Synopsis
Four decades ago Costa and colleagues identified a small, secreted polypeptide in the brain capable of displacing the benzodiazepine diazepam from the GABAA receptor, and thus was coined Diazepam Binding Inhibitor (DBI). Shortly after, an identical polypeptide was identified in liver on its ability to induce termination of fatty acid synthesis and named Acyl-CoA Binding Protein (ACBP). Since then, ACBP/DBI has been studied in parallel without a clear and integrated understanding of its dual role. The first genetic loss-of-function models have revived the field, allowing targeted approaches to better understand the physiological roles of ACBP/DBI in vivo. Here we discuss the roles of ACBP/DBI in central and tissue-specific functions in mammals, with emphasis on metabolism and mechanisms of action.
Keywords: Acyl-CoA binding protein (ACBP), Diazepam binding inhibitor (DBI), acyl-CoA, fatty acid metabolism, GABAA receptor, G-protein coupled receptor
Nearly half a century ago, enkephalins and endorphins were discovered as endogenous agonists of opiate receptors. Together with the discovery of specific benzodiazepine binding sites the putative concept of an endogenous benzodiazepine-type ligand (“endozepine”) was born [1]. Soon after, Guidotti and Costa [2] and Skolnick [3] independently of each other identified that crude extracts from rat cerebral cortex and bovine brain, respectively, contain factors that were able to displace diazepam from binding to specific recognition sites on crude synaptic membranes. Subsequently, Costa and Guidotti successfully purified Diazepam Binding Inhibitor (DBI) from rat brains to homogeneity and found that when injected intracerebroventricularly it completely reversed the anticonflict action of diazepam on unpunished drinking and facilitated shock-induced suppression of drinking [4]. Interestingly, the anxiety-like behavior caused by injection of DBI was blocked by flumazenil, a GABAAR benzodiazepine-binding site antagonist, confirming that DBI binds to the benzodiazepine binding site on GABAAR. Independently of these studies, a polypeptide identical to DBI was also purified to homogeneity from synaptosomes from bovine and human brain by Shoyab and colleagues [5].
Intriguingly, an 18-amino acid tryptic peptide, named octadecaneuropeptide (ODN), derived from DBI was found to be significantly more potent than DBI to displace binding of the benzodiazepine receptor inverse agonist β-carboline-3-carboxylate methyl ester and in its pro-conflict action [6]. Subsequently, an ODN complementary oligonucleotide was used to screen cDNA libraries and thus clone the mature mRNA of DBI from both rat and human brain [7, 8]. Concurrently, in an effort to understand how termination of fatty acid synthesis in goat mammary gland was regulated by fatty acid binding protein (FABP), Mogensen et al. found that an impurity in an FABP preparation strongly induced synthesis of medium-chain fatty acids [9]. Further characterization of this protein from both bovine and rat liver demonstrated that ACBP is a 10 kDa cytosolic protein, which specifically binds medium- and long-chain acyl-CoA (LCACoA) esters (and not fatty acids and nucleotides), and was thus termed acyl-CoA binding protein, ACBP. Amino acid sequencing showed that this novel acyl-CoA binding protein was identical to DBI [10–12]. Importantly, ACBP/DBI has no sequence homology or structural similarity to fatty acid binding proteins (FABPs).
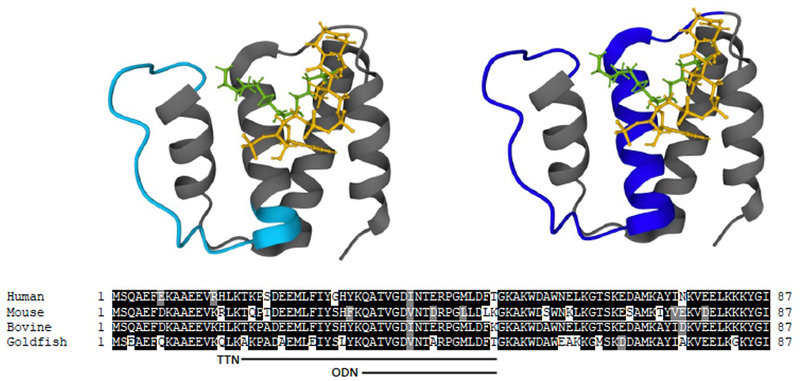
Purification from various phyla and sequencing of numerous prokaryotic and eukaryotic genomes have identified numerous independent proteins containing the ACBP/DBI structural fold; not only as an independent protein but also proteins containing ACBP/DBI as a subdomain, which thus have been coined acyl-CoA binding domain-containing proteins (ACBDs) [13]. Hence, the ACBDs comprise a highly conserved multigene family of proteins, which are found in all eukaryotes and ubiquitously expressed in all metazoan tissues, with distinct expression patterns for individual ACBDs. For example, in humans the ACBP domain is present in a total of 7 different proteins, which include ACBP/DBI (ACBD1/DBI), ECI2 (ACBD2), ACBD3 (GCP60/PAP7), ACBD4, ACBD5 (membrane associated DBI), ACBD6 and ACBD7. In addition, multiple transcript variants of human ACBP/DBI have been identified, including three high abundant and eleven low-abundant transcript variants [13–15]. The structures of apo- and holo forms of ACBP/DBI from both lower and higher eukaryotic species have been solved by NMR spectroscopy and/or X-ray crystallography showing that its overall structure as a four-α-helix bundle protein is highly evolutionarily conserved [16–23] (Figure 1).
Figure 1. Tertiary structure of holo DBI/ACBP (bovine) with bound palmitoyl-CoA.
The peptide backbone is depicted in grey (PDB 1NVL), while the acyl-group and CoA of palmitoyl-CoA are shown in green and yellow, respectively. Two peptides that can be derived from ACBP/DBI, ODN (ACBP/DBI33–50) and TTN (ACBP/DBI17–50), are shown in light blue and blue, respectively. In the lower panel sequences of ACBP/DBI from human, mouse, bovine and goldfish have been aligned and sequences covering ODN and TTN shown.
Acyl-CoA Binding Protein as an Intracellular Protein Regulating Lipid Metabolism
Lessons from in vitro studies
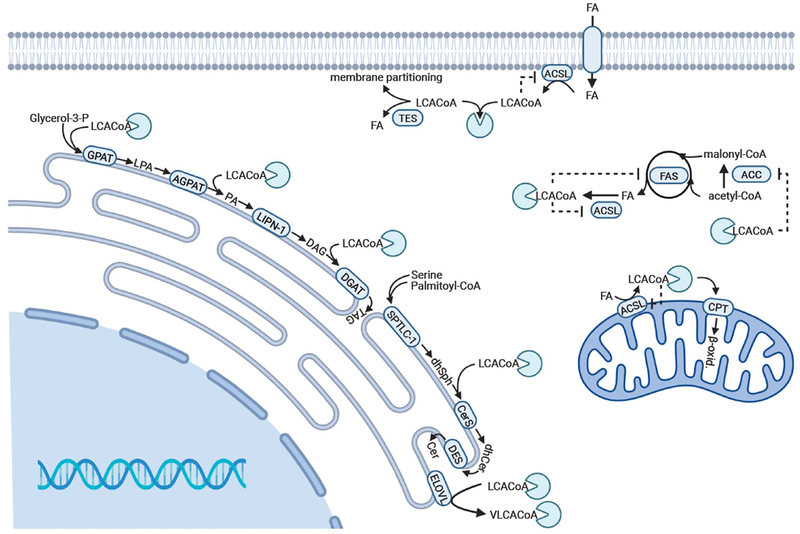
Soon after its identification as an acyl-CoA binding protein, its ability to modulate the regulatory functions or utilization of LCACoAs was examined in vitro. ACBP/DBI can effectively prevent the inhibitory effects of LCACoAs on mitochondrial acyl-CoA synthetase, acetyl-CoA carboxylase and on the mitochondrial adenine nucleotide translocase and can protect LCACoAs from hydrolysis [24]. Rasmussen et al. elegantly showed that ACBP/DBI is capable of desorbing LCACoAs from multilamellar liposomes and donating them to microsomal glycerolipid synthesis [25]. Similarly, ACBP can also stimulate the activities of acyl-CoA-lysophospholipid acyltransferase [26], glycerophosphate acyl-transferase (GPAT) [27–29], acyl-CoA/cholesterol acyltransferase [30], and stimulate synthesis of very-long chain fatty acids [31], ceramides [32] and steroids [33]. Furthermore, ACBP/DBI can also act as an intracellular pool former of LCACoAs [34–37]. Interestingly, ACBP/DBI not only stimulates anabolic pathways, as in vitro kinetic studies also show that LCACoAs can be donated directly to carnitine palmitoyl transferase I (CPTI) by ACBP/DBI augmenting mitochondrial β-oxidation [25, 38, 39]. Consistently, knock down of ACBP/DBI by siRNA in human non-small cell lung cancer cells and in glioblastoma cells significantly impairs mitochondrial fatty acid oxidation [40, 41]. In addition, ACBP/DBI deficiency in mouse astrocytes reduces fatty acid oxidation and esterification into glycerolipids [42] (Figure 2).
Figure 2. Selected intracellular functions of ACBP/DBI.
ACBP/DBI (illustrated as a light green Pacman shape) can act as an acyl-CoA pool former, protecting long-chain acyl-CoA esters (LCACoAs) from hydrolysis by thioesterases (TES), extract LCACoAs from membranes, deliver LCACoAs to glycerolipid, glycerophospholipid, and ceramide synthesis (CerS), to fatty acid elongation by fatty acid elongases (ELOVL) and to mitochondrial β-oxidation. ACBP/DBI also relieves inhibition of the enzymes FAS, ACC and ACS by LCACoAs. Abbreviations: ACAT, acyl-CoA:cholesterol acyltransferase; ACC, acyl-CoA carboxylase; ACSL, acyl-CoA synthetase; AGPAT, acylglycerol-3-phosphate-acyltransferase; Cer, ceramide; CerS, ceramide synthase; CPT1, carnitine palmitoyltransferase 1; dhSph, dihydrosphingosine; dhCer, dihydroceramide; ELOVL, elongation of very long chain fatty acids; FAS, fatty acid synthase; GPAT, glycerol-3-phosphat acyltransferase; LIPN-1, Lipin-1; SPTLC1, Serine Palmitoyltransferase Long Chain Base Subunit 1.
Lessons from in vivo studies
In an effort to understand the function of ACBP/DBI in vivo numerous groups have generated transgenic mouse and rat models overexpressing ACBP/DBI. Transgenic mice overexpressing ACBP/DBI to levels resembling those found after high fat feeding showed no change in body and liver weights compared to wild-type mice, even though LCACoAs levels are increased in livers from transgenic mice relative to wild-type [34]. Similarly, acyl-CoA esters accumulate in liver and adipose tissue in transgenic rats overexpressing ACBP [43], supporting that ACBP/DBI also functions as an acyl-CoA pool former and that ACBP/DBI relieves product inhibition of ACSL and protects LCACoAs from hydrolysis by thioesterases [24]. The LCACoAs accumulated in the microsomal compartment rather than in the cytosol, supporting that ACBP/DBI donates LCACoAs to membrane-bound lipid synthesizing systems. This is consistent with increased GPAT activity and triacylglycerol (TAG) and phospholipid content in livers from ACBP/DBI-overexpressing transgenic mice [34], in keeping with augmented TAG levels with overexpression of ACBP/DBI in McA-RH7777 rat hepatoma cells [44] (Figure 2).
Landrock et al. first reported that loss of ACBP/DBI in mice results in early pre-implantation embryonic lethality at the 8-cell stage [45]. However, numerous subsequent studies have shown that loss of ACBP/DBI does not result in lethality, but indeed leads to significant metabolic phenotypes. Lee and colleagues found that CBA/J mice carrying a 400 kb spontaneous pleiotropic deletion (nm1054), including the Acbp/Dbi gene, are easily distinguishable from their wild-type littermates shortly after birth due to the smaller body size and pale appearance [46]. Interestingly, when bred on a C57BL/6J background the vast majority of live-born mutants die prior to P7, and only few wean successfully [47]. When bred on a mixed background (129S6/SvEvTac x C57BL/6J), however, the nm1054 mice survive to adulthood and are characterized by having sparse, matted, reddish hair with a greasy appearance, along with sebocyte hyperplasia in skin of thorax and nose [46]. Mice with a targeted disruption of the Acbp/Dbi gene (Acbp/Dbi−/−) are viable and fertile and born in a Mendelian ratio [48]. However, the ability of Acbp/Dbi−/− mice to survive weaning is significantly decreased unless the pups are supplied with soaked food and water in the bottom of the cage. Consistent with the phenotypes observed in the nm1054 mice, Acbp/Dbi−/− mice also display tousled and greasy fur, alopecia, and scaling of the skin with age [49]. Interestingly, in comparison to wild-type littermates, Acbp/Dbi−/− mice display delayed hepatic upregulation of SREBP-regulated gene programs at weaning, which is accompanied by a significantly diminished hepatic de novo cholesterol synthesis. The observed suppression of SREBP activity may be due to a transient accumulation of TAG and cholesteryl esters (CE) in the liver of Acbp/Dbi−/− mice caused by increased lipolysis in white adipose tissue [48, 50]. Notably, expression of SREBP target genes is not impaired in liver-specific ACBP/DBI knockout mice but only in mice lacking ACBP/DBI in keratinocytes [50]. Accordingly, application of artificial barriers to the skin of Acbp/Dbi−/− mice rescues hepatic SREBP-regulated gene expression [50]. This finding and the fact that skin-specific loss of ACBP/DBI phenocopy the macroscopic phenotypes observed in Acbp/Dbi−/−, and that transepidermal water loss is significantly increased in both full-body and skin-specific Acbp/Dbi−/− mice [50] collectively show that ACBP/DBI is required for establishing and/or maintaining the epidermal barrier. The impaired epidermal barrier is likely due to decreased synthesis of very long-chain fatty acids and ceramides in the stratum corneum, as ACBP/DBI regulates both synthesis of very long-chain fatty acids and ceramides [31, 32, 49, 51] (Figure 2).
After weaning and during aging Acbp/Dbi−/− mice still have increased trans-epidermal water loss and heat dissipation, but also develop alopecia and depigmentation of the fur. Both full-body ACBP/DBI knockout mice and mice lacking ACBP/DBI in keratinocytes have increased energy expenditure, food intake, and browning of the inguinal white adipose tissue, which all are reversed by housing the mice at thermoneutrality or by blocking β-adrenergic receptor signalling [52]. In line with increased energy expenditure, both full-body and skin-specific Acbp/Dbi−/− mice are completely resistant to diet-induced obesity and the diabetogenic effects of a high-fat diet [52]. Noticeably, metabolic profiling by indirect calorimetry shows that Acbp/Dbi−/− mice have a higher respiratory quotient than wild-type and skin-specific Acbp/Dbi−/− mice [52] arguing that ACBP/DBI facilitates oxidation of fatty acids in other tissues than the skin, and thereby contributes to systemic utilization of fatty acids as energy substrates. Consistent with an in vivo role in fatty acid oxidation, oxidation of unsaturated fatty acids is significantly reduced in both cortical and hypothalamic astrocytes, and in mediobasal hypothalamus slices derived from Acbp/Dbi−/− mice [42]. Besides its role under physiological conditions, intracellular ACBP/DBI also appears to be an important player in tumor growth. Duman et al. recently demonstrated that ACBP/DBI supports proliferation of malignant gliomas by controlling transport of LCACoA to mitochondrial β-oxidation, using both in vitro assays and intracranial xenografts in mice (Figure 2). Accordingly, ACBP/DBI ablation in patient-derived glioblastoma cells or AKT-PDGF-B-driven murine cancer cells increased the survival of tumor-bearing mice [41]. In line with these observations, ACBP/DBI also regulates proliferation and fatty acid oxidation in non-small-cell lung cancer cells in vitro and predicts the outcome for individuals with lung cancer [40]. Given the prominent expression of ACBP/DBI in samples from a wide variety of human cancer types (TCGA Research Network, https://www.cancer.gov/tcga), it is tempting to speculate that ACBP/DBI has a general role in tumor malignancies.
ACBP/DBI as a secreted protein with multiple functions
Unconventional protein secretion and regulation of autophagy by ACBP/DBI
Besides serving crucial intracellular functions in lipid metabolism, ACBP/DBI is also secreted to the extracellular environment as a leaderless protein via the unconventional secretory pathway [53–58]. Accordingly, ACBP/DBI remains in the cytoplasmic compartment after translation and is thus not processed by the prohormone convertases PCSK1 and PCSK2 in the endoplasmic reticulum, the Golgi apparatus and secretory granules, as conventional neuropeptide precursors. Secretion of ACBP/DBI requires Golgi-associated proteins (e.g. GRASP, plasma membrane t-SNARES and formation of autophagosomes) and accordingly its secretion is augmented by autophagy-inducing conditions including starvation and inhibitors of TORC1 signaling [57]. However, the mechanisms involved in ACBP/DBI secretion might be context- and cell type-dependent since it was shown that insulin and glucose, signals known to inhibit autophagy, stimulate ACBP/DBI secretion in hypothalamic explants [59] and cultured tanycytes [60]. Interestingly, ACBP/DBI has also been detected in extracellular vesicles secreted by different breast cancer cell lines [61, 62], but whether this is directly linked to the unconventional secretion of ACBP/DBI is not known.
ACBP/DBI-derived peptides and sites of action
ACBP/DBI comprises several separate basic residues that are prone to processing by trypsin-like endoproteases. Consistently, as mentioned above, tryptic digestion at the C-terminus of lys32 and lys50 of rat ACBP/DBI generates ODN [6]. Besides ODN, several other biologically active peptides from ACBP/DBI have been isolated from brain tissue. Using affinity chromatography with immobilized anti-ODN antibodies, Slobodyansky et al. isolated a number of biologically active ACBP/DBI-derived peptides, including triakontatetraneuropeptide (TTN) (DBI17–50), eicosapentaneuropeptide (EPN) (DBI26–50), and triakontaheptaneuropeptide (THN) (DBI39–75) [63, 64] from rat brain. Interestingly, intracerebroventricular (ICV) injection of TTN produces a pro-conflict behavior, which was prevented by the translocator protein (TSPO) antagonist PK11195 but not by the GABAAR antagonist flumazenil, arguing that TTN binds and signals through TSPO (also known as mitochondrial benzodiazepine receptor), while ODN functions through GABAAR. Moreover, TTN displaces binding of the TSPO ligand Ro5–4864 from rat olfactory bulb synaptic membranes [63]. Like TTN, THN also displaces both Ro5–4864 and PK 11195 binding to crude mitochondrial membranes and stimulates mitochondrial steroid synthesis [65], thus collectively arguing that TTN and THN preferentially bind to specific mitochondrial benzodiazepine receptors, while ODN preferentially binds to GABAAR benzodiazepine-binding sites. An intracellular effect of ACBP/DBI derived peptides would, however, require that the peptides are internalized or ACBP/DBI is cleaved intracellularly. This still remains unexplored.
Role of ACBP/DBI in the brain
ACBP and neurogenesis
In the brain, ACBP/DBI is highly expressed in cells of glial nature, namely mature astrocytes, tanycytes, ependymal cells and undifferentiated neural progenitors, but virtually absent in young or mature neurons [42, 66]. There are in the adult mammalian brain two main neurogenic niches, the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus [67]. Adult neural stem cells (aNSC) proliferate and generate intermediate progenitors that in turn produce either inhibitory or excitatory neurons. ACBP/DBI expression is prominent in aNSCs and progenitors both from the SVZ and SGZ, where it regulates neurogenesis rates via GABA signaling [66, 68]. ACBP/DBI and its cleavage peptide ODN binds to the benzodiazepine binding site of GABAAR, at the interface between alpha and gamma subunits [69], and aNSCs express GABAAR containing a gamma-2 subunit, a specific component of the receptor that provides sensitivity to ACBP/DBI. Experiments using transgenic mice bearing a mutated gamma-2 subunit that prevents ACBP/DBI binding to GABAAR and in vivo overexpression of the short peptide ODN demonstrated that ACBP/DBI acts via GABA signaling to promote cell proliferation in the adult neurogenic niches [66, 68]. Interestingly, ACBP/DBI is also highly expressed in neural stem cells and progenitors in the embryonic brain (Allen Brain Atlas, https://portal.brain-map.org/), where it also modulates neurogenesis rates via its function as a negative modulator of GABA-induced currents (Julieta Alfonso, unpublished). In summary, brain-derived ACBP/DBI regulates neurogenesis both throughout development and in the mature brain primarily via its effects on GABA signaling (Figure 3).
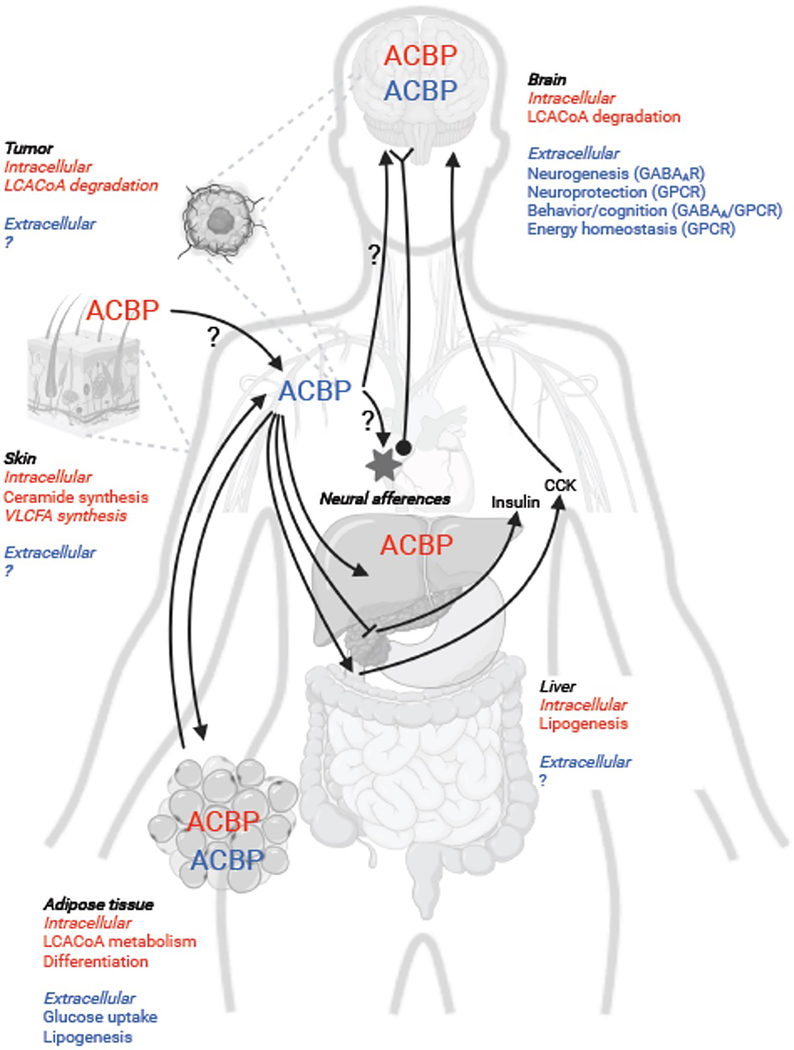
Figure 3. Pleiotropic roles of ACBP/DBI as a secreted extracellular (in blue) and intracellular (in red) protein on peripheral lipid metabolism, hormone secretion and brain functions.
In most cells, intracellular ACBP plays a key role in intracellular fatty acid fluxes and metabolism including LCFACoA oxidation and esterification in neural and tumor cells as well as lipogenesis in fat and liver cells. In the skin, ACBP promotes ceramide and very-long chain fatty acid synthesis which is essential to maintain the trans-epidermal barrier and energy homeostasis. In the brain, secreted ACBP promotes neurogenesis and neuroprotection via GABAAR and ODN-GPCR-dependent pathways respectively. In addition, extracellular ACBP modulates synaptic transmission, neural function, and diverse types of behavior including feeding behavior. Circulating ACBP levels are affected by changes in the metabolic status and fat mass. Extracellular ACBP regulates hormone secretion in the gut (CCK) and endocrine pancreas (insulin) as well as lipid metabolism in adipocytes. Peripheral administration of ACBP promotes feeding and anabolic pathways (lipogenesis in liver and fat cells) though it remains unclear whether the effects of exogenous ACBP on metabolism and food intake are direct (via GABAAR and/or ODN-GPCR) and/or indirect (via hormone secretion and/or afferent neurons).
Anti-seizure and behavior-modifying actions of ACBP/DBI in the brain
Recent evidence also indicates central actions of ACBP/DBI that are sufficiently powerful to modulate distinct forms of cognitive and social behaviors and to suppress certain forms of seizure activity. nm1054 mice, which as noted above lack ACBP/DBI, and α3(H126R) mice, which bear a point mutation in the benzodiazepine binding site of α3 subunit-containing GABAARs, displayed reduced duration of α3-mediated inhibitory postsynaptic currents in the thalamic reticular nucleus (nRT) and insensitivity to flumazenil. These deficits were rescued by viral restoration of ACBP/DBI in the nRT, suggesting a constitutive activation of α3-containing GABAAR benzodiazepine binding sites by an endogenous ACBP/DBI-derived ligand in nRT [70] that appears to be produced primarily in astrocytes [71]. Both nm1054 and α3(H126R) mice also exhibited increased susceptibility to chemical induction of absence seizures, indicating endogenous anti-seizure effects of ACBP/DBI [70]. Furthermore, constitutive ACBP/DBI knockout mice exhibited increased GABAAR-mediated synaptic inhibition in hippocampal CA1 pyramidal cells and decreased inhibitory tone in dentate granule cells compared with wild-type littermates [72], indicating discrete differences in the direction of ACBP/DBI-mediated modulation between subregions. These changes in hippocampal synaptic transmission appear to be reflected in behavioral consequences as well, as ACBP/DBI knockout mice exhibited disrupted hippocampus-dependent spatial learning [73]. Intriguingly, this impairment is similar to that observed in a ACBP/DBI-overexpressing mouse [74], suggesting that disruption of ACBP/DBI signaling in either gain- or loss-of-function directions can lead to cognitive deficits. ACBP/DBI also appears to play a role in contextual fear conditioning [73, 75] and social motivation and interest, but not social recognition [76] or anxiety-like behavior, although it affects the anxiolytic effect of diazepam [77]. Collectively, these studies indicate that ACBP/DBI acts in multiple brain areas to modulate synaptic transmission, neural function, and diverse types of behavior (Figure 3). Notably, it still remains unresolved as to what extent these behavioral effects are mediated by ACBP/DBI or by one or more derived fragment peptides.
ACBP/DBI and neuroprotection
Accumulating evidence demonstrates that ACBP/DBI and ODN exert neuroprotective effects in vitro and in vivo by preventing neuronal cell death induced by neurotoxic molecules targeting dopaminergic neurons e.g. 6-Hydroxydopamine hydrobromide (6-OHDA) and 1-methyl-4phenyl-1,2,3,6-tetrahydropyrine (MPTP)) [78]. ODN protective action in cultured neurons is GABAAR-independent and relies on an unidentified G-protein coupled receptor (GPCR)-mediated activation of PKC and ERK leading to upregulation of the anti-apoptotic factor Bcl-2 [79]. Accordingly, ACBP/DBI deficient mice are more susceptible to neuronal death induced by MPTP [80]. It was further demonstrated that ACBP/DBI and ODN reduced astrogliosis, inflammatory responses and promote astrocyte survival in response to MPTP or oxidative stress through activation of PKA and ERK in an ODN-GPCR-dependent manner [80, 81]. Together, these findings suggest that ODN secreted by astrocytes may have dual protective effects on both astrocytes and neurons through autocrine and paracrine actions, and ODN-GPCR-dependent activation of cell-specific signaling pathways [14]. Further investigations will be required to determine whether impairments in ACBP/DBI expression, secretion and/or signaling plays a role in the etiology of neuroinflammation and neurodegenerative diseases.
Role of brain ACBP/DBI on energy balance
In hypothalamic astrocytes and tanycytes, ACBP/DBI expression is reduced by fasting in a circadian manner [56, 82] while metabolic signals of energy sufficiency (i.e. insulin and glucose) [59, 82] increase its expression, suggesting that ACBP/DBI is implicated in energy sensing in hypothalamic glial cells [42, 56, 77]. Shortly after establishing the anxiogenic and proconflict responses induced by central injection of ACBP/DBI or ODN, it was reported that ICV administration of ODN or its C-terminal octapeptide (OP) exert a strong anorectic action in goldfish [83] and rodents [84, 85]. Interestingly, it was further demonstrated by pharmacological means that the anorectic action of central ODN or OP neither implicates GABAAR nor TSPO but the unidentified ODN-GPCR [85], probably coupled to Gq, phospholipase C, and Ca2+ signaling as previously found in hypothalamic neurons [56]. The anorectic effect of ODN is associated with increased expression of the anorectic neuropeptide pro-opiomelanocortin (POMC) and decreased expression of the orexigenic neuropeptide Y (NPY) in the arcuate nucleus (ARC) suggesting that ODN acts on ARC neurons to reduce food intake [86]. This model is supported by findings showing that ICV administration of ODN or OP increases c-Fos expression in POMC neurons [60] and that treatment of hypothalamic slices with OP, ODN or the cyclic agonist of the ODN GPCR increases the firing of POMC neurons in the ARC in a GABAAR-independent manner [56, 60]. In agreement, the anorectic effect of ODN in goldfish [87] and rodents [56, 59] is dependent on the hypothalamic melanocortin 4 receptor (MC4R). Despite these findings supporting a key role of the hypothalamic melanocortin system in the anorectic action of ODN, it is important to note that ODN or OP administration restricted to the 4V in the brainstem also reduces feeding [88], an effect that may be dependent on the activation of brainstem POMC neurons [61,64]. In addition, it was recently shown that ODN or OP induces c-Fos expression in non-POMC neurons including nesfatin/NUCB2, tyrosine hydroxylase, and GLP-1 neuronal populations in the hypothalamus and brainstem [60], suggesting that anorexia induced by exogenous ODN or OP relies on the recruitment of different neuronal circuits.
The role of endogenously expressed ACBP/DBI in feeding and energy balance was recently reported using targeted deletion of ACBP/DBI in Glial Fibrillary Acidic Protein (GFAP)+ astrocytes or in ependymocytes and ARC neurons [56]. Interestingly, targeted deletion of ACBP in GFAP+ astrocytes (pan-brain astroglial deletion that also targeted ACBP/DBI in some ARC tanycytes), but not in Nkx2.1- neuronal lineage and ependymal cells, increased weight gain in chow fed conditions and the susceptibility to high fat diet-induced obesity and glucose intolerance in both male and female mice. This phenotype was prevented by virally-mediated rescue of ACBP/DBI expression in GFAP+ astrocytes of the ARC but not in tanycytes [56]. Although energy expenditure and locomotor activity were slightly reduced in knockout mice, this obesity-prone phenotype was mostly dependent on hyperphagia. These findings suggest that endozepinergic tone deficiency in ARC astrocytes plays a key role in the development of diet-induced obesity and increasing endozepine signaling in the ARC may thus have beneficial effects on energy homeostasis in obese rodent models. In line with this notion, daily central administration of OP in obese mice fed with a high fat diet reduces body weight and food intake thereby improving glucose intolerance [60]. In leptin-deficient obese (ob/ob) mice, the findings are more discrepant. Indeed, Bouyakdan et al. showed that acute or chronic daily ICV administration of ODN or the ODN-GPCR agonist reduces body weight and food intake in ob/ob mice [56] while Guillebaud et al. found that ob/ob mice are unresponsive to ICV OP [60]. The latter study further showed that OP sensitizes ob/ob mice to the anorectic action of exogenous leptin by promoting leptin transport into the hypothalamus via median eminence and ARC tanycytes. Interestingly, the anorectic action of ICV leptin is not affected in astroglial ACBP/DBI knockout mice, in which ACBP/DBI expression is reduced in some but not all tanycytes [56]. The reason for this discrepancy could implicate differences in the rodent models and means to modulate ACBP/DBI in ARC astrocytes vs. tanycytes. Nonetheless, these recent studies demonstrate that ACBP/DBI in ARC astrocytes and tanycytes is critical for the maintenance of a healthy energy balance [56]. Interestingly, Lanfray et al. recently found that a paralog gene of ACBP/DBI, coding for the acyl-CoA binding domain containing protein (ACBD7), is expressed in some ARC POMC and GABAergic neurons. Similar to ACBP/DBI, ACBD7 expression is increased during states of energy sufficiency in the ARC and processed into an anorexigenic nonadecaneuropeptide, NDN, (ACBD734–52) [89]. Neither injection of flumazenil nor PK11195 blunted NDN-induced anorexia, while both the ODN GPCR antagonist cyclo1–8[DLeu5]ODN(11–18) and a MC4R-specific antagonist (HS024) blocked NDN anorectic action, collectively arguing that NDN does not act via the classical benzodiazepine receptors GABAAR or TSPO but rather via a still uncharacterized GPCR and the melanocortin pathway [89]. Although these studies support a major role of secreted ACBP/DBI and its processed peptides in the central control of energy balance, one cannot rule out the possibility that regulation of LCACoA metabolism in glial cells by intracellular ACBP/DBI [41, 42] contributes to the observed phenotypes. In agreement, astroglial ACBP/DBI knockout mice are unresponsive to the anorectic effect of ICV oleate [56]. Additional investigations will be required to decipher the contribution of glial intra- vs. extracellular glial ACBP/DBI in the central regulation of energy homeostasis.
Collectively, targeted deletion of ACBP/DBI in the brain or periphery reveals that ACBP/DBI is indispensable for whole-body energy homeostasis, and that ACBP/DBI regulates fatty acid oxidation, complex lipid synthesis and specific signaling pathways in a tissue-specific manner (Figure 3).
Is ACBP a novel lipogenic factor?
Recently, Kroemer and colleagues found that ACBP/DBI is secreted to the circulation in response to starvation in an autophagy-dependent manner [57]. Interestingly, addition of neutralizing ACBP antibodies or recombinant ACBP/DBI to cultured human cells stimulated and inhibited, respectively, cellular starvation-induced autophagy [57]. Moreover, knockdown of ACBP/DBI in human neuroglioma cells inhibited starvation-induced autophagy, but interestingly augmented autophagy in ACBP/DBI-expressing cells, collectively arguing that extracellular ACBP/DBI may impede cellular autophagy by acting in an autocrine or paracrine manner [57]. Accordingly, this study also reported that systemic injection of recombinant ACBP/DBI stimulates food intake, lowers glucose levels, activates orexigenic neurons in the lateral hypothalamus, and augments expression of lipogenic genes like fatty acid synthase and PPARγ in liver and white adipose tissue. Conversely, neutralizing antibodies or induced autoimmunity towards ACBP/DBI in mice triggers hyperglycemia, hypophagia, and weight loss, and reduced adiposity of white adipose tissue and the adverse diabetogenic effects of a high-fat diet. Consistently with this notion, adult mice with a conditional systemic loss of ACBP/DBI gain less weight upon a high-fat diet and are highly sensitive to fasting-induced weight loss [57]. Interestingly, the orexigenic effect of ACBP/DBI injection is mediated via GABAAR but independently of its ability to bind LCACoAs [90]. Altogether, these observations imply that ACBP/DBI exerts an extracellular role modulating food intake and lipid and carbohydrate metabolism. Interestingly, conditional loss of ACBP/DBI in white adipocytes is sufficient to render mice resistant to high-fat diet induced weight gain [91].
It should be noted, however, that regulation of circulatory ACBP/DBI levels is complex, as starvation, high-fat diet, and hyperphagia all manifest in increased expression of ACBP/DBI in adipose tissue and liver in mice, correlating with its levels in the circulation. Accordingly, ACBP/DBI has been suggested to function as a hunger factor that induces appetite and anabolic functions. Consistent with this postulate, ACBP/DBI levels have been reported to be significantly elevated in severely obese individuals and conversely reduced in anorexic individuals, thus correlating positively with BMI [57, 90]. However, circulatory ACBP/DBI levels seem to follow a dual pattern, since voluntary fasting or disease-associated undernutrition augments ACBP/DBI levels [91], which on the other hand is in keeping with starvation- and autophagy-induced increases in ACBP/DBI level as described above. Accordingly, sepsis or systemic inflammation in rodents and human patients, known to induce anorexia, are associated with increased plasma ACBP/DBI levels and expression of ACBP/DBI in the hypothalamus [92, 93].
ACBP/DBI as modulator of exocrine and endocrine pancreas secretion
In 1996 Herzig et al. isolated a tryptic-sensitive peptide from porcine intestinal mucosa on its ability to stimulate cholecystokinin (CCK) secretion when administered intraduodenally in rat. This peptide was found to be identical to ACBP/DBI by mass spectrometry analyses and peptide sequencing of tryptic fragments. Accordingly, it was found that picomolar concentrations of synthetic porcine ACBP/DBI could stimulate pancreatic enzyme secretion and CCK release from porcine intestinal mucosa cells and in rat intestine [94] and later in an intestinal tumor cell line [95]. ODN also stimulated CCK release and pancreatic secretion, but the effect was 100 times less potent than that of ACBP/DBI [94]. In keeping with this observation, Li et al. found that anti-ACBP/DBI serum prevents CCK release induced by intraduodenal infusion of ACBP/DBI [96].
Besides having an effect on exocrine pancreatic functions and intestinal secretion, low picomolar levels of both ACBP/DBI and ODN significantly reduce glucose-induced insulin secretion in vivo and in isolated rat Langerhans islets [97–99]. By similar means, ODN also inhibits glucose-induced insulin secretion from HIT-115 insulinoma cells likely via a signalling pathway that affects cytosolic Ca2+ levels [100]. Consistent with these observations, ACBP/DBI antibodies potentiate glucose-induced insulin release from isolated islets [98]. Interestingly, ACBP/DBI is expressed in all tissues including the intestine and both the endocrine and exocrine pancreas (Human Protein Atlas, http://www.proteinatlas.org), collectively arguing that ACBP/DBI can potently modulate both exocrine and endocrine pancreas functions that control central regulation of food intake and systemic metabolic functions (Figure 3).
Conclusion and future outlook
In this review, we have highlighted and discussed the discovery and multiple functions of Diazepam Binding Inhibitor, also recognized as Acyl-CoA Binding Protein (Figure 3). Clearly, as an intracellular lipid binding protein, ACBP/DBI plays key roles in sequestering and mediating flux and the regulatory properties of LCACoAs (Figure 2). ACBP/DBI binds LCACoAs with very-high affinity (KD 1–10 nM) and thus likely requires a specific interaction with utilizing enzymes to relieve its bound ligand as reported for ceramide synthases [32]. Interestingly, lipid membranes (e.g., anionic lipids) can promote a charged–dependent destabilizing effect that weakens ligand binding, thus enabling ACBP/DBI to mediate transport to membranes [101]. Future mechanistic studies will reveal if interactions with specific utilizing enzymes have similar effects on ligand binding.
As a secreted peptide, ACBP/DBI and its derived peptides have pleiotropic effects including gut and pancreatic hormone secretion, while brain-derived ACBP/DBI regulates neurogenesis, neuron survival, cognition and behavior as well as energy balance neurocircuits (Figure 3). Importantly, the diverse biological effects of central ACBP/DBI rely on specific signaling pathways that implicate GABAAR, TSPO or the unidentified ODN-GPCR in a brain region- and cell-specific manner. The recent discoveries and current hypotheses of ACBP/DBI being a lipogenic and hunger factor raise numerous questions and new interesting challenges that need to be tackled to fully depict and understand its in vivo functions (see Outstanding questions). In keeping with a function as a secreted lipogenic factor, ACBP/DBI has recently been reported to positively correlate with BMI [57]. By contrast, plasma levels of ACBP/DBI have also been reported to be significantly lower in morbidly obese subjects compared to normal weight individuals [102], and that conditions that induce anorexia like sepsis or systemic inflammation are associated with increased plasma ACBP/DBI levels [92, 93]. In addition, overexpression of ACBP/DBI in rats neither affects body weight nor glucose tolerance [43, 103]. Joseph et al. recently reported that adipocyte-specific loss of ACBP/DBI was sufficient to render mice resistant to diet-induced obesity, thus consolidating its function as a secreted lipogenic factor [91]. Although reported not to obscure skin functions [91], adipocyte-specific loss of ACBP/DBI seemingly impairs the insulating dermal fat layer, leaving it plausible that adipocyte-specific loss of ACBP/DBI impairs development of dermal adipocytes thus augmenting heat loss across the dermal barrier ultimately resulting in increased energy expenditure and hence protect against an increased accumulation of calories. In keeping with such notion, ACBP/DBI is required for adipocyte differentiation [104], and loss of ACBP/DBI in keratinocytes (which likely does not affect circulating levels of ACBP/DBI) specifically impairs the epidermal barrier and hence increases systemic energy expenditure and protects against the diabetogenic effects of a high fat diet [52].
Depending on the metabolic state, circulatory levels of ACBP/DBI in humans range from 10–300 ng/ml [57, 91], corresponding to approximately 1–30 nM. Notably, picomolar levels of ACBP/DBI stimulate CCK secretion (well known to promote satiety) and inhibit insulin secretion (an anorectic hormone) in vivo, while the orexigenic effects of ACBP/DBI in mice was obtained by i.v. injection of 0.5 mg/kg recombinant ACBP/DBI [57, 90], corresponding approximately to 0.75 μM, likely are mediated via GABAAR and by activation of orexigenic neurons in the lateral hypothalamus [57, 90]. Although inactivation of ACBP/DBI in the circulation by neutralizing antibodies reduces food intake post-starvation, the orexigenic effects are obtained by abnormally high concentrations of ACBP/DBI. Further, in contrast to previous findings [97–99], injection of recombinant ACBP did not affect insulin levels [57]. These discrepancies call for additional careful investigations of the concentrations required to obtain the orexigenic effects of ACBP/DBI. In addition, it still remains elusive whether ACBP/DBI is cleaved after administration thereby changing ODN and/TTN levels and their respective effects, and if ACBP/DBI neutralizing antibodies also neutralize the biological effects of endogenous systemic ODN and/or TTN. Finally, it cannot be excluded that ACBP/DBI-neutralizing antibodies reach and penetrate circumventricular organs (CVO), including the median eminence and ARC where the blood-brain barrier is leaky, and thus inhibit the anorectic effect of glia-derived ACBP/DBI. In the same line, high levels of recombinant ACBP entering the brain through CVO could desensitize the ODN GPCR to promote feeding.
Although ACBP/DBI has been reported to serve intracellular as well as extracellular functions, it cannot be excluded that these functionalities somehow are coordinated. Although ACBP/DBI is secreted via the unconventional secretory pathway that involves the autophagic machinery, its regulation remains elusive. Thus, as extracellular ACBP/DBI may regulate autophagy in an autocrine or paracrine manner, intracellular ACBP/DBI and/or LCACoAs may also regulate autophagy as well as the activity of the unconventional secretory pathway and therefore also ACBP/DBI secretion. Finally, the extent to which the extracellular effects are mediated by full-length ACBP/DBI or by one of its derived peptides remains elusive, emphasizing the importance of studying the underlying molecular mechanisms of ACBP/DBI functions in further details.
ACKNOWLEDGEMENTS
We apologize for not citing other relevant publications due to space limitations. This work was supported by grants from the Novo Nordisk Foundation (NNF20OC0064744) (N.J.F), NIH R01 NS105825 and Brain and Behavior Research Foundation NARSAD Young Investigator Grant 24086 (C.A.C.-H.), Chica and Heinz Schaller Foundation (J.A.), Canadian Institutes of Health Research (CIHR) PJT153035 (T.A.). T.A. was supported by a salary award from Fonds de Recherche Québec-Santé (FRQS). We thank Ditte Neess for valuable graphical help.
GLOSSARY
- ACBP
Acyl-CoA binding protein. It belongs to the family of acyl-CoA-binding domain-containing proteins and binds long-chain acyl-CoA esters with high affinity and specificity. By mediating intracellular transport of long-chain acyl-CoA esters, ACBP directionally channels these substrates to utilizing enzymes
- Anorectic
causing a loss of appetite, e.g. an anorectic drug reduces appetite, resulting in lower food consumption, ultimately leading to weight loss
- Autophagy
a highly regulated cellular mechanism that degrades and recycles unnecessary or dysfunctional components in an ordered fashion. Besides being a pathway that is induced to protect against starvation, autophagy also plays a major role in cellular homeostasis of non-starved cells
- β-oxidation
the catabolic process by which two carbon atoms are removed from fatty acids in the form of acetyl-CoA from an acyl-CoA ester at the carboxyl terminal. Besides acetyl-CoA, FADH2 and NADH are also generated. Acetyl-CoA subsequently enters the TCA cycle while FADH2 and NADH are oxidized via the electron transport chain to generate ATP. In eukaryotes, β-oxidation of medium- and long-chain acyl-CoA esters primarily takes place in the mitochondria
- DBI
diazepam binding inhibitor. DBI and ACBP are identical proteins discovered independently of each other. DBI is capable of displacing benzodiazepines from binding to the type-A γ-aminobutyric acid receptors (GABAARs) and of binding to translocator protein (TSPO) on the outer mitochondrial membrane
- GABA
γ-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the mature mammalian central nervous system. GABA binds to either GABAA or GABAC receptors, which are ligand-activated chloride channels, or GABAB metabotropic receptors, which are G-protein coupled receptors, that open or close potassium channels via G-protein signaling
- GPCR
G protein-coupled receptors, also known as seven-transmembrane domain receptors, which comprise a vast group of evolutionarily conserved surface receptors that respond to extracellular cues to regulate the activity of various effector proteins to produce 2nd messengers including cAMP and Ins-1,4,5P3
- Lipogenic
involved in the metabolic process through which acetyl-CoA is converted to fatty acids and triglycerides. Fatty acids are produced in the cytosol via fatty acid synthase by repeatedly adding two-carbon units to acetyl-CoA. Triglycerides are synthesized in the endoplasmic reticulum via a series of esterifications of fatty acids to a glycerol backbone. Both processes take place mainly in liver and adipose tissue
- Long chain acyl-CoA
LCACoA, activated long-chain fatty acids and key intermediates in numerous lipid metabolic pathways, and recognized as important signaling molecules. Acyl-CoA esters are synthesized by acyl-CoA synthetases in an energetically costly reaction that uses the equivalent of two high-energy bonds
- Melanocortin pathway
plays a crucial role in systemic energy homeostasis. The fed state is signaled by abundance of circulating hormones such as leptin and insulin, which bind to receptors expressed at the surface of POMC neurons to promote processing of POMC to α-MSH. After release, α-MSH then signals to decrease energy intake by binding to melanocortin-4 receptor (MC4R) expressed by MC4R neurons in the paraventricular nucleus of the hypothalamus
- Neurogenesis
the process by which new neurons are produced from neural stem cells. Neurogenesis is most active during embryonic development and is responsible for producing all types of neurons of an organism. Neurogenesis also continues in certain brain regions after birth in the mammalian brain
- POMC
pro-opiomelanocortin is the precursor of a number of biologically active peptides including circulating melanocyte stimulating hormone (α-MSH), adrenocorticotropin hormone (ACTH), β-lipotropin and β-endorphin. Differential cleavage of POMC by proprotein convertases occur in the different tissues where it is expressed, resulting in diverse biological functions e.g. POMC-expressing neurons in the brain play a role in the control of pain via the formation of β-endorphin
- TTN and ODN
posttranslational processing of DBI/ACBP gives rise to TTN (DBI17–50) and ODN (DBI33–50). DBI/ACBP binds to TSPO and to the benzodiazepine binding site on GABAAR, while ODN is a more selective ligand for the binding site on GABAAR, and TTN is more selective towards binding to TSPO
- Unconventional secretory pathway
a secretory pathway comprising either extracellular secretion of cytosolic proteins without a signal peptide (e.g. leaderless proteins) or cell-surface trafficking of signal-peptide-containing transmembrane proteins independent of the endoplasmic reticulum or Golgi apparatus
BIBLIOGRAPHY
- 1.Iversen L (1977) Anti-anxiety receptors in the brain? Nature 266 (5604), 678–678. [Google Scholar]
- 2.Guidotti A et al. (1978) An endogenous protein modulates the affinity of GABA and benzodiazepine receptors in rat brain. Nature 275 (5680), 553–5. [DOI] [PubMed] [Google Scholar]
- 3.Skolnick P et al. (1978) Identification of inosine and hypoxanthine as endogenous inhibitors of [3H] diazepam binding in the central nervous system. Life Sci 23 (14), 1473–80. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti A et al. (1983) Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A 80 (11), 3531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoyab M et al. (1986) Isolation and characterization of a putative endogenous benzodiazepineoid (endozepine) from bovine and human brain. J Biol Chem 261 (26), 11968–73. [PubMed] [Google Scholar]
- 6.Ferrero P et al. (1986) Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci U S A 83 (3), 827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocchetti I et al. (1986) Putative diazepam binding inhibitor peptide: cDNA clones from rat. Proc Natl Acad Sci U S A 83 (19), 7221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray PW et al. (1986) Cloning and expression of cDNA for human diazepam binding inhibitor, a natural ligand of an allosteric regulatory site of the gamma-aminobutyric acid type A receptor. Proc Natl Acad Sci U S A 83 (19), 7547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogensen IB et al. (1987) A novel acyl-CoA-binding protein from bovine liver. Effect on fatty acid synthesis. Biochem J 241 (1), 189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsen J et al. (1987) Amino acid sequence of acyl-CoA-binding protein from cow liver. Biochem J 245 (3), 857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen J and Knudsen J (1987) Acyl-CoA-binding protein from cow. Binding characteristics and cellular and tissue distribution. Biochem J 248 (3), 709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen J et al. (1989) Acyl-CoA-binding protein in the rat. Purification, binding characteristics, tissue concentrations and amino acid sequence. Biochem J 262 (2), 513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neess D et al. (2015) Long-chain acyl-CoA esters in metabolism and signaling: Role of acyl-CoA binding proteins. Prog Lipid Res 59, 1–25. [DOI] [PubMed] [Google Scholar]
- 14.Tonon MC et al. (2020) Endozepines and their receptors: Structure, functions and pathophysiological significance. Pharmacol Ther 208, 107386. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun B et al. (2021) Glial endozepines and energy balance: Old peptides with new tricks. Glia 69 (5), 1079–1093. [DOI] [PubMed] [Google Scholar]
- 16.Andersen KV and Poulsen FM (1992) Three-dimensional structure in solution of acyl-coenzyme A binding protein from bovine liver. J Mol Biol 226 (4), 1131–41. [DOI] [PubMed] [Google Scholar]
- 17.Kragelund BB et al. (1993) Three-dimensional structure of the complex between acyl-coenzyme A binding protein and palmitoyl-coenzyme A. J Mol Biol 230 (4), 1260–77. [DOI] [PubMed] [Google Scholar]
- 18.Teilum K et al. (2005) Different secondary structure elements as scaffolds for protein folding transition states of two homologous four-helix bundles. Proteins 59 (1), 80–90. [DOI] [PubMed] [Google Scholar]
- 19.van Aalten DM et al. (2001) Binding site differences revealed by crystal structures of Plasmodium falciparum and bovine acyl-CoA binding protein. J Mol Biol 309 (1), 181–92. [DOI] [PubMed] [Google Scholar]
- 20.Taskinen JP et al. (2007) High resolution crystal structures of unliganded and liganded human liver ACBP reveal a new mode of binding for the acyl-CoA ligand. Proteins 66 (1), 229–38. [DOI] [PubMed] [Google Scholar]
- 21.Monzani PS et al. (2010) A new topology of ACBP from Moniliophthora perniciosa. Biochim Biophys Acta 1804 (1), 115–23. [DOI] [PubMed] [Google Scholar]
- 22.Costabel MD et al. (2006) Structure of armadillo ACBP: a new member of the acyl-CoA-binding protein family. Acta Crystallogr Sect F Struct Biol Cryst Commun 62 (Pt 10), 958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin J et al. (2020) Crystal structure of the rice acyl-CoA-binding protein OsACBP2 in complex with C18:3-CoA reveals a novel pattern of binding to acyl-CoA esters. FEBS Lett 594 (21), 3568–3575. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen JT et al. (1993) Interaction of acyl-CoA binding protein (ACBP) on processes for which acyl-CoA is a substrate, product or inhibitor. Biochem J 292 ( Pt 3), 907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen JT et al. (1994) Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for beta-oxidation and glycerolipid synthesis. Biochem J 299 ( Pt 1), 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fyrst H et al. (1995) Detection of acyl-CoA-binding protein in human red blood cells and investigation of its role in membrane phospholipid renewal. Biochem J 306 ( Pt 3), 793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossett RE et al. (1998) Structure and function of normal and transformed murine acyl-CoA binding proteins. Arch Biochem Biophys 350 (2), 201–13. [DOI] [PubMed] [Google Scholar]
- 28.Jolly CA et al. (2000) Microsomal fatty acyl-CoA transacylation and hydrolysis: fatty acyl-CoA species dependent modulation by liver fatty acyl-CoA binding proteins. Biochim Biophys Acta 1483 (1), 185–97. [DOI] [PubMed] [Google Scholar]
- 29.Kannan L et al. (2003) Aging and acyl-CoA binding protein alter mitochondrial glycerol-3-phosphate acyltransferase activity. Biochim Biophys Acta 1631 (1), 12–6. [DOI] [PubMed] [Google Scholar]
- 30.Chao H et al. (2003) ACBP and cholesterol differentially alter fatty acyl CoA utilization by microsomal ACAT. J Lipid Res 44 (1), 72–83. [DOI] [PubMed] [Google Scholar]
- 31.Gaigg B et al. (2005) Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J Biol Chem 280 (23), 22515–22. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira NS et al. (2017) Regulation of very-long acyl chain ceramide synthesis by acyl-CoA-binding protein. J Biol Chem 292 (18), 7588–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boujrad N et al. (1993) Inhibition of hormone-stimulated steroidogenesis in cultured Leydig tumor cells by a cholesterol-linked phosphorothioate oligodeoxynucleotide antisense to diazepam-binding inhibitor. Proc Natl Acad Sci U S A 90 (12), 5728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H et al. (2005) Acyl-coenzyme A binding protein expression alters liver fatty acyl-coenzyme A metabolism. Biochemistry 44 (30), 10282–97. [DOI] [PubMed] [Google Scholar]
- 35.Mandrup S et al. (1993) Effect of heterologous expression of acyl-CoA-binding protein on acyl-CoA level and composition in yeast. Biochem J 290 ( Pt 2), 369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schjerling CK et al. (1996) Disruption of the gene encoding the acyl-CoA-binding protein (ACB1) perturbs acyl-CoA metabolism in Saccharomyces cerevisiae. J Biol Chem 271 (37), 22514–21. [DOI] [PubMed] [Google Scholar]
- 37.Wang W et al. (2021) A Ratiometric Fluorescent Biosensor Reveals Dynamic Regulation of Long-Chain Fatty Acyl-CoA Esters Metabolism. Angew Chem Int Ed Engl 60 (25), 13996–14004. [DOI] [PubMed] [Google Scholar]
- 38.Abo-Hashema KA et al. (2001) The interaction of acyl-CoA with acyl-CoA binding protein and carnitine palmitoyltransferase I. Int J Biochem Cell Biol 33 (8), 807–15. [DOI] [PubMed] [Google Scholar]
- 39.Hostetler HA et al. (2011) Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyl transferase I. Mol Cell Biochem 355 (1–2), 135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris FT et al. (2014) Acyl-coenzyme A-binding protein regulates Beta-oxidation required for growth and survival of non-small cell lung cancer. Cancer Prev Res (Phila) 7 (7), 748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duman C et al. (2019) Acyl-CoA-Binding Protein Drives Glioblastoma Tumorigenesis by Sustaining Fatty Acid Oxidation. Cell Metab 30 (2), 274–289 e5. [DOI] [PubMed] [Google Scholar]
- 42.Bouyakdan K et al. (2015) A novel role for central ACBP/DBI as a regulator of long-chain fatty acid metabolism in astrocytes. J Neurochem 133 (2), 253–65. [DOI] [PubMed] [Google Scholar]
- 43.Oikari S et al. (2008) Downregulation of PPARs and SREBP by acyl-CoA-binding protein overexpression in transgenic rats. Pflugers Arch 456 (2), 369–77. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y et al. (2001) Overexpression of acyl-coA binding protein and its effects on the flux of free fatty acids in McA-RH 7777 cells. Lipids 36 (6), 595–600. [DOI] [PubMed] [Google Scholar]
- 45.Landrock D et al. (2010) Acyl-CoA binding protein gene ablation induces pre-implantation embryonic lethality in mice. Lipids 45 (7), 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee L et al. (2007) Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J Invest Dermatol 127 (1), 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohgami RS et al. (2005) nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood 106 (10), 3625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neess D et al. (2011) Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. The Journal of biological chemistry 286 (5), 3460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bloksgaard M et al. (2012) The acyl-CoA binding protein is required for normal epidermal barrier function in mice. J Lipid Res 53 (10), 2162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neess D et al. (2013) Delayed hepatic adaptation to weaning in ACBP−/− mice is caused by disruption of the epidermal barrier. Cell Rep 5 (5), 1403–12. [DOI] [PubMed] [Google Scholar]
- 51.Gaigg B et al. (2001) Depletion of acyl-coenzyme A-binding protein affects sphingolipid synthesis and causes vesicle accumulation and membrane defects in Saccharomyces cerevisiae. Mol Biol Cell 12 (4), 1147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neess D et al. (2021) Epidermal Acyl-CoA-binding protein is indispensable for systemic energy homeostasis. Mol Metab 44, 101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anjard C and Loomis WF (2005) Peptide signaling during terminal differentiation of Dictyostelium. Proc Natl Acad Sci U S A 102 (21), 7607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duran JM et al. (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 188 (4), 527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manjithaya R et al. (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188 (4), 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouyakdan K et al. (2019) The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J Clin Invest 129 (6), 2417–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo-San Pedro JM et al. (2019) Acyl-CoA-Binding Protein Is a Lipogenic Factor that Triggers Food Intake and Obesity. Cell Metab 30 (6), 1171. [DOI] [PubMed] [Google Scholar]
- 58.Loomis WF et al. (2010) Pregnenolone sulfate and cortisol induce secretion of acyl-CoA-binding protein and its conversion into endozepines from astrocytes. J Biol Chem 285 (28), 21359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanfray D et al. (2013) Gliotransmission and brain glucose sensing: critical role of endozepines. Diabetes 62 (3), 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guillebaud F et al. (2020) Glial Endozepines Reverse High-Fat Diet-Induced Obesity by Enhancing Hypothalamic Response to Peripheral Leptin. Mol Neurobiol 57 (8), 3307–3333. [DOI] [PubMed] [Google Scholar]
- 61.Rontogianni S et al. (2019) Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun Biol 2, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhondt B et al. (2020) Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J Extracell Vesicles 9 (1), 1736935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slobodyansky E et al. (1989) Isolation and characterization of a rat brain triakontatetraneuropeptide, a posttranslational product of diazepam binding inhibitor: specific action at the Ro 5–4864 recognition site. J Neurochem 53 (4), 1276–84. [DOI] [PubMed] [Google Scholar]
- 64.Slobodyansky E et al. (1994) Purification of a novel DBI processing product, DBI39–75, and characterization of its binding site in rat brain. Regul Pept 50 (1), 29–35. [DOI] [PubMed] [Google Scholar]
- 65.Papadopoulos V et al. (1991) Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology 129 (3), 1481–8. [DOI] [PubMed] [Google Scholar]
- 66.Dumitru I et al. (2017) Diazepam Binding Inhibitor Promotes Stem Cell Expansion Controlling Environment-Dependent Neurogenesis. Neuron 94 (1), 125–137 e5. [DOI] [PubMed] [Google Scholar]
- 67.Obernier K and Alvarez-Buylla A (2019) Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alfonso J et al. (2012) Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10 (1), 76–87. [DOI] [PubMed] [Google Scholar]
- 69.Sigel E and Ernst M (2018) The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol Sci 39 (7), 659–671. [DOI] [PubMed] [Google Scholar]
- 70.Christian CA et al. (2013) Endogenous positive allosteric modulation of GABA(A) receptors by diazepam binding inhibitor. Neuron 78 (6), 1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christian CA and Huguenard JR (2013) Astrocytes potentiate GABAergic transmission in the thalamic reticular nucleus via endozepine signaling. Proc Natl Acad Sci U S A 110 (50), 20278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Courtney CD and Christian CA (2018) Subregion-Specific Impacts of Genetic Loss of Diazepam Binding Inhibitor on Synaptic Inhibition in the Murine Hippocampus. Neuroscience 388, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ujjainwala AL et al. (2019) Differential impacts on multiple forms of spatial and contextual memory in diazepam binding inhibitor knockout mice. J Neurosci Res 97 (6), 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siiskonen H et al. (2007) Diazepam binding inhibitor overexpression in mice causes hydrocephalus, decreases plasticity in excitatory synapses and impairs hippocampus-dependent learning. Mol Cell Neurosci 34 (2), 199–208. [DOI] [PubMed] [Google Scholar]
- 75.Sherrin T et al. (2009) Region specific gene expression profile in mouse brain after chronic corticotropin releasing factor receptor 1 activation: the novel role for diazepam binding inhibitor in contextual fear conditioning. Neuroscience 162 (1), 14–22. [DOI] [PubMed] [Google Scholar]
- 76.Ujjainwala AL et al. (2018) Genetic loss of diazepam binding inhibitor in mice impairs social interest. Genes Brain Behav 17 (5), e12442. [DOI] [PubMed] [Google Scholar]
- 77.Budry L et al. (2016) DBI/ACBP loss-of-function does not affect anxiety-like behaviour but reduces anxiolytic responses to diazepam in mice. Behav Brain Res 313, 201–207. [DOI] [PubMed] [Google Scholar]
- 78.Masmoudi-Kouki O et al. (2018) Neuroprotection with the Endozepine Octadecaneuropeptide, ODN. Curr Pharm Des 24 (33), 3918–3925. [DOI] [PubMed] [Google Scholar]
- 79.Kaddour H et al. (2013) The octadecaneuropeptide ODN prevents 6-hydroxydopamine-induced apoptosis of cerebellar granule neurons through a PKC-MAPK-dependent pathway. J Neurochem 125 (4), 620–33. [DOI] [PubMed] [Google Scholar]
- 80.Bahdoudi S et al. (2018) Neuroprotective effects of the gliopeptide ODN in an in vivo model of Parkinson’s disease. Cell Mol Life Sci 75 (11), 2075–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghouili I et al. (2018) Endogenous Expression of ODN-Related Peptides in Astrocytes Contributes to Cell Protection Against Oxidative Stress: Astrocyte-Neuron Crosstalk Relevance for Neuronal Survival. Mol Neurobiol 55 (6), 4596–4611. [DOI] [PubMed] [Google Scholar]
- 82.Compere V et al. (2010) Acute food deprivation reduces expression of diazepam-binding inhibitor, the precursor of the anorexigenic octadecaneuropeptide ODN, in mouse glial cells. J Mol Endocrinol 44 (5), 295–9. [DOI] [PubMed] [Google Scholar]
- 83.Matsuda K et al. (2007) Effect of the diazepam-binding inhibitor-derived peptide, octadecaneuropeptide, on food intake in goldfish. Neuroscience 150 (2), 425–32. [DOI] [PubMed] [Google Scholar]
- 84.de Mateos-Verchere JG et al. (2001) The octadecaneuropeptide [diazepam-binding inhibitor (33–50)] exerts potent anorexigenic effects in rodents. Eur J Pharmacol 414 (2–3), 225–31. [DOI] [PubMed] [Google Scholar]
- 85.do Rego JC et al. (2007) Pharmacological characterization of the receptor mediating the anorexigenic action of the octadecaneuropeptide: evidence for an endozepinergic tone regulating food intake. Neuropsychopharmacology 32 (7), 1641–8. [DOI] [PubMed] [Google Scholar]
- 86.Compere V et al. (2003) Effect of intracerebroventricular administration of the octadecaneuropeptide on the expression of pro-opiomelanocortin, neuropeptide Y and corticotropin-releasing hormone mRNAs in rat hypothalamus. J Neuroendocrinol 15 (2), 197–203. [DOI] [PubMed] [Google Scholar]
- 87.Matsuda K et al. (2010) The anorexigenic action of the octadecaneuropeptide (ODN) in goldfish is mediated through the MC4R- and subsequently the CRH receptor-signaling pathways. J Mol Neurosci 42 (1), 74–9. [DOI] [PubMed] [Google Scholar]
- 88.Cavaillon JM and Haeffner-Cavaillon N (1985) The role of serum in interleukin 1 production by human monocytes activated by endotoxins and their polysaccharide moieties. Immunol Lett 10 (1), 35–41. [DOI] [PubMed] [Google Scholar]
- 89.Lanfray D et al. (2016) Involvement of the Acyl-CoA binding domain containing 7 in the control of food intake and energy expenditure in mice. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joseph A et al. (2020) Metabolic and psychiatric effects of acyl coenzyme A binding protein (ACBP)/diazepam binding inhibitor (DBI). Cell Death Dis 11 (7), 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joseph A et al. (2021) Effects of acyl-coenzyme A binding protein (ACBP)/diazepam-binding inhibitor (DBI) on body mass index. Cell Death Dis 12 (6), 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clavier T et al. (2014) Increased plasma levels of endozepines, endogenous ligands of benzodiazepine receptors, during systemic inflammation: a prospective observational study. Crit Care 18 (6), 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clavier T et al. (2016) Increased Hypothalamic Levels of Endozepines, Endogenous Ligands of Benzodiazepine Receptors, in a Rat Model of Sepsis. Shock 45 (6), 653–9. [DOI] [PubMed] [Google Scholar]
- 94.Herzig KH et al. (1996) Diazepam binding inhibitor is a potent cholecystokinin-releasing peptide in the intestine. Proc Natl Acad Sci U S A 93 (15), 7927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida H et al. (1999) Diazepam-binding inhibitor33–50 elicits Ca2+ oscillation and CCK secretion in STC-1 cells via L-type Ca2+ channels. Am J Physiol 276 (3 Pt 1), G694–702. [DOI] [PubMed] [Google Scholar]
- 96.Li Y et al. (2000) Diazepam-binding inhibitor mediates feedback regulation of pancreatic secretion and postprandial release of cholecystokinin. J Clin Invest 105 (3), 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borboni P et al. (1991) Modulation of insulin secretion by diazepam binding inhibitor and its processing products. Neuropharmacology 30 (12B), 1399–403. [DOI] [PubMed] [Google Scholar]
- 98.Ostenson CG et al. (1994) Inhibition by rat diazepam-binding inhibitor/acyl-CoA-binding protein of glucose-induced insulin secretion in the rat. Eur J Endocrinol 131 (2), 201–4. [DOI] [PubMed] [Google Scholar]
- 99.Ostenson CG et al. (1990) Effects of porcine diazepam-binding inhibitor on insulin and glucagon secretion in vitro from the rat endocrine pancreas. Regul Pept 29 (2–3), 143–51. [DOI] [PubMed] [Google Scholar]
- 100.De Stefanis P et al. (1995) Inhibitory effect of ODN, a naturally occurring processing product of diazepam binding inhibitor, on secretagogues-induced insulin secretion. Regul Pept 56 (2–3), 153–65. [DOI] [PubMed] [Google Scholar]
- 101.Micheletto MC et al. (2017) Lipid membranes and acyl-CoA esters promote opposing effects on acyl-CoA binding protein structure and stability. Int J Biol Macromol 102, 284–296. [DOI] [PubMed] [Google Scholar]
- 102.Siejka A et al. (2015) Reduced plasma level of diazepam-binding inhibitor (DBI) in patients with morbid obesity. Endocrine 49 (3), 859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oikari S et al. (2008) Effect of medium- and long-chain fatty acid diets on PPAR and SREBP-1 expression and glucose homeostasis in ACBP-overexpressing transgenic rats. Acta Physiol (Oxf) 194 (1), 57–65. [DOI] [PubMed] [Google Scholar]
- 104.Mandrup S et al. (1998) Inhibition of 3T3-L1 adipocyte differentiation by expression of acyl-CoA-binding protein antisense RNA. J Biol Chem 273 (37), 23897–903. [DOI] [PubMed] [Google Scholar]