Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, cohort study, ischemic stroke, risk factors, white matter
Abstract
Background and Purpose:
The aim of this study was to assess the rate of chronic covert brain infarctions (CBIs) in patients with acute ischemic stroke (AIS) and to describe their phenotypes and diagnostic value.
Methods:
This is a single-center cohort study including 1546 consecutive patients with first-ever AIS on magnetic resonance imaging imaging from January 2015 to December 2017. The main study outcomes were CBI phenotypes, their relative frequencies, location, and association with vascular risk factors.
Results:
Any CBI was present in 574/1546 (37% [95% CI, 35%–40%]) of patients with a total of 950 CBI lesions. The most frequent locations of CBI were cerebellar in 295/950 (31%), subcortical supratentorial in 292/950 (31%), and cortical in 213/950 (24%). CBI phenotypes included lacunes (49%), combined gray and white matter lesions (30%), gray matter lesions (13%), and large subcortical infarcts (7%). Vascular risk profile and white matter hyperintensities severity (19% if no white matter hyperintensity, 63% in severe white matter hyperintensity, P<0.001) were associated with presence of any CBI. Atrial fibrillation was associated with cortical lesions (adjusted odds ratio, 2.032 [95% CI, 1.041–3.967]). Median National Institutes of Health Stroke Scale scores on admission were higher in patients with an embolic CBI phenotype (median National Institutes of Health Stroke Scale, 5 [2–10], P=0.025).
Conclusions:
CBIs were present in more than a third of patients with first AIS. Their location and phenotypes as determined by MRI were different from previous studies using computed tomography imaging. Among patients suffering from AIS, those with additional CBI represent a vascular high-risk subgroup and the association of different phenotypes of CBIs with differing risk factor profiles potentially points toward discriminative AIS etiologies.
Chronic covert brain infarctions (CBIs) are focal, presumably postischemic lesions detected on brain imaging in patients without a history of an attributable obvious acute neurological dysfunction.1,2 Patients with CBIs have a 2- to 3-fold increased risk of manifest acute ischemic stroke (AIS).2–4 Additionally, 1 in 3 patients with first-ever AIS has an additional chronic CBI on acute imaging.5–9
Previous studies addressing the role of CBI in patients with AIS are limited by use of computed tomography (CT) imaging, which is neither sensitive in detecting small CBIs nor specific in differentiating CBI from other cerebral lesions.10 Some studies used magnetic resonance imaging (MRI), but reliable imaging criteria for CBI diagnosis have only recently been proposed.11 Most available studies compared patients with any CBI to patients without CBI but did not consider the imaging phenotype of the CBI lesion. Potentially relevant phenotypes include subcortical lacunes (=cavitatory lesions), large subcortical infarcts, isolated gray matter lesions, and combined gray and white matter lesions.11 In patients with AIS, CBI might convey clues to underlying vascular risk factors and stroke etiology. As a consequence of the methodological weaknesses of the existing studies, guidelines for AIS work-up do not include information on concomitant CBI to guide the diagnostic work-up in patients with AIS.12
The aim of this study was to describe the frequency, imaging phenotypes, and distribution of CBI—defined according to established imaging criteria as evidence of any prior brain infarct in patients with first clinically evident ischemic stroke. CBI included cortical infarcts as well as lacunes (cavitatory), large subcortical (noncavitatory) infarcts and cerebellar and brain stem lesions. In addition, we sought to determine the vascular risk factor profile according to CBI phenotypes and disentangle the association of CBI and undifferentiated white matter hyperintensities (WMHs).
Methods
For the conceptualization of this project, we adhered to the Strengthening the reporting of observational studies in epidemiology international collaborative initiative guidelines.
Data Availability
Anonymized data and statistical analyses will be shared upon motivated request from any qualified investigator after clearance from the ethics committee.
Patients
This is a single center cohort study with 90 days follow-up. All consecutive patients treated at our comprehensive stroke center were enrolled in a prospective stroke registry. Among these patients, we applied the following inclusion criteria: (1) first clinically evident ischemic stroke, (2) MR-imaging on admission (MRI is the preferred primary imaging modality for patients with suspected stroke in the absence of contraindications), (3) admission between January 1, 2015 and December 31, 2017.
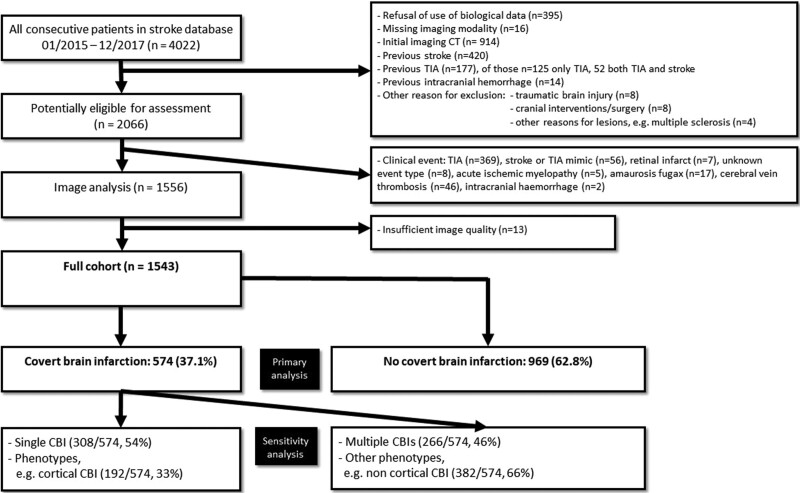
We excluded patients with CT imaging as first imaging modality and diagnoses potentially leading to brain lesions that could mimic CBI. These included previous AIS and/or transient ischemic attack defined as any transient neurological event of presumably vascular etiology, previous intracranial hemorrhage, traumatic brain injury, cranial interventions/surgery, and neuroinflammatory diseases. Additionally, patients with stroke types other than AIS and insufficient image quality were excluded (see Figure 1 for full exclusion criteria).
Figure 1.
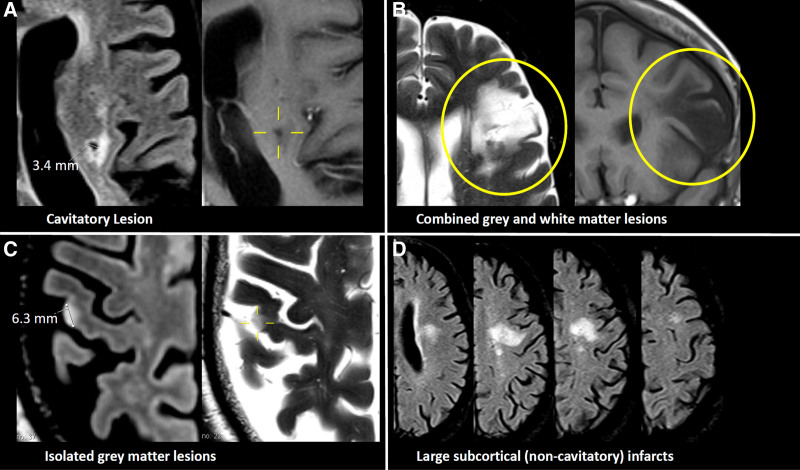
Examples of phenotypes of covert brain infarction. A, Lacune. B, Combined gray and white matter lesions. C, Isolated gray matter lesions. D, Large subcortical (noncavitatory) infarcts.
Neuroimaging by Brain MRI
Type of baseline MRI, used protocols, and review by 2 raters is described in detail in the Data Supplement.
Location
The CBIs were mapped in the following anatomic locations: basal ganglia (defined as involvement of deep nuclei, including the thalamus), subcortical supratentorial (white matter involvement only), cortical supratentorial, cerebellar, and brain stem. In addition to CBIs, the presence of vascular subcortical MRI WMH was graded according to the age-related white matter change (ARWMC) Rating Scale.13
Phenotypes
The following imaging phenotypes were included according to established criteria,10,11,14 (see Figure 1 for examples):
Lacunes (Of Presumed Vascular Origin)
1. Lesions ≥3 mm in size with central cavity formation with imaging intensities compatible with cerebrospinal fluid. A fluid-attenuated inversion recovery/T2 hyperintensity reflecting adjacent gliosis, symmetry as well as anatomic location was used to differentiate them from perivascular spaces. In the cerebellum, parenchymal defects ≤15 mm were rated in this category, whereas larger defects were rated as combined gray and white matter lesions.
Focal, Irregularly Shaped Hyperintensities on Fluid-Attenuated Inversion Recovery and/or T2-Weighted Imaging With Corresponding Hypointensity on T1-Weighted Imaging
These were subdivided as follows:
2. Combined gray and white matter lesions: involvement of cortical and subcortical areas.
3. Isolated gray matter lesions: isolated involvement of gray matter either in the supratentorial cortex or basal ganglia.
4. Large subcortical (noncavitatory) infarcts: for differentiation of this phenotype from unspecific lesions, the lesion had to match a clear vascular supply territory in the distribution of the medial or lateral lenticular striatal arteries, with a size of 15 mm or more, and/or be new compared with a previous MRI performed within 3 months. Additionally, other diagnoses potentially explaining the lesion such as multiple sclerosis were excluded based on the medical history.11
Clinical Baseline and Follow-Up Data Collection
Dedicated stroke research fellows collected standardized and prespecified variables including patient demographics (age, sex, body mass index), medical history (eg, history of prior AIS, prior intracerebral hemorrhage, hypertension, dyslipidemia, diabetes, smoking), medication at onset, clinical features (stroke severity, blood glucose, and blood pressure on admission), atrial fibrillation (AF: known before stroke or diagnosed up to 90 days after stroke with a work-up of ECG of 7 days duration starting 1 to 3 days after admission) as well as diagnostic tests performed (eg, imaging modality, presence of large vessel occlusion). Information on recanalization treatments included use of intravenous thrombolysis and/or mechanical thrombectomy.
Outcomes
The main outcomes for this analysis were (1) frequency of any CBI, (2) phenotypes of CBI, (3) anatomic distribution of CBIs, and (4) vascular risk profile according to CBI phenotype.
Statistical Analysis
We used medians (interquartile ranges or means [SD]), as appropriate, as well as percentages (95% CI by Clopper-Pearson method) to present the distribution of continuous, ordinal and categorical variables, respectively. We compared baseline characteristics between groups using the Pearson χ2 test for categorical variables and the Wilcoxon rank-sum or Kruskal-Wallis test for continuous and ordinal variables. We used Cohen’s kappa statistics to report interrater agreement.
Backward elimination (significance level of 0.15) was used for selection of potentially causal covariates associated with CBI presence in logistic regression analysis. Those potentially causal vascular risk factors included age, sex, obesity, admission serum creatinine levels, hypertension, hyperlipidemia, AF, smoking, diabetes, and congestive heart failure. The analysis was done separately for each CBI phenotype as compared with patients without CBI and patients with isolated moderate or severe (ARWMC score ≥2) subcortical WMH without any CBI as compared with patients without subcortical WMH (with a sensitivity analysis considering only isolated CBIs). Logistic regression for the association of any cortical involvement and AF was done unadjusted and adjusted for the CHA2DS2-VASc score.
Stroke severity was analyzed using quantile regression analysis with CBI number as independent variable. All statistical analyses were performed using STATA (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). All P are 2-sided, with P<0.05 considered statistically significant, and without adjustments for multiple testing. Because of limited information on CBI phenotypes, no study size calculation was possible.
Ethics
The local stroke database was approved by the ethics committee for quality control and research. ICF was waived by the ethics committee, and patients were informed about the registry and the potential use of their data for research. After information, patients may object the use of their data for research (but not for quality control). In accordance to Swiss law, patients who refused the use of their data for research were excluded from this analysis.
Results
Seventy-five percent (2992/4022) of patients underwent MRI on admission during the study timeframe. Overall, of 4022 patients considered, 1543 patients with first clinically evident ischemic stroke fulfilled the in- and exclusion criteria (Figure 2). The median age was 71 years (interquartile range, 60–81), 40% (95% CI, 38%–43%) were female, the median score on the National Institutes of Health Stroke Scale was 3 points (1–8).
Figure 2.
Study flow chart. CBI indicates covert brain infarction; CT, computed tomography; and TIA, transient ischemic attack.
Frequency, Agreement
In total, 574 patients (37% [95% CI, 35%–40%]) had a total of 950 CBIs. Seventeen percent of patients had multiple CBIs: 10% with 2 CBIs, 3% with 3 CBIs, and 4% with >3 CBIs. The interrater agreement was substantial for presence of any CBI (agreement 86%, κ=0.72, P<0.0001) and moderate to severe WMH (agreement 88%, κ=0.75, P<0.0001).
Location
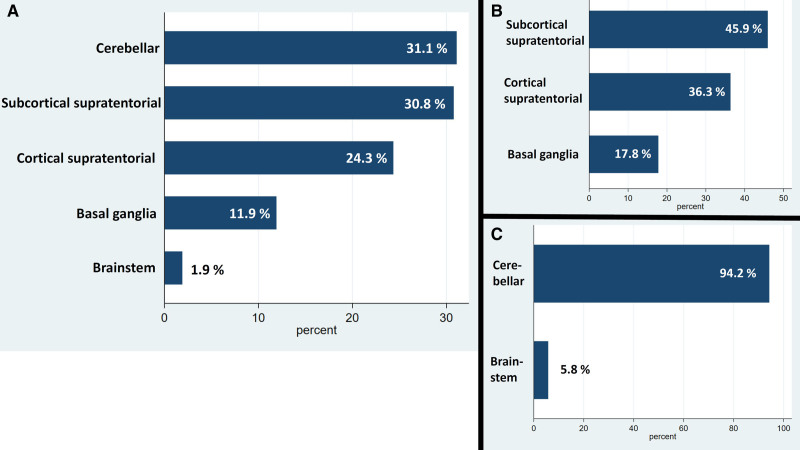
Overall, 67% of CBIs were located supratentorially, 36% of which had cortical involvement, 46% extended purely subcortically, and 18% involved the basal ganglia. Of the 33% infratentorial lesions, almost all (94%) were cerebellar lesions, making it the most common location overall (Figure 3). There was a trend for supratentorial lesions to be in the right rather than the left hemisphere (53% [95% CI, 49%–57%]), whereas for infratentorial lesions no such trend was observed (50% [95% CI, 44%–56%]).
Figure 3.
Location of covert brain infarction. A, All lesions. B, Supratentorial lesions. C, Infratentorial lesions.
Phenotypes
The distribution of CBI phenotypes is shown in Figure 4. In supratentorial location, the most frequent phenotypes of CBI included lacunes 298/637 (47%), combined gray and white matter lesions 145/637 (23%), and pure gray matter lesions 126/637 (20%, Figure 4). In infratentorial location, phenotype frequency of CBI were mostly lacunes 165/313 (53%), and combined gray and white matter lesions 145/313 (46%, Figure 4).
Figure 4.
Frequency of phenotypes of covert brain infarction. A, All lesions. B, Supratentorial lesions. C, Infratentorial lesions.
Risk Factors for Any CBI
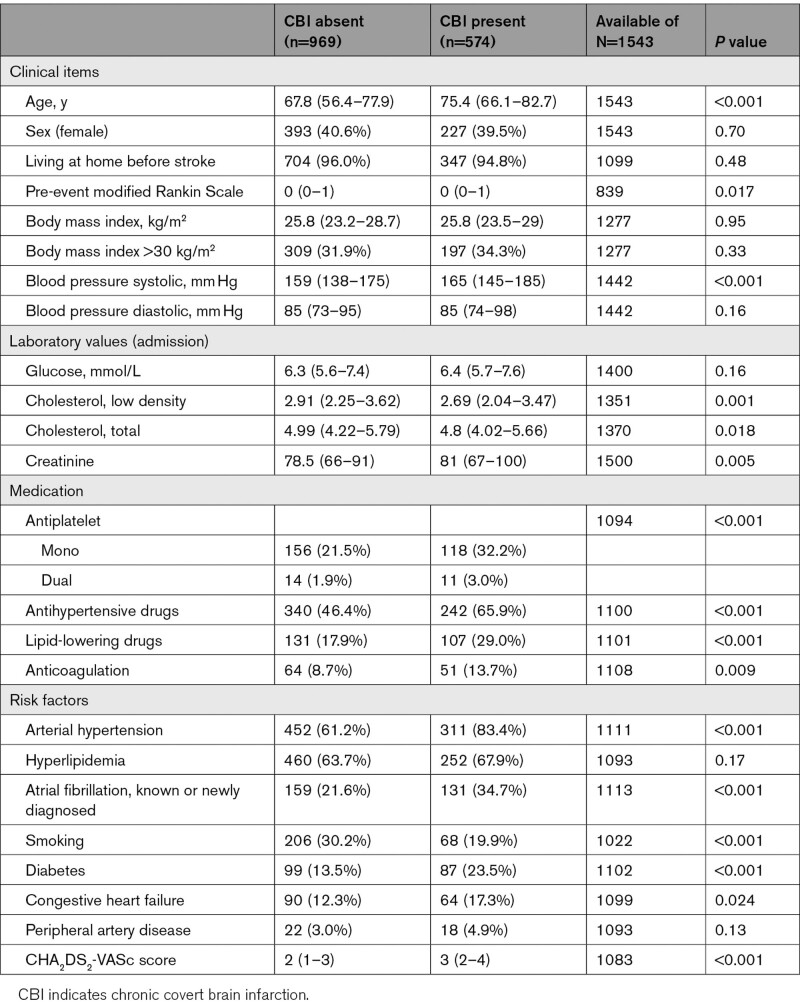
Univariable analysis showed that the following potentially causal vascular risk factors were significantly associated with presence of any CBI (Table 1).
Table 1.
Baseline Characteristics of Patients According to Presence and Number of Covert Brain Infarctions
Age
Whereas in patients younger than 50 years only 13% had any CBI and 4% multiple CBIs, the numbers increased with age as follows: 51 to 60 years 32%/18%, 61 to 70 years 31%/14%, 71 to 80 years 43%/20%, 81 to 90 years 49%/22%, >90 years 57%/22%, P<0.001.
Additionally, systolic blood pressure, hypertension, creatinine level, diabetes, AF, and congestive heart failure were associated with any CBI. There was an inverse association of cholesterol levels and active smoking with CBI; however, the intake of lipid-lowering drugs, antiplatelets, oral anticoagulation, and antihypertensives was more frequent in patients with any CBI. Prestroke mRS was slightly higher in patients with any CBI.
There was a pronounced association of WMH and CBI: whereas in patients without signs of WMH (ARWMC score 0) only 19% had any CBI and 7% multiple CBIs, the numbers increased as follows: ARWMC score 1, 36%/15%; ARWMC score 2, 53%/26%, and ARWMC score 3, 63%/40%, P<0.001.
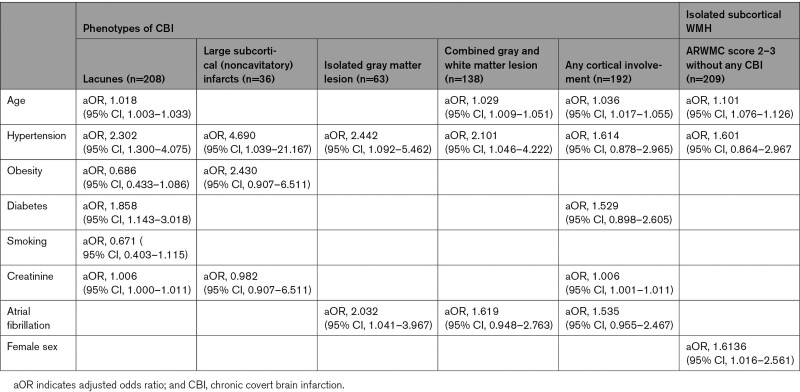
Risk Factors According to CBI Phenotype
Distribution of risk factors differed among CBI phenotypes (Table 2): risk factors associated with lacunes as compared with patients without any CBI were age, hypertension, diabetes, and higher creatinine. Risk factors associated with cortical involvement of gray matter were different: for isolated gray matter lesions, associations were significant for hypertension and AF. For combined gray and white matter lesions, increasing age, and hypertension were significant predictors whereas a borderline significance was found for AF. A similar trend for the association of any cortical involvement and AF was present. There was an association of any cortical involvement with AF in unadjusted (odds ratio, 2.4 [95% CI, 1.6–3.5]) and after adjustment for CHA2DS2-VASc score (adjusted odds ratio, 1.8 [95% CI, 1.2–2.8]).
Table 2.
Vascular Risk Factor Analysis According to Phenotype of Covert Brain Infarction
Subcortical WMH
There was a linear association of WMH with AF: whereas in patients without signs of WMH (ARWMC score 0) only 16% had AF, the numbers increased as follows: ARWMC score 1, 28%; ARWMC score 2, 33%; and ARWMC score 3, 43%; P<0.001. However, in patients with AF, CBI also was present more frequently with increasing subcortical WMH: in patients with AF with ARWMC score 0 already 30% had any CBI, the numbers increased as follows: ARWMC score 1, 41%; ARWMC score 2, 56%; and ARWMC score 3, 61%, P=0.004. Therefore, AF was eliminated in the stepwise risk factor selection and risk factors associated with isolated moderate or severe subcortical WMH without presence of any CBI were age and female sex (Table 2). The association of WMH remained stable in a sensitivity analysis for isolated cortical CBIs (OR, 1.34 [1.05–1.73]).
Characteristics of AIS
The characteristics of the AIS according to number of CBI and their phenotypes is described in the Data Supplement.
Discussion
In this large dataset of patients with a first-ever AIS, we provide an MRI-based analysis of CBI frequency, phenotypes, and risk factor profiles. Various manifestations of cerebrovascular disease (CBI, white matter lesions, microbleeds, enlarged perivascular spaces) share common risk factors, but studies have not yet addressed different phenotypes of CBI in patients with AIS separately. This is the first study to apply harmonized radiological definitions for CBI to patients with AIS, in contract with previously used heterogeneous diagnostic criteria.15
The main findings of this study are (1) 1 in 3 patients with first evident AIS had an additional chronic CBI. (2) Several vascular risk factors and WMH severity were associated with presence of CBI, hence those with additional CBI represent a vascular high-risk subgroup. (3) Different phenotypes of CBIs were associated with differing risk factor profiles (eg, consistent association of cortical CBI with AF).
Frequency of CBI
The overall rate of 37% of patients with AIS with any CBI found in our study is similar to other contemporary studies using MRI.5–7 Only one study using MRI and a somewhat different definition of CBI reported a lower rate of CBI.16 Because of the lower sensitivity of CT imaging to detect CBI, most outdated studies using CT found a lower frequency of CBI in patients with AIS.17–21 However, in the more recent IST-3 study, which used CT in 98% of patients with AIS, a similar frequency of CBI (44%) was found.8 Also, in a substudy of the ENOS trial using CT in 92% of patients, 42% of patients showed any CBI.9 Because of the difficulties of CT imaging to differentiate Virchow-Robin spaces from lacunes, however, some of the CBIs might have been misclassified.10 We more frequently found multiple CBIs than in a previous study,7 probably because in that study, the frequency of cerebellar CBIs was very low. Our reported rate of CBI (13%) in patients with AIS <50 years is similar to published studies.22–24
Location
In our study, the most frequent location of almost 1 in 3 CBIs was within the cerebellum. One in 4 CBIs involved supratentorial cortical structures with roughly 40% isolated cortical lesions. This distribution is very different as compared with previous reports.7 Hence, one of the main findings of this study is that—taken together—CBIs within the basal ganglia and supratentorial subcortical white matter did not even account for half of the CBIs observed.
In population-based studies of asymptomatic patients, most CBIs had a subcortical supratentorial location. In contrast, in this AIS population, CBIs with cortical or infratentorial location were much more frequently found.25,26 This can be interpreted as a hint towards an increased risk of AIS in patients with such CBI locations. Another possible explanation is that selection bias in this population of hospital admitted patients with AIS accounts for the difference in distribution and prevalence of the lesions in our study as compared with population based studies. Hence, prospective studies need to confirm this finding. Lesions in the cerebellum can be very small and invisible on some sequences. Thus, they may be easily overlooked and future studies need to reliably take them into account.
The trend toward a right-sided location of supratentorial CBIs is in line with previous research19 and fits flawlessly the more frequent left-sided location of AIS.27 Coherently to the presumed eloquence of the left supratentorial hemisphere, no such trend was seen for infratentorial lesions.
Phenotypes
The most common phenotype of CBI in our study was a lacune. This was true for both supra- and infratentorial location. Our finding that 20% of all patients with AIS have a lacunar CBI is within the reported range.28 However, the reported frequency of lacunes accounting for only roughly half of the observed CBIs is much lower than previously reported.6,16,29 This probably has to do with the fact that most studies—especially studies using CT—were not able to detect or disregarded other phenotypes of CBI.
Most studies did not discern CBI location and phenotype, which hampers comparisons. Additionally, in infratentorial location, it is difficult to differentiate lacunes presumably caused by small-vessel disease and embolic lesions, since the STRIVE criteria were developed for supratentorial lesions and the hyperintense fluid-attenuated inversion recovery-rim was almost never present in our cohort.
The AHA/ASA guidelines on the prevention of stroke in patients with silent cerebrovascular disease recommend to use the STRIVE criteria to describe CBIs and other forms of cerebrovascular lesions. However, the authors acknowledge themselves that those criteria were not developed to classify cortical involvement, subcortical infarcts larger than 15 mm, subcortical infarcts involving gray matter and it is unclear, if the definitions for lacunes should be the same for infratentorial lesions.30–36
Taken together, at least 25% of the CBIs found in this AIS population were not part of the small-vessel disease spectrum for which the STRIVE classification was developed. Furthermore, STRIVE was developed for supratentorial lesions, since at the time, there was not much info on cerebellar small-vessel disease lesions. Hence, an additional 30% of cerebellar CBIs present in this study was also not clear-cut classifiable. We think that the combination of STRIVE and other CBI phenotypes11 to combine the concept of small-vessel disease2,28 as well as embolic causes of brain frailty, as performed in this study, is important to capture all information to optimally manage patients with AIS.
Risk Factors for Any CBI
Most importantly, the associations of many vascular risk factors with CBI shows that among patients suffering AIS, those with additional CBI represent a vascular high-risk subgroup. Previous studies using CT imaging found no association of AF with any CBI or number of CBIs.18 Our findings of increased rates of AF in patients with any CBI (35% versus 22%, P<0.001) is consistent with other contemporary studies, that embolic sources including AF more frequently showed CBI (58% versus 34%, P=0.008).5 This discrepancy probably has to do with the fact that CT imaging primarily detects lacunes in which AF is less important.18,20,37–39
We also show that several other vascular risk factors are associated with presence of any CBI, of which the most important ones seem to be age and arterial hypertension. We explain the paradoxical association of lower cholesterol levels and nonsmoking with CBI by the fact that the intake of lipid lowering drugs was much more frequent in patients with CBI (reflecting the overall higher cerebrovascular risk profile of CBI-patients) and that active smokers were much younger. Since we found no associations of CBI with sex, and BMI, those risk factors might be less important for CBI occurrence.
Risk Factors According to CBI Phenotype
Because the risk factor profile differs according to CBI phenotype in a pathophysiologically plausible way, it suggests that the underlying etiology of AIS might differ.
An embolic cortical pattern of CBI was reported more often in patients with AF using CT imaging.18,40 Congruently, the stepwise selection model included AF for patients with isolated cortical CBI phenotype, combined cortical and subcortical CBI phenotype or the combination with any cortical involvement in our dataset with an overall consistent association of cortical lesions with AF. Importantly, when we accounted for covariates the apparent association of increasing WMH with AF disappeared. Hence, CBI phenotype seems more useful in identifying patients with AIS with covert AF than mere CBI presence or number. Further research needs to determine whether addition of cortical CBIs refines strategies to optimize indications for long-term monitoring in patients with AIS.41
Relevance for Clinical Practice
As those patients with acute stroke and presence of CBI represent a vascular high-risk subgroup, it might well be considered that this might impact future trials on therapeutic strategies investigating the best secondary prevention strategies, stratifying patients accordingly (eg, more aggressive secondary prevention in patients with AIS with additional CBIs). Also, if the present results would be confirmed in patients with transient ischemic attack, it could be used as an adjunct to existing scales to guide hospitalization and need of acute diagnostics. The phenotype of CBI might also guide the clinician in tailoring the specific diagnostic workup to the presumed underlying etiology of not only the acute lesion, but also that of the CBIs (eg, prolonged monitoring to detect AF in patients with AIS with additional cortical CBIs). Prospective studies are needed, however, to test the hypothesis that such a tailored diagnostic work-up and precision treatment results in reduced rates of vascular events, disability, dementia, and death.
Strengths and Limitations
Our study has several strengths: (1) this is a large, prospectively collected sample giving sufficient power to identify associations of vascular risk factors with phenotypes of CBI. (2) MRI reading was performed according to harmonized recent definitions, was structured, and with blinded assessors skilled in stroke imaging. (3) We used data from consecutive patients in a prospective database with predefined variables, which limits selection bias. (4) Data completeness was good to excellent for most outcomes including imaging (100%) and National Institutes of Health Stroke Scale (91%).
Our study has several limitations: () although data were collected prospectively, the statistical analysis was retrospective and thus prone to bias. (2) The current study was underpowered to prove associations in multiple subgroups. However, we consider this study hypothesis generating. (3) Because of the low rate of 3T MRI use, we could not analyze cortical micro-infarcts. (4) Imaging acquisition parameters were heterogeneous throughout the study period, and some lesions might have been misclassified. However, the use of routine clinical MR imaging assures feasibility and generalizability in external cohorts. (5) Although Bern is a multiethnic city, racial and ethnic groups other than White participants are likely underrepresented and race and ethnicity are not available within the dataset. (6) The covert nature of the MRI lesion was retrospectively adjudicated based on patient history and without a standardized questionnaire. Nevertheless, in clinical practice in our hospital, this information is thoroughly assessed in patients with AIS.
Conclusions
Using MRI, more than a third of patients with first-evident AIS had an additional chronic CBI. Several vascular risk factors and WMH severity were associated with presence of CBI. Thus, among patients suffering AIS, those with additional CBI represent a vascular high-risk subgroup. The location and phenotypes as determined by MRI definitions are different from previous studies using CT imaging with a frequent cerebellar location. Different phenotypes of CBIs with differing risk factor profiles (eg, consistent association of cortical CBI with AF) exist, potentially pointing toward discriminative AIS etiologies.
Article Information
Sources of Funding
The Swiss National Foundation (Grant number 32003B_189077) supports a multicentre study on the incidence of silent atrial fibrillation in patients with covert brain infarction, led by our group.
Disclosures
Dr Kaesmacher reports grants from SAMW/Bangerter and grants from Swiss Stroke Society outside the submitted work. Dr Wardlaw reports grants from Medical Research Council during the conduct of the study, grants from Fondation Leducq, grants from British Heart Foundation, grants from EU H2020, and grants from UK Stroke Association outside the submitted work. Dr Roten reports personal fees from Abbott and personal fees from Medtronic outside the submitted work. Dr Gralla reports grants from Medtronic and grants from Swiss National Funds outside the submitted work. Dr Heldner reports grants from the Swiss Heart Foundation, grants from Bangerter Foundation, and grants from SITEM-Insel Support Funds (SISF) outside the submitted work. Dr Goeldlin reports grants from Swiss Academy of Medical Sciences & Bangerter-Rhyner Foundation, other from Swiss Stroke Society, grants from Mittelbauvereinigung der Universität Bern, and other from Pfizer outside the submitted work. Dr Arnold reports personal fees from Amgen, personal fees from Bayer, personal fees from BMS, personal fees from Covidien, personal fees from Medtronic, personal fees from Daiichi Sankyo, personal fees from Nestlé Health Science, and personal fees from Novartis during the conduct of the study, grants from Swiss National Science Foundation and grants from the Swiss Heart Foundation and scientific advisory board honoraria from, Sanofi outside the submitted work. Dr Meinel reports grants from Bangerter-Rhyner-Foundation and Swiss Academy of Medical Sciences, and other from Swiss Stroke Society outside the submitted work. Dr Fischer reports grants from Medtronic, other from Medtronic, other from Stryker, other from CSL Behring, grants from Swiss Heart Foundation, and grants from Swiss National Science Foundation outside the submitted work.
Supplemental Material
Expanded Methods and Results
Online Tables I and II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AIS
- acute ischemic stroke
- ARWMC
- age-related white matter change
- CBI
- chronic covert brain infarction
- CT
- computed tomography
- MRI
- magnetic resonance imaging
- NIHSS
- National Institutes of Health Stroke Scale
- WMH
- white matter hyperintensity
J. Vynckier and J. Kaesmacher contributed equally.
U. Fischer and T.R. Meinel contributed equally.
This manuscript was sent to Liping Liu, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.034347.
For Sources of Funding and Disclosures, see page 566.
Contributor Information
Johannes Kaesmacher, Email: johannes.kaesmacher@insel.ch.
Joanna Marguerite Wardlaw, Email: joanna.wardlaw@ed.ac.uk.
Laurent Roten, Email: laurent.roten@insel.ch.
Morin Beyeler, Email: morin.beyeler@insel.ch.
Nebiyat Filate Belachew, Email: nebiyatfilate.belachew@insel.ch.
Lorenz Grunder, Email: lorenz.grunder@insel.ch.
David Julian Seiffge, Email: david.seiffge@insel.ch.
Simon Jung, Email: simon.jung@insel.ch.
Jan Gralla, Email: jan.gralla@insel.ch.
Tomas Dobrocky, Email: tomas.dobrocky@insel.ch.
Mirjam Rachel Heldner, Email: mirjam.heldner@insel.ch.
Ulrike Prange, Email: ulrike.prange@insel.ch.
Martina Béatrice Goeldlin, Email: MartinaBeatrice.Goeldlin@insel.ch.
Marcel Arnold, Email: marcel.arnold@insel.ch.
Urs Fischer, Email: urs.fischer@insel.ch.
Thomas Raphael Meinel, Email: thomas.meinel@insel.ch.
References
- 1.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, et al. Silent brain infarction and risk of future stroke: a systematic review and meta-analysis. Stroke. 2016;47:719–725. doi: 10.1161/STROKEAHA.115.011889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho AH, Kwon SU, Kim TW, Lee SJ, Shon YM, Kim BS, Yang DW. High prevalence of unrecognized cerebral infarcts in first-ever stroke patients with cardioembolic sources. Eur J Neurol. 2009;16:838–842. doi: 10.1111/j.1468-1331.2009.02604.x [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Park SS, Lee SH, Yoon BW. Reduced severity of strokes in patients with silent brain infarctions. Eur J Neurol. 2011;18:962–971. doi: 10.1111/j.1468-1331.2010.03282.x [DOI] [PubMed] [Google Scholar]
- 7.Oh SH, Kim NK, Kim SH, Kim JK, Kim HS, Kim WC, Kim OJ. The prevalence and risk factor analysis of silent brain infarction in patients with first-ever ischemic stroke. J Neurol Sci. 2010;293:97–101. doi: 10.1016/j.jns.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw JM, Sandercock P, Cohen G, Farrall A, Lindley RI, von Kummer R, von Heijne A, Bradey N, Peeters A, Cala L, et al. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third international stroke trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol. 2015;14:485–496. doi: 10.1016/S1474-4422(15)00012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleton JP, Woodhouse LJ, Adami A, Becker JL, Berge E, Cala LA, Casado AM, Caso V, Christensen HK, Dineen RA, et al. ; ENOS Investigators. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology. 2020;94:e439–e452. doi: 10.1212/WNL.0000000000008881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014;45:3461–3471. doi: 10.1161/STROKEAHA.114.005919 [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 13.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, et al. ; European Task Force on Age-Related White Matter Changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318 [DOI] [PubMed] [Google Scholar]
- 14.De Cocker LJ, Kloppenborg RP, van der Graaf Y, Luijten PR, Hendrikse J, Geerlings MI; SMART Study Group. Cerebellar cortical infarct cavities: correlation with risk factors and MRI markers of cerebrovascular disease. Stroke. 2015;46:3154–3160. doi: 10.1161/STROKEAHA.115.010093 [DOI] [PubMed] [Google Scholar]
- 15.Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42:1140–1145. doi: 10.1161/STROKEAHA.110.600114 [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Weimar C, Wanke I, Möller-Hartmann C, Gizewski ER, Blatchford J, Hermansson K, Demchuk AM, Forsting M, Sacco RL, et al. ; PRoFESS Imaging Substudy Group. Risk of recurrent stroke in patients with silent brain infarction in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) imaging substudy. Stroke. 2012;43:350–355. doi: 10.1161/STROKEAHA.111.631739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kase CS, Wolf PA, Chodosh EH, Zacker HB, Kelly-Hayes M, Kannel WB, D’Agostino RB, Scampini L. Prevalence of silent stroke in patients presenting with initial stroke: the Framingham Study. Stroke. 1989;20:850–852. doi: 10.1161/01.str.20.7.850 [DOI] [PubMed] [Google Scholar]
- 18.Boon A, Lodder J, Heuts-van Raak L, Kessels F. Silent brain infarcts in 755 consecutive patients with a first-ever supratentorial ischemic stroke. Relationship with index-stroke subtype, vascular risk factors, and mortality. Stroke. 1994;25:2384–2390. doi: 10.1161/01.str.25.12.2384 [DOI] [PubMed] [Google Scholar]
- 19.Chodosh EH, Foulkes MA, Kase CS, Wolf PA, Mohr JP, Hier DB, Price TR, Furtado JG., Jr. Silent stroke in the NINCDS Stroke Data Bank. Neurology. 1988;38:1674–1679. doi: 10.1212/wnl.38.11.1674 [DOI] [PubMed] [Google Scholar]
- 20.Davis PH, Clarke WR, Bendixen BH, Adams HP, Jr, Woolson RF, Culebras A. Silent cerebral infarction in patients enrolled in the TOAST Study. Neurology. 1996;46:942–948. doi: 10.1212/wnl.46.4.942 [DOI] [PubMed] [Google Scholar]
- 21.Mounier-Vehier F, Leys D, Rondepierre P, Godefroy O, Pruvo JP. Silent infarcts in patients with ischemic stroke are related to age and size of the left atrium. Stroke. 1993;24:1347–1351. doi: 10.1161/01.str.24.9.1347 [DOI] [PubMed] [Google Scholar]
- 22.Putaala J, Kurkinen M, Tarvos V, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts and leukoaraiosis in young adults with first-ever ischemic stroke. Neurology. 2009;72:1823–1829. doi: 10.1212/WNL.0b013e3181a711df [DOI] [PubMed] [Google Scholar]
- 23.Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol. 2012;259:653–659. doi: 10.1007/s00415-011-6234-3 [DOI] [PubMed] [Google Scholar]
- 24.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–1203. doi: 10.1161/STROKEAHA.108.529883 [DOI] [PubMed] [Google Scholar]
- 25.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM; Rotterdam Scan Study. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15 [DOI] [PubMed] [Google Scholar]
- 27.Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T; Arbeitsgruppe Schlaganfall Hessen. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366:392–393. doi: 10.1016/S0140-6736(05)67024-9 [DOI] [PubMed] [Google Scholar]
- 28.Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–1234. doi: 10.1212/WNL.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi T, Kobayashi S, Yamaguchi S. Frequency and pathogenesis of silent subcortical brain infarction in acute first-ever ischemic stroke. Intern Med. 2002;41:103–108. doi: 10.2169/internalmedicine.41.103 [DOI] [PubMed] [Google Scholar]
- 30.Kakkos SK, Sabetai M, Tegos T, Stevens J, Thomas D, Griffin M, Geroulakos G, Nicolaides AN; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg. 2009;49:902–909. doi: 10.1016/j.jvs.2008.10.059 [DOI] [PubMed] [Google Scholar]
- 31.Ter Schiphorst A, Tatu L, Thijs V, Demattei C, Thouvenot E, Renard D. Small obliquely oriented cortical cerebellar infarctions are associated with cardioembolic stroke. BMC Neurol. 2019;19:100. doi: 10.1186/s12883-019-1328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Merwijk G, Lodder J, Bamford J, Kester AD. How often is non-valvular atrial fibrillation the cause of brain infarction? J Neurol. 1990;237:205–207. doi: 10.1007/BF00314595 [DOI] [PubMed] [Google Scholar]
- 33.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646–2648. doi: 10.1161/01.str.29.12.2646 [DOI] [PubMed] [Google Scholar]
- 34.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, Kay R, Ringelstein EB. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Nagakane Y, Tomii Y, Toda S. Infarct pattern of cortical and subcortical multiple small infarctions in terms of potential source of embolism. J Neurol Sci. 2017;381:184. doi: 10.1016/j.jns.2017.08.529 [Google Scholar]
- 36.Bogousslavsky J. Topographic patterns of cerebral infarcts. Rev Ecuatoriana Neurol. 1993;2:59–62. doi: 10.1159/000108899 [Google Scholar]
- 37.Jørgensen HS, Nakayama H, Raaschou HO, Gam J, Olsen TS. Silent infarction in acute stroke patients. Prevalence, localization, risk factors, and clinical significance: the Copenhagen Stroke Study. Stroke. 1994;25:97–104. doi: 10.1161/01.str.25.1.97 [DOI] [PubMed] [Google Scholar]
- 38.Herderscheê D, Hijdra A, Algra A, Koudstaal PJ, Kappelle LJ, van Gijn J. Silent stroke in patients with transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. Stroke. 1992;23:1220–1224. doi: 10.1161/01.str.23.9.1220 [DOI] [PubMed] [Google Scholar]
- 39.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, Anderson CS, Hankey GJ, Jamrozik K, Appelros P, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. doi: 10.1161/STROKEAHA.109.558809 [DOI] [PubMed] [Google Scholar]
- 40.Kempster PA, Gerraty RP, Gates PC. Asymptomatic cerebral infarction in patients with chronic atrial fibrillation. Stroke. 1988;19:955–957. doi: 10.1161/01.str.19.8.955 [DOI] [PubMed] [Google Scholar]
- 41.Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns HJGM, et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration. Circulation. 2019;140:1834–1850. doi: 10.1161/CIRCULATIONAHA.119.040267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data and statistical analyses will be shared upon motivated request from any qualified investigator after clearance from the ethics committee.