, samples were taken from washrooms used by COVID-19 patients and showed 23.8% of coronavirus contamination [70]. In a single washroom with no ventilation in Wuhan, China, a high concentration of coronavirus particles has been reported [71]. There have not been any reports on the fecal-oral transmission of the coronavirus as well as SARS-CoV-2 clusters are related to public washroom use [72,73].
3. Controlling the transmission of SARS-CoV-2
Coronavirus is mainly transmitted by direct contact routes (droplets). However, indirect contact via surfaces is also an important source for viruses spreading among the community. In some cases, it's the most effective route for virus transmission [74]. The use of copper alloy surfaces is a successful measure to prevent the spread of viruses on public metal surfaces (door handles) [75]. Copper alloys quickly inactivate viruses on their surfaces [75]. Therefore, it is recommended in crowded places, gatherings to prevent the virus from spreading to the community. Disinfectants have also been found to limit viruses that exist significantly and spread through public surfaces (toilet, elevator) as well as body surfaces. However, use with appropriate concentration and contact time, do not use organic materials to achieve the highest efficiency [76,77]. An example of disinfectant: the 70% ethanol acts as an antiseptic solution which is commonly used in hospitals or medical centers. It begins to inactivate SARS-CoV-2 after 30 seconds of exposure [78]. Prevention has been applied worldwide since the early days of COVID-19 disease because coronavirus is easily transmitted from human to crowded human places or on highly infectious surfaces. So, when the COVID-19 epidemic broke out in China, the government has implemented an emergency [79]. The fact that these measures reduce the rate of spreading and the size of epidemic significantly [79]. The hospitals and health centers were mentioned too [80,81]. Health workers play a major role in disease prevention. All prevention activities at the health care facilities must be centered on these subjects [30]. In health care facilities, personal protective equipment such as gloves, facemask, gown, eye protection is promoted with effective prevention of disease transmission [82,83]. Social distancing also plays a significant in reducing the transmission of viruses in hospitals, especially in hospitals with few single rooms. As soon as there was the first case in Singapore, in respiratory surveillance wards, the facility renovation was quickly conducted to create good conditions for social spacing [84]. Outside people are not allowed in these quarantine facilities. The University of Chicago Medicine, United States has introduced [85] meetings and conferences should be conducted online. In clinical workrooms, redistributing distance between computers for a minimum of 6 feet is essential. While it is also realigning the working space and time of doctors to reduce the density of people in the room simultaneously. On call rooms, the distance between patients, cleaning tools, disinfectants, and sanitary conditions must be guaranteed. In addition, the methods of disinfecting with alcohol gel, controlling information and the number of people entering medical facilities, checking the temperature and symptoms here have significantly reduced the incidence of disease for both medical staff and patients [83]. Currently, social distancing and isolation measures are used to effectively prevent transmission. Deactivating public facilities, manage gatherings in markets, avoiding crowding are activities that enhance the effectiveness of the above measures [86]. Furthermore, the increment of single rooms in hospitals and quarantine areas helps to increase the effectiveness of quarantine measures [87]. Table 1 shows us the half-life of the SARS-CoV-2 in different materials for various points and surfaces.
Table 1.
Half-life of the SARS-CoV-2 in the different materials, objects and tools.
| Materials, objects and tools | Ref | Half-life of the SARS-CoV-2 | The possible surfaces or points which are made by the materials or include the objects and toolsa |
|---|---|---|---|
| Plastics | [89,90] | 5.3–6.8 hours | Door handles, public toilets, hospitals or medical centers, factories and industrial zones, public transports, public elevators, and public washrooms. |
| Polypropylene | [91] | 9.02, 4.51, 28.75, 75.54 hours under indoor, summer, spring/fall and winter conditions, respectively | Door handles, public toilets, hospitals or medical centers, factories and industrial zones, public transports, public elevators, and public washrooms. |
| Stainless steel | [91,92] | 3.41–70.6 hours. In 54,5 °C, the half-life is 10.8 ± 3.0 min | Door handles, public toilets, hospitals or medical centers, factories and industrial zones, public transports, public elevators, and public washrooms. |
| Galvanized steel | [91] | 6.93, 4.19, 24.22, 67.21 hours under indoor, summer, spring/fall and winter conditions, respectively. | Door handles, public toilets, hospitals or medical centers, factories and industrial zones, public transports, public elevators, and public washrooms. |
| Urine | [93] | 7.89–57.73 hours | Public toilets, hospitals or medical centers, and public washrooms. |
| Glass | [91] | 9.6, 5.58, 27.34, 92.03 hours under indoor, summer, spring/fall, and winter, respectively | Public toilets, hospitals or medical centers, factories and industrial zones, public transports, and public washrooms. |
| Aerosol | [89] | 1.1–1.2 hours | Public toilets, hospitals or medical centers, factories and industrial zones, public transports, local markets, and public washrooms. |
| Nitrile gloves – outer surface | [91] | 11.56, 4.42, 22.94, and 85.71 hours under indoor, summer, spring/fall, and winter condition, respectively | Hospitals or medical centers |
| Tyvek | [91] | 9.36, 4.57, 31.82, and 90.59 hours under indoor, summer, spring/fall, and winter condition, respectively | Public toilets, hospitals or medical centers, factories and industrial zones, public transports, and public washrooms. |
| N95 mask | [91] | 9.01, 4.4, 27.77, and 106.37 hours under indoor, summer, spring/fall, and winter condition, respectively | Hospitals and medical centers |
| Cloth | [91] | 3.5, 2.99, 19.94, and 47,94 hours under indoor, summer, spring/fall, and winter condition, respectively | Public toilets, hospitals or medical centers, factories and industrial zones, public transports, local markets, and public washrooms. |
| Rubber | [91] | 11.33, 5.03, 28.27, and 115.74 hours under indoor, summer, spring/fall, and winter condition, respectively | Hospitals or medical centers |
| Wastewater | [94] | 0.49 day (high titer), 0.64 day (low titer). 4.6 minutes (high titer) on 50 °C wastewater and 0.6 minute (high titer) on 70 °C wastewater | Wastewater plants, public washrooms |
| Tap water | [94] | 0.59 day (high titer) | Public toilets, hospitals or medical centers, factories and industrial zones, and public washrooms |
Listed by the authors of this paper.
4. Conclusions
This paper shows a need for essential studies on the existence and transmissibility of SARS-CoV-2 and its RNA on highly infectious surfaces and public places and measures to limit it. Prophylaxis based on highly contagious surfaces and public transmission places should be considered. Centralized spaces such as hospitals, local markets, elevators, public transport, untreated and treated wastewaters, and wastewater plants, and public washrooms with the presence of many contaminated surfaces and points (door handle and public toilet) create favorable conditions for SARS-CoV-2 to spread (Table 2 and Fig. 1 ). Therefore, disinfecting and washing hands immediately after touching surfaces as well as before and after using the toilet is essential to minimize the risk of SARS-CoV-2 infection from surfaces. In closed or crowded spaces such as hospitals, local markets, and elevators, it is necessary to wear masks properly and keep a safe distance from others to prevent infection from droplets or aerosols. In addition to the sources stated, wastewater plants can also be a vector that is especially common for countries with inadequate wastewater treatment facilities and low-income countries such as Africa [88]. Moreover, open defecation habits can also cause additional complications and increase the risk of water pollution, which makes fecal-oral a necessary disease transmission to look out for [88]. Poor urban planning systems, large populations, and transportation difficulties make the problem of wastewater treatment facilities in these countries even more profound [88]. Furthermore, The half-life of SARS-CoV-2 on different materials, objects, and tools are varied (Table 2). Therefore, it is necessary to establish suitable wastewater treatment facilities as well as educate the community to eliminate open defecation. On-site sanitation as an alternative to squat toilets is an effective means of waste treatment in these countries [88]. In summary, focusing on interventions related to the highest-risk-associated points for spreading and transmission of SARS-CoV-2 infections is an effective strategy to prevent and reduce the risk of COVID-19 spread. In addition, our study may be the basis for studies of SARS-CoV-2 transmission and persistence on surfaces and public places. It contributes to preventing and minimizing the harmful effects of this disease.
Table 2.
The possible highest-risk-contaminated points and surfaces of SARS-CoV-2.
| Points and surfaces | Ref | Important messages |
|---|---|---|
| Door handles | [9] | The highest risk – contaminated: drawer handles, refrigerators, sink faucets in the break room, soap dispensers in the women's restroom, push bar on the main exit |
| [10] | Door handles have been reported due to the presence of SARS-CoV-2 | |
| [11] | 60% toilet sites (sink, toilet bowl, door handles) giving positive results (Patient C) | |
| [95] | The toilet handle's contamination rate: 5.3% | |
| [96] | Positive sample can be taken from doorknobs at the rate of 8.3% - the fourth highest rate of contamination within a general COVID-19 prevention ward in a hospital | |
| Public toilets | [12] | 4 out of 107 surface samples in isolation rooms were tested positive: two-ward door handles, one bathroom toilet – seat cover, one bathroom door handle. |
| [97] | Outside the bathrooms of hospitals, a case of transmission between 9 people in public bathroom was reported | |
| [13] | There are three transmissions of SARS-CoV-2 mechanisms: inhalation of feces and/or aerosol urine from an individual contains SARS-CoV-2; airborne respiratory transmission face-to-face shortly after use among users; or from transmission of fomite through sites of frequent contact such as doorknobs, washbasin faucets, ball balls or toilet roll dispensers | |
| [30] | A current systematic review attributed the coronavirus prevalence in hospitals and recorded that 24% of air samples collected from toilets were tested positive, with mean viral RNA concentrations per m3. Air is obviously higher than in any other areas that were sampled (Birgand et al., 2020) | |
| [31] | Destitute adherence to hand cleanliness encourages the survival and determination of hand infections for transmission to self or other planes | |
| [14,15] | SARS-CoV-2 can survive on indoor natural textures, for example, plastic, glass, stainless steel, ceramics, elastic gloves, wood, and surgical veils | |
| [14] | Infection endures for many hours in feces and 3–4 days in pee | |
| Hospitals or medical centers | [30] | In China, more than 3,000 health workers were infected by late February 2020. In hospital, SARS-CoV-2 is easily transferred through endotracheal intubation, and bronchoscopy |
| [38] | In hospitals, coronavirus is easily transmitted through endotracheal intubation and bronchoscopy | |
| [96] | SARS-CoV-2 was detected on floor, trash cans, computer mice, sickbed handrails and in air around patient | |
| Factories and industrial zones | [46] |
|
| [47] | The meat processing workers in Nebraska, USA with 5002 cases out of 26,000 suspected cases (19% attack rate) | |
| [8] | The food processing and agricultural companies in the US, 8,978 employees have confirmed COVID-19, 55 workers were reported dead | |
| Public transport | [16] | Use of underground trains and bus were associated with illness onset |
| [48] | The number of COVID-19 cases in the destination cities was associated with the frequency of flights and high-speed- trains. The presence of an airport or high-speed-train station is related to the rate of infection |
|
| [98] | A 51-year-old male taxi driver infected with SARS-CoV-2 in Thailand | |
| [50] | A number of COVID-19 cases in other cities with progressively increased correlations for trains and buses | |
| Public elevators | [17] | The elevator is a space with a simple ventilation system that leads to unsafe ventilation (especially in hospitals). |
| [18] | Infection cases from shopping malls could have been caused by elevator | |
| [55] | Positive results rate of elevator buttons in medical areas is 42.86% | |
| Traditional/Local market | [19] | At the beginning of the epidemic, the rate of market-to-human transmission was 2–34 times higher than that of human-to-human. |
| Wastewaters and wastewater plants | [62] | Aerosol viruses can be transmitted through the wind during wastewater treatment of sewage ponds for transmission in buildings |
| Public washroom | [66] | There are three potential transmission pathways in the washroom, which are (1) the facial-oral route, when the contaminated hands touch the food of face, (2) the respiratory route, when an individual is exposed to droplets or particles containing the virus, and (3) transmission through direct contact with infected surfaces |
Fig. 1.
Summary of the transmission of COVID-19 in public areas (Figure created withBioRender.com).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Nguyen Manh Long (Center for Biomedicine and Community Health, Vietnam National University) and Khanh Linh Hoang (Department of Life Sciences, University of Science and Technology of Hanoi, Hanoi, Vietnam) for partially collecting references and critical reading and checking to improve the manuscript. We especially thank Dr. Le Bui Minh (NTT Hi-tech Institute, Nguyen Tat Thanh University, 300A Nguyen Tat Thanh St., Ward 13, District 4, Ho Chi Minh City, Vietnam) for the license to use BioRender to create the figures in this work.
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabari P., Amini M., Moghadami M., Moosavi M. International public health responses to COVID-19 outbreak: a rapid review. Iran. J. Med. Sci. 2020;45(3):157–169. doi: 10.30476/ijms.2020.85810.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Coronavirus disease (COVID-19): Variants of SARS-COV-2. 2021 11/01/2022]; Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-%28covid-19%29-variants-of-sars-cov-2?gclid=CjwKCAiA5t-OBhByEiwAhR-hm2IUdumgypJJKxkfenwlJm9nph9_llmJzIk-VlIFXXXYeCbOdzie7xoCM2wQAvD_BwE.
- 5.WHO. Tracking SARS-CoV-2 Variants. 2022 11/01/2022]; Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 6.CDC. SARS-CoV-2 Variant Classifications and Definitions. 2021 110/01/2022]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html#anchor_1632158885160.
- 7.CDC . 2021. How COVID-19 Spreads.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html Available from: [Google Scholar]
- 8.Waltenburg M.A., Rose C.E., Victoroff T., Butterfield M., Dillaha J.A., Heinzerling A., Chuey M., Fierro M., Jervis R.H., Fedak K.M., Leapley A., Gabel J.A., Feldpausch A., Dunne E.M., Austin C., Pedati C.S., Ahmed F.S., Tubach S., Rhea C., Tonzel J., Krueger A., Crum D.A., Vostok J., Moore M.J., Kempher H., Scheftel J., Turabelidze G., Stover D., Donahue M., Thomas D., Edge K., Gutierrez B., Berl E., McLafferty M., Kline K.E., Martz N., Rajotte J.C., Julian E., Diedhiou A., Radcliffe R., Clayton J.L., Ortbahn D., Cummins J., Barbeau B., Carpenter S., Pringle J.C., Murphy J., Darby B., Graff N.R., Dostal T.K.H., Pray I.W., Tillman C., Rose D.A., Honein M.A., Cdc C., Emergency Response T. Coronavirus disease among workers in food processing, food manufacturing, and agriculture workplaces. Emerg. Infect. Dis. 2021;27(1):243–249. doi: 10.3201/eid2701.203821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurgat E.K., Sexton J.D., Garavito F., Reynolds A., Contreras R.D., Gerba C.P., Leslie R.A., Edmonds-Wilson S.L., Reynolds K.A. Impact of a hygiene intervention on virus spread in an office building. Int. J. Hyg. Environ. Health. 2019;222(3):479–485. doi: 10.1016/j.ijheh.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Jakhar D., Kaur I., Kaul S. Art of performing dermoscopy during the times of coronavirus disease (COVID‐19): simple change in approach can save the day. J. Eur. Acad. Dermatol. Venereol. 2020:242–244. doi: 10.1111/jdv.16412. [DOI] [PubMed] [Google Scholar]
- 11.Sean Wei Xiang Ong Y.K.T., Chia Po Ying, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama Network. 2020 March 4;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhen Ding H.Q., Xu Bin, Huang Ying, Miao Te, Yen Hui-Ling, Xiao Shenglan, Cui Lunbiao, Wu Xiaosong, Shao Wei, Song Yan, Li Sha, Zhou Lian, Xu Yan, Zhu Baoli, Li Yuguo. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. Sci Total Environ. 2021 Jan 20;753:141710. doi: 10.1016/j.scitotenv.2020.141710. https://www.sciencedirect.com/science/article/pii/S0048969720352396?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dancer S.J., Li Y., Hart A., Tang J.W., Jones D.L. What is the risk of acquiring SARS-CoV-2 from the use of public toilets? Sci. Total Environ. 2021;792:148341. doi: 10.1016/j.scitotenv.2021.148341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Li T., Deng Y., Liu S., Zhang D., Li H., Wang X., Jia L., Han J., Bei Z., Li L., Li J. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021;107:105–107. doi: 10.1016/j.jhin.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayward A.C., Beale S., Johnson A.M., Fragaszy E.B. Public activities preceding the onset of acute respiratory infection syndromes in adults in England - implications for the use of social distancing to control pandemic respiratory infections. Wellcome Open Res. 2020;5:54. doi: 10.12688/wellcomeopenres.15795.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., Tanabe S.I., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing Cai W.S., Huang Jianping. Centers for Disease Control and Prevention; Wenzhou, China: 2020. Michelle Gamber, Jing WuComments to Author , and Guiqing He, Indirect Virus Transmission in Cluster of COVID-19 Cases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizumoto K., Kagaya K., Chowell G. Effect of the Wet Market on the coronavirus disease (COVID-19) transmission dynamics in China, 2019-2020. Int. J. Infect. Dis. 2020:96–101. doi: 10.1016/j.ijid.2020.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitol A.K., Julian T.R. Community transmission of SARS-CoV-2 by surfaces: risks and risk reduction strategies. Environ. Sci. Technol. Lett. 2021;8(3):263–269. doi: 10.1021/acs.estlett.0c00966. [DOI] [PubMed] [Google Scholar]
- 21.Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies Chem. Environ. Eng. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S., Pierson E., Koh P.W., Gerardin J., Redbird B., Grusky D., Leskovec J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2021;589(7840):82–87. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhen-Dong Y., Gao-Jun Z., Run-Ming J., Zhi-Sheng L., Zong-Qi D., Xiong X., Guo-Wei S. Clinical and transmission dynamics characteristics of 406 children with coronavirus disease 2019 in China: a review. J. Infect. 2020;81(2):e11–e15. doi: 10.1016/j.jinf.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley J.E., Vellas B. Editorial: COVID-19 and older adults. J. Nutr. Health Aging. 2020;24(4):364–365. doi: 10.1007/s12603-020-1349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima K., Kato H., Yamashiro T., Izumi T., Takeuchi I., Nakajima H., Utsunomiya D. COVID-19 pneumonia: infection control protocol inside computed tomography suites. Jpn. J. Radiol. 2020:1–3. doi: 10.1007/s11604-020-00948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szerlip M., Anwaruddin S., Aronow H.D., Cohen M.G., Daniels M.J., Dehghani P., Drachman D.E., Elmariah S., Feldman D.N., Garcia S. Catheter Cardiovasc Interv; 2020. Considerations for Cardiac Catheterization Laboratory Procedures during the COVID-19 Pandemic. [DOI] [PubMed] [Google Scholar]
- 29.D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long‐term care: the ABCDs of COVID‐19. J. Am. Geriatr. Soc. 2020;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 30.Birgand G., Peiffer-Smadja N., Fournier S., Kerneis S., Lescure F.X., Lucet J.C. Assessment of air contamination by SARS-CoV-2 in hospital settings. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam S.C., Cheung M.M.F., Au J.K.L., Suen L.K.P. Bioluminescence-based hygiene evaluation of public washroom environment: repeated measurement of posthandwashing facilities on baseline and before and after cleaning schedule. Am. J. Infect. Control. 2021;49(6):746–752. doi: 10.1016/j.ajic.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Marcenac P., Kim S., Molinari N., Person M., Frankson R., Berendes D., McDonald C., Yoder J., Hill V., Garcia-Williams A. Knowledge, attitudes, and practices around hand drying in public bathrooms during the COVID-19 pandemic in the United States. Am. J. Infect. Control. 2021:1186–1188. doi: 10.1016/j.ajic.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dancer S.J., Li Y., Hart A., Tang J.W., Jones D.L. What is the risk of acquiring SARS-CoV-2 from the use of public toilets? Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148341. 148341-148341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.-L., Xiao S., Cui L., Wu X., Shao W., Song Y., Sha L., Zhou L., Xu Y., Zhu B., Li Y. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141710. 141710-141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao P., Chan P.-T., Gao Y., Lai H.-W., Zhang T., Li Y. Physical factors that affect microbial transfer during surface touch. Build. Environ. 2019;158:28–38. [Google Scholar]
- 36.Luo C., Yao L., Zhang L., Yao M., Chen X., Wang Q., Shen H. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai’an, Jiangsu Province, China. JAMA Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.4583. e204583-e204583. [DOI] [PubMed] [Google Scholar]
- 37.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong J., Goh Q.Y., Tan Z., Lie S.A., Tay Y.C., Ng S.Y., Soh C.R. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can. J. Anaesth. 2020;67(6):732–745. doi: 10.1007/s12630-020-01620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lie S.A., Wong S.W., Wong L.T., Wong T.G.L., Chong S.Y. Practical considerations for performing regional anesthesia: lessons learned from the COVID-19 pandemic. Can. J. Anesthesia/J. Canadien d'anesthésie. 2020:1–8. doi: 10.1007/s12630-020-01637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otter J.A., Yezli S., French G.L. Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections. Springer; 2014. The role of contaminated surfaces in the transmission of nosocomial pathogens; pp. 27–58. [Google Scholar]
- 41.Ki M. MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol. Health. 2015;37(2015) doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M., Wolff R.A., Ibrahim N.K., Chua M.L.K., Hotte S.J., Meyers B.M., Elfiki T., Curigliano G., Eng C., Grothey A., Xie C. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncol. 2020;25(6):e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S.S., Zhou X., Lin X.G., Liu Y.Y., Wu J.L., Sharifu L.M., Hu X.L., Rong Z.H., Liu W., Luo X.P., Chen Z., Zeng W.J., Chen S.H., Ma D., Chen L., Feng L. Experience of clinical management for pregnant women and newborns with novel coronavirus pneumonia in Tongji hospital, China. Curr. Med. Sci. 2020;40(2):285–289. doi: 10.1007/s11596-020-2174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 2020;222(5):415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H.Q., Peng L., Chen Y., Peng P., Zhang P., Chu Q., Shen Q., Wang Y., Xu S.Y., Zhao J.P., Zhou M. Ann Oncol; China: 2020. Clinical Characteristics of COVID-19-Infected Cancer Patients: a Retrospective Case Study in Three Hospitals within Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Occupational Safety and Health Administration . 2021. Protecting Workers: Guidance on Mitigating and Preventing the Spread of COVID-19 in the Workplace.https://www.osha.gov/coronavirus/safework Available from: [Google Scholar]
- 47.Herstein J., Degarege A., Stover D., Austin C., Schwedhelm M., Lawler J., Lowe J., Ramos A., Donahue M. Characteristics of SARS-CoV-2 transmission among meat processing workers in Nebraska, USA, and effectiveness of risk mitigation measures. Emerg. Infect. Dis. J. 2021;27(4):1032. doi: 10.3201/eid2704.204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Zhang A., Wang J. Exploring the roles of high-speed train, air and coach services in the spread of COVID-19 in China. Transport Pol. 2020;94:34–42. doi: 10.1016/j.tranpol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pongpirul W.A., Pongpirul K., Ratnarathon A.C., Prasithsirikul W. Journey of a Thai taxi driver and novel coronavirus. N. Engl. J. Med. 2020;382(11):1067–1068. doi: 10.1056/NEJMc2001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng R., Xu Y., Wang W., Ning G., Bi Y. Spatial transmission of COVID-19 via public and private transportation in China. Trav. Med. Infect. Dis. 2020;34:101626. doi: 10.1016/j.tmaid.2020.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunthe S.S., Patra S.S. Impact of international travel dynamics on domestic spread of 2019-nCoV in India: origin-based risk assessment in importation of infected travelers. Glob. Health. 2020;16(1):45. doi: 10.1186/s12992-020-00575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahl E. Coronavirus (Covid-19) outbreak on the cruise ship Diamond Princess. Int. Marit. Health. 2020;71(1):5–8. doi: 10.5603/MH.2020.0003. [DOI] [PubMed] [Google Scholar]
- 53.Kenji Mizumoto a.G.C. Transmission potential of the novel coronavirus (COVID-19) onboard the Diamond princess cruises ship, 2020. Infect. Dis. Model. 2020 February 29:264–270. doi: 10.1016/j.idm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D., Liu R., Wei S., Li T., Cai J., Ge H. Infection prevention and control measures during COVID-19 from medical physics perspective: a single institution experience from China. J. Appl. Clin. Med. Phys. 2020;21(7):221–222. doi: 10.1002/acm2.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu S., Wang Y., Jin X., Tian J., Liu J., Mao Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen W., Zhang N., Wei J., Yen H.-L., Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build. Environ. 2020:106859. doi: 10.1016/j.buildenv.2022.109166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng W., Zong W., Wang F., Ju S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review. Mol. Cancer. 2020;19(1):1–14. doi: 10.1186/s12943-020-01218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristian A.R., Andersen G., Ian Lipkin W., Holmes Edward C., Garry Robert F. The proximal origin of SARS-CoV-2. Nature. 2020 March 17:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogler A., Packman A., Furman A., Gross A., Kushmaro A., Ronen A., Dagot C., Hill C., Vaizel-Ohayon D., Morgenroth E. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nat. Sustain. 2020;3(12):981–990. [Google Scholar]
- 63.Barceló D. Wastewater-based epidemiology to monitor COVID-19 outbreak: present and future diagnostic methods to be in your radar. Case Studies in Chemical and Environmental Engineering. 2020;2:100042. doi: 10.1016/j.cscee.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8(4):104006. doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buonerba A., Corpuz M.V.A., Ballesteros F., Choo K.-H., Hasan S.W., Korshin G.V., Belgiorno V., Barceló D., Naddeo V. Coronavirus in water media: analysis, fate, disinfection and epidemiological applications. J. Hazard Mater. 2021:125580. doi: 10.1016/j.jhazmat.2021.125580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vardoulakis S., Oyarce D.A.E., Donner E. Transmission of COVID-19 and other infectious diseases in public washrooms: a systematic review. Sci. Total Environ. 2021:149932. doi: 10.1016/j.scitotenv.2021.149932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’accolti M., Soffritti I., Passaro A., Zuliani G., Antonioli P., Mazzacane S., Manfredini R., Caselli E. SARS-CoV-2 RNA contamination on surfaces of a COVID-19 ward in a hospital of Northern Italy: what risk of transmission. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9202–9207. doi: 10.26355/eurrev_202009_22872. [DOI] [PubMed] [Google Scholar]
- 68.Wei L., Huang W., Lu X., Wang Y., Cheng L., Deng R., Long H., Zong Z. Contamination of SARS-CoV-2 in patient surroundings and on personal protective equipment in a non-ICU isolation ward for COVID-19 patients with prolonged PCR positive status. Antimicrob. Resist. Infect. Control. 2020;9(1):1–5. doi: 10.1186/s13756-020-00839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.-L., Xiao S., Cui L., Wu X., Shao W. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753:141710. doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo L., Liu D., Zhang H., Li Z., Zhen R., Zhang X., Xie H., Song W., Liu J., Huang Q. Air and surface contamination in non-health care settings among 641 environmental specimens of 39 COVID-19 cases. PLoS Neglected Trop. Dis. 2020;14(10) doi: 10.1371/journal.pntd.0008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 72.Vardoulakis S., Sheel M., Lal A., Gray D. Australian and New Zealand Journal of Public Health; 2020. COVID‐19 Environmental Transmission and Preventive Public Health Measures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicol A. National Collaborating Centre for Environmental Health; Vancouver, BC: 2020 Sep 22. Public Washrooms in the Time of COVID-19: Facility Features and User Behaviours Can Influence Safety [blog] [Google Scholar]
- 74.Otter J., Donskey C., Yezli S., Douthwaite S., Goldenberg S., Weber D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6(6):e01697–15. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4(11):3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabenau H., Kampf G., Cinatl J., Doerr H. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61(2):107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in‐vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) using Povidone‐Iodine oral antiseptic rinse. J. Prosthodont. 2020:529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian H., Liu Y., Li Y., Wu C.-H., Chen B., Kraemer M.U., Li B., Cai J., Xu B., Yang Q. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S., Alkhaldi K.Z., Almohammadi E.L., Alraddadi B.M., Gerber S.I. MERS-CoV outbreak in Jeddah—a link to health care facilities. N. Engl. J. Med. 2014;372(9):846–854. doi: 10.1056/NEJMoa1408636. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plummer F. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chughtai A.A., Seale H., Dung T.C., Maher L., Nga P.T., MacIntyre C.R. Current practices and barriers to the use of facemasks and respirators among hospital-based health care workers in Vietnam. Am. J. Infect. Control. 2015;43(1):72–77. doi: 10.1016/j.ajic.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Odor P.M., Neun M., Bampoe S., Clark S., Heaton D., Hoogenboom E.M., Brown M., Patel A., Kamming D. British Journal of Anaesthesia; 2020. Anaesthesia and COVID-19: Infection Control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wee L.E., Conceicao E.P., Sim X.Y.J., Aung M.K., Tan K.Y., Wong H.M., Wijaya L., Tan B.H., Ling M.L., Venkatachalam I. Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J. Hosp. Infect. 2020;105(2):113–115. doi: 10.1016/j.jhin.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arora V.M., Chivu M., Schram A., Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J. Hosp. Med. 2020;15(5):290–291. doi: 10.12788/jhm.3434. [DOI] [PubMed] [Google Scholar]
- 86.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Trav. Med. 2020;27(2) doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Otter J.A., Yezli S., Salkeld J.A., French G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control. 2013;41(5):S6–S11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Panchal D., Prakash O., Bobde P., Pal S. SARS-CoV-2: sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Control Ser. 2021:1–20. doi: 10.1007/s11356-021-13170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gidari A., Sabbatini S., Bastianelli S., Pierucci S., Busti C., Bartolini D., Stabile A.M., Monari C., Galli F., Rende M. SARS-CoV-2 survival on surfaces and the effect of UV-C light. Viruses. 2021;13(3):408. doi: 10.3390/v13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwon T., Gaudreault N.N., Richt J.A. Environmental stability of SARS-CoV-2 on different types of surfaces under indoor and seasonal climate conditions. Pathogens. 2021;10(2):227. doi: 10.3390/pathogens10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Ferris A., Miller D., Weaver W., Zeitouni N.E., Freeburger D. SARS-CoV-2 is rapidly inactivated at high temperature. Environ. Chem. Lett. 2021;19(2):1773–1777. doi: 10.1007/s10311-021-01187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon T., Gaudreault N.N., Richt J.A. Seasonal stability of SARS-CoV-2 in biological fluids. Pathogens. 2021;10(5):540. doi: 10.3390/pathogens10050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(12):937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng V.C., Wong S.C., Chan V.W., So S.Y., Chen J.H., Yip C.C., Chan K.H., Chu H., Chung T.W., Sridhar S., To K.K., Chan J.F., Hung I.F., Ho P.L., Yuen K.Y. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19) Infect. Control Hosp. Epidemiol. 2020:1–32. doi: 10.1017/ice.2020.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhen-Dong Guo Z.-Y.W., Zhang Shou-Feng, Xiao Li, Lin Li, Li Chao, Cui Yan, et al. vol. 26. Centers for Disease Control and Prevention; Wuhan, China: 2020. (Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chao Luo M., Yao Lun, Zhang Li, et al. 2020 March 30. Possible Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a Public Bath Center in Huai’an. Jiangsu Province, China. [DOI] [PubMed] [Google Scholar]
- 98.Wannarat K.P., Pongpirul A., Ratnarathon Anuttra C. Wisit Prasithsirikul, Journey of a Thai taxi driver and novel coronavirus. N. Engl. J. Med. 2020 March 12 doi: 10.1056/NEJMc2001621. https://www.nejm.org/doi/full/10.1056/NEJMc2001621 [DOI] [PMC free article] [PubMed] [Google Scholar]