Abstract
Activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors increases phrenic motor output. Ampakines are a class of drugs that are positive allosteric modulators of AMPA receptors. We hypothesized that 1) ampakines can stimulate phrenic activity after incomplete cervical spinal cord injury (SCI), and 2) pairing ampakines with brief hypoxia could enable sustained facilitation of phrenic bursting. Phrenic activity was recorded ipsilateral (IL) and contralateral (CL) to C2 spinal cord hemisection (C2Hx) in anesthetized adult rats. Two weeks after C2Hx, ampakine CX717 (15 mg/kg, i.v.) increased IL (61±46% baseline, BL) and CL burst amplitude (47±26%BL) in 8 of 8 rats. After 90 minutes, IL and CL bursting remained above baseline (BL) in 7 of 8 rats. Pairing ampakine with a single bout of acute hypoxia (5-minutes, arterial partial pressure of O2 ~ 50 mmHg) had a variable impact on phrenic bursting, with some rats showing a large facilitation that exceeded the response of the ampakine alone group. At 8 weeks post-C2Hx, 7 of 8 rats increased IL (115±117%BL) and CL burst amplitude (45±27%BL) after ampakine. The IL burst amplitude remained above BL for 90-minutes in 7 of 8 rats; CL bursting remained elevated in 6 of 8 rats. The sustained impact of ampakine at 8 weeks was not enhanced by hypoxia exposure. Intravenous vehicle (10% 2-Hydroxypropyl-β-cyclodextrin) did not increase phrenic bursting at either time point. We conclude that ampakines effectively stimulate neural drive to the diaphragm after cervical SCI. Pairing ampakines with a single hypoxic exposure did not consistently enhance phrenic motor facilitation.
Keywords: Ampakine, respiratory, neuroplasticity, spinal cord injury, phrenic, hypoxia
Introduction
Most spinal cord injuries (SCI) are anatomically incomplete leaving at least some spared tissue at the lesion epicenter. Accordingly, strategies for enhancing the efficacy of spared synaptic pathways to spinal motor pools caudal to the lesion have potential to improve motor function after SCI (Goshgarian, 2003). Ampakines are allosteric modulators of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channel kinetics that enhance glutamatergic synaptic transmission (Arai and Kessler, 2007; Lynch, 2006). AMPA receptors are prominent on phrenic motoneurons (Rana et al., 2019a; Rana et al., 2019b) as well as the premotor respiratory neural circuits in the brainstem (Shao et al., 2003). Ampakines can stimulate respiratory motor output and are particularly effective in conditions where respiratory motor output is impaired or reduced (ElMallah et al., 2015; Ren et al., 2012). Accordingly, we reasoned that ampakines may be able to increase neural drive to the diaphragm muscle that has been impaired by cervical SCI. Using an established model of SCI in the adult rat (cervical hemisection at C2, C2Hx) (Goshgarian, 2003; Sandhu et al., 2009), we hypothesized that systemic (intravenous) delivery of a low impact ampakine (CX717) could increase the inspiratory related discharge of the phrenic nerve ipsilateral to the C2Hx lesion.
In addition to acting as a respiratory stimulant, prior work from our laboratory indicates that ampakines can augment the capacity for respiratory neuroplasticity (Turner et al., 2018; Turner et al., 2016; Wollman et al., 2020). Studies of hippocampal long-term potentiation also indicate that ampakines can enhance synaptic plasticity (Baudry et al., 2012; Rex et al., 2006). Our studies have focused on pairing ampakine treatment with exposure to brief bouts of hypoxia. Repeated bouts of hypoxia (“acute intermittent hypoxia”) can produce a sustained increase in respiratory motor output known as long term facilitation (Fuller et al., 2000). This response can be augmented by pretreatment with the low impact ampakine CX717 (Turner et al., 2016). We also recently reported that pairing low dose ampakine treatment with a single, brief hypoxic exposure could evoke sustained (> 60 minute) increases in phrenic motor output in spinal intact rats (Wollman et al. 2020). This observation is significant because a single brief episode of hypoxia is not expected to evoke post-hypoxia respiratory motor facilitation (Baker and Mitchell, 2000; Wilkerson et al., 2018). Rather, sustained increases in phrenic motor output typically require repeated hypoxic exposures to activate the spinal molecular mechanisms driving this form of respiratory neuroplasticity (Gonzalez-Rothi et al., 2015).
Building upon prior work in spinal-intact rats (Wollman et al., 2020) and mice (Turner et al., 2016), the second goal of our study was to test the hypothesis that following C2Hx injury, pairing low dose ampakine with a single brief hypoxic exposure can cause a sustained in phrenic inspiratory bursting beyond the impact of ampakine alone. The C2Hx injury causes dynamic changes in molecules involved in the initiation and/or maintenance of hypoxia-induced phrenic motor plasticity including serotonin (Golder and Mitchell, 2005), serotonin receptors (Fuller et al., 2005), and trophic factors (Sieck and Mantilla, 2009). The dynamic nature of these post-SCI changes may mean that potential ampakine-hypoxia interactions are variable depending on the amount of time that has passed post-injury. Accordingly, separate groups of rats were studied at 2- or 8-weeks post-C2Hx. We did not have a specific a priori hypothesis regarding the impact of hypoxia and/or ampakine treatment at 2 versus 8 weeks, but nevertheless felt it was important to evaluate at least 2 post-injury time points.
Materials and Methods
Animals
For all experiments, we used adult male Sprague-Dawley rats (Colony 206, ENVIGO Laboratories). Age and weight of animals in each experimental group can be found in Table 1. Rats were housed in pairs in a controlled environment at the University of Florida (12:12 light/dark cycle, 22 ˚C, 20–30% relative humidity) with food and water available ad libitum. All procedures and protocols described were approved by the University of Florida Institutional Animal Care and Use Committee, and are in accordance with National Institutes of Health Guidelines.
Table 1.
Age, weight, phrenic nerve inspiratory burst ʃamplitude, burst frequency, heart rate, and mean arterial pressure during the baseline recordings. There were no differences in any of these parameters when comparing across treatment groups.
| CX717 | CX717 + Hypoxia | HPCD + Hypoxia | |
|---|---|---|---|
|
| |||
| Age at experiment (weeks) | |||
| 2 wk post-Hx | 13.9±1.6 | 13.4±1.4 | 14.0±1.5 |
| 8 wk post-Hx | 18.6±1.5 | 18.6±1.8 | 18.2±1.7 |
| Age at time of surgery (weeks) | |||
| 2 wk post-Hx | 11.8±1.7 | 11.1±1.3 | 11.8±1.5 |
| 8 wk post-Hx | 10.4±1.4 | 10.6±1.6 | 10.2±1.6 |
| Weeks post injury | |||
| 2 wk post-Hx | 2.1±0.2 | 2.3±0.4 | 2.3±0.3 |
| 8 wk post-Hx | 8.2±0.6 | 7.9±0.6 | 8.0±0.5 |
|
| |||
| Weight at experiment (grams) | |||
| 2 wk post-Hx | 331 ± 32 | 317 ± 34 | 318 ± 28 |
| 8 wk post-Hx | 393±28 | 409±22 | 392±35 |
| Weight at Hx (grams) | |||
| 2 wk post-Hx | 301±38 | 301±33 | 307±15 |
| 8 wk post-Hx | 329±37 | 320±20 | 391±33 |
|
| |||
| ʃBurst Amplitude (mV) | |||
| Ipsilateral, 2 wk post-Hx | 1.6±1.1 | 1.9±0.1.2+ | 2.5±1.4+ |
| Contralateral, 2 wk post-Hx | 9.1±3.3 | 9.1±6.7 | 7.8±2.2 |
| Ipsilateral, 8 wk post-Hx | 1.4±0.6 | 2.8±2.1 | 2.0±1.7 |
| Contralateral, 8 wk post-Hx | 7.8±2.6 | 8.7±5.3 | 12.0±4.4 |
|
| |||
| Frequency (per minute) | |||
| 2 wk post-Hx | 52±5 | 52±6 | 50±5 |
| 8 wk post-Hx | 51±6 | 53±6 | 54±3 |
|
| |||
| Heart Rate (per minute) | |||
| 2 wk post-Hx | 438±11 | 436±23 | 451±17 |
| 8 wk post-Hx | 417±21* | 423±14 | 411±14* |
|
| |||
| Mean Arterial Pressure (mmHg) | |||
| 2 wk post-Hx | 114±9 | 126±20 | 114±15 |
| 8 wk post-Hx | 141±20* | 145±23 | 141±25* |
Data are presented as Mean ± SD.
different than the corresponding 2-week time point within the treatment group, p<0.05;
does not include experiments were the baseline phrenic burst amplitude was zero
Spinal cord injury
The general procedures have been described in recent publications from our laboratory (Smuder et al., 2016; Streeter et al., 2020).Anesthesia was induced with 3% isoflurane in 100% O2, and rats were transferred to a heated pad and anesthesia was maintained through a nose cone with 1.5–2% isoflurane. The surgical area was cleaned with three, alternating rounds of betadine surgical scrub followed by 70% ethanol and a dorsal incision was made over the spinal midline from the base of the skull to the fifth cervical segment. A C2 laminectomy was performed to expose the spinal cord and a left, C2 hemisection (C2Hx) was performed using a micro-scalpel. The overlying muscles, and separately the skin, were sutured with sterile 4–0 Vicryl suture. Rats received buprenorphine (0.03 mg/kg, s.q.) for the initial 48 hours post-injury, and Lactated Ringer’s solution (10ml/day, s.q.) and oral Nutri-cal supplements (1–3 ml, Webster Veterinary, MA, USA) until adequate volitional eating and drinking resumed.
Drugs
Ampakine CX717 was provided by RespireRX and was mixed in 10% 2-Hydroxypropyl-β-cyclodextrin (HPCD) solution (Sigma) at highest soluble concentration (5 mg/ml) and stored in 1.5 mL aliquots at −18 ˚C. Aliquots were thawed and allowed to warm to room temperature prior to use on the day of each experiment. The CX717 was used at a concentration (15 mg/kg) that can stimulate breathing in neuromuscular disease (ElMallah et al., 2015) and enhance hypoglossal LTF in anesthetized mice (Turner et al., 2016). HPCD solution (10% HPCD in 0.45% saline solution), used for vehicle injection, was stored at 4 ˚C and allowed to warm to room temperature prior to use on the day of each experiment. All drugs were administered intravenously over the course of 1 minute via a tail vein catheter.
In vivo Neurophysiology
Rats were studied using established in vivo electrophysiology methods, the fundamentals of which have been recently described (Streeter et al., 2017; Wollman et al., 2020). Rats were anesthetized with 3% isoflurane (in 100% O2) and a tail vein catheter was placed for intravenous delivery of urethane and fluids. Rats were placed on an electric heating pad on medium heat, and maintained under 1% isoflurane as urethane (1.7 g/kg, i.v.; 0.7 g/ml in distilled water) was slowly infused (6 ml/h; Harvard Apparatus syringe pump) until the absence of hindlimb withdrawal after toe pinch. Rats were then transferred to a heated surgical station where core body temperature was maintained at 37 ± 0.2 ˚C (model 700 TC-1000, CWE). The trachea was cannulated, rats were pump-ventilated (Rodent Ventilator 683, Harvard Apparatus), and a bilateral vagotomy was performed. Inspired CO2 was added to maintain end-tidal CO2 between ~45 and 50 mmHg (Capnogard, Respironics, Inc.). Tracheal pressure was continuously monitored and lungs were periodically hyperinflated (~ 1/hour, 2–3 breaths) via expiratory line occlusions. Femoral arterial catheter was placed to monitor blood pressure and sample blood gasses (ABL90 FLEX, Radiometer).
Using a dorsal approach, the left and right phrenic nerves were isolated and cut distally. The epineurium was removed at the distal end of the nerve to enable suction electrode recordings. Animals received a neuromuscular paralytic, pancuronium bromide (2.5 mg/kg, i.v., Hospira) to prevent respiratory muscle contraction during nerve recordings. A continuous infusion (1 ml/h) of a 1:4 solution (8.4% sodium bicarbonate/lactated Ringer’s solution, i.v.) was maintained during the experiment. Adequacy of anesthesia was monitored prior to the start of each experimental protocol by assessing arterial blood pressure response to toe-pinch. Urethane supplements (0.2 ml bolus) were given in the event of toe pinch response. Experiments were started at least 20 minutes after the discontinuation of I.V. urethane and once the amplitude and frequency of inspiratory phrenic bursts were stable.
Bilateral phrenic nerve output was recorded using custom-made bipolar suction electrodes filled with 0.9% saline. Compound action potentials were amplified (x10 kHz, Grass Instruments, P511), analog bandpass filtered (3 Hz- 3 kHz), digitized [16 bit, 25 k samples/s/channel; Power1401, Cambridge Electronic Design, (CED)], and integrated (time constant: 20 ms) with Spike2 software (CED).
Experimental protocols
For each experimental time point, rats were exposed to one of three treatments (N=8 per group for both 2-week and 8-week time points): Ampakine alone (15 mg/kg, i.v.; 5 mg/ml in 10% HPCD solution), Ampakine followed by hypoxia (12.5% inspired O2) (ampakine + hypoxia), or vehicle (10% HPCD, matched to CX717 volume, i.v.) followed by hypoxia (HPCD + hypoxia) as a control. In preliminary studies we observed that the acute effects of ampakine CX717 on phrenic motor output are evident rapidly following the i.v. infusion, with a modest increase in burst amplitude observed after approximately 2 minutes. Our a priori goal was to provide the acute hypoxic exposure during the period associated with the peak effects of ampakine. Previous work has shown that this protocol produces a sustained increase in phrenic motor amplitude in spinal-intact rats (Wollman et al., 2020). Accordingly, a single episode of hypoxia (5-min duration) was initiated 2-minutes following the ampakine or vehicle infusions. Recordings were maintained for 90-minutes following each treatment. The primary hypotheses focused on phrenic motor output during the stable conditions associated with the baseline recordings (which could be considered as “eupneic” breathing in the anesthetized state). However, we also evaluated phrenic motor output during acute exposure to hypoxia as well an even stronger chemoreceptor challenge by briefly stopping the mechanical ventilator. This latter test produces a few “gasp-like” intense respiratory efforts (Wollman et al., 2020), after which the ventilator was immediately turned back on. The purpose of this was to determine if the ampakine or ampakine + hypoxia treatments had influenced the output of the phrenic system during periods of intense respiratory drive.
Arterial blood samples were obtained at baseline, during the first episode of hypoxia (for treatments ampakine + hypoxia and HPCD + hypoxia), and at approximately the 20-, 40-, 60-, and 90-minute time points. If baseline PaCO2 was not between 40–50 mmHg or if subsequent PaCO2 values were not within 2 mmHg of baseline, inspired CO2 was adjusted to bring the values back to the isocapnic range. If adjustments were made, data for that respective time point was collected during the minute prior to the acceptable blood sample.
Analyses
All data were collected using Spike2.v8 software (CED) and statistical analyses were performed using GraphPad Prism 7. Phrenic nerve burst amplitude and frequency, and mean arterial pressure and heart rate were measured. Integrated phrenic nerve amplitude was reported as absolute amplitude (at baseline) and % above baseline and phrenic burst frequency was expressed as bursts per minute. Heart rate was expressed as beats per minute and mean arterial pressure (MAP), expressed as mmHg, was calculated using the following equation: MAP = [Systolic Blood Pressure + (2 x Diastolic Blood Pressure)] / 3. Arterial partial pressure CO2 (PaCO2), O2 (PaO2), and pH were obtained from arterial blood samples. The cardiovascular and respiratory response to acute i.v. ampakine (or vehicle solution) consistently peaked ~ 2 minutes after the start of i.v. infusion. Therefore, data representing the peak response to ampakine or vehicle were averaged over the third minute after the start of infusion.
Data from time points associated with arterial blood gas measurements were averaged over the 1-minute period prior withdrawal of the blood sample. Data from the final hypoxia challenge was averaged over the third minute after the onset of hypoxia. The peak response to maximal chemoreceptor activation was measured from the largest single burst amplitude that occurred during the ventilator off challenge (usually the last burst prior to apnea).
Data from 48 rats (n=8 in each experimental group). Age, body weight, and baseline phrenic burst amplitude and frequency, heart rate and mean arterial pressure (MAP) were all evaluated using one-way analysis of variance (ANOVA). Fisher’s exact test was used to statically compare the proportion of rats which responded to ampakine or vehicle with an increase in phrenic burst amplitude. We did this because in several cases the baseline phrenic output recorded ipsilateral to the C2Hx lesion was either absent or too small to reliably quantify. Such data points could not be included in the calculation of the phrenic output as “% baseline” and could therefore not be included in the associated statistical tests. The Fisher’s exact test allowed us to include all the data points in the statistical testing. Phrenic nerve responses to acute hypoxia and the brief period of withholding ventilator support to maximally stimulate chemoreceptors were evaluated using one-way ANOVA. The response to hypoxia or maximal chemoreceptor stimulation in ampakine versus vehicle treated rats was compared using Student’s unpaired t-test. The acute responses to ampakine or vehicle solution (HPCD) were compared using two-way ANOVA. Two-way repeated measures ANOVA was used to compare inspiratory phrenic burst amplitude, frequency, heart rate, MAP, and arterial blood gas data between the three treatment groups. When appropriate, individual multiple comparisons were made using Tukey’s multiple comparison post hoc test. Statistical significance was assumed if P ≤ 0.05. Mean data are presented with ± 1 standard deviation.
Results
Blood gases, heart rate and mean arterial pressure (MAP)
Age and body weight were similar across the treatment groups at the time of the neurophysiological experiment and at the time of the C2Hx surgery (Table 1). No differences heart rate or MAP were detected across the three experimental groups during baseline recording conditions at either post-injury time point. All three experimental groups tended to have higher heart rate and mean arterial pressure at 8- compared to 2-weeks post-injury, and this was statistically significant in the ampakine and HPCD + hypoxia groups (Table 1).
Baseline PaCO2, PaO2, and pH were similar between experimental groups at both post-injury time points (Tables 2 and 3). Within each experimental group, PaCO2 and PaO2 were stable over the course of the experimental protocols with the exception of the expected reduction in PaO2 when inspired oxygen was reduced. All rats were well oxygenated with PaO2 values > 200 mmHg throughout the baseline and post-hypoxic recording periods. Some differences in arterial blood chemistry were noted between experimental groups, as follows. At 2-weeks (Table 2) and 8-weeks post-injury (Table 3), the ampakine time control group, which did not receive the first hypoxia exposure, had greater PaO2 at the 20-minute time point (vs. ampakine + hypoxia and HPCD + hypoxia). Tables 2 and 3 show that arterial pH was stable within and across each experimental group with a few small differences (e.g., 0.01–0.02 pH units) as indicated.
Table 2.
Arterial blood gas values at 2-weeks post-injury. Blood gases were similar between the three treatment groups at baseline. Within each treatment group, values of each parameter were stable and not statistically different across the 90-minute experimental paradigm (with the exception of reduced PaO2 during hypoxia). PaO2 values were slightly lower at 20-min following hypoxia as compared to the ampakine alone group which did not have an initial hypoxia bout.
| 2 weeks post injury | PCO2 | PO2 | pH |
|---|---|---|---|
|
| |||
| CX717 | |||
| Baseline | 46.1±1.6 | 316.8±19.6 | 7.36±0.02 |
| 20 min | 45.9±1.7 | 315.1±10.5 | 7.35±0.01 |
| 40 min | 46.1±1.5 | 312.6±19.5 | 7.36±0.02 |
| 60 min | 46.8±0.9 | 317.7±10.3 | b7.35±0.02 |
| 90 min | 46.2±2.1 | 317.7±7.6 | b7.35±0.02 |
|
| |||
| CX717 + Hypoxia | |||
| Baseline | 45.9±1.4 | 314.1±14.5 | 7.37±0.01 |
| Hypoxia 1 | 45.9±2.8 | *48.8±5.9 | 7.36±0.02 |
| 20 min | 46.7±1.3 | a272.3±34.3 | 7.37±0.02 |
| 40 min | 46.1±1.8 | 309.3±32.7 | 7.38±0.03 |
| 60 min | 46.4±1.7 | 327.3±21.4 | 7.38±0.02 |
| 90 min | 46.1±2.6 | 320.1±20.2 | 7.37±0.31 |
|
| |||
| HPCD + Hypoxia | |||
| Baseline | 46.2±2.1 | 323.1±17.2 | 7.35±0.02 |
| Hypoxia 1 | 46.6±2.7 | *48.6±6.1 | 7.36±0.02 |
| 20 min | 46.7±2.5 | a265.7±47.1 | 7.36±0.02 |
| 40 min | 46.1±2.6 | 316.6±17.8 | 7.37±0.02 |
| 60 min | 46.2±1.9 | 316.6±24.6 | 7.36±0.02 |
| 90 min | 46.3±2.1 | 321.5±23.9 | 7.36±0.03 |
Data are presented as Mean ± SD.
p<0.05 compared to CX717 group;
p<0.05 compared to CX717 + Hypoxia group;
p<0.05 compared to baseline
Table 3.
Arterial blood gas values at 8-weeks post-injury. Blood gases were similar between the three treatment groups at baseline. Within each treatment group, values of each parameter were stable and not statistically different across the 90-minute experimental paradigm (with the exception of reduced PaO2 during hypoxia).
| 8 weeks post injury | PCO2 | PO2 | pH |
|---|---|---|---|
|
| |||
| CX717 | |||
| Baseline | 46.1±2.3 | 337.7±13.4 | 7.37±0.02 |
| 20 min | 46.1±2.9 | 330.5±10.6 | 7.36±0.02 |
| 40 min | 45.9±2.1 | 327.8±14.3 | 7.36±0.01 |
| 60 min | 46.5±2.5 | 327±11.1 | 7.36±0.01 |
| 90 min | 47.2±3.1 | 325.5±17.9 | 7.34±0.02 |
|
| |||
| CX717 + Hypoxia | |||
| Baseline | 46.1±1.5 | 330.9±9.3 | 7.37±0.02 |
| Hypoxia 1 | 45.6±1.7 | #50.7±7.6 | 7.37±0.02 |
| 20 min | 46.3±1.9 | a297.3±32.7 | 7.36±0.02 |
| 40 min | 46.3±1.5 | 319.4±12.8 | 7.35±0.02 |
| 60 min | 46.7±1.6 | 324±10.3 | #7.36±0.01 |
| 90 min | 46.2±1.7 | 321.6±7.8 | 7.35±0.02 |
|
| |||
| HPCD + Hypoxia | |||
| Baseline | 45.6±2.1 | 336.1±31.7 | 7.37±0.02 |
| Hypoxia 1 | 46.3±2.8 | #49.1±7.4 | 7.37±0.03 |
| 20 min | 45.9±1.7 | a281±38.7 | 7.36±0.02 |
| 40 min | 45.4±2.1 | 327.3±25.8 | 7.36±0.03 |
| 60 min | 46.1±2.6 | 331.3±29.7 | #7.36±0.03 |
| 90 min | 46.2±1.2 | 329.5±32.2 | 7.35±0.02 |
p<0.05 compared to CX717 group.
p<0.05 compared to the baseline value.
Data are presented as Mean ± SD.
Acute effects of ampakine CX717 on phrenic motor output, heart rate, and MAP
During baseline recordings, prior to ampakine or vehicle (HPCD) treatment, no differences in phrenic nerve burst amplitude or frequency were detected between the three experimental groups (Table 1). Intravenous ampakine infusion caused a rapid increase in ipsilateral (to C2Hx) and contralateral inspiratory phrenic burst amplitude in >95% of experiments (Table 4). Injection of the vehicle solution (HPCD) did not impact on phrenic inspiratory bursting (Table 4).
Table 4.
Contingency tables showing the proportion of experimental animals that responded to ampakine CX717 or HPCD with an acute increase in phrenic bursting. This was defined as an increase in the amplitude of the integrated phrenic inspiratory burst that was at least 10% above the baseline (pre-infusion) values. The CX717 group includes all animals that received CX717 (e.g., n=8 ampakine and n=8 ampakine + hypoxia). For the latter group, data were evaluated immediately prior to the hypoxia exposure. P-values represent the results of Fisher’s exact test of proportions in small sample sizes.
| Phrenic | Treatment | Time | Increase (n) | No Change (n) | P-value |
|---|---|---|---|---|---|
| Ipsilateral | CX717 | 2 week | 16 | 0 | <0.001 |
| HPCD | 2 week | 0 | 8 | ||
| CX717 | 8 week | 15 | 1 | <0.001 | |
| HPCD | 8 week | 0 | 8 | ||
| Contralateral | CX717 | 2 week | 15 | 1 | <0.001 |
| HPCD | 2 week | 0 | 8 | ||
| CX717 | 8 week | 15 | 1 | <0.001 | |
| HPCD | 8 week | 0 | 8 |
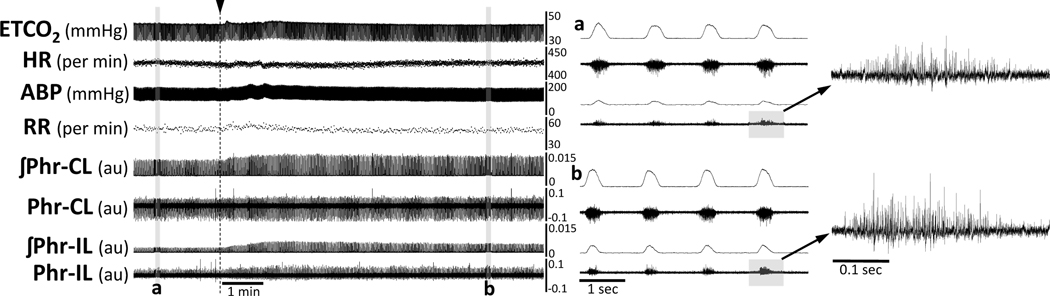
A representative example of the acute impact of ampakine on phrenic motor output is shown in Figure 1. Very shortly following ampakine infusion there is a rapid increase in the amplitude of the inspiratory burst recorded in both the ipsilateral and contralateral phrenic nerves. A small and transient increase in the frequency of inspiratory bursts can also be seen, and this rapidly returns to pre-infusion values. Figure 2 shows the average acute response to ampakine at 2- and 8-weeks following C2Hx injury. Note that at 2-weeks post-C2Hx, inspiratory phrenic bursting could be detected at baseline in 5 of 8 animals. These 5 data points are included in the normalized (% baseline) data shown in Figure 2A. The data from the remaining n=3 could not be normalized to a baseline value, but the data are included in Table 4. Statistical analyses (two-way ANOVA) of the acute impact of ampakine indicated an effect of treatment (ampakine vs. vehicle, P<0.001) but no impact of the side of the recording (ipsilateral vs. contralateral, P=0.580).
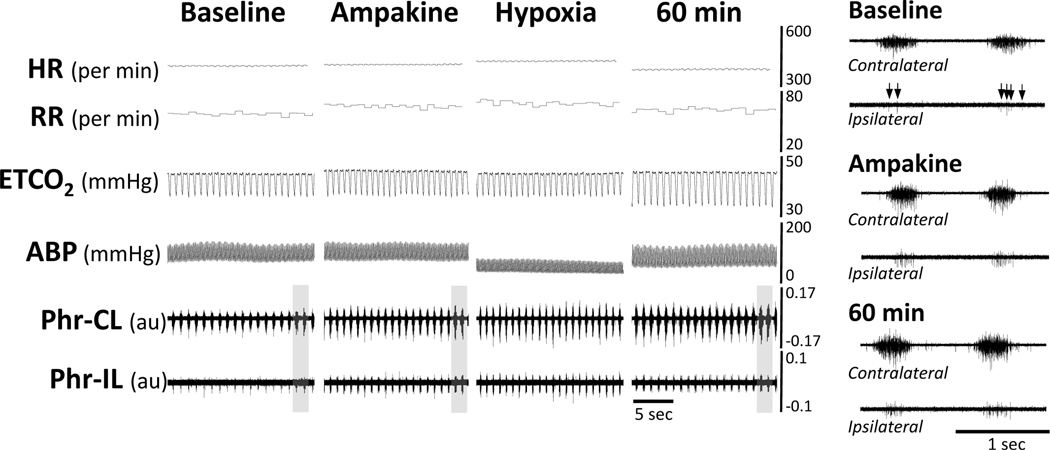
Figure 1. Representative data showing the acute impact of intravenous delivery of ampakine CX717 on phrenic motor output, heart rate, and MAP at 8 weeks following C2Hx injury.
The traces show that infusion of ampakine solution (indicated by the arrow and dashed line) evoked a rapid and bilateral increase in phrenic motor output with relatively little impact on respiratory rate. Panels a and b provide expanded time scale traces which highlight the increase of inspiratory burst amplitude recorded ipsilateral to the C2Hx injury. ABP = arterial blood pressure (ABP); HR = heart rate; ETCO2 = end-tidal CO2; RR = respiratory rate; ʃPhr = integrated phrenic signal; CL = contralateral to C2Hx; IL = ipsilateral to C2Hx. The phrenic nerve burst amplitude can be considered as arbitrary units (au).
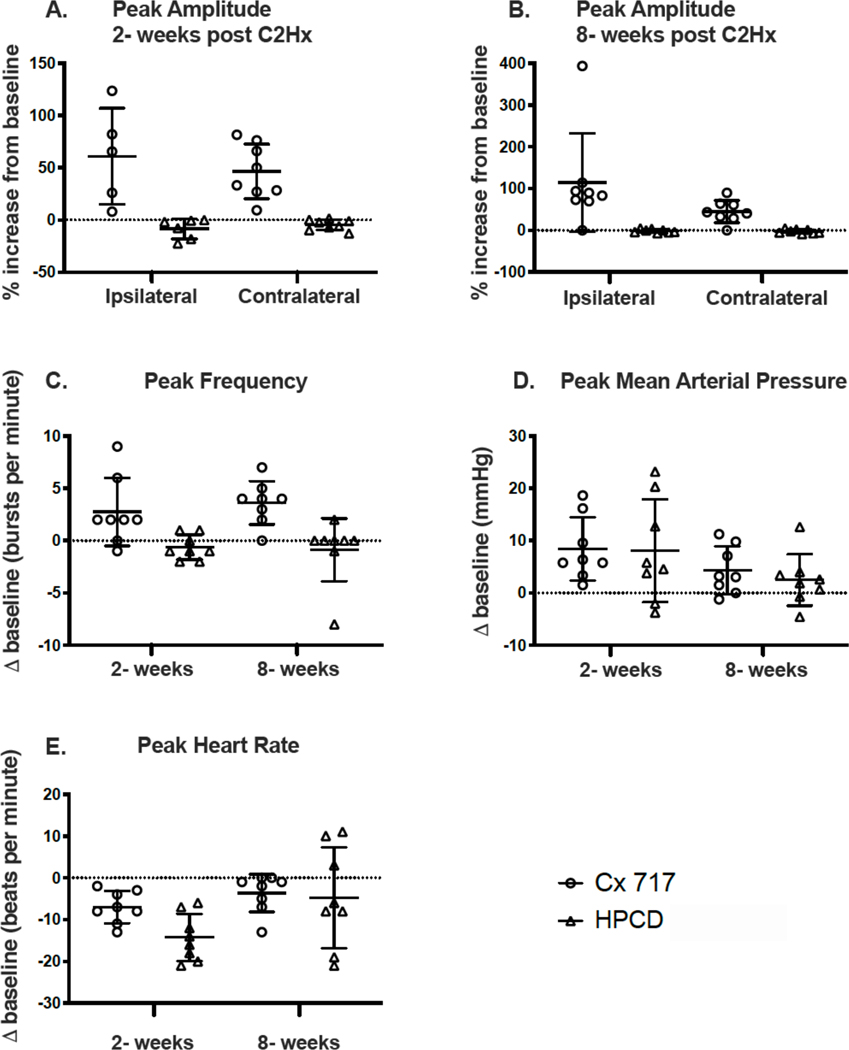
Figure 2. The peak response to intravenous delivery of ampakine CX717 or HPCD.
The peak change in phrenic burst amplitude, burst frequency, mean arterial pressure and heart rate were evaluated at three minutes following infusion of CX717 or vehicle solution (HPCD). N=8 for all groups with the following exception. In panel A, for the phrenic nerve recordings ipsilateral to C2Hx, n=5 for the CX717 group and n=6 for the HPCD group. This is because data points with “zero baseline” (i.e., no quantifiable inspiratory phrenic bursting) could not be included in these normalized data plots. Statistical results are reported in the main text.
At 8-weeks post-injury (Figure 2B), ipsilateral and contralateral phrenic burst amplitude increased after intravenous ampakine in all experiments, and once again the vehicle had no discernable impact on bursting (treatment effect, P<0.001). The impact of ampakine showed a tendency to be greater on ipsilateral as compared to contralateral phrenic output 8 weeks, but this was not statistically significant (P=0.101).
Ampakine CX717 treatment caused an acute increase in the inspiratory burst frequency at both 2- and 8-weeks post-injury (Figure 2C). In contrast, the HPCD vehicle injection had no discernable impact on burst frequency. ANOVA confirmed that ampakine but not vehicle increased burst frequency (treatment, P<0.001), and this response was similar at 2- and 8-weeks post-injury (time, P=0.787).
An acute increase in MAP occurred in response to intravenous injection of the ampakine but also the vehicle solution (Figure 2D). No statistical difference in the acute MAP response was present between ampakine vs. vehicle (treatment, P=0.962) or between 2- and 8-weeks post injury (time, P=0.145). The acute change in heart rate following ampakine or vehicle is shown in Figure 2E. The heart rate response was variable, particularly to the vehicle injection, and there was not a statistically significant difference between ampakine and vehicle injection (treatment, P=0.115). However, there was a significant difference in the response to injection at 2- vs. 8- weeks (time, P=0.005). Inspection of the Figure 2E data indicates that injection of both ampakine and vehicle caused a reduction in heart rate that was more prominent at 2-weeks post-injury.
Sustained impact of ampakine or ampakine + hypoxia on phrenic motor output
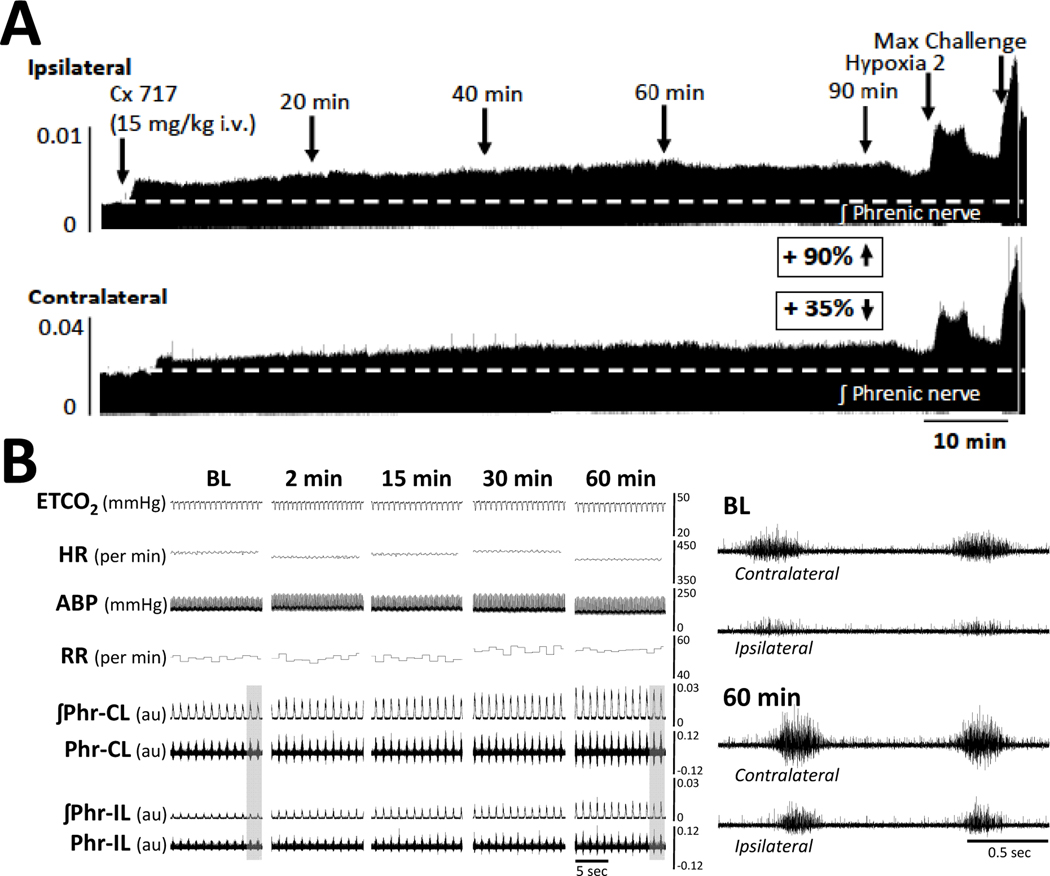
Intravenous ampakine infusion caused a sustained (90-minute) increase of >10 % baseline in ipsilateral (to C2Hx) inspiratory phrenic burst amplitude in 15 of 16 of experiments (7/8 at 2 weeks, and 8/8 at 8 weeks post-C2Hx). For the contralateral phrenic bursting, ampakine caused sustained increases in 13 of 16 experiments (7/8 at 2 weeks, and 6/8 at 8 weeks post-C2Hx). These values include the subset of experiments in which baseline phrenic output was absent or too small to quantitate, and ampakine induced the appearance of inspiratory bursting. Representative data that illustrate sustained changes in phrenic nerve output after ampakine are provided in Figure 3 (ampakine alone; example from 8-weeks post injury)
Figure 3. A representative example of the sustained impact of intravenous delivery of ampakine CX717 on phrenic nerve activity, arterial blood pressure and heart rate at 8 weeks following C2Hx injury.
Approximately 15-seconds of data are shown at baseline (BL), and 2-, 15-, 30-, and 60-minutes following CX717 administration. Note the sustained and bilateral increase in inspiratory phrenic burst amplitude. Panels a (baseline) and b (60-minutes post-CX717) provide expanded time scale traces taken from the areas indicated by the gray box. ETCO2 = end-tidal CO2, HR = heart rate; ABP = arterial blood pressure (ABP), RR = respiratory rate; ʃPhr = integrated phrenic signal; CL = contralateral to C2Hx; IL = ipsilateral to C2Hx. The phrenic nerve burst amplitude can be considered as arbitrary units (au).
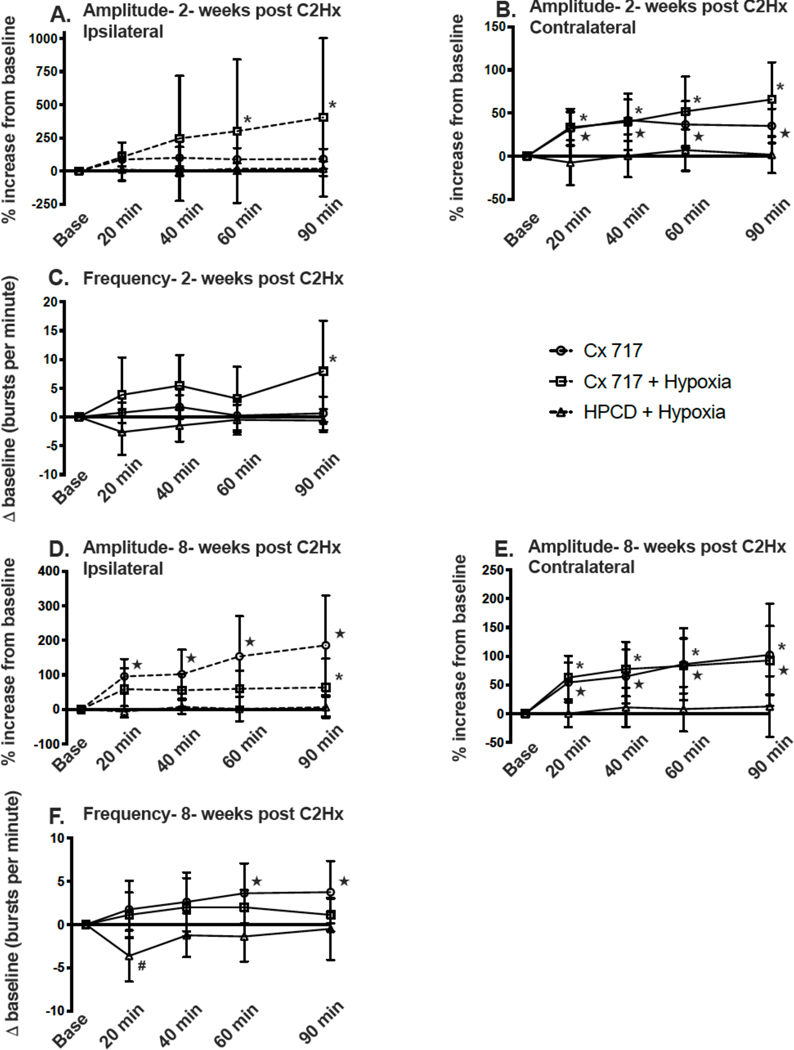
The average ipsilateral phrenic burst amplitude (% baseline) over the 90-minute recording period in rats studied 2-weeks after C2Hx is shown in Figure 4A. The two-way repeated measures ANOVA did not indicate a statistically significant overall impact of treatment (P=0.237); however the ipsilateral phrenic burst amplitude values were statistically different across time (P=0.022). Further evaluation of the time effect with post-hoc tests suggests that phrenic motor facilitation in the ipsilateral nerve was strongest with the combination of ampakine + hypoxia. Specifically, the ipsilateral phrenic burst amplitude (%baseline) was elevated above the baseline values in the ampakine + hypoxia group at 40- (P=0.026), 60- (P=0.010), and 90-minutes following the acute hypoxia exposure (P<0.001). The relative magnitude of the response to ampakine+hypoxia was highly variable, however, and at 90-minutes post treatment the increase of ipsilateral phrenic bursting ranged from relatively modest (~50%baseline) to substantial (~1600 %baseline).
Figure 4. The sustained impact of ampakine CX717 alone, CX717+hypoxia, or vehicle (HPCD)+hypoxia on phrenic motor output.
Data are shown from 2-weeks post-C2Hx (panels A-C) and 8-wks post-C2Hx (panels D-F). Data were collected in separate cohorts of rats as described in the text. In panel A, ipsilateral phrenic nerve output was absent during baseline recordings in several animals within each group. Thus, for Panel A, n=5 for CX717, n=6 for CX717+hypoxia, and n=6 for HPCD+hypoxia. The sample size is n=8 for all groups in Panels B-F. *, indicates that the CX717+hypoxia data point is statistically greater than baseline; ★, indicates that the CX717 alone data point is statistically greater than baseline. ANOVA results are presented in the main text.
Contralateral phrenic output (%baseline) recorded at 2-weeks post-C2Hx showed a statistical interaction between time and treatment (Figure 4B, P<0.001). Post-hoc tests showed that contralateral phrenic burst amplitude was greater in the ampakine + hypoxia group (P=0.003) and ampakine alone group (P=0.023) when compared to HPCD + hypoxia. In addition, both the ampakine and ampakine + hypoxia groups had contralateral bursting above baseline values at each time point following the ampakine treatment (all P<0.05). Thus, both ampakine and ampakine + hypoxia caused a sustained increase in phrenic inspiratory bursting recorded contralateral to the C2Hx lesion at 2-weeks post-injury (Figure 4B).
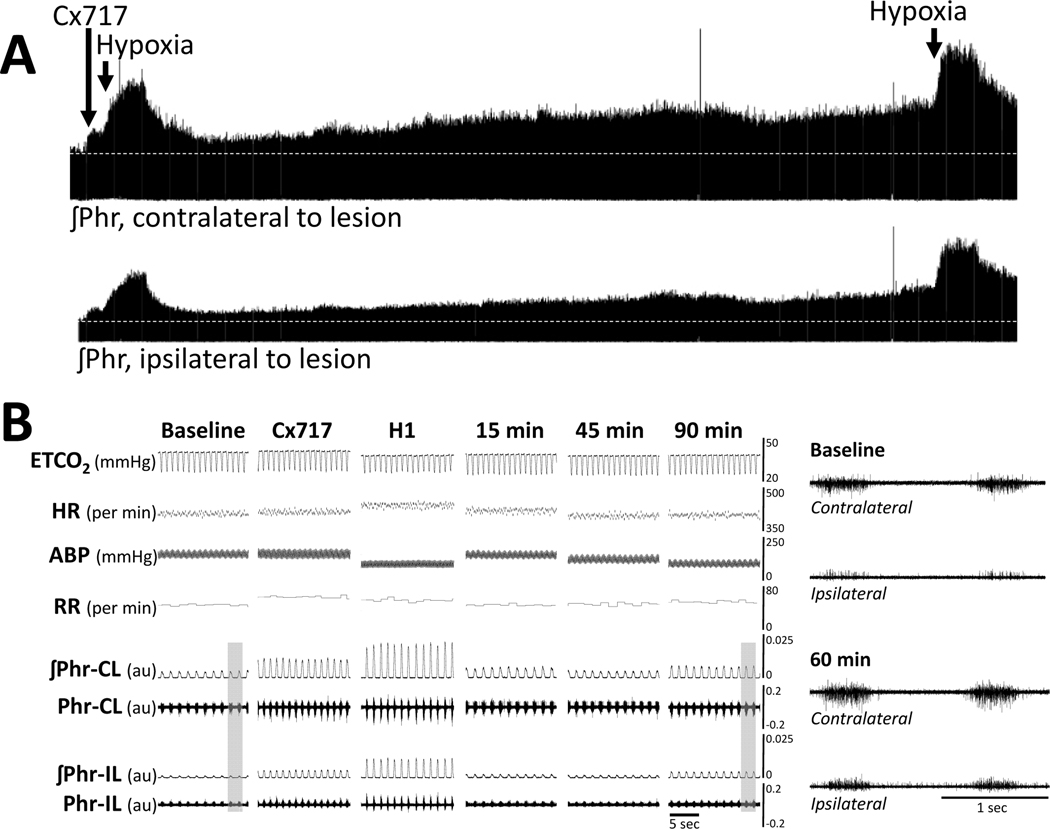
Figure 4C shows the change in phrenic inspiratory burst frequency (bursts per minute) over the course of the experimental protocol at 2-weeks post-C2Hx. Statistical analyses showed an interaction between time and treatment (P=0.015), and review of the data indicates that the combination of ampakine + hypoxia induced a small but persistent increase in inspiratory burst frequency. Post-hoc tests indicated that the Δ burst frequency was greater in ampakine + hypoxia group as compared to the HPCD + hypoxia group (P=0.006) but not the ampakine alone group (P=0.300). An example of the impact of ampakine + hypoxia on phrenic nerve output at 2-weeks post injury is shown in Figure 5.
Figure 5. A representative example of the sustained impact of intravenous delivery of ampakine CX717 following a few minutes later by exposure to hypoxia on phrenic nerve activity, arterial blood pressure and heart rate at 2 weeks following C2Hx injury.
Approximately 15-seconds of data are shown at baseline (BL), 3-minutes after CX717 infusion, during hypoxia (H1), and 15-, 45-, and 90-minutes following hypoxia. Panels a (baseline) and b (90-minutes post-hypoxia) provide expanded time scale traces taken from the areas indicated by the gray box. ETCO2 = end-tidal CO2, HR = heart rate; ABP = arterial blood pressure (ABP), RR = respiratory rate. The phrenic nerve burst amplitude can be considered as arbitrary units (au).
The average ipsilateral phrenic burst amplitude in rats studied 8-weeks after C2Hx is shown in Figure 4D. Statistical evaluation revealed that the effect of treatment was dependent on the time of the measurement (time x treatment interaction, P<0.001). The Tukey all pairwise comparison indicated that the group receiving ampakine alone had greater ipsilateral burst amplitude as compared to the HPCD + hypoxia group (P=0.002) and a strong tendency for elevated output when compared to the ampakine + hypoxia group (P=0.066). Burst amplitude in the contralateral phrenic nerve also showed an interaction between time and treatment (P=0.012). Figure 4E shows that contralateral bursting was similar in the ampakine alone and ampakine + hypoxia groups over the course of the experiment. The change in inspiratory burst frequency during the 8-week post-injury experimental protocol is shown in Figure 4F. Statistical analyses showed an effect of treatment (P<0.001) with ampakine (P<0.001) and ampakine + hypoxia (P=0.014) both different than the HPCD + hypoxia group.
Impact of ampakine or ampakine + hypoxia on response to respiratory challenge
Hypoxia 1 was completed at the beginning of the experiment (immediately after ampakine or vehicle treatment). The first hypoxic episode was not completed in the group receiving ampakine only (i.e., the ampakine only “time control”). Hypoxia 2 was done at the end of the 90-minute recording period and was completed in all three treatment groups. The maximal chemoreceptor challenge accomplished by briefly stopping the mechanical ventilator and was completed in all three treatment groups at the conclusion of the experiment.
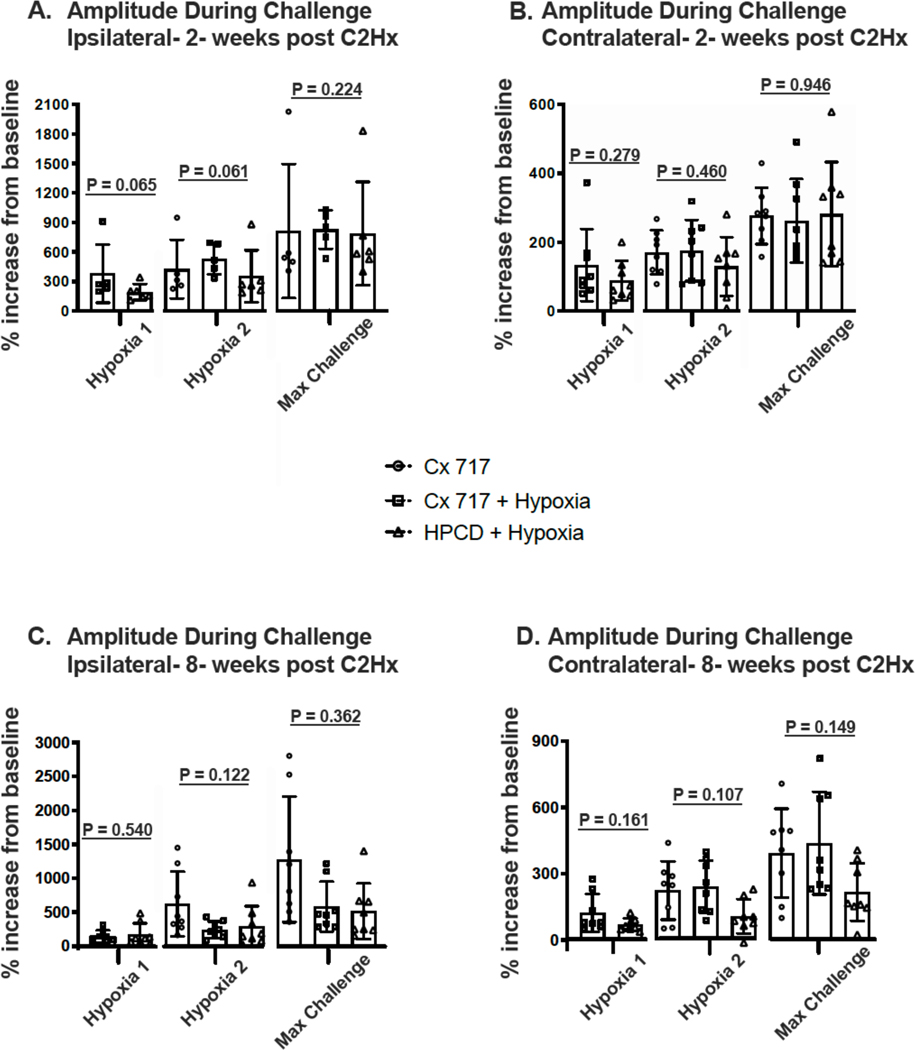
Figure 6A shows a strong trend for the ampakine + hypoxia group to have a greater increase in ipsilateral phrenic burst amplitude in response to hypoxia at 2-weeks post-injury (p-values are presented in Figure 6A). No such trend was observed in maximum chemoreceptor challenge with similar values across the 3 treatment groups. Contralateral phrenic output at 2-weeks (Figure 6B) was similar across the three groups during hypoxia 1, hypoxia 2, and the maximum challenge. Ipsilateral and contralateral phrenic responses to respiratory challenge at 8-weeks post-injury are presented in Figures 6C and 6D, respectively. During hypoxia 1, ipsilateral output was similar between the groups receiving ampakine or vehicle. During hypoxia 2 and maximal challenge, several of the ampakine treated rats had particularly large ipsilateral phrenic responses, but on average the outputs were not different across groups. Contralateral output during respiratory challenge at 8-weeks post-injury showed a tendency to be elevated after ampakine treatment (Figure 6D), but values were not statistically different across groups.
Figure 6. Phrenic inspiratory burst amplitude during respiratory challenges.
Bilateral phrenic nerve recordings were made during an initial hypoxic exposure (H1) in two treatment groups: CX717+hypoxia and HPCD+hypoxia. The group receiving CX717 alone was not exposed to H1. An additional hypoxic episode (H2) and a maximum chemoreceptor challenge (Max Challenge) were made at the conclusion of the experiment in all three groups. Data were collected at 2-weeks (panels A-B) and 8-weeks post C2Hx (panels C-D). The P-values from t-test (H1) and 1-way ANOVA (H2 and Max Challenge) are presented on the figure.
Influence of ampakine or ampakine + hypoxia on heart rate and blood pressure
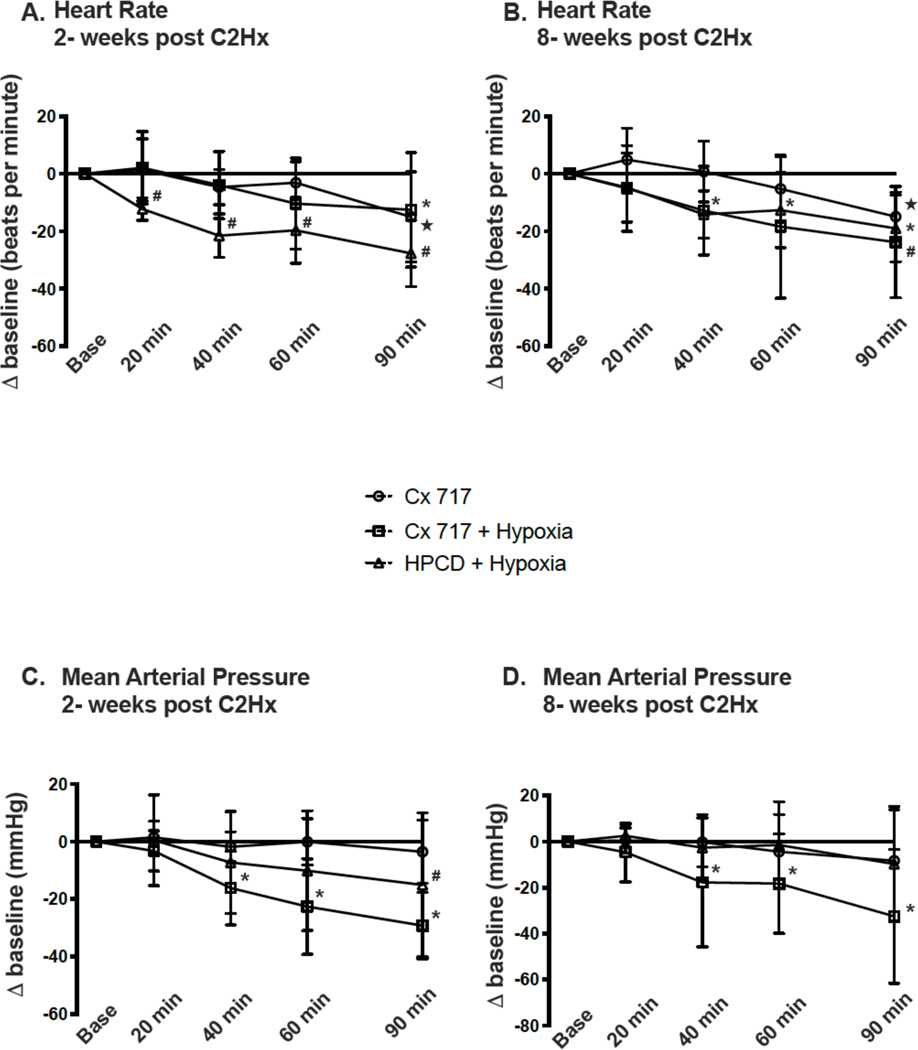
Figure 7A-B shows the effects of each treatment on heart rate, and Figure 7C-D shows mean arterial pressure over the course of the experimental protocol. At 2-weeks post-injury, evaluation of Δ heart rate showed a statistical interaction between time and treatment (P=0.028, Figure 7A). All groups showed a decline in heart rate as the 90 minute experiment progressed, and post-hoc comparison (Tukey) indicated that the Δ heart rate was different between ampakine vs. HPCD + hypoxia (P=0.019) and ampakine + hypoxia vs. HPCD + hypoxia (P=0.027). At 8-weeks post-injury, evaluation of heart rate showed a significant effect of time (P<0.001) but not treatment (P=0.201; Figure 7B).
Figure 7. The sustained impact of ampakine CX717 alone, CX717+hypoxia, or vehicle (HPCD)+hypoxia on heart rate and mean arterial pressure.
Data are shown from 2-weeks post-C2Hx (panels A and C) and 8-wks post-C2Hx (panels B and D). The sample size is n=8 for all groups. *, indicates that the CX717+hypoxia data point is statistically greater than baseline; ★, indicates that the CX717 alone data point is statistically greater than baseline; #, indicates that the HPCD+hypoxia data point is greater than baseline. ANOVA results are presented in the main text.
The change in mean arterial pressure at 2-weeks post-injury (Figure 7C) showed an interaction between time and treatment (P=0.001). Post-hoc comparison (Tukey) indicated that the decline in mean arterial pressure was greater in the ampakine + hypoxia group as compared to the ampakine alone group (P=0.047). A progressive decline in mean arterial pressure was also observed in the ampakine + hypoxia group studied at 8-weeks post-injury (Figure 7D). These data showed a statistical interaction between time and treatment (P=0.048). Post-hoc multiple comparisons (Tukey) did not indicate statistical differences between groups (all P>0.100), but compared to baseline, only the ampakine + hypoxia group showed a decline in values (Figure 7D).
Ampakine can restore phrenic bursting after spinal cord injury
At 2-weeks post C2Hx injury, some animals had no quantifiable nerve output at baseline recorded from the side ipsilateral to injury, and accordingly these experiments could not be included in the data shown in Figures 2 or 4. That is, the absence of a baseline value prevented the phrenic data from being normalized as % baseline. This occurred for n=3 in the ampakine only group, n=2 for ampakine + hypoxia, and n=2 for HPCD + hypoxia. Importantly, we observed that acute administration of ampakine restored a degree of ipsilateral phrenic bursting in 5 of 5 animals with absent (or unquantifiable) bursting at baseline (example data shown in Figure 8). This was in sharp contrast to vehicle treatment, with 0 of 2 showing bursting after HPCD treatment.
Figure 8. A representative example obtained 2 weeks following C2Hx injury in which the baseline phrenic output was too small to quantitate.
Presented in the figure are heart rate (HR), respiratory rate (RR), end-tidal CO2 (ETCO2), arterial blood pressure (ABP) and phrenic nerve discharge recorded contralateral (CL) and ipsilateral (IL) to C2Hx. The right panels provide expanded time scale traces taken from the areas indicated by the gray box in the left panel. In this example, the baseline recordings indicate that only a few phrenic motor units were active. This is best appreciated in the expanded time scale traces shown in the right panel (arrows indicate spikes in the recording). In all 5 animals tested in which ipsilateral output was too small to measure reliability (e.g. zero of just a few sporadic motor unit discharges), administration of ampakine CX717 restored some degree of bursting. The phrenic nerve burst amplitude can be considered as arbitrary units (au).
Discussion
Impaired respiratory muscle activation is a significant problem following cervical SCI (Winslow et al., 2002; Winslow and Rozovsky, 2003). Here we introduce a new pharmacologic treatment that may prove useful in that regard. We show that intravenous delivery of a low impact ampakine, CX717, can evoke a sustained and bilateral increase in phrenic inspiratory motor output in a rodent model of incomplete cervical SCI. Translation of ampakine treatment to clinical may be possible since clinical trials related to cognitive and affective disorders show minimal side effects (Doraiswamy and Xiong, 2006; Oertel et al., 2010; Porrino et al., 2005; Wesensten et al., 2007).
Ampakines
In vitro studies confirm that ampakines are not AMPA receptor agonists, but are positive allosteric modulators of AMPA receptor activity (Arai et al., 1996; Arai et al., 2002; Arai et al., 2004). Ampakines do not impact NMDA or kainite receptors directly and this class of compounds can be grouped in two categories based on their impact on AMPA receptor kinetics (Arai and Kessler, 2007). Low impact (or type 1) ampakines have a shorter decay time constant as compared to high impact (or type 2) ampakines. The high impact ampakines can prolong excitatory currents by delaying channel closing and interfering with desensitization, which may be responsible for their poor safety profile (Arai and Kessler, 2007). Both high and low impact ampakines can facilitate hippocampal long-term potentiation, but the high impact form may also promote long-term depression (Arai and Kessler, 2007). For the current study, we chose a low impact ampakine, CX717, due to the success of prior experiments using this drug in spinal-intact rodent models (ElMallah et al., 2015; Turner et al., 2016; Wollman et al., 2020) and also because it has passed through initial clinical studies in humans, particularly demonstrating the ability to reduce opioid induced respiratory depression, an indication of AMPA receptor target site engagement (Boyle et al., 2012; Oertel et al., 2010; Wesensten et al., 2007).
Expression of AMPA receptors in the phrenic motor circuit and the impact of SCI
Glutamatergic synaptic transmission via AMPA receptors is critical in both the generation and transmission of the neural drive to breathe (Liu et al., 1990; Pace et al., 2007), including phrenic motoneuron depolarization (Funk and Feldman, 1995; Funk et al., 1993; Greer et al., 1991; Pace et al., 2007). Recent work from Rana provides a comprehensive description of AMPA as well as NMDA receptor mRNA expression in phrenic motoneurons, with smaller motoneurons having increased density of mRNA transcripts (Rana et al., 2019a; Rana et al., 2019b). It is not surprising, therefore, that microinjection of AMPA into the phrenic motoneuron pool triggers an increase in phrenic motor output (Chitravanshi and Sapru, 1996). Overall, it is well established that phrenic motoneurons have an abundance of AMPA receptors and activation of these receptors contributes to their depolarization during inspiration. Alilain and Goshgarian provided the first exploration of possible changes in phrenic motoneuron AMPA receptors after SCI (Alilain and Goshgarian, 2008). At 6–12 weeks following C2Hx injury in adult rats, homogenates from the mid-cervical spinal cord showed an increase in GluR1 AMPA subunits and a decrease in GluR2 AMPA subunits. Mantilla advanced this work by using laser-dissection methods to verify that phrenic motoneurons expressed AMPA receptors after C2Hx in the rat model (Mantilla et al., 2012). However, no changes in phrenic motoneuron AMPA receptor expression were observed (compared to samples from control, uninjured rats) over 3–21 days post-C2Hx. The Mantilla study is particularly important with regard to the current data since it unequivocally confirmed that AMPA receptors are present on ipsilateral phrenic motoneurons after C2Hx. A more recent report from Gransee also used laser capture of phrenic motoneurons in the rats C2Hx model, and reported that mRNA expression of the AMPA glutamate receptor 2 (GluR2) subunit was decreased at 14-days post-injury (Gransee et al., 2017). Interestingly, however, rats that showed spontaneous diaphragm recovery after C2Hx had greater expression of phrenic motoneuron GluR2 receptors as compared to those that did not. The impact of SCI on brainstem AMPA receptors is less clear. However, studies in the neonatal rat show that C2Hx leads to a rapid decrease in medullary expression of GluR2 AMPA subunits (Zimmer and Goshgarian, 2007).
Acute and sustained impact of ampakine CX717 on respiratory motor output after C2Hx
The C2Hx model used in the current work deprives the ipsilateral phrenic motor pool of descending synaptic inputs, and therefore causes immediate paralysis of the ipsilateral hemidiaphragm. However, “crossed spinal” synaptic pathways that provide respiratory-related synaptic input to ipsilateral phrenic motoneurons are present, but functionally silent immediately post-injury (Fuller et al., 2003). Over time there is a gradual and spontaneous increase in phrenic motor output ipsilateral to C2Hx (Goshgarian, 2003, 2009; Sandhu et al., 2009). Phrenic activity below the injury is minimal or absent during the initial weeks post-injury, and spontaneous recovery does not approach “normal” phrenic activity levels (Fuller et al., 2006), at least when evaluated under anesthesia (Bezdudnaya et al., 2018). Therefore, the current neurophysiology preparation in anesthetized rats enabled us to test how ampakines influence the output of phrenic motoneurons that have considerably reduced baseline output due to SCI (i.e., ipsilateral to C2Hx). Conversely, recording from the contralateral phrenic nerve enabled us to determine how pathways that were not directly altered by the lesion responded to ampakines.
We observed that ampakine caused a substantial and rapid increase in phrenic motor output in the ipsilateral and contralateral nerves at both 2- and 8-weeks post C2Hx injury. Thus, the impact of ampakine on respiratory motor output was not restricted to “impaired” pathways. The increase in ipsilateral phrenic bursting is likely to represent facilitation of “crossed spinal” synaptic inputs to phrenic motoneurons, either via direct monosynaptic inputs (Ellenberger et al., 1990; Moreno et al., 1992) or poly-synaptic pathways using cervical interneurons to relay respiratory motor drive from the medulla (Lane et al., 2009; Satkunendrarajah et al., 2018; Streeter et al., 2020). Ampakine-induced increases in phrenic inspiratory bursting from the nerve contralateral to the C2Hx injury indicate that the neural pathways not directly impacted by the lesion can still increase output via allosteric modulation of AMPA receptors. Our prior study of spinal intact rats reported that phrenic burst amplitude was on average approximately 25% above baseline at 60 min after a similar ampakine dosing (Wollman et al., 2020). Here we found that contralateral phrenic bursting was approximately 35% above baseline in 2-wk post-injury rats after 60 minutes, and 90% above baseline in 8-wk post-injury rats at 60 minutes. Thus, by qualitative comparison, it appears that the impact of ampakine on “intact” neural pathways is somewhat greater after cervical SCI.
At both time points, the acute increase in phrenic burst amplitude was accompanied by an increase in the rate of breathing by a few breaths per minute. These changes in phrenic burst amplitude and frequency were qualitatively similar to what we reported previously for spinal-intact rats (Wollman et al., 2020). We also observed sustained (up to 90-minutes) and bilateral increases in phrenic output following ampakine. In our prior study of spinal-intact rats, inspiratory phrenic burst amplitude returned toward baseline by 60-minutes following ampakine treatment (Wollman et al., 2020). Thus, the sustained increases observed in the current study suggest that ampakines impact phrenic motor output differently after cervical SCI.
The observed increases in breathing rate after ampakine (e.g., Figure 4) suggests that CX717 had some impact on the inspiratory rhythm generating circuits (Ren et al., 2009; Ren et al., 2015). However, it is not possible to tell from the current data set whether the mechanism driving the increased phrenic burst amplitude was primarily spinal (e.g., increased phrenic motoneuron response to synaptic inputs), supra-spinal (e.g., increased “drive” to phrenic motoneurons), or a mixture of both. Work from Greer and colleagues confirms that ampakines can act on premotor “rhythm generating” neurons and also respiratory motoneurons (Lorier et al., 2010; Ren et al., 2009; Ren et al., 2012, 2015; Ren et al., 2013), and both mechanisms may be active in our studies. Regardless of the underlying mechanisms, the current data demonstrate that the low impact ampakine CX717 can stimulate phrenic motoneuron output after incomplete cervical SCI, and the effect is not limited to “injured” neural pathways since increases in motor output were observed bilaterally.
Pairing ampakine CX717 with hypoxia
A recent publication from our group showed that pre-treatment with ampakine CX717 (15 mg/kg) created preconditions which enabled a single brief bout of hypoxia to produce sustained facilitation of phrenic motor output in the spinal intact rat (Wollman et al., 2020). Similar data were also obtained in a study of inspiratory motor output from the hypoglossal nerve in anesthetized mice (Turner et al., 2018). These data are noteworthy since prior studies had found that multiple episodes of hypoxia are required to evoke sustained respiratory motor facilitation (reviewed in (Gonzalez-Rothi et al., 2015)). Therefore, at least in spinal intact conditions, ampakines can reduce the number of hypoxia episodes necessary to evoke mechanisms which can trigger sustained increases in respiratory motor output.
Here we explored whether a single bout of hypoxia could enhance the sustained impact of intravenous ampakine treatment following cervical SCI. At 2-weeks following C2Hx injury, there was evidence that the combination of ampakine + hypoxia could enhance phrenic motor facilitation ipsilateral to the lesion. However, the response was variable with some animals showing greatly enhanced facilitation and others showing responses similar to the ampakine alone treatment. At 8-weeks after the C2Hx injury, the results unequivocally showed that the addition of a single bout of hypoxia did not enhance ampakine-induced facilitation of ipsilateral phrenic bursting. Rather, the sustained impact of ampakine on the ipsilateral phrenic inspiratory burst was attenuated in rats exposed to the single hypoxic episode. For the contralateral phrenic recordings, ampakine alone or ampakine + hypoxia evoked similar sustained increases in inspiratory burst amplitude at 2- and 8-week post-injury. Qualitatively, the magnitude of the phrenic motor facilitation induced by ampakine + hypoxia in the prior study of spinal intact rats (Wollman et al., 2020) (~100% baseline at 60 minutes) was similar to what was observed for phrenic output contralateral to C2Hx in the current study at 8-weeks post-injury (~80% baseline at 60 minutes).
Our a priori experimental intent was not to statistically compare the response to ampakine treatment at 2- vs. 8-weeks post-injury. Nevertheless, qualitative comparison of the ipsilateral phrenic nerve response between these time points suggests that the phrenic motor response may be different. Certainly the injured spinal cord will be undergoing considerable neuroplastic changes during progression from the sub-acute to chronic phases. Dynamic changes in expression of serotonin (Fuller et al., 2005; Golder and Mitchell, 2005), neurotrophic molecules such as brain-derived neurotrophic factor (BDNF) (Gill et al., 2016; Hernandez-Torres et al., 2017), or the neuroinflammatory response (Kiernan et al., 2016; Lynch et al., 2016) all have the potential to impact the response to ampakine and/or hypoxia. We suggest that probing the potential differences in the phrenic motor response to ampakine at early and late time points following SCI is an important target of future investigations.
Significance
These data show that the low impact ampakine CX717 can stimulate a bilateral increase in neural drive to the diaphragm after incomplete cervical SCI. The impact of CX717 was most evident during baseline recording conditions associated with steady but not high “respiratory drive” to the phrenic motor system. There was no suggestion of an impact of CX717 on the response to intense chemoreceptor stimulation (i.e., “max challenge”, Figure 6). On the other hand, there was potential indication of an enhanced response to moderate hypoxia following CX717, but the p-value (0.06) fell short of the typically used 0.05 criteria. Collectively, the data indicate that during conditions of low to moderate respiratory drive, ampakines can stimulate neural drive to the diaphragm following cervical SCI.
The current work also indicates that a single bout of moderate, isocapnic hypoxia (arterial PO2 ~ 50 mmHg) is not sufficient to induce a consistent and robust enhancement of ampakine-induced phrenic motor facilitation in rats with C2Hx injury. It may be that the mechanisms enabling the previously reported hypoxia-ampakine interactions in the spinal intact condition (Turner et al., 2018; Wollman et al., 2020) may not be effectively activated after C2Hx. Alternatively, additional bouts of hypoxia (e.g., 3 – 10 short episodes, (Tester et al., 2014; Turner et al., 2016)) may enable more robust and reproducible responses to the combination of ampakine + hypoxia.
Translation of ampakine treatment to clinical use in the SCI population may be possible since initial clinical trials related to cognitive and affective disorders show minimal side effects at therapeutic doses (Doraiswamy and Xiong, 2006; Oertel et al., 2010; Porrino et al., 2005; Wesensten et al., 2007). In specific, ampakine CX717 has been administered to spinally intact humans without any reported adverse events (Boyle et al., 2012; Oertel et al., 2010; Wesensten et al., 2007). However, autonomic and cardiovascular dysfunction are of paramount concern in persons with SCI and have the potential to be impacted by ampakines. We therefore recommend additional testing of ampakines in awake (non-anesthetized) rodent models of SCI in which arterial blood pressure and heart rate and breathing can be monitored in real time.
Highlights.
Cervical spinal cord injury (cSCI) impairs phrenic motor output (PMO)
Ampakines are positive allosteric modulators of AMPA receptors
Ampakine CX717 increased bilateral PMO at 2- and 8 weeks post-cSCI
Ampakines may help restore respiratory muscle activation after cSCI
Acknowledgements:
This work was supported by funding from the National Institute of Health, grant numbers: 1T32HL134621-01A1 (LBW), K99 HL143207-01 (KS), F32NS095620-01 (KS), 1R01HL139708-01A1 (DDF). We are grateful to Dr. Arnold Lippa of RespireRx for providing the ampakine CX717 used in these experiments.
References
- Alilain WJ, Goshgarian HG, 2008. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Experimental neurology 212, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Kessler M, Rogers G, Lynch G, 1996. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. The Journal of pharmacology and experimental therapeutics 278, 627–638. [PubMed] [Google Scholar]
- Arai AC, Kessler M, 2007. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Current drug targets 8, 583–602. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Rogers G, Lynch G, Kessler M, 2002. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. The Journal of pharmacology and experimental therapeutics 303, 1075–1085. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E, 2004. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience 123, 1011–1024. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. The Journal of physiology 529 Pt 1, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X, 2012. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of disease 47, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Hormigo KM, Marchenko V, Lane MA, 2018. Spontaneous respiratory plasticity following unilateral high cervical spinal cord injury in behaving rats. Experimental neurology 305, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk DJ, 2012. Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep. Journal of psychopharmacology 26, 1047–1057. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN, 1996. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain research 715, 104–112. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Xiong GL, 2006. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert opinion on pharmacotherapy 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG, 1990. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. The Journal of comparative neurology 302, 707–714. [DOI] [PubMed] [Google Scholar]
- ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, Greer JJ, Fuller DD, 2015. Stimulation of Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease by Ampakines. Am J Respir Cell Mol Biol 53, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS, 2000. Long term facilitation of phrenic motor output. Respiration physiology 121, 135–146. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS, 2005. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. Journal of neurotrauma 22, 203–213. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB Jr., Mitchell GS, 2006. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol 100, 800–806. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr., Mitchell GS, 2003. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Feldman JL, 1995. Generation of respiratory rhythm and pattern in mammals: insights from developmental studies. Current opinion in neurobiology 5, 778–785. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL, 1993. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol 70, 1497–1515. [DOI] [PubMed] [Google Scholar]
- Gill LC, Gransee HM, Sieck GC, Mantilla CB, 2016. Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons. Respiratory physiology & neurobiology 226, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS, 2005. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. Journal of applied physiology 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, 2003. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol 94, 795–810. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, 2009. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respiratory physiology & neurobiology 169, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Gonzalez Porras MA, Zhan WZ, Sieck GC, Mantilla CB, 2017. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. The Journal of comparative neurology 525, 1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL, 1991. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. The Journal of physiology 437, 727–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC, 2017. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. Journal of neurophysiology 117, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan EA, Smith SM, Mitchell GS, Watters JJ, 2016. Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia. The Journal of physiology 594, 1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ, 2009. Spinal circuitry and respiratory recovery following spinal cord injury. Respiratory physiology & neurobiology 169, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC, 1990. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. Journal of neurophysiology 64, 423–436. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Funk GD, Greer JJ, 2010. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PloS one 5, e8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, 2006. Glutamate-based therapeutic approaches: ampakines. Current opinion in pharmacology 6, 82–88. [DOI] [PubMed] [Google Scholar]
- Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ, 2016. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. The journal of spinal cord medicine, 1–9. [DOI] [PMC free article] [PubMed]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC, 2012. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Experimental neurology 234, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG, 1992. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Experimental neurology 116, 219–228. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lotsch J, 2010. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clinical pharmacology and therapeutics 87, 204–211. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA, 2007. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol 582, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA, 2005. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS biology 3, e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Mantilla CB, Sieck GC, 2019a. Glutamatergic input varies with phrenic motor neuron size. Journal of neurophysiology 122, 1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Sieck GC, Mantilla CB, 2019b. Heterogeneous glutamatergic receptor mRNA expression across phrenic motor neurons in rats. Journal of neurochemistry. [DOI] [PMC free article] [PubMed]
- Ren J, Ding X, Funk GD, Greer JJ, 2009. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110, 1364–1370. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ, 2012. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. Journal of applied physiology 113, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ, 2015. Ampakines enhance weak endogenous respiratory drive and alleviate apnea in perinatal rats. American journal of respiratory and critical care medicine 191, 704–710. [DOI] [PubMed] [Google Scholar]
- Ren J, Lenal F, Yang M, Ding X, Greer JJ, 2013. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 118, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G, 2006. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. Journal of neurophysiology 96, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD, 2009. Respiratory recovery following high cervical hemisection. Respiratory physiology & neurobiology 169, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG, 2018. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 562, 419–422. [DOI] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL, 2003. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. The Journal of physiology 547, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB, 2009. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respiratory physiology & neurobiology 169, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, Fuller DD, 2016. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. Journal of applied physiology 120, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel S, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD, 2017. Intermittent Hypoxia Enhances Functional Connectivity of Midcervical Spinal Interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 8349–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel SR, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD, 2020. Mid-cervical interneuron networks following high cervical spinal cord injury. Respiratory physiology & neurobiology 271, 103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, 2014. Long-term facilitation of ventilation in humans with chronic spinal cord injury. American journal of respiratory and critical care medicine 189, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Streeter KA, Greer J, Mitchell GS, Fuller DD, 2018. Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respiratory physiology & neurobiology 256, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, ElMallah MK, Hoyt AK, Greer JJ, Fuller DD, 2016. Ampakine CX717 potentiates intermittent hypoxia-induced hypoglossal long-term facilitation. Journal of neurophysiology 116, 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesensten NJ, Reichardt RM, Balkin TJ, 2007. Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviation, space, and environmental medicine 78, 937–943. [DOI] [PubMed] [Google Scholar]
- Wilkerson JER, Devinney M, Mitchell GS, 2018. Intermittent but not sustained moderate hypoxia elicits long-term facilitation of hypoglossal motor output. Respiratory physiology & neurobiology 256, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Bode RK, Felton D, Chen D, Meyer PR Jr., 2002. Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest 121, 1548–1554. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J, 2003. Effect of spinal cord injury on the respiratory system. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 82, 803–814. [DOI] [PubMed] [Google Scholar]
- Wollman LB, Streeter KA, Fuller DD, 2020. Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation. Journal of neurophysiology 123, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG, 2007. Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Experimental neurology 206, 137–145. [DOI] [PubMed] [Google Scholar]