Abstract
Background
Antibody responses to SARS-CoV-2 vaccination are impaired in people with multiple sclerosis (MS) under anti-CD20 therapies. It is however unclear, whether patients who received the basic immunization prior to anti-B cell medication start respond to the COVID-19 booster dose, once B cells are depleted.
Aim
To investigate the humoral response to recall antigen by COVID-19 booster vaccines in people with MS (pwMS), who recently started an anti-CD20 therapy compared to people with long-term B cell depletion.
Methods
We enrolled 15 pwMS who had received booster vaccination on anti-CD20 therapy. Of these, 11 had established anti-CD20 medications and were therefore vaccinated during a continuous state of B cell depletion (CD20-vaccine cohort). Four pwMS had received the basic immunization prior to anti-CD20 therapy commencement and only the booster dose (vaccine-CD20-vaccine cohort) under conditions of B cell depletion. We assessed SARS-CoV-2 specific antibody responses after booster vaccination among both groups and evaluated accompanying B cell numbers and proportions from the peripheral circulation.
Results
The booster dose of SARS-CoV-2 vaccination elicited measurable antibody responses in 18% of individuals from the CD20-vaccine cohort compared to 100% from the vaccine-CD20-vaccine cohort. Antibody-levels were significantly higher among patients from the vaccine-CD20-vaccine cohort compared to the CD20-vaccine cohort (mean 951.25 ± 1137.96 BAU/ml, vs mean 12.36 ± 11.94 BAU/ml; mean difference 938 BAU/ml (95% CI: 249–1629 BAU/ml), p <0.0001). Among the vaccine-CD20-vaccine cohort, the booster immunization led to augmentation of spike antibody levels in 75% despite concomitant B cell depletion, and values increased by 3.8 – 9.4-fold compared to basic immunization. We observed no correlation of B cell kinetics and SARS-CoV-2 antibody levels.
Conclusion
Our study suggests that antibody production to recall COVID-19 antigens is preserved in pwMS despite concomitant anti-CD20 therapy. If corroborated in bigger cohorts, this could have implications in the management of individuals about to start B cell medications.
Keywords: SARS-CoV-2, Antibody levels, Antibody titers, B cell depletion, Vaccination, Immunization
1. Introduction
Multiple sclerosis (MS) is considered an inflammatory disorder of the central nervous system (CNS), presumably triggered by pathogenic memory B and T-helper 17 (Th17) cells. Consequently, approved immunomodulatory drugs (IMDs) target these lymphocyte lineages to different extents (Baker et al., 2017; Moser et al., 2020a). An ideal therapy for MS would therefore only hamper auto-aggressive clones with encephalitogenic potential, and spare immune processes important for pathogen control.
Anti-CD20 agents induce a rapid and profound depletion of peripheral B cells and have been proven very efficacious and generally safe for the treatment of relapsing and progressive forms of MS (Hauser et al., 2017; Montalban et al., 2017). However, their mode of action appears to confer increased vulnerability for COVID-19 regarding at least two major concerns. First, several studies have recently confirmed that antibody responses to SARS-CoV-2 vaccines are blunted under B cell depleting antibodies (Novak et al., 2021; Tallantyre et al., 2021). Second, anti-CD20 medication has been associated with an increased risk for a poor outcome following SARS-CoV-2 infection (Sormani et al., 2021). COVID-19 is an emerging disorder that has caused a devastating pandemic affecting daily lives across the globe over the last two years. Pathogen-specific vaccines, consisting of a truly new biological antigen (neoantigen), have shown high levels of efficacy (Baden et al., 2021; Polack et al., 2020). However, reports on the durability of antibody levels following infection or vaccination have observed a substantial decay of humoral immunity already in the initial months (Ibarrondo et al., 2021). As a result, people have recently been recommended a third injection after three to six months (EMA recommendations, 2021). In contrast to the basic immunization, booster doses are intended to elicit a recall antigen response. The impact of the booster shots in people with autoimmune disorders, in particular regarding sequelae of immunomodulation, has not sufficiently been established and more data is required to guide people with MS (pwMS) receiving immunosuppressant agents regarding risk of contagion.
The purpose of this study was to investigate whether B cell depletion impacted on a previously achieved humoral immunologic memory to COVID-19 immunization. We therefore compared the antibody response to recall-SARS-CoV-2 spike antigen in people receiving the booster vaccination shortly after their first cycle of anti-CD20 therapy to individuals with long-term B cell depletion.
2. Methods
2.1. Recruitment and serological assessments
We invited patients with MS (age ≥18 years) under monoclonal-B cell therapy to participate in this observational, monocentric pilot study. All individuals were required to have received 3 doses of approved SARS-CoV-2 vaccinations at study start. Patients were divided into two cohorts. The first cohort consisted of participants with established anti-CD20 medication. Therefore, they had received full SARS-CoV-2 immunization while under long-term B cell depletion (CD20-vaccine cohort). The second group consisted of patients who were vaccinated twice before starting a monoclonal anti-B cell treatment. Therefore, they were assumed to have achieved humoral immunity to SARS-CoV-2 at the time of anti-CD20 commencement. The booster vaccination was then given under concomitant B cell depletion (vaccine-CD20-vaccine cohort). All participants had the SARS-CoV-2 antibody evaluation after the booster dose. For the vaccine-CD20-vaccine cohort, assessment of SARS-CoV-2 spike antibody levels between the second vaccine dose (basic immunization) and the first anti-CD20 infusion was a prerequisite to participate and was used as a comparator to the antibody levels reached after the third dose.
SARS-CoV-2 specific IgG antibody levels to the spike receptor–binding domain (RBD) were evaluated in the peripheral blood using the SARS-CoV-2 IgG II Quant assay (Abbott Laboratories). The assay was measured on the Architect i 2000 SR in accordance with manufacturer's instructions. The detection limit of the antibody assay is 7 BAU/ml. Samples with values below this limit were counted as 7 BAU/ml for statistical calculations. In addition, we assessed corresponding lymphocyte proportions and absolute B cell counts. For this reason, EDTA whole blood was measured on the Sysmex XN system (Sysmex, Kōbe, Prefecture Hyōgo, Japan). Lymphocyte phenotyping was performed by flow cytometry (BD FACSLyric, Becton Dickinson). Analyses were performed by standard routine laboratory methods at the local Department of Laboratory Medicine, certified according to the ISO-9001 standard and working according to ISO-15,189 standards.
2.2. Statistics and ethics
Data were cleaned and screened for correctness. Due to the small sample sizes, bootstrap t-tests with and without the assumption of variance homogeneity were also used for continuously distributed variables based on 5000 Monte Carlo simulations. Crosstabulation tables were analyzed using Fisher's exact, Pearson's Chi-Square test and Wilcoxon-Mann Whitney for two multinomials (both based exact distributions). All reported tests were two-sided, and p-values < 0.05 were considered statistically significant. All statistical analyses in this report were performed by use of STATISTICA 13 (Hill, T. & Lewicki, P. Statistics: Methods and Applications. StatSoft, Tulsa, OK) and StatXact (2013), Version 10.0.0, Cytel software cooperation (Cambridge, MA, USA).
All patients gave written informed consent to participate in this investigation. The study was conducted according to the Declaration of Helsinki and has received Ethics Committee approval (Landesethikkommission Salzburg 415-E/1612/11–2018).
3. Results
We enrolled 15 participants (54% women) with a mean age of 45.9 years (SD ± 11.8 years), a median expanded disability status scale (EDSS) of 4.5 (IQR: 2.75–6.25) and a mean MS duration of 9.7 years (SD ± 7.9 years). Demographic features for the respective cohorts are given in Table 1 . The CD20-vaccine cohort was older and clinically more disabled. Vaccine types were similarly distributed among the two cohorts. MRNA-based vaccines were administered in 83% and 88% and vector immunization used in 17% and 12% of the vaccine-CD20-vaccine and CD20-vaccine group, respectively.
Table 1.
Demographics of the participating cohorts.
| Variable | vaccine-CD20-vaccine cohort (n = 4) | CD20-vaccine cohort (n = 11) | p-value |
|---|---|---|---|
| age, y mean ± SD | 35.0 ± 11.4 | 49.8 ± 9.5 | 0.025 |
| female% | 55% | 50% | n.s. |
| EDSS, median (IQR) | 2.25 (1.75–3.5) | 5.5 (3.75–6.5) | 0.006 |
| No. cycles, median (range) | 1 (1–2) | 5.0 (2–7) | 0.0015 |
| MS duration, y mean ± SD | 5.75 ± 6.2 | 11.2 ± 8.2 | n.s. |
| No. IMDs, mean ± SD | 2.25 ± 2.6 | 1.1 ± 1.2 | n.s. |
y = years; SD = standard deviation; EDSS = expanded disability status scale; IQR = interquartile range; No. = number; nr. cycles = number of anti-CD20 infusions received; MS = multiple sclerosis; No. IMDs = immunomodulating drugs before anti-CD20 medication; n.s. = statistically not significant.
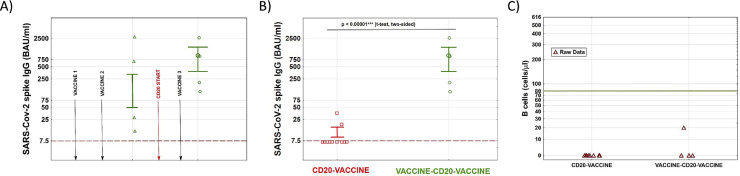
As expected, participants among the vaccine-CD20-vaccine cohort had achieved relevant SARS-CoV-2 spike antibody levels (mean 861 BAU/ml, SD ± 1279 BAU/ml) at mean 79 days (SD ± 53 days) from the basic immunization (Fig. 1 A). The time between third vaccination and follow-up antibody assessment for the vaccine-CD20-vaccine and CD20-vaccine cohort were 24 days (SD ± 8 days) and 32 days (SD ± 13 days), respectively.
Fig. 1.
Antibody levels to SARS-CoV-2 spike antigen after immunization and B cell counts of the two participating cohorts. A) Scheme shows time of the three COVID-19 vaccinations (vaccine 1–3) and temporal relationship of anti-CD20 therapy start (CD20 start) as well as longitudinal antibody assessment among the vaccine-CD20-vaccine cohort. We observed a relevant (3.8 – 9.4-fold) increase in anti-SARS-CoV-2 antibody levels after the booster vaccine in 3/4 patients despite B cell depletion. B) SARS-CoV-2 spike antibody levels to the COVID-19 booster dose. Antibody levels are significantly higher among the vaccine-CD20-vaccine cohort compared to the CD20-vaccine cohort. Only two individuals among the latter group had detectable antibodies, compared to 100% of the vaccine-CD20-vaccine individuals. C) Corresponding B cell counts from the peripheral circulation did not statistically differ among the two cohorts. Legend: Dashed lines in A) and B) represent the detection limit of the antibody assay; green vertical line in C) represents lower limit of normal for B cell counts. Data are shown as raw data and mean +/- standard error.
After the booster dose, 2/11 (18%) patients among the CD20-vaccine cohort had measurable SARS-CoV-2 spike specific antibodies, compared to 100% of the vaccine-CD20-vaccine cohort. The mean antibody levels were significantly higher among the vaccine-CD20-vaccine cohort (mean 951.25 ± 1137.96 BAU/ml vs. mean 12.36 ± 11.94 BAU/ml; mean difference 938 BAU/ml (95% CI: 249–1629 BAU/ml); p <0.0001, Fig. 1B). Moreover, the booster vaccination led to a substantial increase in SARS-CoV-2 spike antibody levels (3.8 – 9.4- fold) compared to post-basic immunization values in 75% of the vaccine-CD20-vaccine cohort (Fig. 1A). Among the vaccine-CD20-vaccine cohort, the time between first (after basic immunization) and second (after the booster dose) antibody evaluation were in mean 182 days (SD ± 79 days) and 178 days (SD ± 44 days) had elapsed between first anti-CD20 infusion and third vaccination.
B cells were profoundly depleted in the majority of participants (Fig. 1C). We found no differences in B cell proportions and counts among the two cohorts and no correlation between number of B cells and antibody responses to vaccination.
4. Discussion
In times of a global pandemic, where anti-CD20 therapies substantially attenuate humoral responses as of COVID-19 vaccines (Novak et al., 2021; Tallantyre et al., 2021), our pilot project reports encouraging data on efficacy of booster vaccination in people who had received the basic immunization prior to starting B cell depletion. The third anti-SARS-CoV-2 dose elicited a robust humoral immune response in all patients with recently initiated monoclonal B cell medication and the majority achieved substantial increases compared to the antibody levels measured after basic immunization. Since booster responses were compared to antibody levels assessed soon after the basic immunization and not directly prior to the third dose, we suggest that the net effect of the booster dose may be even higher than reported here in this study. This is supported by the kinetics of humoral immunology following vaccination or natural infection, which suggests that approximately 90% of antibodies wane within 90 days (Ibarrondo et al., 2021). Our data therefore offers hope for immunized pwMS who are about to commence anti-CD20 medications, that additional COVID-19 booster vaccines, if necessary, may result in preserved humoral responses.
Our results also align with previous studies indicating that vaccine-induced antibody production is substantially blunted in pwMS with long-term B cell depletion, if the first antigen contact occurs after anti-CD20 therapy commencement. This inhibition of eliciting a humoral immune response to novel antigens is likely attributed to the rapid and profound depletion of B cells which, in the majority of cases, do not recover upon the next infusion but remain constantly depleted for the period of ongoing treatment and beyond. Data from the pivotal trial have even suggested that B cells only repopulate after 72 weeks in people receiving ocrelizumab (Kappos et al., 2011).
We propose the following explanation for discrepant responses to neo and recall antigens in people with concomitant anti-CD20 therapy, based on observations from mouse models (DiLillo et al., 2008): Long-lived plasma cells are the main source of circulating antibodies. Once antigen-specific plasma cells have been formed within lymphoid tissues, they assemble in survival niches mainly within the bone marrow. Even though monoclonal antibodies appear to reach these sites of plasma cell shelter (Tabrizi et al., 2010), terminally differentiated B cell phenotypes are not accessible to depletion, because CD20 expression is downregulated during maturation. Their precursors, including memory B cells, however express CD20. CD20+ B cells have important roles during exposure to novel antigens, and their depletion following anti-CD20 therapy likely explains the inhibition of humoral responses to neoantigens, whilst recall immunization remains preserved. As to whether humoral immune responses remain intact despite possible antigen variations with exposure to new COVID-19 variants remains to be seen. The hypothesis that pre-treatment humoral immunity is not affected by B cell depletion is supported by a pooled safety analysis from the pivotal ocrelizumab trials OPERA I and OPERA II (Ziemssen et al., 2017). Among 825 ocrelizumab treated patients, antibody levels to common pathogens were maintained throughout a 96 week follow up. Similarly, a recent study reported sustained humoral responses to COVID-19 vaccination in the context of anti-CD20 therapy over a follow-up of four months in lymphoma patients, corroborating that pre-established humoral immunity is spared despite peripheral B cell depletion (Shree et al., 2022). These findings are in line with data from a cladribine trial, another IMD used in MS resulting in profound reduction of peripheral B cells (Moser et al., 2020b). Antibody titers to several common pathogens were retained 24 months after immune reconstitution via cladribine (Moser et al., 2021), suggesting again no substantial impact of B cell depletion on the pre-existing humoral immunological memory.
Importantly, despite reduced humoral responses under B cell depletion, vaccines appear to elicit an increased T cell reaction in patients with concomitant anti-CD20 therapies (Apostolidis et al., 2021; Gadani et al., 2021). Whether vaccine- induced T cell response would be increased during recall antigen presentation in people under anti-B cell therapies should be the subject of future studies.
Lastly, our findings could have implications for patients who have extended their therapy intervals to reach sufficient humoral responses (Baker et al., 2020; van Lierop et al., 2021). According to our observations, they are likely to exhibit intact recall responses to the booster dose even when the recommended dosing interval of anti-CD20 medication is resumed.
Even though we have measured robust antibody levels (mean 951.25 BAU/ml ± 1137.96 BAU/ml) to the booster dose among the vaccine-CD20-vaccine cohort, we cannot exclude that the humoral response is relatively blunted compared to people with no or other immunomodulatory drugs. However, antibody levels were not inferior compared to two studies which assessed the humoral response after the second COVID-19 vaccination using the same antibody detection method. The first work evaluated antibody levels among 50 participants, including two with immunosuppressive medication, 3 weeks after the second dose of AstraZeneca vaccination and found a median value of 171 BAU/ml (interquartile range: 123.40‐278.70 BAU/ml) (Perkmann et al., 2021). The second report found that among 60 pwMS with concomitant anti-CD20 therapies, only 28.3% developed antibody levels higher than 54 BAU/ml two-four weeks after the second dose of a mRNA-based COVID-19 vaccination (Novak et al., 2021), again underscoring our hypothesis of a spared vaccine response among the vaccine-CD20-vaccine cohort recommending a policy of immunizing prior to anti-CD20 therapy start whenever possible.
The main limitation of this study is the small number of patients and the heterogeneity of the cohorts. Preservation of recall responses to booster doses and eventually to SARS-CoV-2-variant antigens has to be confirmed in bigger cohorts. This pilot-study was not intended for intragroup-comparisons but to provide early insights into recall responses once anti-CD20 therapies have been initiated. An important advantage of this pilot study concerns the fact that based on the effect sizes and standard deviations in this pilot study - later on - an a priori power analysis can be done for planning of a new study with a larger sample size. Another limitation is that the antibody responses were not assessed in a close temporal relationship to the booster vaccine. This was as we were required to compare post-booster levels to the only previously performed antibody assessment which had occurred at some timepoint between basic immunization and anti-CD20 therapy start. As discussed above, this however argues in favor of a robust recall response in patients with previously achieved humoral memory. Of serological note, the exact cut-off for protective antibody levels has not yet been established, even though higher titers and more durable responses have been associated with virus neutralization and faster recoveries from COVID-19 infections (Khoury et al., 2021).
In conclusion, our pilot study corroborates that vaccine efficacy to COVID-19 is generally reduced in people receiving immunization under anti-CD20 therapy but appears to be preserved if the first antigen contact has occurred before treatment start. This could have implications for clinical decision-making in patients about to start an anti-CD20 therapy.
Author contributions
Conceptualization: T.M.; data acquisition: T.M., F.O., C.O., G.P., P.W.; data curation: T.M., W.H.; writing/original draft preparation: T.M., C.O; visualization: T.M. and W.H.; supervision: E.T. and P.W; writing/review and editing: all authors; All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data that support the findings of this study are available on reasonable request from the corresponding author.
Declaration of Competing Interest
TM received travel support, honoraria for presentations or participation on advisory boards from Biogen Idec, Celgene, Novartis, Roche, Sanofi, Merck and Teva. FO received travel support, honoraria for presentations or participation on advisory boards from Biogen Idec, Celgene, Novartis, Roche, Sanofi, Merck and Teva. ET has received consultation fees and/or speakers honoraria from Arvelle, Argenx, Angelini, Bial, Bio-gen-Idec, Boehringer Ingelheim, Eisai, Epilog, GL Pharma, GW Pharmaceuticals, Ever Pharma, Hikma, LivaNova, Marinus, Medtronics, Newbridge, Novartis, Sanofi, Genzyme, and UCB Pharma. PW has received consultation fees and/or speakers honoraria from Bayer, Biogen Idec, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi Genzyme, Teva Pharmaceutical Industries Ltd. He received research grants from Biogen Idec and Merck. The remaining authors have no competing interests.
References
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., C E.M., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L.R., ElSahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., Group C.S. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Marta M., Pryce G., Giovannoni G., Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41–50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Pryce G., James L.K., Marta M., Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- DiLillo D.J., Hamaguchi Y., Ueda Y., Yang K., Uchida J., Haas K.M., Kelsoe G., Tedder T.F. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 2008;180(1):361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- EMA recommendations. 2021 https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters Accessed 01.01. 2022. [Google Scholar]
- Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., Calabresi P.A., Mowry E.M., Fitzgerald K.C., Bhargava P. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Bar-Or A., Comi G., Giovannoni G., Hartung H.P., Hemmer B., Lublin F., Montalban X., Rammohan K.W., Selmaj K., Traboulsee A., Wolinsky J.S., Arnold D.L., Klingelschmitt G., Masterman D., Fontoura P., Belachew S., Chin P., Mairon N., Garren H., Kappos L., Opera I., Investigators O.I.C. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- Ibarrondo F.J., Hofmann C., Fulcher J.A., Goodman-Meza D., Mu W., Hausner M.A., Ali A., Balamurugan A., Taus E., Elliott J., Krogstad P., Tobin N.H., Ferbas K.G., Kitchen S.G., Aldrovandi G.M., Rimoin A.W., Yang O.O. Primary, recall, and decay kinetics of SARS-CoV-2 vaccine antibody responses. ACS Nano. 2021 doi: 10.1021/acsnano.1c03972. [DOI] [PubMed] [Google Scholar]
- Kappos L., Li D., Calabresi P.A., O'Connor P., Bar-Or A., Barkhof F., Yin M., Leppert D., Glanzman R., Tinbergen J., Hauser S.L. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G., de Seze J., Giovannoni G., Hartung H.P., Hemmer B., Lublin F., Rammohan K.W., Selmaj K., Traboulsee A., Sauter A., Masterman D., Fontoura P., Belachew S., Garren H., Mairon N., Chin P., Wolinsky J.S., Investigators O.C. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Moser T., Akgun K., Proschmann U., Sellner J., Ziemssen T. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun. Rev. 2020;19(10) doi: 10.1016/j.autrev.2020.102647. [DOI] [PubMed] [Google Scholar]
- Moser T., O'Sullivan C., Puttinger C., Feige J., Pilz G., Haschke-Becher E., Cadamuro J., Oberkofler H., Hitzl W., Harrer A., Kraus J., Trinka E., Wipfler P. Pre-existing humoral immunological memory is retained in patients with multiple sclerosis receiving cladribine therapy. Biomedicines. 2021;9(11) doi: 10.3390/biomedicines9111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T., Schwenker K., Seiberl M., Feige J., Akgun K., Haschke-Becher E., Ziemssen T., Sellner J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020;7(11):2199–2212. doi: 10.1002/acn3.51206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak F., Nilsson A.C., Nielsen C., Holm D.K., Ostergaard K., Bystrup A., Byg K.E., Johansen I.S., Mittl K., Rowles W., McPolin K., Spencer C., Sagan S., Gerungan C., Wilson M.R., Zamvil S.S., Bove R., Sabatino J.J., Sejbaek T. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;56 doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkmann T., Mucher P., Perkmann-Nagele N., Radakovics A., Repl M., Koller T., Schmetterer K.G., Bigenzahn J.W., Leitner F., Jordakieva G., Wagner O.F., Binder C.J., Haslacher H. The comparability of Anti-Spike SARS-CoV-2 antibody tests is time-dependent: a prospective observational study. medRxiv. 2021 doi: 10.1128/spectrum.01402-21. 2021.2008.2026.21262426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Group, C.C.T. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree T., Shankar V., Lohmeyer J.J., Czerwinski D.K., Schroers-Martin J.G., Rodriguez G.M., Beygi S., Kanegai A.M., Corbelli K.S., Gabriel E., Kurtz D.M., Khodadoust M.S., Gupta N.K., Maeda L.S., Advani R.H., Alizadeh A.A., Levy R. CD20-targeted therapy ablates de novo antibody response to vaccination but spares pre-established immunity. Blood Cancer Discov. 2022 doi: 10.1158/2643-3230.BCD-21-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study, G Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi M., Bornstein G.G., Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., Bramhall K., Chance R., Evangelou N., George K., Giovannoni G., Godkin A., Grant L., Harding K.E., Hibbert A., Ingram G., Jones M., Kang A.S., Loveless S., Moat S.J., Robertson N.P., Schmierer K., Scurr M.J., Shah S.N., Simmons J., Upcott M., Willis M., Jolles S., Dobson R. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2021 doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lierop Z.Y., Toorop A.A., van Ballegoij W.J., Olde Dubbelink T.B., Strijbis E.M., de Jong B.A., van Oosten B.W., Moraal B., Teunissen C.E., Uitdehaag B.M., Killestein J., Kempen Z.L.V. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult. Scler. 2021 doi: 10.1177/13524585211028833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T., Bar-Or A., Arnold D., Comi G., Hartung H.-.P., Hauser S.L., Lublin F., Selmaj K., Traboulsee A., Chin P., Fontoura P., Garren H., Masterman D., Kappos L. P 2 Effect of ocrelizumab on humoral immunity markers in the phase iii, double-blind, double-dummy, IFN β -1a–controlled OPERA I and OPERA II studies. Clin. Neurophysiol. 2017;128:e326–e327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.