Supplemental Digital Content is available in the text.
Keywords: brain, hypoxia, ischemia, regulatory T cells, sex-difference
Abstract
Background and Purpose:
Neonatal encephalopathy caused by hypoxia-ischemia (HI) is a major cause of death and disability in newborns. Clinical and experimental studies suggest a sexual dimorphism in HI-induced brain injury and therapy responses. A major hallmark of HI pathophysiology is the infiltration of peripheral immune cells into the injured brain. However, the specific role of regulatory T cells (Tregs) in neonatal HI is still unknown.
Methods:
Nine-day-old mice were exposed to HI by ligation of the right common carotid artery followed by 1 hour hypoxia (10% oxygen). Using immunohistochemistry, flow cytometry, and microarray analyses, Tregs were investigated in the brain, spleen, and blood 24 hours post HI. The functional role of Tregs was evaluated by acute Treg depletion in depletion of regulatory T cells transgenic mice. Brain injury, neuroinflammatory responses, and vascular injury were analyzed via immunohistochemistry and Western blot 48 hours and 7 days after HI. Functional outcome was assessed 3 days and 5 weeks after HI.
Results:
Female mice revealed an increased cerebral Treg infiltration, coinciding with elevated chemokine receptor expression. Treg depletion in females aggravated HI-induced brain tissue injury, short-term motor deficits, and long-term deficits in exploratory activity, paralleled by an increased microglia and endothelial activation and leukocyte infiltration. Treg depletion in male mice reduced HI-induced brain injury, short-term motor, and long-term cognitive deficits, associated with reduced vascular injury. Ex vivo isolated female Tregs displayed an increased immunosuppressive activity on effector T cell proliferation and an increased gene enrichment in pathways related to enhanced Treg activity.
Conclusions:
Tregs from neonatal female mice provide endogenous neuroprotection, whereas Tregs from male mice increase secondary neurodegeneration. As potential mechanisms, we identified intrinsic transcriptional differences associated with enhanced anti-inflammatory activity of female Tregs. Our study emphasizes the urgent need for sex-stratified clinical and preclinical analyses.
Neonatal encephalopathy caused by hypoxia-ischemia (HI) is a major cause of long-term morbidity and mortality in newborns. The only available therapy, therapeutic hypothermia is limited with 40% to 50% of surviving infants still suffering from major neurological problems.1 In spite of tremendous efforts to identify new and/or additional therapies, translation into clinical practice failed. A major reason might be our incomplete understanding of complex HI-pathophysiology, involving not only cells of the central nervous but also of the peripheral immune system. Furthermore, clinical and experimental studies suggested a sexual dimorphism in HI pathophysiology and inflammatory responses.2,3
A major hallmark of HI pathophysiology is the infiltration of peripheral immune cells into the injured brain. Previous studies reported sex-differences in outcomes after depletion of peripheral immune cell subsets and in the extent of immune cell infiltration.4,5 Depletion of peripheral myeloid cells was neuroprotective in males, while no differences were observed in females.5 With regard to lymphocytes, an increased infiltration into the injured brain was observed in male mice 3 days after HI.4 However, previous reports indicated different functions of different lymphocyte populations, that is, the total lack of T and B cells in Rag−/− was neuroprotective,6 while the selective depletion of T cells enhanced HI-induced tissue injury.7 Furthermore, increased HI-induced injury after immunomodulatory therapy was associated with a reduced amount of regulatory T cells (Treg) in the brain,7 suggesting a neuroprotective function of these cells in neonatal HI.
A large body of evidence from studies in adult brain ischemia models related neuroprotective functions of Tregs to their anti-inflammatory capacity, that is, reducing infiltration of peripheral leukocytes and inhibiting micro- and astrogliosis.8–10 However, far less is known about Tregs in neonatal HI. Considering pronounced differences between the adult and neonatal immune system, findings in adult ischemic brain injury may not be translated to neonates. For example, perinatally developed Tregs are supposed to be more proliferative with a higher suppressive activity compared with adult Tregs.11 Nevertheless, common to both, adult and neonatal brain injury, is the lack of information about the impact of sex on Treg function in the pathophysiology of brain ischemia.
Sex-dependent differences in Treg functionality and phenotype have been reported in different disease paradigms.12 Besides sex-hormone dependent mechanisms, differences in the transcriptomic and metabolic profile between female and male Tregs have been recently discussed in the context of chronic intestine and visceral adipose tissue inflammation.13,14 With regard to ischemic brain injury, data are limited, because the majority of studies targeting Tregs in adult stroke models used male mice. In neonatal HI, Tregs have not been investigated to date.
In the present study, we characterized Treg infiltration and function in neonatal HI in sex-stratified analyses. We further investigated the impact of endogenous Tregs on the development of HI-induced brain injury and identified sex-specific cellular targets and signaling pathways.
Methods
Detailed data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Care and Group Allocation
Experiments were performed in accordance to the ARRIVE guidelines with government approval by the State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia. C57BL/6J mice, initially ordered from Charles River, and transgenic depletion of regulatory T cells transgenic (DEREG) mice,15 initially provided by Tim Sparwasser, were bred in house and kept under a 12 hours light/dark cycle with food and water ad libitum. Genotyping of DEREG mice is described in the Supplemental Methods. Bodyweight was recorded at postnatal day 9 (P9), P10, P11, P16 and weekly after weaning. A total of 424 animals (215 female, 209 male) were enrolled. Eleven transgenic naïve DEREG mice (5 female, 6 male) were used to exclude potential confounding effects by Treg depletion in neonates. Fifty-two transgenic DEREG mice (27 female, 25 male) were exposed to sham-operation. Out of the remaining 291 transgenic DEREG (146 female, 145 male), 42 wild-type littermate DEREG (21 female, 21 male) and 28 C57/BL6 mice (16 female, 12 male) that were exposed to HI, 35 (9.7%, 17 female, 18 male) died. Detailed information on methods of randomization, blinding, sample sizes, sex, and mortality are provided in the Supplemental Methods and Table S1.
Neonatal HI and Treg Depletion
Hypoxic-ischemic brain injury was induced in 9-day-old animals as previously described.7,16,17 Briefly, the right common carotid artery was occluded through cauterization (high temperature cauter, 1200 °C, Bovie) under isoflurane anesthesia (1.5–2.5 Vol%) followed by 1 hour hypoxia (10% O2) in an air-tight oxygen chamber (OxyCycler, Biospherix) after 1 hour recovery with their dams. Perioperative analgesia was ensured by subcutaneous administration of 0.1 mg/kg buprenorphine. According to our previous studies,18 Tregs were depleted in DEREG mice by intraperitoneal (IP) administration of 30 ng/g body weight diphtheria toxin (DTX, Calbiochem, Germany) 24 hours before and 24 hours after HI. Control animals received the same amount of PBS. Previous work showed that repetitive daily Treg depletion in neonatal mice is associated with weight loss at P16 and development of autoimmune pathology.11,15 However, in our experimental setting with 2 single injections at P8 and P10, we did not detect signs of autoimmunity in peripheral organs 5 weeks after DTX injection (Figure S1A and S1B) or weight loss in HI-injured animals at P16 (Figure S1C).
Processing of Peripheral Blood, Spleen, and Brain Tissues for Flow Cytometry and Fluorescent-Activated Cell Sorting
Isolation of single cell suspension for flow cytometry analysis was performed as previously described.7 Briefly, animals were deeply anesthetized by IP injections of an overdose chloralhydrate followed by transcardial perfusion with ice-cold PBS and removal of spleens and brains. Blood specimens were collected with EDTA-coated capillaries (Radiometer, Denmark) by snipping the right atrium of the heart immediately before perfusion via the left ventricle. Blood samples were transferred into EDTA-coated collection tubes (Minicollect, Greiner Bio One, Germany) until further processing. Details on cell isolation, antibody staining, flow cytometry, and cell sorting are provided in the Supplemental Methods and Table S2. Suppressive activity of Tregs was analyzed in T cell proliferation assays and by assessment of their impact on myeloid cell activation as described in the Supplemental Methods.
Tissue Preparation, Histology, and Immunohistochemistry
One, 2, or 7 days after HI, mice were deeply anesthetized with chloralhydrate and transcardially perfused with ice-cold PBS. Brains were removed and snap-frozen on dry ice. Histopathology and immunohistochemistry were performed on 20 μm cryostat sections according to published protocols.7,16 A detailed description of staining and quantifications is given in the Supplemental Methods and Table S3.
Behavioral Assessment
The impact of HI, sex, and Treg depletion on short-term motor coordination was assessed in front limb suspension tests 3 days after HI. Long-term neurodevelopmental outcome was determined in Elevated Plus Maze and Novel Object Recognition tests 5 weeks after HI. Details are provided in Supplemental Methods.
Western Blot
For western blot analysis, whole hemisphere tissue specimens were collected at the hippocampal level between −1.8 to −2.4 mm from bregma. Tissue processing and immunoblotting were performed according to our previous studies7,16 and are specified in the Supplemental Methods.
Microarray and Gene Set Enrichment Analysis
Total RNA of sorted splenic Tregs was isolated with QiaShredder columns and the RNeasy Micro Kit (Qiagen, Hilden, Germany) according to manufacturers’ instructions. RNA samples were evaluated for RNA integrity with the Agilent 2100 Bioanalyzer System (Agilent Technologies, Waldbronn, Germany). Microarray analysis and Gene Set Enrichment Analysis are specified in the Supplemental Methods.
Statistical Analysis
Sample sizes were calculated a priori with the G*Power (version 3.1.9.2) software for the primary outcomes histological brain injury and behavioral outcome. We assumed a Cohen’s d ES of 0.6 to be biologically relevant, a highly conservative assumption based on the model-associated variability.7,16,17 An α-level of 0.05 and a power of 0.8 were required and a mortality rate of 10% was expected,7,16,17 yielding a final sample size of 10 animals per group. Exploratory analyses without prestudy sample size calculation included flow cytometry, Western blot, in vitro assays with ex vivo isolated Tregs, and microarray analyses. Results are expressed as box plots with individual data points including median values, the 25% and the 75% percentile. For statistical analysis, the GraphPad Prism 6.0 software package (GraphPad Software) was used. Continuous data were tested for gaussian distribution with the D’Agostino & Pearson omnibus normality test or, for small sample sizes, with the Shapiro-Wilk normality test. In case of normal distribution either unpaired Students t Test or ordinal 1-way ANOVA with post hoc Sidak’s were applied. For ordinal and nonparametrically distributed continuous data Mann-Whitney or Kruskal-Wallis with Dunn’s were applied. Details on applied tests for each data set are provided in Table S4. In all analyses, P<0.05 was considered statistically significant.
Results
Neonatal HI Induces Enhanced and Selective Infiltration of Tregs Into the Female Brain
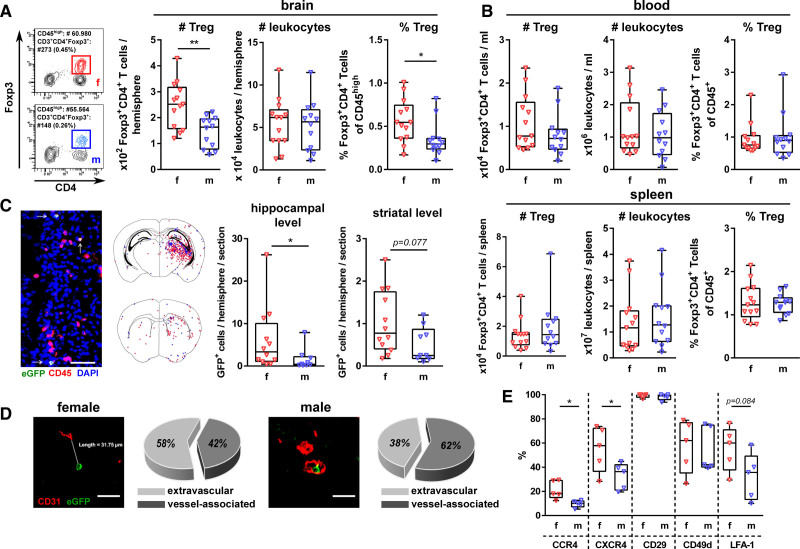
Analyses of Treg infiltration via flow cytometry demonstrated a significant increase in the total amount and frequency of Tregs in female brains compared with male brains 24 hours after HI (Figure 1A). No differences in Treg numbers and frequencies were observed in the spleen and the peripheral blood (Figure 1B). Immunohistochemistry analysis confirmed the increased infiltration of female Tregs with most pronounced infiltration at the hippocampal level in both sexes (Figure 1C). Of note, the majority of male Tregs (62%) was associated with the vasculature (ie, intra- or perivascular) compared with 42% of female Tregs (Figure 1D). Distances of intraparenchymal infiltrated Tregs to the vasculature did not differ between sexes (Figure S2A). Compared with the periphery (Figure S2B), chemokine receptor and integrin expression on brain-infiltrated Tregs (Figure 1E) was elevated for all analyzed proteins, except for CCR4. However, the frequency of CCR4+ and CXCR4+ Tregs was significantly increased in female compared with male brains (Figure 1E). Furthermore, the frequency of LFA-1+ Tregs was higher in females than in males, while the percentage of CD49d+ and CD29+ did not differ between sexes (Figure 1E). In the blood and spleen, we detected no differences between female and male Tregs (Figure S2C).
Figure 1.
Increased infiltration of female Tregs into the hypoxia-ischemia (HI)-injured brain. Postnatal day 9 (P9) C57BL/6 (A and B) and depletion of regulatory T cells transgenic (DEREG) (C and D) mice were exposed to HI. Tregs and total leukocytes were quantified via flow cytometry in the brain (A), blood, and spleen (B) 24 h after HI. Localization of brain-infiltrated Tregs was analyzed via immunohistochemistry in eGFP/CD45 (C, Scale bar: 50 μm, arrows indicate Tregs) and eGFP/CD31 double staining (D, Scale bar: 30 μm). Chemokine receptor and integrin expression on brain-infiltrated Tregs were determined via flow cytometry (E). *P<0.05, **P<0.01. n=12–13/group in (A, B), n=9–12/group in (C, graphs), n=sum of Tregs from 5 animals/group (16 sections/animal) in C, n=sum of Tregs from 5 animals/group (8 sections/animal) in D, n=5/group in E.
Treg Depletion in Females Exacerbates Neuronal Injury, Early Motor Deficits, and Long-Term Exploratory Activity, While Treg-Depleted Males Are Protected From HI-Induced Neuronal Loss, Motor Deficits, and Long-Term Cognitive Dysfunction
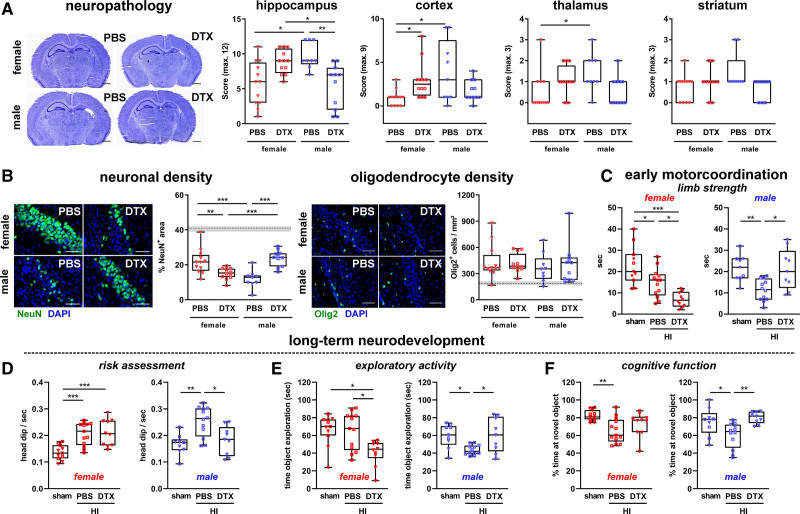
To selectively investigate the functional role of Tregs in the acute phase of neonatal HI, Tregs were depleted by DTX injection in DEREG mice 24 hours prior and 24 hours after HI. A single DTX injection resulted in efficient Treg depletion for 48 hours in the blood, spleen, and HI-injured brain tissues, independent of sex (Figure S3). Histopathologic evaluation in cresyl violet stained brain sections revealed an increased tissue injury in Treg-depleted females in the hippocampus and cortex (Figure 2A). Treg depletion in males resulted in a significantly reduced hippocampal brain injury (Figure 2A). Opposing effects of Treg depletion were confirmed via immunohistochemistry, demonstrating a significantly reduced neuronal density in the hippocampus of Treg-depleted females, while males were protected from HI-induced neuronal loss (Figure 2B). For both, histopathologic changes and neuronal loss, PBS-treated male control mice showed an increased brain injury compared with female control mice (Figure 2A and 2B). HI-induced increase of total oligodendrocytes was not modulated, neither by sex nor by Treg depletion (Figure 2B). Confounding effects by DTX injection per se could be excluded as assessed in wild type littermate HI-injured and sham-operated transgenic DEREG animals (Figure S4). Of note, different subacute responses to early Treg depletion were also not caused by sex-differences in Treg recovery (Figure S5).
Figure 2.
Acute Treg depletion increases subacute neurodegeneration in females and is protective in males associated with altered short- and long-term functional outcome. P9 depletion of regulatory T cells transgenic (DEREG) mice were exposed to hypoxia-ischemia (HI). Tregs were depleted by IP injection of 30 ng/g bodyweight DTX at P8 and P10. At P16 histological brain injury (A), neuronal and oligodendrocyte densities (B) were analyzed in cresyl violet stained tissue sections and via immunohistochemistry for NeuN and Olig2 (B), respectively. Scale bars (A): 1 mm, (B): 50 μm (both CA1 region of the hippocampus). Dashed lines and gray shadows indicate quantiles of sham-operated animals. Short-term motor coordination deficits were assessed in forelimb suspension tests 3 d after HI; the time to fall of the wire was measured (C). Long-term neurobehavioral development was evaluated in the EPM (D) and the NOR test (E and F) 5 wks after HI. The number of head dips in the open and center area of the EPM (D), the time spent at objects in the training trial of the NOR test (E) and % time of novel object exploration compared with total object exploration in the testing trial of the NOR test (F) were measured. C–F, Rectangles and triangles in sham groups indicate PBS and DTX treatment, respectively; left graphs: female, right graphs: male. *P<0.05, **P<0.01, ***P<0.001. n=9–12/group (A and B), n=9–13 (D–F).
Histological findings were confirmed on a functional level, as demonstrated by significantly increased impairment of forelimb strength in Treg-depleted females, while males were protected from this early motor coordination deficit (Figure 2C). These early behavioral differences translated into long-lasting alterations of neurodevelopmental functions in young adult mice (Figure 2D through 2F). HI-induced increase of head dipping in open arm and center regions of the Elevated Plus Maze (as a measure of risk assessment and escape behavior), reduction of exploratory activity in the object recognition task and decreased novel object recognition memory in the NOR task, were significantly improved in Treg-depleted males (Figure 2D through 2F). Furthermore, Treg depletion in females resulted in a significantly reduced exploratory activity (Figure 2D). HI-induced alterations of impulsivity/escape behavior, reflected by an enhanced time in the open arms of the Elevated Plus Maze, was slightly increased in Treg-depleted females but not in males (Figure S6A). Enhanced escape behavior in HI-injured animals is also reflected by an increased mean velocity, which was, however, not modulated by Treg depletion (Figure S6B).
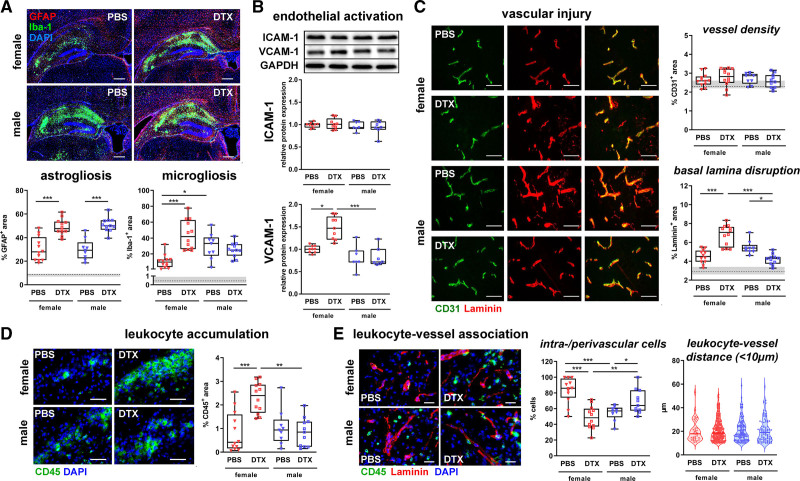
Female Tregs Inhibit HI-Induced Neuroinflammatory Processes and Male Tregs Promote Vascular Injury
To get deeper insights into the mechanisms underlying the divergent function of Tregs, we analyzed glial and endothelial activation, vascular damage, and peripheral immune cell infiltration, major hallmarks of HI pathophysiology. Both, neuropathological assessment and immunohistochemistry demonstrated most pronounced effects in the hippocampus (Figure 2A and 2B, Figure S7). Therefore, further analyses focused on this brain region. Treg depletion led to an increased astrocyte activation, independent of sex (Figure 3A). Microglia activation was significantly increased in Treg-depleted females compared with PBS-treated controls (Figure 3A). Except of an increased microglia activation per se, no differences were observed by Treg depletion in males (Figure 3A). Similarly, Treg depletion in females but not in males led to a significantly enhanced endothelial activation, as demonstrated by elevated VCAM-1 (vascular adhesion molecule-1) expression (Figure 3B). ICAM-1 (intercellular adhesion molecule-1) expression was not modulated, neither by sex nor by Treg depletion (Figure 3B). In addition to endothelial activation, neonatal HI leads to damage of the basal lamina.17 While Treg depletion resulted in an increased basal lamina disruption in females, Treg-depleted male mice were protected from HI-induced vascular injury (Figure 3C). Concomitant with increased VCAM-1 expression and vascular injury, Treg-depleted female mice revealed a significant increase in peripheral leukocyte accumulation compared with PBS-treated controls, whereas no differences were observed in males (Figure 3D). To determine the impact of Tregs on infiltration patterns of peripheral leukocytes, we characterized leukocyte localization, demonstrating that the amount of vessel-associated leukocytes was significantly reduced in Treg-depleted females, while a large proportion of peripheral leukocytes was either located in the vessels or in the perivascular space in Treg-depleted males (Figure 3D). Distances of clearly extravasated leukocytes (<10 μm apart from the vessel) were not modulated by sex or Treg depletion (Figure 3D).
Figure 3.
Sex-dependent effects of Treg depletion on glial and endothelial activation, leukocyte infiltration and vascular injury. P9 depletion of regulatory T cells transgenic (DEREG) mice were exposed to hypoxia-ischemia (HI). Tregs were depleted by IP injection of 30 ng/g bodyweight DTX at P8 and P10. Analysis was performed in the hippocampus at P16. Micro- and astrogliosis (A) were analyzed by immunohistochemistry for Iba-1 and GFAP, respectively (A). Endothelial activation was assessed by quantification of ICAM-1 (intercellular adhesion molecule-1) and VCAM-1 (vascular adhesion molecule-1) expression via Western blot, data were normalized to the reference protein GAPDH and to PBS-treated female mice (B). Vascular injury and leukocyte accumulation were determined in CD31/Laminin (C) and CD45 (D) stained tissue sections by quantification of positively stained areas. Leukocyte localization was characterized in high resolution images of CD45/Laminin staining, quantifying the distance of leukocytes to the vasculature in the peri-infarct area (E). Scale bars (A): 250 μm, (C and D): 50 μm, (E): 20 μm. *P<0.05, **P<0.01, ***P<0.001. n=9–12/group in A, C–E, n=7–9/group in B. Dashed lines and gray shadows indicate quantiles of sham-operated animals.
Except of increased inflammatory responses and vascular injury (ie, microglia activation, leukocyte accumulation, basal lamina disruption) in HI-injured males, no differences were detected in DTX-treated wild-type littermate and sham-operated transgenic DEREG mice (Figure S8).
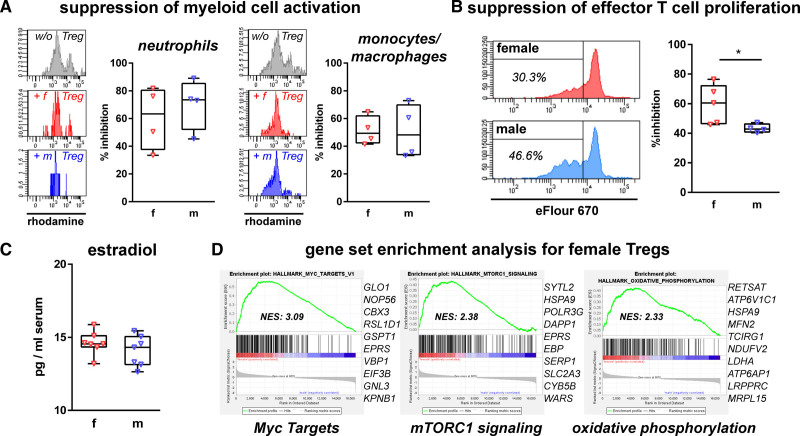
Tregs of Female Mice Display Increased Immunosuppressive Activity
The observed sex-differences in early and late outcome after acute Treg depletion suggested early differences in Treg function. To characterize Treg functionality in the acute disease phase, that is, 24 hours after HI, we compared their inhibitory potential on myeloid cell activation (Figure 4A) and effector T cell proliferation (Figure 4B) demonstrating no sex-differences on myeloid cell activation. However, female Tregs revealed an increased immunosuppressive activity on effector T cell proliferation compared with male Tregs (Figure 4B). According to previous reports in adults, indicating that the female sex hormone estradiol is important for Treg activity,19 we compared serum estradiol levels, which did not differ (Figure 4C). To get first insights into the molecular pathways involved, we performed microarray analysis of ex vivo sorted Tregs. Gene set enrichment analyses in female Treg revealed significant enrichments in the hallmark pathways Myc Targets, mTORC1 signaling and oxidative phosphorylation (Figure 4D; Table S5), which were recently identified as important pathways modulating Treg activity.20,21
Figure 4.
Enhanced immunosuppressive activity in female Tregs is associated with intrinsic transcriptional differences. Tregs were sorted via FACS from spleens of depletion of regulatory T cells transgenic (DEREG) mice 24 h after hypoxia-ischemia (HI) to assess their inhibitory capacity on myeloid cell activation measured by quantification of reactive oxygen species production upon stimulation in the absence and presence of Tregs (A). Suppressive activity of Tregs on effector T cells was measured in proliferation assays (B). Serum estradiol levels were analyzed via ELISA 48 h after HI (C). Sorted splenic Tregs were used for microarray analyses, gene enrichment plots including the 10 leading edge genes of the 3 highest enriched gene sets in females compared with males are shown (C). *P<0.05. n=4–5/group in A and B, n=7/group in C, n=3 samples per group (2 animals pooled per sample) in D.
Sex-Dependent Differences in Treg Function Develop Over Time
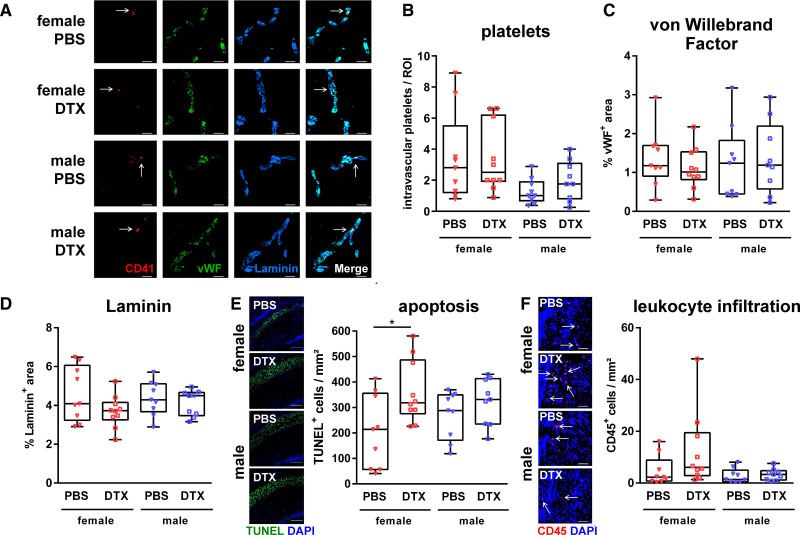
Previous reports in adult ischemic brain injury showed that Tregs contribute to ischemic brain injury via induction of secondary micro-thrombosis.22 According to the early enhanced vessel association of male Tregs (Figure 1D), associated with subacute vascular injury (Figure 3D), we analyzed endothelial (VWF [von Willebrand Factor]) and platelet (glycoprotein IIb/IIa [CD41]) proteins, both involved in the complex cascade of thromboinflammation. Analyses were combined with Laminin staining to quantify vessel-associated platelets and to evaluate the early degree of vascular injury 48 hours after HI, that is, 24 hours after the second DTX injection. (Figure 5A). Neither the number of intravascular platelets nor immunoreactivity of VWF were modulated by Treg depletion independent of sex (Figure 5B and 5C). Of note, in contrast to results obtained 7 days after HI (Figure 3D), early assessment of basal laminar integrity revealed no differences (Figure 5D). Similarly, though Treg depletion in females significantly increased acute apoptotic cell death, no differences were detected in males at this early time point (Figure 5E). Furthermore, except of a trend towards increased leukocyte infiltration, microglia activation and VCAM-1 expression in Treg-depleted females, we detected no significant group differences (Figure 5F, Figure S9).
Figure 5.
Tregs protect from acute hypoxia-ischemia (HI)-induced apoptotic cell death in females but do not modulate early vascular injury and neuroinflammatory responses. P9 depletion of regulatory T cells transgenic mice were exposed to HI. Tregs were depleted by IP injection of 30 ng/g bodyweight DTX at P8 and P10. Analyses were performed 48 hours after HI Characteristics of thromboinflammation and vascular injury were analyzed via immunohistochemistry for CD41, VWF (von Willebrand Factor), and laminin (A, Scale bar: 20 μm, arrows indicate intravascular platelets). Intravascular platelets were counted manually (B). The expression of VWF (C) and degree of vascular injury (D) was assessed by quantification of VWF+ and Laminin+ areas. Acute apoptotic cell death and leukocyte infiltration were analyzed by TUNEL staining (E, scale bar: 200 μm) and CD45 staining (F, scale bar: 100 μm, arrows indicate leukocytes), respectively. *P<0.05. n=9–10/group.
Discussion
Sex differences are increasingly recognized in neonatal HI-induced brain injury. While differences in proinflammatory responses have been discussed to modulate outcome and therapy effects, the impact of sex on immunoregulatory and potentially cerebroprotective cells in neonatal HI was unknown. The main finding of the present study is that female Tregs provide endogenous neuroprotection, whereas male Tregs enhance secondary neurodegeneration. As potential underlying mechanisms, we identified an increased intracerebral infiltration and anti-inflammatory activity of female Tregs, which were associated with intrinsic transcriptional differences. Increased vascular interaction of male Tregs and reduced basal lamina disruption after Treg depletion suggest that male Tregs induce vascular injury, thereby contributing to secondary neurodegeneration.
First clinical and preclinical studies imply an increased risk for males to experience an HI insult and/or to develop more severe brain injury.2 In line with these observations, we detected an increased HI-induced brain injury in males, which was associated with elevated inflammatory responses (ie, microglia activation) 7 but not 2 days after the insult. These data suggest that sex-differences become particularly evident in the phase of secondary neurodegeneration and inflammation. This is supported by Mirza et al,4 who observed worse damage and increased pro-inflammatory cytokine expression in brain tissues of males 3 days but not 1 day after HI. While different innate immune responses are widely discussed to contribute to sex-based differences in outcome and treatment responses,3,5 the impact of sex on cells of the adaptive immune system in neonatal HI was unknown. Here, we show an increased cerebral Treg infiltration in females, which was selective for Tregs. In support of clinical findings,23 Treg number and frequency did not differ in the spleen and blood. However, elevated Treg numbers in female HI-injured brains were associated with increased chemokine receptor expression, which was only observed in the brain. These results may not only explain enhanced intraparenchymal localization of female Tregs but also indicate that female Tregs acquire enhanced migratory capacity during the complex cascade of transmigration through the blood brain barrier. In contrast to neonatal mice, an increased Treg infiltration was observed in aged male mice when compared with females.24 These differences might be explained by different time points of analyses but also emphasize that findings cannot be extrapolated between different ages.
The different Treg infiltration pattern between females and males provides a plausible explanation for the observed increased acute apoptosis in Treg-depleted females 48 hours after HI and a lack of differences in males. However, different amounts of infiltrated Tregs would not explain subacute neuroprotection in males after acute Treg depletion. Previous kinetic studies of depletion efficiency showed a gradual increase of Tregs after 48 hours and full recovery at 72 hours.25 Since Treg recovery was similar in males and females, different outcomes between the early (24 hours after DTX) and the late analysis time point (144 hours after DTX) suggest that male and female Tregs prime different cellular targets in the acute disease phase, thereby modulating secondary injury processes. Indeed, early Treg depletion resulted in long-lasting effects on neurodevelopmental outcome, reflected by improvements of risk assessment/escape behavior, exploratory activity and cognitive function in Treg-depleted males. Nevertheless, these results seem to contradict recent findings in adult males, demonstrating a clear neuroprotective effect associated with reduced neuroinflammatory responses (ie, micro and astrogliosis) and improved white matter integrity.8,10 In addition to different experimental models and developmental stages, differences in experimental design may account for this discrepancy. The aforementioned studies in adult animals particularly focused on the role of Tregs in the chronic disease phase with repetitive DTX injections between 8 and 21 days and tissue analyses at 14 and 21 days after injury.8,10 In the present work, we investigated the role of Tregs in the early injury phase with 2 single DTX injections at 1 day before and 1 day after HI. Further studies with delayed Treg depletion after neonatal HI will be needed.
Being aware about the difficulty to separate cause and consequence, our systematic analyses of different pathophysiological processes provide first evidence for selective cellular targets of female and male Tregs in neonatal HI. The association between increased brain injury and enhanced microglia and endothelial activation as well as elevated leukocyte infiltration in females, imply an increased anti-inflammatory activity of female Tregs compared with male Tregs. In addition to brain cells, other peripheral leukocytes might be target of Tregs thereby contributing to different outcomes. This is supported by the finding of an increased suppression of effector T cell proliferation by female Tregs and an enhanced intraparenchymal infiltration of leukocytes in Treg-depleted females. Diminished vascular injury in Treg-depleted males combined with a strong vessel association of male Tregs indicate that they induce injury to the neurovascular unit through interaction with the vasculature. According to that, previous work in models of adult stroke discovered nonimmunologic detrimental effects of Tregs by inducing secondary micro-thrombosis.22 In the present experimental model, however, we did not detect any differences in the amount of intravascular platelets and VWF immunoreactivity, as markers for thromboinflammation. The different age of animals and the selective analysis of 2 proteins might explain this discrepancy.
Our data suggest intrinsic differences between neonatal female and male Tregs per se. The female sex hormone estradiol has been described to increase Treg cell numbers and function.19 However, in line with previous data,4 estradiol levels did not differ between neonatal females and males, most likely because of prepubertal age. Our first exploratory mRNA expression analyses indicate that Treg function differs between neonatal females and males, which is supported by a pronounced gene enrichment for the mTORC1 and Myc signaling pathways, recently linked to increased Treg activity.20,21 These studies also provided a causal link between these pathways and metabolic activity of Tregs.20,21 Of note, in addition to mTORC1 and Myc signaling, the third most enriched gene set was oxidative phosphorylation, indicating that increased Treg functionality in neonatal females may be attributed to an increased metabolic fitness.21,26 Nevertheless, further analyses should specifically characterize brain-infiltrated Tregs, which was beyond the scope of the present study and is challenging because of small cell numbers, as recently demonstrated in a model of adult brain ischemia.8
While sex differences in treatment and outcomes in adult stroke are often related to steroid hormones, sex-hormone independent differences may also play a role. Our study adds important knowledge to the field, uncovering pronounced intrinsic differences between female and male Treg function in ischemic brain injury. The identification of important molecular pathways provides an ideal starting point for comprehensive proteomic and metabolic analyses to determine sex-specific therapeutic targets, not only for neonatal HI but possibly also for adult stroke.
Article Information
Acknowledgments
We thank M. Rizazad, K. Kempe, S. Luppus, W. Bartosik, S. Senkel, A. Hyla for excellent technical assistance. We thank M. Möllmann and J. Göthert for the possibility to perform flow cytometry measurements, R. Hermann for discussion of microarray data and T. Sparwasser for providing DEREG mice.
Sources of Funding
This work was supported by the Else-Kröner-Fresenius-Stiftung (ELAN), the C.D.-Stiftung, the Karl-Heinz-Frenzen Stiftung and the German Research Foundation (389030878, 405358801, 428668629, all within FOR-2879).
Disclosures
None.
Supplemental Material
Supplemental Methods
Figures S1–S9
Tables S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- DEREG
- depletion of regulatory T cells
- DTX
- diphtheria toxin
- HI
- hypoxia-ischemia
- ICAM-1
- intercellular adhesion molecule-1
- Treg
- regulatory T cell
- VCAM-1
- vascular adhesion molecule-1
- VWF
- von Willebrand Factor
L.B., S.O., and N.L. contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.037537.
For Sources of Funding and Disclosures, see page 389.
Contributor Information
Lucia Beckmann, Email: LuciaBeckmann@gmx.de.
Stefanie Obst, Email: stefanie.obst@uk-essen.de.
Nicole Labusek, Email: nicole.labusek@uk-essen.de.
Hanna Abberger, Email: hanna.abberger@uk-essen.de.
Christian Köster, Email: christian.koester@uk-essen.de.
Ludger Klein-Hitpass, Email: ludger.klein-hitpass@uk-essen.de.
Sven Schumann, Email: sven.schumann@uni-mainz.de.
Christoph Kleinschnitz, Email: christoph.kleinschnitz@uk-essen.de.
Dirk M. Hermann, Email: dirk.hermann@uk-essen.de.
Ursula Felderhoff-Müser, Email: ursula.felderhoff@uk-essen.de.
Ivo Bendix, Email: Ivo.Bendix@uk-essen.de.
Wiebke Hansen, Email: Wiebke.Hansen@uk-essen.de.
References
- 1.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, et al. ; TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788 [DOI] [PubMed] [Google Scholar]
- 2.Charriaut-Marlangue C, Besson VC, Baud O. Sexually dimorphic outcomes after neonatal stroke and hypoxia-ischemia. Int J Mol Sci. 2017;19:E61. doi: 10.3390/ijms19010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villapol S, Faivre V, Joshi P, Moretti R, Besson VC, Charriaut-Marlangue C. Early sex differences in the immune-inflammatory responses to neonatal ischemic stroke. Int J Mol Sci. 2019;20:E3809. doi: 10.3390/ijms20153809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith PLP, Mottahedin A, Svedin P, Mohn CJ, Hagberg H, Ek J, Mallard C. Peripheral myeloid cells contribute to brain injury in male neonatal mice. J Neuroinflammation. 2018;15:301. doi: 10.1186/s12974-018-1344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazmi A, Albertsson AM, Rocha-Ferreira E, Zhang X, Vontell R, Zelco A, Rutherford M, Zhu C, Nilsson G, Mallard C, et al. Lymphocytes Contribute to the Pathophysiology of Neonatal Brain Injury. Front Neurol. 2018;9:159. doi: 10.3389/fneur.2018.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herz J, Köster C, Crasmöller M, Abberger H, Hansen W, Felderhoff-Müser U, Bendix I. Peripheral T cell depletion by FTY720 exacerbates hypoxic-ischemic brain injury in neonatal mice. Front Immunol. 2018;9:1696. doi: 10.3389/fimmu.2018.01696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5 [DOI] [PubMed] [Google Scholar]
- 9.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927 [DOI] [PubMed] [Google Scholar]
- 10.Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. 2021;54:1527–1542.e8. doi: 10.1016/j.immuni.2021.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 13.Goodman WA, Bedoyan SM, Havran HL, Richardson B, Cameron MJ, Pizarro TT. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc Natl Acad Sci USA. 2020;117:17166–17176. doi: 10.1073/pnas.2002266117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasanthakumar A, Chisanga D, Blume J, Gloury R, Britt K, Henstridge DC, Zhan Y, Torres SV, Liene S, Collins N, et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature. 2020;579:581–585. doi: 10.1038/s41586-020-2040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz J, Köster C, Reinboth BS, Dzietko M, Hansen W, Sabir H, van Velthoven C, Bendix I, Felderhoff-Müser U. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav Immun. 2018;70:118–130. doi: 10.1016/j.bbi.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 17.Mülling K, Fischer AJ, Siakaeva E, Richter M, Bordbari S, Spyra I, Köster C, Hermann DM, Gunzer M, Felderhoff-Müser U, et al. Neutrophil dynamics, plasticity and function in acute neurodegeneration following neonatal hypoxia-ischemia. Brain Behav Immun. 2021;92:234–244. doi: 10.1016/j.bbi.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Abel S, Lückheide N, Westendorf AM, Geffers R, Roers A, Müller W, Sparwasser T, Matuschewski K, Buer J, Hansen W. Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J Immunol. 2012;188:5467–5477. doi: 10.4049/jimmunol.1102223 [DOI] [PubMed] [Google Scholar]
- 19.Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol. 2011;8:50–58. doi: 10.1038/cmi.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman NM, Zeng H, Nguyen TM, Wang Y, Vogel P, Dhungana Y, Liu X, Neale G, Locasale JW, Chi H. mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat Commun. 2018;9:2095. doi: 10.1038/s41467-018-04392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saravia J, Zeng H, Dhungana Y, Bastardo Blanco D, Nguyen TM, Chapman NM, Wang Y, Kanneganti A, Liu S, Raynor JL, et al. Homeostasis and transitional activation of regulatory T cells require c-Myc. Sci Adv. 2020;6:eaaw6443. doi: 10.1126/sciadv.aaw6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier FM, Tang ML, Martino D, Saffery R, Carlin J, Jachno K, Ranganathan S, Burgner D, Allen KJ, Vuillermin P, et al. The ontogeny of naïve and regulatory CD4(+) T-cell subsets during the first postnatal year: a cohort study. Clin Transl Immunology. 2015;4:e34. doi: 10.1038/cti.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, D’Aigle J, Blixt FW, Zhu L, Bravo Alegria J, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun. 2020;87:556–567. doi: 10.1016/j.bbi.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinoza Mora MR, Steeg C, Tartz S, Heussler V, Sparwasser T, Link A, Fleischer B, Jacobs T. Depletion of regulatory T cells augments a vaccine-induced T effector cell response against the liver-stage of malaria but fails to increase memory. PLoS One. 2014;9:e104627. doi: 10.1371/journal.pone.0104627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17:618–625. doi: 10.1038/ni.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feather-Schussler DN, Ferguson TS. A Battery of Motor Tests in a Neonatal Mouse Model of Cerebral Palsy. J Vis Exp. 2016;117:e53569. doi: 10.3791/53569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.