Abstract
Mas-related G protein-coupled receptors (Mrgprs) are a family of receptors implicated in a diverse array of human diseases. Since their discovery in 2001, great progress has been made in determining their relation to human disease. Vital for Mrgprs therapeutic efforts across all disease disciplines is a thorough understanding of Mrgprs signal transduction pathways and polymorphisms, as these offer insights into new drug candidates, existing discrepancies in drug response, and differences in disease susceptibility. In this review, we discuss the current state of knowledge regarding Mrgprs signaling pathways and polymorphisms.
Keywords: Mrgprs, signaling pathways, polymorphism
Introduction
Mas-related G protein-coupled receptors (Mrgprs) are a family of receptors expressed primarily in small-diameter sensory neurons of the dorsal root ganglia (DRG) and trigeminal ganglia (TG) [18]. Due to this concentration in sensory neurons, Mrgprs have been widely studied for their role in somatosensation, with most research focused on itch and pain sensation [17, 26, 46–48, 72]. Later studies have shown that Mrgprs are expressed in a variety of other tissues and are implicated in a growing variety of disease pathologies and processes, such as bronchoconstriction, anaphylaxis, antibacterial immunity, and tumorigenesis [20, 44, 49, 53]. Understanding Mrgprs signal pathways and accompanying polymorphisms will greatly improve therapeutic efforts across disease disciplines by leading to the identification of new drug candidates and elucidating discrepancies in both existing drug response and disease susceptibility. Thus, herein we attempt to provide a brief but concise overview of the changing state of knowledge regarding Mrgprs signaling pathways and polymorphisms.
Overview of Mrgprs Signaling Pathways
Canonically, when an agonist molecule binds inactive G protein-coupled receptors (GPCRs) it induces the exchange of GDP for GTP on the bound Gα subunit. This induces a conformation change in the GPCR as the transmembrane helices V and VI move to create an opening on the cytoplasmic side, freeing the Gβγ dimer from being bound to the Gα protein. Newly activated Gα and Gβγ subunits can then bind downstream effectors, amplifying the GPCR signal. There exist four known families of Gα subunits (Gαstimulatory or Gαs, Gαinhibitory or Gαi/o, Gαq/11, and Gα12/13), each with a different signaling pathway [8, 40, 51, 65]. To date, Mrgprs have been found to utilize Gαq/11, Gαi/o, Gαs, and Gβγ but not Gα12/13 pathways.
Most known Mrgprs are associated with Gαq/11-dependent signaling. The typical downstream signaling pathway for Gαq/11 involves the activation of β-phosphodipase C (PLC), which then cleave phosphatidylinositol 4,5-biphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 can then activate calcium channels in the endoplasmic reticulum (ER), which along with DAG can culminate in protein kinase C (PKC) signaling [41].
Only MrgprD has been shown to utilize Gαs signaling. Activated Gαs binds adenylyl cyclase (AC) to produce cAMP, which in turn activates Protein Kinase A (PKA). PKA then performs a variety of functions including the phosphorylation of CREB proteins and TRP channels [41]. Specifically, PKA has been shown to sensitize both TRPV1 and TRPA1 [7, 45]. Many Mrgprs have been found to utilize the Gαi/o pathway, which directly inhibits adenylyl cyclase (AC) from producing cAMP, thus inhibiting the Gαs pathway [41]. Finally, several Mrgprs including MrgprA3, MrgprC11, and hMrgprX1 have been shown to utilize Gβγ-signaling. Similar to many Gα signaling pathways, Gβγ can activate PLC-β, PKC, and PKA [41].
MrgprA3
MrgprA3, which is expressed in about 6-7% of DRG neurons, mediates chloroquine-induced sensory neuron activation and itch sensation [37]. Controversial evidence has been presented arguing the key molecules required for MrgprA3 intracellular signaling. For instance, Wilson et al. demonstrated that both chloroquine-evoked calcium signals and action potentials in cultured mouse DRG sensory neurons are abolished with application of the Gβγ inhibitor gallein, suggesting that MrgprA3 couples to Gβγ in DRG sensory neurons [80]. They also found that chloroquine-induced neuronal responses were not affected by U73211, an inhibitor for the PLC family, suggesting that MrgprA3 does not couple to Gαq/11. However, Than et al. have shown that chloroquine induced depletion of PIP2 in HEK293 cells transfected with MrgprA3, suggesting the involvement of PLC in MrgprA3 downstream signaling in HEK293 cells [71]. It is possible that MrgprA3 couples to different signaling pathways in different cell types. Since in vitro heterologous systems are frequently used to investigate the GPCR signaling pathway, caution should be taken when interpreting the results and comparing them to the in vivo physiological condition.
Chloroquine-induced calcium responses in sensory neurons were completely blocked in the absence of extracellular Ca2+, suggesting that MrgprA3 activation by chloroquine leads to the opening of transduction channels. Several TRP channel family members including TRPC3, TRPVI, and TRPA1 have been tested for their role in MrgprA3 downstream signaling since they coexpress with MrgprA3 and are key transduction channels mediating the function of nociceptors [74, 81]. A possible role for TRPC3 has been suggested by Than et al., since both the general TRPC family blocker BTP2 and the TRPC3 specific inhibitor Pyr3 blocked chloroquine-induced sensory neuron excitation [71]. Sharif et al. found that while the TRPC3 blocker pyrazole 10, did not reduce chloroquine-induced itch-behavior suggesting that TRPC3 may not play a key role in MrgprA3 signaling [61].
The role of TRPV1 in MrgprA3 signaling is not clear. Studies have suggested that TRPV1 is not required for MrgprA3 signaling because both sensory neuron excitation and mouse scratching behavior induced by chloroquine were unaffected in TRPV1 knockout mice [58, 80]. However, Sharif et al. showed that TRPV1 inhibitor significantly reduced chloroquine-inducted itch behavior suggesting a potential role of TRPV1 in MrgprA3 downstream signaling pathway [61].
HC-030031, a TRPA1 inhibitor, successfully blocked Ca2+ responses in sensory neurons and scratching responses induced by chloroquine, suggesting the role of TRPA1 in MrgprA3 signaling [55, 61, 71, 80]. Liu et al. also found that application of ruthenium red, a broad TRP channel blocker, could reduce the calcium response elicited by chloroquine in primary sensory neurons [37]. However, recent evidence has shown that both ruthenium red and HC-030031 are not specific to TRP channels. They can induce inhibition of voltage-gated calcium channels (VGCC) in sensory neurons [13, 73], raising the possibility that the inhibitions of chloroquine signaling by ruthenium red and HC-030031 are independent of TRPA1.
Wilson et al. also demonstrated that chloroquine-induced intracellular Ca2+ signals in NG108 cells transfected with both TRPA1 and MrgprA3, but not in cells transfected with MrgprA3 alone. These data suggest that MrgprA3 couples to TRPA1 in NG108 cells to induce calcium influx. They also found that both sensory neuron activation and mouse scratching behaviors induced by chloroquine were abolished in TRPA1 knockout mice, leading to the conclusion that TRPA1 is the main downstream target for MrgprA3 [80].
However, recent evidence from Ru et al. argues against the requirement of TRPA1 for MrgprA3 signaling in sensory neurons [58]. Using ex vivo DRG nerve–skin preparation, Ru et al. performed extracellular recordings from cell bodies of sensory neurons while tested chemicals were applied to the skin. They found that chloroquine-induced action potential discharge in sensory neurons was not affected by HC-030031, ruthenium red, or the Gβγ inhibitor gallein. The behavioral analysis from Ru et al. is consistent with their cellular recording results, showing that chloroquine-induced scratching behavior was not affected in TRPA1 knockout mice. They also found that sensory neurons in TRPC3/TRPC6 double knockout mice exhibit similar action potential discharge induced by chloroquine in the WT neurons, suggesting that TRPC3 is not required for MrgprA3 signaling, which is consistent with Sharif et al.’s findings [61]. The discrepancy between these results and other studies may be due to differences in the subtleties of experimental design. It may also represent potential different mechanisms at the cell soma vs nerve terminals in the skin.
Ru et al. suggest the role of PLCβ3 in MrgprA3 signaling [58]. PLCβ3 is the only one among the 13 PLC family members that are highly expressed in MrgprA3+ neurons [74, 81]. Both chloroquine-induce scratching behavior and neuronal activities in the ex vivo nerve-skin preparation were abolished in PLCβ3 knockout mice [58], whereas Imamachi et al. showed that PLCβ3 knockout mice exhibited normal chloroquine-induced scratching responses [27].
Recent work from Sharif et al. has showed the multimodality of MrgprA3+ neurons [61]. Their results suggest that metabotropic Gq-linked stimulation of MrgprA3+ neurons (chloroquine or Dreadd) induces itch, whereas ionotropic stimulation (ChR2 or ATP gated P2X3 channels) induces pain sensation. They found that blockers for TRP channels attenuated itch behavior evoked by chloroquine, but did not affect pain behavior evoked by optical activation of MrgprA3+ neurons. These interesting results suggest that MrgprA3 can engage distinct intracellular signaling to exert different somatosensory effects.
MrgprC11
MrgprC11 is expressed in a subset of nociceptors that constitute approximately 18% of DRG neurons and includes almost all MrgprA3+ neurons [21, 22, 39]. It can be activated by multiple agonists including Bam8-22, SLIGRL, Cathepsin S, and RF-amide peptide family characterized by common C-terminal -RF(Y)G or -RF(Y) amide motif [22, 37, 38, 54]. Activation of MrgprC11+ nerves at different locations exert different somatosensory effects. Excitation of peripheral MrgprC11+ nerves in the skin by multiple pruritogens evokes itch sensation [17], whereas stimulation of central MrgprC11+ nerves by intrathecally delivered agonists induces analgesic effects [19, 35].
Results from several groups suggest that MrgprC11 mediated itch signaling involve Gαq/11. TRPA1, and Nav1.9. Han et al. utilized mouse embryotic fibroblast (MEF) cells derived from Gαq/11 or Gα12/13 KO mice to examine the required G proteins for downstream signaling of both MrgprC11 and MrgprA1 [22]. γ2-MSH and FLRFa were used to activate MrgprC11 and MrgprA1 transfected in MEFs respectively and induce calcium responses. They observed normal calcium responses in Gα12/13 KO MEFs. However, calcium responses were completely abolished in Gαq/11 KO MEFs, and the calcium responses were then rescued in Gαq/11 KO MEFs co-transfected with wildtype Gαq, suggesting that MrgprC11 and MrgprA1 utilize Gαq/11-dependent signaling in MEFs. Additionally, Han et al. found that γ2-MSH and FLRFa activation of HEK293 cells expressing MrgprC11 or MrgprA1 was not affected by pretreatment with pertussis toxin (PTX), which inhibits the Gαi/o [22], suggesting that Gαi/o is not required for MrgprC11 signaling.
Consistent with Han et al.’s finding, Wilson et al. later showed that activation of DRG sensory neurons by Bam8-22 was blocked by the PLC-inhibitor U73122, but not by the Gβγ inhibitor gallein, suggesting that MrgprC11 couples to Gαq/11 and activates PLC to induce neuronal excitation [80]. Their results also suggest the role of TRPA1 for MrgprC11-mediated neuronal activation, as TRPA1 knockout mice exhibited reduced sensory neuron responses and scratching behavior in response to Bam8-22. However, recent results from Tseng et al showed that TRPA1 knockout mice exhibited similar scratching bouts as WT mice in response to Bam8-22 [73]. Therefore, whether TRPA1 is required for MrgprC11 signaling is still unclear.
Recent results from Salvatinerra et al. demonstrate the role of Nav1.9 in itch sensation mediated by both MrgprA3 and MrgprC11 [59]. Nav1.9 is a voltage-gated Na+ channel that is critical for the propagation of action potentials in nociceptors [15]. Salvatinerra et al. has shown the expression of Nav1.9 in both MrgprA3+ and MrgprC11+neurons. Nav1.9 knockout mice exhibited a significant reduction of scratching behaviors induced by chloroquine and Bam8-22, suggesting that Nav1.9 is required for MrgprA3 and MrgprC11-mediated neuronal excitation. Intriguingly, Nav1.9L799P/WT mice, which carry a Nav1.9 gain-of-function mutation, exhibited robust spontaneous scratching, demonstrating the critical role of Nav1.9 in itch signaling. It is unclear how Nav1.9 is activated by MrgprA3 and MrgprC11 signaling.
The analgesic effect that results from activation of MrgprC11+central nerves has been demonstrated by several groups independently and shown in a variety of rodent pain behavioral models including inflammatory pain, neuropathic pain, and cancer pain [19, 23, 29, 60, 69, 76–79]. This inhibitory effect was abolished in Mrgpr-cluster knockout mice, suggesting the role of MrgprC11 in pain inhibition [19]. Several reports from Guan’s group have investigated the molecular mechanisms underlying these MrgprC11-mediated analgesic effects. They found that activation of MrgprC11 by Bam8-22 or JHU58, a dipeptide and MrgprC11 selective agonist, inhibits N-type high-voltage-activated (HVA) calcium channels [34, 35], a critical molecule for neurotransmitter release in sensory neurons [42], and eventually leads to reduced evoked excitatory postsynaptic currents in the spinal neurons [19]. The inhibition of HVA current can be blocked by the PLC inhibitor U73122 and the Gβγ blocker gallein, but not by the Gαi pathway blocker pertussis toxin or the Gαs pathway inhibitor cholera toxin, suggesting that MrgprC11 couples to PLC to attenuate HVA current [34].
They later found that MrgprC11 can also positively regulate the analgesic effect of the μ-opioid receptor (MOR) by forming a heteromeric complex with MOR and promote MOR recycling. Intrathecal administration of Bam8-22 potentiated acute morphine analgesia and inhibit chronic morphine tolerance in WT mice. This effect was not observed in Mrgpr-cluster knockout mice. These results suggest the possibility to achieve efficient pain relief with a low dose of morphine without tolerance by targeting MrgprC11.
MrgprD
MrgprD was initially thought to be a sensory neuron-specific GPCR mediating somatosensation [18, 84]. Later extensive studies identified multiple potent agonists including β-alanine, almandine, and angiotensin-(1-7), as well as its expression in a variety of tissues such as testes, artery, uterus, and adipose [5]. MrgprD has been shown to couple to different signaling pathways in different cell types.
Several cellular studies have provided evidence demonstrating that MrgprD is coupled to both Gαi/o and Gαq/11 subunits. Specifically, Shinohara et al. found β-alanine activation of MrgprD induced elevated intracellular calcium signaling in Chinse Hamster Ovary (CHO) cells [63], while Ajit et al. demonstrated that direct activation of MrgprD by β-alanine was completely abolished in CHO cells pre-treated with the PLC inhibitor U73122 [1]. Consistently, almandine activation of MrgprD was inhibited by the PLC inhibitor U73122 in adipose cells collected from rats [16]. These results are indirect indicators Gαq/11 signaling because PLC and elevated intracellular calcium signaling are also downstream of Gβγ. Providing more direct evidence of Gαq/11 signaling, Uchiyama et al. demonstrated that leptin mRNA expression induced by almandine activation of MrgprD was reversed in the presence of the Gαq/11 specific-inhibitor YM25490 within adipose cells [16].
Although almandine-induced MrgprD signaling was unchanged by PTX in adipose cells [16], both Ajit et al. and Shinohara et al. found evidence of PTX-sensitivity of MrgprD signaling in CHO cells, implicating Gαi/o. Ajit et al. found that β-alanine induced calcium mobilization in CHO cells expressing MrgprD was inhibited by PTX [1], while Shinohara et al. found that PTX reversed β-alanine suppression of forskolin-induced cAMP production [63]. This difference in PTX-sensitivity may be due to different signaling pathways employed by different ligands (β-alanine vs alamandine) or different cell types (CHO vs adipose cells).
Moreover, Crozier et al. found that β-alanine-induced activation of MrgD leads to inhibition of KCNQ2/3 subunits, an M-type potassium channel, in both CHO cells and DRG sensory neurons [14]. This effect can be effectively blocked by both PLC inhibitor U73122 and PTX, suggesting the involvement of Gαi/o and Gαq/11 signaling.
In addition to utilizing Gαi/o and Gαq/11 pathways, Tetzner et al. has also linked Angiotensin-(1-7)-induced MrgprD responses with Gαs. Angiotensin-(1-7) activated MrgprD expressed in HEK293 cells and induced a dose-dependent increase in cAMP and PKA, downstream molecules of Gαs, which was decreased by the adenylyl cyclase inhibitor SQ22536 [70].
Similar to MrgprA3 and MrgprC11, Wang et al. suggested that MrgprD also couples to TRPA1 in DRG sensory neurons [75]. Specifically, they demonstrate that the TRPA1 inhibitor HC-030031 suppresses β-alanine-induced responses in DRG neurons. β-alanine-induced neuronal responses are also decreased in TRPA1 knockout mice. Consistent with the results from Tetzner et al., they demonstrate that β-alanine-induced Ca2+ response in DRG sensory neurons can be inhibited by the PKA inhibitor H89 dihydrochloride, but not by PKC inhibitor chelerythrine chloride or the PLC inhibitor U73122 [70], suggesting that MrgprD couples to Gαs and PKA in DRG sensory neurons.
Finally, using Xenopus oocytes transfected with MrgprD Zhao et al. showed that calcium-activated chloride channels (CaCCs) are downstream effectors for MrgprD signaling [83]. They found that β-alanine-induced currents were blocked by EGTA, PLC inhibitor U73122, IP3 receptor antisense oligoneucleotide, and CaCCs inhibitor FFA. The PKC inhibitor, calphostin, did not induce any change in β-alanine-induced currents. Their data suggest that activation of MrgprD induces the opening of CaCC via the Gαq/11-PLC-IP3-Ca2+ pathway [83].
hMrgprX1
hMrgprX1 is expressed in human DRG sensory neurons and mediates itch sensation [10, 64]. Based on the ligand binding affinity, hMrgprX1 is considered as a functional orthologue for both MrgprA3 and MrgprC11 [43]. Several heterologous systems including cell lines, rat primary neuron culture, and transgenic mice have been used to understand the intracellular signaling pathways that can be recruited by hMrgprX1, although it is not clear if the discovered results are true in human cells naturally expressing hMrgprX1. HEK293 cells expressing hMrgprX1 exhibit calcium signals upon Bam8-22 activation [33]. Tseng et al. examined hMrgprX1 expressed in DRG sensory neurons using a “humanized” transgenic mouse line in which endogenous mouse Mrgprs was replaced with hMrgprX1 [73]. They found that hMrgprX1 mediated excitation of sensory neurons are mediated by PLC pathways in a Gβγ dependent manner, and is also sensitive to Gαi/o inhibitor pertussis toxin.
Chen & Ikeda used rat primary neurons overexpressed with hMrgprX1 to examine hMrgprX1 signaling [11]. Similar to MrgprC11, they found that Bam8-22 activation of hMrgprX1 expressed in both rat superior cervical ganglion neurons and DRG neurons inhibited high-voltage-activated (HVA) calcium current. Bam8-22 also induced inhibition of evoked excitatory postsynaptic currents in hippocampal neurons expressing hMrgprX1. Consistent results were presented by Li et al. showing that Bam8-22 inhibits HVA calcium current and attenuates synaptic transmission in the “humanized” mouse sensory neurons expressing hMrgprX1 [36]. However, the inhibition of HVA current by MrgprC11 in native DRG sensory neurons and hMrgprX1 in tested heterologous neuron systems seems to utilize different signaling pathways. MrgprC11 couples to Gβγ and PLC signaling, and is insensitive to Gαi pathway blocker pertussis toxin [34]. hMrgprX1 mediated inhibition of HVA calcium current is insensitive to the PLC inhibitor U73122, but is Gαi/o and Gβγ-dependent [36].
Solinski et al. have shown that TRPV1 is a downstream component of hMrgprX1 when overexpressed in F11 cells, a rat neuron cell line [66]. They found that Bam8-22 stimulation of hMrgprX1 in F11 cells both directly activate TRPV1 via DAG and PIP2 and sensitize TRPV1 via PKC phosphorylation [66]. However, using the “humanized” mouse sensory neurons expressing hMrgprX1, Tseng et al. did not observe the involvement of TRPA1 or TRPV1 as the TRPA1 inhibitor HC030031 and TRP channels blocker ruthenium red had no inhibitory effect on Bam8-22 evoked action potentials or firing rates [73]. They suggested the involvement of Nav1.8 and Nav1.9 since TTX reduced the number of action potentials induced by Bam8-22.
MrgprB2/hMrgprX2
MrgprB2 and its human homolog MrgprX2 are mainly expressed in mast cells. They can be activated by basic secretagogues, a group of positively charged molecules, to induce IgE-independent mast cell degranulation, a reaction to release numerous bioactive molecules involved in various processes including immune responses, vascular tone, and nociception [44, 52].
Subramanian et al. demonstrate that MrgprX2 serves as the receptor to mediate mast cell activation by both antimicrobial peptide LL-37 and β-defensins [67, 68]. Mast cells exhibit calcium response and degranulation often via a pertussis toxin-sensitive Gαi/o pathway upon stimulation by an agonist. However, they found that pertussis toxin inhibited degranulation, but not calcium mobilization in human mast cells in response to both LL-37 and β-defensins. These data suggest that MrgprX2 couples to a Gαi/o-independent pathway for calcium response and Gαi/o-dependent pathway for degranulation in human mast cells. In support of these findings, Substance P induced degranulation and calcium mobilization in rat basil leukemia (RBL-2H3) cells expressing MrgprX2 were found to be abolished when exposed to both PTX and the Gαq/11 inhibitor YM-254890 [12].
GPCRs are known to activate a G proteins-independent pathway which involves the adaptor proteins β-arrestins [28]. β-arrestins not only serve as inhibitors of GPCRs by promoting GPCR desensitization, but also play an important role in G protein-independent signaling to regulate a variety of cellular events. Assays examining β-arrestins recruitment and translocation have been widely used to identify agonists for GPCRs [33]. Although extensive studies have not been performed to examine the role of β-arrestins in Mrgprs signaling, several agonists for MrgprX2 and MrgprX4 have been identified using β-arrestins recruitment assays [4, 33], suggesting that β-arrestins are activated by Mrgprs. Roy et al. have demonstrated that MrgprX2 in mast cells, when stimulated by different agonists, recruits different downstream signaling pathways [56]. For example, while MrgprX2 recruits both G protein signaling and β-arrestin signaling when stimulated by compound 48/80, it only recruits G protein signaling when activated by AG-30/5C, an angiogenic host defense peptide. They later showed that MrgprB2-mediated degranulation of peritoneal mast cells induced by both ciprofloxacin and compound 48/80 were significantly enhanced in β-arrestin-2 knockout mice, demonstrating the involvement of β-arrestin-2 in MrgprB2 signaling [57]. Finally, Babina et al. recently showed that silencing of β-arrestin-1, but not β-arrestin-2, by siRNA attenuated MrgprX2 internalization in skin mast cells induced by Codeine [4].
Mast cell degranulation follows the intracellular calcium mobilization. Ample evidence has demonstrated that IgE-dependent mast cell activation is mediated by the store-operated Ca2+ entry (SOCE) via stromal interaction molecule 1 (STIM1) [3, 6, 62]. STIM1 is the central proponent of SOCE and contains Ca2+-binding domains oriented towards the lumen of the endoplasmic reticulum (ER). When cellular activation results in the depletion of Ca2+ in the ER, ER-lumen oriented Ca2+ binding domains of STIM1 become unbound, resulting in the formation of a complex with calcium release-activated calcium (CRAC) channels and TRPC channels to allow for Ca2+ influx into the cytoplasm. Occhiuto et al. showed that hMrgprX2 utilizes a similar mechanism for IgE-independent mast cell activation [50]. They demonstrate that MrgprX2 agonist CST-14 induced calcium signal and degranulation in LAD2 human mast cells and these effects were decreased by the CRAC channel antagonist YM 58483 and the SOCE inhibitor SKF 96365 HC1. In contrast, the TRP channel inhibitors Nifedipine and A425619 had no effect on either degranulation or calcium signal. These conclusions are supported by evidence from an in vivo mouse model of pseudo-allergy. SOCE inhibitor treated mice were injected with MrgprB2 agonist compound 48/40 to induce mast cell degranulation. Compared to control, the SOCE inhibitor treated mice were found to exhibit decreased paw swelling and decreased histamine levels in serum, indicating a reduction in mast cell degranulation.
MrgprA1/hMrgprX4
MrgprA1 and hMrgprX4 mediate bilirubin-induced itch and have been implicated in cholestatic pruritus [47]. Consistent with Han et al.’s finding that MrgprA1 utilizes Gαq/11-dependent pathways in MEFs that we discussed above [22], Meixiong et al. have shown that bilirubin-induced calcium responses in HEK293 cells expressing mMrgprA1 can be abolished by the Gαq specific-inhibitor YM-254890 or the PLC inhibitor U73122 [47].
Similar to MrgprA1, hMrgprX4 couples to Gαq in HEK293 cells. Yu et al. has demonstrated that bile acid can activate hMrgprX4 expressed in a Gαq/11-dependent reporter assay, but not in a Gαs-dependent reporter assay [82]. Both deoxycholic acid and bilirubin induced activation of hMrgprX4 were inhibited by the PLC inhibitor U73122 or the Gαq inhibitor YM254890 [47, 48]. Additionally, Kroeze et al. found that nateglinide induced calcium mobilization and phosphatidylinositol hydrolysis in HEK293 cells expressing hMrgprX4 [33].
Polymorphisms in Mrgprs
Single nucleotide polymorphisms (SNPs) are among the most common types of genetic variation, with missense SNPs often cited as the main explanatory cause for disease variation due to their effects on the structure of the protein, and thus its ability to transduce signals [9]. Many SNPs have been identified in human Mrgpr family members and can be found in publicly available human exome sequencing databases such as NCBI dbSNP, NHLBI GO Exome Sequencing Project, and 1000 Genomes. However, research investigating how Mrgprs polymorphisms contribute to human diseases is in its infancy. There are only a few reports showing the possible functional implications for naturally occurring missense variants of Mrgprs.
Heller et al. identifies two MrgprX1 mutations with allele frequency exceeding 0.1%, R131S and H133R, that result in loss and gain of function respectively with regard to their ability to bind Bam8-22, based on the results of luciferase reporter assays utilizing transfected HEK cells [24]. Whether these mutations have a human clinical relevance is not clear.
Alkanfari et al. tested several missense variants of MrgprX2 overexpressed in RBL-2H3 cells, a rat basophilic leukemia cell line, to examine if any of them display abnormal function [2]. The results showed that four of them including G165E, D184H, W243R, and H259Y disrupted RBL-2H3 cell degranulation in response to MrgprX2 ligands including substance P, hemokinin-1, β-defensin-3, and icatibant. Another study reported four loss of function naturally occurring MrgprX2 variants including V123F, R138C, R141C, and V282M [12]. These variants exhibit disrupted calcium mobilization and degranulation in RBL-2H3 cells in response to substance P. These results suggest that these missense mutations might protect the carrying individuals from MrgprX2-mediated pseudoallergy and chronic inflammatory diseases. Two gain of function MrgprX2 variants, S325L and L329Q, have also been identified since they showed enhanced mast cell activation upon substance P stimulation [12].
Using an exome-wide association study [32], Kozlitifna et al. identified two MrgprX4 variants, N245S and T43T, that are expressed exclusively within African American participants and associated with a 5-8 fold increase in the preference of menthol cigarette among smokers. They found that these MrgprX4 variants exhibit reduced signaling in HEK293 cells based on both β-arrestin-based and Gαq-based reporter assays. They also identified menthol as a negative modulator for MrgprX4 since it reduced the MrgprX4 signaling in HEK293 cells in response to Nateglinide. However, it remains unknown how these MrgprX4 variants contribute to the preference for menthol cigarette use.
Conclusions
Mrgprs utilize multiple endogenous ligands through distinct signaling pathways to encode for and modulate different biological processes. Although many key signaling components have been identified, further research is needed to build upon the findings presented here as our understanding has evolved greatly in the last few decades. In particular, there are emerging caveats to the use of particular inhibitors as well as cell and tissue types that need to be taken into account. For example, the PLC inhibitor U73122 has been shown to have many non-specific effects [30, 31, 73]. Pertussis toxin is utilized almost exclusively for identifying Gαi/o signaling, despite one of the subfamilies, Gαz, being unaffected by PTX [25]. While Tseng et al. found that the HC030031 is not a TRPA1 specific inhibitor since it also induces the inhibition of voltage-gated calcium channels in sensory neurons [73]. As such, evaluating the results from pharmacological inhibitors should be handled with care.
Due to the critical role of Mrgpr family members in a variety of physiological processes, polymorphisms in Mrgprs can have a profound impact on human disease. However, despite the existence of many naturally occurring Mrgprs polymorphisms, few have been extensively studied to date. Future research focusing on Mrgprs polymorphisms will help to explain differences in disease incidence and drug effect across different populations.
Fig. 1.
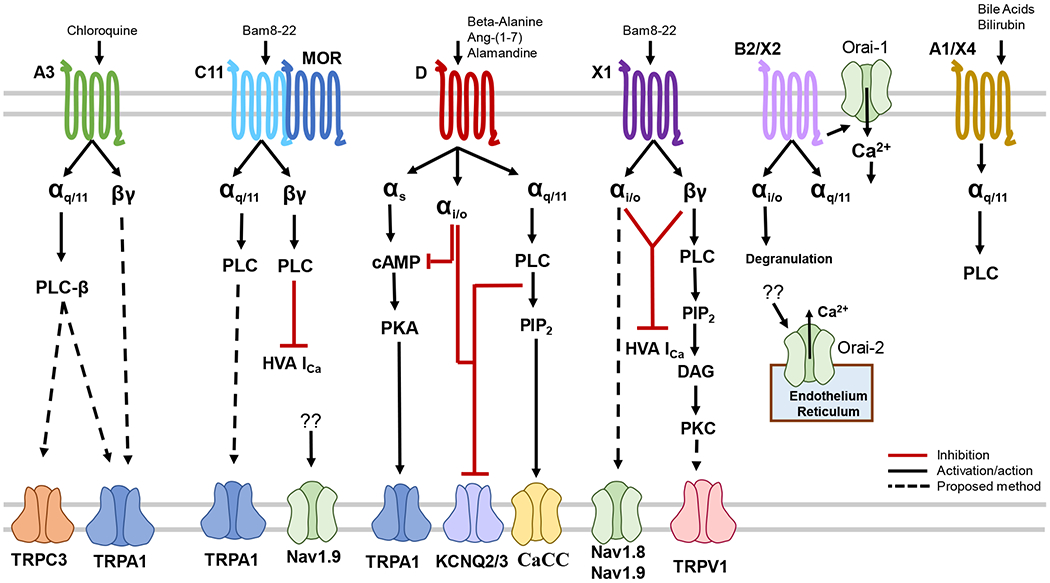
Overview of Mrgprs Signaling Pathways. Mrgprs have been found to utilize Gαq/11, Gαi/o, Gαs, and Gβγ pathways. Activation of G proteins stimulates the intracellular signaling pathways and leads to the opening of transduction channels.
Highlights.
Mrgprs utilize canonical G protein signaling pathways
Mrgprs couple to ion channels to excite neurons
Functional implications of Mrgprs polymorphisms
Acknowledgments
The work was supported by grants from the US National Institutes of Health (NS087088 and HL141269) and Pfizer Aspire Dermatology Award to L.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ajit SK, Pausch MH, Kennedy JD, Kaftan EJ, Development of a FLIPR assay for the simultaneous identification of MrgD agonists and antagonists from a single screen, Journal of biomedicine & biotechnology 2010. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alkanfari I, Gupta K, Jahan T, Ali H, Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human β-Defensin-3, and Icatibant, The Journal of Immunology 201 (2018) 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T, Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses, Nature immunology 9 (2008) 81–88. [DOI] [PubMed] [Google Scholar]
- [4].Babina M, Wang Z, Roy S, Guhl S, Franke K, Artuc M, Ali H, Zuberbier T, MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through beta-Arrestin and Lack of Correlation with the FcepsilonRI Pathway, The Journal of investigative dermatology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bader M, Alenina N, Andrade-Navarro MA, Santos RA, MAS and its related G protein-coupled receptors, Mrgprs, Pharmacol Rev 66 (2014) 1080–1105. [DOI] [PubMed] [Google Scholar]
- [6].Bergmeier W, Weidinger C, Zee I, Feske S, Emerging roles of store-operated Ca(2)(+) entry through STIM and ORAI proteins in immunity, hemostasis and cancer, Channels 7 (2013) 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau R.W.t., cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation, Neuron 35 (2002) 721–731. [DOI] [PubMed] [Google Scholar]
- [8].Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE, Insights into G protein structure, function, and regulation, Endocrine reviews 24 (2003) 765–781. [DOI] [PubMed] [Google Scholar]
- [9].Cargill M, Altshuler D, Ireland J, Sklar P, Ardlie K, Patil N, Shaw N, Lane CR, Lim EP, Kalyanaraman N, Nemesh J, Ziaugra L, Friedland L, Rolfe A, Warrington J, Lipshutz R, Daley GQ, Lander ES, Characterization of single-nucleotide polymorphisms in coding regions of human genes, Nature genetics 22 (1999) 231–238. [DOI] [PubMed] [Google Scholar]
- [10].Castro J, Harrington AM, Lieu T, Garcia-Caraballo S, Maddern J, Schober G, O’Donnell T, Grundy L, Lumsden AL, Miller P, Ghetti A, Steinhoff MS, Poole DP, Dong X, Chang L, Bunnett NW, Brierley SM, Activation of pruritogenic TGR5, MrgprA3, and MrgprC11 on colon-innervating afferents induces visceral hypersensitivity, JCI insight 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen H, Ikeda SR, Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons, J Neurosci 24 (2004) 5044–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chompunud Na Ayudhya C, Roy S, Alkanfari I, Ganguly A, Ali H, Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P, Int J Mol Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cibulsky SM, Sather WA, Block by ruthenium red of cloned neuronal voltage-gated calcium channels, The Journal of pharmacology and experimental therapeutics 289 (1999) 1447–1453. [PubMed] [Google Scholar]
- [14].Crozier RA, Ajit SK, Kaftan EJ, Pausch MH, MrgD Activation Inhibits KCNQ/M-Currents and Contributes to Enhanced Neuronal Excitability, 27 (2007) 4492–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dib-Hajj SD, Cummins TR, Black JA, Waxman SG, Sodium channels in normal and pathological pain, Annual review of neuroscience 33 (2010) 325–347. [DOI] [PubMed] [Google Scholar]
- [16].Dong P, Guo CX, Huang SX, Ma MH, Liu Q, Luo WQ, TRPC3 Is Dispensable for beta-Alanine Triggered Acute Itch, Sci Rep 7 (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dong X, Dong X, Peripheral and Central Mechanisms of Itch, Neuron 98 (2018) 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dong X, Han S-K, Zylka MJ, Simon MI, Anderson DJ, A Diverse Family of GPCRs Expressed in Specific Subsets of Nociceptive Sensory Neurons, 106 (2001) 619–632. [DOI] [PubMed] [Google Scholar]
- [19].Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X, Mas-related G-protein-coupled receptors inhibit pathological pain in mice, Proceedings of the National Academy of Sciences of the United States of America 107 (2010) 15933–15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, Zhu Y, Wilson J, Mitzner W, Kollarik M, Undem BJ, Canning BJ, Dong X, Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness, Nat Neurosci 21 (2018) 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han L, Ma C, Liu Q, Weng H-J, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X, A subpopulation of nociceptors specifically linked to itch, Nature Neuroscience 16 (2013) 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI, Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the G q/11 pathway, 99 (2002) 14740–14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He SQ, Li Z, Chu YX, Han L, Xu Q, Li M, Yang F, Liu Q, Tang Z, Wang Y, Hin N, Tsukamoto T, Slusher B, Tiwari V, Shechter R, Wei F, Raja SN, Dong X, Guan Y, MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain, Pain 155 (2014) 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heller D, Doyle JR, Raman VS, Beinborn M, Kumar K, Kopin AS, Novel Probes Establish Mas-Related G Protein-Coupled Receptor X1 Variants as Receptors with Loss or Gain of Function, Journal of Pharmacology and Experimental Therapeutics 356 (2016) 276–283. [DOI] [PubMed] [Google Scholar]
- [25].Ho MK, Wong YH, G(z) signaling: emerging divergence from G(i) signaling, Oncogene 20 (2001) 1615–1625. [DOI] [PubMed] [Google Scholar]
- [26].Huang CC, Yang W, Guo C, Jiang H, Li F, Xiao M, Davidson S, Yu G, Duan B, Huang T, Huang AJW, Liu Q, Anatomical and functional dichotomy of ocular itch and pain, Nature medicine 24 (2018) 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK, TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms, Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jean-Charles PY, Kaur S, Shenoy SK, G Protein-Coupled Receptor Signaling Through beta-Arrestin-Dependent Mechanisms, Journal of cardiovascular pharmacology 70 (2017) 142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiang J, Wang D, Zhou X, Huo Y, Chen T, Hu F, Quirion R, Hong Y, Effect of Mas-related gene (Mrg) receptors on hyperalgesia in rats with CFA-induced inflammation via direct and indirect mechanisms, British journal of pharmacology 170 (2013) 1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kadamur G, Ross EM, Mammalian phospholipase C, Annual review of physiology 75 (2013) 127–154. [DOI] [PubMed] [Google Scholar]
- [31].Kittaka H, Tominaga M, The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin, Allergology international : official journal of the Japanese Society of Allergology 66 (2017) 22–30. [DOI] [PubMed] [Google Scholar]
- [32].Kozlitina J, Risso D, Lansu K, Olsen RHJ, Sainz E, Luiselli D, Barik A, Frigerio-Domingues C, Pagani L, Wooding S, Kirchner T, Niaura R, Roth B, Drayna D, An African-specific haplotype in MRGPRX4 is associated with menthol cigarette smoking, PLOS Genetics 15 (2019) e1007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kroeze WK, Sassano MF, Huang X-P, Lansu K, McCorvy JD, Giguère PM, Sciaky N, Roth BL, PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome, Nature Structural & Molecular Biology 22 (2015) 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li Z, He SQ, Tseng PY, Xu Q, Tiwari V, Yang F, Shu B, Zhang T, Tang Z, Raja SN, Wang Y, Dong X, Guan Y, The inhibition of high-voltage-activated calcium current by activation of MrgC11 involves phospholipase C-dependent mechanisms, Neuroscience 300 (2015) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Z, He SQ, Xu Q, Yang F, Tiwari V, Liu Q, Tang Z, Han L, Chu YX, Wang Y, Hin N, Tsukamoto T, Slusher B, Guan X, Wei F, Raja SN, Dong X, Guan Y, Activation of MrgC receptor inhibits N-type calcium channels in small-diameter primary sensory neurons in mice, Pain 155 (2014) 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li Z, Tseng PY, Tiwari V, Xu Q, He SQ, Wang Y, Zheng Q, Han L, Wu Z, Blobaum AL, Cui Y, Tiwari V, Sun S, Cheng Y, Huang-Lionnet JH, Geng Y, Xiao B, Peng J, Hopkins C, Raja SN, Guan Y, Dong X, Targeting human Mas-related G protein-coupled receptor X1 to inhibit persistent pain, Proceedings of the National Academy of Sciences of the United States of America 114 (2017) E1996–E2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu Q, Tang ZX, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng YX, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong XZ, Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus, Cell 139 (2009) 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Q, Weng HJ, Patel KN, Tang ZX, Bai HH, Steinhoff M, Dong XZ, The Distinct Roles of Two GPCRs, MrgprC11 and PAR2, in Itch and Hyperalgesia, Sci. Signal 4 (2011) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q, Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors, J Neurosci 28 (2008) 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mahoney JP, Sunahara RK, Mechanistic insights into GPCR-G protein interactions, Current opinion in structural biology 41 (2016) 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS, G-protein signaling: back to the future, Cellular and molecular life sciences : CMLS 62 (2005) 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McGivern JG, Targeting N-type and T-type calcium channels for the treatment of pain, Drug discovery today 11 (2006) 245–253. [DOI] [PubMed] [Google Scholar]
- [43].McNeil B, Dong XZ, Mrgprs as Itch Receptors. In: Carstens E, Akiyama T (Eds.), Itch: Mechanisms and Treatment, Crc Press-Taylor & Francis Group, Boca Raton, 2014, pp. 213–236. [PubMed] [Google Scholar]
- [44].McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong XZ, Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions, Nature 519 (2015) 237-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Meents JE, Fischer MJ, McNaughton PA, Sensitization of TRPA1 by Protein Kinase A, PLoS One 12 (2017) e0170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, Dong X, Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch, Immunity 50 (2019) 1163–1171 e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meixiong J, Vasavda C, Green D, Zheng Q, Qi L, Kwatra SG, Hamilton JP, Snyder SH, Dong X, Identification of a bilirubin receptor that may mediate a component of cholestatic itch, eLife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meixiong J, Vasavda C, Snyder SH, Dong X, MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus, Proceedings of the National Academy of Sciences of the United States of America 116 (2019) 10525–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nishimura S, Uno M, Kaneta Y, Fukuchi K, Nishigohri H, Hasegawa J, Komori H, Takeda S, Enomoto K, Nara F, Agatsuma T, MRGD, a MAS-related G-protein Coupled Receptor, Promotes Tumorigenisis and Is Highly Expressed in Lung Cancer, PLoS ONE 7 (2012) e38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Occhiuto CJ, Kammala AK, Yang C, Nellutla R, Garcia M, Gomez G, Subramanian H, Store-Operated Calcium Entry via STIM1 Contributes to MRGPRX2 Induced Mast Cell Functions, Front Immunol 10 (2019) 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Oldham WM, Hamm HE, Heterotrimeric G protein activation by G-protein-coupled receptors, Nature reviews. Molecular cell biology 9 (2008) 60–71. [DOI] [PubMed] [Google Scholar]
- [52].Olivera A, Beaven MA, Metcalfe DD, Mast cells signal their importance in health and disease, The Journal of allergy and clinical immunology 142 (2018) 381–393. [DOI] [PubMed] [Google Scholar]
- [53].Pundir P, Liu R, Vasavda C, Serhan N, Limjunyawong N, Yee R, Zhan Y, Dong X, Wu X, Zhang Y, Snyder SH, Gaudenzio N, Vidal JE, Dong X, A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity, Cell host & microbe 26 (2019) 114–122 e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA, Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs, Nat Commun 6 (2015) 7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ, Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons, Nat Neurosci 16 (2013) 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Roy S, Ganguly A, Haque M, Ali H, Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B2 Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells, Journal of immunology 202 (2019) 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roy S, Gupta K, Ganguly A, Ali H, beta-Arrestin2 expressed in mast cells regulates ciprofloxacin-induced pseudoallergy and IgE-mediated anaphylaxis, The Journal of allergy and clinical immunology 144 (2019) 603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ru F, Sun H, Jurcakova D, Herbstsomer RA, Meixong J, Dong X, Undem BJ, Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin, The Journal of Physiology 595 (2017) 3651–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Salvatierra J, Diaz-Bustamante M, Meixiong J, Tierney E, Dong X, Bosmans F, A disease mutation reveals a role for NaV1.9 in acute itch, Journal of Clinical Investigation 128 (2018) 5434–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schroder W, Alique M, Herrero JF, Effects of the mas-related gene (Mrg) C receptor agonist BAM6-22 on nociceptive reflex activity in naive, monoarthritic and mononeuropathic rats after intraplantar and intrathecal administration, Eur J Pharmacol 770 (2016) 147–153. [DOI] [PubMed] [Google Scholar]
- [61].Sharif B, Ase AR, Ribeiro-da-Silva A, Seguela P, Differential Coding of Itch and Pain by a Subpopulation of Primary Afferent Neurons, Neuron (2020). [DOI] [PubMed] [Google Scholar]
- [62].Shaw PJ, Feske S, Physiological and pathophysiological functions of SOCE in the immune system, Frontiers in bioscience 4 (2012) 2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S, Identification of a G protein-coupled receptor specifically responsive to beta-alanine, J. Biol. Chem 279 (2004) 23559–23564. [DOI] [PubMed] [Google Scholar]
- [64].Sikand P, Dong X, Lamotte RH, BAM8-22 Peptide Produces Itch and Nociceptive Sensations in Humans Independent of Histamine Release, J. Neurosci 31 (2011) 7563–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Simon MI, Strathmann MP, Gautam N, Diversity of G proteins in signal transduction, Science 252 (1991) 802–808. [DOI] [PubMed] [Google Scholar]
- [66].Solinski HJ, Zierler S, Gudermann T, Breit A, Human sensory neuron-specific Mas-related G protein-coupled receptors-X1 sensitize and directly activate transient receptor potential cation channel V1 via distinct signaling pathways, The Journal of biological chemistry 287 (2012) 40956–40971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Subramanian H, Gupta K, Guo Q, Price R, Ali H, Mas-related Gene X2 (MrgX2) Is a Novel G Protein-coupled Receptor for the Antimicrobial Peptide LL-37 in Human Mast Cells RESISTANCE TO RECEPTOR PHOSPHORYLATION, DESENSITIZATION, AND INTERNALIZATION, J. Biol. Chem 286 (2011) 44739–44749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H, beta-Defensins activate human mast cells via Mas-related gene X2, Journal of immunology 191 (2013) 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sun Y, Jiang M, Hou B, Lu C, Lei Y, Ma Z, Gu X, Mas-Related Gene (Mrg) C Activation Attenuates Bone Cancer Pain via Modulating Gi and NR2B, PLoS One 11 (2016) e0154851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacanas O, Walther T, G-Protein-Coupled Receptor MrgD Is a Receptor for Angiotensin-(1-7) Involving Adenylyl Cyclase, cAMP, and Phosphokinase A, Hypertension 68 (2016) 185–194. [DOI] [PubMed] [Google Scholar]
- [71].Than J, Li L, Hasan R, Zhang XM, Excitation and Modulation of TRPA1, TRPV1, and TRPM8 Channel-expressing Sensory Neurons by the Pruritogen Chloroquine, J. Biol. Chem 288 (2013) 12818–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tiwari V, Tiwari V, He S, Zhang T, Raja SN, Dong X, Guan Y, Mas-Related G Protein-Coupled Receptors Offer Potential New Targets for Pain Therapy, Advances in experimental medicine and biology 904 (2016) 87–103. [DOI] [PubMed] [Google Scholar]
- [73].Tseng PY, Zheng Q, LI Z, Dong X, MrgprX1 mediates neuronal excitability and itch through tetrodotoxin-resistant sodium channels, Itch 4 (2019) e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing, Nat Neurosci 18 (2015) 145–153. [DOI] [PubMed] [Google Scholar]
- [75].Wang CM, Gu LY, Ruan YL, Geng X, Xu M, Yang NN, Yu L, Jiang YC, Zhu C, Yang Y, Zhou Y, Guan XW, Luo WQ, Liu Q, Dong XZ, Yu G, Lan L, Tang ZX, Facilitation of MrgprD by TRP-A1 promotes neuropathic pain, Faseb J. 33 (2019) 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang D, Chen T, Zhou X, Couture R, Hong Y, Activation of Mas oncogene-related gene (Mrg) C receptors enhances morphine-induced analgesia through modulation of coupling of mu-opioid receptor to Gi-protein in rat spinal dorsal horn, Neuroscience 253 (2013) 455–464. [DOI] [PubMed] [Google Scholar]
- [77].Wang D, Wang P, Jiang J, Lv Q, Zeng X, Hong Y, Activation of Mas Oncogene-Related G Protein-Coupled Receptors Inhibits Neurochemical Alterations in the Spinal Dorsal Horn and Dorsal Root Ganglia Associated with Inflammatory Pain in Rats, The Journal of pharmacology and experimental therapeutics 354 (2015) 431–439. [DOI] [PubMed] [Google Scholar]
- [78].Wang D, Xue Y, Chen Y, Ruan L, Hong Y, Mas-related gene (Mrg) C receptors inhibit mechanical allodynia and spinal microglia activation in the early phase of neuropathic pain in rats, Neuroscience letters 618 (2016) 115–121. [DOI] [PubMed] [Google Scholar]
- [79].Wang D, Xue Y, Yan Y, Lin M, Yang J, Huang J, Hong Y, Reversal of neurochemical alterations in the spinal dorsal horn and dorsal root ganglia by Mas-related gene (Mrg) receptors in a rat model of spinal nerve injury, Neurobiology of disease 91 (2016) 274–283. [DOI] [PubMed] [Google Scholar]
- [80].Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM, TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch, Nature Neuroscience 14 (2011) 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xing Y, Chen J, Hilley H, Steele H, Yang J, Han L, Molecular Signature of Pruriceptive MrgprA3(+) Neurons, The Journal of investigative dermatology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yu H, Zhao T, Liu S, Wu Q, Johnson O, Wu Z, Zhuang Z, Shi Y, Peng L, He R, Yang Y, Sun J, Wang X, Xu H, Zeng Z, Zou P, Lei X, Luo W, Li Y, MRGPRX4 is a bile acid receptor for human cholestatic itch, eLife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhuo RG, Ma XY, Zhou PL, Liu XY, Zhang K, Wei XL, Yan HT, Xu JP, Zheng JQ, Mas-related G protein-coupled receptor D is coupled to endogenous calcium-activated chloride channel in Xenopus oocytes, J Physiol Biochem 70 (2014) 185–191. [DOI] [PubMed] [Google Scholar]
- [84].Zylka MJ, Rice FL, Anderson DJ, Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd, Neuron 45 (2005) 17–25. [DOI] [PubMed] [Google Scholar]


